Abstract
Background and Aims
The phylogenetic relationships between species of Coffea and Psilanthus remain poorly understood, owing to low levels of sequence variation recovered in previous studies, coupled with relatively limited species sampling. In this study, the relationships between Coffea and Psilanthus species are assessed based on substantially increased molecular sequence data and greatly improved species sampling.
Method
Phylogenetic relationships are assessed using parsimony, with sequence data from four plastid regions [trnL–F intron, trnL–F intergenic spacer (IGS), rpl16 intron and accD–psa1 IGS], and the internal transcribed spacer (ITS) region of nuclear rDNA (ITS 1/5·8S/ITS 2). Supported lineages in Coffea are discussed within the context of geographical correspondence, biogeography, morphology and systematics.
Key Results
Several major lineages with geographical coherence, as identified in previous studies based on smaller data sets, are supported. Other lineages with either geographical or ecological correspondence are recognized for the first time. Coffea subgenus Baracoffea is shown to be monophyletic, but Coffea subgenus Coffea is paraphyletic. Sequence data do not substantiate the monophyly of either Coffea or Psilanthus. Low levels of sequence divergence do not allow detailed resolution of relationships within Coffea, most notably for species of Coffea subgenus Coffea occurring in Madagascar. The origin of C. arabica by recent hybridization between C. canephora and C. eugenioides is supported. Phylogenetic separation resulting from the presence of the Dahomey Gap is inferred based on sequence data from Coffea.
Key words: Africa, accD–psa1 IGS, Coffea, coffee, Indian Ocean Islands, ITS, Madagascar, molecular phylogeny, rpl16 intron, Rubiaceae, trnL–F intron, trnL–F IGS
INTRODUCTION
The genus Coffea L. comprises 103 species (Davis et al., 2006) and occurs naturally in tropical Africa, Madagascar, the Comoros and the Mascarenes (Mauritius and Reunion). Coffea species are mostly restricted to humid evergreen forest, although some species are found in seasonally dry deciduous forest and/or bushland. The most recent classifications of Coffea (Bridson, 1988a, b, 2003; Davis et al., 2005, 2006) divide the genus into two subgenera: subgenus Coffea (95 spp.) and subgenus Baracoffea (J.-F. Leroy) J.-F. Leroy (eight spp.). Coffea subgenus Coffea occurs throughout the range of the genus, whereas Coffea subgenus Baracoffea is restricted to the seasonally dry forest and scrubland of western Madagascar (Davis et al., 2005) and, according to Leroy (1982), NE Kenya and SE Somalia. Coffea subgenus Coffea includes the species used in the production of coffee, i.e. C. arabica (Arabica coffee), C. canephora (robusta coffee) and C. liberica (Liberian coffee). Coffea arabica is by far the most important traded species, and provides at least 65 % of commercial production. Coffea arabica is the only allotetraploid Coffea species (2n = 4x = 44; Carvalho, 1952; Grassias and Kammacher, 1975); all other Coffea species are diploid (2n = 2x =22). Coffea arabica is also self-compatible (Carvalho et al., 1991), thus far only reported in two other species: C. heterocalyx (Coulibaly et al., 2002) and C. anthonyi ined. (P. Stoffelen, pers. comm.).
It is now well established that Psilanthus Hook.f. is the closest relative of Coffea. Davis et al. (2007) showed that these genera form a well supported monophyletic group within tribe Coffeeae, based on molecular (BP [bootstrap percentage] 100; b [Bremer support value/decay value] = 9) and combined molecular–morphological data (BP 100; b = 13), for example. Coffea and Psilanthus share a unique carpel morphology: the endocarp (pyrene shell) is hard (horny/crustaceous), the pyrene (and seed) has a deep ventral invagination (i.e. ‘coffee bean’ morphology) and the seed coat consists of crushed endotestal cells with ± isolated fibres (Robbrecht and Puff, 1986). Psilanthus comprises 22 species and occurs in tropical Africa, southern and SE Asia, and as far east as tropical northern Australia (Davis, 2003). Psilanthus is also divided into two subgenera (Bridson, 1988b): Psilanthus subgenus Psilanthus (two spp.), and Psilanthus subgenus Afrocoffea (Moens) Bridson (20 spp.). Psilanthus sub-genus Psilanthus is restricted to tropical West and Central Africa, whereas subgenus Afrocoffea occurs throughout the range of the genus. The morphological characterization of Coffea and Psilanthus and their subgenera is reported in detail by Davis et al. (2005).
Coffea and Psilanthus have been the focus of several recent phylogenetic studies, using systematic data from various sources, including morphology (Stoffelen, 1998; Davis et al., 2005), random amplified polymorphic DNA (RAPD) (Lashermes et al., 1993), sequences from plastid DNA (Cros, 1994; Lashermes et al., 1996; Cros et al., 1998) and internal transcribed spacer (ITS) sequences of nuclear rDNA (Lashermes et al., 1997). At the species level, the studies of Lashermes et al. (1997) and Cros et al. (1998) provided the most useful data.
On the basis of ITS2 data, Lashermes et al. (1997: 953, Fig. 4) separated Coffea into four main geographical groups, although the bootstrap support for three of these groups was negligible to weak, i.e. Madagascar (BP 53), East Africa (BP 22) and Central Africa (BP 67), and West and Central Africa formed an unresolved group. Cros et al. (1998) used sequence data from the trnL–trnF intergenic spacer (IGS) region, and separated Coffea into five area groupings, four the same as Lashermes et al. (1997), but with better support values, i.e. Madagascar (BP 82), East Africa (BP 64), Central Africa (BP 100) and West and Central Africa (BP 41), plus a west Africa clade (BP 100). Cros et al. (1998) concluded that there was good agreement between their trnL–trnF data analysis and the ITS study of Lashermes et al. (1997), including the separation of Coffea species into the four major geographical groups as given above, but also noted that there was some conspicuous incongruence between the two data sets. The most notable incongruity was the position of C. arabica within a clade of central African taxa [i.e. C. eugenioides, and C. sp. ‘Moloundou’ (= C. anthonyi ined.); see Davis et al., 2006] in their plastid DNA analysis (BP 100), compared with placement within a ‘canephoroid’ species group (C. canephora, C. brevipes, C. congensis; BP 53) with ITS2 (Lashermes et al., 1997). Further to this observation (Cros et al., 1998), Raina et al. (1998) used genomic in situ hybridization (GISH) and fluorescent in situ hybridization (FISH) to study the genome organization and evolution of C. arabica, and they concluded that C. congensis and C. eugenioides are the diploid progenitors of C. arabica. Using restriction fragment length polymorphism (RFLP) markers in combination with GISH data, Lashermes et al. (1999) suggested that C. arabica is an amphidiploid formed by hybridization between C. eugenioides and C. canephora, or ecotypes related to these diploid species. Although Raina et al. (1998) did not take into account the diversity of C. canephora, C. congensis and C. arabica (they used only one sample per species), their result is not in any real conflict given that C. congensis and C. canephora are genetically very similar (Lashermes et al., 1997; Prakesh et al., 2005).
Fig. 4.
Strict consensus tree generated from combined molecular (plastid–ITS) analysis. Bootstrap values of >50 % are placed above the branches, followed by Bremer support (decay) values. See Table 1 for species authorities and provenance. EA-IO clade = East Africa-Indian Ocean clade; EA clade = East Africa clade; E-CA clade = East-Central Africa clade; LG/C clade = Lower Guinea/Congolian clade; UG clade = Upper Guinea clade; MAS clade = Mascarene clade. Regions: EA = East Africa; E-CA = East Central Africa; Com = Comoros; In = India; Mad = Madagascar; Mau = Mauritius; WA = West Africa. *Denotes progenitor species for C. arabica. (B) = species belonging to Coffea subgenus Baracoffea.
The other topological inconsistencies identified by Cros et al. (1998) involved two taxa from West Africa, C. stenophylla and C. humilis, and C. sp. ‘X’ (= C. heterocalyx; Coulibaly et al., 2002, 2003), which were placed in different African groups for ITS and trnL–trnF. The plastid analysis of Cros et al. (1998) placed C. stenophylla as sister to C. humilis, and with the Central African, East African and Madagascan species (BP 35). Coffea sp. ‘X’ (= C. heterocalyx) was placed with species from West and Central Africa (BP 32; unresolved in relation to two accessions of C. liberica). The ITS2 analysis of Lashermes et al. (1997) placed C. stenophylla with species from West and Central Africa (BP 4), and C. heterocalyx with their Central Africa clade (BP = 67; unresolved in relation to C. eugenioides and C. sp. ‘Moloundou’ (= C. anthonyi ined.). Cros et al. (1998) suggested that these could be interpreted as the result of interspecies transfer of plastid DNA mediated by hybridization. However, with generally low levels of support, such discussions are speculative.
Owing to limited sequence divergence between Coffea and Psilanthus and the nested position of one Psilanthus species [P. travancorensis (Wight & Arn.) J.-F. Leroy] within Coffea, Lashermes et al. (1997) concluded that their ITS data did not support the recognition of two genera. On the basis of their trnL–trnF data, Cros et al. (1998) concurred with Lashermes et al. (1997) on this matter, although their tree topology shows an unresolved relationship between the two species of Psilanthus sampled (P. mannii and P. ebracteolatus) and Coffea. Cros et al. (1998) and Lashermes et al. (1997) did not include other representatives of Coffeeae (cf. Davis et al., 2007) as outgroups.
Despite recent advances in Coffea systematics, the phylogenetic relationships between species of Coffea and Psilanthus are still poorly understood. This is mostly due to low levels of sequence variation so far recovered, coupled with a relatively small taxon sample size. Lashermes et al. (1997) used 37 samples, including 22 species of Coffea (approx. 21 % of known species diversity) and three species of Psilanthus (approx. 14 % of species diversity); the number of variable characters (nuclear substitutions and indels) was not given. In the study of Cros et al. (1998), 26 samples were used, covering 18 species of Coffea (approx. 17 % of known species diversity) and two species of Psilanthus (approx. 9 % of species diversity); only 32 variable characters (26 nucleotide substitutions and six indels) were found within their study sample. The studies of Lashermes et al. (1997) and Cros et al. (1998) did not include any samples of Coffea subgenus Baracoffea (eight spp.), Mascarene Coffea (at least three spp.) or the morphologically and geographically isolated (Bridson, 1979, 1983; Davis et al., 2005) C. rhamnifolia. Small sample size is mostly a problem of logistics, as wild Coffea and Psilanthus species are not well represented in cultivation, are often difficult to find in the wild and DNA suitable for PCR and sequencing is not easily isolated from herbarium specimens. In addition, numerous new species of Coffea have been discovered and/or described since 1998, including seven species from west and central tropical Africa (Stoffelen et al., 1996a, b, 1997a, b; Cheek et al., 2002; Sonké and Stoffelen, 2004; Sonké et al., 2006b), two from East Africa (Davis and Mvungi, 2004) and 13 from Madagascar (Davis and Rakotonasolo, 2000, 2001a, b, 2003; Davis, 2001). Further new species are in the process of formal description and publication (Davis et al., 2006; Table 1).
Table 1.
