Abstract
Background
The incorporation of silica within the plant cell wall has been well documented by botanists and materials scientists; however, the means by which plants are able to transport silicon and control its polymerization, together with the roles of silica in situ, are not fully understood.
Recent Progress
Recent studies into the mechanisms by which silicification proceeds have identified the following: an energy-dependent Si transporter; Si as a biologically active element triggering natural defence mechanisms; and the means by which abiotic toxicities are alleviated by silica. A full understanding of silica formation in vivo still requires an elucidation of the role played by the environment in which silica formation occurs. Results from in-vitro studies of the effects of cell-wall components associated with polymerized silica on mineral formation illustrate the interactions occurring between the biomolecules and silica, and the effects their presence has on the mineralized structures so formed.
Scope
This Botanical Briefing describes the uptake, storage and function of Si, and discusses the role biomolecules play when incorporated into model systems of silica polymerization as well as future directions for research in this field.
Key words: Silica, biosilicification, stress resistance, silicon transport, silicic acid
INTRODUCTION
Silicon (Si) in a chemically combined form is ubiquitous in nature. The Si content of soils can vary dramatically from <1 to 45 % dry weight (Sommer et al., 2006), and its presence in the form of silicic acid [Si(OH)4] (or its ionized form, Si(OH)3O−, which predominates at pH > 9) allows its uptake by plants. Silicic acid is generally found in soils at concentrations ranging from 0·1 to 0·6 mM (Epstein, 1994) but, to our knowledge, there has been no evidence of the occurrence of biosilicification reactions in the soil. Although not traditionally thought of as an element essential to the life cycle of plants, with the exception of the early-diverging Equisetaceae (Chen and Lewin, 1969), certain algae and diatoms, Si is found in plants at concentrations ranging from 0·1 to 10 % (=103–105 mg kg−1; dry weight basis), an amount equivalent to, or even exceeding, several macronutrients (Epstein, 1994). Plants deprived of Si are often weaker structurally and more prone to abnormalities of growth, development and reproduction and it is the only nutrient which is not detrimental when collected in excess (Epstein, 1999). This Botanical Briefing aims to cover aspects of Si uptake and its role as an alleviator of biotic and abiotic stress, and also to provide insight into how investigations into the surrounding plant cell-wall environment are helping to elucidate the role of chemical influences in the formation of polymeric silica in plants. [‘Si’ is the symbol for the element silicon and is also used as a generic term when the nature of the silicon compound(s) is not being specified. Si(OH)4 is silicic acid, more correctly named orthosilicic acid; it is the fundamental building block of silicas and is itself the simplest silica. Silica is amorphous, hydrated and usually polymerized material produced from Si(OH)4.]
OCCURRENCE AND FORM
The occurrence of Si within the plant is a result of its uptake, in the form of soluble Si(OH)4 or Si(OH)3O−, from the soil and its controlled polymerization at a final location. However, the ability of a plant to accumulate Si varies greatly between species (0·1–10 % of shoot dry weight) and extensive analysis of Si uptake in plants has been carried out (e.g. Simpson and Volcani, 1981; Takahashi et al., 1990; Hodson et al., 2005). Si accumulation has been found to a greater extent, but not exclusively, in monocotyledonous plants. Plants of the families Poaceae, Equisetaceae and Cyperaceae show high Si accumulation (>4 % Si), the Cucurbitales, Urticales and Commelinaceae show intermediate Si accumulation (2–4 % Si), while most other species demonstrate little accumulation. Si concentrations of shoots tend to decline in the order liverworts > horsetails > clubmosses > mosses > angiosperms > gymnosperms > ferns (Hodson et al., 2005). Different parts of the same plant can show large differences in Si accumulation, a good example being the variation from 0·5 g kg−1 in polished rice, 50 g kg−1 in rice bran, 130 g kg−1 in rice straw, 230 g kg−1 in rice hulls to 350 g kg−1 in rice joints (found at the base of the grain) (Van Hoest, 2006). These concentrations are also in distinct contrast to those found for oat and wheat straw, which are at a levels of tens of g kg−1.
On Si uptake into the plant, phytoliths or silica bodies are formed which infill the cell walls and lumina of certain plant cells (Prychid et al., 2004); however, the nature of its association with cell-wall components including polysaccharides, lignins and proteins is not fully understood (Perry and Lu, 1992). The cell-wall environment is a highly complex matrix composed of cellulose and more complex polysaccharides such as hemicelluloses and pectins, the latter being rich in galacturonic acid residues cross-linked by the co-ordination of Ca ions (Fry, 2004). This biochemical environment may be capable of modifying the solubility and binding abilities of the silica phase as it develops (Perry and Keeling-Tucker, 1998).
