Abstract
Background
Cotton is the dominant source of natural textile fibre and a significant oil crop. Cotton fibres, produced by certain species in the genus Gossypium, are seed trichomes derived from individual cells of the epidermal layer of the seed coat. Cotton fibre development is delineated into four distinct and overlapping developmental stages: fibre initiation, elongation, secondary wall biosynthesis and maturation.
Scope
Recent advances in gene expression studies are beginning to provide new insights into a better understanding of early events in cotton fibre development. Fibre cell development is a complex process involving many pathways, including various signal transduction and transcriptional regulation components. Several analyses using expressed sequence tags and microarray have identified transcripts that preferentially accumulate during fibre development. These studies, as well as complementation and overexpression experiments using cotton genes in arabidopsis and tobacco, indicate some similar molecular events between trichome development from the leaf epidermis and fibre development from the ovule epidermis. Specifically, MYB transcription factors regulate leaf trichome development in arabidopsis and may regulate seed trichome development in cotton. In addition, transcript profiling and ovule culture experiments both indicate that several phytohormones and other signalling pathways mediate cotton fibre development. Auxin and gibberellins promote early stages of fibre initiation; ethylene- and brassinosteroid-related genes are up-regulated during the fibre elongation phase; and genes associated with calmodulin and calmodulin-binding proteins are up-regulated in fibre initials. Additional genomic data, mutant and functional analyses, and genome mapping studies promise to reveal the critical factors mediating cotton fibre cell development.
Key words: Gossypium, cotton, fibre, polyploid, ovule, phytohormone, auxin, gibberellin, trichome, gene expression
INTRODUCTION
Cotton is an important crop that is widely grown and is used to produce both natural textile fibre and cottonseed oil. Commercial cotton is grown in >80 countries, including Australia, China, Franco-Africa, India, Pakistan, the USA and Uzbekistan. China is the largest user and producer of raw cotton, while the USA is the second largest producer, with the cotton industry contributing about $5 billion per year to the US economy (Agricultural Outlook 2006, http://www.fapri.org/outlook2006/). Cotton fibres can be used for producing innumerable commodities, ranging from textile fabrics and computer screens to automobile brakes. More than 150 countries are involved in import and export of cotton. Economic impact is estimated to be approx. $500 billion year−1 worldwide (National Cotton Council 2006, http://www.cotton.org/).
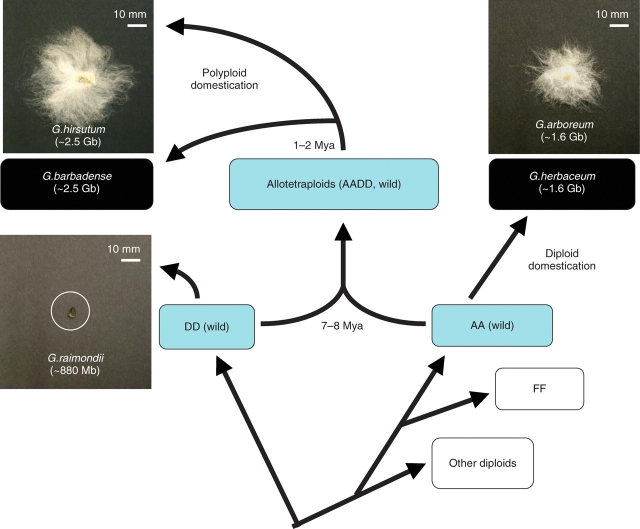
The genus Gossypium occurs naturally throughout tropical and subtropical regions, and includes about 45 species split across two ploidy levels, diploid (2n = 2x = 26) and tetraploid (2n = 4x = 52) (Wendel, 1989; Percival et al., 1999; Wendel and Cronn, 2003). An important event in cotton genome evolution was the spontaneous formation of allopolyploid cotton that has been subsequently selected and domesticated as modern cultivated cotton (Fig. 1). The progenitors of allotetraploid cotton are most closely related to ‘AA’ and ‘DD’ extant diploid species. This polyploidization event occurred approx. 1·5 million years ago (Mya), and the AADD allotetraploids diverged into five species that are distributed throughout the New World and the rest of the globe (Wendel, 1989; Percival et al., 1999; Wendel and Cronn, 2003; Desai et al., 2006). Among the extant diploids resembling the presumed ancestors of tetraploid cotton, the AA progenitor species produce both lint (long) fibres that are spinnable into yarn and shorter fibres called fuzz. In contrast, the DD genome progenitor species produce very few lint fibres that are initiated pre-anthesis, but are much shorter in length than the lint fibres of the AA genome progenitor (Percival et al., 1999; Applequist et al., 2001) (Fig. 1).
Fig. 1.
Evolution of allotetraploid cotton and cotton fibres. Extant diploid progenitors diverge 7–8 millions years ago (Mya), and allotetraploidization occurred naturally 1–2 Mya between a fibre-producing AA-genome extant species and a fibre-poor DD-genome extant species, generating AADD allotetraploid species (Wendel, 1989; Percival et al., 1999; Wendel and Cronn, 2003). Superior fibre yield and quality have been selected in allotetraploid cotton species, as well as the domesticated diploid G. arboreum. There are five allotetraploid species, and two of them, G. hirsutum and G. barbadense, provide >95% of the modern commercial cotton crop. The genome sizes in parentheses are based on published work (Hendrix and Stewart, 2005). Mb, Mega base pairs (106); Gb, gig base pairs (109).
Among the five allotetraploids, upland or American cotton, Gossypium hirsutum, represents over 95 % of annual cotton crop worldwide. Pima or Egyptian cotton, G. barbadense, and Asian cotton (a diploid), G. arboreum, together represent the remaining 5 % (Smith and Cothren, 1999). Interestingly, compared with the AA and DD genome progenitors, the fibre traits in the allotetraploids are dramatically enhanced. The allotetraploids produce more abundant and higher quality fibres than the extant descendant species, suggesting strong selection on polyploid cotton for fibre properties.
Cotton is a model system for the study of cell elongation and cell wall and cellulose biosynthesis (Kim and Triplett, 2001). The fibre is composed of nearly pure cellulose, the largest component of plant biomass. Annual world production of cellulose is approx. 100 million metric tons, primarily in the cell walls of higher plants. The basic study of cellulose biosynthesis in fibre cells is highly pertinent to the applied objectives of renewable resource and bioenergy research.
Cotton fibres are seed trichomes. Cotton fibre development undergoes several distinctive but overlapping steps including fibre initiation, elongation, secondary cell wall biosynthesis, and maturation, leading to mature fibres (Basra and Malik, 1984; Tiwari and Wilkins, 1995; Wilkins and Jernstedt, 1999; Kim and Triplett, 2001) (Fig. 2). In G. hirsutum, lint fibres develop prior to or on the day of anthesis, and fuzz fibres develop a few days later. The process is quasi-synchronized in each developing ovule and among ovules within each ovary (boll). Initiation of fuzz fibre development occurs after initiation of lint fibre development but the timing varies among genotypes (Basra and Malik, 1984).
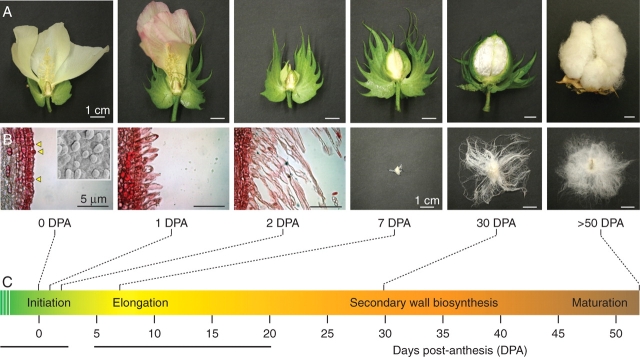
Fig. 2.
Fibre initiation and elongation stages. (A) Cotton boll and fibre development: flowers and bolls at increasing stages of development were partially dissected to show ovules. All scale bars = 1 cm. (B) Fibre development is shown over developmental time. Ovules collected on 0–2 DPA were sectioned, mounted, and stained with safranin O. The ovule epidermis is shown. Yellow triangles indicate emerging 0 DPA fibre. The inset in the 0 DPA fibre panel shows a scanning electron microscope image of the 0 DPA ovule epidermis at 500× magnification as previously described (Lee et al., 2006). Scale bars = 5 µm. Later-stage ovules were isolated and a single fibre-bearing ovule at each stage was photographed. Scale bars = 1 cm. (C) The partially overlapping stages of fibre development include initiation, elongation and secondary wall biosynthesis, and maturation. The initiation and elongation stages are underlined.
