Abstract
Background and Aims
A deviation from the classical beetle pollination syndrome of dull-coloured flowers with an unpleasant scent is found in the Greater Cape Floral Region of South Africa. Here, monkey beetles (Scarabaeidae) visit brightly coloured, odourless flowers with conspicuous dark spots and centres (beetle marks). The role of flower colour and markings in attracting monkey beetles is still poorly understood.
Method
Artificial model flowers with different marking patterns were used to test the effect of beetle marks on visitation by monkey beetles. To test whether monkey beetles are conditioned to the colour of the local matrix species, model flowers of different colours were placed in populations of three differently coloured species of Iridaceae.
Key Results
Among all three matrix species the presence of dark markings of some kind (either centres or spots) increased visitation rates but the different matrix species differed in whether the effect was due to a dark centre or to dark spots. Monkey beetles were not conditioned for the colour of the matrix species: model colour was not significant in the Hesperantha vaginata and in the Romulea monadelpha matrices, whereas yellow model flowers were preferred over orange ones in the orange-flowered Sparaxis elegans matrix.
Conclusions
This study is the first to demonstrate that beetle marks attract pollinating monkey beetles in the Greater Cape Floral Region. In contrast to plants with the classical beetle pollination syndrome that use floral scent as the most important attractant of pollinating beetles, plants with the monkey beetle pollination syndrome rely on visual signals, and, in some areas at least, monkey beetles favour flowers with dark beetle markings over unmarked flowers.
Key words: Beetle marks, beetle pollination syndrome, cantharophily, Greater Cape Floral Region, convergent evolution, Iridaceae, monkey beetles, pollinator attraction
INTRODUCTION
Convergent evolution has resulted in the recurrent development of the same suites of floral characteristics among different plant taxa that are pollinated by the same guild of pollinators (Faegri and van der Pijl, 1979). The value of floral syndromes as predictive tools is still relatively poorly tested and in some instances cannot be substantiated (e.g. Hingston and McQuillan, 2000; Ollerton et al., 2003; Zhang et al., 2005; Valdivia and Niemeyer, 2006). Among families dominated by specialist pollination systems, however, such as Iridaceae in southern Africa, pollination syndromes are more often diagnostic and still form the basis from which much of our understanding of pollination systems is inferred (Goldblatt and Manning, 2006).
Beetles were among the earliest pollinators of angiosperms, and plants that rely on these insects for pollination have classically been characterized by dull-coloured, chamber- or urn-shaped flowers with a strong, often unpleasant scent (Faegri and van der Pijl, 1979). In typical cantharophilous species scent production is often stressed over pigmentation (Dafni et al., 1990). A striking deviation from the classical beetle pollination syndrome is found in the eastern Mediterranean region, where species of Anemone and Ranunculus (Ranunculaceae), Papaver (Papaveraceae) and Tulipa (Liliaceae) are pollinated mainly by Amphicoma scarab beetles. These plants have undergone convergent evolution for bright red, bowl-shaped flowers with a dark centre (Dafni et al., 1990). A remarkably similar syndrome is found in plants of the Greater Cape Floral Region of South Africa, which are pollinated by another group of scarab beetles known as monkey beetles (Scarabaeidae: Rutelinae: Hopliini). Plants that rely on these insects for pollination typically produce odourless, brightly coloured, shallowly bowl-shaped flowers (or inflorescences) (Goldblatt et al., 1998). In addition, many of these species have conspicuous dark (sometimes pale) markings known as beetle marks at the base of the tepals or petals, which give the flowers (or inflorescences) a dark or contrastingly coloured central zone or eye (Fig. 1). Beetle marks have evolved in taxa in numerous different genera, particularly in the Asteraceae and Iridaceae (Picker and Midgley, 1996; Goldblatt et al., 1998).
Fig. 1.

Beetle marks on the three matrix species used to test the effect of marking patterns and flower colour on visitation by monkey beetles. (A) Flower of Hesperantha vaginata. (B) Flower of Hesperantha vaginata with copulating Clania glenlyonensis monkey beetles. (C) Flower of Romulea monadelpha. (D) Flowers of Sparaxis elegans. All flowers are approx. 3–5 cm in diameter.