ROLE OF SILICA IN STRESS RELIEF
The presence of Si in plants has been found to alleviate many abiotic and biotic stresses, leading to the incorporation of silicates into many fertilizers. How Si is able to exert such a protective effect has yet to be fully elucidated although roles including providing a physical and/or biochemical defence system have been proposed. The deposition of silica as a physical barrier to penetration and reduction in the susceptibility to enzymatic degradation by fungal pathogens has been examined (Yoshida et al., 1962). However, there is debate as to whether this increased strength is sufficient to explain the protective effects observed (Fauteux et al., 2005). An alternative explanation for silicon's protective role is as a biologically active element capable of triggering a broad range of natural defences. This was first demonstrated in cucumbers: Si-treated plants demonstrated enhanced activity of chitinases, peroxidases, polyphenol oxidases and flavonoid phytoalexins, all of which may protect against fungal pathogens (Chérif et al., 1994; Fawe et al., 1998). Further investigation of these defence mechanisms by Bélanger et al. (2003) and Rodrigues et al. (2003), studying wheat and rice blast, respectively, indicated that these species were also capable of inducing similar biologically active defence agents, including increased production of glycosylated phenolics and antimicrobial products such as diterpenoid phytoalexins in the presence of silica. Experiments performed on cucumber leaves following fungal infection showed that further resistance to infection is acquired by expression of a proline-rich protein together with the presence of silica at the site of attempted penetration (Kauss et al., 2003). The C-terminus of this protein contained a high density of lysine and arginine residues proposed to catalyse the localized deposition of silica at the site of vulnerability.
In addition, metal toxicity, salinity, drought and temperature stresses can be alleviated by Si application (Liang et al., 2007) and the means by which Si exerts these protective effects is still under investigation. Metal toxicities including Mn, Cd, Al and Zn have been studied and the proposed mechanisms for the action of Si include the accumulation of Zn as a silicate (Neuman and zur Nieden, 2001); reduction of lipid peroxidation and increased enzymatic (e.g. superoxide dismutase; SOD) and non-enzymatic antioxidants (e.g. ascorbate) against Mn toxicity (Shi et al., 2005); and increased release of phenolics with strong chelating ability for Al tolerance (Kidd et al., 2001). Drought tolerance brought about by the application of ‘Si’ may result from decreased transpiration (Epstein, 1999) and the presence of silicified structures in plants suggested a reduction of leaf heat-load, providing an effective cooling mechanism and thereby improving plant tolerance to high temperatures (Wang et al., 2005). The resistance to salt stress has been found to be due to the enhancement of enzymes such as SOD and catalase, preventing membrane oxidative damage (Zhu et al., 2004; Moussa, 2006).
TRANSPORT
Plants differ in their abilities to accumulate silica and initially Takahashi et al. (1990) proposed three methods of uptake: active, passive and rejective. Further examination of Si uptake in several different plant species has resulted in different modes of silicic acid uptake and transport being elucidated. Ma and colleagues have used mutants defective in silicic acid uptake to examine the means by which silicic acid is accumulated in rice, a known Si accumulator.
The uptake of Si from solution by rice was found to be the result of two different transport mechanisms. A low-affinity transporter (Lsi1) found on the lateral roots is responsible for the uptake of silicic acid from the external solution to the root cortical cells (Ma and Yamaji, 2006). The gene responsible, Lsi1, has been mapped to chromosome 2, contains five exons and four introns, and encodes a 298-amino-acid protein. The predicted membrane protein has high homology with the aquaporins, including six transmembrane domains and two Asn–Pro–Ala (NPA) motifs. In particular, alanine 132 was found to be a critical residue as substitution resulted in altered conformation of the protein. The expression of Lsi1 is constitutive and occurs predominantly in the roots; however, it is regulated by silicic acid availability with constant silicic acid supply resulting in decreased expression. The transporter has been localized on the distal cells of exodermis and endodermis, and injection of the cDNA encoding Lsi1 into Xenopus laevis oocytes resulted in increased silicic acid transport (Ma and Yamaji, 2006). No homology of the Lsi1 gene from rice has been found with a gene family responsible for silicic acid transport in the marine diatom Cylindrotheca fusiformis (Hildebrand et al., 1998). Other plants investigated for their uptake of silicic acid are cucumber and tomato, moderate and low-level accumulators, respectively. For both species the uptake of silicic acid from the environment was found to be mediated by a transporter with the same affinity for silicic acid as Lsi1, but differences in transporter density on the lateral roots were detected, accounting for the variation in Si uptake. A second transporter has also been identified in rice which is responsible for xylem loading of Si, but in cucumber and tomato plants xylem loading occurs by passive diffusion (Mitani and Ma, 2005).