Fibre cell initials usually emerge on the day of anthesis [0 d post-anthesis (DPA); see Table 1 for a list of abbreviations used]. Therefore, the chronology of fibre cell development is conventionally monitored relative to the number of DPA (a negative number indicates days before fibre cell emergence). Morphologically distinct fibre cell initials continue to grow rapidly without cell division for 16–25 d during the phase of fibre cell elongation and the biosynthesis of secondary wall cellulose (Basra and Malik, 1984; Tiwari and Wilkins, 1995; Wilkins and Jernstedt, 1999; Kim and Triplett, 2001; Haigler et al., 2005). The elongated fibre cells may reach lengths of nearly 6 cm, or one-third the height of an arabidopsis plant (Kim and Triplett, 2001). Finally, fibre cells mature from 50 to 60 DPA when cotton bolls open, and the long and mature (lint) fibres can be detached from the seeds. Both fibre and seed can be used for industrial applications (Basra and Malik, 1984).
Table 1.
List of common abbreviations used in the text
| ABA | Abscisic acid |
| ACC | 1-Aminocyclopropane-1-carboxylic acid |
| BL | Brassinolide |
| BR | Brassinosteroid |
| BRZ | Brassinazole 2001 |
| DPA | Days post-anthesis |
| EST | Expressed sequence tag |
| GA | Gibberellic acid |
| IAA | Indole-3-acetic acid |
The molecular basis of the fibre initiation stage remains largely mysterious. About 15–25 % of the epidermal layer cells differentiates and develops lint fibres (Basra and Malik, 1984; Tiwari and Wilkins, 1995; Kim and Triplett, 2001). Fibre cell initiation process is rapid and quasi-synchronous. Cell fate determination undoubtedly precedes the formation of morphologically visible fibre cell initials. Phytohormone treatments from 2–3 d pre-anthesis to the day of anthesis induce fibre production on cultured ovules, but few fibres are produced if phytohormones are applied after the day of anthesis (Graves and Stewart, 1988). The fibre elongation phase is perhaps the best-studied period of fibre development. The temporal boundaries separating fibre elongation from the prior initiation phase and the subsequent maturation phase are not discrete, but elongation is generally defined to be from 5 to 20 DPA (Fig. 2).
Following many studies involving gene cloning and expression, recent work using large-scale characterization of expressed sequence tags (ESTs) and gene expression microarrays has documented some trends coincident with the biological process of fibre cell development. In this review, we focus on recent advances in understanding the early events of cotton fibre cell development, including fibre cell initiation and elongation.
COTTON SEED TRICHOMES AND ARABIDOPSIS LEAF TRICHOMES
Cotton fibres are classified as seed trichomes, which share many similarities with leaf trichomes. The models learned from cell fate determination and elongation in arabidopsis leaf trichomes may provide a framework for understanding fibre cell initiation and elongation in cotton (Wang et al., 2004). In spite of striking similarities between two cell types, arabidopsis leaf trichomes are branched (Hülskamp et al., 1994; Marks, 1997; Hülskamp and Schnittger, 1998; Hülskamp, 2004), whereas cotton seed fibres are unbranched. Therefore, putative cotton genes identified in the stages of branch formation and growth directionality may have different functions in cotton fibre development. Moreover, endoreduplication is common in arabidopsis leaf trichomes but has inconsistent results in cotton fibres (Van't Hof, 1999; Taliercio et al., 2005).
Many leaf trichome mutants are available in arabidopsis (Hülskamp, 2004) and have been extensively used for the study of cell fate determination (Marks, 1997) (Table 2). Arabidopsis trichomes are initiated by a ‘trichome promoting complex’ that consists of GLABROUS1 (GL1), TRANSPARENT TESTA GLABRA (TTG) and GL3 (Szymanski et al., 2000). Shoot or leaf epidermal cells containing this complex induce GL2 expression and develop trichomes, whereas the neighbouring cells lacking this complex fail to initiate trichomes and become ‘spacer’ cells between trichomes (Szymanski et al., 2000; Hülskamp, 2004). The physiological changes in arabidopsis leaf trichome development include DNA endoreduplication, rapid growth, and branch formation (Hülskamp, 2004; Schellmann and Hülskamp, 2005).
Table 2.
Arabidopsis trichome-related genes and their putative cotton orthologues
| Trichome developmental stage | Arabidopsis gene | Putative cotton orthologue | E-value* |
|---|---|---|---|
| Pattern formation | GL1 | NP869199 | 1e-60 |
| MYB23 | TC78581 | 7e-64 | |
| TTG1 | TC60020 | 1e-177 | |
| GL3 | TC71002, TC77343 | 1e-152, 1e-141 | |
| EGL3 | TC71002, TC77343 | 1e-162, 1e-149 | |
| TRY | TC72522 | 6e-14 | |
| CPC | TC72522 | 3e-15 | |
| ETC1 | TC80190 | 1e-11 | |
| ETC2 | TC72522 | 1e-11 | |
| GL2 | TC74707 | 0 | |
| Endoreduplication | SIM | N/A | N/A |
| KAK | TC74666 | 9e-28 | |
| SPY | CO122426 | 1e-129 | |
| CPR5 | TC71602 | 8e-13 | |
| ICK/KRP | TC79148 | 5e-6 | |
| RHL2 | TC69621 | e-130 | |
| HYP6 | TC65874 | 4e-40 | |
| Branch formation | AN | TC71768 | 1e-164 |
| ST1 | DN804424 | 4e-66 | |
| FRC | TC76881 | 0 | |
| TFCA | TC75687 | 7e-61 | |
| TFCC | TC78431 | 1e-109 | |
| ZWI | TC70950 | 0 | |
| Growth directionality | GRL | N/A | N/A |
| KLK | TC62633 | 1e-15 | |
| BRICK1 | TC77933 | 7e-34 | |
| ROP2 | TC67109 | 1e-114 | |
| DIS1 | DT561655 | 1e-105 | |
| DIS2 | TC68347 | 1e-134 | |
| WRM | TC61308 | 0 | |
| CRK | TC74982 | 0 |
N/A, Not available.
* E-value was estimated using tBLAST against the Cotton Gene Index CGI 8 (http://compbio.dfci.harvard.edu/tgi/cgi-bin/tgi/gimain.pl?gudb=cotton).
MYB transcription factors
GL1 is a well-characterized MYB transcription factor required for leaf trichome initiation in arabidopsis (Larkin et al., 1993, 1994). GL1 is highly expressed in developing trichomes, and the gl1 mutation results in a trichome-less or glabrous phenotype (Larkin et al., 1993; Serna and Martin, 2006). GL1 and TRIPTYCHON (TRY) synergistically promote endoreduplication in trichome cells (Szymanski and Marks, 1998). Overexpression of a cotton cDNA (L04497) encoding a putative MYB transcription factor GL1 in tobacco produces supernumerary epidermal trichomes on cotyledons and other organs (Payne et al., 1999), suggesting that cotton MYB transcription factors can influence leaf trichome initiation.
Expression studies of six MYB-related genes in G. hirsutum allotetraploids indicate that GhMYB4 and GhMYB6 display ovule-enriched expression patterns, and GhMYB6 expression levels are high in fibres (Loguercio et al., 1999). Another gene, GhMYB109 that encodes an R2R3 MYB-like transcription factor, is expressed specifically in fibre initials and elongating fibres (Suo et al., 2003). In fibre-bearing diploid species G. arboreum L. (AA), a transcript GaMYB2, encoding a putative MYB transcription factor, is predominantly expressed early in fibre development (Wang et al., 2004). Interestingly, overexpression of GaMYB2 complemented the arabidopsis gl1 phenotype, suggesting a role for cotton MYB-like transcription factors in the development of leaf trichomes. Observation of a single seed trichome in arabidopsis transgenic plants suggests that other associated factors are required for producing seed trichomes.
The cotton MYB gene GhMYB25 is expressed early during lint fibre initiation in cotton (Wu et al., 2006). GhMYB25 is similar to Antirrhinum AmMIXTA, a putative R2R3 MYB protein. Overexpression of GhMYB25 increases branches of leaf trichomes in transgenic tobacco, further suggesting a link between cotton fibre initiation and leaf trichome development (Wu et al., 2006). Using laser-capture microdissection, Wu et al. (2006) further demonstrated that MYB25 mRNA is enriched in the fibre initial cells relative to the non-fibre ovular epidermal cells. Expression of MYB25 in young fibre cells has been independently uncovered in another experiment using oligo-gene microarrays (Lee et al., 2006).