By using model flowers, Dafni et al. (1990) demonstrated that the Amphicoma scarab beetles that are the major pollinators of this system in the Mediterranean display a distinct preference for red flowers with dark centres over other coloured flowers without dark centres. A more generalized attraction for beetles by dark markings has also been demonstrated in England, where the addition of ink spots to flowers of Brassica napus (Brassicaceae) increased visitation by the pollen beetle Meligehtes aeneus (Free and Williams, 1978). Futhermore, mordelid beetles prefer inflorescences of Daucus carota with dark central florets over ones without dark florets in its non-native range in eastern North America (Westmoreland and Muntan, 1996). Such an effect was, however, not found for Daucus carota in its native range in Europe (Lamborn and Ollerton, 2000). Overall, these studies indicate that dark markings are used as visual cues by several groups of beetle species.
Although the convergent evolution of beetle marks suggests that they are adaptive, their exact role in floral function remains incompletely understood. Hutchinson (1946) suggested that the beetle marks on the ray florets of Gorteria diffusa mimic herbivorous beetles and thus function as repellents to such beetles. In a test of Hutchinson's hypothesis, Midgley (1993) found no evidence that artificial addition of beetle marks to unmarked inflorescences of another species of Asteraceae repelled herbivorous monkey beetles. Because monkey beetles often carry significant quantities of pollen on their hairy bodies, Midgley (1993) suggested that it is more likely that beetle marks play a role in attracting monkey beetles as potential pollinators rather than deterring them. This is also likely to apply to many other plant species with beetle marks in the Greater Cape Floral Region. Monkey beetles, which feed on the pollen and also use the flowers as mating sites, have been identified as effective pollinators of many species with beetle marks (Fig. 1B; Goldblatt et al., 1998).
The effect of beetle marks on visitation by monkey beetles in the south-western part of the Greater Cape Floral Region was tested by Johnson and Midgley (2001) using artificial model flowers with and without dark markings. Although their study revealed slightly higher visitation rates of monkey beetles on model flowers with dark centres, this effect was not significant. There are, however, more than 200 species of monkey beetles in the Greater Cape Floral Region (Péringuey, 1902; Steiner, 1998), and their assemblages can differ considerably between areas within this region (Colville et al., 2002). Johnson and Midgley's (2001) study was carried out on the Cape Peninsula, which is not known for its high incidence of plants with beetle marks. It is likely, therefore, that the role of beetle marks may be more pronounced in other areas within the Greater Cape Floral Region, particularly those with high concentrations of beetle flowers or which are known regions of monkey beetle diversity.
Therefore the experiments of Johnson and Midgley (2001) were repeated here on the Bokkeveld Escarpment, which is a centre of monkey beetle diversity in the Greater Cape Floral Region (Peringuey, 1902; Colville et al., 2002), by using arrays of plain coloured model flowers and marked model flowers to test the effect of beetle marks on monkey beetle visitation rates. The model flowers were positioned in populations of three differently coloured species of Iridaceae that are known to be pollinated by monkey beetles: Hesperantha vaginata, Romulea monadelpha and Sparaxis elegans (Fig. 1). Because flower colour itself may also play an important role in attracting monkey beetles (Picker and Midgley, 1996; Johnson and Midgley, 2001; Colville et al., 2002), model flowers of different colours were used to test whether monkey beetles showed a preference for the colour of the matrix species.
MATERIALS AND METHODS
Study area and matrix species
The study was performed in 2002 in Northern Cape Province, South Africa, in nature reserves around Nieuwoudtville (31°22′S, 019°07′E) on the Bokkeveld Escarpment. The study area is situated on the border between Namaqualand and the Cape Floral Region, both of which form part of the Greater Cape Floral Region (Born et al., 2007), and is known as a centre of monkey beetle diversity (Peringuey, 1902; Colville et al., 2002). The experiment (see below) was performed in populations of three species of Iridaceae known to be pollinated by monkey beetles (Goldblatt et al., 1998). Hesperantha vaginata (Sweet) Goldblatt has bowl-shaped, yellow flowers with dark brown spots and a dark centre (Fig. 1A, B), and occurs on heavy clay soils derived from dolerite; Romulea monadelpha (Sweet) Baker has bowl-shaped, red flowers with a dark centre (Fig. 1C), and occurs on doleritic clays; and Sparaxis elegans (Sweet) Goldblatt has shallowly bowl-shaped, orange flowers with yellow spots on a black ring (Fig. 1D), and occurs on lighter clay soils derived from tillite. All species flower between early August and late September.