An elegant investigation of silicic acid uptake in wheat has been performed (Casey et al., 2003) using solution nuclear magnetic resonance (NMR) techniques to identify the molecular species containing Si. Developing seedlings were provided with an aqueous growth medium that included 98·7 at. % 29Si and the xylem exudate collected after excision of the shoots was measured for soluble Si by using a colorimetric method; the precise species were identified by solution state 29Si-NMR spectroscopy. The maximum concentration of dissolved silicate recorded in the 90-min sampling period was 8 mm, well above the solubility limit of silicic acid. However, NMR studies of the earliest exudates only detected monomeric and dimeric silicic acid although the concentrations detected by the two methods varied somewhat. It is a great pity that the later exudates were not examined by 29Si-NMR spectroscopy (nor by 1H- or 13C-NMR spectroscopy, which would have indicated whether organic components were present).
BIOGENIC SILICA STRUCTURES: MOLECULES TO MATERIALS
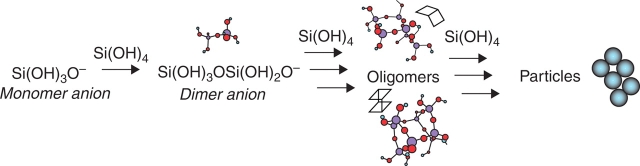
Plants, diatoms and sponges are capable of accumulating, storing and processing Si to create ornate hierarchical patterned biosilicas. The production of silica by organisms is carried out in an aqueous environment from under-saturated solutions of silicic acid, at atmospheric pressure and with temperatures ranging between 4 and 40 °C, and this production, amounting to gigatons per annum, vastly outweighs that produced industrially. The methods by which this can be achieved in organisms have been the subject of immense study and debate; however, it is apparent that the organic environment, including a vast range of proteins, carbohydrates, lipids, metal ions and (in plants) phenolic compounds, is likely to play a fundamental role (Perry and Lu, 1992; Harrison, 1996). The simplest form of silica is the monomer orthosilicic acid, a weakly acidic molecule (pKa 9·8) composed of Si tetrahedrally co-ordinated to four hydroxyl groups (Iler, 1979) that is found ubiquitously in soil at low concentrations (a few mg kg−1). It is in this form that plants are able to take up Si via the processes described above. Where the concentration is in excess of 100–200 mg kg−1, polycondensation reactions lead to: polymerization of monomers to form stable nuclei with critical size; growth of nuclei to form spherical particles; and aggregation of particles to form branched chains or structural motifs (Fig. 1) (Perry et al., 2003). As the silica particles grow and approach 1–3 nm in size (as found in nature), they carry a surface negative charge and these particles then interact with the local environment such as the plant cell wall. Many different factors affect the process of silica condensation, including the concentration of silicic acid, the temperature, the pH, and the presence of other ions, small molecules and polymers (Iler, 1979) but in all cases the materials that form are built up from SiO4 tetrahedra with variable Si–O–Si bond angles and Si–O bond distances. The materials are amorphous at the 1-nm length scale (Mann and Perry, 1986). They contain hydroxyl groups and, according to the reaction environment in which the mineral forms, silica from different organisms or precipitation conditions can vary greatly with regard to density, hardness, solubility, viscosity and composition (Perry et al., 2003).
Fig. 1.
The polymerization of monomeric silicic acid to form larger silica particles proceeds though various condensation reactions with dimers, oligomers and aggregates as intermediates.
The silica structures formed and their localization show great variation between individual plant families. In the Poaceae, silica is deposited as a 2·5-μm layer immediately beneath the cuticle layer with an Si–cuticle double layer being found in the leaf blades of rice. The silicified structures in the Poaceae can be subdivided into silica cells, dumb-bell-shaped cells located on vascular bundles and silica bodies found on bulliform cells of rice leaves. However, the silicification of cells is not restricted to the leaf blades and silicified cells are also found within the epidermis and vascular tissue of the stem, leaf sheath and hull (Prychid et al., 2004). As already discussed, the amount of silica present in the different parts of the rice plant is very variable. The silicified structures of Equisetum, examined by a number of researchers (Kaufman et al., 1971; Perry and Fraser, 1991; Holzhüter et al., 2003), are found on the epidermal surface of the entire cell wall primarily as discrete knobs and rosettes, which are themselves covered in spicules (Fig. 2). The thickness of this silica surface layer is dependent upon the location within the plant: thicknesses of 3–7 µm and 0·2–1·0 µm are observed in the stem and leaf, respectively. Characterization of the silica ultrastructures found in plant hairs from an example of the Poaceae (Perry et al., 1984), Equisetaceae (Perry and Fraser, 1991) and nettle stinging hairs (Hughes, 1989) revealed similar microstructural forms, including globular, fibrous and sheet-like structures with the distribution of these ultrastructural motifs being dependent on the anatomical region studied. The silicas formed in biological systems show a narrow particle size distribution for specific structural motifs.
