Abstract
Background
Rubber trees (Hevea spp.) are perennial crops of Amazonian origin that have been spread over the whole tropical belt to guarantee worldwide production of natural rubber. This crop plant has found its place in many national economies of producing countries, and although its domestication by selection of suitable genotypes was very slow, it contributes a lot to the welfare of small farmers worldwide. Its development is limited by severe diseases. In South America, the main fungal disease of rubber trees is the South American leaf blight (SALB) caused by the ascomycete Microcyclus ulei. This fungus inhibits natural rubber production on a commercial scale in South and Central America.
Scope
The disease is still restricted to its continent of origin, but its potential to be distributed around the world rises with every transcontinental airline connection that directly links tropical regions. The need to develop control measures against the disease is an urgent task and must be carried out on an international scale. All control efforts so far taken since 1910 have ended in a miserable failure. Even the use of modern systemic fungicides and use of greatly improved application techniques have failed to prevent large losses and dieback of trees. The results of research dealing with both the disease and the pathosystem over more than 50 years are summarized and placed into perspective.
Future Prospects
A detailed knowledge of this host–pathogen combination requires understanding of the dynamics of Hevea leaf development, the biochemical potential for cyanide liberation, and molecular data for several types of resistance factors. Resolution of the Hevea–SALB problem may serve as a model for future host–pathogen studies of perennial plants requiring a holistic approach.
Key words: Hevea, South American leaf blight, resistance factors, source–sink relationship, stage-specific resistance, cyanogenesis, defence, resistance screening, scopoletin, marker-assisted breeding
INTRODUCTION
Natural rubber (NR) produced from latex of the rubber tree Hevea brasiliensis nowadays accounts for about 40 % of the world's total rubber consumption; 60 % is delivered by synthetic processes. The annual world demand for natural rubber is constantly growing because of its typical physicochemical properties, which are still not achieved by synthetic products. Worldwide production reached 9·7 million tons in 2004 (FAO, 2006). Most plantation areas are located in South East Asia, especially in Thailand, Indonesia and Malaysia, with growing production areas in Vietnam and China. In contrast to this geographical distribution of successful production of natural rubber, the biological centre of origin of the rubber tree is the Amazon basin in South America. The annual production of natural rubber in this vast area of Brazil is only 97 000 t, which is about 1 % of world production and far below the national needs of Brazil for rubber. The reason for this discrepancy is the fact that natural rubber in Asia, India and Africa can be grown on plantation scale, while in Brazil natural rubber is almost completely produced in extractive production systems in the Amazon basin, i.e. in co-operatives that collect latex from wild-growing rubber trees (e.g. Dean, 1987). Plantations in Central and South America so far have never reached full production because they are destroyed before the trees reach physiological maturity (Table 1).
Table 1.
Impact of South American leaf blight on rubber tree cultivation in Central and South America
| Surinam | 1911 | 40 000 trees planted |
| 1918 | Plantation destroyed, first reported epidemics by M. ulei | |
| Brazil | 1927 | Fordlandia: 3 200 ha, 200 000 trees |
| 1933 | Plantation destroyed by M. ulei attack | |
| 1936 | Belterra: 6 478 ha | |
| 1943 | Plantation destroyed by M. ulei | |
| Panama | 1935 | Plantation created by Goodyear Comp. |
| 1941 | Plantation destroyed | |
| Colombia | 1941 | Epidemic development of M. ulei |
| Costa Rica | 1942 | Epidemic development of M. ulei |
| Brazil | 1967 | Start of SUDHEVEA, a three-step-programme for the development of rubber cultivation in Central Amazonia |
| 1972–1975 | PROBOR I | |
| 1978–1982 | PROBOR II | |
| 1984–1994 | PROBOR III | |
| 1986 | Programme was stopped: about 100 000 ha of the 150 000 ha of plantation were already devastated | |
| Brazil | 2002 | Scientific meeting about new efforts towards rubber cultivation in areas affected by SALB |
| Brazil | 2005 | Plantations only in areas free the fungal pressure: in all other areas latex is collected from wild-growing rubber |
| Colombia | 2006 | Programme on selection of rubber trees resistant to SALB |
The main reason for low production and failure of plantation growth is South American leaf blight (SALB), a rubber tree disease caused by the endemic fungus Microcyclus ulei, an ascosmycete that together with its host plant originates from the Amazon area. Since the beginning of rubber exploitation in the region, all attempts to plant rubber trees at a plantation scale in South an Central America have failed completely (Table 1), and to date all control measures against this disease have been unsuccessful (Smith et al., 1995). Neither chemical protection nor biological control measures have promoted the development of healthy and productive plantations (Chee, 1980). Breeding and selection (Gonçalves et al., 1983; Gasparotto et al., 1997) as well as agronomical measures have not led to successful production on a plantation scale. The last concentrated efforts were taken in the governmental programs PROBOR I, II and III of Brazil from 1966 to 1989 (Smith et al., 1995).
At this point in time, the fungus has not spread to other continents on which rubber is cultivated, at least in part because of intensive control of international air traffic and freight shipments from South America to other tropical areas. At present, there are almost no direct intercontinental flights between Brazil and other humid tropical countries, but future direct intercontinental flights are a probability. Disease control is becoming more complex, and because of these factors the probability of an uncontrolled spread of this pathogen is high.
It has been demonstrated that the majority of cultivated natural rubber tree genotypes outside Brazil are susceptible to the disease (Chee, 1976). This finding, from both intensive field tests and laboratory studies, is not surprising. Rather, if one considers the way rubber tree seeds were distributed historically, the fact that the pathogen has never been introduced into the Old World is remarkable. The complete genetic basis for rubber production outside Brazil can be ascribed to only some hundred genotypes, all based on the Wickham collection. Wickham brought more than 80 000 rubber tree seeds from the Amazon region to Kew Gardens. Only a small number of these recalcitrant seeds survived the transport and storage and germinated at Kew. These seedlings formed the basis of the ‘oriental’ clones on which the whole plantation culture outside the Americas has been based. Some new introductions to the South East Asian production areas were made late in the 1980s, as a result of the first international approach to dealing with the rubber tree germplasm problem.
The introduction of Microcyclus ulei into the South East Asian areas of production is a continuing threat; such an introduction would effect a drastic change in the national economies of the rubber-producing countries as well as the world market for all fine rubber products, especially for high quality tires for aeroplanes and automobiles (Chee and Holliday, 1986). The serious nature of this threat to the world economy has recently led to the decision to include South American leaf blight in a list of biological weapons (United Nations Office on Drugs and Crime, http://www.unodc.org/unodc/terrorism_weapons_mass_destruction_page005.html).
In this paper, the state of knowledge concerning the specific nature of the plant–fungus interaction is analysed with an intent to elucidate the bottlenecks that limit the spread of the pathogen and to highlight factors that need to be given special consideration for the successful future development of natural rubber germplasm. My goal in this review is to facilitate production of plant material tolerant or resistant to M. ulei and to initiate the development of an international management strategy for successful resistance.
DESCRIPTION OF THE HOST PLANT MATERIAL
The genus Hevea, family Euporiaceae, comprises eleven species (Gonçalves et al., 1983). The main source for high quality natural rubber is Hevea brasiliensis, a tree that contains latex vessels in the inner bark and that can be tapped by cutting the bark. Latex flow begins immediately after injury to the bark and stops when internal reactions in the latex cause coagulation of this milky substance. Fused isoprene droplets form a plug in the laticifers. The quality of a rubber tree is correlated with the latex composition and production, most importantly the flow rate of latex after cutting. Selection of trees with superior latex quality has been successful, but almost all of these highly productive trees are also highly susceptible to attack by SALB. Hevea brasiliensis is a tall tree native to the Amazon basin, that may reach heights of more than 20 m within a forest, with a trunk of up to more than 50 cm in diameter at 1·5 m stem height. Since the beginning of the last century there have been numerous attempts to cultivate this tree in plantations throughout the tropics. This species has not been completely domesticated, but selection of useful parameters has been made with respect to latex quality, tapping suitability and stress resistance. Concentrated selection, such as that which has occurred in the domestication of main world crop plants like wheat, maize or potato, remains to be carried out with this technically useful plant.