Experimental set-up
Experimental methodology largely followed that of Johnson and Midgley (2001). Cone-shaped model flowers of different colours were constructed from orange, red and yellow cardboard, with an external diameter of 5 cm and a central opening 2 cm in diameter. Model flowers were taped onto empty film canisters (3 cm in diameter and 5 cm high) that functioned as traps. Model flowers of each colour were marked with three types of patterns, developed to match commonly encountered natural floral marking patterns (see x-axis of Fig. 2) as follows: (1) unmarked model flowers were mounted on a translucent film canister, thus with a pale centre; (2) model flowers with six dark spots (7 mm diameter) at regular intervals at a distance of 5 mm from the edge of the central opening were mounted on a translucent film canister, thus with a pale centre; and (3) model flowers with six dark spots as in (2) above were mounted on a black film canister, thus with a dark centre. Black spots were painted with carbon mixed with a clear acrylic medium. The model flowers were mounted individually on wire stakes inserted in the ground and positioned 10 cm above ground level.
Fig. 2.
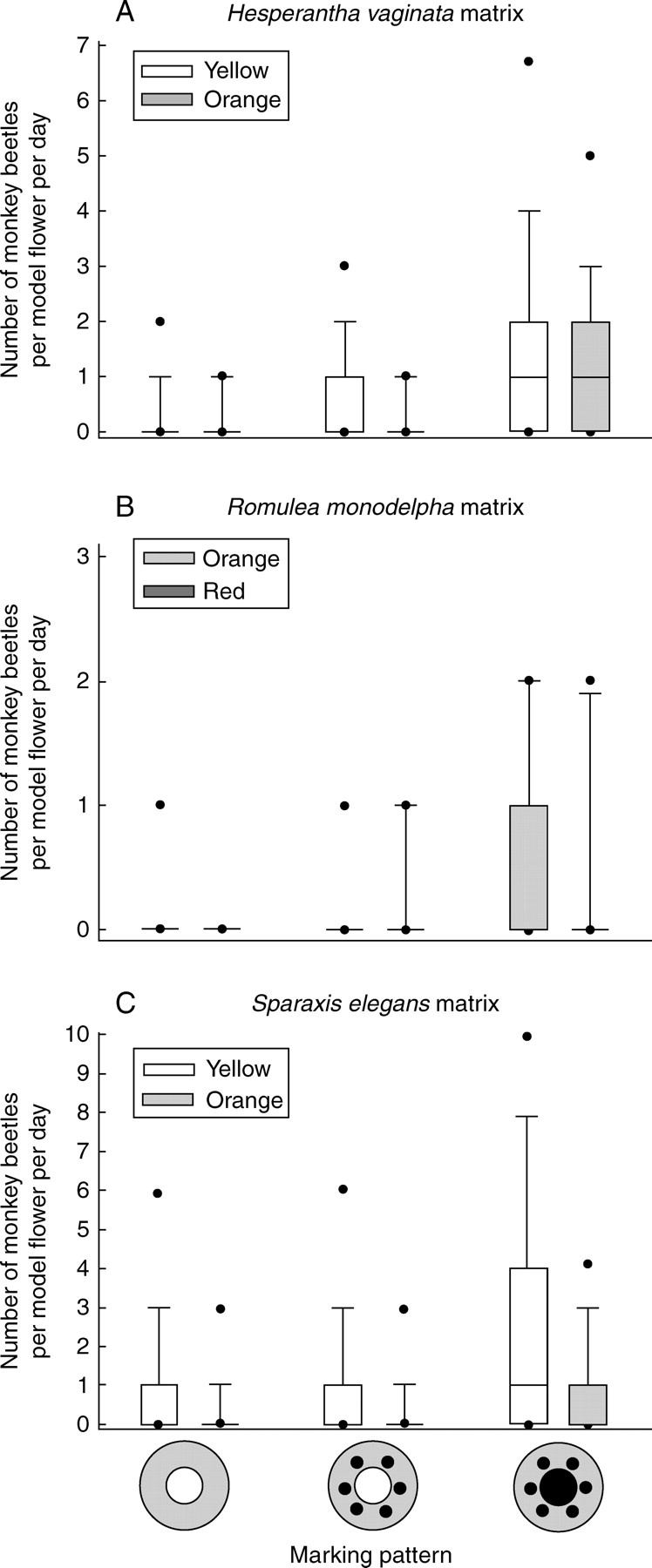
Monkey beetle visitation (number of beetles per day) to model flowers with different colours and marking patterns positioned in populations of the matrix species (A) Hesperantha vaginata, (B) Romulea monadelpha and (C) Sparaxis elegans. The boundaries of the box around the median indicate the 75th and 25th percentiles. The whiskers indicate the 90th and 10th percentiles, and the dots above and below each box indicate the 95th and 5th percentiles, respectively.