Fig. 2.
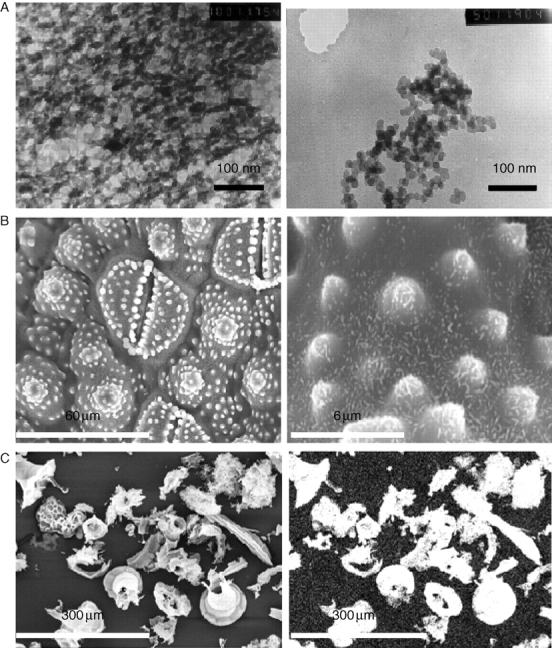
Electron micrographs of silica structures from plants. (A) Gel-like (left) and globular silica (right) from the early-diverging plant Equisetum arvense at very early stages of development of the silica structures. (B) Stomata of mature E. arvense surrounded by pilulae encrusted with rosettes; the observed specimens contain 0·1 % w/w C, with the remainder being silica; left, upper surface; right, lower surface. (C) Inorganic material from acid-digested Cucurbita (marrow) leaves (right-hand image shows the Si elemental map).
ENVIRONMENTAL INFLUENCES ON SILICA POLYMERISATION
Cell-wall extracts of rice shoots have been studied for the presence of Si-binding compounds (Inanaga and Okasaka, 1995). Phenol–carbohydrate complexes (obtained after treatment with cellulase) and lignin–carbohydrate complexes (extracted with dimethyl sulphoxide) subjected to chromatography produced eluates in which any Si present co-eluted with phenolic acids, lignin and carbohydrate in the void volume. Inanaga and Okasaka proposed that Si is combined in some way with the other species present although definitive evidence is yet to be provided. Examination of the silica phase of the early-diverging higher plants Equisetum telmateia and Equisetum arvense along with hairs from the lemma of the grass Phalaris canariensis has revealed an intimate association between the silica phase and the organic matrix (Harrison, 1996; Perry and Keeling-Tucker, 1998, 2003). Extraction and examination of the organic matrix by chemical methods varying in their ability to oxidize the plant cell wall revealed distinctive chemical compositions. The most easily extracted material was rich in hydroxylated amino acids (25 mol. %), acidic amino acids (25 mol. %) and glycine (20 mol. %). The second extract contained much lower levels of serine and threonine, higher levels of lysine, and glycine was largely replaced by proline. The increase in lysine continued in the third extract and high levels of proline and aliphatic amino acids were also observed. This increasingly basic material has been speculated to be involved in the regulation of silica nucleation (Perry and Keeling-Tucker, 1998, 2003). The composition of the cell-wall extracts in some ways mirrors that of the strongly cationic proline-rich protein isolated from systemically resistant cucumber plants (Kauss et al., 2003).
