Abstract
Background and Aims
Leaf responses to environmental conditions have been frequently described in fruit trees, but differences among cultivars have received little attention. This study shows that parameters of Farquhar's photosynthesis and Jarvis' stomatal conductance models differed between two apple cultivars, and examines the consequences of these differences for leaf water use efficiency.
Methods
Leaf stomatal conductance (gsw), net CO2 assimilation rate (An), respiration (Rd) and transpiration (E) were measured during summer in 8-year-old ‘Braeburn’ and ‘Fuji’ apple trees under well-watered field conditions. Parameters of Farquhar's and Jarvis' models were estimated, evaluated and then compared between cultivars. Leaf carbon isotope discrimination (Δ13C) was measured at the end of the growing season.
Key Results
A single positive relationship was established between VCmax (maximum carboxylation rate) and Na (leaf nitrogen concentration per unit area), and between Jmax (maximum light-driven electron transport rate) and Na. A higher leaf Rd was observed in ‘Fuji’. The gsw responded similarly to increasing irradiance and leaf temperature in both cultivars. gsw responded to lower vapour pressure deficit in ‘Fuji’ than in ‘Braeburn’. Maximal conductance (gswmax) was significantly smaller and An was more limited by gsw in ‘Braeburn’ than ‘Fuji’. Lower gsw, E and higher intrinsic water use efficiency were shown in ‘Braeburn’ and confirmed by smaller leaf Δ13C compared with ‘Fuji’ leaves.
Conclusions
The use of functional model parameters allowed comparison of the two cultivars and provided evidence of different water use ‘strategies’: ‘Braeburn’ was more conservative in water use than ‘Fuji’, due to stomatal limitation of An, higher intrinsic water use efficiency and lower Δ13C. These physiological traits need to be considered in relation to climate adaptation, breeding of new cultivars and horticultural practice.
Key words: Apple, carbon isotope discrimination, leaf nitrogen, leaf temperature, irradiance, Malus × domestica, modelling, photosynthesis, stomata, transpiration, vapour pressure deficit, water use efficiency
INTRODUCTION
The responses of fruit tree crops to fluctuating or changing climatic conditions may help in understanding current practical problems in fruit tree management, such as yield variability. The vegetative and reproductive growth of trees depends on assimilate production which is controlled by tree architecture and leaf functions, both modulated by environmental interactions (Flore and Lakso, 1989; Lakso, 1994). At branch scale, Massonnet et al. (2004) showed that two apple cultivars differed in transpiration rate, suggesting that this may result either from variability in branch structure, which affects light interception within the tree crown, or from differences in leaf physiological functions, or from both. Architectural diversity has been characterized among apple cultivars: Lespinasse (1992) and Costes et al. (2003) classified apple cultivars into four groups (types I to IV) based on branching and fruiting patterns. Massonnet (2004) showed that two group IV apple cultivars (‘Fuji’ and a new hybrid ‘X3305’) have a spatial leaf distribution conferring greater light interception by the canopy than two group III cultivars (‘Braeburn’ and ‘Ariane’). The present study addresses the question of leaf functional differences between two of these cultivars, ‘Fuji’ and ‘Braeburn’.
Stomatal conductance (gsw) and net CO2 assimilation rate (An) in C3 fruit species depend upon conditions such as solar irradiance (Marini and Sowers, 1990; Francesconi et al., 1997), leaf temperature (Seeley and Kammereck, 1977; Berry and Bjorkman, 1980), vapour pressure deficit (Watson et al., 1978; Fanjul and Jones, 1982), soil and plant water status (Schulze, 1986) and mineral nutrition. The capacity of cultivars to adjust to environmental variations, and their water use strategy in particular, is affected by differences within a species in leaf biochemistry (e.g. carboxylation rate of Rubisco, electron transport rate) and stomatal responses. Among apple cultivars, stomatal regulation has been related to tree vigour; rapid growth was generally linked to high gsw (Atkinson et al., 2000; Li et al., 2002). Oren et al. (1999) showed that stomatal sensitivity to leaf-to-air vapour pressure deficit (VPD) varies both within and between species. In the short term and at leaf scale, stomatal movements control the trade-off between An and transpiration (E), and hence water use efficiency (WUE) which is the ratio between carbon gain and water loss (Farquhar et al., 1989). The intrinsic water use efficiency (IWUE), which was defined by Comstock and Ehleringer (1992) as the ratio of An rate to gsw, is less dependent upon instantaneous environmental conditions (air temperature and relative humidity) than WUE, and is under tight genetic control in many tree species (Brendel et al., 2002, 2007; Casasoli et al., 2006). WUE can be estimated by carbon isotope discrimination (Δ13C), an integrative variable, which is linearly and negatively correlated with WUE in many C3 species (Farquhar and Richards, 1984; Guehl et al., 1995).
Quilot et al. (2002) and Tardieu (2003) suggested that ecophysiological models could be valuable tools for studying complex processes, i.e. interrelated physiological functions, particularly for comparing cultivars. Each genotype can be represented by a set of response parameters for a given range of environmental conditions (Tardieu, 2003). Thus, we hypothesized that parameters from ecophysiological models could be applied to apple cultivars and used to characterize the differences in leaf function between them.
The objective of this study was to identify the physiological traits that are cultivar-dependent in apple trees. To this end stomatal conductance, photosynthesis and dark respiration were compared in ‘Fuji’ and ‘Braeburn’. The comparison was based on (a) parameters of the empirical stomatal conductance model of Jarvis (1976) and maximal stomatal conductance; and (b) parameters of the biochemical photosynthesis model of Farquhar et al. (1980). The relationship between leaf An and gsw, E and Δ13C was also examined to assess water use strategies of these apple cultivars.
MATERIALS AND METHODS
Plant material
Two apple cultivars belonging to two different architectural types of the Lespinasse's classification (Lespinasse, 1992) were studied: ‘Braeburn’ (type III), and ‘Fuji’ (type IV), grafted on dwarfing M9 rootstock. Three representative 8-year-old trees of ‘Braeburn’ and of ‘Fuji’ planted in December 1994, 6 m × 1·8 m apart, in a north–south orientation and trained using the Solaxe system (Lauri and Lespinasse, 2000) were used. The experimental plot was carefully irrigated using a microjet system monitored by tensiometers to avoid soil water deficits. All trees were unpruned, and the crop loads adjusted by chemical and manual thinning. The annual fruit yields were between 25 kg and 35 kg per individual tree. All experiments were carried out at INRA experimental station (Mauguio, located near Montpellier, South of France) during the 2002 growing season.
Leaf physiological traits
For all the measurements described, leaves were sampled at random within tree crowns at an external position for sunlit leaves and an internal position for shaded leaves. The sample details of each experiment are specified below.
Stomatal conductance
Stomatal responses to PPF (photosynthetic photon flux, μmol m−2 s−1), Tl (leaf temperature, °C) and VPD (leaf to air water vapour pressure deficit, kPa) were measured using a portable infrared photosynthesis system (LI-6400; Li-Cor, Inc., Lincoln, NE, USA). Stomatal responses were measured between early July and early August on different samples of four sunlit leaves, simultaneously in the two cultivars. Each set of measurements was obtained by varying only one environmental parameter, the others being set at standard conditions, i.e. PPF = 1500 µmol m−2 s−1, Tl = 25 °C, VPD ≤ 1·5 kPa. CO2 concentration of the air at the leaf surface (Ca) was maintained at 35 Pa during these measurements. Each gsw value was recorded after equilibration for at least 20 min (steady-state condition) as described by Le Roux et al. (1999). Leaves used for the measurements were located in comparable positions for all cultivars, thus minimizing uncontrolled effects resulting from the presence of fruits near the measured leaf, the length of the bearing shoot or the leaf position on that shoot.