For each colour, two replicate model flowers of each marking pattern type were randomly assigned to positions in rectangular plots (2 × 3 model flowers) with 10 cm between model flowers. Each plot was paired with another plot at 5 m distance that had model flowers of a different colour. Yellow and orange model flowers were positioned in matrices of either yellow Hesperantha vaginata flowers or orange Sparaxis elegans flowers, and orange and red model flowers were positioned in a matrix of red Romulea monadelpha flowers. Pink or blue model flowers were not used because the guild of monkey beetles that visit flowers with these colours are poorly represented at Nieuwoudtville (Colville et al., 2002). The paired plots were replicated five or eight times on the same site or on the same date, and paired plots were >40 m apart. Each of these experiments was repeated at the same site on five days (25, 27 and 28 August, and 6 and 7 September, 2002) for the H. vaginata matrix, on four days (24, 25, 27 and 28 August, 2002) for the R. monadelpha matrix, and at six sites (two sites per date; 24, 26 and 27 September, 2002) for the S. elegans matrix. These different dates and locations are hereafter referred to as repetitions. A total of 31, 20 and 30 paired plots were used for the H. vaginata, R. monadelpha and S. elegans matrices, respectively. A total of 972 model flowers were used, of which three had to be discarded because of wind damage.
All experiments were conducted on sunny days when monkey beetles are most likely to be active (Goldblatt et al., 1998). The traps (model flowers) were set up during the early morning before the flowers opened, and were collected at the end of the same day after the flowers had closed. For R. monadelpha traps were in the field between ±1100 and 1645 h; for H. vaginata between ±1130 and 1700 h; and for S. elegans between ±1000 and 1630 h.
The central film canisters of the model flowers were filled with water to prevent escape of visiting monkey beetles. Model flowers were checked at least every 1–2 h to prevent the monkey beetles from drowning. Trapped beetles were released at some distance away (>600 m) from the study site to reduce the chance of re-trapping the same individuals.
For each model flower, the number of each species of trapped monkey beetles was tallied. Beetles were identified by comparison with a reference collection identified by H. Dombrow (Worms, Germany) and J. Colville (University of Cape Town, South Africa).
Analysis
The count data on the number of monkey beetles per model flower followed a negative-binomial distribution. The data were therefore analysed with a series of generalized linear models with the negative-binomial distribution implemented in the statistical software Genstat (Lawes Agricultural Trust, IACR, Rothamsted, UK). Data for each of the three matrix species, H. vaginata, S. elegans and R. monadelpha, were first analysed separately to test for effects of ‘colour’ (yellow and orange for H. vaginata and S. elegans, and orange and red for R. monadelpha), ‘dark spots’ and ‘dark centre’, and the interactions of ‘colour’ with ‘dark spots’ and ‘dark centre’. ‘Dark centre’ was first fitted in the model to compare the plain model flowers and those with dark spots and a translucent centre with the model flowers with dark spots and a dark centre. Then ‘dark spots’ was fitted to compare plain model flowers with those with dark spots and a translucent centre. The conclusions remained the same when ‘dark spots’ was fitted before ‘dark centre’. Variation due to ‘repetition’ (i.e. date for H. vaginata and R. monadelpha, and date and location for S. elegans), ‘paired plots’ and ‘plots’ was controlled for by including these factors in the model. To test for differences in the effect of marking patterns on monkey beetle visitation between the matrix species, data on all three matrix species were analysed together for the orange model flowers, which were used for all matrix species.
For each factor, ratios of changes in mean deviance (quasi F-values) were calculated after adding the factor to the model. Quasi F-values approximately follow the F-distribution (Payne et al., 2005), enabling us correctly to test differences between colours against variation among plots, and differences between marking patterns (i.e. effects of dark spots and dark centres) against their interactions with ‘repetition’. The canonical link function, log ratio, of the negative binomial distribution was used and the aggregation factor was set to one. However, in the analyses of the data of the S. elegans matrix and the analysis of all three matrix species together, the model could not be fitted because some of the fitted values predicted by the model were out of the valid range for the applied model. For these analyses, the log-link function was used.