The first major steps towards scientifically rational rubber plant development can be linked to the International Rubber Excursion for collection of germplasm material in 1984 (Allen, 1984; Bahia et al., 1985). Many subsequent genetic improvement programs for natural rubber now pursue high latex quality, reduced panel dryness, different canopy forms, good root formation, annual leaf shedding pattern and reflushing of the canopy, etc. (e.g. CIRAD, 2005). However, agricultural and agroforestry breeding research leading to understanding and control of SALB, or management of resistance to it, is still not a focus of research and development activities.
There is no mass production of latex in Brazil; rubber is only obtained from small plantations in areas on the margins of the ranges of the forests, such as in Mato Grosso del Norte, where environmental conditions are unfavourable for fungal development (Gasparotto et al., 1997). In the Amazon region, latex is still extracted from wild-grown trees in the same manner as it was 100 years ago. Rubber trees in the primary forest occur in low abundance, about two trees per hectare. For the related species Hevea guianensis, a density of 1·8 trees (>10 cm at 1·50 m stem height) per hectare has been reported (Degen, 1999). Rubber gatherers can normally collect latex from 100–120 trees per day. This number of trees constitutes a ‘seringal’, a tapping trail in the rain forest.
Hevea brasiliensis, a perennial tree that can have 50 years of commercial life, has a distinctive juvenile stage in which the growth and developmental pattern is quite distinct from that of adult trees (Hallé and Martin, 1968). The growing rubber seedling is characterized by a rhythmic-flush growth pattern. Whorls of leaves in successive cycles are produced throughout the year. These whorls are separated from each other by pronounced elongation of the internodes following the formation of a new whorl. Each whorl consists of 9–15 leaves, inserted in a 3/8 spiral phyllotaxy pattern. This process leads to rapid development of thin, but elongated, stems. After 5 or 6 years the crown develops the first lateral branches, and successive whorl formation is replaced by annual leaf shedding. Each year, the entire canopy is shed at the beginning of the dry season. Refoliation occurs at the beginning of the new rainy season (Chee and Holliday, 1986). Like many other members of the Euphorbiaceae, rubber tree inflorescences are made up of male, female and bisexual flowers (Dornelas and Rodríguez, 2005). Pollination is primarily dependent on small insects such as midges and thrips, but self-fertilization and inbreeding occurs to various degrees. Self-incompatibility has been observed and established by experiment (Gonçalves, 1986), but male-sterile clones (such as GT-1) also occur (Gonçalves, 1986). In recent breeding projects, cross-pollination or outcrossing has been shown to lead to better fruit set (Hamzah et al., 2002). Unfertilized pistillate flowers quickly wilt and die (Hamzah et al., 2002). The seeds produced in the three-lobed fruit capsules are liberated by explosive dehiscence, a mechanical ‘explosion’ of the fruit shell. Seeds are short-lived and loose their potential to germinate within 2 months of storage under ambient conditions (Holliday, 1970).
THE FUNGUS: BIOLOGY AND RACE STRUCTURE
The fungus is known by several names. The teleomorph of this fungus is Microcyclus ulei (Henn.) von Arx, a member of the bitunicate ascomycetes. Synonyms are Dothidella ulei Henn. 1904 and Melanopsammopsis ulei (Henn.) Stahel 1917. The conidial state is Fusicladium macrosporum Kuyper 1912 and the pycnidial state is Aposphaeria ulei Henn. A thorough account of the fungal systematics is given by Chee and Holliday (1986). In 1962, Muller and von Arx revised the systematics of the fungus and transferred the genus Dothidella to Microcyclus. An examination of the morphology and an updated taxonomic description of this species, documented with excellent illustrations, has appeared in print (Chee and Holliday, 1986).
The first report of M. ulei on wild Hevea spp. was made by Ule (1905), who collected samples in 1900 and 1901 from Brazil near the river Juruà and in 1902 from Peru near Iquitos. In subsequent years, outbreaks of severe infection in densely planted plots of H. brasiliensis occurred in many places in South and Central America (Table 1). In all cases, the plantations were destroyed when the trees reached the maturation stage between 6 to 7 years and changed from rhythmic-flush growth to annual leaf-shedding patterns. Belgrave (1922) and Weir (1929) gave early warnings of the extreme danger of SALB to rubber plantations, especially those in South East Asia.
Mass production of conidiospores and the rapid build-up of inoculum is the causal step in the epidemiological development of fungal infection in flushing canopies. The conidia are short lived, whereas the ascospores produced in black stromatic structures survive for several months. These ascospores can initiate new infection cycles as soon as susceptible young leaf tissues are produced by the host (Chee and Holliday, 1986). It is important to note that the initial infection in flushing tree canopies often starts from conidia that are present throughout the year on young leaf stages of rhythmic flushes of the seedlings or young, freshly budded trees.
The pathogen can be grown on complex media (Chee, 1978; Junqueira, 1985). Sexual stages do not appear to be formed in vitro, but mass production of conidia can be induced by synchronization of spore formation using appropriate light conditions (e.g. Lieberei et al., 1983; Junqueira et al., 1988; Rivano, 1992). New research activities involving examination of fungal races and virulence variation have been undertaken.
Mattos et al. (2003) recently reported the results of studies based on 12 rubber plant clones. The differentiation set of rubber tree genotypes used by these authors for race identification was virtually based on the genotypes selected for the studies of Junqueira et al. (1988). Thirty-six profiles of virulence have been identified among fungal isolates. Host ranges and quantitative ranges of aggressiveness of the isolates varied strongly. None of the isolates was able to infect all host genotypes. These results were combined with previous screening data of Junqueira et al. (1988), Rivano (1997), Hashim and Almeida (1987) and Chee (1976) and, in summary, a total of 68 races with different host reaction spectra have now been identified. The susceptible host-genotype range is very large.
Furthermore, the physiological tolerance of the isolates to temperature and relative humidity, as well as to complex environmental factors (Gasparotto et al., 1989, 1991), suggests that the potential for adaptation of fungal isolates to new environments is high. This variability underscores the potential threat of this fungus to new rubber growing areas outside South and Central America.
Important steps for analysis of the diversity of the fungus and its genetic polymorphism have also been carried out using molecular approaches (Le Guen et al., 2004). In fungal isolates from Brazil and from French Guiana, microsatellite markers were described and assessed with respect to their variability within the isolates. This approach offers a way to group the highly variable complex isolate groups on a genetic information basis.
THE DISEASE: PLANT REACTIONS TO FUNGAL ATTACK
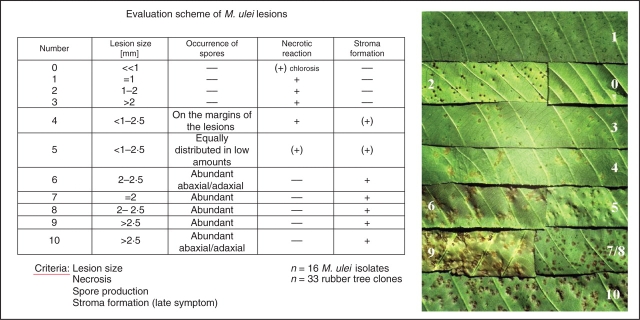
Microcyclus ulei attacks young leaves, stems or fruit tissue of five or more species within the genus Hevea (H. brasiliensis, H. benthamiana, H. spruceana, H. guianensis, H. camporum; Chee and Holliday, 1986). Early breeding concepts for resistance were based on the observation that, in addition to highly susceptible genotypes, plants also occurred that seemed to be immune or completely resistant to M. ulei (Bos and McIndoe, 1965; Gonçalves, 1968; Chee, 1976). These resistant plants were used in crossings with high-yielding individuals of the ‘oriental’ (Wickham) clones. Unfortunately, most of the offspring of these crossings were susceptible to at least some of the fungal strains, and with progressive identification of the fungal virulence sprectrum it became clear that the concept of specific or complete resistance sensu Parlevliet (1975) was not applicable to the rubber tree. Subsequent research was redirected to identify factors that contribute to general resistance (Simmonds, 1989). These consist of many interactive factors in the pathogen–host plant relationship that minimize disease development. Important studies have addressed the physiological aspects of resistance with the aim of identifying processes involved in the plant–fungus interaction (Hashim et al., 1980; Hashim and Pereira, 1989; Mevenkamp, 1992; García et al., 1995) and, finally, to develop biochemical markers for marker-assisted breeding programs. Based on a broad range of observations, a comprehensive view of many of the various stages of the plant–fungus interaction has been achieved. Junqueira et al. (1988) prepared detailed descriptions of lesion types produced by a diverse set of host genotypes. Combinations of various host genotypes with selected fungal isolates varying in virulence spectra led to the formation of a sequence of lesion types with graded differences in the occurrence of necrotic tissue, lesion size, number of spores formed, distribution of conidial layers in the lesion, and stroma formation as a late symptom (Figs 1 and 2). Five main developmental levels in the host–pathogen interaction, which together contribute to the formation of phenotype resistance, were identified (Giesemann, 1986; Junqueira et al., 1988).
