Abstract
Background and Aims
Species of the genus Burkholderia, from the Betaproteobacteria, have been isolated from legume nodules, but so far they have only been shown to form symbioses with species of Mimosa, sub-family Mimosoideae. This work investigates whether Burkholderia tuberum strains STM678 (isolated from Aspalathus carnosa) and DUS833 (from Aspalathus callosa) can nodulate species of the South African endemic papilionoid genera Cyclopia (tribe Podalyrieae) and Aspalathus (Crotalarieae) as well as the promiscuous legume Macroptilium atropurpureum (Phaseoleae).
Method
Bacterial strains and the phylogeny of their symbiosis-related (nod) genes were examined via 16S rRNA gene sequencing. Seedlings were grown in liquid culture and inoculated with one of the two strains of B. tuberum or with Sinorhizobium strain NGR 234 (from Lablab purpureus), Mesorhizobium strain DUS835 (from Aspalathus linearis) or Methylobacterium nodulans (from Crotalaria podocarpa). Some nodules, inoculated with green fluorescence protein (GFP)-tagged strains, were examined by light and electron microscopy coupled with immunogold labelling with a Burkholderia-specific antibody. The presence of active nitrogenase was checked by immunolabelling of nitrogenase and by the acetylene reduction assay. B. tuberum STM678 was also tested on a wide range of legumes from all three sub-families.
Key Results
Nodules were not formed on any of the Aspalathus spp. Only B. tuberum nodulated Cyclopia falcata, C. galioides, C. genistoides, C. intermedia and C. pubescens. It also effectively nodulated M. atropurpureum but no other species tested. GFP-expressing inoculant strains were located inside infected cells of C. genistoides, and bacteroids in both Cyclopia spp. and M. atropurpureum were immunogold-labelled with antibodies against Burkholderia and nitrogenase. Nitrogenase activity was also shown using the acetylene reduction assay. This is the first demonstration that a β-rhizobial strain can effectively nodulate papilioinoid legumes.
Conclusions
Papilionoid legumes from widely different tribes can be nodulated by β-rhizobia, forming both indeterminate (Cyclopia) and determinate (Macroptilium) nodules.
Key words: β-rhizobia, nitrogen fixation, fynbos, Macroptilium atropurpureum, ‘Siratro’, Mimosa, Aspalathus
INTRODUCTION
Several species of bacteria in the genus Burkholderia are known to be diazotrophs, particularly those that are isolated from the rhizosphere and endorhizosphere of gramineous plants in the tropics and sub-tropics (Gillis et al., 1995; Estrada de Los Santos et al., 2001; Reis et al., 2004; Perin et al., 2006). Burkholderia strains that contain nodA as well as nifH genes have been isolated from legume nodules (Moulin et al., 2001; Vandamme et al., 2002), and it is now well established that so-called ‘β-rhizobia’ in the genus Burkholderia can nodulate and form effective N2-fixing symbioses with legumes, most particularly those in the large mimosoid genus Mimosa (Chen et al., 2003a, 2005a, b; Barrett and Parker, 2005, 2006; Elliott et al., 2007). Burkholderia strains have also been isolated from nodules on papilionoid legumes (Moulin et al., 2001; Vandamme et al., 2002; Rasolomampianina et al., 2005), as well as from other mimosoid legumes (Abarema macradenia, Pithecellobium hymenaeafolium; Barrett and Parker, 2005), but there has been no evidence presented as yet that they can nodulate the plants from which they were isolated. Indeed, in the case of Burkholderia phymatum STM815, which was originally isolated from nodules on the papilionoid species Machaerium lunatum, Elliott et al. (2007) failed to nodulate Machaerium spp. with this strain, but it was found to be a highly effective nodulator of several Mimosa spp., thus suggesting that this isolate was at most a ‘passenger’ in the Machaerium nodule it was isolated from, and most likely originated instead from a Mimosa nodule in the vicinity. This contention is supported by the nodA gene sequence from B. phymatum, which has high similarity to other Mimosa-nodulating Burkholderia strains (Chen et al., 2005a, b), and also by the subsequent identification of another B. phymatum strain, NGR195A, originally isolated from Mimosa nodules in Papua New Guinea (Elliott et al., 2007). Together with the recent description of Burkholderia mimosarum and Burkholderia nodosa (Chen et al., 2006, 2007), this brings the number of formally described Mimosa-nodulating Burkholderia strains to three, but are there any Burkholderia spp. that can effectively nodulate papilionoid legumes?
The nodA gene of Burkholderia tuberum STM678 is distinct in phylogenetic terms from the Mimosa-nodulating β-rhizobia, including B. mimosarum, B. nodosa, B. phymatum, and Cupriavidus (Ralstonia) taiwanensis (Chen et al., 2003a, 2005a, b), being more similar to that of the alphaproteobacterium Methylobacterium nodulans (isolated from nodules on Crotalaria spp.; Sy et al., 2001a, b) and to Bradyrhizobium (Chen et al., 2003a, 2005a, b). B. tuberum STM678 was originally isolated from the papilionoid legume Aspalathus carnosa, which is native to the seasonally burnt and acid soils supporting the fynbos vegetation of South Africa (Deschodt and Strijdom, 1976; Dakora, 1998; Muofhe and Dakora, 1999; Cocks and Stock, 2001). The present paper describes another strain of B. tuberum, DUS833, which was also isolated from Aspalathus nodules, and tests various Aspalathus spp. for nodulation with both STM678 and DUS833. In view of reports of B. tuberum-like strains isolated from Cyclopia nodules (Kock, 2004; Kock and Steyn, 2004), B. tuberum STM678 and DUS833 were also tested for nodulation on species of this papilionoid genus, which is also endemic to the South African cape region (Spriggs et al., 2003; Spriggs and Dakora, 2007), as well as on Macroptilium atropurpureum, a papilionoid legume well known to be highly promiscuous with regard to microsymbionts (Trinick et al., 1991). Nodulation of Aspalathus and Cyclopia spp. by the two B. tuberum strains was compared with that of Mesorhizobium sp. strain DUS835, Methylobacterium nodulans strain ORS2060 (Sy et al., 2001a) and the broad host-range Sinorhizobium (Ensifer) strain NGR234 (Trinick, 1980; Pueppke and Broughton, 1999).
