Abstract
Background and Aims
Most plant scientists, in contrast to animal scientists, study only half the organism, namely above-ground stems, leaves, flowers and fruits, and neglect below-ground roots. Yet all acknowledge roots are important for anchorage, water and nutrient uptake, and presumably components of yield. This paper investigates the relationship between domestication, and the root systems of landraces, and the parents of early, mid- and late green-revolution bread wheat cultivars. It compares the root system of bread wheat and ‘Veery’-type wheat containing the 1RS translocation from rye.
Methods
Wheat germplasm was grown in large pots in sand culture in replicated experiments. This allowed roots to be washed free to study root characters.
Key Results
The three bread wheat parents of early green-revolution wheats have root biomass less than two-thirds the mean of some landrace wheats. Crossing early green-revolution wheat to an F2 of ‘Norin 10’ and ‘Brevor’, further reduced root biomass in mid-generation semi-dwarf and dwarf wheats. Later-generation semi-dwarf wheats show genetic variation for root biomass, but some exhibit further reduction in root size. This is so for some California and UK wheats. The wheat–rye translocation in ‘Kavkaz’ for the short arm of chromosome 1 (1RS) increased root biomass and branching in cultivars that contained it.
Conclusions
Root size of modern cultivars is small compared with that of landraces. Their root system may be too small for optimum uptake of water and nutrients and maximum grain yield. Optimum root size for grain yield has not been investigated in wheat or most crop plants. Use of 1RS and similar alien translocations may increase root biomass and grain yield significantly in irrigated and rain-fed conditions. Root characters may be integrated into components of yield analysis in wheat. Plant breeders may need to select directly for root characters.
Key words: Root biomass, root branching, unconscious selection, Triticum, Mexican wheat, breeding, ‘Veery’ wheat, wheat–rye translocation, 1RS translocation, components of grain yield
INTRODUCTION
Domestication of plant species by humans in the last 10 000 years proceeded with observation and direct selection of mostly above-ground organs, namely stems, leaves, flowers and fruits. Below-ground organs, such as roots, were little observed, unless the root comprised a food-storage organ that was selected for directly. This general lack of interest in plant root systems, where up to half the plant was neglected, contrasts strongly with animals where the whole organism was observed and selected during domestication. Most plant scientists acknowledge roots are important for anchorage, and water and nutrient uptake from the soil solution. A small number consider the root system important for components of yield analysis (Bazzaz et al., 2000).
Bread wheat (Triticum aestivum L.) is an allohexaploid (2n = 6x = 42) with the genome formula BBAADD. It was formed from hybridization of domesticated tetraploid wheat [T. turgidum L. ssp. dicoccum (Schrank) Thell. genome formula BBAA] with diploid weedy goat grass (Aegilops tauschii Coss. DD) in north-west Iran or Armenia (Dvorak et al., 1998). Triticum turgidum ssp. dicoccum is a domesticated race of wild emmer T. turgidum L. ssp. dicoccoides (Korn. ex Asch. & Graebn.) Thell. native to the fertile crescent of the Near East, and itself an allotetraploid formed from an ancestor of Aegilops speltoides Tausch as female parent (BB) and T. urartu Tum. ex Gandil. as male parent (AA) (Waines and Barnhart, 1992).
The history of the green revolution in wheat in the mid-20th century was outlined by Dr Norman Borlaug (Borlaug, 1968). This internationally important plant breeding programme was conducted largely by selection of above-ground organs. Wheat roots were rarely studied by breeders in the CIMMYT or national programmes. This paper investigates the relationship between domestication and the root system of bread wheat. It looks at root biomass of landraces and of the parent lines hybridized to select early-, mid- and late-generation green-revolution cultivars of bread wheat in Mexico, California and the UK.
This paper also compares root biomass of pure bread wheat and of ‘Veery’-type wheat that contains the 1RS translocation from rye (Secale cereale L.). ‘Veery’ wheats are popular under drought or heat-stressed conditions for, on average, they may produce a 7 % grain yield advantage (Rajaram et al., 1983). They are favoured where grain yield is more important than bread-making quality. Up to 50 % of all wheat cultivars in China may now carry the ‘Veery’ 1RS translocation (Zhou et al., 2003). ‘Veery’ wheats may have the 1RS arm from two sources: the more common 1RS(K) is from ‘Kavkaz’ winter wheat, and the original translocation was selected in Germany from crosses of wheat with a ‘Petkus’ rye for resistance to fungal diseases (Schlegel and Korzun, 1997), while the more recent 1RS(A) arm is from ‘Amigo’ winter wheat and the translocation was selected in Oklahoma from crosses with Brazilian ‘Insave’ rye for aphid resistance (Sebesta and Wood, 1978).