Fig. 1.
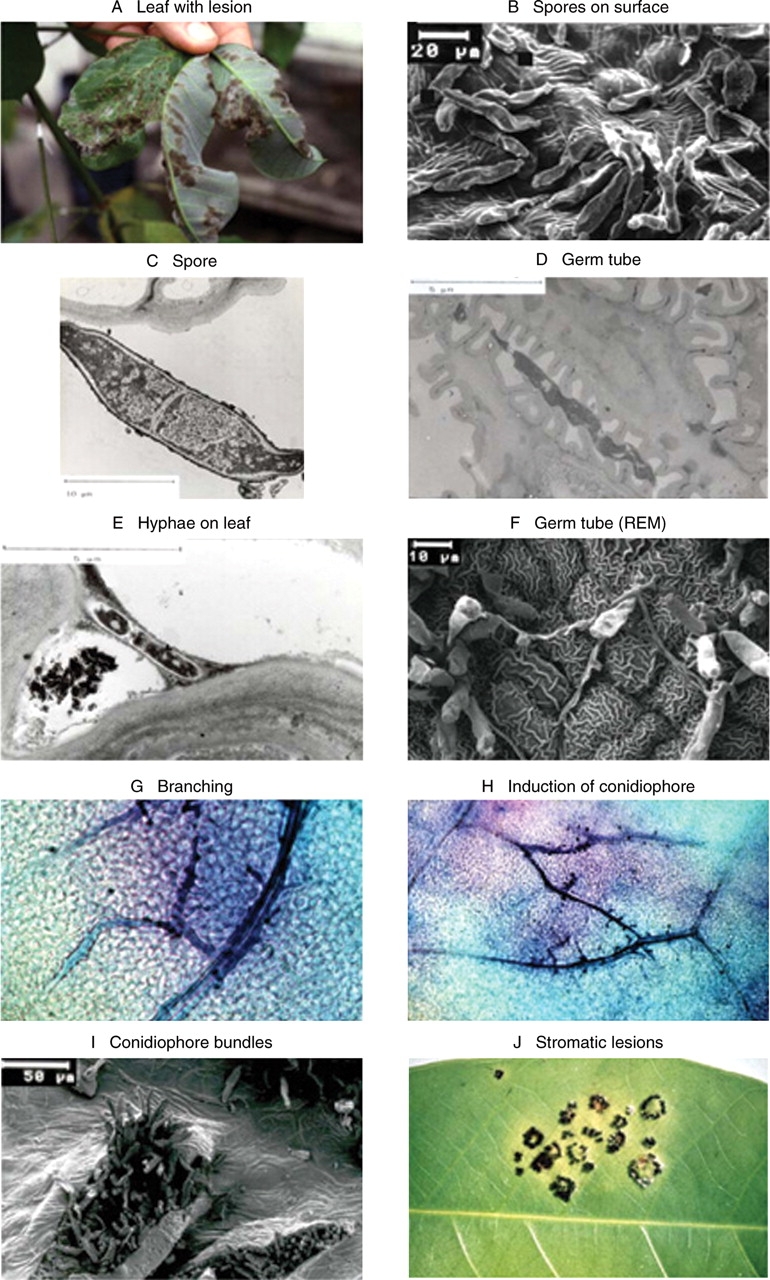
Anatomical and morphological aspects of M. ulei attack. (A) Heavyly infected leaflets of H. brasiliensis with greyish conidiospore layers on upper and lower leaf surfaces. (B) Dried conidiospores on lower leaf surface with reticulate surface structure. (C) Germinating conidiospore, section. (D) Germ tube adjacent to the wax layers on the reticulate surface structures of epidermal cells. The dark grey contrasted germtube is very flexible and polymorphic. (E) Section through a germ tube that is fixed to epidermal cells by a slimy and sticky compound, which is set free from the conidiospore surface after intensive washing. (F) Germinating conidiospores forming penetration structures: a penetration hyphae is entering the leaf at the junction of three adjacent cells (middle). (G) Blue-stained hyphae are spreading through the leaf tissue, often parallel to the vessels (24–36 h post-infection). (H) Blue-stained hyphae; branching hyphae growing back to the surface form dark-blue points (60–72 h post-infection). (I) Conidiospores break through the lower leaf surface. (J) Black pigmented globose structures form ring-like stromata.
Fig. 2.
Evaluation scheme of M. ulei lesions. The classification of lesions follows the description of Junqueira (1985). On leaf section ‘0’ only very tiny necrotic spots become visible. The lesions develop within 3–4 d after infection and incubation in a humid chamber under controlled conditions.
Germination and pre-penetration phase with early impact of the plant on the spores' infection potential.
Penetration and initial infection phase with induction of early resistance responses.
Leaf colonization, intercellular growth and ramification of hyphae; a phase that is considerably influenced by cell wall modifications and leaf maturation processes.
Formation of conidiophores, which leads to differences in the spore number and spore distribution in and around the lesion.
Stroma production and ascospore formation.
Quantitative variations of each of these were observed; they ultimately combine to produce the phenotype of incomplete or partial resistance. In addition to these highly variable pathosystem features, there exists a stage-specific resistance. Only young, developing leaves (Fig. 1A), immature fruits and young stems of this tree species are susceptible. When infection pressure is low and leaves are not infected during the young stages of ontogenetic development, they develop to highly resistant leaf stages. Only a few studies have addressed these various aspects of physiological resistance (Blasquez and Owen, 1957; Junqueira et al., 1990).
Germination and hyphae formation, pre-penetration phase
When the conidiospores of M. ulei come into contact with young leaf tissues of susceptible genotypes, the spores germinate on the reticulate surface of the rubber tree leaves (Fig. 1B–E). Once in contact with a wetted leaf surface, the spores germinate and are not washed off by rain, as they are tightly affixed to the cuticle by an electron-dense substance (Fig. 1E) (Giesemann, 1986). Infection hyphae penetrate the epidermal layer of the lower leaf side (Fig. 1F) in the contact zone of three epidermal cells with or without formation of appressoria. The degree of formation of branched germ tubes and the formation of appressioria is dependent on interaction in the pre-penetration phase. (Hashim et al., 1978; Giesemann, 1986).
Spore germination and germ-tube formation occur immediately after contact of the conidiospores with the leaf. The morphology of the germ tube varies with the reticulate leaf surface structure of the young rubber tree leaves. In Fig. 1D and F, the flexibility of the germ tube morphology is seen and in Fig. 1E the formation of dark, contrasting material between fungus and plant is shown. Germ-tube diameter, germ-tube length, branching and formation of appressoria are strongly influenced by leaf surface constituents, which modify the infection process (Table 2; Blasquez and Owen, 1963; Hashim et al., 1978; Giesemann, 1986).
Table 2.
Germination of M. ulei conidiospores on leaves of selected rubber tree clones: influence of host–pathogen combination in the pre-germination phase. On resistant-reacting leaves the germ tubes are shorter than on susceptible leaves, the germ tube diameter is bigger, and the amount of appresion formed is higher on resistant leaf surfaces. On susceptible leaves, germ tubes are found to form branches
| Control |
HS |
R/S |
R |
|||||
|---|---|---|---|---|---|---|---|---|
| H2O | GT 1 | RRIM 600 | IAN 573 | Fx 25 | F 4542 | PuA 7 | F 2261 | |
| Germination (% in 24 h) | 96 | 95·5 | 95 | 95·8 | 76 | 95·5 | 95·8 | 95·5 |
| Germ tube length (μm in 40 h) | 124 | 114 | 127 | 56 | 62 | 70 | 66 | 73 |
| Germ tube width (μm in 40 h) | 4·2 | 3·6 | 3·4 | 6·0 | 6·0 | 5·8 | 6·1 | 6·2 |
| Appressoria formed (% in 40 h) | 5·2 | 5·8 | 5·5 | 19 | 17·5 | 14·5 | 22·2 | 20 |
| Branched germ tubes (%) | 22 | n.t | 12 | n.t | n.t | 8 | n.t | |
Data taken from Giesemann (1986), n.t: not tested.