MATERIALS AND METHODS
Bacterial strains and culture conditions
All bacterial strains used in this study are listed in Table 1. In addition to these wild-type strains, B. tuberum STM678 was transformed using green fluorescent protein (TnGFP) to form STM678GFP as described in Chen et al. (2003b). STM678 was also transformed using the nodDGUS-containing plasmid pRG960SD-32 (Van den Eede et al., 1992) to form STM678nodDGUS using the same method, except that a spontaneous chloramphenicol-resistant STM678 mutant was developed and used as the recipient, with chloramphenicol (34 µg mL−1) and streptomycin (20 µg mL−1) used for final transformant selection.
Table 1.
Bacterial strains used in this study
| Strain | Identification from 16S rRNA gene sequence | Original host | Geographical origin | Source/Reference |
|---|---|---|---|---|
| STM678 | Burkholderia tuberum | Aspalathus carnosa | South Africa | Moulin et al. (2001), Vandamme et al., (2002) |
| DUS833 | Burkholderia tuberum* | Aspalathus callosa | South Africa | Muofhe and Dakora (1998) |
| DUS835 | Mesorhizobium sp.* | Aspalathus linearis | South Africa | Muofhe and Dakora (1998) |
| ORS2060 | Methylobacterium nodulans | Crotalaria podocarpa | Senegal | Sy et al. (2001) |
| NGR234 | Sinorhizobium sp. | Lablab purpureus | Papua New Guinea | Trinick et al. (1980) |
*Previously named Bradyrhizobium aspalati (Boone et al., 1999).
Further identification of strains and phylogeny of symbiosis-related genes
The nearly full-length 16S rRNA gene from DUS833 and DUS835 was amplified and sequenced using primers fD1 (5′-AGAGTTTGATCCTGGCTCAG-3′) and rD1 (5′-AAGGAGGTGATCCAGCC-3′) (Weisburg et al., 1991). Accession numbers for the 16S rRNA gene sequence of DUS833 and DUS835 are EF566975 and EF566978, respectively. For DUS833 and DUS835, a 501-bp fragment of the nodA gene was amplified and sequenced with primers 5′-TGCRGTGGARDCTRYGCTGGGAAA-3′ and 5′-GNCCGTCRTCRAASGTCARGTA-3′ and a 585-bp nifH gene fragment with primers 5′-CGCIWTYTACGGIAARGGIGG-3′ and 5′-GGIKCRTAYTSGATIACIGTCAT-3′ (Chen et al., 2003a). For DUS833 a 461-bp nodC gene fragment was amplified with the reverse primer 5′-CTCAATGTACACARNGCRTA-3′ and the tagged primer 5′-H1-GAYATGGARTAYTGGYT-3′ and sequenced with the tag H1 (5′-GGTTCCACGTAAGCTTCC-3′). Accession numbers for partial nodA and nodC gene sequences of DUS833 are EF566976 and EF566977, respectively, and EF566979 for the nodA gene sequence of DUS835.
Plant species, germination and inoculation
Seeds of five species each of Aspalathus (A. chortophila, A. linearis, A. nivea, A. subtingens, A. teres) and Cyclopia spp. (as listed in Table 2) were obtained from Silver Hills Seeds (Capetown, South Africa) or from B and T World Seeds (Paguignan, France). Macroptilium atropurpureum ‘Siratro’ seeds were obtained from the Australian Tropical Crops and Forages Collection, Biloela, Queensland, Australia. All seeds were germinated by immersion in concentrated H2SO4 for 10 min, then washed five times in sterile distilled H2O and placed onto 1 % water agar and left at room temperature in the dark. Resulting seedlings were grown under sterile conditions in 30-mL tubes filled with modified Jensens N-free plant nutrient medium (Somasegaran and Hoben, 1994), and were inoculated with the appropriate bacterial inoculum as previously described (Chen et al., 2003b). Plants of all species were inoculated with one each of the five wild-type strains (Table 1), with additional plants inoculated with STM678GFP and STM678nodDGUS for microscopical studies. All plants were inoculated (with controls) in quadruplicate on two or more separate occasions, and were grown at 26 °C under Triplus T5 triphosphor plant growth lamps at 339·5 (± 28·2) μE m−2 s−1 with a 12-h daylength.
Table 2.
Effect of inoculation with Burkholderia tuberum strains STM678 and DUS833 on nodulation of Cyclopia spp.
| Host plant species | Inoculant strain | Mean (±s.d.) number of nodules |
|---|---|---|
| Cyclopia falcata | STM678 | 3·3 (±0·9) |
| DUS833 | 0·5 (±0·4) | |
| Cyclopia galioides | STM678 | 5·2 (±1·0) |
| DUS833 | 8·0 (±5·6) | |
| Cyclopia genistoides | STM678 | 5·3 (±0·9) |
| DUS833 | 5·5 (±3·9) | |
| Cyclopia intermedia | STM678 | 1·5 (±0·5) |
| DUS833 | No nodulation | |
| Cyclopia pubescens | STM678 | 3·8 (±0·8) |
| DUS833 | No nodulation |
All data were collected at 60 d after initial inoculation.
A separate inoculation experiment was performed in Taiwan on a wide range of plants from all three sub-families of the Leguminosae as follows: Canavalia gladiata, C. rosea, Chamaecrista mimosoides, Glycine max, Macroptilium atropurpureum, Medicago polymorpha, Mimosa diplotricha, M. pigra, M. pudica, Phaseolus vulgaris, Prosopis africana, Vigna angularis, V. radiata and Sesbania cannabina.