Taxon accession data
| Taxon | Voucher | Source | accD–psa1 | rpl16 | trnL–F | ITS |
|---|---|---|---|---|---|---|
| Coffea abbayesii J.-F. Leroy | Davis 2334 (K) | Madagascar | DQ153438 | DQ153687 | DQ153805 | DQ153566 |
| Coffea ambongensis J.-F. Leroy ex A.P. Davis & Rakotonas., ined. | Davis 2509 (K) | Madagascar | DQ153419 | DQ153668 | DQ153786 | DQ153539/DQ153540/DQ153541 |
| Coffea andrambovatensis J.-F. Leroy | Davis 2322 (K) | Madagascar | DQ153422 | DQ153671 | DQ153789 | DQ153545 |
| Coffea anthonyi Stoff. & F. Anthony, ined. (C. sp. ‘Moloundou’) | IRD-Montpelier OE 53 (K) | DR Congo | DQ153489 | DQ153738 | DQ153856 | DQ153620 |
| Coffea ankaranensis J.-F. Leroy ex A.P. Davis & Rakotonas. | Davis 2331 (K) | Madagascar | DQ153407 | DQ153656 | DQ153774 | DQ153527 |
| Coffea arabica L. | Jaufeerally-Fakim 29 (K) | Mascarenes (Introduced) | DQ153478 | DQ153727 | DQ153845 | DQ153609 |
| Coffea arenesiana J.-F. Leroy | Davis 2207 (K) | Madagascar | DQ153440 | DQ153689 | DQ153807 | DQ153568 |
| Coffea augagneuri Dubard | Davis 2220 (K) | Madagascar | DQ153433 | DQ153682 | DQ153800 | DQ153561 |
| Coffea bakossii Cheek & Bridson | Lane 361 (K) | Cameroon | DQ153468 | DQ153717 | DQ153835 | DQ153599 |
| Coffea bertrandii A. Chev. | Davis 2348 (K) | Madagascar | DQ153424 | DQ153673 | DQ153791 | DQ153549 |
| Coffea betamponensis Portères & J.-F. Leroy | Davis 2300 (K) | Madagascar | DQ153421 | DQ153670 | DQ153788 | DQ153543/DQ153544 |
| Coffea boinensis A.P. Davis & Rakotonas., ined. | Davis 2502 (K) | Madagascar | DQ153408 | DQ153657 | DQ153775 | DQ153528 |
| Coffea boiviniana (Baill.) Drake | Davis 2231 (K) | Madagascar | DQ153426 | DQ153675 | DQ153793 | DQ153551/DQ153552/DQ153553 |
| Coffea brevipes Heirn | Maurin 8 (K) | Cameroon | DQ153460 | DQ153709 | DQ153827 | DQ153591 |
| Coffea bridsoniae A.P. Davis & Mvungi | Davis 2904 (K) | Tanzania | DQ153455 | DQ153704 | DQ153822 | DQ153584/DQ153585/DQ153586 |
| Coffea buxifolia A. Chev. | Rakotonasolo 69 (K, TAN) | Madagascar | DQ153442 | DQ153691 | DQ153809 | DQ153570 |
| Coffea campaniensis J.-F. Leroy | Leroy 55 (K) | Mascarenes (Mauritius) | DQ153470 | DQ153719 | DQ153837 | DQ153601 |
| Coffea canephora Pierre ex Froehn. | Maurin 21 (K) | Cameroon (cultivated) | DQ153462 | DQ153711 | DQ153829 | DQ153593 |
| Coffea commersoniana (Baill.) A. Chev. | Davis 2715 (K) | Madagascar | DQ153432 | DQ153681 | DQ153799 | DQ153560 |
| Coffea congensis A. Froehn. | Harris & Fay 1507 (BR, K, MO) | Cameroon | DQ153467 | DQ153716 | DQ153834 | DQ153598 |
| Coffea costatifructa Bridson | ORSTOM 08 117 (K) | Tanzania | DQ153473 | DQ153722 | DQ153840 | DQ153604 |
| Coffea coursiana J.-F. Leroy | Davis 2278 (K) | Madagascar | DQ153417 | DQ153666 | DQ153784 | DQ153537 |
| Coffea decaryana J.-F. Leroy | Davis 1537 (K) | Madagascar | DQ153429 | DQ153678 | DQ153796 | DQ153556 |
| Coffea dubardii Jum. | Davis 2216 (K) | Madagascar | DQ153435 | DQ153684 | DQ153802 | DQ153563 |
| Coffea eugeniodes S.Moore | Harley 9332 (BR, K) | Tanzania | DQ153457 | DQ153706 | DQ153824 | DQ153588 |
| Coffea fadenii Bridson | Mvungi 9 (DSM, K) | Tanzania | DQ153446 | DQ153695 | DQ153813 | DQ153574 |
| Coffea farafanganensis J.-F. Leroy | Davis 2317 (K) | Madagascar | DQ153405 | DQ153654 | DQ153772 | DQ153525 |
| Coffea grevei Drake ex A. Chev. | Davis 2566 (K) | Madagascar | DQ153414 | DQ153663 | DQ153781 | DQ153534 |
| Coffea heimii J.-F. Leroy | Davis 2241 (K) | Madagascar | DQ153431 | DQ153680 | DQ153798 | DQ153558/DQ153559 |
| Coffea heterocalyx Stoff. | Maurin 23 (K) | Cameroon | DQ153463 | DQ153712 | DQ153830 | DQ153594 |
| Coffea heterocalyx Stoff. | IRD-Montpelier JC 66 (K) | ?Cameroon/ DR Congo) | DQ153492 | DQ153741 | DQ153859 | DQ153623 |
| Coffea homollei J.-F. Leroy | Davis 2305 (K) | Madagascar | DQ153402 | DQ153651 | DQ153769 | DQ153521 |
| Coffea humbertii J.-F. Leroy | Rakotonasolo 50 (K, TAN) | Madagascar | DQ153437 | DQ153686 | DQ153804 | DQ153565 |
| Coffea humblotiana Baill. | Davis 2327 (K) | Madagascar | DQ153411 | DQ153660 | DQ153778 | DQ153531 |
| Coffea humilis A. Chev. | Bamps 1967 (BR) | Ivory Coast | DQ153480 | DQ153729 | DQ153847 | DQ153611 |
| Coffea kapakata (A. Chev.) Bridson | Hepper & Maley 7723 (K) | Angola | DQ153465 | DQ153714 | DQ153832 | DQ153596 |
| Coffea kianjavatensis J.-F. Leroy | Davis 2313 (K) | Madagascar | DQ153482 | DQ153731 | DQ153849 | DQ153613 |
| Coffea kihansiensis A.P. Davis & Mvungi | Mvungi 21 (DSM, K) | Tanzania | DQ153454 | DQ153703 | DQ153821 | DQ153583 |
| Coffea kimbozensis Bridson | Mvungi 6 (DSM, K) | Tanzania | DQ153447 | DQ153696 | DQ153814 | DQ153575 |
| Coffea kivuensis Lebrun | Lebrun 5539 (BR) | Zaire | DQ153481 | DQ153730 | DQ153848 | DQ153612 |
| Coffea pterocarpa A.P. Davis & Rakotonas., ined. | Davis 2519 (K) | Madagascar | DQ153425 | DQ153674 | DQ153792 | DQ153550 |
| Coffea labatii A.P. Davis & Rakotonas., ined. | Davis 3069 (K) | Madagascar | DQ153499 | DQ153748 | DQ153866 | DQ153630 |
| Coffea lancifolia A. Chev. | Davis 2307 (K) | Madagascar | DQ153403 | DQ153652 | DQ153770 | DQ153522 |
| Coffea leroyi A.P. Davis | Davis 2311 (K) | Madagascar | DQ153404 | DQ153653 | DQ153771 | DQ153523/DQ153524 |
| Coffea liaudii J.-F. Leroy ex A.P. Davis | Rakotonasolo 61 (K, TAN) | Madagascar | DQ153434 | DQ153683 | DQ153801 | DQ153562 |
| Coffea liberica var. liberica Bull. ex Hiern | Billiet 19370062 (BR) | DR Congo | DQ153479 | DQ153728 | DQ153846 | DQ153610 |
| Coffea liberica var. dewerei (De Wild. &T. Durand) Lebrun | Hepper & Maley 7729 (BR, K, MO) | Central African Republic | DQ153472 | DQ153721 | DQ153839 | DQ153603 |
| Coffea littoralis A.P. Davis & Rakotonas. | Rakotonasolo 261 (K) | Madagascar | DQ153441 | DQ153690 | DQ153808 | DQ153569 |
| Coffea lulandoensis Bridson | Mvungi 2 (DSM, K) | Tanzania | DQ153452 | DQ153701 | DQ153819 | DQ153580 |
| Coffea macrocarpa A. Rich. | Gueho 18555 (K) | Mascarenes (Mauritius) | DQ153471 | DQ153720 | DQ153838 | DQ153602 |
| Coffea mapiana Sonké, Nguembou & A.P. Davis | Sonké 3694 (K, YA) | Cameroon | DQ153509 | DQ153758 | DQ153876 | DQ153640 |
| Coffea mangoroensis Portères | Rakotonasolo 41 (K, TAN) | Madagascar | DQ153503 | DQ153752 | DQ153870 | DQ153634 |
| Coffea manombensis A.P. Davis | Davis 2141 (K) | Madagascar | DQ153445 | DQ153694 | DQ153812 | DQ153573 |
| Coffea mauritiana Lam. | Friedmann 1267 (K) | Mascarenes (Reunion) | DQ153469 | DQ153718 | DQ153836 | DQ153600 |
| Coffea mayombensis A. Chev. | Maurin 16 (K) | Cameroon | DQ153461 | DQ153710 | DQ153828 | DQ153592 |
| Coffea mcphersonii A.P. Davis & Rakotonas. | Davis 2339 (K) | Madagascar | DQ153423 | DQ153672 | DQ153790 | DQ153546/DQ153547/DQ153548 |
| Coffea millotii J.-F. Leroy | Davis 2306 (K) | Madagascar | DQ153409 | DQ153658 | DQ153776 | DQ153529 |
| Coffea mongensis Bridson | Mvungi 11 (DSM, K) | Tanzania | DQ153448 | DQ153697 | DQ153815 | DQ153576 |
| Coffea montekupensis Stoff. | Davis 3010 (K) | Cameroon | DQ153459 | DQ153708 | DQ153826 | DQ153590 |
| Coffea montis-sacri A.P. Davis | Davis 2308 (K) | Madagascar | DQ153430 | DQ153679 | DQ153797 | DQ153557 |
| Coffea moratii J.-F. Leroy ex A.P. Davis & Rakotonas. | Davis 2326 (K) | Madagascar | DQ153502 | DQ153751 | DQ153869 | DQ153633 |
| Coffea mufindiensis Hutch. ex Bridson | Mvungi 19 (DSM, K) | Tanzania | DQ153449 | DQ153698 | DQ153816 | DQ153577 |
| Coffea myrtifolia (A. Rich. ex DC.) J.-F. Leroy | Jaufeerally-Fakim 22 (K) | Mascarenes (Mauritius) | DQ153477 | DQ153726 | DQ153844 | DQ153608 |
| Coffea perrieri Drake ex Jum. & H.Perrier | Davis 1174 (K) | Madagascar | DQ153500 | DQ153749 | DQ153794 | DQ153631 |
| Coffea pervillenana (Baill.) Drake | Davis 2328 (K) | Madagascar | DQ153412 | DQ153661 | DQ153779 | DQ153532 |
| Coffea pocsii Bridson | Mvungi 7 (DSM, K) | Tanzania | DQ153453 | DQ153702 | DQ153820 | DQ153581/DQ153582 |
| Coffea pseudozanguebariae Bridson | Mvungi 16 (DSM, K) | Tanzania | DQ153450 | DQ153699 | DQ153817 | DQ153578 |
| Coffea racemosa Lour. | Hepper & Maley 7717 (BR, K) | Mozambique | DQ153464 | DQ153713 | DQ153831 | DQ153595 |
| Coffea rakotonasoloi A.P. Davis | Davis 2265 (K) | Madagascar | DQ153416 | DQ153665 | DQ153783 | DQ153536 |
| Coffea ratsimamangae J.-F. Leroy ex A.P. Davis & Rakotonas. | Davis 2240 (K) | Madagascar | DQ153444 | DQ153693 | DQ153811 | DQ153572 |
| Coffea resinosa (Hook.f.) Radlk. | Davis 1103 (K) | Madagascar | DQ153428 | DQ153677 | DQ153795 | DQ153555 |
| Coffea rhamnifolia (Chiov.) Bridson | Friis et al. 4908(K, BR, P) | Somalia | DQ153458 | DQ153707 | DQ153825 | DQ153589 |
| Coffea richardii J.-F. Leroy | Davis 2253 (K) | Madagascar | DQ153415 | DQ153664 | DQ153782 | DQ153535 |
| Coffea sahafaryensis J.-F. Leroy | Davis 2345 (K) | Madagascar | DQ153413 | DQ153662 | DQ153780 | DQ153533 |
| Coffea sakarahae J.-F. Leroy | Davis 2167 (K) | Madagascar | DQ153439 | DQ153688 | DQ153806 | DQ153567 |
| Coffea salvatrix Swynn. & Philipson | IRD-Montpelier LA 51 (K) | Mozambique | DQ153491 | DQ153740 | DQ153858 | DQ153622 |
| Coffea sambavensis J.-F. Leroy ex A.P. Davis & Rakotonas. | Davis 2323 (K) | Madagascar | DQ153418 | DQ153667 | DQ153785 | DQ153538 |
| Coffea schliebenii Bridson | Mbago 2256 (DSM) | Tanzania | DQ153456 | DQ153705 | DQ153823 | DQ153587 |
| Coffea sessiliflora Bridson | Mvungi 25 (DSM, K) | Tanzania | DQ153451 | DQ153700 | DQ153818 | DQ153579 |
| Coffea anthonyi Stoff. & F. Anthony, ined. | IRD-Montpelier OE 53 (K) | DR Congo | DQ153489 | DQ153738 | DQ153856 | DQ153620 |
| Coffea sp. ‘G’ (FTEA) | Mabberley 1417 (K) | Tanzania | DQ153474 | DQ153723 | DQ153841 | DQ153605 |
| Coffea stenophylla G.Don | Hepper & Maley 7723 (K) | Ivory Coast | DQ153466 | DQ153715 | DQ153833 | DQ153597 |
| Coffea tetragona Jum. & H.Perrier | Davis 2318 (K) | Madagascar | DQ153406 | DQ153655 | DQ153773 | DQ153526 |
| Coffea togoensis Jum. & H.Perrier | Hall & Abbins 43367 (K) | Togo | DQ153476 | DQ153725 | DQ153843 | DQ153607 |
| Coffea tsirananae J.-F. Leroy | Davis 2215 (K) | Madagascar | DQ153443 | DQ153692 | DQ153810 | DQ153571 |
| Coffea vianneyi J.-F. Leroy | Davis 2320 (K) | Madagascar | DQ153436 | DQ153685 | DQ153803 | DQ153564 |
| Coffea vatovavyensis J.-F. Leroy | Davis 2316 (K) | Madagascar | DQ153410 | DQ153659 | DQ153777 | DQ153530 |
| Coffea zangueberiae Lour. | Groenendijk 884 (K) | Mozambique | DQ153475 | DQ153724 | DQ153842 | DQ153606 |
| Psilanthus bridsoniae Sivar., Biju & P.Mathew | Biju & Sasi 44800 (K) | India | DQ153397 | DQ153646 | DQ153764 | DQ153516 |
| Psilanthus ebracteolatus Heirn | Davis 3008 (K) | Cameroon | DQ153392 | DQ153641 | DQ153759 | DQ153510 |
| Psilanthus mannii Hook.f. | Maurin 1 (K) | Cameroon | DQ153393 | DQ153642 | DQ153760 | DQ153511 |
| Psilanthus sapinii De Wild. | Sapin s.n. (BR 0856914) | Congo-Kinshasa | DQ153394 | DQ153643 | DQ153761 | DQ153512 |
| Psilanthus semsei Bridson | Kisera 1473 (K) | Tanzania | DQ153395 | DQ153644 | DQ153762 | DQ153513 |
| Psilanthus sp. ‘A’ (FTEA) | Luke 10197 (K) | Tanzania | DQ153399 | DQ153648 | DQ153766 | DQ153518 |
| Psilanthus travancorensis (Wight & Arn.) J.-F. Leroy | Biju s.n. (K) | India | DQ153398 | DQ153647 | DQ153765 | DQ153517 |
| Tricalysia sp. | Davis 2173 (K) | Madagascar | DQ153400 | DQ153649 | DQ153767 | DQ153519 |
| Tricalysia verdcourtiana Robbr. | Mvungi 43 (DSM, K) | Tanzania | DQ153401 | DQ153650 | DQ153768 | DQ153520 |
Herbarium abbreviations after Holmgren et al. (1990). Where several ITS types were isolated these are listed below with multiple GenBank accesion numbers.