Penetration and initial infection stages
After penetration of the leaf surface, the hyphae colonize the underlying tissue by intercellular growth. They often enter the tissue layers adjacent to the leaf vascular bundles and spread rapidly along the veins into the leaves (Fig. 1G, H; Giesemann, 1986). In this biotrophic phase, compatible combinations do not show cell death. However, in resistant clones, the cells in direct contact with the penetration hyphae collapse. Hashim et al. (1978) ascribed this to a hypersensitive reaction and to pre-formed resistance factors (Blasquez and Owen, 1957; Figari, 1965), and proof was also given for induced defence compounds such as scopoletin (Tan and Low, 1975; Giesemann et al., 1986; García et al., 1995b). This early detection process of the fungal presence in the attacked tissue of resistant plants leading to a hypersensitive response is a typical defensive reaction (Breton et al., 1997). This reaction is regarded as an indicator for complete or vertical resistance, but this concept is applicable only to mature leaves of H. brasiliensis and occurs with most genotypes of the previously uninvestigated host species Hevea pauciflora (Junqueira et al., 1988). At the biochemical level of host reactions, a hypersensitive response is often associated with well-described defence reactions such as formation of reactive oxygen-type compounds (García et al., 1999), deposition of autofluorescent compounds in the cell wall (Mevenkamp, 1992), synthesis of callose, occurrence of scopoletin as phytoalexin (Giesemann et al., 1986; García et al., 1995b), and finally cell death in a restricted area surrounding the penetrating hyphae. Detailed and quantified descriptions have been given by García et al. (1999).
Fast hypersensitive response reactions typically lead to tiny necrotic lesions or to yellow microlesions in controlled inoculation experiments (Fig. 2) and they have also been reported as field symptoms associated with both young tissues and with mature rubber tree leaves. Thus, mature leaves express the well-described stage-specific resistance. All adult leaves (Blasquez and Owen, 1963; Junqueira et al., 1988), irrespective of the clonal origin, are highly resistant. This transient and stage-specific enhancement of biochemical resistance patterns needs to be considered in any new strategy of plant selection for broad-based resistance against M. ulei.
In the early phase of leaf penetration and colonization, cyanide liberation occurs as a consequence of cell breakdown (Lieberei, 1986). Cyanide liberation is due to the contact of vacuolar cyanogenic precursors with an apoplastic β-glucosidase. HCN, depending on the velocity of liberation and on precursor concentration, can be inhibitory to plant defence reactions or simply diluted out.
Leaf colonization, intercellular hyphal growth and ramification
In susceptible host–pathogen combinations, the intercellular spread of the fungal hyphae takes place along the leaf vein (Fig. 1G) and freely into the apoplastic regions of the tissue between the veins (Fig. 1H; Giesemann, 1986). This phase ends after 3–5 d with a remarkable morphogenetic change in hyphal growth: the hyphae then form side hyphae, which grow perpendicular to the original hyphae. This new type of hyphae can be seen as coloured points adjacent to the leaf-colonizing hyphae (Fig. 1H). The external or internal factors that regulate these morphogenetic changes remain to be studied. These factors appear to be linked to the leaf maturation process. In the intercellular spaces around the growing hyphae, lignin-like material is deposited that can lead to reduction of the lesion size.
Formation of conidiophores
The branching hyphae are directed towards the lower epidermis and within 24 h after formation they break through the lower leaf surface and form conidiophores and conidia (García et al., 1995a). Small lesions that can fuse laterally to form ring-like structures often lead to large lesions (Fig. 2). The fact that the lesion formation patterns occur in defined types and in a reproducible manner indicates that intercellular growth and ramification of the hyphae underlies a plant genotype–fungal race interdependence (Junqueira et al., 1988).
Stroma production and ascospore formation
Stromatic structures with pseudothecia and ascospores are formed on infected leaves with low infection incidence. Leaves with low levels of infection remain on the trees, whereas heavily infected leaves fall from the tree after conidiospore formation and degrade on the ground. When less-infected leaves remain in the crown 2 months or more after infection, stromata on the upper leaf side are formed in small globose structures that may fuse to form rings (Fig. 1J). In these structures, the sexual stages of fungal development are completed with formation of ascospores. In many plant–fungus combinations, the formation of stromata does not occur. Junqueira et al. (1990) reported that conidial formation in lesions is dependent on the leaf age when infection takes place. The older the developing leaves, the smaller the number of spores formed. Subsequently, formation of stromata is delayed.
Description of the plant–pathogen interaction between Hevea species and SALB and the occurrence of host-specific reactions to various fungal pathogen races establishes that the large set of biochemical defence events known to occur in many other plants also occur in rubber trees and, furthermore, knowledge of these steps underscores the fact that a genetic precondition for biochemical defence is present in all rubber tree genotypes (Hashim et al., 1978, 1980; Mevenkamp, 1992; García et al., 1995a, b). Initiation and function of host defence processes depends on plant/pathogen signalling between host genotypes and fungal isolates. These processes are linked to successful and rapid detection of signals and to activation of the complete biochemical signalling chain for both vertical and complete resistance systems. In addition to this general defence based on major resistance genes, many other variables and patterns in the formation of lesion size and of spore formation are expressed (see Figs 2 and 3). Some host plant genotypes are susceptible to all fungal isolates that have been examined. These variations in expression of partial or horizontal resistance must be based on genetic factors, but can be modified by transient factors on the physiological level of plant hosts (Lieberei et al., 1989).
Fig. 3.
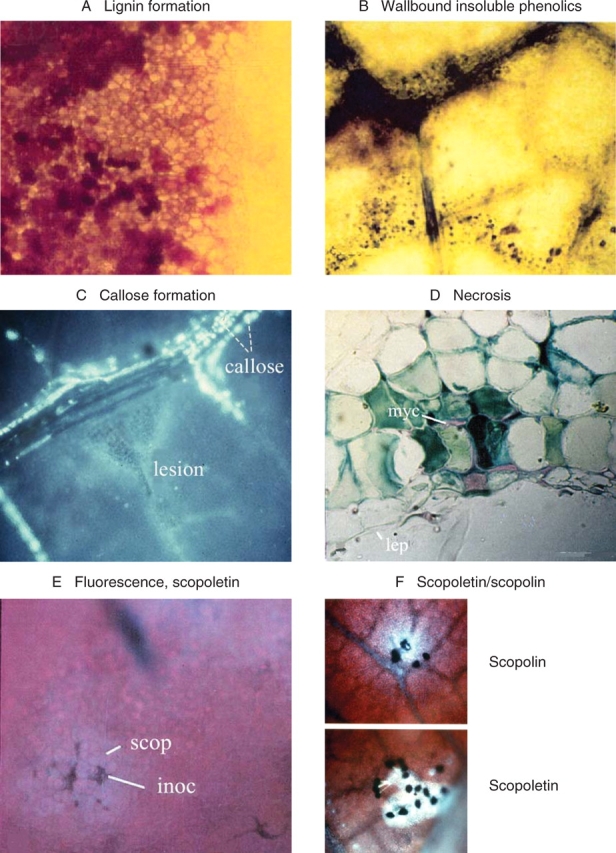
Biochemical resistance reactions in rubber tree leaves. (A) Formation of lignin in cell walls around lesions of intermediately resistant leaves infected by M. ulei. There is a progressing impregnation of the cell walls with lignin (Vance et al., 1980). (B) Resistance reactions are accompanied by the formation of wallbound insoluble phenolics, which become visible after treatment of leaves with Toluidin blue (Feder and O'Brien, 1968). (C) Callose formation around localized lesions (triangular zone in centre of image), detected with Aniline blue (Eschrich and Currier, 1964). (D) Necrotic spot in a hypersensitively reacting leaf adjacent to mycelium (myc); lep = lower epidermis. (E) View of an infected leaf surface under UV-irradiation, red chlorophyll fluorescence in the background and faint blue fluorescence of scopoletin (scop); inoc = inoculation site. (F) Scopoletin in a reticulate distribution under UV irradiation. The blue fluorescing field surrounds black germinating spores of Periconia manihoticola; leaf incubation 24 h under humid conditions in the dark in Petri dishes. Scopolin is formed when the leaves are left attached to the plant and are covered by black plastic bags. The fluorescent compound is located in the vacuoles.