Plant assessment and harvest
Plants were harvested at 40 or 60 d after inoculation (dai). Acetylene reduction assays (ARAs) were conducted on intact plants according to Chen et al. (2003b). Nodules were then harvested for light microscopy, transmission electron microscopy (TEM) and immunogold labelling with antibodies raised against the genus Burkholderia or against the nifH (Fe-) protein according to Chen et al. (2003b, 2005b). A separate experiment was also conducted to examine the infection of Cyclopia genistoides and C. pubescens by B. tuberum STM678GFP and STM678nodDGUS; this involved harvesting the plants at 7 and 15 dai and examining them for signs of infection using light and epifluorescence microscopy, as well as for more detailed studies using confocal laser scanning microscopy (CLSM) and TEM according to Chen et al. (2003b).
RESULTS
The 16S rRNA, nodA and nodC gene sequences of strain DUS833 were identical to Burkholderia tuberum STM678 (100 % homology). The 16S rRNA gene sequence of DUS835 showed highest sequence similarity (>99·9 %) to Mesorhizobium spp. The nodA gene sequence of DUS835 shared 73·8 % DNA identity with those of DUS833, with the most similar sequences within sequence databanks belonging to species of the genera Sinorhizobium and Mesorhizobium.
Burkholderia tuberum STM678 formed nodules with all five species of Cyclopia (Table 2, Fig. 1A–C), whereas DUS833 formed nodules on C. falcata, C. galioides and C. genistoides (Table 2). No nodules formed on any of the five Aspalathus species inoculated with either B. tuberum strain, and all plants subsequently died from apparent N-limitation. None of the other three inoculant strains (DUS835, NGR234, ORS2060) formed nodules on any of the Cyclopia or Aspalathus spp. In the case of C. genistoides, the inoculated plants were green (Fig. 1A) and gave significant ARA activity with both strains of B. tuberum (146·2 ± 72·1 and 140·7 ± 33·8 nmol C2H4 plant−1 h−1 for STM678 and DUS833, respectively). Nitrogenase activity and immunogold labelling of bacteroids of STM678 were also observed in C. galioides and C. pubescens, but the number of effectively nodulated plants was very low, and these had lower ARA values (8·4 and 11·4 nmol C2H4 plant−1 h−1, respectively), and no ARA activity was detected with C. falcata and C. intermedia. Therefore, as nodulation and nitrogen fixation by C. genistoides was more consistent compared with the other Cyclopia species, it was selected for further studies with GFP- and nodDGUS-marked variants of STM678, and the nodules formed by these (Fig. 1C) showed GFP (Fig. 1D, E) and GUS (β-glucuronidase) activity (Fig. 1F), respectively. Macroptilium atropurpureum plants were also green and healthy compared with uninoculated control plants after they had been inoculated with either of the B. tuberum strains, and were clearly nodulated (Fig. 1G), but the plants inoculated with Mesorhizobium sp. strain DUS835 were similar in appearance to the uninoculated controls, even though they were also nodulated (Fig. 1G).
Fig. 1.
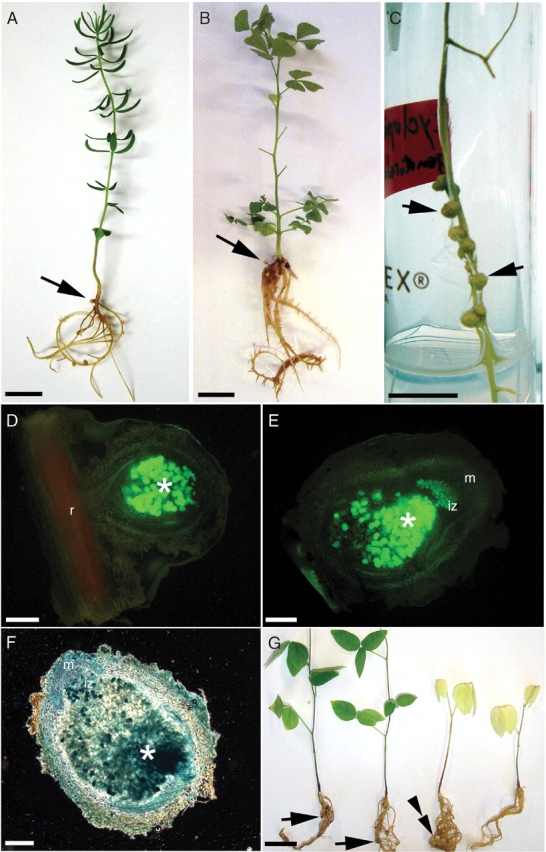
(A) Cyclopia genistoides inoculated with Burkholderia tuberum STM678 and (B) C. galioides inoculated with B. tuberum DUS833. Nodules are indicated by arrows. Nodules (arrows) on a root of C. genistoides 50 d after inoculation with B. tuberum STM678GFP (C). Sections of (D) young and (E) mature nodules of C. genistoides 20 and 50 d after inoculation with B. tuberum STM678GFP viewed under epifluorescence. The central infected tissue of the nodules fluoresces green (*) indicating the presence of the GFP-expressing B. tuberum cells, but the vascular cylinder of the root (r) subtending the young nodule fluoresces red. (E) The mature C. genistoides nodule has a pronounced meristem, thus indicating that it is indeterminate. (F) Section of a mature C. genistoides nodule at 50 d after inoculation with B. tuberum STM678nodDGUS showing high expression of glucuronidase in the infected cells (*), and in the invasion zone (iz) close to the meristem (m). (G) Macroptilium atropurpureum ‘Siratro’ plants which were either inoculated with B. tuberum STM678 (first left), B. tuberum DUS833 (second left), Mesorhizobium sp. DUS835 (third left) or left uninoculated (right). Nodules on the B. tuberum-inoculated plants are indicated by arrows, and the white nodules on the Mesorhizobium-inoculated plant by a double arrowhead. Scale bars = 1 cm (A–C), 100 µm (D), 200 µm (E, F), 2 cm (G).