One of the first scientists to excavate and illustrate the root system of bread wheat and rye was Weaver (1926) who noted differences in length of seminal roots and lateral roots between the two species, but did not investigate cultivar differences within a species. Root growth differences among seven cultivars of Canadian spring bread wheat were reported by Hurd (1968) who hybridized parents with large, deep root systems and other desirable characters to select new cultivars with larger root systems and higher grain yield. Mac Key (1973) was the first to observe roots and shoots of F1 hybrid plants of spring tall cultivar ‘Prins’ and tall winter cultivar ‘Starke’ with ‘Norin 10’ and ‘Tom Thumb’ the source of the Rht1, Rht2 and Rht3 alleles used by Borlaug (1968) in his Mexican semi-dwarf wheat breeding programme. Mac Key (1973) noted ‘Prins’ and ‘Starke’ had larger root dry weights than the F1 hybrids with ‘Norin 10’ or ‘Tom Thumb’. He concluded that ‘a tall wheat plant tends to have a deep, and a short wheat plant a shallow, root system’. This raised the possibility that breeding for semi-dwarf stems, controlled by the Rht1 and Rht2 alleles on homoeologous group 4 chromosomes, might condition semi-dwarf root systems that might affect adversely the amount of water and nutrients absorbed by the plant, and hence grain yield. This was investigated in isogenic lines of UK winter wheat by Lupton et al. (1974) and found not to be so. Ehdaie and Waines (1994) in California, and Mirrales et al. (1997) in Argentina, used isogenic lines of the Brazilian spring cultivar ‘Maringa’. The semi-dwarf Rht1 and Rht 2 isolines had larger root biomass than the tall rht line. The dwarf Rht3 isoline with the shortest stems also had the smallest root system. In the semi-dwarf Rht1 and Rht2 isolines, assimilates not used to develop a large shoot system might be diverted to the roots to develop a larger root system. Secondly, Mac Key (1973, table 5) observed large differences in root biomass among old and modern wheats. Old varieties such as German ‘Brown Schlanstedt’ had three times larger root biomass than dwarf Mexican ‘Mayo 64’ or dwarf Japanese ‘Kohnosu 25’ while Brazilian ‘Frontana’ and Mexican ‘Pictic 62’ were intermediate. Troughton and Whittington (1968) using cultivar chromosome substitution lines, observed that differences in root size between tall landrace ‘Chinese Spring’ with large root biomass, and cultivar ‘Hope’, with small root biomass, were largely governed by genetic factor(s) on chromosome 1A, which is a different genetic system from that controlling the Rht stem dwarfing genes on chromosome 4, which have a smaller effect on root size. (See Appendix for a full list of cultivars mentioned in the text, together with brief descriptions.)
Similarly, three old tall landraces from China and Iran had 2- to 4-fold larger root biomass than four semi-dwarf Mexican, Iraqi, Pakistani and California cultivars, all of which were descended from CIMMYT breeding material (Ehdaie et al., 1991; Ehdaie and Waines, 1993, 1997; Ehdaie, 1995). The root biomass differences between the landraces and the CIMMYT-derived modern cultivars were always significantly different. The root size of other CIMMYT-derived bread wheats have since been determined and they are always significantly smaller than drought-tolerant landraces (J. G. Waines and B. Ehdaie, unpubl. res.), though admittedly only a few accessions have been observed. This tends to support the view that direct selection for only above-ground organs might also indirectly select for a small root system, especially under well-irrigated and well-fertilized growing conditions, as at CIMMYT breeding stations, where there would be no selection advantage for a larger root system.
What is lacking in this review of the few published observations on bread wheat root systems is knowledge of what happened, in a historic sense, in the progression from tall landraces to semi-dwarf and dwarf cultivars in the last 100 years. In barley (Hordeum vulgare L.) seedlings, Grando and Cecarelli (1995) noted an initial increase in root size from ten wild barleys to ten landrace barleys, then a marked decrease in root size from landraces to ten modern barley cultivars. Wild collections of bread wheat do not exist, only landraces. As far as we are aware, there is no report in the literature of the root systems of the three spring wheat parents which Borlaug (1968) hybridized to select the first-generation, tall Mexican wheats such as ‘Nainari 60’ or ‘Lerma Rojo’, or the root system of the Japanese two-gene-dwarfed wheat ‘Norin 10’ that contributed the Rht1 and Rht2 stem-dwarfing genes to mid-generation semi-dwarf and dwarf cultivars released by CIMMYT. This research was undertaken to fill this void.
MATERIALS AND METHODS
Kernels of the three spring wheat parental cultivars, namely ‘Gabo’ from Australia, and ‘Marroqui’ and ‘Mentana’ from the Mediterranean region, hybridized by Borlaug (1968) to form early green-revolution bread wheats, and of Japanese, tall ‘Aka Komugi’ were obtained from Dr Harold Bockelman, USDA Small Grains Collection, Aberdeen, ID, USA. Kernels of early Mexican tall ‘Nainari 60’ were obtained from Dr M. D. Gale, John Innes Centre, Norwich, UK, and mid-generation Mexican semi-dwarf ‘Anza’, ‘Pavon 76’ and ‘Yecora Rojo’ cultivars were obtained from Dr C. O. Qualset, Department of Plant Sciences, University of California, Davis. Kernels of late-generation Mexican wheats ‘Bacanora’, ‘Pastor’ and ‘Rayon’ were obtained from CIMMYT. Grains of the Pavon 1RS near isogenic lines, namely ‘Pavon 76’, ‘Pavon 1RS(K).1AL’, ‘Pavon 1RS(K).1BL’ and ‘Pavon 1RS(K).1DL’ (Lukaszewski, 1993, 2000) and other Pavon translocation stocks were donated by Dr A. J. Lukaszewski, University of California, Riverside. Wheat kernels of similar weight were surface sterilized in a 10 % solution of commercial sodium hypochlorite, washed in distilled water, and allowed to germinate in Petri dishes. Seedling plants of similar primary leaf length and seedling root number were transplanted to black plastic pots containing a polyethylene bag with 5 kg washed sand #30 (Ehdaie et al., 2003). A small hole in the bag allowed excess water to drain without sand being lost.
In an early experiment (Table 1; Ehdaie, 1995) plants were watered every 2–3 d and returned to field capacity in a closed sand culture system. CIMMYT semi-dwarf and dwarf wheats had root biomass values between 2·0 g and 2·5 g per plant in the sand-culture experiments, and ‘Anza’ with 2·35 g per plant was intermediate. Root biomass was expressed as a percentage of mid-generation CIMMYT wheat ‘Anza’.
Table 1.