Other trichome-related genes
In addition to GL1, trichome development in arabidopsis leaves is mediated through several positive and negative regulators such as TTG1, GL3, TRY, CAPRICE (CPC), and GL2 (Hülskamp, 2004; Schellmann and Hülskamp, 2005). TTG1 and GL3 are considered to be positive regulators along with GL1, because mutation in any of these genes reduces the number of trichomes (Schellmann and Hülskamp, 2005). GL3 has some functional redundancy with its close homologue ENHANCER OF GLABRA3 (EGL3), and mutation in GL3 alone causes smaller and less-branched trichomes (Esch et al., 2003). Sequence analyses have identified putative cotton orthologues to some of the arabidopsis genes (Table 2). Two cotton genes encoding arabidopsis CPC orthologues were identified in the ESTs derived from fibre initials at 1 DPA, and one of them is down-regulated in fibre initials at 1 DPA (Taliercio and Boykin, 2007), suggesting a negative role for putative cotton CPC orthologues in fibre development, which is reminiscent of the negative role of arabidopsis CPC in the differentiation of leaf trichomes (Schellmann et al., 2002). The functions of these putative cotton genes (Table 2) in cotton fibre development should be tested further.
GL2 is expressed in trichomes and encodes a homeodomain transcription factor (Rerie et al., 1994; Pesch and Hülskamp, 2004). Genetic tests indicate that GL2 acts downstream of TTG1 and GL1 because gl2 gl1 and gl2 ttg double mutants lack trichomes, whereas plants with only the gl2 mutation initiate trichomes normally (Hülskamp et al., 1994; Hülskamp, 2004). In arabidopsis, TTG1 functions in trichome formation and trichome spacing through lateral inhibition of differentiation in neighbouring epidermal cells. Four tetraploid cotton genes homologous to arabidopsis TTG1 have been identified. Arabidopsis mutant is rescued by a cotton orthologue, suggesting that equivalent TTG1 functionality mediates both arabidopsis leaf trichome and cotton fibre development (Humphries et al., 2005).
FIDDLEHEAD (FDH) encodes an enzyme involved in the synthesis of long-chain lipids found in the cuticle (Lolle et al., 1992; Pruitt et al., 2000), and mutations in FDH suppress epidermal cell interactions in arabidopsis and display a deleterious effect on trichome development (Yephremov et al., 1999). In cotton, the FDH-like gene is highly expressed in developing fibres (Li et al., 2002) and is repressed in the fibreless naked seed mutant (N1N1), suggesting that down-regulation of the FDH-like gene in the N1N1 background might be associated with abnormal development of fibre cell initials (Lee et al., 2006).
ADDITIONAL GENES INVOLVED IN FIBRE CELL INITIATION
Recent studies have uncovered a set of genes that may regulate fibre cell initiation (Table 3). A cotton sucrose synthase gene (SuSy) plays a role in carbohydrate partitioning and ovule development (Hendrix, 1990). At 1 DPA, SuSy protein is immunolocalized to the basal area of epidermal layer cells, coincident with the high accumulation level of SuSy mRNAs. This localization pattern indicates a close relationship between SuSy and fibre cell differentiation (Notle et al., 1995). Comparative analysis of a fibreless (fl, fibre cell defective) mutant and wild-type cotton shows that SuSy mRNA was abundant in the fibre cell initials of normal ovules relative to that of mutant ovules (Ruan and Chourey, 1998). Furthermore, down-regulation of SuSy mRNA levels in the ovular epidermis by RNAi is associated with adverse phenotypes, including impairment of fibre initiation, fibre elongation and seed development (Ruan et al., 2001, 2003).
Table 3.
Selected genes that are shown to be associated with fibre initiation and elongation
| Fibre developmental stages | Genes | Accession | Annotation | References |
|---|---|---|---|---|
| Fibre initiation | GaMYB2 | AY626160 | G. arboreum myb family transcription factor 2/fibre factor 1 | Wang et al., 2004 |
| GaRDL1 | AY641990 | G. arboreum dehydration-induced protein RD22-like protein 1 | Wang et al., 2004 | |
| PDF1 | AF141375 | Protodermal factor 1 (A. thaliana) | Lee et al., 2006 | |
| GhMYB25 | AF336283 | GhMYB25 transcription factor | Lee et al., 2006; Wu et al., 2006; Wu et al., 2007 | |
| GhHD1 | AY464063 | Homeodomain-leucine zipper transcription factor | Wu et al., 2006; Wu et al., 2007 | |
| ON035C9* | AY464064 | Putative cell wall protein | Wu et al., 2006; Wu et al., 2007 | |
| ON035N9* | AY464065 | Fatty acid elongase | Wu et al., 2007 | |
| ON033M19* | AY464062 | Lipid transfer protein | Wu et al., 2007 | |
| ON035N6* | N/A | Small glycine rich protein | Wu et al., 2007 | |
| Fibre initiation and elongation | GhMYB1 | L04497 | MYB1 | Loguercio et al., 1999 |
| GhMYB2-6 | AF034130, AF034131, AF034132, AF034133, AF034134 | MYB-like DNA-binding domain proteins | Loguercio et al., 1999; Taliercio and Boykin, 2007 | |
| GhMyb109 | AJ549758 | MYB109 transcription factor | Suo et al., 2003; Taliercio and Boykin, 2007 | |
| GhRDL | AY072821 | Dehydration-induced protein RD22-like protein | Li et al., 2002; Lee et al., 2006, Taliercio and Boykin, 2007 | |
| GhACY | AY072824 | Acyltransferase-like protein | Li et al., 2002; Taliercio and Boykin, 2007 | |
| GhFDH | AY072823 | Fiddlehead-like protein | Li et al., 2002; Lee et al., 2006; Taliercio and Boykin, 2007 | |
| GhSCP | AY072822 | Putative serine carboxypeptidase precursor | Li et al., 2002; Taliercio and Boykin, 2007 | |
| GhTUA6 | BM356394 | Tubulin alpha-2/alpha-4 chain (A. thaliana) | Li et al., 2002 | |
| GhTUB1 | BM356393 | Beta tubulin 1 (Lupinus albus) | Li et al., 2002; Taliercio and Boykin, 2007 | |
| GhCesA-5 | BM356396 | Cellulose synthase catalytic subunit (RWS1) (A. thaliana) | Li et al., 2002 | |
| GhACT | BM356395 | Actin 2/7 (A. thaliana) | Li et al., 2002 | |
| GhSMT | BM356397 | Sterol-C-methyltransferase | Li et al., 2002 | |
| GhSuSy | U73588 | Sucrose synthase | Notle et al., 1995; Ruan and Chourey, 1998; Ruan et al., 2003 | |
| Contig13* | N/A | Homeobox1 (Picea abies) | Taliercio and Boykin, 2007 | |
| Contig15340* | N/A | Calmodulin (Nicotiana attenuata) | Taliercio and Boykin, 2007 | |
| Contig10804* | N/A | ER lumen protein retaining receptor | Taliercio and Boykin, 2007 | |
| Fibre elongation | GhSUT1 | AF191025 | Alonsoa meridionalis sucrose transporter 1 | Ruan et al., 2001 |
| GhKT1 | AJ224961 | Ricinus communis sucrose carrier | Ruan et al., 2001 | |
| GhEXP1 | AF512539 | Alpha-expansin precursor | Ruan et al., 2001 | |
| GhWBC1 | AY255521 | ABC transporter | Zhu et al., 2003 | |
| GhKCBP | AY216263 | Kinesin-like calmodulin binding protein | Ji et al., 2003 | |
| GhABP | AY189968 | Auxin binding protein | Ji et al., 2003 | |
| GhMAPK | AY207316 | MAP kinase-like protein | Ji et al., 2003 | |
| GhRac1 | AF165925 | RAC-like G-protein Rac 1 | Kim and Triplett, 2004 | |
| GhKCH1 | AY695833 | Kinesin (KCH1) | Preuss et al., 2004 | |
| GhACT1 | AY305723 | Actin (ACT1) | Li et al., 2005 | |
| GhPFN1 | AI729533 | Similar to profilin (Ricinus communis) | Wang et al., 2005 | |
| GhGlcAT1 | AY346330 | Glycuronosyltransferase-like protein | Wu and Liu, 2005 | |
| GhCER6 | DQ122189 | 3-ketoacyl-CoA synthase | Qin et al., 2007 | |
| GhEF1A4 | DQ174250, DQ174251, DQ174253, DQ174254, DQ174258 | Translation elongation factor 1A-1, 2, 4, 5 and 9, respectively | Xu et al., 2007 | |
| GhCER6 | DQ122189 | 3-Ketoacyl-CoA synthase | Qin et al., 2007 | |
| Fibre elongation and secondary wall biosynthesis | GhE6 | BM356398 | Fibre protein E6 | John and Crow, 1992; John, 1996; Li et al., 2002 |
| GhGlu1 | D88416 | Endo-1,3-beta-glucanase, clone CF922 | Ruan et al., 2004 |
N/A, Not available.