Light and electron microscopy coupled with immunogold labelling with antibodies against Burkholderia (Chen et al., 2005b) confirmed that the bacteroids in the host cells of C. genistoides nodules formed by either B. tuberum strain were Burkholderia (Fig. 2A–D). Negative control sections incubated in non-immune serum gave little or no signal (Fig. 2E, F). Nodules formed by either B. tuberum STM678 or DUS833 on C. genistoides were effective in appearance and were typically indeterminate with a meristem, invasion zone and nitrogen-fixing zone (Fig. 3A–D), and hence were generally as described by Vasse et al. (1990) for alfalfa (Medicago sativa) nodules. Nodules formed on C. galioides (Figs 1B and 3E), C. pubescens (Fig. 3F) and C. falcata (not shown) by B. tuberum were essentially similar to those formed on C. genistoides. Immunogold labelling with antibodies against the nitrogenase Fe-protein (nifH protein) confirmed that the bacteroids in the host cells of Cyclopia nodules formed by the B. tuberum strains expressed the nitrogenase enzyme complex, for example in C. genistoides (Fig. 3D).
Fig. 2.
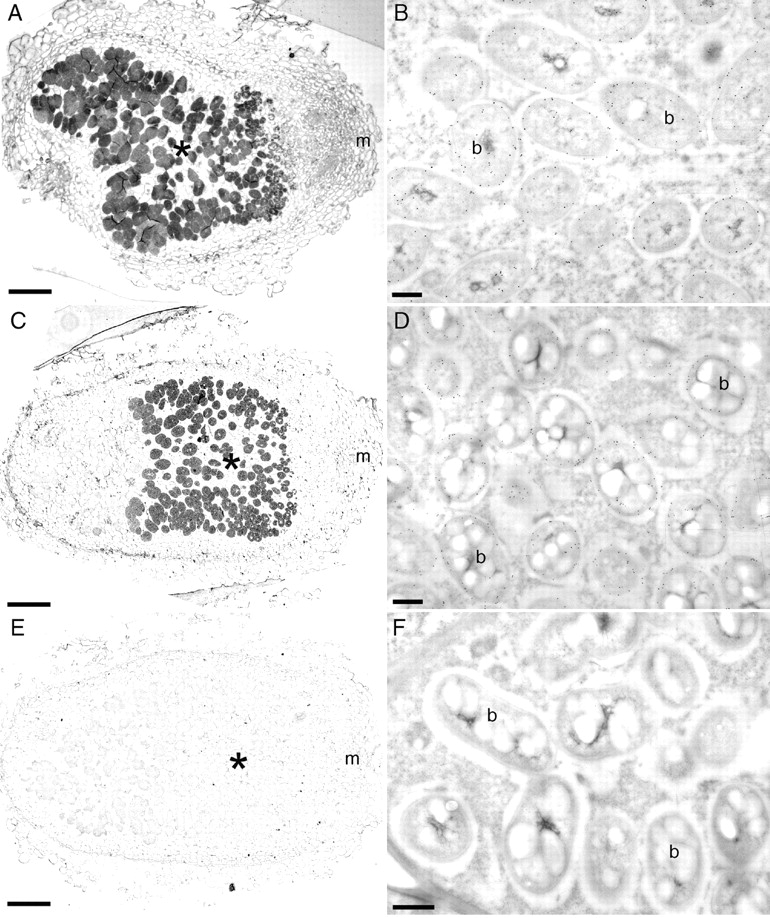
Light microscopy (A, C, E) and transmission electron microscopy (TEM) (B, D, F) of sections of Cyclopia genistoides nodules infected with B. tuberum STM678 (A, B) or DUS833 (C–F). The sections were immunogold labelled with either an antibody raised against B. phymatum STM815 (A–D), or with pre-immune serum (E, F). The infected zone (*) is clearly labelled in A and C, but there is no signal in E. The meristem (m) is indicated at the right-hand side of each of A, C and E. Similarly, bacteroids (b) in B and D are labelled with gold whereas those in F are not. Scale bars = 100 µm (A, C, E), 500 nm (B, D, F).
Fig. 3.
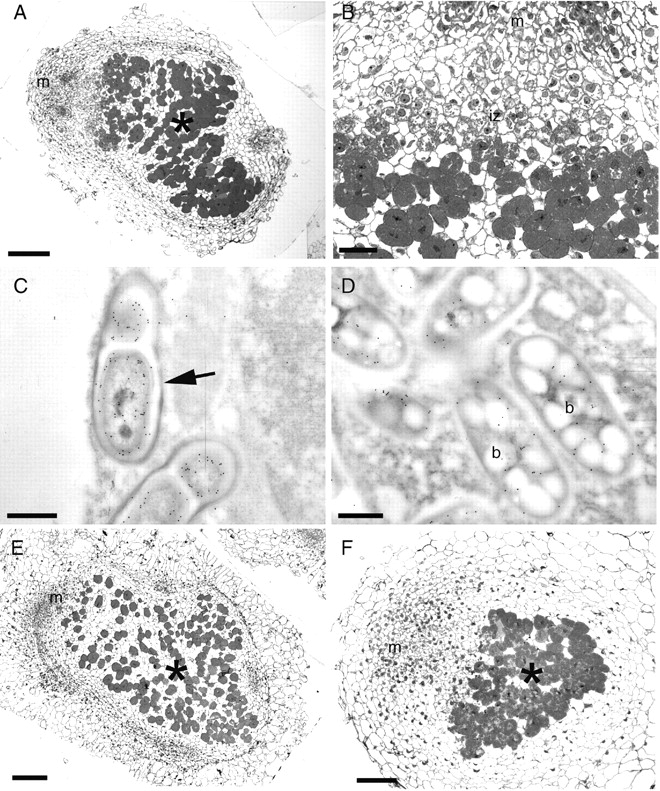
Light microscopy (A, B, D, E) and TEM (C, F) of sections of nodules of Cyclopia genistoides (A–D), C. galioides (E) and C. pubescens (F) infected with B. tuberum STM678. Cyclopia nodules are typically indeterminate with a distinct apical meristem (m), an invasion zone (iz) of newly divided cells being penetrated by infection threads containing rhizobia [which in this case have been immunogold labelled with an antibody raised against B. phymatum STM815 (arrow in C)], and a central zone of infected cells (*) containing bacteroids (b) that express the enzyme nitrogenase, as indicated in D by immunogold labelling with an antibody against the nifH protein. Scale bars = 100 µm (A, E, F), 25 µm (B), 500 nm (C, D).