RESULTS
In total, 688 monkey beetles belonging to eight species were trapped during the experiment (Table 1). In the H. vaginata matrix, the model flowers were visited by three monkey beetle species, of which Clania glenlyonensis was by far the most frequent (94·6 %). In the R. monadelpha matrix, the model flowers were visited by four monkey beetle species, of which C. glenlyonensis again predominated (91·1 %). In these two matrix species, therefore, a single beetle species accounted for the great majority of all visits. In the S. elegans matrix, the model flowers were visited by six monkey beetle species, of which Anisochelus hilaris was the most frequent (37·4 %), followed by Pachynema calviniana (23·3 %), Lepithrix freudei (20·7 %) and Anisochelus inornatus (17·6 %). In S. elegans, therefore, four beetle species together accounted for 99·0 % of all visits.
Table 1.
Numbers of different monkey beetle species visiting model flowers in populations of the monkey-beetle-pollinated species Hesperantha vaginata, Romulea monadelpha and Sparaxis elegans
| Monkey beetle species | H. vaginata | R. monadelpha | S. elegans |
|---|---|---|---|
| Anisochelus hilaris Burmeister, 1844 | 1 | 0 | 144 |
| Anisochelus inornatus Burmeister, 1844 | 0 | 0 | 68 |
| Clania glenlyonensis Dombrow, 1997 | 243 | 41 | 0 |
| Female | 11 | 2 | 0 |
| Male | 232 | 39 | 0 |
| Clania macgregorii Dombrow, 1997 | 13 | 2 | 0 |
| Heterochelus bicolour Dombrow, 2001 | 0 | 1 | 2 |
| Female | 0 | 1 | 0 |
| Male | 0 | 0 | 2 |
| Heterochelus pickeri Dombrow, 1997 | 0 | 0 | 2 |
| Female | 0 | 0 | 2 |
| Male | 0 | 0 | 0 |
| Lepithrix freudei Schein, 1959 | 0 | 1 | 80 |
| Pachycnema calviniana Schein, 1959 | 0 | 0 | 90 |
| Female* | 0 | 0 | 49 |
| Male | 0 | 0 | 41 |
| Total | 257 | 45 | 386 |
For dimorphic species, the numbers of female and male beetles are given in italics.
* Females of P. calviniana might have been mixed up with the morphologically similar females of P. multiguttata Thunberg, 1888.
Four of the eight observed monkey beetles in the experiment were dimorphic, allowing us to distinguish easily between females and males (Table 1). Male monkey beetles were the most frequent visitors (82·8 %) but this was mainly accounted for by a single species, C. glenlyonensis (Table 1).
Monkey beetle visitation rates differed significantly between dates for the H. vaginata and R. monadelpha matrices and between locations and dates for S. elegans (significant ‘repetition’ effects in Table 2). There was also significant variation among paired plots within the replications for the H. vaginata and S. elegans matrices, which indicates spatial variation in visitation rate at each location.
Table 2.
Summary of generalized linear models based on a negative binomial distribution testing the effects of colour and presence of dark spots and dark centres of model flowers on the number of visiting monkey beetles in populations of Hesperantha vaginata, Romulea monadelpha and Sparaxis elegans
|
H. vaginata matrix |
R. monadelpha matrix |
S. elegans matrix |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Effect | d.f. | Mean deviance | Quasi-F | d.f. | Mean deviance | Quasi-F | d.f. | Mean deviance | Quasi-F |
| Repetition | 4 | 8·3192 | 2·92* | 3 | 13·7611 | 13·43*** | 5 | 52·4810 | 14·63*** |
| Paired plots | 26 | 2·8483 | 2·94** | 16 | 1·0244 | 1·40 | 24 | 3·5884 | 3·06** |
| Colour | 1 | 2·9412 | 1·23 | 1 | 0·7463 | 0·40 | 1 | 27·9636 | 33·26** |
| Rep. × Colour | 4 | 2·3980 | 2·48 | 3 | 1·8518 | 2·53 | 5 | 0·8408 | 0·72 |
| Plot | 26 | 0·9685 | 1·95** | 16 | 0·7332 | 3·77*** | 24 | 1·1718 | 3·50*** |
| Centre | 1 | 58·8884 | 28·74** | 1 | 13·0775 | 7·37 | 1 | 27·2918 | 17·32** |
| Spots | 1 | 1·4632 | 1·47 | 1 | 1·3630 | 24·97* | 1 | 0·5123 | 0·61 |
| Rep. × Centre | 4 | 2·0489 | 1·58 | 3 | 1·7751 | 5·49** | 5 | 1·5757 | 2·61 |
| Rep. × Spots | 4 | 0·9944 | 0·93 | 3 | 0·0546 | 0·09 | 5 | 0·8436 | 4·75** |
| Pair × Centre | 26 | 1·2962 | 1·83 | 16 | 0·3233 | 0·60 | 24 | 0·6031 | 1·05 |
| Pair × Spots | 26 | 1·0663 | 2·53* | 16 | 0·5761 | 23044** | 24 | 0·1775 | 0·48 |
| Colour × Centre | 1 | 1·3541 | 1·91 | 1 | 0·5443 | 1·01 | 1 | 0·0172 | 0·03 |