Root biomass of landrace bread wheats from Iran and China, an old Californian cultivar and mid-green revolution cultivars from California, Iraq and Pakistan (data from Ehdaie, 1995, Table 2)
| Genotype | Dwarfing gene | Group | Root biomass (g) | % Anza | Height (cm) |
|---|---|---|---|---|---|
| Iran 49 | rht | Landrace | 6·43a* | 273 | 115 |
| Chinese Spring | rht | Landrace | 4·38b | 186 | 112 |
| Iran 14 | rht | Landrace | 3·35c | 138 | 111 |
| Ramona 50 | ? | CA cultivar | 2·70cd | 114 | 108 |
| Anza | Rht1 | mid-gr† | 2·35d | 100 | 88 |
| Chenab 70 | Rht1 | mid-gr | 2·43cd | 103 | 92 |
| Sholeh | Rht1 | mid-gr | 2·30d | 97 | 100 |
| Yecora Rojo | Rht1/Rht2 | mid-gr | 2·00d | 85 | 76 |
* Means followed by the same letters within a column are not significantly different at P ≤ 0·05, using an LSD test.
† mid-gr = mid-green revolution cultivar.
A second experiment was started in mid-January 2006, with ‘Pavon 76’ as the standard cultivar. UK winter cultivar ‘Zion 19’ requiring vernalization was given cold temperatures of 5 °C and 8 h light, for 6 weeks beginning 1 December 2005. Two ancestors of CIMMYT wheats, namely ‘Norin 10’ and ‘Brevor’ were initially included in the experiment, but their data were later removed because plants were extremely long-day photoperiodic and did not head, in the case of ‘Brevor’ at Riverside, until the end of July 2005, and even then poorly. Both wheats, which contributed the Rht1 and Rht2 stem dwarfing genes, were bred to be grown in northern Japan or in Washington State where summer day lengths may exceed 16 h. The genotypes grown in pots and for which data are reported are listed in Table 2. At physiological maturity, irrigation ceased and plants were allowed to dry. Shoots were separated from roots at the soil surface. Roots were gently washed free of sand and allowed to dry in a forced air oven at 60 °C for 7 d, then weighed.
Table 2.
Root biomass of a Japanese bread wheat landrace, parents of early, mid- and late green revolution cultivars and two modern cultivars from California and Europe
| Genotype | Dwarfing gene | Group | Root biomass (g) | % Pavon 76 | Height (cm) |
|---|---|---|---|---|---|
| Aka Komugi | rht | Landrace | 9·33a* | 186 | 130 |
| Marroqui | rht? | Tunisia | 6·38bcd | 127 | 91 |
| Mentana | rht? | Italy | 6·19bcd | 123 | 122 |
| Gabo | rht? | Australia | 4·00cd | 80 | 90 |
| Nainari 60 | rht | early-gr† | 7·15ab | 142 | 100 |
| Pavon 76 | Rht1 | mid-gr | 5·00bcd | 100 | 76 |
| Bacanora | Rht1 | late-gr | 6·22bc | 124 | 87 |
| Rayon | Rht1 | late-gr | 4·17cd | 83 | 76 |
| Pastor | Rht1 | late-gr | 3·69d | 74 | 70 |
| Summit | Rht1 | late CA | 4·49cd | 90 | 68 |
| Zion 19 | Rht8 | late UK | 4·61cd | 92 | 72 |
* Means followed by the same letters within a column are not significantly different at P ≤ 0·05, using an LSD test.
† early-gr = early-green revolution cultivar; mid-gr = mid-green revolution cultivar; late-gr = late-green revolution cultivar.
In the second experiment (Table 2) plants were watered generally each day as needed and allowed to drain. All root biomass values were expressed as a percentage of ‘Pavon 76’, also a mid-green-revolution wheat similar to ‘Anza’. Further, ‘Pavon 76’ was chosen as the standard CIMMYT cultivar by Dr A. J. Lukaszewski and has replaced landrace ‘Chinese Spring’ as the cytogenetic-stock wheat. Wheat breeders do not like the morphological or quality characters of ‘Chinese Spring’, whereas they do like ‘Pavon 76’, which is a semi-dwarf CIMMYT wheat, of high grain quality that is also cytogenetically tractable. The rye 1RS translocations are in the background of ‘Pavon 76’ wheat.
Root biomass and grain yield of the ‘Pavon 76’ near-isogenic line series that compared bread wheat with 1RS(K) ‘Veery’ wheats, in glasshouse and field experiments, were reported by Ehdaie et al. (2003). Experiments that investigated the effect of the 1RS(K) arm in tetraploid wheat and compared root biomass of ‘Aconchi’ durum and ‘Aconchi 1RS(K).1BL’, and that investigated the effect of different sources of the 1RS arm on root biomass in ‘Pavon 1RS(A).1AL’ and ‘Pavon 1RS(K).1AL’ were reported earlier (Waines et al., 2004; Waines and Ehdaie, 2005).
RESULTS
Mexican wheats
In the first experiment conducted in 1993, the three tall bread wheat landraces, presumed to carry a rht allele, though not necessarily the same one, had root biomass ranging from 3·25 g (Iran 14) to 6·43 g (Iran 49) with a mean root biomass of 4·68 g (Table 1). Plant height for the same genotypes ranged from 111 cm to 115 cm. ‘Ramona 50’, a popular California wheat released before the introduction of Mexican wheats, had root biomass smaller than the landraces, but larger than four mid-generation, green-revolution wheats released in California, Iraq and Pakistan. The cultivar with the smallest root biomass was the two-gene (Rht1/Rht2) dwarf ‘Yecora Rojo’, which also had the shortest stem height at 76 cm.