Macroptilium atropurpureum nodulated by the wild-type, GFP- and GUS-marked variant strains of B. tuberum STM678, and by B. tuberum DUS833, had significant (albeit highly variable) acetylene reduction activities (Table 3), with some of the plants inoculated with the GFP-marked strain having particularly high activity (up to 1181 nmol C2H4 plant−1 h−1). None of the plants nodulated by Mesorhizobium sp. DUS835 had any activity. The M. atropurpureum nodules formed by the B. tuberum strains were effective in appearance and typically determinate (Fig. 4A), with the bacteroids immunogold-labelled with antibodies against Burkholderia (Fig. 4B) and the nitrogenase Fe- (nifH) protein (Fig. 4C). Bacteroids incubated in non-immune serum gave a negligible immunogold signal (Fig. 4D). In contrast to the effective B. tuberum-induced nodules, the ineffective nodules formed by Mesorhizobium sp. DUS835 on M. atropurpureum were white in colour (Fig. 1F) and disorganized in structure (not shown).
Table 3.
Nodulation and nitrogenase (acetylene reduction) activities of Macroptilium atropurpureum inoculated with wild-type and genetically modified variant strains of Burkholderia tuberum STM678, B. tuberum DUS833 and Mesorhizobium sp. DUS835, and nodulation and nitrogenase activities of Phaseolus vulgaris and Vigna angularis inoculated with B. tuberum STM678
| Strain | Number of nodules | Nitrogenase activity (nmol C2H4 plant−1 h−1) |
|---|---|---|
| M. atropurpureum | ||
| STM678 | 18·9 ± 7·0 | 125·6 ± 41·5 |
| STM678gfp | 17·6 ± 4·8 | 444·4 ± 461·8 |
| STM678(pSD32) | 11·6 ± 5·4 | 60·4 ± 73·0 |
| DUS833 | 12·8 ± 3·0 | 45·3 ± 52·4 |
| DUS835 | 9·8 ± 3·4 | 0 |
| Control | 0 | 0 |
| P. vulgaris | 14·6 ± 4·6 | 0 |
| V. angularis | 17·6 ± 5·5 | 0 |
Values are given as mean ± s.d. All data were collected at 40 d after initial inoculation.
Fig. 4.
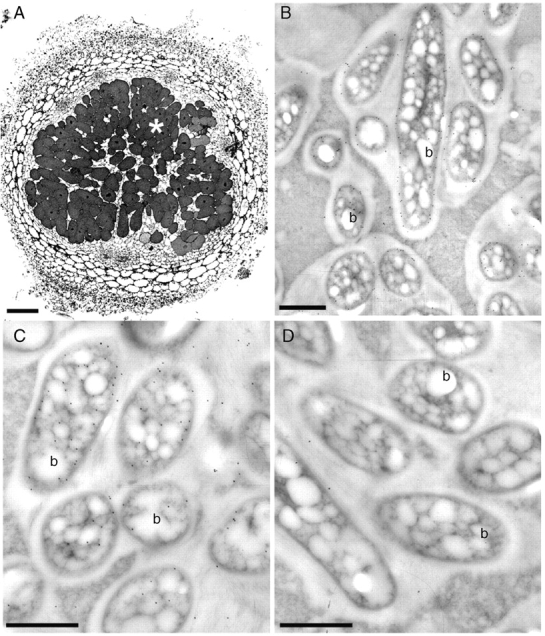
Sections of Macroptilium atropurpureum ‘Siratro’ nodules after inoculation with B. tuberum STM678 viewed under the light microscope (A) or the TEM (B–D). The mature nodule (A) is effective in appearance with densely stained infected cells (*), but it is spherical and without a meristem, and hence is typically determinate. Bacteroids (b) immunogold-labelled with antibodies against either B. phymatum STM815 (B) or the nifH protein (C). Bacteroids in serial sections from the same nodules incubated in pre-immune serum had little or no gold labelling (D). Scale bars = 200 µm (A), 1 µm (B–D).
B. tuberum STM678 failed to nodulate a wider range of legumes, including Canavalia gladiata, C. rosea, Chamaecrista mimosoides, Glycine max, Medicago polymorpha, Mimosa diplotricha, M. pigra, M. pudica, Prosopis africana and Sesbania cannabina, but it could ineffectively nodulate Phaseolus vulgaris and Vigna angularis (Table 3).
DISCUSSION
The 16S rRNA gene sequence of strain DUS833 clearly places it in the species Burkholderia tuberum, thereby doubling the number of strains recorded in this species. Interestingly, several 16S rRNA gene sequences from rhizobia isolated from Cyclopia spp. and deposited by Kock (2004) and Kock and Steyn (2004) also have a very high similarity to B. tuberum STM678 (as well as other Burkholderia spp.) and so there is probably a much larger number of strains of this species, particularly in nodules of Cyclopia spp. native to the fynbos vegetation of South Africa. The nodA and nodC genes of DUS833 were also very similar to those of B. tuberum STM678, as are those of the strains identified by Kock (2004), and the most similar α-rhizobia (in terms of nodA gene sequence) are Methylobacterium nodulans ORS2060, with the next closest being members of the large genus Bradyrhizobium (Chen et al., 2003a, 2005a, b). The 16S rRNA gene sequence of the other DUS isolate, DUS835, was most similar to those of members of the genus Mesorhizobium. Note that both of these strains were originally named as representing ‘Bradyrhizobium aspalati’ (Boone et al., 1999) yet neither isolate proved to be a bradyrhizobium. It is assumed that this nomenclature was adopted in the absence of any identifying sequence data on the preposition that these isolates were similar to those identified using now somewhat outdated methodology and published some years previously (Dakora, 1998).