| Colour×Spots | 1 | 0·0411 | 0·10 | 1 | 0·8513 | 34052*** | 1 | 1·0330 | 2·78 |
| Plot × Centre | 26 | 0·7092 | 1·43 | 16 | 0·5389 | 2·77*** | 24 | 0·5734 | 1·71* |
| Plot × Spots | 26 | 0·4210 | 0·85 | 16 | 0·00003 | 0·00 | 24 | 0·3718 | 1·11 |
| Residual | 194 | 0·4976 | 126 | 0·1946 | 187 | 0·3352 | |||
*P<0·05, **P<0·01, ***P<0·001.
Colour of the model flowers had no significant effect on the number of monkey beetles within the H. vaginata matrix (149 and 108 beetles in total on yellow and orange model flowers, respectively) and R. monadelpha matrix (26 and 19 beetles in total on orange and red model flowers, respectively) but yellow model flowers (284 beetles in total) were visited more frequently than orange model flowers (102 beetles in total) in the S. elegans matrix (Fig. 2; Table 2). The total numbers of monkey beetles on plain, spotted and spotted plus dark-centred model flowers were, respectively, 33, 46 and 178 for the H. vaginata matrix, five, ten and 30 for the R. monadelpha matrix, and 89, 105 and 192 for the S. elegans matrix. For the H. vaginata and S. elegans matrices, the effect of the dark centres was significant but the effect of the spots was not (Fig. 2; Table 2). This contrasts with the R. monadelpha matrix, in which the effect of dark centres was only marginally significant (quasi-F1,3 = 7·37, P = 0·073) but the effect of the spots was significant (Table 2). The effect of the marking pattern was independent of the colour of the model flower for the H. vaginata and S. elegans matrices (i.e. there were no significant interactions between colour and marking pattern parameters; Fig. 2; Table 2). For the R. monadelpha matrix there was a significant colour-by-spots interaction due to a stronger positive effect of the presence of spots on red model flowers than on orange model flowers (Fig. 2; Table 2), but this was based on a total of only 15 visiting monkey beetles.
On average, the most monkey beetles per model flower were found in the S. elegans matrix, followed by the H. vaginata matrix and the R. monadelpha matrix (Fig. 2; Table 1). However, owing to the large variation in number of monkey beetles among dates and locations (significant ‘repetition’ effect: quasi-F12,66 = 5·41, P < 0·001), the differences among matrix species were not significant (quasi-F2,12 = 1·04, P = 0·384). The magnitude of the positive effect of a dark centre on monkey beetle visitation also did not differ between the species matrices (quasi-F2,12 = 1·20, P = 0·336).
DISCUSSION
This study is the first to demonstrate experimentally that beetle marks play a role in the attraction of pollinating beetles in the Greater Cape Floral Region. This finding contrasts with a previous similar study by Johnson and Midgley (2001) that found no significant effect of beetle marks on visitation of model flowers by monkey beetles in the south-western part of the Greater Cape Floral Region, which has a less diverse monkey beetle fauna than the present study area. Even though the effect of dark centres in the study by Johnson and Midgley (2001) was not statistically significant, model flowers with dark centres were visited more frequently than plain models in all the experiments undertaken. The available evidence thus indicates that flowers with dark centres are generally more attractive to monkey beetles than flowers without dark centres. Further research is required to demonstrate whether monkey beetles have an innate preference for dark centres or whether they have been conditioned to associate dark centres with a reward.
Although the current experiments show that monkey beetles prefer flowers with dark centres and dark spots, the presence of dark spots alone (i.e. without an associated dark centre) was positively associated with visitation rates only in the R. monadelpha matrix. Other plant species that are pollinated by monkey beetles, such as the peacock irises in Moraea subgenus Vieusseuxia (Steiner, 1998), have dark markings on the tepals but lack a dark centre. Similar studies in areas with a high frequency of these species should reveal whether the local monkey beetles have a preference for dark spots in the absence of an associated dark centre.