In the investigation reported here, plastid sequence data from the trnL–F intron, trnL–F IGS, rpl16 intron and accD–psa1 IGS, and ITS sequences of nuclear rDNA were analysed in an attempt to increase the number of molecular characters available for phylogenetic reconstruction. Better sampling of Coffea has been made possible due to recent collecting activities, and it was possible to examine approx. 83 % of total species diversity for the genus, and 32 % of total species diversity for Psilanthus (see Materials and Methods). The main objective of the study was to identify well-supported lineages within Coffea, and to discuss these within the contexts of geographical coherence, morphology, systematics and biogeography. Secondarily, the aim was to elucidate consistently retrieved lineages within Coffea and well-supported lineages within Psilanthus, and to assess the relationship between Coffea and Psilanthus.
MATERIALS AND METHODS
Taxon sampling and plant material
As this study is concerned with assessing relationships above the rank of species, multiple species samples or infraspecific taxa were not used. Only two exceptions were made: a second sample of C. heterocalyx (IRD-Montpellier JC 66; voucher K) was included, which it is believed is the same as (or very similar to) that used by Lashermes et al. (1997) and Cros et al. (1998), and C. liberica var. dewevrei. Coffea heterocalyx is of considerable interest because it is reported to be self-compatible (Coulibaly et al., 2002). N'Diaye et al. (2005) report that C. liberica var. liberica and C. liberica var. dewevrei have a high genetic differentiation (Gst 0·25) with AFLP markers, and the pollen viability of F1 hybrids between them is low (44·2 %) and similar to interspecific hybrids, indicating that there are marked reproductive barriers between the two varieties. For these reasons, one sample of each variety of C. liberica was included.
The samples used in this study, with accepted taxon names, voucher information and GenBank accession numbers for the sequences, are given in Table 1. Most samples are of wild origin, with many collected during recent field expeditions to Madagascar (1997–2004), Tanzania (2001–2003) and Cameroon (in 2002). To complete the sampling, living material held in botanical gardens and coffee research stations, and some herbarium material (leaf samples or single seeds) taken from specimens held at K and BR (abbreviations after Holmgren et al., 1990) were included. In total, 88 samples (86 spp.) of Coffea and seven samples (seven spp.) of Psilanthus were analysed. The sample includes representatives of all subgenera of Coffea and Psilanthus. Two species of the genus Tricalysia A. Rich ex DC. were used as outgroups, one from Madagascar and the other from Tanzania. Tricalysia is a close relative of Coffea and a member of Coffeeae (Andreasen and Bremer, 2000; Davis et al., 2007).
The present Coffea taxon sampling includes a number of undescribed taxa, although these entities are well documented (Davis et al., 2006) and appear to represent clearly defined species based on morphological data. Inclusion of undescribed species belonging to Coffea subgenus Baracoffea (Davis et al., 2005) was necessary for testing the monophyly and systematic placement of the subgenus. The floristic study of Mascarene Coffea species by Leroy (1989) enumerated three native species of Coffea, C. macrocarpa, C. myrtifolia and C. mauritiana. A sample of C. campaniensis was also included, which Leroy (1989) placed in the synonymy of C. mauritiana.
Taxonomic details for all taxa (below generic rank) mentioned herein (such as author, place and date of publication, synonyms, distribution) follow the World Rubiaceae Checklist (www.kew.org/wcsp/rubiaceae). More detailed information for Coffea species is given in Davis et al. (2006).
DNA extraction, amplification and sequencing
Most of the DNA samples were obtained from silica-dried leaf material (Chase and Hills, 1991). The other DNA samples were extracted from seeds obtained from herbarium specimens; a single seed was used for each sample. DNA extraction was performed from a maximum of 0·3 g of silica-dried leaf material (or from one seed) using the 2 × CTAB method of Doyle and Doyle (1987). The DNA was purified on caesium chloride/ethidium bromide gradients (1·55 g mL–1 density) and dialysed before inclusion in the DNA Bank at the Royal Botanic Gardens, Kew (http://www.kew.org/data/dnaBank/homepage.html). To avoid problems of PCR inhibition, all DNA samples were further purified using QIAquick purification columns (QIAgen) following the manufacturer's protocol.
Amplification of the trnL–F region (the trnL intron and the trnL–trnF IGS), the rpl16 intron, the accD–psa1 (plastid DNA) IGS and ITS (nuclear encoded internal transcribed spacer) was performed using the primers listed in Table 2. Any ITS trace files showing evidence of heterogeneous ITS copies were cloned to isolate single sequences using the Promega pGem-T Easy Vector kit (catalogue no. A1360). The ITS region was then re-amplified from the transformed bacterial colonies using the M13 primers contained in the kit and a small portion of the colony as the DNA template. Amplification of trnL–F was carried out using primers c and f of Taberlet et al. (1991). For many taxa, the internal primers d and e also had to be used because of difficulty in amplifying the region as a single piece. Amplification of rpl16 was carried out using primers 71F and 1661R of Jordan et al. (1996). For many taxa, the amplification of DNA using these primers was not satisfactory and so internal primers were designed on the basis of the first sequences in a conserved and GC-rich region suitable for amplification of rpl16 in two fragments for Rubiaceae. The accD–psa1 region was amplified using the primers ACCD-769 forward and PSA1-75 reverse from Mendenhall (1994). Two internal primers were again designed to obtain satisfactory PCR products for the recalcitrant specimens.
Table 2.
Amplification primers for trnL–F, ITS, rpl1 6 and accD–psa1
| Locus | Primer | Primer sequence | Reference |
|---|---|---|---|
| trnL intron | Forward (c) | 5′-CGAAATCGGTAGACGCTACG-3′ | Taberlet et al. (1991) |
| Reverse (d) | 5′-GGGGATAGAGGGACTTGAAC-3′ | ||
| trnL–F IGS | Forward (e) | 5′-GGTTCAAGTCCCTCTATCCC-3′ | Taberlet et al. (1991) |
| Reverse (f) | 5′-ATTTGAACTGGTGACACGAG-3′ | ||
| ITS | Forward (17SE or 101) | 5′-ACGAATTCATGGTCCGGTGAAGTGTTCG-3′ | Sun et al. (1994) |
| Reverse (26SE or 102) | 5′-TAGAATTCCCCGGTTCGCTCGCCGTTAC-3′ | ||
| Internal reverse (ITS 2) | 5′-GCTGCGTTCTTCATCGATGC-3′ | White (1990) | |
| Internal forward (ITS 3) | 5′-GCATCGATGAAGAACGCAGC-3′ | ||
| rpl16 | Forward (F71) | 5′-GCTATGCTTAGTGTGTGACTCGTTG-3′ | Jordan et al. (1996) |
| Reverse (R1661) | 5′-CGTACCCATATTTTTCCACCECGAC-3′ | ||
| Internal forward | 5′-GTAAGAAGTGATGGGAACGA-3′ | Designed at Kew | |
| Internal reverse | 5′-TCGTTCCCATCACTTCTTAC-3′ | ||
| accD–psa1 IGS | Forward (ACCD 769 F) | 5′-GGAAGTTTGAGCTTTATGCAAATGG-3′ | Mendenhall (1994) |
| Reverse (PSA1 75 R) | 5′-AGAAGCCATTGCAATTGCCGGAAA-3′ | ||
| Internal forward Coffeeae | 5′-GCTAAAAATCTCTSTTGGTTCGG-3′ | Designed at Kew | |
| Internal reverse Coffeeae | 5′-CCGAACCAASAGAGATTTTTAGC-3′ |
The PCR program for trnL–F consisted of 2 min at 94 °C followed by 28 cycles of 1 min at 94 °C (denaturation), 1 min at 50 °C (annealing) and 1 min at 72 °C (extension), followed by a final 7 min extension (72 °C). For rpl16, the PCR program used was 2 min at 94 °C followed by 30 cycles of 1 min 94 °C, 1 min at 52 °C and 3 min at 72 °C, with a final extension at 72 °C for 7 min. The accD–psa1 region was amplified using the PCR program: a denaturation phase of 3 min (94 °C), followed by 30 cycles of 1 min at 94 °C, 1 min at 51 °C and 1 min at 72 °C, and a final extension of 72 °C for 5 min. The ITS region was amplified using primer 17SE forward and 26SE reverse from Sun et al. (1994). Dimethylsulfoxide (DMSO; 4·5%) was used to reduce secondary structure problems common with nuclear DNA.
A PCR mastermix containing 2·5 mm MgCl2 (Advanced Biotechnologies, Ltd) was used for trnL–F and rpl16 amplifications. For accD–psa1, commercial mastermix did not give good amplifications, and so a pre-mix was prepared using Biotaq DNA polymerase (Bioline, UK), 10× NH4 reaction buffer (Bioline, UK), 50 mm MgCl2 and dNTPs (Promega, Maddison, WI, USA). Amplified products were purified using QIAquick purification columns (QIAgen) as described in the manufacturer's protocol. Cycle sequencing reactions were carried out using BigDye™ Terminator Mix (Applied Biosystems, Inc., Warrington, Cheshire, UK). The program consisted of 26 cycles of: 10 s denaturation (96 °C), 5 s annealing (50 °C) and 4 min elongation (60 °C). PCR and sequencing reactions were run using a Perkin-Elmer GenAMP™ model 9600 or 9700 PCR system, and sequencing products were run on either an ABI 3100 Genetic Analyzer or an ABI 377 automated sequencer according to the manufacturer's protocols (Applied Biosystems, Inc.). Electropherograms were edited and assembled into contigs using Sequencher version 3·2·2. (Gene Codes Corp., Ann Arbor, MI, USA). The sequences generated were submitted to GenBank using the Sequin Application (version 5·26; available from http://www.ncbi.nlm.nih.gov/Sequin/).
Data matrix composition and parsimony analysis
All sequences were aligned manually in PAUP* (version 4·0b10; Swofford, 2002) without difficulty due to low levels of sequence variation. Areas of ambiguous alignment were excluded from the analysis, as were regions with missing sequences, for example the beginning and end of sequences and around the internal primer-binding sites.
Maximum parsimony was implemented to analyse (a) trnL–F, (b) rpl16, (c) accD–psa1, (d) combined plastid data, (e) ITS and (f) combined sequence data, using PAUP*. In all analyses, gaps were treated as missing data and characters were equally weighted and unordered (Fitch, 1971). All data sets were analysed separately and examined by eye in order to identify topological conflict, i.e. moderate to strong support for placement of a taxon in different clades. Thirteen insertions/deletions were identified and scored for the plastid DNA regions.
Tree searches were conducted using 10 000 replicates of random taxon sequence addition, retaining ten trees at each step, with tree-bisection-reconnection (TBR) branch swapping, delayed transformation (DELTRAN) optimization, MulTrees in effect, and saving a maximum of ten trees per replicate. Support for clades in all analyses was estimated using bootstrap analysis (Felsenstein, 1985), with 10 000 replicates of full heuristic search, simple sequence addition, TBR swapping, with MulTrees in effect and saving a maximum of ten trees per replicate. BPs are described as strongly/well supported (85–100 %), moderate (75–84 %) or low (50–74 %). Support was also estimated by calculating Bremer support values (b) (Bremer, 1988, 1994; Källersjö et al., 1992), otherwise known as decay values. These were obtained using PAUP* (Swofford, 2002), in conjunction with AutoDecay 4·0·2 (Eriksson, 1999), with 100 replicates of random addition for each constraint tree.