There is evidence provided by the sequences deposited by Kock (2004) that B. tuberum could nodulate Cyclopia. For example, many of the Cyclopia strains that were shown to be similar to B. tuberum STM678 had been authenticated as being capable of nodulating Cyclopia by an earlier study by A. C. Spriggs and F. D. Dakora (unpubl. res.). However, the present study is the first to show conclusively via ASAs and microscopy that two B. tuberum strains (STM678 and DUS833) can nodulate Cyclopia species effectively, and is the first study to show that B. tuberum can effectively nodulate any legume. Nodules on this genus have previously been reported to be indeterminate (Sprent, 2001), but there has been no report of their structure. Taxonomically the tribe Podalyrieae is considered to be close to the tribes Genisteae and Crotalarieae, both of which appear to have nodules that are not formed following root hair infection and where the infected tissue contains no uninfected cells (Sprent, 2007; Sprent and James, 2007). The internal structure of Cyclopia nodules is common to many legumes, but not in this part of legume phylogeny (van Wyk, 2005). However, arguably the most interesting aspect of nodulation by Cyclopia is that it seems to be so specific in its preference for B. tuberum as a symbiont. For example, even though Sinorhizobium (Ensifer) sp. NGR234 can nodulate a huge range of legumes, including those in the same tribe as Cyclopia (the Podylareae) (Pueppke and Broughton, 1999), it failed to nodulate any of the Cyclopia spp. Small bumps were formed by Methylobacterium nodulans on C. galioides, but they were symbiotically ineffective and disorganized structures (G. N. Elliott, J. I. James and E. K. Sprent, unpubl. res.), and so at present it must be concluded from the results of the present study and that of Kock (2004) that B. tuberum is the dominant symbiont of this genus. Finally with regard to Cyclopia, Sprent (2001) lists only four out of the nine Cyclopia species as nodulated, including C. genistoides, so the reports from the present study of nodulation of C. galioides, C. intermedia and C. pubescens are new, and, together with the recent report of nodulation of C. falcata (syn. C. subternata) by Spriggs and Dakora (2007), brings the total number of confirmed nodulated Cyclopia spp. to eight.
Although Moulin et al. (2001) and Vandamme et al. (2002) have described B. tuberum STM678 as a symbiont of Aspalathus carnosa, the present study failed to demonstrate nodulation of any of the five Aspalathus spp. tested under the growth conditions that allowed for nodulation of Cyclopia spp. and M. atropurpureum. It is possible that these conditions were simply not conducive to nodulation of Aspalathus spp., although the plants grew well until they succumbed to N-limitation, so the conditions were not considered as inherently bad for plant growth. Unfortunately, seeds of A. carnosa, the plant from which STM678 is reported to have been isolated, could not be obtained and so it could not be confirmed if this particular species could be nodulated under the conditions used in the present study. Therefore, the only conclusions that can be reached at present with regard to nodulation of Aspalathus spp. by B. tuberum or by any rhizobial strain, including NGR234 and the DUS strains, is that these conditions may not allow for it. Indeed, the question as to whether B. tuberum can nodulate A. carnosa will not be resolved until it is tested on this species, but such tests will need to be done under sterile conditions similar to those used in the present study, and in comparison with positive control strains known to nodulate Aspalathus spp. Definitive Aspalathus-nodulating rhizobia are uncommon in the literature even though the genus is large (245 species), and it contains 57 known nodulated species (Sprent, 2001), which appear to make a positive contribution to the post-fire N-cycle of the fynbos (Cocks and Stock, 2001). This makes it all the more surprising that, apart from B. tuberum STM678, most isolates from Aspalathus spp. either remain unidentified (Deschodt and Strijdom, 1976; Allen and Allen, 1981) or have been only provisionally described as Bradyrhizobium spp. based solely on their slow growth rates many years previously (Dakora, 1998; Muofhe and Dakora, 1998; Boone et al., 1999).
Unlike the other nodulating species of β-rhizobia so far described (Chen et al., 2001, 2006, 2007; Vandamme et al., 2002), B. tuberum STM678 failed to nodulate any of the three invasive Mimosa spp. This was expected, as nodulation gene sequences were dissimilar from the known Mimosa-nodulators (see Introduction), although experiments are currently underway to determine if selected Mimosa-nodulating β-rhizobia can nodulate Cyclopia spp. (G. N. Elliott, unpubl. res.). B. tuberum STM678 also failed to nodulate a range of legumes in all three sub-families, but could ineffectively nodulate the promiscuous papilionoid legumes Phaseolus vulgaris and Vigna angularis, and effectively nodulate Macroptilium atropurpureum. Indeed, both strains of B. tuberum showed a particular ability to nodulate M. atropurpureum effectively, a species known to be very promiscuous with regard to symbionts (Trinick et al., 1991), but which in spite of its promiscuity could not be nodulated effectively by other β-rhizobia, such as Cupriavidus taiwanensis or B. phymatum (Moulin et al., 2001; Elliott et al., 2007). However, the nitrogenase activity of M. atropurpureum nodulated by the B. tuberum strains was highly variable, ranging from undetectable to over 1000 nmol C2H4 plant−1 h−1 in the case of the GFP-marked STM678, but only up to just over 100 nmol C2H4 plant−1 h−1 for the wild-type and GUS-marked strains. There is no clear explanation for either the variability of the nitrogenase activity by plants inoculated by wild-type STM678 and its genetically modified variants, or the particularly high activity by some of the plants nodulated with the GFP-marked strain compared with those nodulated by the wild-type and GUS-marked strains, but TnGFP insertion position effects on symbiotic nitrogen fixation in the Burkholderia genome are currently being investigated (W-M. Chen, unpubl. res.). However, the high variability in nitrogenase activity of M. atropurpureum nodulated by B. tuberum (by both wild-type and genetically modified strains) may help to explain why the present study demonstrated an effective symbiois between this plant and strain STM678 whereas the earlier study of Moulin et al. (2001) showed an ineffective one.
ACKNOWLEDGEMENTS
Work in Dundee and York was funded by NERC, grant references NE/B505038/1 and NE/B505046/1. Work in Taiwan was funded by the National Science Council, Taipei, Taiwan, Republic of China (NSC 95-2313-B-022-001 and 95-2320-B-022-001-MY2). We thank Alan Prescott and Sam Swift for assistance with confocal microscopy, and Pete Rowell for use of his gas chromatograph. We also thank Bill Broughton, Lionel Moulin and Herman Spaink for bacterial strains.