In the second experiment conducted in 2006 (Table 2) root biomass values were larger than those reported in Table 1, presumably because the plants received twice as much water (25 kg vs. 12·74 kg). However, the same trend was observed. The tall (137 cm) Japanese landrace ‘Aka Komugi’ also had the largest root biomass (9·33 g). The parents of early-generation Mexican wheats had root biomass that ranged from 4·01 g (‘Gabo’) to 6·38 g (‘Marroqui’) with a mean of 5·52 g. Plant height of these lines was similar (119–122 cm). The tall (100 cm) (rht) early-generation Mexican wheat ‘Nainari 60’, with root biomass of 7·15 g was selected from segregating hybrid populations of the three parents, which root biomass, but not plant height, it exceeded. Early-generation Mexican wheats were crossed to segregating F2 lines of ‘Norin 10’ בBrevor’, whose root biomass it was not possible to measure because both cultivars were day-length sensitive for flowering at Riverside. The mid-generation green-revolution wheat, ‘Pavon 76’, that carried the semi-dwarf Rht1 allele had root biomass of 5·01 g and plant height of 87 cm. Late-generation semi-dwarf green-revolution wheats that carried the Rht1 allele had variable root biomass that ranged from ‘Bacanora’ with 6·22 g and 86 cm, to ‘Pastor’ with 3·69 g and 70 cm and a mean root biomass of 4·69 g. A recent California semi-dwarf (Rht1) wheat ‘Summit’ had root biomass of 4·49 g and height of 68 cm, and a recent semi-dwarf UK winter wheat ‘Zion 19’ had root biomass of 4·60 g and height of 72 cm.
‘Veery’ wheats
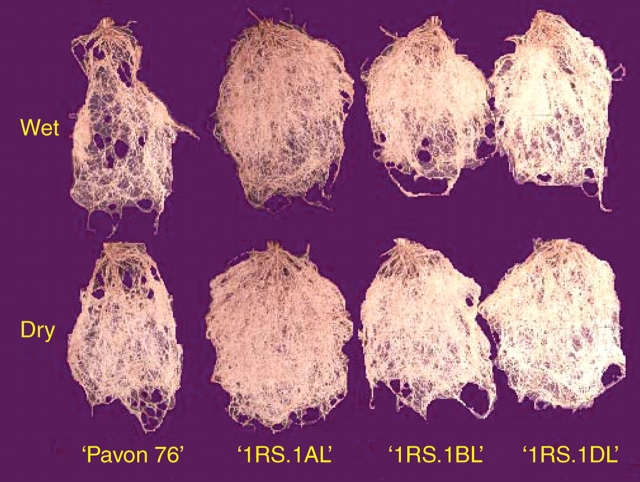
Earlier, experiments were undertaken to elucidate the morphological and/or physiological basis of ‘wide adaptation’ in ‘Veery’-type wheats carrying the 1RS arm translocated for wheat chromosome arm 1BS or 1AS. That this might involve differences in root biomass was supported by use of near-isogenic lines where plants with the 1RS translocation always had larger root biomass and branching in pot cultures (Fig. 1). In this experiment, where pots were weighed every 2–3 d and returned to field capacity, CIMMYT bread wheat ‘Pavon 76’ had root biomass of 2·5 g, while near-isogenic lines for the 1RSK arm had in ‘Pavon 1RS.1AL’ 3·4 g, ‘Pavon 1RS.1BL’ 3·0 g and ‘Pavon 1RS.1DL’ 3·4 g root biomass, respectively, in well-watered pot cultures. Root biomass was similar to results obtained in Table 1. In drought stressed pot cultures root biomass showed a similar trend. In field experiments, grain yield under irrigation was positively associated with root biomass in glasshouse sand cultures (Ehdaie et al., 2003).
Fig. 1.
Root biomass of green-revolution bread wheat ‘Pavon 76’ and its near isogenic rye 1RS translocation lines ‘Pavon 1RS.1AL’, ‘Pavon 1RS.1BL’ and ‘Pavon 1RS.1DL’ grown under well-watered (wet) and drought-stressed (dry) pot sand-culture conditions in a cooled glasshouse.
In another experiment, the root biomass of ‘Aconchi’ durum was 3·17 g per plant whereas that of ‘Aconchi 1RS(K).1BL’ was 4·41 g per plant and showed a 39 % increase (Waines et al., 2004). In a separate experiment, root biomass of ‘Pavon 1RS(A).1AL(A)’ showed a 9 % increase over that of ‘Pavon 76’, while ‘Pavon 1RS(K).1AL(P)’ showed a 31 % increase over the ‘Pavon 76’ control (Waines et al., 2004; Waines and Ehdaie, 2005).
DISCUSSION
In these pot, sand culture studies, tall landrace bread wheats had larger root biomass than mid-green revolution wheats (Tables 1 and 2). These results were confirmed independently using field soil and sand cultures in Indiana by Crowley et al. (2005, 2006) who found ‘Iran 49’ always had more root biomass than ‘Iran 14’, and some local bread wheat cultivars. In contrast, the three parents of Mexican wheat listed by Borlaug (1968) have a mean root biomass of 5·52 g well below landrace ‘Aka Komugi’. ‘Gabo’ from Australia had an especially small root system, relative to the other parents ‘Mentana’ and ‘Marroqui’. CIMMYT's early-generation ‘Nainari 60’ had root biomass (7·15 g) that exceeded its parents.
Crossing the early-generation Mexican wheats with F2 segregants of ‘Norin 10’ and ‘Brevor’ to introduce the stem-dwarfing Rht1 and Rht2 alleles did not increase the root biomass of the mid-generation green-revolution wheat such as ‘Pavon 76’ (Table 2), which has root biomass below the mean of the parents (5·52 g). The results are, by implication, similar in Table 1. Further hybridization and selection of late green-revolution wheats from the CIMMYT programme did introduce some variability in the case of ‘Bacanora’, but ‘Rayon’ and ‘Pastor’ show a continued decrease in root biomass. A similar trend of unconsciously selecting wheats with small root biomass, as a consequence of selection under optimum growing conditions, is evident in two randomly chosen cultivars, modern Californian ‘Summit’ and United Kingdom ‘Zion 19’ bread wheat. As ‘Zion 19’ is a winter wheat, there may have been similar unconscious selection of small root biomass in winter wheat as well as spring wheat.