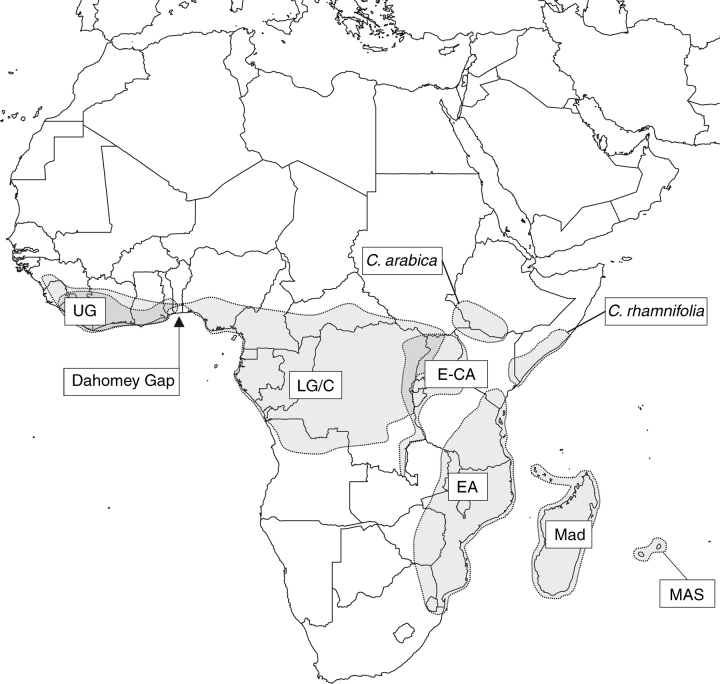
Map construction
Figure 1 is based on the distribution of individual species as recorded in an African Coffea specimen database (approx. 2400 records; P. Stoffelen and A. Davis, unpubl. data) and Madagascan/Mascarene Coffea specimen database (approx. 1000 records; A. Davis and S. Dawson, unpubl. data). Species distributions maps were plotted and then a generalized map was drawn by hand.
Fig. 1.
Distribution map of Coffea showing location of clades and groups. UG = Upper Guinea clade; LG/C = Lower Guinea/Congolian clade; E-CA = East-Central Africa clade; EA = East Africa clade; Mad = Madagascan species; MAS = Mascarene clade. The map does not indicate the distribution of the poorly known C. anthonyi ined. (see Materials and Methods).
RESULTS
Tree data and statistics for individual and combined analyses using (a) trnL–F, (b) rpl16, (c) accD–psa1, (d) combined plastid data, (e) ITS and (f) combined sequence data are given in Table 3. Individual plastid sequence analyses were topologically consistent (negligible to zero incongruence) and for the purpose of the results and discussion were combined and treated as a single analysis. The combined plastid analysis is largely congruent with the ITS analysis, apart from the placement of C. arabica, an accession of C. heterocalyx [IRD-Montpellier JC 66 (K); see Table 1], and three species from the Upper Guinea region (C. humilis, C. stenophylla, C. togoensis). Coffea arabica and the accession of C. heterocalyx were the only strongly supported points of incongruence. It is now generally accepted that C. arabica is of hybrid origin, as discussed in the Introduction. On the basis of the present results, it is believed that the accession of C. heterocalyx [IRD-Montpellier JC 66 (K)] used here is the same as that sampled by Lashermes et al. (1997) and Cros et al. (1998). The combined plastid data analysis places C. heterocalyx with C. liberica var. dewevrei, and the ITS analysis with C. eugenioides. Examination of the plastid sequences shows that there are only 3 bp differences and one 11 bp deletion (for C. liberica var. dewevrei) between this accession of C. heterocalyx and C. liberica var. dewevrei [Hepper & Maley 7729 (BR, K, MO)] across the three plastid regions. Cros et al. (1988) reported that their trnL–trnF sequence of C. heterocalyx (no accession data) was identical to one of their samples of C. liberica. The present ITS sequence data show that there is only 1 bp difference between this sample of C. heterocalyx and C. eugenioides [Harley 9332 (BR, K)]. The ITS2 data of Lashermes et al. (1997) placed C. heterocalyx in an unresolved position within a clade containing four samples of C. eugenioides and two of C. sp. Moloundou (= C. anthonyi ined.). Based on these data, it is believed that this accession of C. heterocalyx is a hybrid between C. eugenioides and C. liberica, resulting from either introgression in the wild or a chance crossing in cultivation. The natural distributions of these taxa overlap in the wild [Democratic Republic of Congo, Sudan, Uganda (Davis et al., 2006; Fig. 1)], but there do not appear to be field data indicating wild hybrids between C. liberica and C. eugenioides. The sequences of the sample of C. heterocalyx taken directly from the wild [Maurin 23 (K)] in Cameroon do not closely match those of the accession IRD-Montpellier JC 66 (K), as the former is consistently placed with species from West Africa (see Fig. 4). The sample IRD-Montpellier JC 66 (K) was originally held at the IRD coffee breeding station of Divo, Ivory Coast (ex IRD-IFCC station) and then transferred to IRD-Montpellier, France. The accession data imply that it was collected from either Cameroon or the Democratic Republic of Congo during the 1960s; it has been maintained in cultivation for >40 years (F. Anthony, pers. comm.).
Table 3.
Description of trees for each plastid region, combined plastid region and combined molecular data sets
| Characteristics | trnL–F | rpl16 | accD–psaI | Combined plastid data | ITS | Combined molecular data |
|---|---|---|---|---|---|---|
| Number of taxa | 95 | 95 | 95 | 95 | 107* | 106† |
| Total number of characters | 915 | 1120 | 1187 | 3222 | 831 | 3901 |
| Invariable characters | 837 | 995 | 1052 | 2884 | 652 | 3395 |
| Parsimony uninformative characters | 43 | 73 | 82 | 198 | 72 | 251 |
| Parsimony informative characters | 35 | 52 | 53 | 198 | 107 | 255 |
| Tree length | 90 | 106 | 199 | 499 | 415 | 952 |
| Consistency index (CI)‡ | 0·808 | 0·677 | 0·594 | 0·563 | 0·438 | 0·467 |
| Retention index (RI) | 0·941 | 0·867 | 0·849 | 0·809 | 0·764 | 0·761 |
| Number of trees | 18 753 | 6860 | 6290 | 75 120 | 7760 | 11 520 |
* Including ITS clones.
† Including ITS clones but C. arabica removed (see Materials and Methods and Results).
‡ Calculated without uninformative sites.
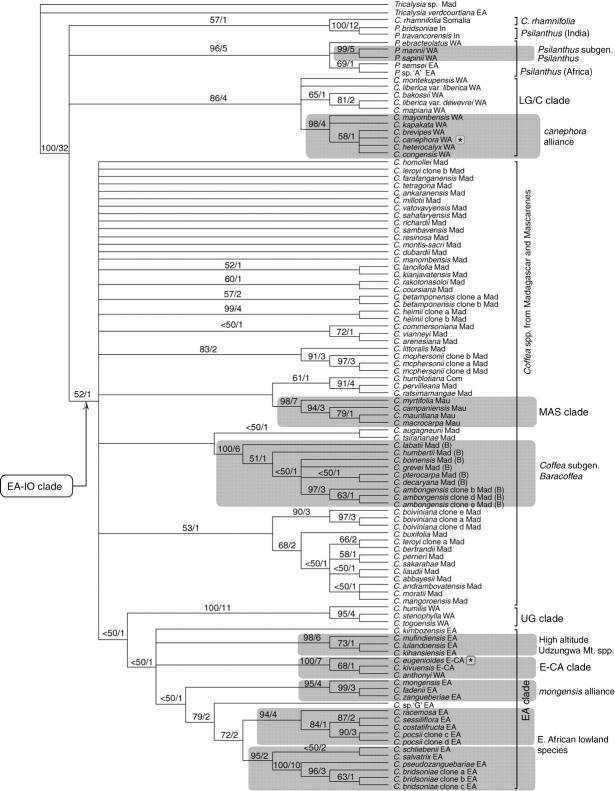
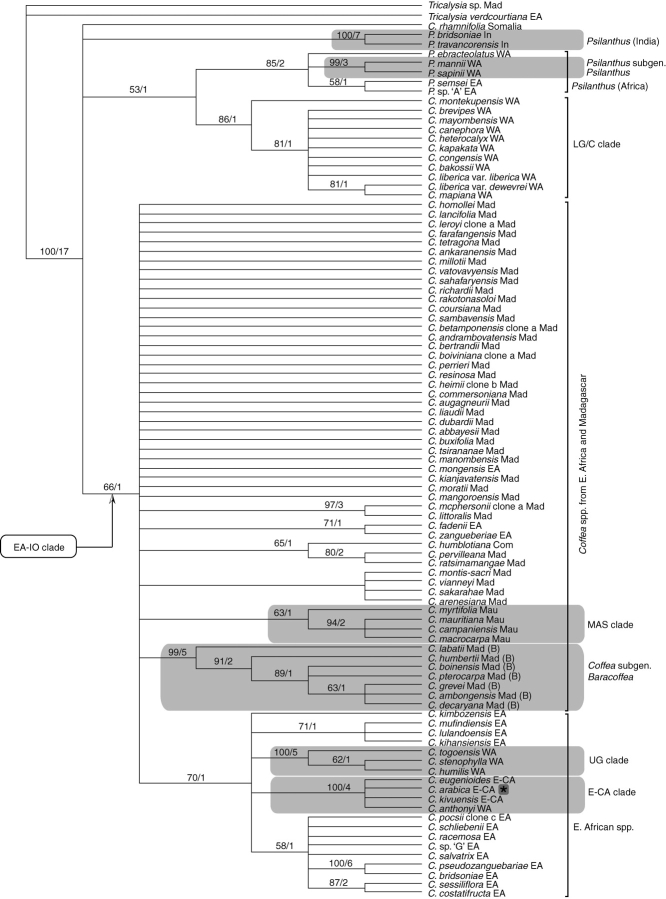
Based on the evidence given above, the Montpellier accession of C. heterocalyx was excluded from the analyses, and C. arabica was removed from the final combined analysis (combined plastid plus ITS). After the deletion of these species, any highly supported incongruence between the combined plastid analysis and the ITS analysis was removed, enabling these two data sets to be combined. The position of three species (C. humilis, C. stenophylla and C. togoensis) from the Upper Guinea region is different for the combined plastid analysis and ITS analysis. In the combined plastid analysis, they form a well-supported clade (the UG clade; BP 100, b = 5; Fig. 2), which falls within a clade of species from East and East-Central Africa (BP 70, b = 1; Fig. 2). In the ITS analysis, these three species do not form a clade, but are all positioned within a clade of species that contains species from the Lower Guinea/Congo region (the ‘canephora alliance’; see below) and C. arabica (BP 74, b = 1; Fig. 3). Similar results were reported by Cros et al. (1998) based on their trnL–trnF data for C. humilis and C. stenophylla, and by comparison with the ITS data of Lashermes et al. (1987) for C. stenophylla (see Introduction). The combined molecular analysis places the UG clade in approximately the same position as the combined plastid analysis, although this relationship is very weakly supported (BP 23, b = 1; Fig. 4). The incongruence identified in the present investigation is not well supported, and the above three species were retained in combined plastid and ITS analysis, where their inclusion does not significantly influence either the topology of the tree or support values. In the combined molecular (plastid–ITS) analysis, the UG clade is in approximately the same position as the combined plastid analysis, although this relationship is only weakly supported (BP 23, b = 1; Fig. 4).
Fig. 2.
Strict consensus tree generated from combined plastid analysis. Bootstrap values of >50 % are placed above the branches, followed by Bremer support (decay) values. See Table 1 for species authorities and provenance. EA-IO clade = East Africa-Indian Ocean clade; E-CA = East-Central Africa clade; LG/C clade = Lower Guinea/Congolian clade; UG clade = Upper Guinea clade; MAS clade = Mascarene clade. Regions, given after species names: EA = East Africa; E-CA = East Central Africa; Com = Comoros; In = India; Mad = Madagascar; Mau = Mauritius; WA = West Africa. * = Reference symbol for C. arabica. (B) = species belonging to Coffea subgenus Baracoffea.
Fig. 3.