LITERATURE CITED
- Allen ON, Allen EK. Madison, WI: The University of Wisconsin Press; 1981. The Leguminosae. A source book of characteristics, uses and nodulation. [Google Scholar]
- Barrett CF, Parker MA. Prevalence of Burkholderia sp. nodule symbionts on four mimosoid legumes from Barro Colorado Island, Panama. Systematic and Applied Microbiology. 2005;28:57–65. doi: 10.1016/j.syapm.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Barrett CF, Parker MA. Coexistence of Burkholderia, Cupriavidus, and Rhizobium sp. nodule bacteria on two Mimosa spp. in Costa Rica. Applied and Environmental Microbiology. 2006;72:1198–1206. doi: 10.1128/AEM.72.2.1198-1206.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone CM, Olsthoorn MMA, Dakora FD, Spaink HP, Thomas-Oates JE. Structural characterisation of lipo-chitin oligosaccharides isolated from Bradyrhizobium aspalati, microsymbionts of commercially important South African legumes. Carbohydrates Research. 1999;317:155–163. doi: 10.1016/s0008-6215(99)00083-x. [DOI] [PubMed] [Google Scholar]
- Chen W-M, Laevens S, Lee TM, Coenye T, de Vos P, Mergeay M, Vandamme P. Ralstonia taiwanensis sp. nov., isolated from root nodules of Mimosa species and sputum of a cystic fibrosis patient. International Journal of Systematic and Evolutionary Microbiology. 2001;51:1729–1735. doi: 10.1099/00207713-51-5-1729. [DOI] [PubMed] [Google Scholar]
- Chen W-M, Moulin L, Bontemps C, Vandamme P, Béna G, Boivin-Masson C. Legume symbiotic nitrogen fixation by β-Proteobacteria is widespread in nature. Journal of Bacteriology. 2003;185(a):7266–7272. doi: 10.1128/JB.185.24.7266-7272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W-M, James EK, Prescott AR, Kierans M, Sprent JI. Nodulation of Mimosa spp. by the β-proteobacterium Ralstonia taiwanensis. Molecular Plant–Microbe Interactions. 2003;16(b):1051–1061. doi: 10.1094/MPMI.2003.16.12.1051. [DOI] [PubMed] [Google Scholar]
- Chen W-M, de Faria SM, Straliotto R, Pitard RM, Simões-Araùjo JL, Chou Y-J, et al. Proof that Burkholderia forms effective symbioses with legumes: a study of novel Mimosa-nodulating strains from South America. Applied and Environmental Microbiology. 2005;71(a):7461–7471. doi: 10.1128/AEM.71.11.7461-7471.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W-M, James EK, Chou J-H, Sheu S-Y, Yang S-Z, Sprent JI. Beta-rhizobia from Mimosa pigra, a newly-discovered invasive plant in Taiwan. New Phytologist. 2005;168(b):661–675. doi: 10.1111/j.1469-8137.2005.01533.x. [DOI] [PubMed] [Google Scholar]
- Chen W-M, James EK, Coenye T, Chou J-H, Barrios E, de Faria SM, et al. Burkholderia mimosarum sp. nov., isolated from root nodules of Mimosa spp. from Taiwan and South America. International Journal of Systematic and Evolutionary Microbiology. 2006;56:1847–1851. doi: 10.1099/ijs.0.64325-0. [DOI] [PubMed] [Google Scholar]
- Chen W-M, de Faria SM, James EK, Elliott GN, Lin K-Y, Chou J-H, et al. Burkholderia nodosa sp. nov., isolated from root nodules of the woody Brazilian legumes Mimosa bimucronata and Mimosa scabrella. International Journal of Systematic and Evolutionary Microbiology. 2007;57:1055–1059. doi: 10.1099/ijs.0.64873-0. [DOI] [PubMed] [Google Scholar]
- Cocks MP, Stock WD. Field patterns of nodulation in fifteen Aspalathus species and their ecological role in the fynbos vegetation of southern Africa. Basic Applied Ecology. 2001;2:115–125. [Google Scholar]
- Dakora FD. Nodulation specificity of Aspalathus linearis subsp. linearis, a shrub tea legume indigenous to the Western Cape. In: Elmerich C, Kondorosi A, Newton WE, editors. Biological nitrogen fixation for the 21st century. Dordrecht: Kluwer; 1998. pp. 671–672. [Google Scholar]
- Deschodt CC, Strijdom BW. Effective nodulation of Aspalathus linearis by rhizobia from other Aspalathus species. Phytophylactica. 1976;8:103–104. [Google Scholar]
- Elliott GN, Chen W-M, Chou J-H, Wang H-C, Sheu S-Y, Perin L, et al. Burkholderia phymatum is a highly effective nitrogen-fixing symbiont of Mimosa spp. and fixes nitrogen ex planta. New Phytologist. 2007;173:168–180. doi: 10.1111/j.1469-8137.2006.01894.x. [DOI] [PubMed] [Google Scholar]
- Estrada de Los Santos P, Bustillos-Cristales R, Caballero-Mellado J. Burkholderia, a genus rich in plant-associated nitrogen fixers with wide environmental and geographic distribution. Applied and Environmental Microbiology. 2001;67:2790–2798. doi: 10.1128/AEM.67.6.2790-2798.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis M, Tran Van V, Bardin R, Goor M, Hebbar P, Willems A, et al. Polyphasic taxonomy in the genus Burkholderia leading to an emended description of the genus and proposition of Burkholderia vietnamiensis sp. nov. for N2-fixing isolates from rice in Vietnam. International Journal of Systematic Bacteriology. 1995;45:274–289. [Google Scholar]
- Kock M. South Africa: University of Pretoria; 2004. Diversity of root nodule bacteria associated with Cyclopia species. PhD thesis. [Google Scholar]
- Kock M, Steyn PL. Determination of the diversity of root-nodulating bacteria associated with Cyclopia spp. Proceedings of the 6th European Nitrogen Fixation Conference; Toulouse, France. 2004. Abstract P4·3, 60. [Google Scholar]