Stomatal responses to PPF (1500, 1000, 600, 400, 200, 100, 50, 0 µmol m−2 s−1) were obtained by varying the intensity of a red/blue LED light source (LI-6400-02B; Li-Cor, Inc.). Stomatal responses to Tl [20 °C, 25 °C, 30 °C (±1 °C)] were measured early in the morning with leaf chamber temperature regulated by integrated Peltier coolers that allowed the temperature to be controlled to within ±6 °C of the air temperature. Stomatal responses to increasing VPD (1·0, 1·5, 2·0, 2·5, 3·0 and 3·5 kPa) were obtained by regulating the relative humidity of the air in the LI 6400 cuvette chamber either by scrubbing a fraction of the inlet air through a desiccating column or by humidifying it through a series of 25-L cans containing water.
The stomatal response to the different environmental factors was expressed as a fraction of the highest gsw value reached for each leaf (Le Roux et al., 1999).
Maximal stomatal conductance (gswmax, mol m−2 s−1) was estimated on an independent sample of 12–17 sunlit or shaded leaves of each cultivar under the standardized environmental conditions previously described, simultaneously with the measurements of stomatal response to the environment.
Leaf photosynthesis and respiration
Net CO2 assimilation rate (An) in relation to internal concentration of CO2 (Ci) was measured in situ during the same period (early July) with the LI-6400 system, by changing Ca, in the following order: 0, 180, 2·5, 150, 5, 100, 7·5, 60, 10, 40, 15, 20 and 30 Pa. The alternating high and low Ca values avoided leaf saturation by assimilates resulting from persistently high CO2. All measurements were made under standard environmental conditions (Tl = 25 °C, PPF = 1500 µmol m−2 s−1, VPD ≤ 1·5 kPa). Eight sunlit and shaded leaves were measured in both cultivars.
Respiration (Rd) was estimated simultaneously over the same period (early July) by measuring CO2 production rate at the end of the night, before sunrise (0600–0800 h in solar time), for eight sunlit and shaded leaves.
Leaf nitrogen concentration
All leaves measured for stomatal conductance or photosynthesis were immediately collected and placed in a cool box. Leaf area (Al, m2) was recorded with a flatbed scanner (CanoScan 4400F, Canon) coupled to the Optimas software (V6·5; Media Cybernetics, Silver Spring, MD, USA). Leaves were then rapidly frozen in liquid nitrogen and stored in a freezer at −26 °C before freeze drying (Heto VR1 CT110, Denmark) for dry mass (W, g) determination. Leaf nitrogen content (N) was measured by catharometry after dry ignition (Agronomy Laboratory Cirad Montpellier, France), and leaf nitrogen concentration expressed per unit area (Na, g m−2).
Leaf carbon isotope discrimination
The primary growth of 73–81 long shoots per cultivar was monitored at weekly intervals from April to June. Coloured labels were positioned below the apex of each growing shoot, indicating the leaf positioned below the label, the period of its expansion during the 2002 growing season. In October, three different sets of ten sunlit leaves per cultivar were collected on the basis of leaf expansion date (3 April, 17 April and 3 May) from three trees per cultivar. These samples were placed immediately on ice, frozen in liquid nitrogen and lyophilized. Leaves were crushed and homogenized using a centrifugal crusher. Leaf discrimination for 13C (Δ) was calculated as proposed in Farquhar et al. (1989):
| 1 |
where δa and δl are the carbon isotopic compositions of air and leaf, respectively, expressed in δ units (‰) relative to the international Pee Dee Belemnite (PDB) standard. Measurements of δl were obtained on a flow isotope ratio mass spectrometer (Delta S; Finnigan MAT, Bremen, Germany) and a constant value of −8 ‰ was used for δa as described by Farquhar et al. (1989).
Estimation of model parameters
Leaf stomatal conductance model
Jarvis' empirical model (Jarvis, 1976), modified by Stewart (1988), describes stomatal conductance as a combination of independent relative functions (varying from 0 to 1) characterizing leaf response to PPF, Tl, VPD, Ca and Ψ (bulk soil water potential):
| 2 |
In this equation, gswmax (mol m−2 s−1) is expressed as a linear function of leaf nitrogen concentration per unit area (Na), as proposed by Le Roux et al. (1999), and f1 to f5 are empirical functions whose best fit values for these parameters, i.e. those presenting the closest correlation, were estimated by linear or non-linear least squares regression using SigmaPlot software (2001, SPSS Inc., Chicago, IL, USA). Relative gsw (dimensionless) is drawn from eqn (2) as the ratio of gsw to gswmax.
To simplify the Jarvis-Stewart model, the functions f4 and f5 which describe the responses of relative gsw to Ca and to Ψ, respectively, were neglected. Indeed, Ca variations in the orchard during the day are negligible in comparison to other sources of variation. Regarding the f5 function, it was considered that soil water potential showed little variation in the irrigated conditions used.
Leaf photosynthesis model
The parameters of Farquhar's model (Farquhar et al., 1980), modified by Le Roux et al. (1999), were estimated in shaded and sunlit leaves of the two cultivars, assuming that mesophyll CO2 conductance (gi) was infinite. This assumption has been questioned (Evans and von Caemmerer, 1996; Ethier and Livingston, 2004; Warren and Dreyer, 2006) because gi generally limits photosynthesis and leads to the underestimation of VCmax (maximum carboxylation rate). Estimating gi is still a matter of controversy, and it was decided not to take this parameter into account. All values of VCmax presented are therefore ‘apparent’ values that underestimate the real values.
Apparent VCmax and maximum electron transport rate (Jmax) were estimated from An–Ci curves, and dark respiration rate (Rd) from direct field measurements (see above). The best fit by non-linear least squares regression (SAS macro; P. Montpied, EEF, INRA Champenoux, pers. comm.) resulted from measurements made with Ca at <30 Pa for VCmax and >50 Pa for Jmax. Four to seven leaf data sets were retained for the determination of VCmax and Jmax, and six or seven for Rd. Farquhar's parameters were expressed in relation to the individual leaf Na value, this variable being closely correlated to intercepted irradiance (DeJong and Doyle, 1985).
Comparison of model parameters between the cultivars
All statistical analyses were performed using Statistica 6 software (StatSoft, Inc., Tulsa, OK, USA). The cultivar effect was computed by a one-way ANOVA after ensuring that the data were normally distributed and that the variances were homogeneous. When the parameters also depended on another variable, the cultivar effect was determined by a covariance analysis (ANCOVA), using the continuous variable as covariate. Mean values for both cultivars were compared by the Newman–Keuls test for parametric analyses (more than ten replicates) or the Mann–Whitney test for non-parametric analyses (less than ten replicates).
Evaluation of model predictions
According to the similarity or dissimilarity of cultivar response to environmental variables, single or cultivar-specific sets of parameters were adopted for each model. Given that independent measurements were used to parameterize Jarvis' and Farquhar's models, the quality of model outputs was tested by cross-validation between the two data sets. Leaf gas exchange measurements made to parameterize Farquhar's model were used to evaluate the quality of Jarvis' model. For this purpose, the environmental conditions prevailing during these measurements (PPF, Tl and VPD) were used as inputs into the Jarvis equation to calculate gsw values. Reciprocally, the independent measurements made to parameterize Jarvis' model and the resulting gsw estimations were used to evaluate the quality of Farquhar's model, inferring Ci values from the equation drawn from Harley et al. (1992):
| 3 |
The quality of these estimations, by comparison with measured values, was determined by two parameters: root mean square error (RMSE) and bias (b). These were calculated as follows:
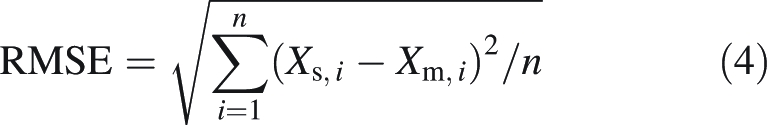 |
4 |
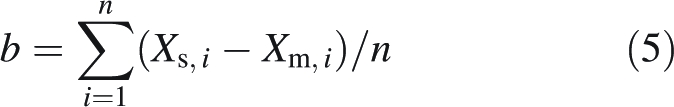 |
5 |
where Xs,i are the estimated values, Xm,i the measured values and n the number of observations.