‘Ramona 50’ bread wheat was released in California in 1950 before the introduction of CIMMYT germplasm. It has a root biomass of 2·70 g per plant. Thus the selection of one cultivar with a small root system occurred in the USA before the introduction of germplasm from the CIMMYT programme in Mexico. Gabo bread wheat from Australia is another example of a cultivar released around 1945 that has a small root system and is adapted to Australia's summer rainfall regime.
One of the first reports of genetic control of cultivar differences for root biomass in bread wheat was by Troughton and Whittington (1968), and Monyo and Whittington (1970) who observed landrace ‘Chinese Spring’ had root biomass twice that of 1930 cultivar ‘Hope’. The difference, discerned by use of cultivar whole-chromosome substitution lines, was controlled by gene(s) on chromosome 1A. The controlling factor(s) were not mapped to chromosome arms, nor does this information appear to have been much used in wheat breeding programmes with the possible exception of that of Hurd (1971) in Canada.
No evidence was found that Borlaug (1968) and the CIMMYT wheat breeding team considered root size or other root characters in the parents or offspring of their breeding programmes in the 1940s through 1970s. In this respect, plant breeding is different from animal breeding where healthy feet and strong legs were observed and selected for in breeding programs throughout animal domestication. Most wheat breeding programmes involve only above-ground plant organs that are readily observed and selected. With a few exceptions (Hurd et al., 1972; Hurd, 1974; Richards and Passioura, 1989) root characters were not observed or selected for. The need to breed for root characters was advocated by Mac Key (1973, 1978) who reported a positive relationship between use of Rht stem dwarfing alleles and small root size. Even though later workers did not confirm this relationship (Lupton et al., 1974; Mirrales et al., 1997), investigation of a possible grain yield advantage by selecting for a larger root system has been slow. Part of the problem was that there are few near-isogenic line series in bread wheat with known differences in root size that might be used to demonstrate the advantage of a larger root size. Another reason may be that many plant scientists do not like to work with roots because root measurements are time consuming and it is difficult to measure root traits for a large number of breeding lines.
One near-isogenic set is material developed at CIMMYT which differs for presence and absence of the ‘Veery 1RS(K).1BL’ translocation in the same genetic background. This translocation from winter wheat ‘Kavkaz’, involves the short arm of chromosome 1 of rye translocated for the short arm of chromosome 1B or 1A of bread wheat, and hence may involve the same genetic system identified by Troughton and Whittington (1968). Field research by Manske and Vlek (2002) at CIMMYT in Mexico was the first to report that wheat genotypes containing 1RS had thinner roots and higher root-length density compared with their 1BS checks. In glasshouse pot studies involving 1RS isolines of ‘Pavon 76’, the lines containing 1RS(K).1AL, 1RS(K).1BL and 1RS(K).1DL always had more root biomass and root branching than ‘Pavon 76’ (Fig. 1; Ehdaie et al., 2003). Root biomass and grain yield were positively correlated under well-watered and droughted pot conditions. In companion field studies, lines containing 1RS also yielded more grain in a well-irrigated treatment, but not in a drought-stressed treatment. The stress tolerance index, calculated from field grain yield and kernel weight, indicated ‘Pavon 76’ isolines for 1RS(K) had greater tolerance to stressful environments than ‘Pavon 76’ (Ehdaie et al., 2003). Based on these pot and field studies those authors concluded the greater adaptability of certain 1RS.1BL translocation lines reported in the literature may be due to their greater root biomass and higher transpiration rate. Root biomass was not investigated in the field experiments. However, grain yield measured in the field experiments was positively correlated with root biomass measured in the pot experiments (Ehdaie and Waines, 2003). If root biomass is increased in the field, these results might be in agreement with the model developed for winter wheat in the UK by King et al. (2003), who predicted more grain yield from a wheat plant with a deep root system that was able to mine a larger soil profile for more water and nutrients than a shallow root system mining a smaller soil profile.
Comparing CIMMYT semi-dwarf durum ‘Aconchi 1BS.1BL’ with the near isogenic durum ‘Aconchi 1RS(K).1BL’ indicated 1RS increased root biomass by 39 % in tetraploid wheat. Hence, the 1RS effect has similar root biomass results in tetraploid and hexaploid wheat. However, the 1RS arm, in ‘Aconchi’ durum background, carries a gene that also conditions partial floret sterility (Waines et al., 2004).
Comparing different sources of the 1RS arm from ‘Kavkaz’ or from ‘Amigo’ in ‘Pavon 76’ bread wheat background, root biomass of ‘Pavon 1RS(A).1AL(A)’ was 9 % more than ‘Pavon 76’, whereas ‘Pavon 1RS(K).1AL(P)’ was 31 % more than ‘Pavon 76’ (Waines et al., 2004). Therefore in ‘Pavon 76’ background, the ‘Kavkaz’ 1RS arm has a 3·4 times larger effect on root biomass than the ‘Amigo’ 1RS arm, which suggests there may be genetic variation for root size within rye.
It was assumed that genotypes containing 1RS that increase root biomass in sand cultures will also increase root biomass in field soils, though not necessarily to the same extent. This is supported by the field results of Manske and Vlek (2002) and the sand pot and field results of Ehdaie et al. (2003). The general increase in grain yield reported in ‘Veery’ wheats containing 1RS may not be attributed solely to increase in root biomass. Research in the UK reports isogenic lines with the 1RS arm may also store more water-soluble carbohydrates in the stems before anthesis and mobilize more water-soluble carbohydrates to grains during grain filling (Shearman et al., 2005). The increased production of water-soluble carbohydrates may be due to increased uptake of water and nutrients, as the model of King et al. (2003) suggests, and/or to increased photosynthesis, with carbohydrates produced before flowering stored in the stem before being re-mobilized after flowering to fill the grains.