*No gene name or accession number was given in the published papers (Taliercio and Boykin, 2007; Wu et al., 2007).
Microarrays provide a high throughput tool for studying temporal expression patterns of many genes during fibre cell development. Using a filter array containing 1536 cDNAs, Li et al. (2002) compared expression patterns of the genes in the ovules at 5 DPA between the wild-type and a different fl mutant and identified ten genes that were highly enriched in wild-type cotton. Those include the genes encoding a RESPONSIVE TO DEHYDRATION22 (RD22)-like protein (GhRDL), a putative acetyltransferase (GhACY), a FIDDLEHEAD homologue (GhFDH), a serine carboxypeptidase-like protein (GhSCP), two tubulin components (GhTUA6 and GhTUB1) and fibre protein E6 (GhE6) (Table 3).
Using spotted oligo-gene microarrays, Lee et al. (2006) identified >20 genes that were expressed at higher levels in the fibre-bearing ovules (3 DPA) than in other tissues (leaves and petals). These genes were also expressed at higher levels in the fibre-bearing ovules (3 DPA) of the wild-type than those of the N1N1 mutant. Several genes including RDL, FDH and E6 were detected in both studies, indicating reliability of cDNA and oligo-gene microarrays. Using microarray analysis of laser-captured tissues, Wu et al. (2007) identified up-regulated genes in the cotton fibre initials relative to epidermal cells, many of which encode putative proteins of cell membrane and primary cell wall and DNA metabolism.
Yang et al. (2006) compared approx. 211 000 cotton ESTs derived from elongating fibres and non-fibre tissues with approx. 32 800 ESTs derived from an ovular EST library using an equal mixture of RNA isolated from −3, 0 and +3 DPA ovules of G. hirsutum ‘Texas Marker-1’ (TM-1) (Yang et al., 2006). The comparative data revealed many additional genes potentially involved in complex biological networks leading to fibre cell development. The genes encoding putative transcription factors, including MYB and WRKY family members, are enriched in early stages of fibre and ovule development. The data agree with the known roles of MYB and WRKY transcription factors in arabidopsis leaf trichome development. The data have also shown that AA-subgenome-specific ESTs are selectively enriched in young ovules (Yang et al., 2006) relative to DD-subgenome transcripts. Enrichment of AA-subgenome mRNAs in fibre-bearing ovules is consistent with the production of long lint fibres in AA-genome species and the absence of such fibres in DD species.
ADDITIONAL GENES INVOLVED IN FIBRE CELL ELONGATION
Physiologically, the process of fibre cell elongation is associated with strong cell turgor pressure and plasmodesmatal dynamics (Ruan et al., 2001). During fibre cell development, plasmodesmata are open from 0 to 9 DPA, close at 10 DPA, and re-open at 16 DPA. Significantly, differences in the duration of plasmodesmatal closure between cotton genotypes correlate with fibre length (Ruan et al., 2004).
Rapid cell elongation is also associated with transporter activities. Expression levels of the genes encoding sucrose and K+ transporters are very high in elongating fibre cells (Ruan et al., 2004). These transcripts are relatively abundant at 10 DPA, presumably causing osmotic and turgor potentials sufficient to allow fibre elongation (Ruan et al., 2004). An ATP-binding cassette transporter (GhWBC1) is also active in developing fibre cells (Zhu et al., 2003), which may suggest that molecular transport and cell-to-cell communication are important for fibre elongation.
The mRNA encoding fibre protein E6 is the one of the predominantly expressed fibre-specific transcripts (John, 1996). Accumulation of E6 transcript is relatively low in young ovules and highest in elongating fibres at 15–22 DPA (John and Crow, 1992). Overexpression of E6 antisense transcripts in transgenic cotton showed no visible fibre defects, indicating that this gene may not be an essential factor for fibre growth (John, 1996).
Subtractive hybridization and cDNA arrays have revealed fibre-related genes by comparing wild-type fibres with fibreless fl mutant ovules at 10 DPA (Ji et al., 2003). More than 100 genes are up-regulated in elongating fibres. Those include a putative vacuolar (H+)-ATPase catalytic subunit, a kinesin-like calmodulin-binding protein, and several arabinogalactan-like proteins. These genes are highly enriched (up to 50-fold) in 10 DPA fibre cells compared with 0 DPA ovules in wild-type cotton, emphasizing their potential roles in the fibre elongation phase. This study also identified several genes related to various potential upstream pathways such as auxin signal transduction and cell wall loosening (Ji et al., 2003).
Additional studies have explored the roles of specific transcripts in cotton fibre elongation. To become fibres, cells must grow anisotropically and expand during the fibre elongation phase. Thus, one question is how structural genes and enzymes mediate cotton fibre cells growth. In one case, a germin-like protein accumulates highly during fibre cell expansion in G. hirsutum (Kim and Triplett, 2001). Although the protein resembles an enzyme, a specific function for the germin-like protein has not yet been identified. Kim and Triplett (2004) also reported abundant GhRac1 expression during the fibre elongation stage. GhRac1 is a member of Rac/Rop GTPase family involved in cytoskeleton assembly in cells (Kim and Triplett, 2004).
A kinesin, GhKCH1, is a member of a plant-specific kinesin family that may act in microtubule organization by facilitating interaction between microtubules and actin microfilaments during fibre development (Preuss et al., 2004). Related to the actin cytoskeleton, 15 GhACT cDNAs encoding putative actins are differentially expressed in various tissues (Li et al., 2005). Specifically, GhACT1 is predominantly expressed in fibre cells, and suppression of GhACT1 was able to disrupt the actin cytoskeleton, causing reduced fibre elongation. Similarly, GhPFN1 (profilin 1) is associated with actin polymerization. When GhPFN1 is overexpressed in tobacco cells, cell cycle progression is delayed, and the mitotic index is slightly lower than the control plant (Wang et al., 2005).
GhGlcAT1, a gene encoding putative cotton glucuronosyltransferase, is abundant in 15 DPA fibres relative to other tissues, with the exception of moderate expression levels in the stem (Wu and Liu, 2005). GhGlcAT1 is hypothesized to be involved in the biosynthesis of non-cellulosic cell wall components during fibre elongation.
Qin et al. (2007) identified elongating fibre-enriched and tissue-specific gene expression of the GhCER6 transcript. Biochemical assays suggested that GhCER6 encodes a functional 3-ketoacyl-CoA synthase that functions in fatty acid elongation.
Another type of gene involved in fibre elongation is translation elongation factor 1A (eEF1A) that acts in protein synthesis to catalyse the binding of aminoacyl-tRNA to the A-site of the ribosome. EF-1 alpha is also known to have a role as a microtubule bundling protein so it may have a role in regulating cytoskeleton (Durso and Cyr, 1994). Several cotton eEF1A transcripts showed differential expression in various tissues and were relatively high in young fibres (Xu et al., 2007). Enrichment of a protein synthesis gene may reflect the need for increased protein synthesis capacity required for the rapid elongation of cotton fibres.
In a microarray study using 12 227 fibre (7–10 DPA) ESTs derived from fibre-producing diploid G. arboreum, Arpat et al. (2004) found that 81 genes were up-regulated, and 2553 ‘expansion-associated’ genes were down-regulated during the developmental switch from primary to secondary cell wall biosynthesis, suggesting a trend of global gene repression during the fibre elongation stage in the allotetraploids.
