Abstract
Background and Aims
Plant cells undergo cell expansion when a temporary imbalance between the hydraulic pressure of the vacuole and the extensibility of the cell wall makes the cell volume increase dramatically. The primary cell walls of most seed plants consist of cellulose microfibrils tethered mainly by xyloglucans and embedded in a highly hydrated pectin matrix. During cell expansion the wall stress is decreased by the highly controlled rearrangement of the load-bearing tethers in the wall so that the microfibrils can move relative to each other. Here the effect was studied of a purified recombinant xyloglucan endotransglucosylase/hydrolase (XTH) on the extension of isolated cell walls.
Method
The epidermis of growing onion (Allium cepa) bulb scales is a one-cell-thick model tissue that is structurally and mechanically highly anisotropic. In constant load experiments, the effect of purified recombinant XTH proteins of Selaginella kraussiana on the extension of isolated onion epidermis was recorded.
Key Results
Fluorescent xyloglucan endotransglucosylase (XET) assays demonstrate that exogeneous XTH can act on isolated onion epidermis cell walls. Furthermore, cell wall extension was significantly increased upon addition of XTH to the isolated epidermis, but only transverse to the net orientation of cellulose microfibrils.
Conclusions
The results provide evidence that XTHs can act as cell wall-loosening enzymes.
Key words: XTH, XET activity, primary cell wall, xyloglucan, cellulose microfibrils, cellulose orientation, cell expansion, cell wall loosening, extensiometry, onion (Allium cepa), Selaginella kraussiana
INTRODUCTION
The indeterminate growth of plants results from the continuous formation of new cells by division in groups of stem cells or meristems, followed by a considerable increase in volume (Lyndon, 1990). A young cell may grow to >1000 times its original volume, thus making cell expansion a central player in the primary production of biomass. Directionality in expansion leads to the variety in shapes of plants and plant parts.
Theoretically, the biophysics of cell expansion are rather simple (Lockhart, 1965). The turgor or hydrostatic pressure of a plant cell is opposed by its surrounding primary cell wall, which consists of cellulose fibrils tethered by hemicelluloses, principally xyloglucans in the majority of seed plant species. This load-bearing cellulose/xyloglucan network is embedded in an amorphous matrix of pectins and glycoproteins (Carpita and Gibeaut, 1993; Brett and Waldron, 1996). Expansion results from a carefully controlled, turgor-driven polymer creep, during which the cellulose microfibrils move relative to each other (Marga et al., 2005). The control of this process lies in the selective loosening and rearrangement of the load-bearing cellulose/xyloglucan network.
In most plant tissues, cell expansion is anisotropic, i.e. it occurs in a preferential direction. Experimental evidence confirms that the predominant cellulose microfibril orientation in the wall defines the direction of growth anisotropy (Kerstens and Verbelen, 2003; Baskin, 2005), and that cells usually grow transversely to this orientation. The precise molecular basis of wall loosening and expansion remains, however, elusive (Thompson, 2005).
Expansins were proposed as a class of proteins that act as wall-loosening agents (Cosgrove, 2000) and are believed to break the load-bearing hydrogen bonds between xyloglucans and cellulose. The evidence is manifold: they restore the acid growth of heat-inactivated isolated cell walls; their exogenous application and ectopic expression stimulates growth; silencing them decreases growth; and their expression correlates well in time and space with growth (reviewed in Cosgrove, 2005).
Besides expansins, xyloglucan endotransglucosylase/endohydrolases (XTHs) were also suggested to loosen cell walls (Fry et al., 1992; Nishitani and Tominaga, 1992). Most XTHs cut and rejoin the xyloglucans that tether adjacent cellulose microfibrils [=xyloglucan endotransglucosylase (XET) action], whereas only some XTHs use water as acceptor substrate, resulting in xyloglucan hydrolysis [=xyloglucan hydrolase (XEH) action] (Rose et al., 2002; Nishitani and Vissenberg, 2007). Thus XET action enables xyloglucan restructuring (Thompson and Fry, 2001), potentially allowing cellulose microfibrils to move apart but then reinstating the mechanical strength of the wall to withstand lysis. As for expansin, XTH gene expression coincides with growth (Vissenberg et al., 2005), as does the enzymatic action in situ (Vissenberg et al., 2000, 2001, 2003). Also lower expression of AtXTH18 and AtXTH27 results in phenotypic changes (Matsui et al., 2005; Osato et al., 2006). However, direct unambiguous proof of XTHs inducing wall stress relaxation and extension is still lacking. The mechanical properties of frozen/thawed cucumber hypocotyl segments were not affected by exogenous tomato XTH (Saladie et al., 2006), whereas XTHs were reported to induce extension in two other systems. A tomato pericarp XTH induced creep in biaxial strain assays of artificial cellulose–xyloglucan composites (Chanliaud et al., 2004). The relevance was, however, criticized because the behaviour of artificial composites cannot always be extrapolated to the behaviour of real plant cell walls (Cosgrove, 2005). On the other hand, the XTH-induced extensibility of isolated azuki bean hypocotyl walls in force/extension assays (Kaku et al., 2002) was disputed (Saladie et al., 2006), given the extreme experimental conditions (very long incubation time of 48 h at 37 °C) and the expected activity of endogenous expansins and XTHs (because no heat inactivation had been applied).