The preference of monkey beetles for flowers with dark centres demonstrates that beetle marks do not function as deterrents of herbivorous monkey beetles as was first suggested by Hutchinson (1946) for Gorteria diffusa. The frequent use of flowers as mating sites by monkey beetles (Fig. 1B; Johnson and Midgley 2001) led Midgley (1993) to propose that beetle marks are mimetic, attracting beetles in search of a mating partner. The study by Johnson and Midgley (2001), however, did not find that model flowers with dead female or male monkey beetles glued to them attracted more visiting ones than plain model flowers but their study was carried out in a region of the south-western Cape that is relatively poor in monkey beetle diversity.
Johnson and Midgley (2001) found a very strong preference of monkey beetles on the Cape Peninsula for orange over red, yellow and blue. The present study, however, revealed only a slight preference for yellow over orange model flowers in the matrix of S. elegans, and no colour preference in the matrices of H. vaginata and R. monadelpha. Whereas both H. vaginata and R. monadelpha are mainly visited by the monkey beetle Clania glenlyonensis, S. elegans was visited by a suite of other monkey beetle species dominated by four different species (Table 1). It is clear that species of monkey beetle may differ in their colour preference, a conclusion that was also drawn by Picker and Midgley (1996), who used colour preference (among other features) to distinguish between three guilds of monkey beetles. Their study, on the West Coast near Darling, South Africa, did not include the species of monkey beetles observed in the current study.
Sparaxis elegans has orange flowers, and the preference for yellow over orange model flowers indicates that monkey beetles in the Nieuwoudtville area are not conditioned to associate the orange colour with pollen reward. Similarly, Picker and Midgley (1996) found that Lepithrix sp. preferred red traps although it was most frequently seen on yellow flowers. This indicates that monkey beetles may have an innate preference for certain colours and are not conditioned by the colour of the locally most frequent matrix species. This has significant implications for the evolution of precise pollinator–plant relationships by favouring strong convergence for specific flower colours in certain guilds.
CONCLUSIONS
Our understanding of specialized beetle pollination has improved in recent years, because a number of recent studies have documented specialized beetle pollination of species that do not have the classical beetle pollination syndrome (Dafni et al., 1990; Sakai and Inoue, 1999; Ollerton et al., 2003; Peter and Johnson, 2006; Johnson et al., 2007). The monkey beetle syndrome is another major exception to the classical beetle pollination syndrome. In contrast to plants with the classical beetle pollination syndrome that use floral scent as the primary attractant of pollinating beetles, plants with the monkey beetle pollination syndrome rely primarily on visual signals. The present study, together with others (Picker and Midgley, 1996; Johnson and Midgley, 2001; Colvillle et al., 2002), demonstrates that some species of monkey beetle use colour as the primary visual cue while others use beetle marks as the primary visual cue. In addition, monkey beetles, in some regions at least, prefer marked flowers to unmarked flowers, providing convincing evidence for an adaptive role of beetle marks in increasing visitation rates and pollination success.
ACKNOWLEDGEMENTS
This work formed part of the GEF Conservation Farming Project co-ordinated by the South African National Biodiversity Institute. We thank the Northern Cape Department of Nature and Environmental Conservation for permits, Neil McGregor and Nieuwoudtville Municipality for access permission, Holger Dombrow and Jonathan Colville for identification of monkey beetle specimens, and Jeff Ollerton and an anonymous reviewer for helpful comments on an earlier version of the manuscript.