PHYTOHORMONAL REGULATION OF FIBRE CELL DEVELOPMENT
Various studies on cotton fibre cell development have implicated plant hormones as critical regulators of fibre development and boll retention. In vitro culture of cotton ovules allows exogenous supplementation with individual hormones or regimes of hormone combinations to allow examination of the physiological effects on cotton fibre development. In addition, endogenous levels of various hormones have been quantified to reveal in vivo correlations with fibre cell initiation and elongation.
Ovule EST analysis revealed many putative phytohormonal regulators related to auxin, brassinosteroid (BR), gibberellic acid (GA) and abscisic acid are enriched in early stages of fibre development. Cotton homologues related to MIXTA, MYB5, GL2 and eight genes in the auxin, BR, GA and ethylene pathways were induced during fibre cell initiation but were repressed in the N1N1 mutant that was impaired in fibre formation. The data are consistent with well-documented phytohormonal effects on fibre cell development in immature cotton ovules cultured in vitro (Yang et al., 2006). Additional ESTs derived from fibre initials at 1 DPA have recently been characterized (Taliercio and Boykin, 2007). In addition to CPC genes, many genes associated with regulation of brassinosterols, GTP-mediated signal transduction and cell cycle control and components of a Ca+2 mediated signalling pathway were identified in 1 DPA fibre, supporting a role for Ca+2 and signalling pathways in fibre development. Furthermore, gene expression studies have uncovered transcripts related to phytohormone biosynthesis and signalling that are correlated with fibre cell development. The phytohormonal pathway-related genes are induced prior to the activation of MYB-like genes, suggesting an important role for phytohormones in cell fate determination (Yang et al., 2006).
Auxin and gibberellic acid
For several decades, combinations of auxin and gibberellin have been known to promote fibre cell development on in vitro-cultured cotton ovules (Fig. 3). Whereas ovules cultured after fertilization produce fibres in vitro (Beasley, 1971), unfertilized ovules require exogenous auxin and gibberellin for maximal fibre growth (Beasley and Ting, 1974). In pioneering experiments, a combination of 500 nM auxin [indole-3-acetic acid (IAA)] with 5·0 µM gibberellin (GA3) was found to be optimal for fibre production (Beasley and Ting, 1974). A more recent study revealed that the timing of hormone treatment is critical, and that pre-anthesis treatment with IAA alone, or post-anthesis treatment with GA3 alone, each can lead to efficient in vitro fibre production (Gialvalis and Seagull, 2001). The technical ability to grow cotton fibres in vitro provides a useful model system both for cotton fibre development in specific and for cell wall biosynthesis in general (Kim and Triplett, 2001).
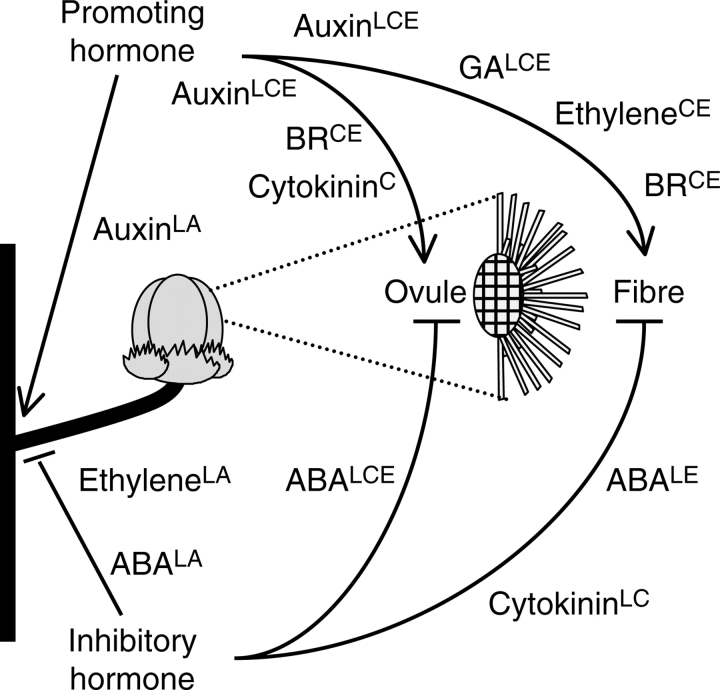
Fig. 3.
Hormones impacting fibre development, ovule growth and boll retention. A schematic depiction of a cotton plant secondary stem, fruiting stem, and boll (left), enlarged view of a fibre-bearing ovule (right) are shown. Hormones shown to promote boll retention, ovule growth and fibre initiation or growth (left to right) are shown at the top with arrows; inhibitory hormones are shown at the bottom with blocked blunted lines. The study types that revealed a role for each hormone in each process are indicated in superscript: L, endogenous level quantification; A, application in vivo; C, application to cultured embryos; E, gene expression profiling. Note that abscission between the fruit and peduncle, as well as the depicted zone between peduncle and stem, causes boll loss. See text for references.
Demonstrating further roles for auxin in cotton fibre development, in vivo quantification has revealed a spike in flower bud auxin levels preceding fibre initiation, declining after anthesis, and rebounding after 4 DPA (Guinn and Brummett, 1988). Within ovules, IAA and related auxin metabolites were detected at 8 DPA (Shindy and Smith, 1975). Similarly, various gibberellins, especially GA13, were detected in 8 DPA ovules. In vivo levels of certain GAs, such as GA3, are low during fibre elongation (Chen et al., 1996; Gokani and Thaker, 2002).
In addition to in vitro ovule culture and in vivo quantification experiments, gene expression studies have revealed apparent roles for auxin and gibberellin in fibre development. A cotton mRNA encoding a cupin superfamily protein was shown to be up-regulated in 10 DPA ovules relative to 0 DPA in a cDNA array (Ji et al., 2003). Though a specific function for a cotton cupin superfamily protein correlated with fibre production has not been demonstrated (Kim et al., 2004), the arabidopsis cupin superfamily member ABP1 is necessary for development, and tobacco ABP1 knockdown causes deficient auxin-induced cell elongation (Chen et al., 2001). Moreover, sequencing of ESTs from −3, 0 and +3 DPA ovules revealed an enrichment of Auxin Response Factor (ARF)-family transcripts relative to the cotton EST assembly and the arabidopsis genome (Yang et al., 2006). The study also revealed an increased abundance of transcripts associated with GA biosynthesis relative to other EST libraries.
Auxin also regulates abscission, thereby influencing boll retention, which is necessary for fibre maturation and harvest. Auxin promotes boll retention; endogenous IAA levels drop at anthesis, when abscission is common, then increase again as abscission frequency declines (Guinn and Brummett, 1988). The endogenous ratio of abscission-promoting abscisic acid to abscission-inhibiting IAA correlates closely with abscission frequency through boll development. In addition, application of auxins inhibits cotton leaf abscission, while application of an auxin transport inhibitor promotes abscission (Suttle and Hultstrand, 1991).
Brassinosteroid
Like auxin and gibberellin, BR positively influences fibre cell development. Application of brassinolide (BL) to cultured cotton ovules increases fibre length and ovule growth, and application of the BR biosynthesis inhibitor brassinazole 2001 (BRZ) inhibits fibre elongation and ovule size (Sun et al., 2004, 2005; Shi et al., 2006). Inhibitory effects of BRZ are reversed by simultaneous BL application, confirming that BRZ represses fibre development by causing BL deficiency.
Expression of genes related to BL biosynthesis and signalling are also correlated with fibre cell development. Cotton mRNAs encoding apparent orthologues of the transmembrane BL receptor BRI1 are enriched in 5–8 DPA fibre-bearing ovules (Sun et al., 2004). Intriguingly, mRNA levels of two differentially expressed cotton BIN2 genes that are presumed negative regulators of BL signalling are also high in 5–8 DPA fibre-bearing ovules (Sun and Allen, 2005), which may reflect a negative feedback loop or some other phenomenon. In addition to these BL signalling genes, transcript levels of the BL biosynthesis genes SMT1 and DET2 have been found to correlate with fibre growth in cDNA microarray experiments (Shi et al., 2006). Cotton ovule SMT1 and DET2 mRNA levels increase from the day of anthesis to about 10 DPA and then decline at 20 DPA; levels of both mRNAs are greatly reduced in 10 DPA ovules of the fibreless fl mutant relative to wild type (Shi et al., 2006).