RESULTS
Stomatal responses to environmental variables
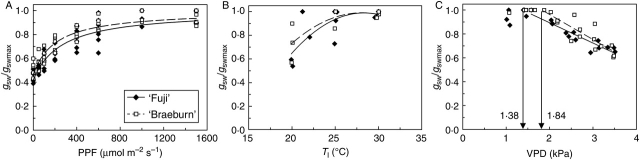
‘Braeburn’ and ‘Fuji’ cultivars showed a similar stomatal response to PPF when fitted by an equilateral hyperbola (Fig. 1A). Non-parametric statistical analysis detected a significant difference (P = 0·034) between the two cultivars only at 100 µmol m−2 s−1. Relative gsw was at a maximum when PPF exceeded 1000 µmol m−2 s−1.
Fig. 1.
Response of stomatal conductance, gsw, normalized by the maximum stomatal conductance value, gswmax, to (A) irradiance (PPF), (B) leaf temperature (Tl) and (C) leaf-to-air water vapour pressure deficit (VPD) in ‘Fuji’ and ‘Braeburn’ apple cultivars. In (A), [gsw/gswmax = (aP PPF + bP)/(cP PPF + dP)] fitting parameters: ‘Fuji’: aP = 66·10−4, bP = 0·716, cP = 66·10−4, dP = 1·716 (R2 = 0·93); ‘Braeburn’: aP = 90·10−4, bP = 0·856, cP = 90·10−4, dP = 1·856 (R2 = 0·94). In (B), (gsw/gswmax = aT Tl2 + bT Tl + cT) fitting parameters: ‘Fuji’: aT = –49·10−4, bT = 0·28, cT = –2·94 (R2 = 0·57); ‘Braeburn’: aT = –39·10−4, bT = 0·22, cT = –2·12 (R2 = 0·61). In (C), the threshold VPD value ensuring gsw = gswmax is noted. Equation beyond this value (gsw/gswmax = aD VPD + bD) uses fitting parameters: ‘Fuji’: aD = –0·17, bD = 1·23 (R2 = 0·89); ‘Braeburn’: aD = –0·21, bD = 1·39 (R2 = 0·75). Results of the Mann–Whitney test: in (A) P = 0·034 at PPF = 100 µmol m−2 s−1 but P > 0·05 at the other PPF levels; in (B) P > 0·05 at three temperatures; in (C) P = 0·029 between the threshold values. The ANCOVA procedure using VPD as the covariate detected no significant differences (P > 0·05) between the cultivars. Responses to PPF, Tl and VPD were recorded under standard conditions (see Materials and Methods).
The response of relative gsw to leaf temperature (Tl) was represented by a second order polynomial in ‘Fuji’ and ‘Braeburn’ (Fig. 1B). For both cultivars, the optimal temperature for gsw was close to 29 °C, without significant differences (P > 0·05) between the two cultivars whatever Tl.
The response of gsw to VPD displayed two stages in both cultivars (Fig. 1C): at low VPD, relative gsw was around 1, but it decreased linearly as VPD increased beyond a given threshold. The VPD threshold was significantly higher in ‘Braeburn’ (1·84 kPa) than in ‘Fuji’ (1·38 kPa) according to the Mann–Whitney test (P = 0·028). The rate of gsw decrease that resulted from VPD increase was slightly faster in ‘Braeburn’ than in ‘Fuji’, although the slopes were not significantly different (P > 0·10; Fig. 1C).
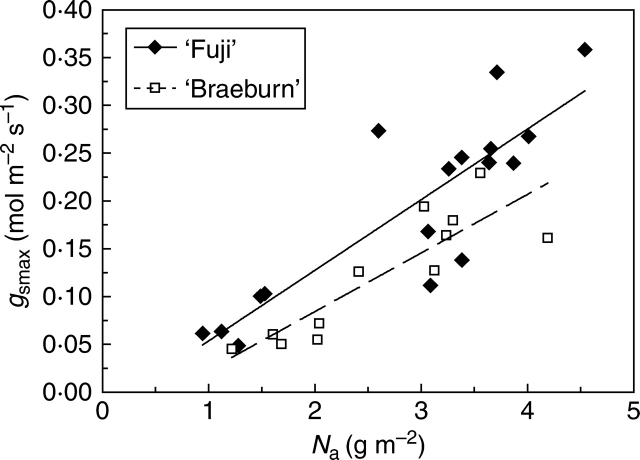
Maximal stomatal conductance
A positive linear relationship was fitted between maximal stomatal conductance (gswmax) and leaf nitrogen concentration per leaf area (Na) for each cultivar (Fig. 2). The covariance analysis with Na as the covariate showed a significant difference (P = 0·005) in mean gswmax with larger values in ‘Fuji’ than in'Braeburn', although the slopes were similar (0·074 and 0·062, respectively). Moreover, the range of gswmax variations was different between cultivars: maximal gswmax was significantly larger in ‘Fuji’ (0·36 mol m−2 s−1) than in ‘Braeburn’ (0·23 mol m−2 s−1; Fig. 2).
Fig. 2.
Relationships between maximum stomatal conductance, gswmax, and leaf nitrogen per unit area, Na, determined in ‘Fuji’ and ‘Braeburn’ apple cultivars. In the linear equation (gswmax = aN Na + bN) the fitting parameters are: ‘Fuji’: aN = 0·074, bN = –0·020 (R2 = 0·74); ‘Braeburn’: aN = 0·062, bN = –0·038 (R2 = 0·77). The ANCOVA with Na as the covariate detected significant differences (P = 0·005) between the cultivars. Mean gswmax values followed by different letters are significantly different: ‘Fuji’, 0·19a, ‘Braeburn’, 0·12b.
Leaf photosynthesis and respiration
A unique positive and linear relationship between VCmax and Na, and between Jmax and Na, was observed in both cultivars, as shown by the ANCOVA analyses (Fig. 3A, B). Of the two variables combined in Na, i.e. nitrogen concentration on a mass basis (Nw) and leaf mass to area ratio (Wa), Wa played the most important role in Jmax and VCmax variations. Indeed, the correlation was higher between VCmax, Jmax and Wa (R2 = 0·56 and 0·54, respectively) than between VCmax, Jmax and Nw (R2 = 0·23 and 0·29, respectively; data not shown). The mean Jmax : VCmax ratio was 1·2 at 25 °C.
Fig. 3.