An XTH, SkXTH1, from the lycopodiophyte Selaginella, that belongs to the group I XTHs from seed plants, has recently been cloned. Enzymatic characterization of the purified heterologously expressed protein revealed that it exclusively performs XET activity over a broad pH and temperature range (Van Sandt et al., 2006).
The epidermis of onion bulb scales is an adequate model to study anisotropy in extension (Kerstens et al., 2001; Suslov and Verbelen, 2006). Its thick outer periclinal wall defines the tissue's mechanical strength and has a clearly defined mean or net cellulose microfibril orientation.
The effect of SkXTH1 on the mechanical properties of the epidermis of growing onion bulb scales in constant-load experiments was studied, avoiding the criticisms raised against the other studies.
MATERIALS AND METHODS
Plant material and sample preparation
Onion (Allium cepa L. ‘Bonkajuin’) bulbs were collected in a field at the beginning of June at the stage of active growth. These bulbs had an average diameter of 27 mm, whereas they can reach 100 mm at the moment of harvest. Strips of epidermis, 20–25 mm long and 4 mm wide, were peeled from the equatorial part of the adaxial side of the fifth (from the outside) live scale in directions parallel and transverse to the axis of the bulb. The epidermal strips were immediately frozen in liquid nitrogen and stored at −20 °C.
Determination of the main orientation of cellulose microfibrils
Visualization of the net orientation of cellulose microfibrils was based on the fluorescence dichroism of Congo Red and was erformed according to Verbelen and Kerstens (2000). Onion epidermal strips were incubated in a 1 % (w/v) solution of Congo Red (Merck CI22120) in water for 2 h and rinsed with water. The stained samples were studied with a confocal laser scanning microscope (Nikon C1) with a 20× (NA 0·5) dry objective.
SkXTH1 and crude expansin extract
SkXTH1 (accession no. AY580314) was produced and purified as described before (Van Sandt et al., 2006); a crude expansin extract was prepared as in McQueen-Mason et al. (1992).
In vivo XET assay
The in vivo localization of XET action in onion epidermal strips was determined (Vissenberg et al., 2000) in a 50 mM sodium acetate pH 4·5, 300 mm NaCl and 10 % glycerol buffer. SkXTH1 was added to a final concentration of 125 ng µL−1. Control samples contained the same concentration of bovine serum albumin (BSA). All assays were conducted in the dark at 22 °C. Imaging was done with a Zeiss Axioskop equipped for epifluorescence using 20× (NA 0·40) and 40× (NA 0·75) dry objective lens and a Nikon DXM1200 digital camera. For valid comparison, images were recorded at identical settings of the microscope and camera.
Constant-load extensiometry
In vitro extension of onion epidermal cell walls was measured with a custom-built constant-load extensiometer as described in Suslov and Verbelen (2006).
A 5 mm segment of an epidermal strip was secured between the clamps and pre-incubated in the experimental buffer/enzyme solution for 5 min. Two buffers were used: 50 mm sodium acetate at pH 4·5 containing 300 mm sodium chloride and 10 % glycerol, or 50 mm sodium phosphate at pH 6 with 300 mM sodium chloride and 10 % glycerol. SkXTH1, when present, was at a final concentration of 300 ng µL−1. High concentrations of NaCl and glycerol in the buffers were needed for preservation of SkXTH1 activity. These components had no effect on the cell wall extensibility on their own. The crude expansin extract was assayed under the same conditions as for SkXTH1.
Taking into account the cell dimensions and the wall thickness of the onion epidermis tissue used, a load of 10 g was appropriate to generate wall stress similar to that reported in experiments on tomato and sunflower epidermis (Hejnowicz and Sievers, 1996; Thompson, 2001) and maize coleoptiles (Schopfer, 2001), and slightly below the natural stresses produced by turgor (Wei and Lintilhac, 2003). Application of a 10 g load resulted in a practically instantaneous cell wall extension, which was followed by a slow time-dependent deformation (creep). Unless stated otherwise, the interval from 10 to 30 min after loading was used for comparison of the effects of different treatments. Earlier deformations (from 0 to 10 min after the load application) were usually not shown since they mostly characterize internal material properties of onion cell walls having no relationship to growth regulation (Suslov and Verbelen, 2006).
Statistical analyses
The significance of differences between transverse and longitudinal load application under each condition was determined by the Student's two-tailed t-test. Differences between the effects of the pH 4·5 and the pH 6 buffers on native or heat-inactivated cell walls were revealed separately in the group of transverse and longitudinal epidermal strips by the Fisher test (P < 0·01) and were subsequently analysed using the Newman–Keuls test for multiple comparisons. The significance of the XTH effect on longitudinal or transverse strips was determined using the paired two-tailed t-test.
RESULTS
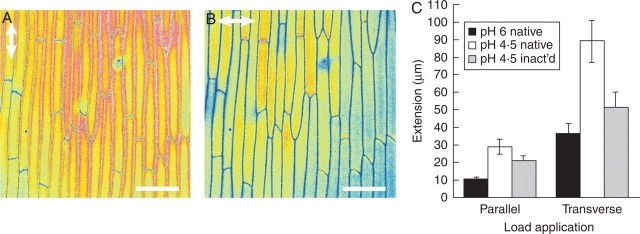
The net orientation of cellulose microfibrils in isolated adaxial epidermal cell walls was analysed using the fluorescence of Congo Red coupled to polarization confocal microscopy (Verbelen and Kerstens, 2000). The intensity of fluorescence was higher when the vector of the excitation light (see double arrowhead) was parallel to the long axis of the cells than when it was transverse to it (Fig. 1A, B), indicating a net longitudinal cellulose orientation.