Ethylene
A recent physiology and gene expression study revealed an apparent role for ethylene in promoting fibre development (Shi et al., 2006). Application of ethylene stimulated fibre growth on cultured ovules in a dose-dependent manner; conversely, the ethylene inhibitor AVG inhibited fibre growth (Shi et al., 2006). In gene expression experiments, cDNA microarrays demonstrated a several-fold increase in three cotton ACC-OXIDASE (ACO) ovule mRNAs peaking during fibre elongation at 10–15 DPA. Further, these transcripts were shown to be specifically enriched in 10 DPA fibre tissue compared with 10-DPA ovule (Shi et al., 2006). The presumed ACC oxidase activity of the encoded cotton proteins was confirmed biochemically.
Ethylene also promotes abscission, sometimes causing boll loss. Production of the gaseous hormone peaks at a developmental stage coincident with peak fruit abscission (Guinn, 1982). A 3-d dim-light treatment or dehydration stress increases the rate of fruit abscission, and these conditions also stimulate cotton fruit ethylene evolution (Guinn, 1976, 1982).
Abscisic acid
Abscisic acid (ABA) is an inhibitor of fibre growth. ABA application to cultured unfertilized ovules causes retardation of fibre development (Beasley and Ting, 1974). The inhibitory effect of ABA is partially compensated by addition of cytokinin, which in the absence of ABA also inhibits fibre development.
Within the cotton boll, abscisic acid concentrations increase from the day of anthesis to 10 DPA, then decline until 20 DPA, only to resurge again from 30 to 50 DPA (Davis and Addicott, 1972). High levels of endogenous ABA are correlated with shorter fibre among cotton cultivars (Gokani et al., 1998). The ratio of ABA to auxin levels fits the regulation of secondary cell wall thickening (Yang et al., 2001). Perhaps the most thoroughly studied aspect of ABA regulation of cotton fruit development regards boll abscission. The ratio of endogenous abscission-promoting ABA to abscission-inhibiting IAA correlates closely with abscission frequency over developmental time (Guinn and Brummett, 1988).
Cytokinin
Cytokinins play essential roles in cell division and vascular tissue development. Cytokinin produces conflicting results on ovule and fibre growth. When applied to in vitro-cultured ovules, cytokinin promotes ovule growth, but inhibits fibre development (Beasley and Ting, 1974). In contrast, cytokinin partially alleviates the inhibition of fibre growth by ABA. Thus, it has been proposed that cytokinin exists at an optimal level for fibre growth in vivo (Beasley and Ting, 1974). Examination of cytokinin levels over developmental time revealed a trend of increasing cytokinin in ovules from various fibreless mutants (n2, H10 and Xu142) after anthesis, over which time cytokinin levels decrease in wild type (Chen et al., 1997). The data suggest that cytokinins promote fibre initiation prior to flowering but inhibit fibre growth after flowering. Several cytokinins, particularly 6-(3-methyl- 2-butenylamino)-9-β-d-ribofuranosylpurine (2iPA), have been quantified from 8 DPA cotton ovules (Shindy and Smith, 1975).
FUTURE PROSPECTS
Cotton fibre cell development is a fundamental biological process that is poorly understood. Initiation of an epidermal layer cell into fibre requires a change in cell fate, involving genetic, physiological and developmental ‘switches’. Genetic mutations, genotypes and phytohormonal regulation can affect the number of cells developing into fibres or alter fibre cell properties. Little is known, however, about the developmental and physiological processes leading to the production of lint (long) and fuzz (short) fibres in the same ovules. Arabidopsis trichome development may provide a useful reference for investigating the functions of fibre-related genes in cotton seed-trichome development. Sequencing of EST libraries from tetraploid cotton, as well as other members of the Gossypium genus, reveals new transcripts for further characterization. To understand the precise mechanisms of fibre cell development, preliminary research should be followed by additional genetic and biochemical studies. Phytohormone accumulation and signalling including Ca2+ may be critical for fibre initiation and elongation. The fibre growth-promoting hormones such as auxin, BR and gibberellin may be most attractive for manipulation. Ethylene may promote fruit loss and negatively regulate the abundance or action of the fibre growth-inhibitory hormones ABA or cytokinin.
In the future, the endogenous levels of phytohormones and Ca2+ should be carefully measured using isotope-labelled standards, and the roles for transcription regulators and phytohormonal biosynthetic genes in fibre cell development should be tested using transgenic approaches in cotton. Genome sequence information of Gossypium species may aid exploration of new research interests. Various microarray platforms continue to provide a complete picture of transcriptional changes accompanying fibre development, both expanding the number of genes examined and the tissue types and fibre-mutants compared. Interactions between homoeologous loci derived from extant progenitors may stimulate fibre cell growth and development in allotetraploid cotton. To date, the role of small RNAs, especially microRNAs, in cotton fibre cell development is under-explored. Early results hold promise for producing candidate genes that can be further characterized and may eventually lead to the improvement of fibre quality and yield.
ACKNOWLEDGEMENTS
We thank Barbara Triplett (USDA-ARS, Southern Regional Research Center) and David Stelly (Texas A&M University) for critical suggestions on improving the manuscript. This work is supported by a grant from the National Science Foundation Plant Genome Research Program (DBI0624077) and Cotton Incorporated (04-5555 and 07‐161).
LITERATURE CITED
- Applequist WL, Cronn R, Wendel JF. Comparative development of fiber in wild and cultivated cotton. Evolution and Development. 2001;3:3–17. doi: 10.1046/j.1525-142x.2001.00079.x. [DOI] [PubMed] [Google Scholar]
- Arpat AB, Waugh M, Sullivan JP, Gonzales M, Frisch D, Main D, et al. Functional genomics of cell elongation in developing cotton fibers. Plant Molecular Biology. 2004;54:911–929. doi: 10.1007/s11103-004-0392-y. [DOI] [PubMed] [Google Scholar]
- Basra A, Malik CP. Development of the cotton fiber. International Review of Cytology. 1984;89:65–113. [Google Scholar]
- Beasley CA. In vitro culture of fertilized cotton ovules. BioScience. 1971;21:906–907. [Google Scholar]
- Beasley CA, Ting IP. The effects of plant growth substances on in vitro fiber development from unfertilized cotton ovules. American Journal of Botany. 1974;61:188–194. [Google Scholar]
- Chen JG, Du XM, Zhao HY, Zhou X. Fluctuation in levels of endogenous plant hormones in ovules of normal and mutant cotton during flowering and their relation to fiber development. Journal of Plant Growth Regulation. 1996;15:173–177. [Google Scholar]
- Chen JG, Du XM, Zhou X, Zhao HY. Levels of cytokinins in the ovules of cotton mutants with altered fiber development. Journal of Plant Growth Regulation. 1997;16:181–185. [Google Scholar]
- Chen JG, Ullah H, Young JC, Sussman MR, Jones AM. ABP1 is required for organized cell elongation and division in Arabidopsis embryogenesis. Genes and Development. 2001;15:902–911. doi: 10.1101/gad.866201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LA, Addicott FT. Abscisic acid: correlations with abscission and with development in the cotton fruit. Plant Physiology. 1972;49:644–648. doi: 10.1104/pp.49.4.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A, Chee PW, Rong J, May OL, Paterson AH. Chromosome structural changes in diploid and tetraploid A genomes of Gossypium. Genome. 2006;49:336–345. doi: 10.1139/g05-116. [DOI] [PubMed] [Google Scholar]
- Durso Na, Cyr RJ. Beyond translation: elongation factor-1-alpha and the cytoskeleton. Protoplasma. 1994;180:99–105. [Google Scholar]
- Esch JJ, Chen M, Sanders M, Hillestad M, Ndkium S, Idelkope B, et al. A contradictory GLABRA3 allele helps define gene interactions controlling trichome development in Arabidopsis. Development. 2003;130:5885–5894. doi: 10.1242/dev.00812. [DOI] [PubMed] [Google Scholar]