Investigation into the modes by which Si polymerization proceeds in vivo and the effects of the surrounding environment has led to the development of several laboratory-based model systems to monitor the processes of silicification in vitro. A variety of silicic acid precursors, including salts of silicates (e.g. diluted and buffered Na2SiO3; Coradin and Livage, 2001), salts of Si complexes (e.g. potassium silicon catecholate, K2[Si(C6H4O2)3]·xH2O; (Perry and Lu, 1992), alkoxysilanes (e.g. Si(OCH3)4 (TMOS) and Si(OC2H5)4 (TEOS)) and alkylsilanes (e.g. CH3Si(OCH3)3; Cha et al., 1999; Zhou et al., 1999), have been examined. All silicic acid precursor systems have their disadvantages: the presence of methanol, ethanol or catechol as breakdown products; very fast kinetics preventing detailed analysis of kinetics (alkoxysilanes); or incomplete breakdown to monomeric silicic acid (silicate solutions). The silicon catecholate complex (solubility up to about 100 mm) can be taken to and maintained at roughly physiological pH (approx. 7) for long periods of time without the need for additional buffers and the system exhibits relatively slow reactions thereby allowing the early stages of the condensation process as well as particle growth, etc., to be readily monitored. Colorimetric methods [molybdenum yellow and blue (the latter being more sensitive to low concentrations)], atomic absorption and inductively coupled plasma analysis are all methods that have been used to measure Si concentrations in solution (Iler, 1979; Perry, 2003). The colorimetric methods are most appropriate if changes in concentration with time are to be monitored but care must be taken in the preparation of the reagent solutions to ensure that the pH is optimized to ‘stop’ reactions (i.e. pH must be 1–2) and that the ‘Si’ species then measured result from the breakdown of specific Si-containing oligomers only (e.g. a 10-min delay between the addition of the two required reagents results in analysis of Si present in monomers or dimers only). Longer delay times allow larger oligomers to break down. Analysis of the materials formed is possible by a number of methods, including dynamic light scattering, gas adsorption and electron microscopy in order to determine particle sizes, aggregate characteristics, and porosity and surface area characteristics.
THE EFFECT OF BIOLOGICALLY RELEVANT SOLUTION ADDITIVES
Investigating the polymerization process in vitro allows the further elucidation of chemical processes involved in silicification in vivo. The effects of biomolecules extracted from plant systems and chemically ‘simpler’ compounds within a model system of Si polymerization allow the examination of chemical interactions between Si and functional groups in the formation of well-defined hierarchical silica structures. Cellulose, although a major cell-wall polymer, has little effect on reaction kinetics but affects the aggregation of particles into large thin sheets, indicating some degree of interaction between the organic and inorganic phases (Perry and Lu, 1992). Tetramers of glucose were the smallest oligosaccharides able to regulate particle growth and aggregation (Harrison and Lu, 1994). Studies on proteins, peptides and amino acids have been more extensive although few experiments have been conducted using protein-containing isolates from plants. Isolates from Equisetum telmateia and plant hairs from Phalaris canariensis obtained by acid digestion of the cell wall followed by solubilization of the protein-containing materials by buffered HF treatment have been assessed for their effects on polymerization, particle growth and aggregation (Perry and Keeling-Tucker, 1998, 2003). Acceleration of the condensation process, the formation of smaller-diameter particles and organization into unusual silica structures at early stages of reaction were observed, indicating that the bio-derived material has an influence early in the silica formation process.
Complementary in-vitro studies using peptides and amino acids have also been performed (e.g. Coradin and Livage, 2001; Belton et al., 2004). Coradin and Livage studied the effect of four amino acids (lysine, serine, aspartic acid and proline) and their homopeptides (approx. 50 amino-acid units for poly-l-lysine, poly l-aspartic acid and poly-l-proline and approx. 130 amino-acid units for poly-l-serine) on silica formation using buffered solutions of diluted sodium silicate over a range of pH values; Belton et al. (2004) explored the effect of 11 amino acids (lysine, arginine, asparagine, glutamine, threonine, serine, tyrosine, glycine, alanine, glutamic acid and proline) together with homopeptides of glycine (1–5 amino-acid units) and lysine (1–5 amino-acids units) on silica formation from solutions containing monomeric silicic acid derived from an Si–catecholate complex. The nature of the side-chain and the length of homopeptide both affect the silica formation process. Positively charged side-chains showed the largest effect via electrostatic interactions between positively charged amino-acid side chains and negatively charged silica species. The isoelectric point (pI) of the amino acids correlates positively with acceleration of the early-stage reaction kinetics, whereas the hydrophobicity of the side-chain correlates with the surface area of the silica material formed. The addition of l-lysine homopeptides of increasing length (n = 1–5) to the model reaction system showed increases in rates of oligomer formation and aggregate growth, together with reduced silica surface area as n increased. The small positively charged peptides are thought to be able to bridge particles to produce extended structures.
Other macromolecules found in plants that have been examined in model reaction systems are the polyamines spermine, spermidine and putrescine, which are sometimes found conjugated to phenolic acids. Putrescine, a simple alkyldiamine, was able to increase the rate of condensation and the rate of particle aggregation (Belton et al., 2005a). The longer-chain polyamines, spermidine and spermine, influenced aggregation according to molecule length. Although only two natural polyamines were examined, these conclusions were verified by the analysis of a number of synthetically produced homologues (Belton et al., 2005b).