Fig. 1.
Structural and mechanical differences between longitudinal and transverse onion epidermal strips. (A, B) Polarization confocal micrographs of the same Congo Red-stained onion epidermal cell walls with colour-coded fluorescence intensity (low = blue, high = red). The vector of polarization of the laser is indicated by the arrows. The scale bar is 100 mm. (C) Extension of isolated cell walls (between 10 and 30 min) when load is applied at pH 4·5 and 6 and of native and heat-inactivated specimens parallel and transverse to the net cellulose orientation. Data are mean ± s.e., n = 6–9.
To characterize the mechanical properties of the onion epidermis, a load was applied transverse and parallel to the net orientation of the cellulose microfibrils and the resulting extension was analysed. The deformation (see Table 1) occurring from 0 to 10 min after load application was not taken into account as it mostly reflects intrinsic wall properties not related to growth regulation (Büntemeyer et al., 1998; Suslov and Verbelen, 2006). Extensions are therefore expressed as the creep occurring between 10 and 30 min after load application. Figure 1C indicates that the magnitude of extension of frozen/thawed epidermal strips was clearly always higher when load was applied transversely to the cellulose orientation. Extension of the epidermis was pH dependent in both directions as it was nearly three times higher at pH 4·5 than at pH 6. The results indicate an acid growth behaviour of the cell wall samples.
Table 1.
Time course of the effect of exogenous SkXTH1 on the transverse extension of heat-inactivated onion epidermal strips
| Extension (mm) |
||||
|---|---|---|---|---|
| Time after load application | Control | SkXTH1 | SkXTH1 to control, % | P-value (paired t-test) |
| 0 s–1 s | 1968·0 ± 226·2 | 2073·9 ± 167·7 | 105·4* | 0·46 |
| 1 s–5 min | 112·4 ± 21·7 | 107·9 ± 15·9 | 96·0* | 0·79 |
| 5–10 min | 22·3 ± 4·2 | 28·0 ± 4·8 | 125·7* | 0·07 |
| 10–15 min | 15·9 ± 3·0 | 22·5 ± 3·7 | 141·5 | 0·0092 |
| 15–20 min | 13·2 ± 2·4 | 19·8 ± 3·3 | 149·8 | 0·0037 |
| 20–25 min | 11·7 ± 2·0 | 17·7 ± 3·0 | 151·6 | 0·0027 |
| 25–30 min | 10·4 ± 1·7 | 16·0 ± 2·7 | 153·8 | 0·0014 |
Onion epidermal strips were extended transverse to the net cellulose orientation by a 10 g load (after a 5 min pre-incubation) in the pH 4·5 buffer alone (control) or in the pH 4·5 buffer with SkXTH1 (300 ng µL−1). Data are means ± s.e. (n = 9).
* The difference was not significant.
In order to define the possible involvement of endogenous enzymes and other proteins in the acid-induced extension, the effect of heat inactivation was studied. Immersion of the epidermis in boiling water for 15 s strongly reduced extension transverse to the cellulose orientation (Fig. 1C). However, no significant difference in extension was found between native and heat-inactivated samples extended parallel to the long axis. Thus heat inactivation had a directional effect on extension. Increasing the time of heat inactivation to 30 s gave no further inhibitory effect on extension, and boiling for 60 s was damaging the wall samples, as they curled up and eventually broke after loading (results not shown). Therefore, 15 s of heat inactivation is sufficient to denature endogenous wall proteins without any damaging effect on the cell wall structure. The fact that creep of the heat-inactivated epidermis extended parallel to the cellulose orientation was still highly pH dependent indicates that the acid-induced extension in that direction is essentially not protein mediated. This finding is quite unexpected for higher plants; however, similar observations were made for algae (Tepfer and Cleland, 1979).
The ability of exogenous SkXTH1 to penetrate and act upon the onion epidermal cell wall was tested using a fluorescent in muro assay for XET action (Vissenberg et al., 2000). In Fig. 2 the fluorescent signal is caused by the incorporation of fluorescently labelled xyloglucan oligosaccharides into the onion cell wall by specific XET action of XTHs. Compared with control samples (Fig. 2A), addition of exogenous SkXTH1 caused a much brighter fluorescence (Fig. 2B). Both observations confirm that SkXTH1 penetrates the model cell walls and effectively displays XET action. The absence of clear fluorescence when non-XET substrates were used proves the specificity of the assay (Fig. 2C). Parallel experiments proved that the enzyme's action was not specific for onion epidermis, as SkXTH1 was found to penetrate and act upon Arabidopsis roots as well (results not shown).
Fig. 2.
Effect of exogenous SkXTH1 on XET action in onion epidermal strips. (A) Orange fluorescence is indicative of the incorporation of fluorescent xyloglucan oligosaccharides into the onion epidermal cell walls by native cell wall XTHs. (B) Addition of SkXTH1 resulted in a much brighter signal, confirming the penetration and action of exogenous SkXTH1 into onion cell walls. (C) Control assay with a non-XET substrate (fluorescent trisaccharide) shows no incorporated fluorescence, proving the specificity of the assay. The scale bar is 100 µm.