This research demonstrated the parental genotypes used to breed early-generation green-revolution wheats have small root biomass in sand cultures, especially ‘Gabo’ from Australia. This may be controlled by genes on homoeologous chromosome group 1, rather than by the Rht dwarfing genes on homoeologous group 4. This interpretation is supported by the F2 segregation data from a cross of tall ‘Chinese Spring’ with large root biomass and dwarf ‘Yecora Rojo’ with small root biomass where plants with small root biomass and tall stems or large roots and dwarf stems were obtained (Ehdaie and Waines, 1993).
Some semi-dwarf mid- and later-generation green-revolution cultivars selected in Mexico, California and the UK also have small root systems in sand cultures. The root systems of some modern bread wheat cultivars appear to be getting smaller or at least not increasing. The reduction in root size began before introduction of green-revolution wheats and may be a general result of domestication and breeding (Mac Key, 1978). In the case of Mexican wheats, this may also be the result of unconscious selection for increased grain yield in irrigated and well-fertilized conditions. Small root systems may account for why some green-revolution wheats perform well in optimum conditions, but poorly in drought and heat-stressed conditions. Alternatively, the parents presently used in crossing blocks may all have similar small root size, and with little available genetic variation, there is no ability to select for a larger root system. There may be reluctance on the part of crop physiologists and breeders to experiment with near-isogenic variation in root size and to investigate its effect on water and fertilizer uptake and grain yield. The lack of root studies contrasts with the many experiments on the effect of near-isogenic variation for shoot height on grain yield in bread wheat.
There has been little characterization of differences in the root systems of wild diploid and tetraploid wheats and their relatives, or of landraces of diploid, tetraploid or hexaploid wheats by germplasm curators. However, this germplasm is recognized as a source of potential drought-adaptive traits for wheat breeding programmes (Reynolds et al., 2007). Also, the possibility of using plant-adaptive mechanism, including genetic variation in wheat root systems, to breed crops for drought and salinity-prone environments has been considered (Reynolds et al., 2005).
In conclusion, presence of 1RSK, from ‘Petkus’ rye, and 1RSA from ‘Insave’ rye, associates with larger root biomass and branching than ‘Pavon 76’ hexaploid wheat. Similarly, presence of the 1RSK.1BL translocation in ‘Aconchi’ increased root biomass over that of ‘Aconchi’ tetraploid wheat. The 1RS arm may increase root biomass in other tetraploid and hexaploid wheats. Grain yield was associated with increased root biomass in the ‘Pavon 76’ near-isogenic lines with or without the 1RS arm. This may open a way to improve root characters, grain yield and ultimately grain quality in bread wheat. If a larger root biomass increases water and nitrogen absorption, this may also suggest a way to reduce nitrate pollution. These results raise the question is the root size of modern wheat large enough for maximum yield? Would more root biomass and branching increase grain yield of pure wheats with semi-dwarf stems in irrigated and rain-fed conditions? Has unconscious selection for small root characters been taken too far in wheat domestication and breeding? The shallow soils reported in Australia, with high boron in the subsoil and largely spring–summer rainfall, suggests not all environments might benefit from a larger root system. However, wheat grown in deeper soils and in other areas such as California and Western Europe, may benefit from a larger root system. To optimize grain yield, it may be necessary to tailor the root system to the soil. At present, root characters are not considered important for components of grain yield analysis in wheat (Donald, 1968), even though a general model for all plants that includes both shoot and root biomass has been published (Bazzaz et al., 2000). Crop physiologists and plant breeders may need to design the complete wheat plant, including roots and shoots, not only the organs observed above ground.
ACKNOWLEDGEMENTS
Funding to pay the Open Access publication charges for this article was provided by the OECD.
APPENDIX
List of species and/or of bread wheat cultivars mentioned in the text, their origin and pertinent comments.
‘Aconchi’: semi-dwarf (Rht1), spring, CIMMYT, durum released in Mexico.
‘Aconchi 1RSK.1BL’: durum ‘Veery’ near-isogenic line.
‘Akakomugi Tall’: very tall (rht) plants in seed of dwarf Japanese ‘Akakomugi’ received from USDA Small-Grains Germplasm Collection, Aberdeen, ID.
‘Amigo’: Oklahoma winter wheat contains 1RS.1AL ‘Veery’ translocation from ‘Insave’ rye.
‘Anza’: semi-dwarf (Rht1) CIMMYT-derived bread wheat released in California and Australia.
‘Bacanora’: semi-dwarf (Rht1), spring, late-generation CIMMYT bread wheat, Mexico.
‘Brevor 51’: Vogel's semi-dwarf, winter long-day responsive cultivar with stiff straw that allowed increased nitrogen rates and higher grain yields in Pacific Northwest, USA.
‘Brown Schlanstedt’: selected from landrace, end of 19th century, Germany.
‘Chenab 70’: semi-dwarf (Rht1) CIMMYT-derived bread wheat released in Pakistan.
‘Chinese Spring’: 1940 selected from Chinese wheat landrace by E. R. Sears.
‘Frontana’: 1945, Brazilian bread wheat cultivar tolerant of soil acidity.
‘Gabo’: 1945, tall bread wheat, Australia.
‘Hope’: 1935, winter wheat cultivar, USA, with small root system relative to ‘Chinese Spring’.
‘Insave’: rye (Secale cereale) cultivar released in Brazil.
‘Iran 14’: tall (rht) selection out of Iranian Khouzistan bread wheat landrace by Ehdaie.
‘Iran 49’: tall (rht) selection out of Iranian Baluchistan bread wheat landrace by Ehdaie.
‘Kavkaz’: Siberian winter bread wheat contains 1RS.1BL ‘Veery’ translocation from ‘Petkus’ rye.
‘Kohnosu 25’: bread wheat cultivar released by the Kohnosu station near Tokyo, Japan.
‘Lerma Rojo 64’: early tall (rht) Mexican spring bread wheat cultivar released by CIMMYT.