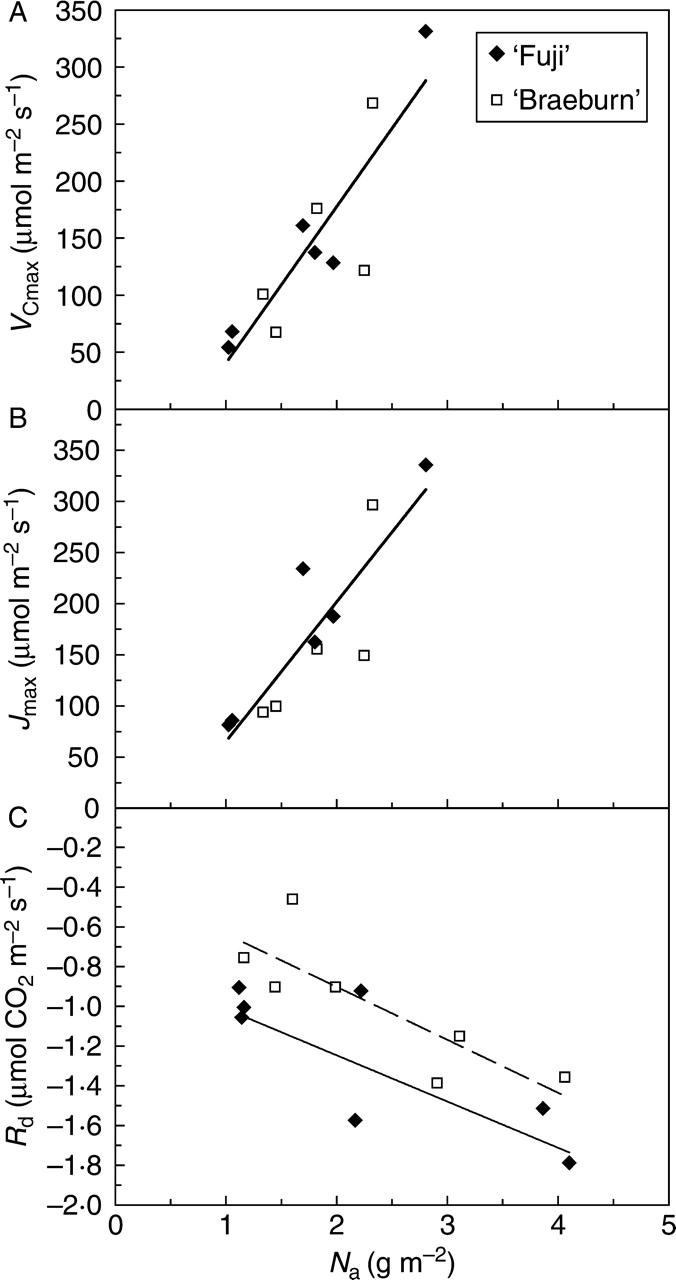
Relationships between (A) maximum carboxylation rate, VCmax, (B) maximum electron transport rate, Jmax, and (C) dark respiration rate, Rd, and leaf nitrogen per unit area, Na, in ‘Fuji’ and ‘Braeburn’ apple cultivars. In (A), linear equation (VCmax = aV Na + bV) and fitting parameters aV = 137·3, bV = –96·94 (R2 = 0·77). In (B), linear equation (Jmax = aJ Na + bJ) and fitting parameters: aJ = 136·4, bJ = –70·98 (R2 = 0·76). In (C), linear equation (Rd = aR Na + bR) and fitting parameters: ‘Fuji’: aR = –0·23, bR = –0·78 (R2 = 0·79); ‘Braeburn’: aR = –0·27, bR = –0·37 (R2 = 0·67). The covariance analysis using Na as covariate detected no significant differences between the cultivars for VCmax and Jmax, but significant differences (P = 0·007) for Rd. The Rd means followed by different letters are significantly different: ‘Fuji’, –1·31a, ‘Braeburn’, –0·96b. Conditions for the estimation of VCmax, Jmax and Rd are given in Materials and Methods.
The relationship between leaf respiration (Rd) and leaf nitrogen per mass area (Na) differed significantly, with ‘Fuji’ greater Rd than ‘Braeburn’ (ANCOVA analysis, P = 0·009; Fig. 3C).
Evaluation of model predictions
The gsw values predicted by Jarvis' model satisfactorily matched the measured values in both cultivars, as shown by the RMSE (0·04) and bias (–0·008; Fig. 4A). The different points were close to the 1:1 line, particularly for ‘Braeburn’. Some of the highest gsw values were underestimated by the model for ‘Fuji’.
Fig. 4.
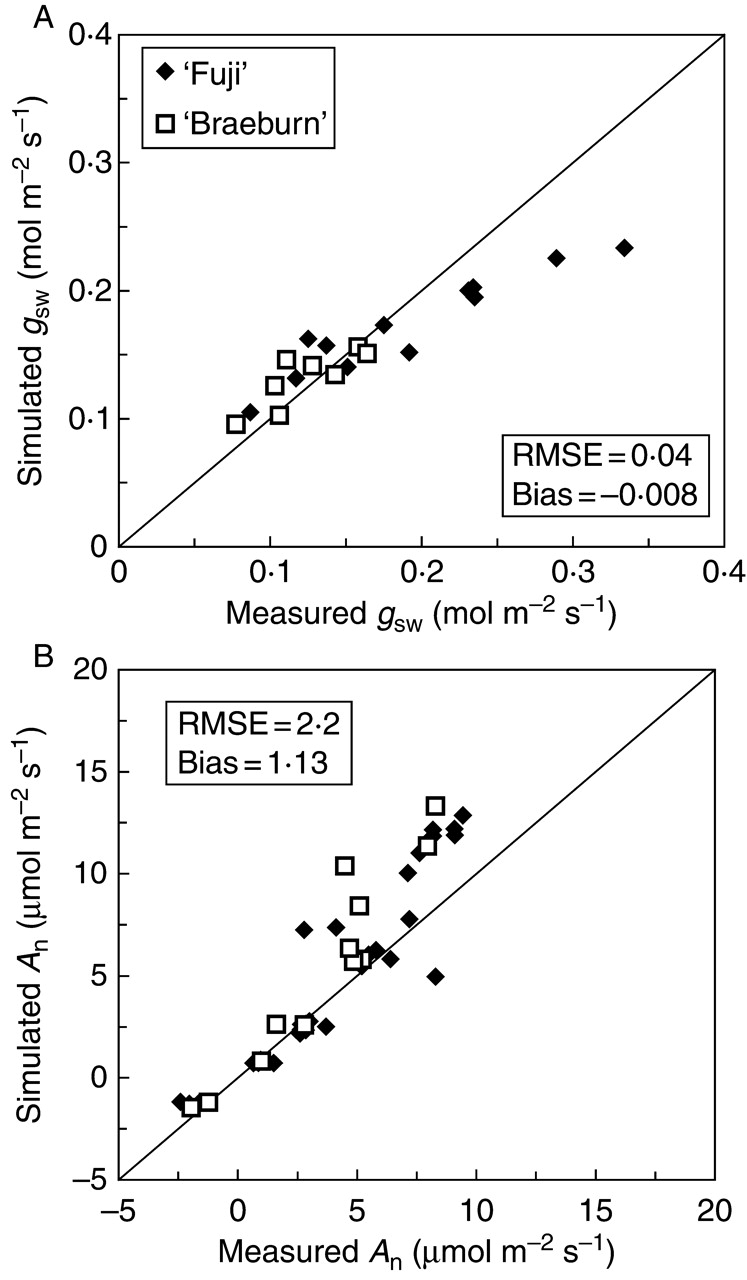
Comparison of (A) stomatal conductance and (B) photosynthetic rate measured in situ on leaves of ‘Fuji’ and ‘Braeburn’ apple cultivars, and simulated by the Jarvis and Farquhar sub-models, respectively. The root mean square error (RMSE), the bias and the 1:1 line are indicated.
Predictions of leaf photosynthesis using Farquhar's model resulted in a fairly good match between estimated and measured values for all cultivars as shown by the RMSE (2·2) and bias (1·13; Fig. 4B). However, the model overestimated (2-fold) some of the highest values for leaf photosynthesis, particularly at high PPF (>600 µmol m−2 s−1, data not shown).