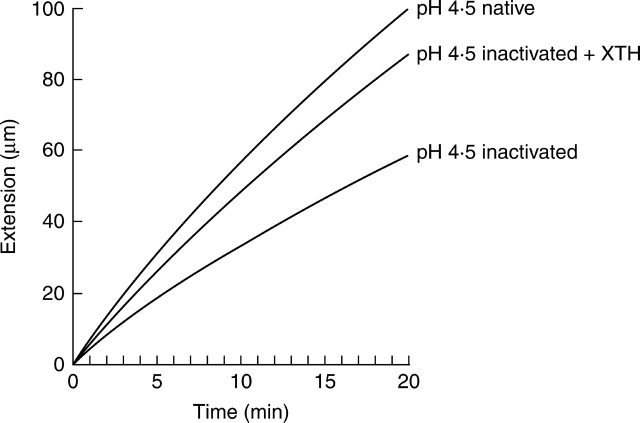
To monitor the specific effect of SkXTH1 on the extensibility of cell walls, heat-inactivated cell wall samples were used exclusively in extensiometric experiments at pH 4·5. Addition of SkXTH1 (300 ng µL−1 in 200 µL of buffer) caused a substantial increase in cell wall extensibility transverse to the net cellulose orientation (Fig. 3). Parallel to the cellulose orientation, no significant change in extensibility was measured. Table 1 shows the dynamics of the enzyme effect from the moment of load application. SkXTH1 started stimulating the epidermis extension after a lag period of about 5 min. This stimulation gradually increased in time. The enzyme effect was also checked at later time points. It was found that the SkXTH1-induced stimulation of wall extension continued at least from 30 to 60 min after load application (results not shown). For ease of comparison, the time course of the extensions transverse to the cellulose orientation under the different experimental conditions is given for a representative sample in Fig. 4. Heat inactivation of the cell walls clearly reduced the extension, but addition of SkXTH1 restored on average 66 % of the protein-dependent creep that was lost during boiling.
Fig. 3.
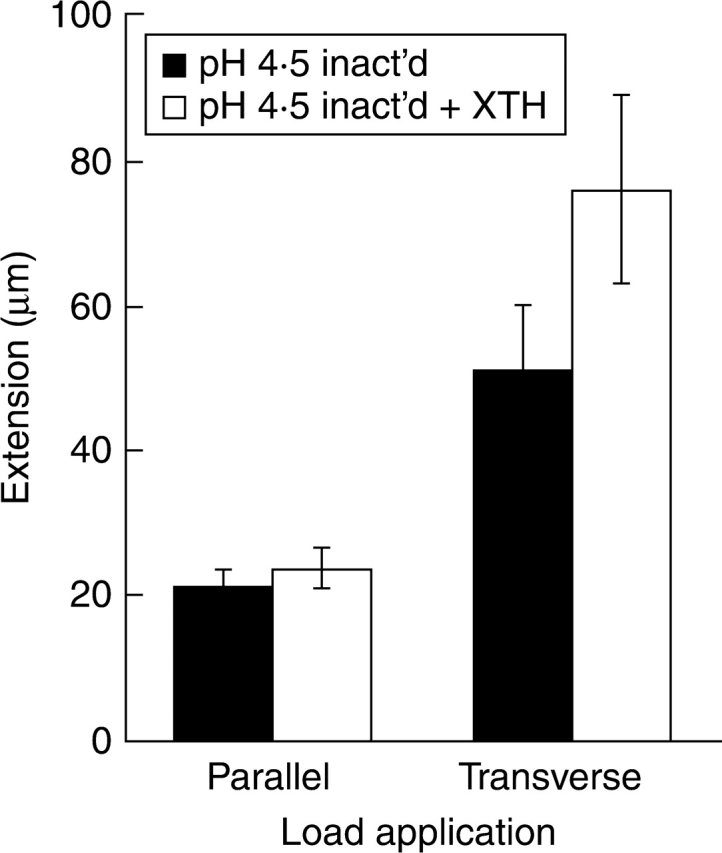
Effect of exogenous SkXTH1 (300 ng mL−1) on the extension of heat-inactivated (boiling in water for 15 s) longitudinal and transverse onion epidermal strips at pH 4·5. The enzyme stimulated extension transverse to the net cellulose orientation (P < 0·01, paired two-tailed t-test) but not parallel to it. Data are mean ± s.e., n = 9.
Fig. 4.
Time course of the extension during load application transverse to the net cellulose orientation. The deformation, which occurs during the first 10 min of load application, is not shown. The graphs represent typical curves for the extension between 10 and 30 min under the different experimental conditions. Heat inactivation greatly decreases the extensibility. Addition of SkXTH1 to the inactivated transverse wall samples largely restores the extensibility at pH 4·5.
In a control experiment (n = 3), a crude cucumber expansin extract in the same buffer conditions as used for SkXTH1 increased the extension of heat-inactivated cell walls by about 20 % (results not shown), confirming the adequacy of the model system.