- Gialvalis S, Seagull RW. Plant hormones alter fiber initiation in unfertilized cultured ovules of Gossypium hirsutum. Journal of Cotton Science. 2001;5:252–258. [Google Scholar]
- Gokani SJ, Thaker VS. Role of gibberellic acid in cotton fibre development. Journal of Agricultural Science. 2002;138:255–260. [Google Scholar]
- Gokani SJ, Kumar R, Thaker VS. Potential role of abscisic acid in cotton fiber and ovule development. Journal of Plant Growth Regulation. 1998;17:1–5. [Google Scholar]
- Graves DA, Stewart JM. Chronology of the differentiation of cotton (Gossypium hirsutum L. fiber cells. Planta. 1988;175:254–258. doi: 10.1007/BF00392435. [DOI] [PubMed] [Google Scholar]
- Guinn G. Water deficit and ethylene evolution by young cotton bolls. Plant Physiology. 1976;57:403–405. doi: 10.1104/pp.57.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinn G. Fruit age and changes in abscisic acid content, ethylene production, and abscission rate of cotton fruits. Plant Physiology. 1982;69:349–352. doi: 10.1104/pp.69.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinn G, Brummett DL. Changes in abscisic acid and indoleacetic acid before and after anthesis relative to changes in abscission rates of cotton fruiting forms. Plant Physiology. 1988;87:629–631. doi: 10.1104/pp.87.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler CH, Zhang DH, Wilkerson CG. Biotechnological improvement of cotton fibre maturity. Physiologia Plantarum. 2005;124:285–294. [Google Scholar]
- Hendrix B, Stewart JM. Estimation of the nuclear DNA content of Gossypium species. Annals of Botany. 2005;95:789–797. doi: 10.1093/aob/mci078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix DL. Carbohydrates and carbohydrate-enzymes in developing cotton ovules. Physiologia Plantarum. 1990;78:85–92. [Google Scholar]
- Humphries JA, Walker AR, Timmis JN, Orford SJ. Two WD-repeat genes from cotton are functional homologues of the Arabidopsis thaliana TRANSPARENT TESTA GLABRA1 (TTG1) gene. Plant Molecular Biology. 2005;57:67–81. doi: 10.1007/s11103-004-6768-1. [DOI] [PubMed] [Google Scholar]
- Hülskamp M. Plant trichomes: a model for cell differentiation. Nature Reviews. Molecular Cell Biology. 2004;5:471–480. doi: 10.1038/nrm1404. [DOI] [PubMed] [Google Scholar]
- Hülskamp M, Schnittger AT. Spatial regulation of trichome formation in Arabidopsis thaliana. Seminars in Cell and Developmental Biology. 1998;9:213–220. doi: 10.1006/scdb.1997.0209. [DOI] [PubMed] [Google Scholar]
- Hülskamp M, Misera S, Jurgens G. Genetic dissection of trichome cell development in Arabidopsis. Cell. 1994;76:555–566. doi: 10.1016/0092-8674(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Ji SJ, Lu YC, Feng JX, Wei G, Li J, Shi YH, et al. Isolation and analyses of gene preferentially expressed during early cotton fiber development by subtractive PCR and cDNA array. Nucleic Acids Research. 2003;31:2534–2543. doi: 10.1093/nar/gkg358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John ME. Structural characterization of genes corresponding to cotton fiber mRNA, E6: reduced E6 protein in transgenic plants by antisense gene. Plant Molecular Biology. 1996;30:297–306. doi: 10.1007/BF00020115. [DOI] [PubMed] [Google Scholar]
- John ME, Crow LJ. Gene expression in cotton (Gossypium hirsutum L.) fiber: cloning of the mRNAs. Proceedings of the National Academy of Sciences of the USA. 1992;89:5769–5773. doi: 10.1073/pnas.89.13.5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Triplett BA. Cotton fiber growth in planta and in vitro: models for plant cell elongation and cell wall biogenesis. Plant Physiology. 2001;127:1361–1366. [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Triplett BA. Characterization of GhRac1 GTPase expressed in developing cotton (Gossypium hirsutum L.) fibers. Biochimica et Biophysica Acta. 2004;1679:214–221. doi: 10.1016/j.bbaexp.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Pesacreta TC, Triplett BA. Cotton-fiber germin-like protein. II. Immunolocalization, purification, and functional analysis. Planta. 2004;218:525–535. doi: 10.1007/s00425-003-1134-0. [DOI] [PubMed] [Google Scholar]
- Larkin JC, Oppenheimer DG, Pollock S, Marks MD. Arabidopsis GLABROUS1 gene requires downstream sequences for function. The Plant Cell. 1993;5:1739–1748. doi: 10.1105/tpc.5.12.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin JC, Oppenheimer DG, Lloyd AM, Paparozzi ET, Marks MD. Roles of the GLABROUS1 and TRANSPARENT TESTA GLABRA genes in Arabidopsis trichome development. The Plant Cell. 1994;6:1065–1076. doi: 10.1105/tpc.6.8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JJ, Hassan OSS, Gao W, Wang J, Wei EN, Russel JK, et al. Developmental and gene expression analyses of a cotton naked seed mutant. Planta. 2006;223:418–432. doi: 10.1007/s00425-005-0098-7. [DOI] [PubMed] [Google Scholar]
- Li CH, Zhu YP, Meng YL, Wang JW, Xu KX, Zhang TZ, et al. Isolation of genes preferentially expressed in cotton fibers by cDNA filter arrays and RT-PCR. Plant Science. 2002;163:1113–1120. [Google Scholar]
- Li XB, Fan XP, Wang XL, Cai L, Yang WC. The cotton ACTIN1 gene is functionally expressed in fibers and participates in fiber elongation. The Plant Cell. 2005;17:859–875. doi: 10.1105/tpc.104.029629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loguercio LL, Zhang JQ, Wilkins TA. Differential regulation of six novel MYB-domian genes defines two distinct expression patterns in allotetraploid cotton (Gossypium hirsutum L.) Molecular and General Genetics. 1999;261:660–671. doi: 10.1007/s004380050009. [DOI] [PubMed] [Google Scholar]
- Lolle SJ, Cheung AY, Sussex IM. Fiddlehead: an Arabidopsis mutant constitutively expressing an organ fusion program that involves interactions between epidermal cells. Developmental Biology. 1992;152:383–392. doi: 10.1016/0012-1606(92)90145-7. [DOI] [PubMed] [Google Scholar]
- Marks MD. Molecular genetic analysis of trichome development in Arabidopsis. Annual Review of Plant Physiology and Plant Molecular Biology. 1997;48:137–163. doi: 10.1146/annurev.arplant.48.1.137. [DOI] [PubMed] [Google Scholar]
- Notle KD, Hendrix DL, Radin JW, Koch KE. Sucrose synthase localization during initiation of seed development and trichome differentiation in cotton ovules. Plant Physiology. 1995;109:1285–1293. doi: 10.1104/pp.109.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne T, Clement J, Arnold D, Lloyd A. Heterologous myb genes distinct from GL1 enhance trichome production when overexpressed in Nicotiana tabacum. Development. 1999;126:671–682. doi: 10.1242/dev.126.4.671. [DOI] [PubMed] [Google Scholar]
- Percival AE, Wendel JF, Stewart JM. Taxonomy and germplasm resources. In: Smith CW, Cothren JT,, editors. Cotton: origin, history, technology, and production. New York, NY: John Wiley & Sons; 1999. pp. 33–63. [Google Scholar]
- Pesch M, Hülskamp M. Creating a two-dimensional pattern de novo during Arabidopsis trichome and root hair initiation. Current Opinion in Genetics & Development. 2004;14:422–427. doi: 10.1016/j.gde.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Preuss ML, Kovar DR, Lee YR, Staiger CJ, Delmer DP, Liu B. A plant-specific kinesin binds to actin microfilaments and interacts with cortical microtubules in cotton fibers. Plant Physiology. 2004;136:3945–3955. doi: 10.1104/pp.104.052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt RE, Vielle-Calzada JP, Ploense SE, Grossniklaus U, Lolle SJ. FIDDLEHEAD, a gene required to suppress epidermal cell interactions in Arabidopsis, encodes a putative lipid biosynthetic enzyme. Proceedings of the National Academy of Sciences of the USA. 2000;97:1311–1316. doi: 10.1073/pnas.97.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin YM, Pujol FM, Hu CY, Feng JX, Kastaniotis AJ, Hiltunen JK, et al. Genetic and biochemical studies in yeast reveal that the cotton fibre-specific GhCER6 gene functions in fatty acid elongation. Journal of Experimental Botany. 2007;58:473–481. doi: 10.1093/jxb/erl218. [DOI] [PubMed] [Google Scholar]
- Rerie WG, Feldmann KA, Marks MD. The GLABRA2 gene encodes a homeo domain protein required for normal trichome development in Arabidopsis. Genes & Development. 1994;8:1388–1399. doi: 10.1101/gad.8.12.1388. [DOI] [PubMed] [Google Scholar]