- Moulin L, Munive A, Dreyfus B, Boivin-Masson C. Nodulation of legumes by members of the β-subclass of Proteobacteria. Nature. 2001;411:948–950. doi: 10.1038/35082070. [DOI] [PubMed] [Google Scholar]
- Muofhe ML, Dakora FD. Bradyrhizobium species isolated from indigenous legumes of the Western Cape exhibit high tolerance of low pH. In: Elmerich C, Kondorosi A, Newton WE, editors. Biological nitrogen fixation for the 21st century. Dordrecht: Kluwer; 1998. p. 519. [Google Scholar]
- Muofhe ML, Dakora FD. Nitrogen nutrition in nodulated field plants of the shrub tea legume Aspalathus linearis assessed using 15N natural abundance. Plant and Soil. 1999;209:181–186. [Google Scholar]
- Perin L, Martínez-Aguilar L, Castro-González R, Estrada-de los Santos P, Cabellos-Avelar T, Guedes HV, et al. Diazotrophic Burkholderia species associated with field-grown maize and sugarcane. Applied and Environmental Microbiology. 2006;72:3103–3110. doi: 10.1128/AEM.72.5.3103-3110.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pueppke SG, Broughton WJ. Rhizobium sp. strain NGR234 and R. fredii USDA257 share exceptionally broad, nested host ranges. Molecular Plant–Microbe Interactions. 1999;12:293–318. doi: 10.1094/MPMI.1999.12.4.293. [DOI] [PubMed] [Google Scholar]
- Rasolomampianina R, Bailly X, Fetiarison R, Rabevohitra R, Béna G, Ramaroson L, et al. Nitrogen-fixing nodules from rose wood legume trees (Dalbergia spp.) endemic to Madagascar host seven different genera belonging to α- and β-Proteobacteria. Molecular Ecology. 2005;14:4135–4146. doi: 10.1111/j.1365-294X.2005.02730.x. [DOI] [PubMed] [Google Scholar]
- Reis VM, Estrada-de los Santos P, Tenorio-Salgado S, Vogel J, Stoffels M, Guyon S, et al. Burkholderia tropica sp. nov., a novel nitrogen-fixing, plant-associated bacterium. International Journal of Systematic and Evolutionary Microbiology. 2004;54:2155–2162. doi: 10.1099/ijs.0.02879-0. [DOI] [PubMed] [Google Scholar]
- Somasegaran P, Hoben HJ. New York: Springer; 1994. Handbook for rhizobia: methods in legume–rhizobium technology. [Google Scholar]
- Sprent JI. Nodulation in legumes. Kew: Royal Botanic Gardens; 2001. [Google Scholar]
- Sprent JI. Evolving ideas of legume evolution and diversity: a taxonomic perspective on the occurrence of nodulation. New Phytologist. 2007;174:11–25. doi: 10.1111/j.1469-8137.2007.02015.x. [DOI] [PubMed] [Google Scholar]
- Sprent JI, James EK. Legume evolution: where do nodules and mycorrhizas fit in? Plant Physiology. 2007;144:575–581. doi: 10.1104/pp.107.096156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs AC, Dakora FD. Competitive ability of selected Cyclopia Vent. rhizobia under glasshouse and field conditions. Soil Biology Biochemistry. 2007;39:58–67. doi: 10.1186/1471-2180-9-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs AC, Stock WD, Dakora FD. Influence of mycorrhizal associations on foliar δ15N values of legume and non-legume shrubs and trees in the fynbos of South Africa: implications for estimating N2 fixation using the 15N natural abundance method. Plant and Soil. 2003;255:495–502. [Google Scholar]
- Sy A, Giraud E, Jourand P, Garcia N, Willems A, de Lajudie P, et al. Methylotrophic Methylobacterium bacteria nodulate fix nitrogen in symbiosis with legumes. Journal of Bacteriology. 2001;183(a):214–220. doi: 10.1128/JB.183.1.214-220.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sy A, Giraud E, Samba R, de Lajudie P, Gillis M, Dreyfus B. Certaines légumineuses du genre Crotalaria sont spécifiquement nodulées par une nouvelle espèce de. Methylobacterium. Canadian Journal of Microbiology. 2001;47(b):503–508. [PubMed] [Google Scholar]
- Trinick MJ. Relationships amongst the fast-growing rhizobia of Lablab purpureus, Leucaena leucocephala, Mimosa spp., Acacia farnesiana and Sesbania grandiflora and their affinities with other rhizobial groups. Journal of Applied Bacteriology. 1980;49:39–53. [Google Scholar]
- Trinick MJ, Miller M, Hadobas PA. Formation and structure of root nodules induced on Macroptilium atropurpureum inoculated with various species of Rhizobium. Canadian Journal of Botany. 1991;69:1520–1532. [Google Scholar]
- Vandamme P, Goris J, Chen WM, de Vos P, Willems A. Burkholderia tuberum sp. nov. and Burkholderia phymatum sp. nov., nodulate the roots of tropical legumes. Systematic and Applied Microbiology. 2002;25:507–512. doi: 10.1078/07232020260517634. [DOI] [PubMed] [Google Scholar]
- Van den Eede G, Deblaere R, Goethals K, Van Montagu M, Holsters M. Broad host range and promoter selection vectors for bacteria that interact with plants. Molecular Plant–Microbe Interactions. 1992;5:228–234. doi: 10.1094/mpmi-5-228. [DOI] [PubMed] [Google Scholar]
- Vasse J, de Billy F, Camut S, Truchet G. Correlation between ultrastructural differentiation of bacteroids and nitrogen fixation in alfalfa nodules. Journal of Bacteriology. 1990;172:4295–4306. doi: 10.1128/jb.172.8.4295-4306.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. Journal of Bacteriology. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wyk B-E. Podalyrieae. In: Lewis GP, Schrire B, Mackinder B, Lock M, editors. Legumes of the World. Kew: Royal Botanic Gardens; 2005. pp. 267–271. [Google Scholar]