FUTURE ASPECTS
In order fully to understand silica formation in vivo, it is necessary to study the environment in which silica formation occurs. Cell-wall proteins are most easily examined owing to their direct link to the plant genetic code. One approach includes extraction and characterization of amino-acid sequence and structure; correlation to proteins with defined functions, if known; followed by exploration of these proteins in the formation of silica in vitro. An alternative approach is to determine the genes responsible for Si polymerization by use of molecular biology techniques such as subtractive cDNA libraries or microarrays [as carried out by Fauteux et al. (2006) to determine the defence-related genes expressed in Arabidopsis after treatment with Si]. The use of such molecular approaches should allow the rapid elimination of proteins involved in general regulatory functions rather than silica formation.
Analysis of the more abundant carbohydrate phase is more difficult because of the lack of a direct genetic link, making molecular techniques ineffective. The only current approach is the careful extraction of cell-wall polysaccharides with their analysis and quantification being performed by well-established techniques such as high-pH anion-exchange chromatography, mass spectroscopy and NMR. The extraction and analysis of glycosylated proteins is more difficult because of the sensitive nature of the oligosaccharide component and requires the use of non-aggressive techniques. The effect of the isolated biomolecules on silica polymerization can be tested in a range of model systems as described above.
Examination of the biomolecules involved in the controlled polymerization of silica within the plant is a complex process requiring their careful extraction and purification. This may best be achieved through standardization of techniques designed to look at the plant system as a whole, allowing each different component to be isolated and examined in its natural state. The use of defined biochemical techniques for this type of study [for which Fry (1988) is an excellent source] would help researchers to avoid methods of extraction and purification that result in the alteration of the biomolecule from its state in vivo.
In summary, we probably do not know all the functions and roles that ‘Si’ may play within higher plants. It is also reasonable to suggest that if plant growth studies where ‘Si’ has previously been ignored were revisited it is likely that the element in some form will be found to be involved in a range of cellular processes. Understanding of the effect of the ‘element’ on plant growth may lead to improved yields and resistance to disease for a wide range of higher plants.
ACKNOWLEDGEMENTS
We wish to thank Dr D. Belton and Mr G. Tilburey for the SEM images and the AFOSR for financial support.
LITERATURE CITED
- Bélanger RR, Benhamou N, Menzies JG. Cytological evidence of an active role of silicon in wheat resistance to powdery mildew (Blumeria graminis f. sp tritici) Phytopathology. 2003;93:402–412. doi: 10.1094/PHYTO.2003.93.4.402. [DOI] [PubMed] [Google Scholar]
- Belton D, Paine G, Patwardhan SV, Perry CC. Towards an understanding of (bio)silicification: the role of amino acids and lysine oligomers in silicification. Journal of Materials Chemistry. 2004;14:2231–2241. [Google Scholar]
- Belton D, Patwardhan SV, Perry CC. Putrescine homologues control silica morphogenesis by electrostatic interactions and the hydrophobic effect. Chemical Communications. 2005;27:3475–3477. doi: 10.1039/b504310g. [DOI] [PubMed] [Google Scholar]
- Belton DJ, Patwardhan SV, Perry CC. Spermine, spermidine and their analogues generate tailored silcas. Journal of Materials Chemistry. 2005;15:4629–4638. [Google Scholar]
- Casey WH, Kinrade SD, Knight CTG, Rains DW, Epstein E. Aqueous silicate complexes in wheat. Triticum aestivum L. Plant, Cell and Environment. 2003;27:49–52. [Google Scholar]
- Cha JN, Shimizu K, Zhou Y, Christiansen SC, Chmelka BF, Stcuky GD, Morse DE. Silicatein filaments and subunits from a marine sponge direct the polymerization of silica and silicones in vitro. Proceedings of the Natural Academy of Sciences. 1999;96:361–365. doi: 10.1073/pnas.96.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-H, Lewin J. Silicon as a nutrient element for Equisetum arvense. Canadian Journal of Botany. 1969;47:125–131. [Google Scholar]