DISCUSSION
The precise mechanism of plant cell wall expansion still remains to be elucidated. Despite the fact that expansins are sometimes considered as the only primary cell wall-loosening factors (Cosgrove, 2000, 2005), other enzymes were suggested for this function as well. Among them, XTHs were put forward as strong candidates (Fry et al., 1992). Extractable XET activity, XTH gene expression and enzymatic action were often correlated with growth (Fry et al., 1992; Hetherington and Fry, 1993; Pritchard et al., 1993; Potter and Fry, 1994; Xu et al., 1995; Palmer and Davies, 1996; Antosiewicz et al., 1997; Catalá et al., 1997; Vissenberg et al., 2000, 2003, 2005; Van Sandt et al., 2007), and lower expression of AtXTH18 and AtXTH27 resulted in phenotypic changes (Matsui et al., 2005; Osato et al., 2006). These observations support the wall-loosening hypothesis of XTHs. On the other hand, XET activity was also detected in non-expanding tissues (Pritchard et al., 1993; Palmer and Davies, 1996). In this case, cell wall-tightening processes could over-ride the wall-loosening effect of active XTHs.
The effect of exogenous XTH on the extensibility of isolated onion epidermis was studied, avoiding the criticisms (Cosgrove, 2005) that were raised against two other studies on mechanical effects of exogenous XTHs (Kaku et al., 2002; Chanliaud et al., 2004). The XTH used in the present study, SkXTH1, is from S. kraussiana and belongs to the group I XTHs which, together with group II XTHs of angiosperms, are thought to mediate exclusively XET activity, whereas members of group III are shown to mediate XEH activity (Rose et al., 2002). SkXTH1 was heterologously expressed in Pichia, purified and found to display XET activity but, indeed, no XEH activity (Van Sandt et al., 2006). Recently the epidermis of onion bulb scales was introduced as a one-cell-layer model tissue to define the relationship between anisotropic cellulose orientation and cell wall creep rate (Suslov and Verbelen, 2006). These epidermal cells have a type I primary cell wall, which is typical of all flowering plants except the grasses, the sedges, the rushes, the palms and the gingers (Carpita and Gibeaut, 1993; Carpita, 1996).
The onion cell walls used had a net longitudinal cellulose orientation as indicated by Congo Red staining coupled to polarization confocal microscopy. In agreement with this, the magnitude of extension of frozen/thawed epidermal strips was clearly higher when load was applied transversely to the cellulose orientation, irrespective of the treatment used. Extensibility was greater in both directions at pH 4·5 than at pH 6, indicating that the cell walls exhibited acid-induced extension which is typical for walls isolated from growing tissues (Cosgrove and Li, 1993). The amount of acid-induced extension exceeds the values reported for bulbs close to maturity (Suslov and Verbelen, 2006). Apparently at the stage of active growth the ability of onion epidermal walls to be extended by a pH 4·5 buffer is much higher than at the subsequent stages. The effects of heat inactivation clearly suggest that the mechanisms of extension in the two directions are different. Heat inactivation strongly reduced extension transverse to the cellulose orientation, suggesting its dependence on undenatured proteins. The absence of a statistically significant effect of heat inactivation on extension parallel to the microfibril orientation means that in this direction the extension is essentially protein independent. These results demonstrate the mechanical anisotropy of the cell wall samples and the protein dependence of extension transverse to the net cellulose orientation. The higher extensibility of the walls transverse to the microfibril orientation agrees with the direction of expansion of these cells in planta, as the bulb scales used were indeed increasing in girth.
In absolute values, the rate of extension of the onion epidermis is only a fraction of that of cucumber hypocotyls, a frequently used model system. In the plant life cycle, the latter organ performs a transient but critically important function of exposing the cotyledons and the plumule to the aerial light environment. In darkness, the hypocotyls have extreme growth rates based on very fast cell elongation, and therefore they were used as models. The onion bulb, on the contrary, is a reserve organ consisting of relatively slowly growing dome-shaped transformed leaves. Consequently, the two experimental systems cannot be considered or compared as equals. The major concern should be that the rate of extension measured in vitro was comparable with the growth rate of the epidermis in vivo. During growth in the field, the fifth scale, as used in the experiments, increased in girth on average by 0·16 % every 20 min. The extension in vitro, in the same direction, was on average 1·25 % per 20 min. The higher values in vitro are not surprising. It is well documented that uniaxial stresses resulting from load application in vitro cause considerably higher extensions than equal multiaxial stresses (similar to those generated by turgor in vivo). The difference is explained by the cellulose microfibril reorientation in the direction of strain in the former case which is constrained in the latter case (Richmond et al., 1980). The in vitro extension rate values were, hence, of physiologically relevant size.