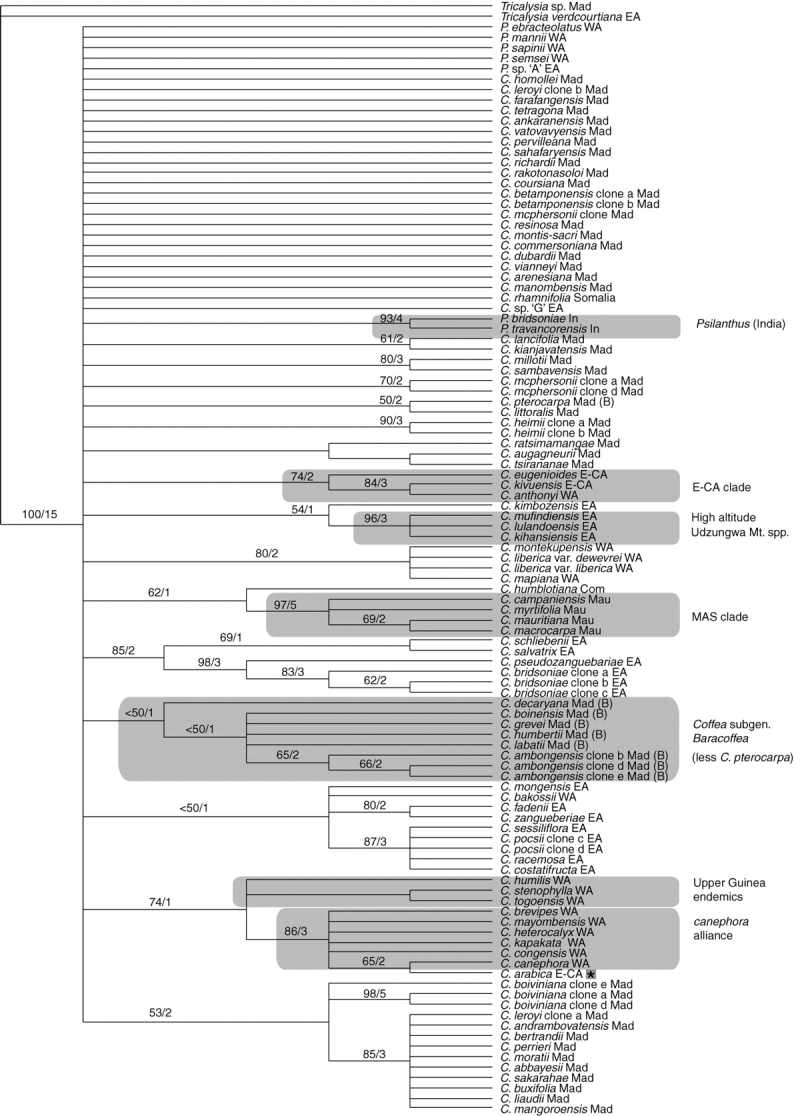
Strict consensus tree generated from ITS analysis. Bootstrap values of >50 % are placed above the branches, followed by Bremer support (decay) values. See Table 1 for species authorities and provenance. E-CA clade = East-Central Africa clade; MAS clade = Mascarene clade. Regions: EA = East Africa; E-CA = East Central Africa; Com = Comoros; In = India; Mad = Madagascar; Mau = Mauritius; WA = West Africa. * = Reference symbol for C. arabica. (B) = species belonging to Coffea subgenus Baracoffea.
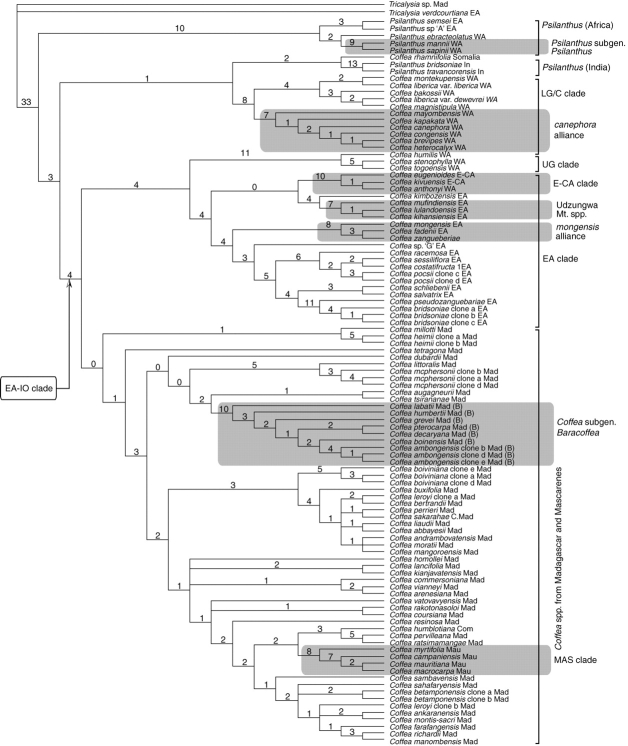
Negligible to moderate resolution was found in some parts of analyses, particularly for the Madagascan taxa. This lack of resolution was mostly due to low levels of sequence divergence, as indicated by the values for the consistency index (CI) and retention index (RI), and review of branch lengths (see Fig. 5).
Fig. 5.
One tree of 11 520 trees from the combined molecular (plastid–ITS) data analysis, with branch lengths. See Table 1 for species authorities and provenance. EA-IO clade = East Africa-Indian Ocean clade; E-CA clade = East-Central Africa clade; EA clade = East Africa clade; LG/C clade = Lower Guinea/Congolian clade; UG clade = Upper Guinea clade; MAS clade = Mascarene clade. Regions: EA = East Africa; E-CA = East Central Africa; Com = Comoros; In = India; Mad = Madagascar; Mau = Mauritius; WA = West Africa. (B) = species belonging to Coffea subgenus Baracoffea.
Terminology of clades
Many of the species groups recovered in the present analyses are consistent, or nearly so, with geographical or phytogeographical regions, and geographical abbreviations have been used for clades (cf. Lashermes et al., 1997; Cros et al., 1998). Where the analyses recovered groups congruent with the most recent infrageneric classification of Coffea and Psilanthus (Davis et al., 2005, 2006), these taxonomic groupings were retained. The following terminology was used for the geographical groupings: Upper Guinea (UG) clade, Lower Guinea/Congolian (LG/C) clade, East Africa-Indian Ocean (EA-IO) clade, East-Central Africa (E-CA) clade, East Africa (EA) clade, and Mascarenes (MAS) clade (see Fig. 1). The EA-IO clade includes the E-CA, EA and MAS clades, and species from Madagascar. The humid Central and West African forests are contained within the Guineo-Congolian Regional Centre of Endemism (White, 1983). Within this major region there are three subcentres of endemism for humid forest species: (1) Upper Guinea; (2) Lower Guinea; and (3) Congolian (White, 1979). For practical purposes, the subcentres (2) and (3) are often put together as the Lower Guinean/Congolian region, and this convention has been followed here. The distribution and systematic positions of C. arabica and C. rhamnifolia are isolated and are treated independently.
There is considerable agreement between the geographical distribution of species and their placement within clades or assumed species groupings. There is 100 % endemicity for the MAS, and EA clades. The UG, LG/C and E-CA clades do not have complete endemicity owing to the inclusion of widespread species: C. canephora and C. liberica occur in both the Upper Guinea (Portères, 1937) and Lower Guinea/Congolian regions and central-east Africa (see Fig. 1); C. anthonyi ined. occurs in the East Central Africa and the Lower Guinea/Congolian regions. However, the natural range of C. canephora and C. liberica in West Africa has no doubt been obscured by introduction and naturalization, and the distribution of C. anthonyi is still poorly known. The Madagascan species do not form a clade, but there is 100 % endemicity for Madagascan Coffea species. Owing to the relatively low sample size for Psilanthus, and because this study is focused on Coffea, abbreviations have not been provided for well-supported clades within Psilanthus.
Single plastid analyses
The accD–psa1 data set yielded the most potentially parsimony informative sites and trnL–F the least. Tree statistics for each separate plastid analysis are given in Table 3.
Combined plastid analysis (Fig. 2)
Several well-supported geographical groupings are revealed within Coffea, including the UG clade (BP 100; b = 5), LG/C clade (BP 86; b = 1), E-CA clade (including C. arabica) (BP 100; b = 4), Coffea subgenus Baracoffea (BP 99; b = 5) and clades within this subgenus. There are also several well-supported Coffea species pairs. The MAS clade is weakly supported (BP 63; b = 1), although three species within the MAS clade receive better support (BP 94; b = 2). The EA-IO clade is weakly supported (BP 66; b = 1). Coffea arabica is placed within the E-CA clade (BP = 100; b = 4), which is consistent with the placement in the Central Africa clade of Cros et al. (1998). The relationship of C. arabica to the other species in this clade (C. eugenioides, C. kivuensis, C. anthonyi ined.) is unresolved, although the sequences of C. arabica and C. eugenioides are identical. The monophyly of Coffea is not supported: C. rhamnifolia is unresolved in relation to other Coffea and Psilanthus species, and species from the Lower Guinea/Congolian region are sister to African Psilanthus (BP 53; b = 1). Psilanthus is unresolved, although African Psilanthus (BP 85; b = 2) and Indian Psilanthus (BP 100; b = 7) are both well-supported.
ITS analysis (Fig. 3)
The cloned ITS sequences from each species showing evidence of heterogeneous copies grouped together, with the exception of the two cloned sequences from C. leroyi. The problems associated with direct sequencing in these taxa thus appear to be due to incomplete homogenization of the ITS copies, rather than hybridization or other causes. Groupings within Coffea receiving high support include a group of species from the Lower Guinea/Congolian region (C. brevipes, C. mayombensis, C. heterocalyx, C. congensis and C. canephora), C. arabica (originating from Ethiopia) and C. kapakata (Angola) (BP 86; b = 3). These six species (less C. arabica) are here referred to as the canephora alliance. Other well-supported clades include a group of species from the Udzungwa Mountains of East Africa (BP 96; b = 3) and the MAS clade (BP 97; b = 5). There are also some well-supported species pairs and well-supported groups of ITS clones. The ITS analysis places C. arabica within the canephora alliance, sister to C. canephora (BP 65; b = 2), and with only 2 bp differences between these species. The two species of Indian Psilanthus are well supported (BP 93; b = 4). The relationship between Coffea and Psilanthus is unresolved.
Combined plastid and ITS analysis (Fig. 4)
Several well-supported groupings within Coffea are recovered, including the UG clade (BP 100; b = 11), LG/C clade (BP 86; b = 4), E-CA clade (BP 100; b = 7), the MAS clade (BP 98; b = 7), Coffea subgenus Baracoffea (BP 100; b = 6) and the canephora alliance (BP 98;b = 4). The EA-IO clade (BP 52; b = 1) and EA clade (BP <50; b = 1) are consistently recovered, but only weakly supported. There are a number of strongly supported lineages of East African species [e.g. Udzungwa Mountains (BP 98; b = 6), and the mongensis alliance (BP 95; b = 4)], several well-supported species pairs and several strongly supported groupings of ITS clones. Indian Psilanthus (BP 100; b = 12) and African Psilanthus (BP 96; b = 5) form well-supported clades. Apart from Coffea subgenus Baracoffea (BP 100; b = 6) and some small groupings of species, the relationships between most Madagascan species are unresolved. In contrast to the well-supported monophyly of Coffea subgenus Baracoffea, subgenus Coffea is paraphyletic. Psilanthus subgenus Psilanthus (BP 99; b = 5) is well supported, but the monophyly of Psilanthus subgenus Afrocoffea was not substantiated. Coffea rhamnifolia is placed with the two species of Indian Psilanthus, but this is only weakly supported (BP 57; b = 1). As in the combined plastid analysis and ITS analysis, the relationship between Coffea and Psilanthus is largely unresolved.
DISCUSSION
West African Coffea: the Upper Guinea (UG) clade
The UG clade, comprising C. humilis, C. stenophylla and C. togoensis, is one of the most strongly supported groups within the combined analysis (BP 100, b = 11; Fig. 4). Cros et al. (1998) found good support (BP 100) for C. stenophylla and C. humilis, which they recognized as the west Africa (W) clade. The convincing phylogenetic support for this clade may well be due to isolation of the Upper Guinea forests, as they are separated from those of the Lower Guinea/Congo region by the Dahomey Gap (Fig. 1), otherwise known as the Dahomey Interval (White, 1979, 1983). The gap is an extension of the woodland savannah of the Sahel to the Gulf of Guinea (Poorter et al., 2004) presently some 250 km wide (White, 1979), which reaches the coast of southern eastern Ghana, Togo and Benin. Booth (1958) suggested that the Dahomey Gap was much wider during periods of glacial aridification, and this is generally supported by more recent studies (e.g. Maley, 1987). The present-day distribution of Coffea species does not show complete separation between the Lower Guinea/Congo region and the Upper Guinea region, as C. canephora and C. liberica occur in both regions, and C. togoensis occurs in isolated humid forest patches in Ghana, Togo and Benin (Sonké et al., 2006a). However, the Dahomey Gap has clearly played a role in the evolution of plant (White, 1983) and animal species (e.g. Booth, 1958; Murphy and Collier, 1997) and populations (Sehgal et al., 2005) in the Upper Guinea region. It is proposed that this is first phylogenetic study to support the evolutionary influence of the Dahomey Gap within the flowering plants.
The incongruent position of the UG clade (see Results; Figs 2 and 3) is most probably due to (maternal) plastid genome transfer, which may have pre-dated speciation in this clade. The data imply plastid capture from a species or species lineage progenitor of East African origin or affiliation. Given the geographical location of the UG clade, the movement of a plastid genome from East to West Africa is most probable, perhaps either via long-distance dispersal or through dispersal via a once-continuous forest link between East and West Africa.