‘Maringa’ (IAC-5): 1970, tall Brazilian bread wheat cultivar
‘Marroqui’ (‘Florence Aurore’): Tunisia, spring bread wheat grown in North Africa.
‘Mayo 64’: CIMMYT semi-dwarf bread wheat.
‘Mentana’: 1930, tall, disease-resistant, Italian bread wheat.
‘Nainari 60’: early tall (rht) Mexican spring bread wheat cultivar released by CIMMYT.
‘Norin 10’: dwarf Japanese cultivar, used as source of Rht1 and Rht2 genes in USA after 1946 and Mexican wheat breeding programmes after 1950.
‘Pavon 76’: semi-dwarf (Rht1) spring CIMMYT bread wheat released in Mexico.
‘Pavon 1RSK.1AL’: Veery (Kavkaz) near-isogenic line of ‘Pavon 76’.
‘Pavon 1RSK.1BL’: Veery (Kavkaz) near-isogenic line of ‘Pavon 76’.
‘Pavon 1RSK.1DL’: Veery (Kavkaz) near-isogenic line of ‘Pavon 76’.
‘Pavon 1RSA.1AL’: Veery (Amigo) near-isogenic line of ‘Pavon 76’.
‘Pastor’: semi-dwarf (Rht1) spring, late-generation CIMMYT bread wheat, Mexico.
‘Petkus’: rye (Secale cereale) one of several cultivars released commercially in Germany.
‘Pitic 62’: CIMMYT semi-dwarf bread wheat cultivar.
‘Prins’: tall Swedish spring bread wheat.
‘Ramona 50’: tall (rht) spring bread wheat from California.
‘Rayon’: semi-dwarf (Rht1) spring, late-generation CIMMYT bread wheat, Mexico.
‘Sholeh’: semi-dwarf, CIMMYT-derived bread wheat introduced to Khouzistan from Iraq.
‘Starke’: tall Swedish winter bread wheat.
‘Summit’: modern commercial semi-dwarf spring bread wheat released in California.
‘Tom Thumb’: very dwarf cultivar, source of Rht3 gene. Not used commercially.
‘Yecora Rojo 70’: two-gene dwarf (Rht1, Rht2) CYMMYT spring bread wheat released in Mexico, still grown in southern California/Arizona, with small root biomass.
‘Zion 19’: modern UK commercial semi-dwarf (Rht8) winter bread wheat.
LITERATURE CITED
- Bazzaz FA, Ackerly DD, Reekie EG. Reproductive allocation in plants. In: Fenner M, editor. Seeds: the ecology and regeneration of plant communities. 2nd edn. Wallingford: CAB International; 2000. pp. 1–29. [Google Scholar]
- Borlaug NE. Wheat breeding and its impact on world food supply. In: Finlay KW, Shepherd KW, editors. Proceedings of the 3rd International Wheat Genetics Symposium. Canberra. Sydney: Australian Academy of Sciences/Butterworths; 1968. pp. 1–36. [Google Scholar]
- Crowley NA, Uphause J, Ohm H. Agronomy Abstracts 8–5. Salt Lake City, UT, USA: 2005. Wheat lines with different root volume. 6 November. [Google Scholar]
- Crowley NA, Uphause J, Ohm H. Agronomy Abstracts 10–4. Indianapolis, IN, USA: 2006. Identification of wheat genotypes with large root volume to potentially increase drought tolerance. 12 November. [Google Scholar]
- Donald CM. The design of wheat ideotypes. In: Finlay KW, Shepherd KW, editors. Proceedings of the 3rd International Wheat Genetics Symposium. Sydney, Australia: Butterworths; 1968. pp. 377–387. [Google Scholar]
- Dvorak J, Luo MC, Yang Z-L. Genetic evidence on the origin of Triticum aestivum. In: Damania AB, Valkoun J, Willcox G, Qualset CO, editors. The origins of agriculture and crop domestication. Aleppo, Syria: ICARDA; 1998. pp. 235–251. [Google Scholar]
- Grando S, Cecarelli S. Seminal root morphology and coleoptile length in wild (Hordeum vulgare ssp. spontaneum) and cultivated (H. vulgare ssp. vulgare) barley. Euphytica. 1995;86:73–80. [Google Scholar]
- Ehdaie B. Variation in water-use efficiency and its components in wheat. II. Pot and field experiments. Crop Science. 1995;35:1617–1626. [Google Scholar]
- Ehdaie B, Waines JG. Variation in water-use efficiency and its components in wheat. I. Well-watered pot experiment. Crop Science. 1993;33:294–299. [Google Scholar]
- Ehdaie B, Waines JG. Growth and transpiration efficiency of near isogenic lines for height in a spring wheat. Crop Science. 1994;34:1443–1451. [Google Scholar]
- Ehdaie B, Waines JG. Growth and evapotranspiration efficiency in landrace and dwarf spring wheats. Journal of Genetics and Breeding. 1997;51:201–209. [Google Scholar]
- Ehdaie B, Waines JG. 1RS translocation increases root biomass in Veery-type wheat isogenic lines and associates with grain yield. In: Pogna NE, Romano M, Pogna EA, Galterio G, editors. Proceedings of the 10th International Wheat Genetics Symposium. Paestum, Italy: Rome. ISC; 2003. pp. 693–695. [Google Scholar]
- Ehdaie B, Hall AE, Farquhar GD, Nguyen HT, Waines JG. Water-use efficiency and carbon isotope discrimination in wheat. Crop Science. 1991;31:1282–1288. [Google Scholar]
- Ehdaie B, Whitkus RW, Waines JG. Root biomass, water-use efficiency and performance of wheat–rye translocations of chromosome 1 and 2 in spring bread wheat Pavon. Crop Science. 2003;43:710–717. [Google Scholar]
- Hurd EA. Growth of roots of seven varieties of spring wheat at high and low moisture levels. Agronomy Journal. 1968;60:201–205. [Google Scholar]
- Hurd EA. Can we breed for drought resistance? In: Larson KL, Easton JD, editors. Drought injury and resistance in crops. 1971. pp. 77–88. Crop Science Society Publication no. 2. [Google Scholar]