LITERATURE CITED
- Born J, Liner HP, Desmet P. The Greater Cape Floristic Region. Journal of Biogeography. 2007;34:147–162. [Google Scholar]
- Colville J, Picker MD, Cowling RM. Species turnover of monkey beetles (Scarabaeidae: Hopliini) along environmental and disturbance gradients in the Namaqualand region of the succulent Karoo, South Africa. Biodiversity and Conservation. 2002;11:243–264. [Google Scholar]
- Dafni A, Bernhardt P, Schmida A, Ivri Y, Greenbaum S, O'Toole C, Losito L. Red bowl-shaped flowers: convergence for beetle pollination in the Mediterranean region. Israel Journal of Botany. 1990;39:81–92. [Google Scholar]
- Faegri K, van der Pijl L. The principles of pollination ecology. Oxford: Pergamon Press; 1979. [Google Scholar]
- Free JP, Williams IH. The responses of the pollen beetle, Meligethes aeneus, and the seed weevil, Ceuthorhynchus assimilis, to oil-seed rape, Brassica napus, and other plants. Journal of Applied Ecology. 1978;15:761–774. [Google Scholar]
- Goldblatt P, Manning JC. Radiation of pollination systems in the Iridaceae of sub-Saharan Africa. Annals of Botany. 2006;97:317–344. doi: 10.1093/aob/mcj040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldblatt P, Bernhardt P, Manning JC. Pollination of petaloid geophytes by monkey beetles (Scarabaeidae: Rutelinae: Hopliini) in southern Africa. Annals of the Missouri Botanical Garden. 1998;85:215–230. [Google Scholar]
- Hingston AB, McQuillan PB. Are pollination syndromes useful predictors of floral visitors in Tasmania? Austral Ecology. 2000;25:600–609. [Google Scholar]
- Hutchinson J. A botanist in South Africa. London: Crawthorn; 1946. [Google Scholar]
- Johnson SD, Midgley JJ. Pollination by monkey beetles (Scarabaeidae: Hopliini): do colour and dark centres of flowers influence alighting behavior? Environmental Entomology. 2001;30:861–868. [Google Scholar]
- Johnson SD, Ellis A, Dotterl S. Specialization for pollination by beetles and wasps: the role of lollipop hairs and fragrance in Satyrium microrrhynchum (Orchidaceae) American Journal of Botany. 2007;94:47–55. doi: 10.3732/ajb.94.1.47. [DOI] [PubMed] [Google Scholar]
- Lamborn E, Ollerton J. Experimental assessment of the functional morphology of inflorescences of Daucus carota (Apiaceae): testing the “fly catcher effect”. Functional Ecology. 2000;14:445–454. [Google Scholar]
- Midgley JJ. An evaluation of Hutchinson's beetle–daisy hypothesis. Bothalia. 1993;23:70–72. [Google Scholar]
- Ollerton J, Johnson SD, Cranmer L, Kellie S. The pollination ecology of an assemblage of grassland asclepiads in South Africa. Annals of Botany. 2003;92:807–834. doi: 10.1093/aob/mcg206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne RW, Harding SA, Murray DA, Soutar DM, Baird DB, Welham SJ, et al. The guide to Genstat release 8. Oxford: VSN International; 2005. [Google Scholar]
- Peter CI, Johnson SD. Anther cap retention prevents self-pollination by elaterid beetles in the South African orchid Eulophia foliosa. Annals of Botany. 2006;97:345–355. doi: 10.1093/aob/mcj041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péringuey L. Descriptive catalogue of the Coleoptera of South Africa (Lucanidae and Scarabaeidae) Transactions of the South African Philosophical Society. 1902;12:1–90. [Google Scholar]
- Picker MD, Midgley JJ. Pollination by monkey beetles (Coleoptera: Scarabaeidae: Hopliini): flower and colour preferences. African Entomology. 1996;4:7–14. [Google Scholar]
- Sakai S, Inoue T. A new pollination system: dung-beetle pollination discovered in Orchidantha inouei (Lowiaceae, Zingiberales) in Sarawak, Malaysia. American Journal of Botany. 1999;86:56–61. [PubMed] [Google Scholar]
- Steiner KE. Beetle pollination of peacock moraeas (Iridaceae) in South Africa. Plant Systematics and Evolution. 1998;209:47–65. [Google Scholar]
- Valdivia CE, Niemeyer HM. Do floral syndromes predict specialization in plant pollination systems? Assessment of diurnal and nocturnal pollination of Escallonia myrtoidea. New Zealand Journal of Botany. 2006;44:135–141. [Google Scholar]
- Westmoreland D, Muntan C. The influence of dark central florets on insect attraction and fruit production in Queen Anne's lace (Daucus carota L.) American Midland Naturalist. 1996;135:122–129. [Google Scholar]
- Zhang L, Barrett SCH, Gao JY, Chen J, Cole WW, Liu Y, et al. Predicting mating patterns from pollination syndromes: the case of “sapromyiophily” in Tacca chantrieri (Taccaceae) American Journal of Botany. 2005;92:517–524. doi: 10.3732/ajb.92.3.517. [DOI] [PubMed] [Google Scholar]