West Africa Coffea: Lower Guinea/Congo (LG/C) clade
The LG/C clade (BP 86, b = 1, Fig. 2; BP 86, b = 4, Fig. 4) is a group of ten predominantly lowland rainforest species, largely restricted to the Lower Guinea/Congolian region (Fig. 1). It does, however, include the widespread C. canephora and C. liberica, which also occur in the Upper Guinea region, and C. kapakata from Angola. Coffea kapakata occurs in the humid evergreen forests enclaves of the Guinea–Congolian/Zambezia Transition Zone (White, 1979), which is otherwise generally covered by non-forest vegetation. These enclaves are composed almost exclusively of humid forest Guineo-Congolian species (White, 1979), and thus the systematic position of C. kapakata within the present analysis is concomitant with humid forest distribution patterns in West Africa (White, 1979, 1983). The LG/C clade supports the findings of Cros et al. (1998, Fig. 2), who recognized a west and central African (WC) clade (BP 41), which included five species and four provisional/unknown taxa from the Lower Guinea/Congolian region.
Within the Lower Guinea/Congo region, the canephora alliance [C. brevipes, C. mayombensis, C. heterocalyx, C. congensis, C. canephora, and C. kapakata (BP 98, b = 4; Fig. 4)] represents an expansion of the ‘canephoroid group’ (C. brevipes, C. canephora, and C. congensis) as enumerated by Cros et al. (1998). Members of this alliance are all very similar morphologically, and (based on single species samples) appear closely related. Recognition of the canephora alliance is important as it provides a well-circumscribed group for further study of the economically important species C. canephora and C. arabica. Other Lower Guinea/Congo Coffea species (C. carrissoi A.Chev., C. dactylifera Robbr. & Stoff., C. fotsoana Stoff. & Sonké and C. leonimontana Stoff.) may belong in the canephora alliance; material of these species were not sampled in the present analyses.
Humid forest West African species and species groups have often been considered to represent the earliest diverging lineages within plant genera (Harris et al., 2000; Davis et al., 2002; Plana et al., 2004) or to include species that are phylogenetically isolated (Malcomber, 2002), although systematic studies for African plants are relatively few (Plana et al., 2004). On the basis of the present data, it is not possible to support these assumptions: the combined plastid analysis places the LG/C clade as sister to African Psilanthus (BP 53, b = 1; Fig. 2) but in the combined molecular analysis (Fig. 4) its position is unresolved. Davis et al. (2002) posited that the response of ancient African plant communities to climate change should be detectable through phylogenetic analysis of plants that span both humid and lowland xeric regions of the African continent. This is based on the significant evidence that lowland rainforest dominated much of Africa in the late Cretaceous and was replaced by xeric vegetation as a response to continental uplift (and other Earth events) and consequent widespread aridification beginning in the late Palaeogene. They suggest that if aridification induced a relatively recent period of diversification, then species that inhabit the humid relict forests of West Africa should represent the earliest diverging lineages of these African radiations, whereas species restricted to arid regions of East Africa should be phylogenetically nested (Davis et al., 2002). This pattern is not retrieved in the present analyses, although certain xeric species (C. costatifructa, C. zanguebariae, C. racemosa, C. pocsii and C. schliebenii) are convincingly nested within humid-dwelling East African lineages (Fig. 4). Coffea rhamnifolia, a species from SE Somalia and NE Kenya, and occurring in a xericenvironment, is intriguing in this respect as it occupies an isolated and equivocal position within the analyses (Figs 2–5).
In the ITS analysis, C. liberica var. dewevrei is grouped with three other Lower Guinea/Congolian taxa (BP 80, b = 2; Fig. 3): C. liberica var. liberica, C. montekupensis and C. mapiana, but the relationship among these taxa is unresolved. In the combined plastid analysis (BP 81, b = 1; Fig. 2) and combined molecular analysis (BP 81, b = 2; Fig. 4), it groups together with the morphologically unusual C. mapiana (Sonké et al., 2006b). Thus, the results add further evidence in support of the findings of N'Diaye et al. (2005), indicating genetic differentiation between the two varieties of C. liberica. Further data and sampling are required to assess fully the relationships between the two varieties of C. liberica and related taxa in the LG/C clade.
East African–Indian Ocean Coffea: EA-IO clade
The EA-IO clade is consistently retrieved in the combined plastid analysis (BP 66, b = 1; Fig. 2) and combined molecular analysis (BP 52, b = 1; Fig. 4), although the support for this clade is weak. The West African UG clade is placed within the EA-IO clade in these analyses, but this position may be due to (presumably maternal) plastid genome transfer via either dispersal or diffusion (see above). The separation of West African Coffea species (the LG/C clades) and East African and Indian Ocean species (EA-IO clade) would be expected given the geological history of Africa. The formation of the East Albertine African Rift Valley would have provided a climatic and physical obstruction to dispersal, separating a west/east humid forest belt that once spanned the African mainland (Maley, 1987; Davis et al., 2002). In addition, further Neogene aridification would have cyclically separated and fragmented a continuous forest belt or larger forest blocks (Maley, 1987). On the basis of the current analysis, the separation of West Africa and East Africa Coffea would require a single vicariance event, such as a climatic incident or long-distance dispersal.
East Africa Coffea: EA clade
In the combined molecular analysis, all species from East Africa and East-Central Africa are placed within the EA clade (Fig. 4). There is negligible support for this group (BP 23; b = 1), although deleting the UG clade from the combined molecular analysis gives the EA increased support (e.g. BP 52). Lashermes et al. (1997) and Cros et al. (1998) also recognized an East Africa (E) clade (see Introduction), although their analyses included only four and five taxa, respectively.
Within the EA clade there are other well-supported groups that have geographical/ecological or morphological correspondence. The clade formed by C. mufindiensis, C. lulandoensis and C. kihansiensis (BP 98, b = 6; Fig. 4) represents a group of high altitude (800–2300 m) species from the Udzungwa Mountains, one of the mountain groups within the Eastern Arc Mountains (Lovett, 1985, 1988). Coffea kimbozensis is also found in the Udzungwa Mountains, but unlike the species above it is restricted to low elevation (300–450 m) on calcareous rocks (Bridson, 1988a; A. Davis and E. Mvungi, pers. observ.). The clade formed by C. mongensis, C. fadenii and C. zanguebariae (BP 95, b = 4; Fig. 4) does not have a distinct geographical or geological delimitation within East Africa, although they are similar morphologically (Bridson, 1988a), and they are labelled here as the ‘mongensis group’. The E-CA clade (C. eugenioides, C. kivuensis and C. anthonyi ined.) is placed in an unresolved position at the base of the EA clade; further discussion of the E-CA clade is given below. The largest clade within the EA clade is a group of predominantly lowland species, labelled the ‘E. Africa lowland species’ (BP 72, b = 2; Fig. 4), and includes two well-supported groups (BP 94, b = 4; BP 95, b = 2). This clade contains mostly species from low elevations (sea level to approx. 500 m, rarely up to 800 m), in seasonally dry forest (some species in xeric woodland). These species mainly occur within the Indian Ocean Coastal belt (White, 1979). One exception is C. salvatrix, which normally occurs at altitudes of 850–1650 m (Bridson, 1988a) within the ‘E. Africa lowland species’ clade (Fig. 4).
East-Central Africa Coffea: E-CA clade
Coffea eugenioides, C. kivuensis and C. anthonyi ined. (as C. sp. Moloundou, Lashermes et al., 1997; Cros et al., 1998) form the E-CA clade in the combined plastid analysis (BP 100, b = 4; Fig. 2) and combined molecular analysis (BP 100, b = 7; Fig. 4). These results support the findings of Lashermes et al. (1997) and Cros et al. (1998), who received weak (BP 67) and strong (BP 100) support (respectively) for a central Africa (C) clade, based on C. eugenioides and C. anthonyi. The distribution of C. eugenioides and C. kivuensis falls mostly within the Lake Victoria Regional Mosaic (White, 1983), at elevations normally well above 1000 m, whereas C. anthonyi ined. occurs in a few isolated locations at low to mid-elevation [350–650(–900) m] in SE Cameroon and NW Congo. Ex situ material of C. anthonyi ined. was relied on for the DNA analysis, and further sampling would be desirable to test the close association of this species with C. eugenioides and C. kivuensis. The placement of the E-CA clade within the EA clade (Fig. 4) is consistent with the geographical proximity and the geological history of Africa (see above). Furthermore, clear associations between the Lake Victoria Regional Mosaic species and the Afromontane species of East Africa have been identified by White (1979); there are numerous species of Coffea occurring in the mountains of East Africa (Bridson, 1988a; Davis et al., 2006).
Madagascan Coffea species
All Madagascan Coffea species are placed within a weakly supported EA-IO clade (BP 66, b = 1, Fig. 2; BP 52, b = 1, Fig. 4). The position of all Madagascan species and species groups is unresolved, due to low levels of sequence divergence (Fig. 4), which is a problem in species-level analysis of Madagascan Rubiaceae, as exemplified by the study of Malcomber (2002). Coffea subgenus Baracoffea (BP 100, b = 6; Fig. 4) is the only well-supported Madagascan clade, apart from C. pervilleana and C. ratsimamangae (BP 91, b = 4; Fig. 4), two closely allied species from northern Madagascar (Davis and Rakotonasolo, 2001a). When comparing morphological diversity of Coffea species occurring in Madagascar (Davis, 2001; Davis and Rakotonasolo, 2001a, b, 2003; Davis et al., 2005) with those from Africa (Bridson, 1988a, 2003; Stoffelen, 1998), where sequence divergence is higher, the former exhibit far more interspecific differences, even excluding the unusual species of Coffea subgenus Baracoffea from western Madagascar (Davis et al., 2005). The paucity of sequence divergence in Madagascan Coffea subgenus Coffea implies either a rapid evolutionary radiation or slow molecular evolution, or perhaps a combination of both.
Suggestions concerning the origin of Madagascan Coffea species must be tentative with the present data at hand. A single dispersal event from Africa, followed by insular speciation, has been inferred for Begonia L. (Begoniaceae) by Plana et al. (2004) and for Gaertnera Lam. (Rubiaceae) by Malcomber (2002). Lavin et al. (2000) infer that the Madagascan species of the genus Ormocarpum P.Beauv. (Fabaceae) are the result of two dispersal events. Investigation of Streptocarpus Lindl. (Gesneriaceae) shows multiple colonization events for the Madagascan representatives of the genus (Möller and Cronk, 2001). A single dispersal event from Africa seems the most likely scenario for Madagascan Coffea, and one that would not be in conflict with the present data (Figs 3–5).
In the combined plastid (BP 65, b = 1; Fig. 2) and combined molecular (BP 61, b = 1; Fig. 4) analyses there is weak support for a sister relationship between C. humblotiana (the only Coffea species from the Comoros), and two species from Madagascar [C. pervilleana and C. ratsimamangae (northern Madagascar)], providing an indication for a northern Madagascan origin for the Comorian species.
Mascarene Coffea: MAS clade
In all analyses, the four Coffea species from the Mascarene Islands (Fig. 1) are placed within the EA-IO clade and form the MAS clade (BP 63, b = 1, Fig. 2; BP = 97, b = 5, Fig. 3; BP 98, b = 7, Fig. 4). Given that these islands are oceanic with a volcanic origin (the oldest approx. 8 million years old), the progenitor species must have arrived on the Mascarenes Islands via a long-distance dispersal event. The position of the MAS clade within the combined plastid and combined molecular analyses (Figs 2 and 5), coupled with the relative proximity of the Mascarenes to Madagascar, infers an ‘out of Madagascar’ origin for the MAS clade.
Taxonomic groups
The present study clearly shows that Coffea subgenus Baracoffea is a well-supported group (BP 100, b = 6; Fig. 4), restricted to the seasonal drylands (including spiny/xerophytic deciduous forest) of western Madagascar (Davis et al., 2005). Coffea subgenus Baracoffea is by far the most morphologically distinct group within Coffea, having evolved as a response to a seasonally dry environment. Morphological features include congested or shrubby (sympodial growth pattern) habit, indeterminate inflorescences, deciduous leaves and pubescent to densely pubescent leaves and corollas (Davis et al., 2005). In comparison, Coffea subgenus Coffea are trees (monopodial growth pattern), with determinate inflorescences, evergreen leaves (all except three species, see Davis et al., 2005), glabrous or rarely very sparsely puberulous leaves, and glabrous corollas (Davis et al., 2005). If it can be convincingly demonstrated that Coffea subgenus Baracoffea has evolved from humid forest ancestors/progenitors, which is possible (Figs 2, 4 and 5), assumptions about the origin of dryland biomes in Madagascar could be posited.
Coffea rhamnifolia cannot be considered a member of Coffea subgenus Baracoffea, as supposed by Leroy (1982): the combined plastid analysis and ITS analysis (Figs 2 and 3) place C. rhamnifolia in an unresolved position at the base of the ingroup, and the combined molecular analysis places it as sister to the two Indian Psilanthus (BP 57, b = 1; Fig. 4).