- Ruan YL, Chourey PS. A fiberless seed mutation in cotton is associated with lack of fiber cell initiation in ovule epidermis and alterations in sucrose synthase expression and carbon partitioning in developing seeds. Plant Physiology. 1998;118:399–406. doi: 10.1104/pp.118.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Y, Llewellyn D, Furbank R. The control of single-celled cotton fiber elongation by developmentally reversible gating of plasmodesmata and coordinated expression of sucrose and k(+) transporters and expansin. The Plant Cell. 2001;13:47–60. doi: 10.1105/tpc.13.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan YL, Llewellyn DJ, Furbank RT. Suppression of sucrose synthase gene expression represses cotton fiber cell initiation, elongation, and seed development. The Plant Cell. 2003;15:952–964. doi: 10.1105/tpc.010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan YL, Xu SM, White R, Furbank RT. Genotypic and developmental evidence for the role of plasmodesmatal regulation in cotton fiber elongation mediated by callose turnover. Plant Physiology. 2004;136:4104–4113. doi: 10.1104/pp.104.051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellmann S, Hülskamp M. Epidermal differentiation: trichomes in Arabidopsis as a model system. International Journal of Developmental Biology. 2005;49:579–584. doi: 10.1387/ijdb.051983ss. [DOI] [PubMed] [Google Scholar]
- Schellmann S, Schnittger A, Kirik V, Wada T, Okada K, Beermann A, et al. TRIPTYCHON and CAPRICE mediate lateral inhibition during trichome and root hair patterning in Arabidopsis. EMBO Journal. 2002;21:5036–5046. doi: 10.1093/emboj/cdf524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serna L, Martin C. Trichomes: different regulatory networks lead to convergent structures. Trends in Plant Science. 2006;11:274–280. doi: 10.1016/j.tplants.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Shi YH, Zhu SW, Mao XZ, Feng JX, Qin YM, Zhang L, et al. Transcriptome profiling, molecular, biological, and physiological studies reveal a major role for ethylene in cotton fiber cell elongation. The Plant Cell. 2006;18:651–664. doi: 10.1105/tpc.105.040303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindy WW, Smith OE. Identification of plant hormones from cotton ovules. Plant Physiology. 1975;55:550–554. doi: 10.1104/pp.55.3.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CW, Cothren JT. Cotton: origin, history, technology, and production. New York, NY: John Wiley & Sons; 1999. [Google Scholar]
- Sun Y, Allen RD. Functional analysis of the BIN2 genes of cotton. Molecular Genetics and Genomics. 2005;274:51–59. doi: 10.1007/s00438-005-1122-0. [DOI] [PubMed] [Google Scholar]
- Sun Y, Fokar M, Asami T, Yoshida S, Allen RD. Characterization of the brassinosteroid insensitive 1 genes of cotton. Plant Molecular Biology. 2004;54:221–232. doi: 10.1023/B:PLAN.0000028788.96381.47. [DOI] [PubMed] [Google Scholar]
- Sun Y, Veerabomma S, Abdel-Mageed HA, Fokar M, Asami T, Yoshida S, et al. Brassinosteroid regulates fiber development on cultured cotton ovules. Plant Cell Physiology. 2005;46:1384–1391. doi: 10.1093/pcp/pci150. [DOI] [PubMed] [Google Scholar]
- Suo J, Liang X, Pu L, Zhang Y, Xue Y. Identification of GhMYB109 encoding a R2R3 MYB transcription factor that expressed specifically in fiber initials and elongating fibers of cotton (Gossypium hirsutum L.) Biochimica et Biophysica Acta. 2003;1630:25–34. doi: 10.1016/j.bbaexp.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Suttle JC, Hultstrand JF. Ethylene-induced leaf abscission in cotton seedlings: the physiological bases for age-dependent differences in sensitivity. Plant Physiology. 1991;95:29–33. doi: 10.1104/pp.95.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanski DB, Marks MD. GLABROUS1 overexpression and TRIPTYCHON alter the cell cycle and trichome cell fate in Arabidopsis. The Plant Cell. 1998;10:2047–2062. doi: 10.1105/tpc.10.12.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanski DB, Lloyd AM, Marks MD. Progress in the molecular genetic analysis of trichome initiation and morphogenesis in Arabidopsis. Trends in Plant Science. 2000;5:214–219. doi: 10.1016/s1360-1385(00)01597-1. [DOI] [PubMed] [Google Scholar]
- Taliercio EW, Boykin D. Analysis of gene expression in cotton fiber initials. BMC Plant Biology. 2007;7:22. doi: 10.1186/1471-2229-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taliercio E, Hendrix B, Stewart JM. DNA content and expression of genes related to cell cycling in developing Gossypium hirsutum (Malvaceae) fibers. American Journal of Botany. 2005;92:1942–1947. doi: 10.3732/ajb.92.12.1942. [DOI] [PubMed] [Google Scholar]
- Tiwari SC, Wilkins TA. Cotton (Gossypium hirsutum) seed trichomes expand via diffuse growing mechanism. Canadian Journal of Botany. 1995;73:746–757. [Google Scholar]
- Van't Hof J. Increased nuclear DNA content in developing cotton fiber cells. American Journal of Botany. 1999;86:776–779. [PubMed] [Google Scholar]
- Wang HY, Yu Y, Chen ZL, Xia GX. Functional characterization of Gossypium hirsutum profilin 1 gene (GhPFN1. in tobacco suspension cells: characterization of in vivo functions of a cotton profilin gene. Planta. 2005;222:594–603. doi: 10.1007/s00425-005-0005-2. [DOI] [PubMed] [Google Scholar]
- Wang S, Wang JW, Yu N, Li CH, Luo B, Gou JY, et al. Control of plant trichome development by a cotton fiber MYB gene. The Plant Cell. 2004;16:2323–2334. doi: 10.1105/tpc.104.024844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendel JF. New World tetraploid cottons contain Old World cytoplasm. Proceedings of the National Academy of Sciences of the USA. 1989;86:4132–4136. doi: 10.1073/pnas.86.11.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendel JF, Cronn RC. Polyploidy and the evolutionary history of cotton. Advances in Agronomy. 2003;78:139–186. [Google Scholar]
- Wilkins TA, Jernstedt JA. Molecular genetics of developing cotton fibers. In: Basra AM,, editor. Cotton fibers. New York, NY: Hawthorne Press; 1999. pp. 231–267. [Google Scholar]
- Wu Y, Machado AC, White RG, Llewellyn DJ, Dennis ES. Expression profiling identifies genes expressed early during lint fibre initiation in cotton. Plant Cell Physiology. 2006;47:107–127. doi: 10.1093/pcp/pci228. [DOI] [PubMed] [Google Scholar]
- Wu Y, Llewellyn DJ, White R, Ruggiero K, Al-Ghazi Y, Dennis ES. Laser capture microdissection and cDNA microarrays used to generate gene expression profiles of the rapidly expanding fibre initial cells on the surface of cotton ovules. Planta. 2007 doi: 10.1007/s00425-007-0580-5. [DOI] [PubMed] [Google Scholar]
- Wu YT, Liu JY. Molecular cloning and characterization of a cotton glucuronosyltranferase gene. Journal of Plant Physiology. 2005;162:573–582. doi: 10.1016/j.jplph.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Xu WL, Wang XL, Wang H, Li XB. Molecular characterization and expression analysis of nine cotton GhEF1A genes encoding translation elongation factor 1A. Gene. 2007;389:27–35. doi: 10.1016/j.gene.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Yang SS, Cheung F, Lee JJ, Ha M, Wei NE, Sze SH, et al. Accumulation of genome-specific transcripts, transcription factors and phytohormonal regulators during early stages of fiber cell development in allotetraploid cotton. The Plant Journal. 2006;47:761–775. doi: 10.1111/j.1365-313X.2006.02829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YM, Xu CN, Wang BM, Jia JZ. Effects of plant growth regulators on secondary wall thickening of cotton fibres. Plant Growth Regulation. 2001;35:233–237. [Google Scholar]
- Yephremov A, Wisman E, Huijser P, Huijser C, Wellesen K, Saedler H. Characterization of the FIDDLEHEAD gene of Arabidopsis reveals a link between adhesion response and cell differentiation in the epidermis. The Plant Cell. 1999;11:2187–2201. doi: 10.1105/tpc.11.11.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YP, Xu KX, Luo B, Wang JW, Chen XY. An ATP-binding cassette transporter GhWBC1 from elongating cotton fibers. Plant Physiology. 2003;133:580–588. doi: 10.1104/pp.103.027052. [DOI] [PMC free article] [PubMed] [Google Scholar]