PHYSIOLOGICAL LEAF PROPERTIES
Rubber tree leaves are formed in a flush growth pattern. Directly after bud burst, the leaves are thin, have a high respiration rate, no net photosynthesis (Table 3; Lieberei, 1984; Lieberei et al., 1996) and are devoid of any resistance against the virulent isolates of M. ulei. In the course of maturation, rubber tree leaves change from susceptible to completely resistant (Blasquez and Owen, 1963; Holliday, 1970; Chee, 1980). This maturation requires 12–20 d after bud burst to occur; the maturation time is genotype dependent. This parameter is very important for disease management. Based on morphological criteria seen from bud burst until hardening of leaflets or leaves, Dijkman (1951) placed the developing leaves in four groups designated A to D (Fig. 4). Blasquez and Owen (1963), Hallé and Martin (1968) and Chee (1976) used leaf colour and the angle formed between the petiole and stem to place these leaves into groups, whereas Samsuddin et al. (1978) correlated the developmental groups with the angle formed between the leaflets and the petiole. In contrast, Lieberei (1984) used physiological data for the description of leaf developmental stages. Voβ (2001) used leaf length increase (Fig. 4), and Lieberei (1984) chlorophyll per leaf area, the chl a : b ratio, the fresh weight per leaf area, and selected data on gas exchange to describe the sink status (Table 3).
Table 3.
Quantitative characteristics of developmental leaf stages of rubber trees
| Leaf stage |
||||
|---|---|---|---|---|
| A | B | C | D | |
| Chlorophyll (μg cm−2 leaf area) | 26 | 33–38 | 25–33 | 43–51 |
| Chl a/b ratio | 1·2–1·6 | 1·8–2·2 | 2·2–2·9 | 3·3–3·6 |
| Photosynthetic CO2 fixation (μmol CO2 m−2 s−1) | −0·72 | −0·24 | 5·1–11·6 | |
| Photosynthetic CO2 fixation per unit chlorophyll (μmol CO2 mg−1 Chl a s−1) | 16·1 | 15·3 | 12·0 | |
| Respiration (μmol CO2 mg−1 Chl a s−1) | −5·03 | −4·48 | −2·01 | |
| Fresh weight (mg cm−2) | 19 | 16 | 9 | 11 |
| Dry weight (% of fresh weight) | 18·9 | 19·6 | 40·7 | |
| Leaf soluble protein (μg cm−2) | 1150 | 865 | 365 | 740 |
| HCN-p (μmol CN g−1 fresh weight) | 20 | 30 | 37 | 20 |
Fig. 4.
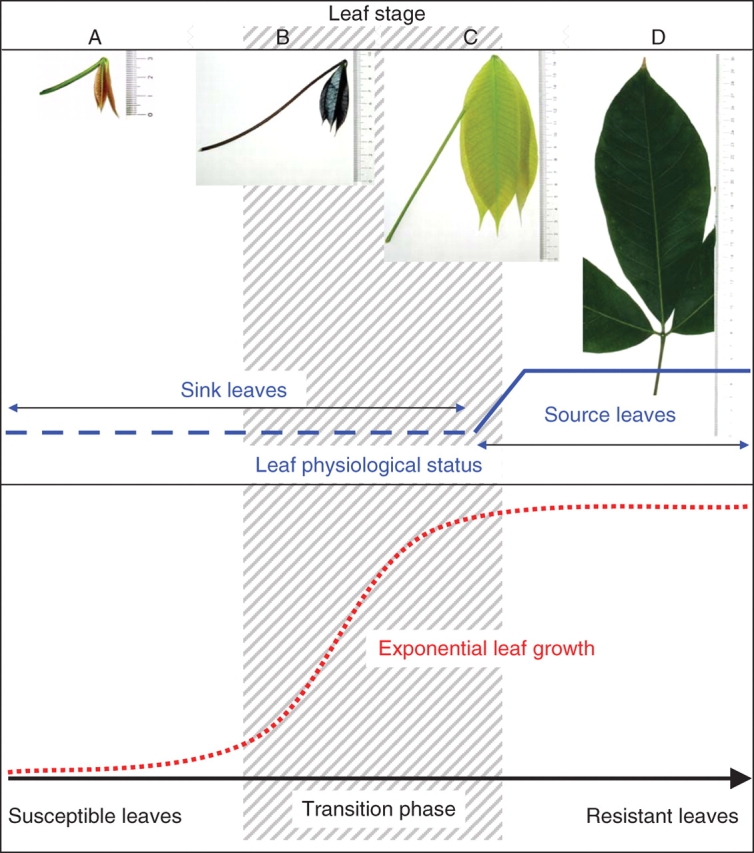
Relationship between exponential leaf growth, transition phase from completely susceptible leaf stage to completely resistant leaf stage, and the short physiological step from sink to source leaves. This qualitative comparison attempts to visualize the different developmental processes that turn a susceptible rubber tree leaf into a completely age-resistant organ. In the hatched area the resistance factors interact in various ways and do not allow the correlation of the QTLs developed with the leaf properties in transition.
Based on these physiological data, leaf stages A, B and C are completely sink leaves with no positive net energy balance (Lieberei et al., 1996). Leaf size increase is almost exponential in the early stages of leaf development. In this phase of rapid cell wall growth and cell enlargement, cell wall hardening processes are retarded; leaves in stages A, B and C are almost free of lignin (Fig. 5A–C; Voβ, 2001).
Fig. 5.
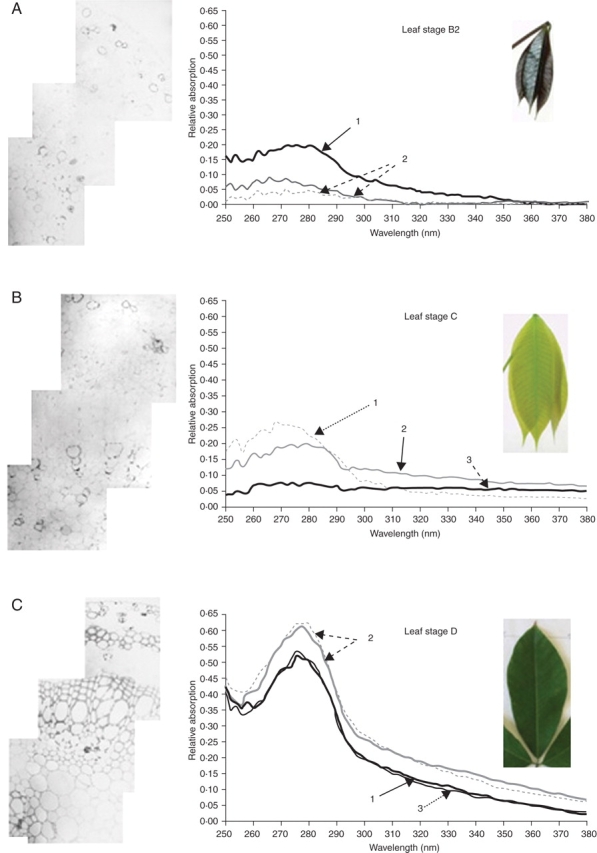
UV photographs and UMSP 80 scans of rubber tree leaves at different developmental stages. Lignin formation was analysed with an ultraviolet (UV) spectrometric device (UMSP 80, Zeiss). Semi-thin sections of acetone-extracted leaves of 1 µm were mounted on quartzglass trays. Spectra were taken in the range of 250–280 nm. Leaves of stages B2, C and D were tested. 1, Middle lamellae and added material; 2, contact layer between three adjacent cells 3; secondary cell wall. The typical absorption spectra for lignin with three combined absorption peaks between 275 nm and 280 nm were detectable in leaf stage D, not before (Voβ, 2001).