Psilanthus subgenus Psilanthus (P. mannii and P. sapinii) is well supported (BP 99, b = 5; Fig. 4) and definable by a single morphological synapomorphy: the presence of accrescent calyx lobes. The monophyly of the most species-rich group of Psilanthus, subgenus Afrocoffea, was not supported in the current analyses (Figs 2–4). Furthermore, the position and strong support for African (BP 85, b = 2, Fig. 2; BP 96, b = 5, Fig. 4) and Indian (BP 100, b = 7, Fig. 2; BP 93, b = 4, Fig. 3; BP 100, b = 12, Fig. 4) Psilanthus implies that the present subgeneric classification of this genus is not consistent with sequence data. Further sequence data and species sampling are required for Psilanthus.
The relationship between Coffea and Psilanthus
On the basis of ITS and trnL–trnF data, Lashermes et al. (1997) and Cros et al. (1998), respectively, concluded that the division of Coffea and Psilanthus into two genera was untenable. The present combined molecular analysis does not resolve the issues of monophyly for Coffea (including C. rhamnifolia) and Psilanthus, as the relationship between these genera is largely unresolved, mainly due to a lack of sequence divergence (Fig. 4). There is some support for inferring that Coffea and Psilanthus are not independent lineages: in the combined plastid analysis there is weak support (BP 53, b = 1, Fig. 2) for a sister relationship between African Psilanthus and West African Coffea, and in the combined molecular analysis C. rhamnifolia is weakly supported as the sister group to the two species of Indian Psilanthus (BP 57, b = 1; Fig. 4).
Three morphological characters separate Coffea from Psilanthus (Davis et al., 2005): short to long filaments (± absent to very short in Psilanthus); sub-medifixed anthers (supra-medifixed in Psilanthus); and (long) emergent style (short and included in Psilanthus). However, P. melanocarpus has short filaments and sub-medifixed anthers, as in Coffea, but an included style, as in Psilanthus. It was not possible to isolate DNA of P. melanocarpus from herbarium samples, and it was not included in the present analyses. If P. melanocarpus is indeed a species of Psilanthus, only one character would separate Coffea and Psilanthus: short vs. long style. If P. melanocarpus nested within Coffea, then the two anther characters would separate Coffea and Psilanthus. The number of pollen apertures (Lobreau-Callen and Leroy, 1980; Chinnappa and Warner, 1981; Stoffelen et al., 1997c) has been used as additional evidence to separate Coffea and Psilanthus (Davis et al., 2005), although considerable polymorphism is evident and there is overlap in the number of apertures between Coffea and Psilanthus and their subgenera (Stoffelen et al., 1997c; Davis et al., 2005). The morphological evidence for the separation of Coffea and Psilanthus is certainly not convincing, and if P. melanocarpus is found to nest within either of these genera there would seem negligible justification for the recognition of two genera on morphological grounds (see above). However, many other genera of Rubiaceae are separated on only one or two morphological characters. Pagamea Aubl. and Gaertnera are well-supported genera based on molecular data, but are separated by a single synapomorphy (pubescent vs. glabrous corolla lobes), for example (Malcomber, 2002).
The robust morphological (Robbrecht and Puff, 1986; Davis et al., 2005) and molecular support for Coffea plus Psilanthus (Davis et al., 2007), low sequence diversity between these genera (see above; Fig. 4) and indications of paraphyly (Figs 2 and 4), may be taken as evidence for accepting Coffea and Psilanthus as a single genus (Lashermes et al., 1997; Cros et al., 1998). However, it is believed that further molecular data are needed to resolve fully the relationship between Coffea and Psilanthus, and in particular sequence data are required for P. melanocarpus and other species of Psilanthus.
Origin of Coffea arabica
The present data support a hybrid origin for C. arabica, following the findings of Lashermes et al. (1997, 1999), Cros et al. (1998) and Raina et al. (1998). Examination of combined plastid and ITS analyses (Figs 2 and 3), and by pair-wise comparison of sequences (see Results), it is concluded that the progenitor species of C. arabica are C. canephora and C. eugenioides. The results concur with those of Lashermes et al. (1999), who found low genetic (RFLP) divergence between the two constituent genomes of C. arabica and those of its progenitor species, suggesting perhaps that the speciation of C. arabica took place relatively recently. The proposed hybrid origin of C. arabica is consistent with the potential recent sympatry of C. canephora and C. eugenioides based on present-day distribution (Davis et al., 2006).
Conclusions
On the basis of sequences from four plastid regions and ITS, it has been possible to identify several well-supported lineages within Coffea that are consistent with major biogeographical regions and geographical areas, including the UG clade (BP 100; b = 11), the LG/C clade (BP 86; b = 4), the E-CA clade (BP 100; b = 7) and the MAS clade (BP 98; b = 7), although the distribution of widespread species (C. canephora and C. liberica) occurs across some of these regions in mainland Africa. The UG, LG/C and E-CA clades are consistent with those either retrieved or identified by Cros et al. (1998) and Lashermes et al. (1997). Within the LG/C clade, it has been possible to substantiate (Cros et al., 1998) and expand on a group of species related to C. canephora, the canephora alliance (BP 98, b = 4; Fig. 4). Other groups were consistently retrieved but received weak support, including the EA-IO clade (BP 52; b = 1) and the EA clade (BP 23; b = 1). Smaller biogeographical groupings were retrieved within the EA clade, including a group of high altitude Udzungwa Mountain species (BP 98; b = 6), and the east African lowland species (BP 72; b = 2). Groups corresponding to geographical distribution were also recovered in Psilanthus, including the two Indian representatives, P. travancorensis and P. bridsoniae (BP 100; b = 12), and species of Psilanthus occurring in Africa (BP 96; b = 5). Two formal taxonomic groups are well supported: Coffea subgenus Baracoffea (BP 100; b = 6) and Psilanthus subgenus Psilanthus (BP 99; b = 5).
The combined sequence data do not substantiate the monophyly of either Coffea or Psilanthus, largely due a lack of resolution (Fig. 4), resulting from low levels of sequence divergence (e.g. see Fig. 5). This evidence, together with weak support for some intergeneric (Coffea–Psilanthus) relationships, and strong molecular and morphological support for a clade comprising Coffea plus Psilanthus [BP 100; b = 32; see also Davis et al. (2007)], could be taken as justification for the recognition of a single genus (i.e. Coffea), although further data and critical sampling are required to resolve this matter fully. The subgeneric classification of Coffea and Psilanthus (Bridson 1988a, b; Davis et al., 2005) is not consistent with phylogenetic data. Most notably, Coffea subgenus Coffea is paraphyletic, due to the nested position of Coffea subgenus Baracoffea.
A recent hybrid origin for C. arabica, with C. canephora and C. eugenioides as the likely progenitor species, is supported (Lashermes et al., 1999). This study provides the first phylogenetic evidence for the influence of the Dahomey Gap (West Africa) on plant speciation.
ACKNOWLEDGEMENTS
We would like to thank the following organizations and individuals who provided plant material for this study, including National Centre for Applied Research on Rural Development (FOFIFA), P. De Block (BR), IRD-Montpellier, Q. Luke (EA), F. Mbago (DSM), F. Rakotonasolo (TAN), B. Sonké (Université de Yaoundé) and P. Stoffelen (BR). Permission to sample and study herbarium and/or living collections from DSM, K and BR is gratefully acknowledged. We also thank the DNA bank managers of the Jodrell Laboratory Royal Botanic Gardens, Kew: L. Csiba, E. Kapinos. In Madagascar fieldwork was carried out under collaborative agreements between the Parc Botanique et Zoologique de Tsimbazaza (PBZT), the University of Antananarivo, the Association Nationale de Gestion des Aires Protegées (ANGAP) and the Royal Botanic Gardens, Kew. We would like to thank J. Andriantiana (TAN), H. Ralimanana (K), and especially F. Rakotonasolo (TAN) for help with fieldwork in Madagascar. We would like to acknowledge the Tanzania Commission for Science and Technology (COSTECH) for providing research clearance to undertake fieldwork in Tanzania. In Tanzania, we are grateful to F. Mbago (DSM), S. Hall, A. Ntemi, R. Hind and Z. Bridger. In Cameroon, we thank the staff and Directors at the Université de Yaoundé, National Herbarium of Cameroon (YA) and Limbe Botanical and Zoological Gardens (SCA), and especially B. Sonké (Université de Yaoundé). At the Royal Botanic Gardens, Kew, we would like to thank W. Baker, D. Bridson, S. Dawson, F. Forest, E. Lucas and J. Moat. At IRD-Montpellier we would like to thank F. Anthony, P. Hamon, A. de Kochko, P. Lashermes and M. Noirot. A.D. would like to thank the staff at the Royal Botanic Gardens, Sydney (NSW) and in particular D. Crayn. E.M. gratefully acknowledges the DANIDA-ENRECA biodiversity project, for their financial support. The funding for this study was largely provided by Kraft Foods (UK), and we are extremely grateful for their financial support for laboratory and herbarium research and the visits of E.M. and Y.J.-F. to Kew.
LITERATURE CITED
- Andreasen K, Bremer B. Combined phylogenetic analysis in the Rubiaceae–Ixoroideae: morphology, nuclear and chloroplast DNA data. American Journal of Botany. 2000;87:1731–1748. [PubMed] [Google Scholar]
- Booth AH. The Niger, the Volta and the Dahomey Gap as geographical barriers. Evolution. 1958;12:48–62. [Google Scholar]
- Bremer K. The limits of amino-acid sequence data in angiosperm phylogenetic reconstruction. Evolution. 1988;42:795–803. doi: 10.1111/j.1558-5646.1988.tb02497.x. [DOI] [PubMed] [Google Scholar]
- Bremer K. Branch support and tree stability. Cladistics. 1994;10:295–304. [Google Scholar]
- Bridson DM. The identity of Paolia (Rubiaceae) Kew Bulletin. 1979;34:376. [Google Scholar]
- Bridson DM. The identities of Plectonia rhamnifolia Chiov. and P. sennii Chiov. (Rubiaceae) Kew Bulletin. 1983;38:320. [Google Scholar]
- Bridson DM. Coffea. In: Polhill RM, editor. Flora of Tropical East Africa, Rubiaceae, 2. a. Balkema: Rotterdam/Brookfield; 1988. pp. 703–723. [Google Scholar]
- Bridson DM. Classification. In: Wrigley G, editor. Coffee. b. New York, NY: Longmans; 1988. pp. 61–75. [Google Scholar]
- Bridson DM. In: Flora Zambesiaca 3. Pope GV, editor. Surrey: Royal Botanic Gardens, Kew; 2003. pp. 452–463. Coffea. [Google Scholar]
- Carvalho A. Taxonomia de Coffea arabica L. Caracteres morfológicos dos haploides. Bragantia. 1952;12:201–212. [Google Scholar]
- Carvalho A, Medina Filho HP, Fazuoli LC, Guerreiro Filho O, Lima MMA. Aspectos genéticos do cafeeiro. Revista Brasileira de Genética. 1991;l4:135–183. [Google Scholar]
- Chase MW, Hills HG. Silica gel: an ideal desiccant for preserving field-collected leaves for use in molecular studies. Taxon. 1991;41:215–220. [Google Scholar]
- Cheek M, Csiba L, Bridson DM. A new species of Coffea (Rubiaceae) from western Cameroon. Kew Bulletin. 2002;57:675–680. [Google Scholar]
- Chinnappa CC, Warner BG. Pollen morphology in the genus Coffea (Rubiaceae) and its taxonomic significance. Botanical Journal of the Linnean Society. 1981;83:221–223. [Google Scholar]
- Coulibaly I, Noirot M, Lorieux M, Charrier A, Hamon S, Louarn J. Introgression of self-compatibility from Coffea heterocalyx to the cultivated species Coffea canephora. Theoretical and Applied Genetics. 2002;105:994–999. doi: 10.1007/s00122-002-1008-z. [DOI] [PubMed] [Google Scholar]
- Coulibaly I, Louarn J, Lorieux M, Charrier A, Hamon S, Noirot M. Pollen viability restoration in a Coffea canephora P. [sic] and C. heterocalyx Stoffelen backcross. QTL identification for marker-assisted selection. Theoretical and Applied Genetics. 2003;106:311–316. doi: 10.1007/s00122-002-1018-x. [DOI] [PubMed] [Google Scholar]