- Chérif M, Asselin A, Bélanger RR. Defense responses induced by soluble silicon in cucumber roots infected by Pythium spp. Phytopathology. 1994;84:236–242. [Google Scholar]
- Coradin T, Livage J. Effect of some amino acids and peptides on silicic acid polymerisation. Colloids and Surfaces B: Biointerfaces. 2001;21:329–336. doi: 10.1016/s0927-7765(01)00143-6. [DOI] [PubMed] [Google Scholar]
- Epstein E. The anomaly of silicon in plant biology. Proceedings of the National Academy of Science. 1994;91:11–17. doi: 10.1073/pnas.91.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E. Silicon. Annual Review of Plant Physiology and Plant Molecular Biology. 1999;50:641–664. doi: 10.1146/annurev.arplant.50.1.641. [DOI] [PubMed] [Google Scholar]
- Fauteux F, Rémus-Borel W, Menzies JG, Bélanger RR. Silicon and plant disease resistance against pathogenic fungi. FEMS Microbiology Letters. 2005;249:1–6. doi: 10.1016/j.femsle.2005.06.034. [DOI] [PubMed] [Google Scholar]
- Fauteux F, Chain F, Belzile F, Menzies JG, Bélanger RR. The protective role of silicon in the Arabidopsis–powdery mildew pathosystem. Proceedings of the National Academy of Science USA. 2006;103:17554–17559. doi: 10.1073/pnas.0606330103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawe A, Abou-Zaid M, Menzies JG, Bélanger RR. Silicon-mediated accumulation of flavinoid phytoalexins in cucumber. Phytopathology. 1998;88:396–401. doi: 10.1094/PHYTO.1998.88.5.396. [DOI] [PubMed] [Google Scholar]
- Fry SC. The growing plant cell wall: chemical and metabolic analysis. Harlow: Longman Scientific and Technical; 1988. [Google Scholar]
- Fry SC. Primary cell wall metabolism: tracking the careers of wall polymers in living plant cells. New Phytologist. 2004;161:641–675. doi: 10.1111/j.1469-8137.2004.00980.x. [DOI] [PubMed] [Google Scholar]
- Harrison CC. Evidence for intramolecular macromolecules containing protein from plant silicas. Phytochemistry. 1996;41:37–42. doi: 10.1016/0031-9422(95)00576-5. [DOI] [PubMed] [Google Scholar]
- Harrison CC, Lu Y. In vivo and in vitro studies of polymer controlled silicification. Bulletin de l'Institute Oceanographique. 1994;14:151–158. [Google Scholar]
- Hildebrand M, Dahlin K, Volcani BE. Characterization of a silicon transporter gene family in Cylindrotheca fusiformis: sequences, expression analysis, and identification of homologs in other diatoms. Molecular and General Genetics. 1998;260:480–486. doi: 10.1007/s004380050920. [DOI] [PubMed] [Google Scholar]
- Hodson MJ, White PJ, Mead A, Broadley MR. Phylogenetic variation in the silicon composition of plants. Annals of Botany. 2005;96:1027–1046. doi: 10.1093/aob/mci255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzhüter G, Narayanan K, Gerber T. Structure of silica in Equisetum arvense. Analytical and Bioanalytical Chemistry. 2003;376:512–517. doi: 10.1007/s00216-003-1905-2. [DOI] [PubMed] [Google Scholar]
- Hughes NP. In vivo and in vitro studies of biomineralization processes. Oxford University; 1989. PhD thesis. [Google Scholar]
- Iler RK. The chemistry of silica. New York: John Wiley & Sons; 1979. [Google Scholar]
- Inanaga S, Okasaka A. Calcium and silicon binding compounds in cell walls of rice shoots. Soil Science and Plant Nutrition. 1995;41:103–110. [Google Scholar]
- Kaufman PB, Bigelow WC, Schmid R, Ghosheh NS. Electron microprobe analysis of silica in epidermal cells of Equisetum. American Journal of Botany. 1971;58:309–316. [Google Scholar]
- Kauss H, Seehaus K, Franke R, Gilbert S, Dietrich RA, Kröger N. Silica deposition by a strongly cationic proline-rich protein from systemically resistant cucumber plants. The Plant Journal. 2003;33:87–95. doi: 10.1046/j.1365-313x.2003.01606.x. [DOI] [PubMed] [Google Scholar]
- Kidd PS, Llugany M, Gunsé B, Barceló J. The role of root exudates in aluminium resistance and silicon induced amelioration of aluminium toxicity in three varieties of maize (Zea mays L.) Journal of Experimental Botany. 2001;52:1339–1352. [PubMed] [Google Scholar]
- Liang Y, Sun W, Zhu Y-G, Christie P. Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: a review. Environmental Pollution. 2007;147:422–428. doi: 10.1016/j.envpol.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Ma J-F, Yamaji N. Silicon uptake and accumulation in higher plants. Trends in Plant Science. 2006;11:392–397. doi: 10.1016/j.tplants.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Mann S, Perry CC. Structural aspects of biogenic silica. Ciba Foundation Symposium. 1986;121:40–58. doi: 10.1002/9780470513323.ch4. [DOI] [PubMed] [Google Scholar]