After visual confirmation that SkXTH1 penetrated the model cell walls and effectively displayed XET action, the effect of exogenous XTH on the extensibility of onion bulb epidermal cell walls was monitored. SkXTH1 restored on average 66 % of the protein-dependent creep that was lost by heat inactivation. This effect occurred only transverse to the net cellulose orientation, which indicates that SkXTH1 catalyses the movement of adjacent cellulose microfibrils relative to each other transverse to their net orientation. This is in agreement with the previous conclusion that the extension in this direction is protein dependent. It needs to be mentioned that besides XTHs, the xyloglucan metabolism itself also controls the elongation of plant cells. Addition of whole xyloglucan suppressed elongation, while addition of xyloglucan oligosaccharides accelerated elongation (Takeda et al., 2002). This could also partially explain why a full restoration of extension was not seen when XTHs were exogeneously added to heat-inactivated onion epidermis. Heat inactivation could influence the availability of oligosaccharides. In addition, the present finding suggests that the XET activity of XTH regulates the degree of growth anisotropy. As there are no data available on the effective concentration of XTH enzymes in the load-bearing part of the cell wall, the high concentration of 300 ng µL−1 was used. The enzymes have to diffuse into the cell wall. They can form enzyme–substrate complexes with or act upon XyG in the non-load-bearing outer layers of the wall but should still reach an effective concentration in the load-bearing inner part of the wall. The effect of SkXTH1 started after a lag period (Table 1), which might suggest that some time is indeed required for the enzyme to reach the load-bearing region. Interestingly, the effect of fungal endoglucanases on the creep in vitro also took place after a distinct lag period (Yuan et al., 2001). In a control experiment, a crude cucumber expansin extract restored part of the extensibility lost by heat inactivation, confirming the adequacy of the model system.
CONCLUSIONS
A purified group I XTH, displaying exclusively XET activity, is thus able to stimulate cell wall extension of a type I cell wall, and can act as a primary cell wall-loosening agent. The results should, however, not be generalized to all XTHs. Diverse XTHs may be functionally specialized, as is the case for expansins (Choi et al., 2006). Functional diversification is suggested by the expression patterns of several XTH genes even in non-expanding tissues (Vissenberg et al., 2005; Becnel et al., 2006). The inability of a tomato XTH to induce the extension of cucumber hypocotyl cell walls (Saladié et al., 2006) could, for example, result from the fact that this enzyme might not be involved in wall loosening at all, but that it could perform other very specialized functions (Maclachlan and Brady, 1994). Each XTH should therefore be analysed individually to reveal its precise role in cell wall mechanics.
ACKNOWLEDGEMENTS
V.V.S. is funded by a PhD-grant of the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT–Vlaanderen). K.V. is a Postdoctoral Fellow of the Research Foundation-Flanders (FWO). The authors acknowledge the financial support of the Research Foundation-Flanders (FWO), grants G.0101·04 and WO038·04 N, and Professor S. C. Fry for critical reading of the manuscript.
LITERATURE CITED
- Antosiewicz DM, Purugganan MM, Polisensky DH, Braam J. Cellular localization of Arabidopsis xyloglucan endotransglycosylase-related proteins during development and after wind stimulation. Plant Physiology. 1997;115:1319–1328. doi: 10.1104/pp.115.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin TI. Anisotropic expansion of the plant cell wall. Annual Review of Cell and Developmental Biology. 2005;21:203–222. doi: 10.1146/annurev.cellbio.20.082503.103053. [DOI] [PubMed] [Google Scholar]
- Becnel J, Natarajan M, Kipp A, Braam J. Developmental expression patterns of Arabidopsis XTH genes reported by transgenes and genevestigator. Plant Molecular Biology. 2006;61:451–467. doi: 10.1007/s11103-006-0021-z. [DOI] [PubMed] [Google Scholar]
- Brett CT, Waldron KW. Physiology and biochemistry of plant cell walls. 2nd edn. London: Chapman and Hall; 1996. [Google Scholar]
- Büntemeyer K, Lüthen H, Böttger M. Auxin-induced changes in cell wall extensibility of maize roots. Planta. 1998;204:515–519. [Google Scholar]
- Carpita NC. Structure and biogenesis of the cell walls of grasses. Annual Review of Plant Physiology and Plant Molecular Biology. 1996;47:445–476. doi: 10.1146/annurev.arplant.47.1.445. [DOI] [PubMed] [Google Scholar]
- Carpita N, Gibeaut DM. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. The Plant Journal. 1993;3:1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Catalá C, Rose JKC, Bennett A. Auxin regulation and spatial localization of an endo-1,4-β-d-glucanase and a xyloglucan endotransglycosylase in expanding tomato hypocotyls. The Plant Journal. 1997;12:417–426. doi: 10.1046/j.1365-313x.1997.12020417.x. [DOI] [PubMed] [Google Scholar]
- Chanliaud E, de Silva J, Strongitharm B, Jeronimidis G, Gidley MJ. Mechanical effects of plant cell wall enzymes on cellulose/xyloglucan composites. The Plant Journal. 2004;38:27–37. doi: 10.1111/j.1365-313X.2004.02018.x. [DOI] [PubMed] [Google Scholar]