- Cros J. France: Université Montpellier II; 1994. Implications phylogénétiques des variations de l'ADN chloroplastique chez les caféiers (genres Coffea L. et Psilanthus Hook.f.). PhD Thesis. [Google Scholar]
- Cros J, Combes MC, Trouslot P, Anthony F, Hamon S, Charrier A, Lashermes P. Phylogenetic analysis of chloroplast DNA variation in Coffea L. Molecular Phylogenetics and Evolution. 1998;9:109–117. doi: 10.1006/mpev.1997.0453. [DOI] [PubMed] [Google Scholar]
- Davis AP. Two new species of Coffea L. (Rubiaceae) from eastern Madagascar. Kew Bulletin. 2001;56:479–489. [Google Scholar]
- Davis AP. A new combination in Psilanthus (Rubiaceae) for Australia, and nomenclatural notes on Paracoffea. Novon. 2003;13:182–184. [Google Scholar]
- Davis AP, Mvungi EF. Two new and endangered species of Coffea (Rubiaceae) from the Eastern Arc Mountains (Tanzania) and notes on associated conservation issues. Botanical Journal of the Linnean Society. 2004;146:237–245. [Google Scholar]
- Davis AP, Rakotonasolo F. Three new species of Coffea L. (Rubiaceae) from Madagascar. Kew Bulletin. 2000;55:405–416. [Google Scholar]
- Davis AP, Rakotonasolo F. Three new species of Coffea L. (Rubiaceae) from NE Madagascar. Adansonia, Sér. 3. (a) 2001;23:137–146. [Google Scholar]
- Davis AP, Rakotonasolo F. Two new species of Coffea L. (Rubiaceae) from Northern Madagascar. Adansonia, Sér. 3. (b) 2001;23:337–345. [Google Scholar]
- Davis AP, Rakotonasolo F. New species of Coffea L. (Rubiaceae) from Madagascar. Botanical Journal of the Linnean Society. 2003;142:111–118. [Google Scholar]
- Davis AP, Bridson DM, Rakotonasolo F. A reexamination of Coffea subgenus Baracoffea and comments on the morphology and classification of Coffea and Psilanthus (Rubiaceae-Coffeeae) In: Keating RC, Hollowell VC, Croat T, editors. Festschrift for William G. D'Arcy: the legacy of a taxonomist. Missouri: MBG Press; 2005. pp. 398–420. (Monograph in Systematic Botany 104) [Google Scholar]
- Davis AP, Govaerts R, Bridson DM, Stoffelen P. An annotated checklist of the genus Coffea L. (Rubiaceae) Botanical Journal of the Linnean Society. 2006;152:465–512. [Google Scholar]
- Davis AP, Chester M, Maurin O, Fay M. Searching for the relatives of Coffea (Rubiaceae, Ixoroideae): the circumscription and phylogeny of Coffeeae based on plastid sequence data and morphology. American Journal of Botany. 2007;94:313–329. doi: 10.3732/ajb.94.3.313. [DOI] [PubMed] [Google Scholar]
- Davis CC, Bell CD, Fritsch PW, Mathews S. Phylogeny of Acridocarpus-Brachylophon (Malpighiaceae): implications for Tertiary tropical floras and Afroasian biogeography. Evolution. 2002;56:2395–2405. doi: 10.1111/j.0014-3820.2002.tb00165.x. [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemistry Bulletin, Botanical Society of America. 1987;19:11–15. [Google Scholar]
- Eriksson T. AutoDecay, version 4·0·2. Stockholm, Sweden: Royal Swedish Academy of Sciences; 1999. Computer program distributed by the author. Bergius Foundation. [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Fitch WM. Toward defining the course of evolution: minimum change for a specific tree topology. Systematic Zoology. 1971;20:406–416. [Google Scholar]
- Grassias M, Kammacher P. Observations sur le conjugaison chromosomique de Coffea arabica L. Café, Cacao, Thé. 1975;19:177–190. [Google Scholar]
- Harris DJ, Poulsen AD, Frimodt-Møller C, Preston J, Cronk QCB. Rapid radiation in Aframomum (Zingiberaceae): evidence from nuclear ribosomal DNA internal transcribed spacer (ITS) sequences. Edinburgh Journal of Botany. 2000;57:377–395. [Google Scholar]
- Holmgren PK, Holmgren NH, Barnett LC. Regnum Vegetabile. 8th edn. New York, NY: New York Botanical Garden; 1990. Index herbariorum. In: Part 1: The herbaria of the world. [Google Scholar]
- Jordan WC, Courtney MW, Neigel JE. Low levels of intraspecific genetic variation at a rapidly evolving chloroplast DNA locus in North American duckweeds (Lemnaceae) American Journal of Botany. 1996;83:430–439. [Google Scholar]
- Källersjö M, Farris JS, Kluge AG, Bult C. Skewness and permutation. Cladistics. 1992;8:275–287. doi: 10.1111/j.1096-0031.1992.tb00071.x. [DOI] [PubMed] [Google Scholar]
- Lashermes P, Cros J, Marmey P, Charrier A. Use of random amplified DNA markers to analyse genetic variability and relationships of Coffea species. Genetic Resources and Crop Evolution. 1993;40:91–99. [Google Scholar]
- Lashermes P, Cros J, Combes M-C, Trouslot P, Anthony F, Hamon S, Charrier A. Inheritance and restriction fragment length polymorphism of chloroplast DNA in the genus Coffea L. Theoretical Applied Genetics. 1996;93:626–632. doi: 10.1007/BF00417958. [DOI] [PubMed] [Google Scholar]
- Lashermes P, Combes M-C, Trouslot P, Charrier A. Phylogenetic relationships of coffee-tree species (Coffea L.) as inferred from ITS sequences of nuclear ribosomal DNA. Theoretical Applied Genetics. 1997;94:947–955. [Google Scholar]
- Lashermes P, Combes M-C, Robert J, Trouslot P, D'Hont A, Anthony F, Charrier A. Molecular characterisation and origin of the Coffea arabica L. genome. Molecular and General Genetics. 1999;261:259–266. doi: 10.1007/s004380050965. [DOI] [PubMed] [Google Scholar]
- Lavin M, Thulin M, Labat J-N, Pennington RT. Africa, the odd man out: molecular biogeography of dalbergioid legumes (Fabaceae) suggests otherwise. Systematic Botany. 2000;25:449–467. [Google Scholar]
- Leroy J-F. L'origine kenyane du genre Coffea L. et la radiation des espèces à Madagascar. Association Scientifique Internationale du Café (ASIC) 1982;10e Colloque:413–420. [Google Scholar]
- Leroy J-F. Coffea L. In: Bosser J, Cadet T, Guého J, Marais W, editors. Flore des Mascareignes, La Réunion, Maurice, Rodrigues. Surrey: Royal Botanic Gardens, Kew; 1989. pp. 93–99. 107. Caprifoliacées à 108bis. Valérianacées. [Google Scholar]
- Lobreau-Callen D, Leroy J-F. Quelques données palynologiques sur le genre Coffea et autres genres du cercle des caféiers. Association Scientifique Internationale du Café (ASIC) 1980:479–506. 9e Colloque. [Google Scholar]
- Lovett JC. Moist forests of Tanzania. Swara. 1985;8:8–9. [Google Scholar]
- Lovett JC. Botanical importance of the Eastern Arc. Journal of the East African Natural History Society. 1988;87:59–74. [Google Scholar]
- Malcomber ST. Phylogeny of Gaertnera Lam. (Rubiaceae) based on multiple DNA markers: evidence of rapid radiation in a widespread morphologically diverse genus. Evolution. 2002;56:42–57. doi: 10.1111/j.0014-3820.2002.tb00848.x. [DOI] [PubMed] [Google Scholar]
- Maley J. Fragmentation de la forêt dense humide africaine et extension des biotopes montagnards au Quaternaire recent: nouvelles données polliniques et chronologiques. Implication palaéoclimatiques et biogéographiques. In: Coetzee JA, editor. Palaeoecology of Africa and the surrounding islands. Rotterdam: A.A. Balkema; 1987. pp. 307–334. [Google Scholar]
- Mendenhall M. Austin: University of Texas; 1994. Phylogeny of Baptisia and Thermopsis (Leguminosae) as inferred from chloroplast DNA and nuclear ribosomal DNA sequences, secondary chemistry, and morphology. PhD Thesis. [Google Scholar]
- Möller M, Cronk QCB. Phylogenetic studies in Streptocarpus: reconstruction of biogeographic history and distribution patterns in Streptocarpus (Gesneriaceae) Systematics and Geography of Plants. 2001;71:545–555. [Google Scholar]
- Murphy WJ, Collier GE. A molecular phylogeny for aplocheiloid fishes (Atherinomorpha, Cyprinodontiformes): the role of vicariance and the origins of annualism. Molecular Biology and Evolution. 1997;14:790–799. doi: 10.1093/oxfordjournals.molbev.a025819. [DOI] [PubMed] [Google Scholar]
- N'Diaye AN, Poncet V, Louran J, Hamon S, Noirot M. Genetic differentiation between Coffea liberica var. liberica and C. liberica var. dewevrei and comparison with C. canephora. Plant Systematics and Evolution. 2005;253:95–104. [Google Scholar]
- Plana V, Gascoigne A, Forrest LL, Harris D, Pennington RT. Pleistocene and pre-Pleistocene Begonia speciation in Africa. Molecular Phylogenetics and Evolution. 2004;31:449–461. doi: 10.1016/j.ympev.2003.08.023. [DOI] [PubMed] [Google Scholar]
- Poorter L, Bongers F, ‘Kouamé FN, Hawthorne WD. Biodiversity of west African forests: an ecological atlas of woody plant species. Wallingford, UK: CABI Publishing; 2004. [Google Scholar]
- Portères R. Étude sur les caféiers spontanés de la section ‘des Eucoffea. Annales Agricoles de L'Afrique Occidentale. 1937;1:68–91. [Google Scholar]
- Prakash NS, Combes M-C, Dussert S, Naveen S, Lashermes P. Analysis of genetic diversity in Indian robusta coffee genepool (Coffea canephora) in comparison with a representative core collection using SSRs and AFLPs. Genetic Resources and Crop Evolution. 2005;52:333–343. [Google Scholar]
- Raina SN, Mukai Y, Yamamoto M. In situ hybridization identifies the diploid progenitor species of Coffea arabica (Rubiaceae) Theoretical and Applied Genetics. 1998;97:1204–1209. [Google Scholar]
- Robbrecht E, Puff C. A survey of the Gardenieae and related tribes (Rubiaceae) Botanische Jahrbücher für Systematik. 1986;108:63–137. [Google Scholar]
- Sehgal RH, Jones HI, Smith TB. Molecular evidence for host specificity of parasitic nematode microfilariae in some African rainforest birds. Molecular Ecology. 2005;14:3977–3988. doi: 10.1111/j.1365-294X.2005.02555.x. [DOI] [PubMed] [Google Scholar]
- Sonké B, Stoffelen P. Une nouvelle espèce de Coffea L. (Rubiaceae, Coffeeae) du Cameroun et quelques notes sue les affinités avec les espèces voisines. Adansonia, Sér. 3. 2004;26:153–160. [Google Scholar]
- Sonké B, Dessein S, Robbrecht E. Rubiaceae. In: Akoègninou A, van der Burg WJ, van der Maesen LJG, editors. Flore analytique du Bénin. a. Leiden: Backhuys; 2006. pp. 869–910. [Google Scholar]
- Sonké B, Nguembou CK, Davis AP. A new dwarf Coffea (Rubiaceae) from southern Cameroon. Botanical Journal of the Linnean Society. (b) 2006;151:425–430. [Google Scholar]
- Stoffelen P. Leuven, Belgium: Katholieke Universiteit; 1998. Coffea and Psilanthus in tropical Africa: a systematic and palynological study, including a revision of the West and Central African species. PhD Thesis. [Google Scholar]
- Stoffelen P, Robbrecht E, Smets E. Coffea (Rubiaceae) in Cameroon: a new species and a nomen recognised as species. Belgian Journal of Botany. (a) 1996;129:71–76. [Google Scholar]
- Stoffelen P, Robbrecht E, Smets E. The ‘arabica–congensis complex’: five Coffea species with a problematic delimitation. Opera Botanica Belgica. (b) 1996;7:237–248. [Google Scholar]
- Stoffelen P, Cheek M, Bridson DM, Robbrecht E. A new species of Coffea (Rubiaceae) and notes on Mount Kupe (Cameroon) Kew Bulletin. (a) 1997;42:989–994. [Google Scholar]
- Stoffelen P, Robbrecht E, Smets E. Adapted to the rain forest floor: a remarkable new dwarf Coffea (Rubiaceae) from Lower Guinea (tropical Africa) Taxon. (b) 1997;46:37–47. [Google Scholar]
- Stoffelen P, Robbrecht E, Smets E. Pollen morphology of Coffea and Psilanthus (Rubiaceae), mainly from Africa. Grana. (c) 1997;36:313–327. [Google Scholar]
- Sun Y, Skinner DZ, Liang GH, Hulbert SH. Phylogenetic analysis of Sorghum and related taxa using internal transcribed spacers of nuclear ribosomal DNA. Theoretical and Applied Genetics. 1994;89:26–32. doi: 10.1007/BF00226978. [DOI] [PubMed] [Google Scholar]
- Swofford DL. PAUP* 4·0b10: Phylogenetic analysis using parsimony (* and other methods, Vers. 4) Sunderland, MA: Sinauer Associates; 2002. [Google Scholar]
- Taberlet P, Gielly L, Pautou G, Bouvet J. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Molecular Biology. 1991;17:1105–1109. doi: 10.1007/BF00037152. [DOI] [PubMed] [Google Scholar]
- White F. The Guineo-Congolian Region and its relationships to other phytochoria. Bulletin du Jardin Botanique National de Belgique. 1979;49:11–55. [Google Scholar]
- White F. The vegetation of Africa. A descriptive memoir to accompany the Unesco/AETFAT/UNSO vegetation map of Africa. Paris: Unesco; 1983. [Google Scholar]