The amount of UV-absorbing compounds in 1-mm leaf sections were analysed with a UV-microspectrophotometer (UMSP 80, Zeiss; Fig. 5). This microspectrophotometric analysis permitted us to prove the occurrence of phenolic compounds in the middle lamellae and primary cell walls. Leaves at stages B and C contain some aromatic compounds, but do not contain lignin. UV-photographs of the leaf sections, taken with UV 280 nm light, show that in developmental stages B and C only lignification in the tracheids of conductive vessels occurs. Other cell walls are devoid of lignin (Voβ, 2001). The typical UV absorption pattern of lignin with three peaks near 280 nm (see Fig. 5C) can only be detected in leaf structures of stage D. This presence of lignin coincides with the onset of leaf hardening in early stage D.
Leaves of developmental stage D possess the physiological and structural parameters of mature leaves (Table 3, Fig. 5C). These leaves are completely resistant to M. ulei. The penetrating hyphae of all strains and isolates of M. ulei tested are stopped by hypersensitive responses (Fig. 3: necrosis as described by Blasquez and Owen 1957, 1963).
With young leaves (stage B), fungal infection leads to incomplete defence reactions. In the course of these reactions, the soluble coumarin scopoletin is formed (Tan and Low, 1975; Giesemann et al., 1986; García et al., 1999). Scopoletin is a fungitoxic compound, but the concentrations reached in Hevea leaf tissue are not very high. According to García et al. (1999), the amount of scopoletin is not directly correlated to resistance.
DYNAMICS OF LEAF RESISTANCE: PERSPECTIVES OF LEAF FLUSH GROWTH IN PLANT DEVELOPMENT
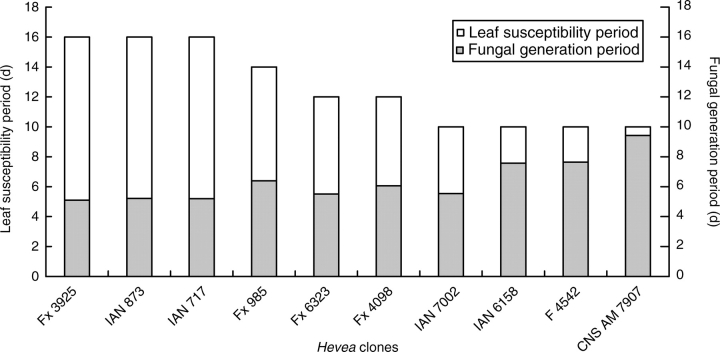
Rubber tree leaves from susceptible Hevea trees are highly susceptible at leaf stage A, but become completely resistant at leaf stage D. The susceptibility of leaves of all Hevea clones to infection with M. ulei generally declines with leaf maturation. This conversion of leaves is a transient and progressive process. The older the leaves are at the time of inoculation, the more distinct is the defence reaction (Junqueira et al., 1990). Within the pathogen isolates, groups have been identified that vary with respect to infection intensity and with respect to their host range (Junqueira et al., 1988; Fig. 2). The variation of resistance of Hevea clones at different stages of leaf development is presented in Figs 6 and 7. Leaves inoculated with virulent isolates at an early leaf age generally form spore-producing lesions a short time after inoculation (Fig. 6). In addition to the short time of the generation period, these lesions are large in diameter. The older the leaves are at the time of inoculation, the smaller in diameter the lesions become. The expressed clonal characteristics of susceptibility are another important factor of disease variability. In clone IAN 717 (Fig. 7), the young leaves produce spores 5 d after inoculation and the lesions reach a size between 2–4 mm in diameter. In clone IAN 6158, spore-producing lesions are less than 2 mm when inoculation takes place 10 d after bud burst. When inoculation is carried out 12 d after bud burst, small necrotic lesions do occur, but no spores are formed in these lesions (Fig. 6). This stage of leaf maturation is characterized by a limited susceptibility to penetration and early fungal spread in the leaf tissue, but later resistance factors such as lignification inhibit the later developmental phases of the pathogen.
Fig. 6.
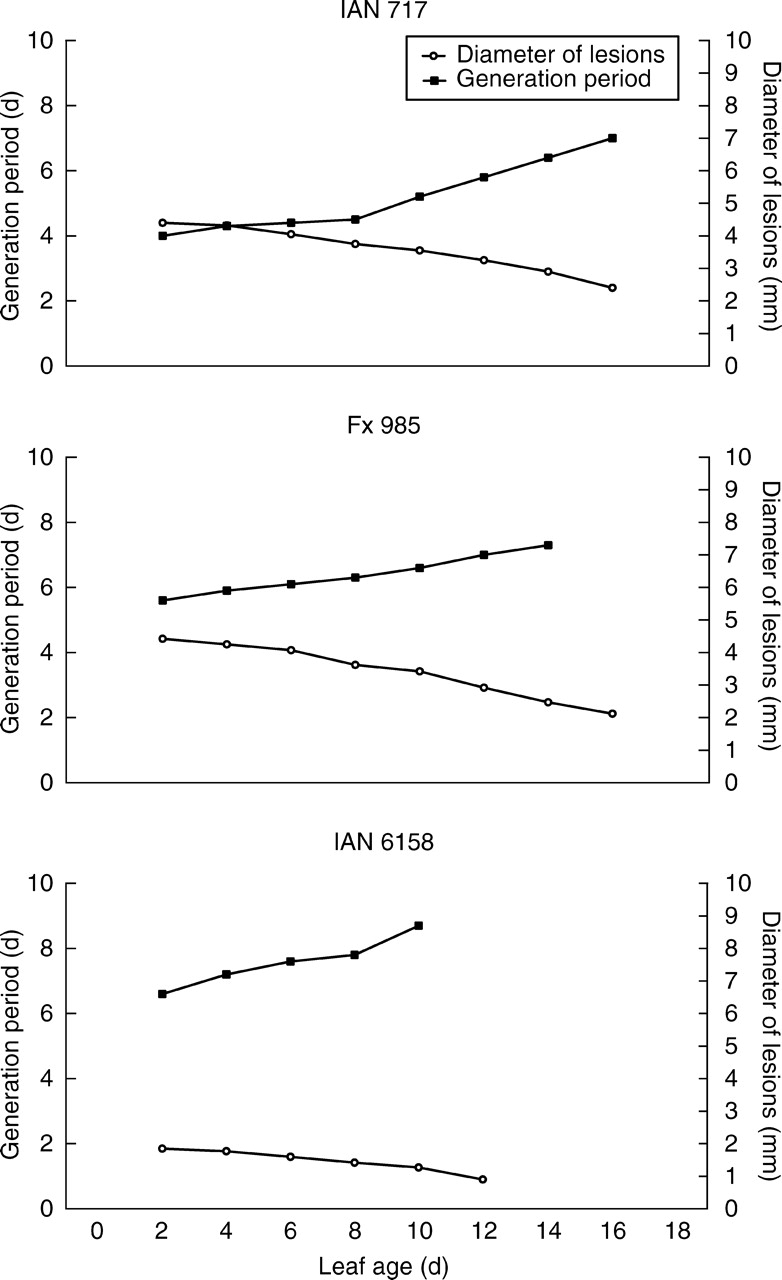
Fungal generation period and lesion diameter on leaves inoculated at different days during the leaf flushing period. Leaves of selected clones were inoculated with M. ulei isolates highly virulent to the respective Hevea clones. The ‘leaf age’ corresponds to the day of inoculation after bud burst, the generation period is given in days from inoculation until spore formation of 50 % of the lesions, and the diameter of lesions are given in mm. The data for the figure have been taken from Junqueira et al. (1990).
Fig. 7.
Fungal generation period in relation to leaf susceptibility period.
The susceptible leaf phases vary considerably with the clones studied (Fig. 7). Clones Fx 7 3925, IAN 873, and IAN 717 are susceptible until a leaf age of 16 d, whereas clones such as IAN 8 6158 or CNS-AM 79 07 become resistant with 10 d. In addition to the clone-specific, leaf-age resistance there is also variation in pathogen development on the clones due to variation in maturation time. In leaves with a long leaf-maturation phase, fungal development is short, i.e. the fungal generation period, defined as the time from inoculation until the formation of new spores (Fig. 7), is shorter than on leaves with short leaf-maturation times. This relation of fungal development to leaf maturation is very important if one considers epidemiological aspects. If the first leaf of a flushing rubber tree canopy is infected and produces spores in the shortest time possible in clone Fx 3925, then by direct reinfection of young leaves at least three fungal generations will be produced, whereas in clones such as F 4542 or CNS AM 7907 the production of spores in infected leaves almost coincides with the onset of leaf-age resistance in the tree crown. Under these conditions reinfection by the newly produced spores in the canopy cannot occur.