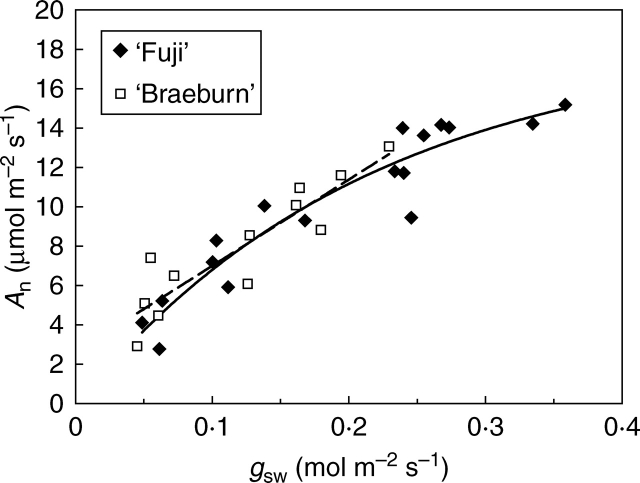
Relationship between stomatal conductance and photosynthesis
The range of gsw variations for sunlit or shaded leaves was narrower in ‘Braeburn’ (between 0·05 and 0·23 mol m−2 s−1) than in ‘Fuji’ (between 0·05 and 0·36 mol m−2 s−1; Fig. 5), in accordance with the lower gswmax found in this cultivar. ‘Braeburn’ also had lower maximal leaf An values (13·1 µmol CO2 m−2 s−1) than ‘Fuji’ (15·2 µmol m−2 s−1, Fig. 5). Differences between cultivars were confirmed by independent measurements made during the same growing season using a Licor LI-6200 system (data not shown). Specific relationships between An and gsw were adjusted by a non-linear fit in ‘Fuji’ and by a linear fit in ‘Braeburn’ (Fig. 5). Analysis with gsw as the covariate yielded significant differences in An between the cultivars (P = 0·007), with lower CO2 net assimilation rates in ‘Braeburn’.
Fig. 5.
Relationships between net assimilation, An, and stomatal conductance, gsw, in ‘Fuji’ and ‘Braeburn’ apple cultivars. Equation for ‘Fuji’: [An = (aA bA gsw)/(aA + bA gsw) + cA]; equation for ‘Braeburn’: (An = aA gsw + bA). Fitting parameters: ‘Fuji’: aA = 18·685, bA = 4·671, cA = –0·183 (R2 = 0·90); ‘Braeburn’: aA = 43·835, bA = 2·611 (R2 = 0·82). The covariance analysis, using gsw as the covariate, revealed significant differences (P < 0·05) between the cultivars for An. The mean An values followed by different letters were significantly different: ‘Fuji’, 10·06a, ‘Braeburn’, 8·84b. An and gsw were measured in sunlit and shaded leaves (n = 12 and 17 in ‘Braeburn’ and ‘Fuji’, respectively) under standardized environmental conditions: PPF = 1500 µmol m−2 s−1, Tl = 25 °C and VPD < 1·5 kPa.
Leaf ecophysiological traits under optimized conditions
The data sets stemming from leaf responses to environmental variables were pooled together, restricting the range of irradiance and air humidity variations to conditions which determine optimal (or sub-optimal) gsw: 600 ≤ PPF ≤ 1500 µmol m−2 s−1 and 0·90 kPa ≤ VPD ≤ 2·1 kPa. On this basis, the average gsw value of sunlit leaves was 25 % smaller in ‘Braeburn’ than in ‘Fuji’ (P = 0·0036; Table 1), and the average An value 8 % smaller in the first cultivar (n.s.). Averaged E was 17 % lower, and WUE 5 % higher, when ‘Braeburn’ sunlit leaves were compared with equivalent ‘Fuji’ leaves, but these differences were not significant (P > 0·10). In contrast, the IWUE of ‘Braeburn’ leaves was 15 % greater than in ‘Fuji’ (P < 0·001).
Table 1.
Averaged (±s.d.; n = 24) ecophysiological traits of sunlit leaves in two apple cultivars
| An (μmol CO2 m−2 s−1) | gsw (mol H2O m−2 s−1) | E (mmol H2O m−2 s−1) | WUE (mmol CO2 mol−1 H2O) | IWUE (μmol CO2 mol−1 H2O) | |
|---|---|---|---|---|---|
| Braeburn | 10·895 ± 1·775 | 0·166 ± 0·051b | 2·034 ± 0·989 | 6·132 ± 2·001 | 68·941 ± 14·210a |
| Fuji | 11·784 ± 2·874 | 0·223 ± 0·074a | 2·458 ± 1·126 | 5·325 ± 1·492 | 55·328 ± 9·281b |
| Cultivar effect | n.s. | ** | n.s. | n.s. | *** |
Measurements were performed during July and August 2002 with the following conditions: CO2 = 35 Pa; 600 ≤ PPF ≤ 1500 µmol m−2 s−1; 20 °C ≤ Tl ≤ 30 °C; 0·90 kPa ≤ VPD ≤ 2·1 kPa.
Values compared by one-way ANOVA procedure for determination of the cultivar effect. **P < 0·01; ***P < 0·001; n.s., not significant. Values followed by different letters are significantly different according to Mann-Whitney test.
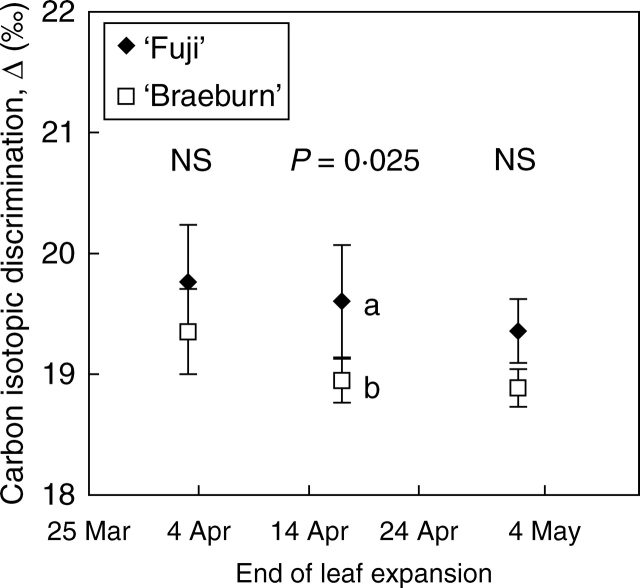
Carbon isotope discrimination of sunlit leaves
Smaller carbon isotope discrimination (Δ13C) occurred in leaves that expanded in May rather than in April for both cultivars (Fig. 6). ‘Fuji’ leaves displayed a higher Δ13C than ‘Braeburn’ leaves irrespective of when they expanded. Non-parametric statistics detected a significant difference between ‘Fuji’ and ‘Braeburn’ during April (P = 0·025; Fig. 6).
Fig. 6.
Carbon isotope discrimination, Δ13C, for sunlit apple leaves which expanded at start, middle and end of extension growth period in ‘Fuji’ and ‘Braeburn’ apple cultivars. All leaves were sampled in October for isotopic analysis. Δ13C of the two cultivars were statistically compared using the Mann–Whitney test. P-values are indicated where the differences are significant and NS where they are not. Values followed by different letters are significantly different.