- Mitani N, Ma J-F. Uptake system of silicon in different plant species. Journal of Experimental Biology. 2005;56:1255–1261. doi: 10.1093/jxb/eri121. [DOI] [PubMed] [Google Scholar]
- Moussa HR. Influence of exogenous application of silicon on physiological response of salt stressed maize (Zea mays L.) International Journal of Agriculture and Biology. 2006;8:293–297. [Google Scholar]
- Neuman D, zur Nieden U. Silicon and heavy metal tolerance of higher plants. Phytochemistry. 2001;56:685–692. doi: 10.1016/s0031-9422(00)00472-6. [DOI] [PubMed] [Google Scholar]
- Perry CC. Silicification: the processes by which organisms capture and mineralize silica. Reviews in Mineralogy and Geochemistry. 2003;54:291–327. [Google Scholar]
- Perry CC, Fraser MA. Silica deposition and ultrastructure in the cell wall of Equisetum arvense: the importance of cell wall structures and flow control in biosilicification? Philosophical Transactions of the Royal Society of London B. 1991;334:149–157. [Google Scholar]
- Perry CC, Keeling-Tucker T. Crystalline silica prepared at room temperature from aqueous solution in the presence of intrasilica bioextracts. Chemical Communications. 1998;23:2587–2588. [Google Scholar]
- Perry CC, Keeling-Tucker T. Model studies of colloidal silica precipitation using biosilica extracts from Equisetum telmatia. Colloid and Polymer Science. 2003;281:652–664. [Google Scholar]
- Perry CC, Lu Y. Preparation of silicas from silicon complexes: role of cellulose in polymerisation and aggregation control. Faraday Transactions. 1992;88:2915–2921. [Google Scholar]
- Perry CC, Mann S, Williams RJP. Structural and analytical studies of the silicified macrohairs from the lemma of the grass Phalaris canariensis L. Proceedings of the Royal Society of London B. 1984;222:427–438. [Google Scholar]
- Perry CC, Belton D, Shafran K. Studies of biosilicas; structural aspects, chemical principles, model studies and the future. Progress in Molecular and Subcellular Biology. 2003;33:269–299. doi: 10.1007/978-3-642-55486-5_11. [DOI] [PubMed] [Google Scholar]
- Prychid CJ, Rudall PJ, Gregory M. Systematics and biology of silica bodies in monocotyledons. The Botanical Review. 2004;69:377–440. [Google Scholar]
- Rodrigues FA, Benhamou N, Datnoff LE, Jones JB, Bélanger RR. Ultrastructural and cytochemical aspects of silicon-mediated rice blast resistance. Phytopathology. 2003;93:535–546. doi: 10.1094/PHYTO.2003.93.5.535. [DOI] [PubMed] [Google Scholar]
- Shi Q, Bao Z, Zhu Z, He Y, Qian Q, Yu J. Silicon mediated alleviation of Mn toxicity in Cucumis sativus in relation to activities of superoxide dismutase and ascorbate peroxidase. Phytochemistry. 2005;66:1551–1559. doi: 10.1016/j.phytochem.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Simpson TL, Volcani BE. Silicon and siliceous structures in biological systems. New York: Springer; 1981. [Google Scholar]
- Sommer M, Kaczorek D, Kuzyakov Y, Breuer J. Silicon pools and fluxes in soils and landscapes – a review. Journal of Plant Nutrition and Soil Science. 2006;169:310–329. [Google Scholar]
- Takahashi E, Ma J-F, Miyake Y. The possibility of silicon as an essential element for higher plants. Comments on Agricultural and Food Chemistry. 1990;2:99–122. [Google Scholar]
- Yoshida S, Ohnishi Y, Kitagishi K. Histochemistry of silicon in rice plant III. The presence of cuticle-silica double layer in the epidermal tissue. Soil Science and Plant Nutrition. 1962;8:1–5. [Google Scholar]
- Van Hoest PJ. Rice straw, the role of silica and treatments to improve quality. Animal Feed Science and Technology. 2006;130:137–171. [Google Scholar]
- Wang L, Nie Q, Li M, Zhang F, Zhuang J, Yang W, et al. Biosilicified structures for cooling plant leaves: a mechanism of highly efficient midinfrared thermal emission. Applied Physics Letters. 2005;87:194105. [Google Scholar]
- Zhou Y, Shimizu K, Cha JN, Stucky GD, Morse DE. Efficient catalysis of polysiloxane synthesis by silicatein α requires specific hydroxyl and imidazole functionalities. Angewandte Chimie International Edition. 1999;38:780–782. doi: 10.1002/(SICI)1521-3773(19990315)38:6<779::AID-ANIE779>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Wei G, Li J, Qian Q, Yu J. Silcon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.) Plant Science. 2004;167:527–533. [Google Scholar]