The variation of lesion size on leaves at various stages of maturation represents a significant observation. The growth of the fungus through the host-leaf apoplastic space is reduced with increasing age of the leaf tissue. In a structural study of infection-induced defence reactions of rubber leaves against M. ulei, García et al. (1995a) reported scopoletin formation and accumulation of lignin-like substances around the infection site. These authors assumed that lignification may stop fungal development. Lignification is an energy-dependent process that is dependent on the availability of precursors and reductive equivalents. Under the assumption that only source leaves are able to carry out defensive reactions, it becomes important to conduct these studies under appropriate light conditions and with leaves attached to the mother plants. In the past many experiments have been carried out by incubation of detached leaves in humid chambers in the dark (e.g. Hashim et al., 1980; Lieberei, 1986; García et al., 1995b; Breton et al., 1997).
PLANT CYANOGENESIS: LEAF INHERENT CONSTITUENTS WITH HIGH INTERACTION POTENTIAL WITH DEFENCE REACTIONS
In addition to the varying activities of phenolic metabolism and lignin formation, Hevea leaves are characterized by liberation of HCN upon infection and tissue injury. The rubber tree belongs to a large group of more than 3 000 species that are cyanogenic, i.e. they release cyanide on damage to the plant. These plants accumulate cyanogenic precursors in their vacuoles. Upon tissue injury the precursors, mostly β-glycosides, are set free from the vacuoles and are mixed with β-glycosidases. Generally, these β-glycosidases are stored separately from the cyanogenic precursors, mostly in the plant cell walls as active apoplastic constituents (Selmar et al., 1988). The pre-formed enzymes cleave the liberated β-glycosides and give rise to a sugar moiety and an aglycone. The aglycone decays and forms HCN and a corresponding carbonyl compound (Seigler, 1998). The time course of HCN liberation from the aglycone depends on the leaf tissue, pH and on the occurrence of another enzyme, an α–hydroxynitrile lyase, which accelerates HCN-liberation (Selmar et al., 1989).
The cyanogenic precursors of the rubber tree are the glucosides linamarin and lotaustralin (Lieberei et al., 1985). In the course of leaf infection by M. ulei or other leaf-attacking fungi, HCN is released from injured leaf tissue. The liberation of HCN follows a reproducible time course. In the case of leaf infection by M. ulei, there is a characteristic HCN peak in the early infection stage (Lieberei, 1986) about 24 h after penetration of the fungus into the tissue.
Cyanogenesis is an obligate feature of rubber trees. All living tissues, including seeds, are strongly cyanogenic and contain both the accumulated cyanogenic precursor and the respective β-glucosidase.
The amount of cyanogenic precursor accumulated per tissue weight permits calculation of the cyanide liberation potential of the tissue and is called the HCN-potential or HCN-p (Loyd and Gray, 1970). The rate of HCN liberation (HCN-c) from an attacked tissue depends on the type of tissue injury and the activity of the β-glucosidases in the given tissue. In many cases this is also linked to the presence and activity of an α-hydroxynitrile lyase (Selmar et al., 1989). Plants of Hevea brasiliensis as well as all other Hevea species studied are cyanogenic, although they vary considerably in their HCN-p and in their HCN-c (Lieberei, 1984). There is a clear relationship of HCN-c of the rubber tree to the susceptibility to M. ulei. The higher the HCN-c, the higher the susceptibility.
In plants with low HCN-c and HCN-p values, the resistance reactions are not impaired by HCN. These plants follow a well-described host–pathogen reaction pattern with the complete spectrum of lesion formation (Lieberei, 1988a, b). These plants have the classical race-specific resistance patterns. A more detailed examination of this plant–pathogen interaction has been conducted with selected pairs of rubber tree genotypes high in HCN-c and low in HCN-c and selected isolates of M. ulei, which were identified by their host range (Lieberei et al., 1989).
When inoculated leaves were incubated in humid chambers, strongly cyanogenic leaves always developed severe lesions, whereas weakly cyanogenic leaves developed the defence patterns shown in Fig. 2. When HCN liberation was experimentally minimized in the incubation systems by resorption of HCN in alkali or by passing a flow of humid air through the incubation system, the highly susceptible, strongly cyanogenic leaves reacted with formation of smaller lesions and with good resistance responses (Lieberei et al., 1989). These results proved that HCN liberation led to impairment of active defence in the leaves, and that leaves of high HCN-p plants after experimental lowering of HCN in the tissue produce the entire set of defence reactions that can be expressed after signal perception.
HCN liberation is a pre-formed trait in plant tissue, which may provide a very good defence against herbivores (e.g. Tattersal et al., 2001; Ballhorn et al., 2005), but which may render strongly cyanogenic plants more susceptible to microbial pathogens (e.g. flax to flax wilt, Trione, 1960). The theoretical background of this controversial reaction needs to be considered in greater detail as a complex phenomenon in quantitative ecology and evolution. For the rubber tree, this phenomenon must be taken into consideration for practical breeding and selection work. The fungal pathogen M. ulei is tolerant to HCN, at least in its vegetative phase (Lieberei et al., 1983). In addition, the fungus produces a soluble extracellular β-glycosidase that has remarkably high affinity to the hosts' cyanogenic compound linamarin (Lieberei, 2006). The quantitative potential in the leaf material varies with leaf age. The youngest and generally most susceptible leaves have pronounced HCN-p and a high activity of β-glucosidase whereas, during maturation, the leaves (stage D) often reveal lower values (Table 3).
MOLECULAR ANALYSIS OF GENETIC DIVERSITY IN RUBBER TREES
In 1981, a germplasm collection excursion to the geographical area of the primary rubber tree germplasm in the Amazon was conducted by an international group, formed under the auspices of the International Rubber Research and Development Board. The resulting IRRDB collection was the first systematic collection of Hevea germplasm in more than 100 years since the rubber seed collection of Wickham in the Amazon basin. This new rubber germplasm collection was carried out in order to broaden the genetic basis for worldwide rubber tree cultivation and domestication. Studies of the genetic and molecular diversity of the selected rubber tree germplasm subsequently began within the Biotrop program from CIRAD, France. Genome organization and molecular diversity were analysed, using 14 isozyme loci, cytoplasmic and nuclear RFLPs and, later, 17 microsatellite loci (Besse et al., 1994; Le Guen et al., 2003). The germplasm accessions could be separated into six genetic groups, which encompass populations of defined geographic origins, spread over the entire Amazon Basin and the western Andean region (1), the eastern region near the Tapajos river (2), the federal state regions of Acre (3,4), Rondonia (5), and Mato Grosso (6) (Besse et al., 1994).
According to detailed studies by Seguin et al. (1996) and Lespinasse et al. (2000b), Hevea brasiliensis has a diploid genomic organization with rare duplicated loci. These studies led to identification of 18 basic linkage groups in a rubber genome of 2150 cM total map length. Using the 195 progenies of the population derived from the crossing of PB 260 × Fx 3899 and their response to six isolated strains of M. ulei, eight quantitative trait loci (QTL) with respect to resistance were identified on seven independent linkage groups.
Le Guen et al. (2003) and Lespinasse et al. (2000b), on the basis of their molecular data, prepared the mapping of genes conferring field resistance to South American leaf blight. For the first time, it was shown that both factors for partial resistance and for complete resistance were quantitatively expressed in the progeny and could be correlated with five loci. The molecular approach to this plant–pathogen combination has greatly enhanced the possibilities of proceeding with marker-assisted breeding and selection in a perennial tropical plant.
Nevertheless, some important questions remained to be answered. The authors debated why the defence mechanisms controlled by a locus termed M 13-lbn differed strongly in behaviour between the field test and that within an inoculation chamber: the efficiency was best under field conditions, but weak or ineffective under controlled conditions. In susceptible progeny, the resistance conferred by M 13-1bn was no longer detectable.
The same question arises for the QTLs that correspond to linkage groups g15 and g12. These factors are observable under controlled conditions but are not found in field experiments. For this reason, whether the lesion-diameter trait is a useful predictor for SALB resistance under field conditions is debated no matter what the environment. The hypothesis of a ‘QTL with a major effect modulated by a few other minor QTLs’ was strengthened by further analyses. In general, the state of development of genetic analysis in this tropical perennial plant is very promising and pursuing the next steps to marker-assisted breeding appears feasible.