DISCUSSION
Quality of model parameter estimations
This study describes the parameterization of Jarvis' stomatal conductance model and of Farquhar's photosynthesis model for leaves of two apple cultivars, and compares the estimated parameters. The stomatal behaviour in response to changing PPF and Tl was similar to that commonly found in other species (Leuning et al., 1995; Le Roux et al., 1999), and previously observed in apple (Warrit et al., 1980; Pretorius and Wand, 2003). The response of gsw to VPD consisted of a two-step process: gsw was close to maximum at low or moderate VPD and showed a severe decrease after a VPD threshold. This response was similar to that observed by Watson et al. (1978) in apples, and by Mediavilla and Escudero (2004) in different oak species. Dragoni et al. (2004) obtained the same result in ‘Royal Empire’ apple trees, and concluded that at low VPD, stomatal conductance was limited by the feedback control exerted by photosynthetic products, while the stomatal response to VPD became a limiting factor at higher VPDs. In other studies (e.g. Winkel and Rambal, 1990; Jones, 1998; Le Roux et al., 1999), the initial plateau was not observed, but this may be due to measurements being started at VPD values close to the threshold (around 1·5 kPa). The gswmax values observed in the present study were smaller than those observed in earlier studies with apple trees (Wünsche et al., 2000; Glenn et al., 2001) but comparable with those described under South African climate (Pretorius and Wand, 2003; Gindaba and Wand, 2007). Indeed, the estimation of gswmax remains difficult. The environmental conditions a priori supposed to maximize gsw were drawn from the literature (e.g. optimal temperature commonly 25 °C; Pretorius and Wand, 2003), but it was observed a posteriori that some of these values were not fully adequate in the present conditions: for the two cultivars analysed here, optimal temperature was near 29 °C. However, as measurements were performed in similar conditions for both cultivars, the comparison discussed below remains valid.
Parameters of leaf photosynthetic capacity were linearly related to nitrogen concentration per unit leaf area (Na), as shown in other deciduous tree species, such as walnut (Le Roux et al., 1999) and peach (Le Roux et al., 2001; Walcroft et al., 2002). VCmax at 25 °C was larger than in a series of forest tree species (Dreyer et al., 2001). In addition, the Jmax : VCmax ratio (1·2) was lower than usually reported even if it is subject to marked variability (Leuning, 1997). Reviewing the literature, Wullschleger (1993) calculated a mean Jmax : VCmax ratio of 1·64 for 109 species including Malus sp. The high nitrogen status of the experimental trees in the present study, indicated by the large Na values (maximum up to 4 g m−2, i.e. Nw of 3·4 %; Fig. 2) of sunlit leaves, could account for the high VCmax values observed. Indeed, Grassi et al. (2002) showed that VCmax values increase and the Jmax : VCmax ratio decreases with high mineral nutrient supply in Eucalyptus. The excess N is preferentially allocated to Rubisco rather than to the proteins that regulate the rate of electron transport. The consequence is therefore an increase in VCmax but not in Jmax, thus decreasing the Jmax/VCmax ratio. The high VCmax values estimated here also suggest a large mesophyll conductance to CO2 in apple leaves; this requires further examination and explicit estimates of mesophyll conductance. Indeed, different studies demonstrated that mesophyll resistance to CO2 transfer imposes limitation to photosynthesis in many species (Warren and Adams, 2006).
Quality of model predictions
In the present study, Jarvis' model produced a fair prediction of leaf stomatal conductance over the range of environmental conditions which are commonly encountered in a Mediterranean climate. As the model underestimated some gsw values, this can result either from the simplification of the Jarvis' model considered here (i.e. Ca and Ψ variations neglected) or from underestimation of gswmax.
Leaf photosynthesis estimated by Farquhar's model was slightly more problematic. Measured values matched predicted values when PPF was below 600 µmol m−2 s−1, but the model overestimated An values at higher irradiance. This was explained by the Jmax module in Farquhar's model which was identified as insufficiently limiting at high PPF. This said, the overestimated photosynthesis rate for strongly sunlit leaves could be considered as having little impact on estimates of photosynthesis at larger scales (e.g. the branch) since most of the leaves on a branch do not receive high irradiance because of within-tree shade and leaf blade orientation (Massonnet, 2004).
Cultivar-dependent leaf functions: differences in water use strategy
The experimental plan used in this study, which included two apple cultivars, allowed the cultivar effect to be explored on leaf photosynthetic and stomatal properties, and also on transpiration rate and water use, in the middle of the growing season.
Based on these instantaneous measurements, the two apple cultivars displayed a similar photosynthetic capacity (VCmax and Jmax) on the basis of normalized leaf Na values. In both cultivars, VCmax, Jmax and Rd increased with Na, as commonly observed in numerous other species (Reich et al., 1998), including walnut (Le Roux et al., 1999), peach (Le Roux et al., 2001) and mango (Urban et al., 2003). The relationship between Rd and Na is interpreted to be a consequence of changed leaf structure resulting from different positions within the canopy, and higher maintenance costs in sunlit leaves considering their carbon budget (Mitchell et al., 1999). This is supported by structural modifications between sunlit and shaded leaves observed in these two cultivars (Massonnet, 2004). The thinner palisade tissue in shaded leaves and lower leaf mass to area ratio could account for the diminished respiration rate. The higher leaf Rd in ‘Fuji’ suggests that the potentially larger photosynthetic rate in this cultivar is partly counterbalanced by greater respiratory losses.
The comparison of the two cultivars revealed different stomatal responses. ‘Braeburn’ and ‘Fuji’ differed with respect to maximal stomatal conductance, ‘Fuji’ having significantly higher gswmax than ‘Braeburn’. The two cultivars showed similar responses to PPF and Tl. The stomatal response to VPD showed some differences between cultivars: a significantly lower VPD threshold value was observed in ‘Fuji’, whereas the rate of relative gsw decrease beyond this threshold was slightly steeper in ‘Braeburn’.
‘Braeburn’ showed a linear relationship between An and gsw, i.e. no apparent saturation of the photosynthesis process even at the highest observed gsw. In this case, carbon gain is likely to be limited more by stomatal conductance than by the photosynthetic apparatus itself. Such a linear relation between An and gsw has already been reported in apple (Lakso, 1979) and mango (Urban et al., 2004), but it was shown here that this cannot be generalized. Indeed, ‘Fuji’ showed some non-stomatal limitation of leaf photosynthesis (rectangular hyperbola fit; Fig. 5). With this curvilinear relationship, An reaches a plateau at the highest observed gsw values, suggesting that An is limited either by the amount of Rubisco or its activity, or by the rate of electron transport. In this case, the trade-off between CO2 assimilation and E is modified, lowering the WUE value. Cultivars with a prevailing stomatal limitation generally appear more water-conserving and consequently exhibit a higher WUE than cultivars with limitation by the photosynthetic machinery (Jones, 1985). The present results are in accordance with this interpretation since instantaneous gas exchange measurements on sunlit leaves indicated slightly higher WUE, and significantly higher IWUE, in ‘Braeburn’ than in ‘Fuji’. The higher water use efficiency in ‘Braeburn’ was confirmed when integrated over a much longer time scale (i.e. the whole growing season), by the smaller values of carbon isotope discrimination (Δ13C) in its leaves. Farquhar and Richards (1984) pointed out that the isotopic composition reflects the effect of plant water status on photosynthesis, with a linear negative relationship between Δ13C values and WUE in various species. In the present study, values of leaf Δ13C were very informative in terms of the physiological properties of a cultivar and complemented instantaneous stomatal conductance measurements, because Δ13C integrated environmental conditions which varied throughout the growing season. The gsw values, predicted by the Jarvis model, result from the response to several environmental variables, without consideration of their interaction. This possible imbalance is overcome by using the more integrative variable, Δ13C, which was therefore useful for comparing functional capacities between cultivars, in addition to the parameters of the ecophysiological models. As the present comparison considered two apple cultivars, it would be worthwhile to examine leaf ecophysiological traits in more depth, among a larger cultivar range. This was performed in a recent study showing a strong variability of leaf ecophysiological traits (e.g. gsw, IWUE, Anmax …) among a recombinant apple F1 population (Regnard et al., 2007), opening new perspectives for breeding.