- Choi D, Cho H-T, Lee Y. Expansins: expanding importance in plant growth and development. Physiologia Plantarum. 2006;126:511–518. [Google Scholar]
- Cosgrove DJ. Loosening of plant cell walls by expansins. Nature. 2000;407:321–326. doi: 10.1038/35030000. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. Growth of the plant cell wall. Nature Reviews Molecular and Cellular Biology. 2005;6:850–861. doi: 10.1038/nrm1746. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ, Li ZC. Role of expansin in cell enlargement of oat coleoptiles (analysis of developmental gradients and photocontrol) Plant Physiology. 1993;103:1321–1328. doi: 10.1104/pp.103.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry SC, Smith RC, Renwick KF, Martin DJ, Hodge SK, Matthews KJ. Xyloglucan endotransglycosylase, a new wall-loosening enzyme activity from plants. Biochemical Journal. 1992;282:821–828. doi: 10.1042/bj2820821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejnowicz Z, Sievers A. Tissue stresses in organs of herbaceous plants. III. Elastic properties of the tissues of sunflower hypocotyl and origin of tissue stresses. Journal of Experimental Botany. 1996;47:519–528. [Google Scholar]
- Hetherington PR, Fry SC. Xyloglucan endotransglycosylase activity in carrot cell suspensions during cell elongation and somatic embryogenesis. Plant Physiology. 1993;103:987–992. doi: 10.1104/pp.103.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaku T, Tabuchi A, Wakabayashi K, Kamisaka S, Hoson T. Action of xyloglucan hydrolase within the native cell wall architecture and its effect on cell wall extensibility in azuki bean epicotyls. Plant and Cell Physiology. 2002;43:21–26. doi: 10.1093/pcp/pcf004. [DOI] [PubMed] [Google Scholar]
- Kerstens S, Verbelen J-P. Cellulose orientation at the surface of the Arabidopsis seedling. Implications for the biomechanics in plant development. Journal of Structural Biology. 2003;144:262–270. doi: 10.1016/j.jsb.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Kerstens S, Decraemer WF, Verbelen J-P. Cell walls at the plant surface behave mechanically like fiber-reinforced composite materials. Plant Physiology. 2001;127:381–385. [PMC free article] [PubMed] [Google Scholar]
- Lockhart JA. An analysis of irreversible plant cell elongation. Journal of Theoretical Biology. 1965;8:264–275. doi: 10.1016/0022-5193(65)90077-9. [DOI] [PubMed] [Google Scholar]
- Lyndon RF. In: Plant development. The cellular basis. Blackman M, Chapman J, editors. London: Unwin Hyman; 1990. Topics in plant physiology: 3. [Google Scholar]
- Maclachlan G, Brady C. Endo-1,4-β-glucanase, xyloglucanase, and xyloglucan endo-transglycosylase activities versus potential substrates in ripening tomatoes. Plant Physiology. 1994;105:965–974. doi: 10.1104/pp.105.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marga F, Grandbois M, Cosgrove DJ, Baskin TI. Cell wall extension results in the coordinate separation of parallel microfibrils: evidence from scanning electron microscopy and atomic force microscopy. The Plant Journal. 2005;43:181–190. doi: 10.1111/j.1365-313X.2005.02447.x. [DOI] [PubMed] [Google Scholar]
- Matsui A, Yokoyama R, Seki M, Ito T, Shinozaki K, Takahashi T, Komeda Y, Nishitani K. AtXTH27 plays an essential role in cell wall modification during the development of tracheary elements. The Plant Journal. 2005;42:525–534. doi: 10.1111/j.1365-313X.2005.02395.x. [DOI] [PubMed] [Google Scholar]
- McQueen-Mason S, Durachko DM, Cosgrove DJ. Two endogenous proteins that induce cell wall extension in plants. Plant Cell. 1992;4:1425–1433. doi: 10.1105/tpc.4.11.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani K, Tominaga R. Endo-xyloglucan transferase, a novel class of glycosyltransferase that catalyzes transfer of a segment of xyloglucan molecule to another xyloglucan molecule. Journal of Biological Chemistry. 1992;267:21058–21064. [PubMed] [Google Scholar]
- Nishitani K, Vissenberg K. Roles of the XTH family in the expanding cell. In: Verbelen JP, Vissenberg K, editors. The expanding cell. Plant Cell Monographs. Vol. 5. Berlin: Springer; 2007. pp. 89–116. [Google Scholar]
- Osato Y, Yokoyama R, Nishitani K. A principal role for AtXTH18 in Arabidopsis thaliana root growth – a functional analysis using RNAi plants. Journal of Plant Research. 2006;119:153–162. doi: 10.1007/s10265-006-0262-6. [DOI] [PubMed] [Google Scholar]
- Palmer SJ, Davies WJ. An analysis of relative elemental growth rate, epidermal cell size and xyloglucan endotransglycosylase activity through the growing zone of ageing maize leaves. Journal of Experimental Botany. 1996;47:339–347. [Google Scholar]
- Potter I, Fry SC. Changes in xyloglucan endotransglycosylase (XET) activity during hormone-induced growth in lettuce and cucumber hypocotyls and spinach cell suspension cultures. Journal of Experimental Botany. 1994;45:1703–1710. [Google Scholar]
- Pritchard J, Hetherington PR, Fry SC, Tomos AD. Xyloglucan endotransglycosylase activity, microfibril orientation and the profiles of cell wall properties along growing regions of maize roots. Journal of Experimental Botany. 1993;44:1281–1289. [Google Scholar]