- Hurd EA. Phenotype and drought tolerance in wheat. Agricultural Meteorology. 1974;14:39–55. [Google Scholar]
- Hurd EA, Townley-Smith TF, Patterson LA, Owen CH. Techniques used in producing Wascana wheat. Canadian Journal of Plant Science. 1972;52:689–691. [Google Scholar]
- King J, Gay A, Sylvester-Bradley R, Bingham I, Foulkes J, Gregory P, et al. Modelling cereal root systems for water and nitrogen capture: towards an economic optimum. Annals of Botany. 2003;91:383–390. doi: 10.1093/aob/mcg033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukaszewski AJ. Reconstruction in wheat of complete chromosome 1B and 1R from 1RS.1BL translocation of Kavkaz origin. Genome. 1993;36:821–824. doi: 10.1139/g93-109. [DOI] [PubMed] [Google Scholar]
- Lukaszewski AJ. Manipulation of the 1RS.1BL translocation in wheat by induced homoeologous recombination. Crop Science. 2000;40:216–225. [Google Scholar]
- Lupton FGH, Oliver RH, Ellis FB. Root and shoot growth in semidwarf and taller winter wheats. Annals of Applied Biology. 1974;77:129–144. [Google Scholar]
- Mac Key J. The wheat root. In: Sears ER, Sears LMS, editors. Proceedings 4th International Wheat Genetics Symposium. Columbia, Missouri, USA: 1973. pp. 827–842. [Google Scholar]
- Mac Key J. Wheat domestication as a shoot : root interrelation process. In: Ramanujam S, editor. Proceedings 5th International Wheat Genetics Symposium. New Delhi: Indian Agricultural Research Institute; 1978. pp. 875–890. [Google Scholar]
- Manske GGB, Vlek PLG. Root architecture-wheat as a model plant. In: Waisel Y, Eshel A, Kafkafi U, editors. Plant roots: the hidden half. 3rd edn. New York, NY: Marcel Dekker; 2002. pp. 249–259. [Google Scholar]
- Mirrales DJ, Slafer GA, Lynch V. Rooting patterns in near isogenic lines of spring wheat for dwarfism. Plant and Soil. 1997;197:79–86. [Google Scholar]
- Monyo JH, Whittington WJ. Genetic analysis of root growth in wheat. Journal of Agricultural Science, Cambridge. 1970;74:329–338. [Google Scholar]
- Rajaram S, Mann CE, Ortiz-Ferrara G, Mujeeb-Kazi A. Adaptation, stability and high yield potential of certain 1B/1R CIMMYT wheats. In: Sakamoto S, editor. Proceedings of the 6th International Wheat Genetics Symposium. Kyoto, Japan: 1983. pp. 613–621. [Google Scholar]
- Reynolds M, Dreccer F, Trethowan R. Drought adaptive traits derived from wheat wild relatives and landraces. Journal of Experimental Botany. 2007;58:177–186. doi: 10.1093/jxb/erl250. [DOI] [PubMed] [Google Scholar]
- Reynolds MP, Mujeeb-Kazi A, Sawkins M. Prospects for utilising plant-adaptive mechanisms to improve wheat and other crops in drought- and salinity-prone environments. Annals of Applied Biology. 2005;146:239–259. [Google Scholar]
- Richards RA, Pasioura JB. A breeding program to reduce the diameter of the major xylem vessel in the seminal roots of wheat and its effect on grain yield in rain-fed environments. Australian Journal of Agricultural Research. 1989;40:943–950. [Google Scholar]
- Schlegel R, Korzun V. About the origin of 1RS.1BL wheat-rye chromosome translocations from Germany. Plant Breeding. 1997;116:537–540. [Google Scholar]
- Sebesta EE, Wood EA. Agronomy Abstracts. Madison, WI: ASA; 1978. Transfer of greenbug resistance from rye to wheat with X-rays; pp. 61–62. [Google Scholar]
- Shearman VJ, Sylvester-Bradley R, Scott RK Foulkes MJ. Physiological processes associated with wheat yield progress in the UK. Crop Science. 2005;45:175–185. [Google Scholar]
- Troughton A, Whittington WJ. The significance of genetic variation in root systems. In: Whittington WJ, editor. Root growth. Proceedings of the 15th Easter School in Agricultural Sciences. New York, NY: University of Nottingham; 1968. pp. 296–314. Plenum. [Google Scholar]
- Waines JG, Barnhart D. Biosystematic research in Aegilops and Triticum. In: Seberg O, Lundquist A, editors. Proceedings of the 1st International Triticeae Symposium. Helsingborg, Sweden: Hereditas Offprint; 1992. pp. 207–212. [Google Scholar]
- Waines JG, Ehdaie B. Optimizing root characters and grain yield in wheat. Czech Journal of Genetics and Breeding; Proceedings of the 5th International Triticeae Symposium; Prague, Czech Republic. 2005. pp. 326–330. [Google Scholar]
- Waines JG, Ehdaie B, Sharma S. Crop Science Society of America Annual Meeting, Poster Abstract 1108. Seattle, WA: 2004. Effect of origin of 1RS translocation on root biomass in wheats. [Google Scholar]
- Weaver JE. Root development of field crops. New York, NY: McGraw-Hill; 1926. [Google Scholar]
- Zhou Y, He ZH, Liu JJ, Liu L. Distribution of 1BL/1RS translocation in Chinese winter wheat and its effect on noodle quality. In: Pogna NE, Romano M, Pogna E, Galterio G, editors. Proceedings 10th International Wheat Genetics Symposium. Paestum Italy: 2003. pp. 1419–1421. [Google Scholar]