CONCLUSIONS
In the present study, the results of both Δ13C analysis and instantaneous responses of gsw to environmental variables indicated different water use strategies in the two apple cultivars analysed. Based on the results, ‘Braeburn’ is more water-conserving than ‘Fuji’. Stomatal responses to environment, and leaf respiration rate, differed between the two cultivars; they also had contrasted crown architectures. As a consequence, combined functional and architectural traits probably play complementary roles in determining the physiological functions of these apple cultivars at the integrated scales of fruiting branches or whole trees. Further examination of a larger range of cultivars is required. The present study also shows that the description of the environmental plasticity of different cultivars is of interest for horticulture in order to determine the potential adaptation of a cultivar to a given climate and consequently the need of finely tuning cultural practices (e.g. irrigation) when the environmental conditions are limiting for growth and productivity.
ACKNOWLEDGEMENTS
The PhD grant awarded to C. Massonnet was funded by INRA and by the Languedoc-Roussillon Region. The authors thank Charles Valancogne (Bioclimatology Unit, INRA Bordeaux) and Michel Ducrey (Mediterranean Forest tree breeding, INRA Avignon) for the loan of portable photosynthesis systems, Pierre Montpied (EEF, INRA Champenoux) for SAS fitting procedures and to Claude Bréchet (EEF, INRA Champenoux) for the isotopic analysis. The authors also thank Mark Jones for revising the English text.
LITERATURE CITED
- Atkinson CJ, Policarpo M, Webster AD, Kingswell G. Drought tolerance of clonal Malus determined from measurements of stomatal conductance and leaf water potential. Tree Physiology. 2000;20:557–563. doi: 10.1093/treephys/20.8.557. [DOI] [PubMed] [Google Scholar]
- Berry J, Bjorkman O. Photosynthetic response and adaptation to temperature in higher plants. Annual Review of Plant Physiology. 1980;31:491–543. [Google Scholar]
- Brendel O, Pot D, Plomion C, Rozenberg P, Guehl JM. Genetic parameters and QTL analysis of δ13C and ring width in maritime pine. Plant, Cell and Environment. 2002;25:945–953. [Google Scholar]
- Brendel O, Le Thiec D, Scotti-Saintagne C, Bodénès C, Kremer A, Guehl JM. Quantitative trait loci controlling water use efficiency and related traits in Quercus robur L. Tree Genetics & Genomes. 2007 doi: 10.1007/s11295-007-0107-z. [Google Scholar]
- Casasoli M, Derory J, Morera-Dutrey C, Brendel O, Porth I, Guehl JM, et al. Comparison of quantitative trait loci for adaptive traits between oak and chestnut based on an expressed sequence tag consensus map. Genetics. 2006;172:533–546. doi: 10.1534/genetics.105.048439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comstock JP, Ehleringer JR. Correlating genetic variation in carbon isotopic composition with complex climatic gradients. Proceedings of the National Academy of Sciences of the USA; 1992. pp. 7747–7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costes E, Sinoquet H, Kelner JJ, Godin C. Exploring within-tree architectural development of two apple tree cultivars over 6 years. Annals of Botany. 2003;91:91–104. doi: 10.1093/aob/mcg010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJong TM, Doyle JF. Seasonal relationships between leaf nitrogen content (photosynthetic capacity) and leaf canopy light exposure in peach (Prunus persica) Plant, Cell and Environment. 1985;8:701–706. [Google Scholar]
- Dragoni D, Lakso AN, Piccioni RM. Transpiration of an apple orchard in a cool humid climate: measurement and modeling. Acta Horticulturae. 2004;664:175–180. [Google Scholar]
- Dreyer E, Le Roux X, Montpied P, Daudet FA, Masson FA. Temperature response of leaf photosynthetic capacity in seedlings from seven temperate tree species. Tree Physiology. 2001;21:223–232. doi: 10.1093/treephys/21.4.223. [DOI] [PubMed] [Google Scholar]
- Ethier GJ, Livingston NJ. On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar-von Caemmerer-Berry leaf photosynthesis model. Plant, Cell and Environment. 2004;27:137–153. [Google Scholar]
- Evans JR, von Caemmerer S. Carbon dioxide diffusion inside leaves. Plant Physiology. 1996;110:339–346. doi: 10.1104/pp.110.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanjul L, Jones HG. Rapid stomatal responses to humidity. Planta. 1982;154:135–138. doi: 10.1007/BF00387906. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Richards RA. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Australian Journal of Plant Physiology. 1984;11:539–552. [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta. 1980;149:78–90. doi: 10.1007/BF00386231. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT. Carbon isotope discrimination and photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology. 1989;40:503–537. [Google Scholar]
- Flore JA, Lakso AN. Environmental and physiological regulation of photosynthesis in fruit crops. Horticultural Reviews. 1989;11:229–287. [Google Scholar]
- Francesconi AHD, Lakso AN, Denning SS. Light and temperature effects on whole-canopy net carbon dioxide exchange rates of apple trees. Acta Horticulturae. 1997;451:287–294. [Google Scholar]
- Gindaba J, Wand SJE. Climate-ameliorating measures influence photosynthetic gas exchange of apple tree. Annals of Applied Biology. 2007;150:75–80. [Google Scholar]
- Glenn DM, Puterka GJ, Drake SR, Unruh TR, Knight AL, Baherle P, Prado E, Baugher TA. Particle film application influences apple leaf physiology, fruit yield, and fruit quality. Journal of the American Society for Horticultural Science. 2001;126:175–181. [Google Scholar]
- Grassi G, Meir P, Cromer R, Tompkins D, Jarvis PG. Photosynthetic parameters in seedlings of Eucalyptus grandis as affected by rate of nitrogen supply. Plant, Cell and Environment. 2002;25:1677–1688. [Google Scholar]
- Guehl JM, Nguyen-Queyrens A, Loustau D, Ferhi A. Genetic and environmental determinants of water use efficiency and carbon isotope discrimination in forest trees. In: Sandermann H, Bonnet-Masimbert M, editors. EUROSILVA Contribution to Forest Tree Physiology. Paris: INRA; 1995. pp. 297–321. [Google Scholar]
- Harley PC, Thomas RB, Reynolds JF, Strain BR. Modeling photosynthesis of cotton grown in elevated CO2. Plant, Cell and Environment. 1992;15:271–282. [Google Scholar]
- Jarvis PG. The interpretation of the variations in leaf water potential and stomatal conductance found in canopies in the field. Philosophical Transactions of the Royal Society of London, B. 1976;273:593–610. doi: 10.1098/rstb.2014.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HG. Partitioning stomatal and non-stomatal limitations to photosynthesis. Plant, Cell and Environment. 1985;8:95–104. [Google Scholar]
- Jones HG. Stomatal control of photosynthesis and transpiration. Journal of Experimental Botany. 1998;49:387–398. (special issue) [Google Scholar]
- Lakso AN. Seasonal changes in stomatal response to leaf water potential in apple. Journal of the American Society for Horticultural Science. 1979;104:58–60. [Google Scholar]