- Richmond PA, Métraux J-P, Taiz L. Cell expansion patterns and directionality of wall mechanical properties in Nitella. Plant Physiology. 1980;65:211–217. doi: 10.1104/pp.65.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JKC, Braam J, Fry SC, Nishitani K. The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: current perspectives and a new unifying nomenclature. Plant and Cell Physiology. 2002;43:1421–1435. doi: 10.1093/pcp/pcf171. [DOI] [PubMed] [Google Scholar]
- Saladie M, Rose JK, Cosgrove DJ, Catala C. Characterization of a new xyloglucan endotransglucosylase/hydrolase (XTH) from ripening tomato fruit and implications for the diverse modes of enzymic action. The Plant Journal. 2006;47:282–295. doi: 10.1111/j.1365-313X.2006.02784.x. [DOI] [PubMed] [Google Scholar]
- Schopfer P. Hydroxyl radical-induced cell-wall loosening in vitro and in vivo: implications for the control of elongation growth. The Plant Journal. 2001;28:679–688. doi: 10.1046/j.1365-313x.2001.01187.x. [DOI] [PubMed] [Google Scholar]
- Suslov D, Verbelen J-P. Cellulose orientation determines mechanical anisotropy in onion epidermis cell walls. Journal of Experimental Botany. 2006;57:2183–2192. doi: 10.1093/jxb/erj177. [DOI] [PubMed] [Google Scholar]
- Takeda T, Furuta Y, Awano T, Mizuno K, Mitsuishi Y, Hayashi T. Suppression and acceleration of cell elongation by integration of xyloglucans in pea stem segments. Proceedings of the National Academy of Sciences, USA. 2002;99:9055–9060. doi: 10.1073/pnas.132080299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepfer M, Cleland RE. A comparison of acid-induced cell wall loosening in Valonia ventricosa and in oat coleoptiles. Plant Physiology. 1979;62:898–902. doi: 10.1104/pp.63.5.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DS. Extensiometric determination of the reological properties of the epidermis of growing tomato fruit. Journal of Experimental Botany. 2001;52:1291–1301. [PubMed] [Google Scholar]
- Thompson DS. How do cell walls regulate plant growth? Journal of Experimental Botany. 2005;56:2275–2285. doi: 10.1093/jxb/eri247. [DOI] [PubMed] [Google Scholar]
- Thompson JE, Fry SC. Restructuring of wall-bound xyloglucan by transglycosylation in living plant cells. The Plant Journal. 2001;26:23–34. doi: 10.1046/j.1365-313x.2001.01005.x. [DOI] [PubMed] [Google Scholar]
- Van Sandt V, Guisez Y, Verbelen J-P, Vissenberg K. Analysis of a xyloglucan endotransglycosylase/hydrolase (XTH) from the lycopodiophyte Selaginella kraussiana suggests that XTH sequence characteristics and function are highly conserved during the evolution of vascular plants. Journal of Experimental Botany. 2006;57:2909–2922. doi: 10.1093/jxb/erl064. [DOI] [PubMed] [Google Scholar]
- Van Sandt V, Stieperaere H, Guisez Y, Verbelen J-P, Vissenberg K. XET activity is found near the sites of growth and cell elongation in bryophytes and some green algae: new insights into the evolution of primary cell wall elongation. Annals of Botany. 2007;99:39–51. doi: 10.1093/aob/mcl232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbelen J-P, Kerstens S. Polarization confocal microscopy and Congo red fluorescence: a simple and rapid method to determine the mean cellulose fibril orientation in plants. Journal of Microscopy. 2000;198:101–107. doi: 10.1046/j.1365-2818.2000.00691.x. [DOI] [PubMed] [Google Scholar]
- Vissenberg K, Martinez-Vilchez IM, Verbelen J-P, Miller JG, Fry SC. In vivo co-localization of xyloglucan endotransglycosylase activity and its donor substrate in the elongation zone of Arabidopsis roots. Plant Cell. 2000;12:1229–1238. doi: 10.1105/tpc.12.7.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissenberg K, Fry SC, Verbelen J-P. Root hair initiation is coupled to a highly localized increase of xyloglucan endotransglycoslyase action in Arabidopsis roots. Plant Physiology. 2001;127:1125–1135. [PMC free article] [PubMed] [Google Scholar]
- Vissenberg K, Van Sandt V, Fry SC, Verbelen J-P. Xyloglucan endotransglucosylase action is high in the root elongation zone and in trichoblasts of all vascular plants from Selaginella to Zea mays. Journal of Experimental Botany. 2003;54:335–344. doi: 10.1093/jxb/erg024. [DOI] [PubMed] [Google Scholar]
- Vissenberg K, Oyama M, Osato Y, Yokoyama R, Verbelen J-P, Nishitani K. Differential expression of AtXTH17, -18, -19 and -20 genes in Arabidopsis roots. Physiological roles in specification in cell wall construction. Plant and Cell Physiology. 2005;46:192–200. doi: 10.1093/pcp/pci013. [DOI] [PubMed] [Google Scholar]
- Wei C, Lintilhac PM. Loss of stability – a new model for stress relaxation in plant cell walls. Journal of Theoretical Biology. 2003;224:305–312. doi: 10.1016/s0022-5193(03)00167-x. [DOI] [PubMed] [Google Scholar]
- Xu W, Purugganan MM, Polisensky DH, Antosiewicz DM, Fry SC, Braam J. Arabidopsis TCH4, regulated by hormones and the environment, encodes a xyloglucan endotransglycosylase. Plant Cell. 1995;7:1555–1567. doi: 10.1105/tpc.7.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Wu Y, Cosgrove DJ. A fungal endoglucanase with plant cell wall extension activity. Plant Physiology. 2001;127:324–333. doi: 10.1104/pp.127.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]