- Lakso AN. Apple. In: Schaffer B, Andersen PC, editors. Handbook of environmental physiology of fruit crops. Boca Raton, FL: University of Florida/CRC Press; 1994. pp. 3–35. [Google Scholar]
- Lauri PE, Lespinasse JM. The vertical axis and solaxe systems in France. Acta Horticulturae. 2000;513:287–296. [Google Scholar]
- Le Roux X, Grand S, Dreyer E, Daudet FA. Parameterization and testing of a biochemically based photosynthesis model for walnut (Juglans regia L.) trees and seedlings. Tree Physiology. 1999;19:481–492. doi: 10.1093/treephys/19.8.481. [DOI] [PubMed] [Google Scholar]
- Le Roux X, Walcroft AS, Daudet FA, Sinoquet H, Chaves MM, Rodrigues A, Osorio L. Photosynthetic light acclimation in peach leaves: importance of changes in mass:area ratio, nitrogen concentration, and leaf nitrogen partitioning. Tree Physiology. 2001;21:377–386. doi: 10.1093/treephys/21.6.377. [DOI] [PubMed] [Google Scholar]
- Lespinasse Y. Le pommier. In: Gallais A, Bannerot H, editors. Amélioration des espèces végétales cultivées. Paris: INRA; 1992. pp. 579–594. [Google Scholar]
- Leuning R. Scaling to a common temperature improves the correlation between the photosynthesis parameters Jmax and Vcmax. Journal of Experimental Botany. 1997;48:345–347. [Google Scholar]
- Leuning R, Kelliher FM, de Pury DGG, Schulze ED. Leaf nitrogen, photosynthesis, conductance and transpiration: scaling from leaves to canopies. Plant, Cell and Environment. 1995;18:1183–1200. [Google Scholar]
- Li F, Cohen S, Naor A, Shaozong K, Erez A. Studies of canopy structure and water use of apple trees on three rootstocks. Agricultural Water Management. 2002;55:1–14. [Google Scholar]
- Marini RP, Sowers DL. Net photosynthesis, specific leaf weight, and flowering of peach as influenced by shade. HortScience. 1990;25:331–334. [Google Scholar]
- Massonnet C. Variabilité architecturale et fonctionnelle du système aérien chez le pommier (Malus × domestica Borkh.): comparaison de quatre cultivars par une approche de modélisation structure-fonction. Montpellier, France: Ecole Nationale Supérieure Agronomique; 2004. PhD Thesis. [Google Scholar]
- Massonnet C, García-Villanueva E, Costes E, Regnard JL. Integrating apple tree aerial and root architecture in a structure–function approach. Acta Horticulturae. 2004;636:601–608. [Google Scholar]
- Mediavilla S, Escudero A. Stomatal responses to drought of mature trees and seedlings of two co-occurring Mediterranean oaks. Forest Ecological Management. 2004;187:281–294. [Google Scholar]
- Mitchell KA, Bolstad PV, Vose JM. Interspecific and environmentally induced variation in foliar dark respiration among eighteen southeastern deciduous tree species. Tree Physiology. 1999;19:861–870. doi: 10.1093/treephys/19.13.861. [DOI] [PubMed] [Google Scholar]
- Oren R, Sperry JS, Katul GG, Pataki DE, Ewers BE, Phillips N, Schafer KVR. Survey and synthesis of intra- and interspecific variation in stomatal sensitivity to vapour pressure deficit. Plant, Cell and Environment. 1999;22:1515–1526. [Google Scholar]
- Pretorius JJB, Wand SJE. Late-season stomatal sensitivity to microclimate is influenced by sink strength and soil moisture stress in ‘Braestar’ apple trees in South Africa. Scientia Horticulturae. 2003;98:1–15. [Google Scholar]
- Quilot B, Génard M, Kervella J, Lescourret F. Ecophysiological analysis of genotypic variation in peach fruit growth. Journal of Experimental Botany. 2002;53:1613–1625. doi: 10.1093/jxb/erf001. [DOI] [PubMed] [Google Scholar]
- Regnard JL, Ducrey M, Porteix E, Segura V, Costes E. Phenotyping apple progeny for ecophysiological traits: how and what for? 27th International Horticultural Congress, Séoul, Korea. Acta Horticulturae. 2007 in press. [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS, Vose JM, Volin JC, Gresham C, Bowman WD. Relationships of leaf dark respiration to leaf nitrogen, specific leaf area and leaf life-span: a test across biomes and functional groups. Oecologia. 1998;114:471–482. doi: 10.1007/s004420050471. [DOI] [PubMed] [Google Scholar]
- Schulze ED. Carbon dioxide and water vapor exchange in response to drought in the atmosphere and in the soil. Annual Review of Plant Physiology. 1986;37:247–274. [Google Scholar]
- Seeley EJ, Kammereck R. Carbon flux in apple trees: the effects of temperature and light intensity on photosynthetic rates. Journal of the American Society for Horticultural Science. 1977;102:731–733. [Google Scholar]
- Stewart JB. Modelling surface conductance of pine forest. Agricultural and Forest Meteorology. 1988;43:19–35. [Google Scholar]
- Tardieu F. Virtual plants: modelling as a tool for the genomics of tolerance to water deficit. Trends in Plant Science. 2003;8:9–14. doi: 10.1016/s1360-1385(02)00008-0. [DOI] [PubMed] [Google Scholar]
- Urban L, Le Roux X, Sinoquet H, Jaffuel S, Jannoyer M. A biochemical model of photosynthesis for mango leaves: evidence for the effect of fruit on photosynthetic capacity of nearby leaves. Tree Physiology. 2003;23:289–300. doi: 10.1093/treephys/23.5.289. [DOI] [PubMed] [Google Scholar]
- Urban L, Lu P, Thibaud R. Inhibitory effect of flowering and early fruit growth on leaf photosynthesis in mango. Tree Physiology. 2004;24:387–399. doi: 10.1093/treephys/24.4.387. [DOI] [PubMed] [Google Scholar]
- Walcroft A, Le Roux X, Diaz-Espejo A, Dones N, Sinoquet H. Effects of crown development on leaf irradiance, leaf morphology and photosynthetic capacity in a peach tree. Tree Physiology. 2002;22:929–938. doi: 10.1093/treephys/22.13.929. [DOI] [PubMed] [Google Scholar]
- Warren CR, Adams MA. Internal conductance does not scale with photosynthetic capacity: implications for carbon isotope discrimination and the economics of water and nitrogen use in photosynthesis. Plant, Cell and Environment. 2006;29:192–201. doi: 10.1111/j.1365-3040.2005.01412.x. [DOI] [PubMed] [Google Scholar]
- Warren CR, Dreyer E. Temperature response of photosynthesis and internal conductance to CO2: results from two independent approaches. Journal of Experimental Botany. 2006;57:3057–3067. doi: 10.1093/jxb/erl067. [DOI] [PubMed] [Google Scholar]
- Warrit B, Landsberg JJ, Thorpe MR. Responses of apple leaf stomata to environmental factors. Plant, Cell and Environment. 1980;3:13–22. [Google Scholar]
- Watson RL, Landsberg JJ, Thorpe MR. Photosynthetic characteristics of the leaves of Golden delicious apple trees. Plant, Cell and Environment. 1978;1:51–58. [Google Scholar]
- Winkel T, Rambal S. Stomatal conductance of some grapevines growing in the field under a Mediterranean environment. Agricultural & Forest Meteorology. 1990;51:107–121. [Google Scholar]
- Wullschleger SD. Biochemical limitations to carbon assimilation in C3 plants – a retrospective analysis of the A/Ci curves from 109 species. Journal of Experimental Botany. 1993;44:907–920. [Google Scholar]
- Wünsche JN, Palmer JW, Greer DH. Effects of crop load on fruiting and gas-exchange characteristics of ‘Braeburn’/M.26 apple trees at full canopy. Journal of the American Society for Horticultural Science. 2000;125:93–99. [Google Scholar]