In a parallel step, characterization of the variability of the ascomycete M. ulei is also proceeding well. Eleven polymorphic microsatellite markers for M. ulei have been characterized and applied to six isolates from Brazil and four isolates from French Guiana.
Recent genetic studies of rubber tree resistance to M. ulei indicate that, in addition to the eight major resistance-conferring QTLs, there must be minor factors that modulate the action of the major components or that interfere with the phenotypic expression of these components. The major resistance factors, when active, result in total expression of defensive reactions and lead to complete resistance, i.e. to control of fungal development in the leaf; this ultimately means that no lesions or no spore-forming lesions occur (lesion types 0, 1 and 2, Fig.2). These lesion types do occur and give proof for the occurrence of major resistance factors.
But the conclusions drawn from the molecular studies also give clear evidence that in addition to the major genes for complete resistance, a set of minor components interfere with the performance of the major resistance factors.
From the results of previous studies on resistance physiology of rubber to M. ulei, the importance of various typical biochemical properties of the rubber tree that can actively interfere with resistance reactions on several levels is known. These reactions occur when the major gene resistance is overcome by pathogen isolates with their respective virulence genes. In these combinations of virulent pathogen and susceptible host, the minor genes or the polyfactorial resistance factors are expressed. Their action is expressed by quantitative variation in lesion size and spore abundance. Some of these polyfactorial reactions have been described with respect to scopoletin and lignin formation by García et al. (1999), but the studies have never been evaluated from the aspect of transient changes of leaf physiology in the course of the infection process. In the same context, resistance impairment by the process of cyanogenesis has never been regarded as interacting with the classical resistance processes. So far, the impact of cyanogenesis has never been considered to be important for the outcome of a disease response, though the studies of Lieberei (1986, 1988a) and Lieberei et al. (1989) clearly established that strongly cyanogenic Hevea genotypes have inefficient or no defence responses. Consequently, this raises the question as to what extent the two properties ‘sink leaves’ and ‘cyanogenesis’ interact with resistance such that they can explain some of the irregularities identified in studies on biochemical defence responses by García et al. (1999).
Leaf flush growth is a remarkable and special feature of tropical plants such as rubber trees (Hallé and Martin, 1968). Leaf stages A, B and C of rubber are metabolic sink leaves and their transition from a metabolic sink to a metabolic source organ is remarkably long. Some of the leaf parameters are summarized in Table 3, which is based on a study by Lieberei (1984). The amount of chlorophyll per leaf area increases from leaf stage A, with 26 mg cm−2, to leaf stage D with 47 mg cm−2, and the Chl a/b ratio shifts from a low value to the typical value found in photosynthetically active plants of about 3. Leaf stage C possesses a remarkably lower chlorophyll amount per area than either stage B or D. The fresh weight per area is lowest in stage C, as is the amount of soluble protein per area. These data are a consequence of exponential leaf growth. The leaf area from stage B to stage C is enlarged mainly by leaf water uptake and cell enlargement. During this phase, the cell walls are very thin and flexible and cell wall hardening is minimal. Lignin is definitely absent from cell walls until stage D (Voβ, 2001). On the other hand, it is remarkable that the vacuolar content of cyanogenic precursors per fresh weight rises from stage A to stage D and the activity of the apoplastic β-glucosidase/linamarase also is very high (Table 3, and Lieberei et al., 1985). Cell wall changes and lignin formation obviously become a predominant reaction with the onset of leaf stage D. The ratio of dry weight to fresh weight doubles from stages B and C to D due to cell wall lignification, and this will also be the reason for the obligate restriction of fungal spread in the wall region of stage D leaves.
The leaves are metabolic sink tissues and dependent on the energy balance of the mother plant for a long time. Energy-dependent synthesis requires transport of assimilate into the leaves to occur. Under these conditions, it seems clear that resistance-screening experiments carried out with detached leaves will result in different results from those with leaves attached to the plants. Processes such as cinnamic acid synthesis or changes in pool sizes of amino acids, active synthesis of scopoletin, lignin and glycosides will be retarded or even stopped in detached leaves due to exhaustion of energy-delivering compounds.
In tests with leaves attached to the plants under controlled conditions, pathogen development will perform differently because these leaves receive their metabolic supply from the adjacent source organs. The production of scopoletin, which is produced as a response to fungal attack in rubber tree leaves (Breton et al., 1997; García et al., 1999), is also dependent on the energy-delivering sources in the inoculated leaves. The amount of scopoletin accumulated in the inoculation droplets, as followed by García et al. (1995b, 1999) and by Breton et al. (1997) is the result of several reactions: the scopoletin formation as an induced response to pathogens and to tissue injury, the elicitor treatment, and other forms of stress to the plant tissue. Scopoletin is exuded from the living cell into the apoplast and can easily be detected under UV excitation as a blue fluorescent compound. Because of its weak antimicrobial action, scopoletin is regarded as a phytoalexin in Hevea species (Giesemann et al., 1986). In the cell wall, apoplastic peroxidases convert scopoletin in the presence of H2O2 to yellow/brown insoluble substances and the fluorescence is quenched (Breton et al., 1997; Chong et al., 1999). A second typical reaction lowering the scopoletin content is the glycosylation of scopoletin to yield the glucoside scopolin. Scopolin is formed by the action of UDP-glucosyl transferase (UGT); the glucoside is located in the vacuole. Recently Langlois-Meurinne et al. (2005) reported on the stress- and pathogen-related expression of UGT in plant–pathogen interactions and, in a general approach, Gachon et al. (2005) emphasized the importance of glycosylation for biosynthesis and storage of secondary compounds. In fact, in most plants that produce scopoletin in response to pathogen attack (e.g. potato, Hughes and Swain, 1960; tobacco, Sequeira, 1969; sunflower, Tal and Robeson, 1986), scopolin co-occurs with scopoletin. In rubber tree experiments, García et al. (1995b, 1999) and Breton et al. (1997) did not detect scopolin. In contrast, Körner (1994) found both scopoletin and scopolin after infection of H. brasiliensis leaves with spores of the weak Hevea pathogen, Periconia manihoticola. Körner (1994) infected rubber tree leaves (stage B) with spores of P. manihoticola and incubated them as detached leaves in darkened Petri dishes on moistened filter paper at 22 °C and as leaves attached to the plant, covered by a black plastic bag that also contained a moistened filter paper. After 2 d, small necrotic lesions about 1 mm in diameter surrounded by a yellow halo had developed under both treatments. When analysed under UV-light, both treatments produced a blue fluorescence in the tissue. The fluorescence in detached leaves was found as a net-like distribution in the apoplast, whereas the fluorescence in the attached leaves was found to be concentrated in the vacuoles (Fig. 3). After methanolic extraction, these two compounds could be verified by TLC analysis as scopoletin in detached leaves and as scopolin with trace amounts of scopoletin in leaves attached to the plants. The general difference between the inoculations that produced scopoletin or scopolin was the fact that leaves attached to the plants appeared to receive sufficient assimilates from the mother plant to form the glucoside, whereas the detached leaves, as isolated sink organs, used reserve compounds to produce limited amounts of scopoletin, but did not possess enough reserve substances to provide energy and substrates for carrying out UDP-glucose formation. In this context, the experimental use of detached leaves for resistance screenings must be re-evaluated. The amount of scopoletin produced in detached leaf systems has also been demonstrated to be highly influenced by incubation conditions (Lieberei et al., 1989). When leaves of strongly cyanogenic genotypes of H. brasiliensis are inoculated and incubated in closed Petri dishes, they form large lesions and large amounts of HCN (38·6 ± 4·4 mg HCN g−1 d. wt) are liberated. The amount of scopoletin is small (0·48 mg Scop g−1 d. wt).
When the incubation conditions are modified in such way that the HCN liberated from the leaves is immediately taken out of the system by absorption in alkali or by passing humid air through the incubation chamber, the lesions in the highly cyanogenic leaves do not develop to larger sizes but remain small, and the amount of scopoletin formed around these lesions is about five times higher than in the strongly cyanogenic condition. Thus, high cyanide liberation during pathogen development in the host tissue can lead to impairment of active defence, or in other words, the experiments indicate that strongly cyanogenic plants may contain all potential resistance factors in the same manner as resistant plants but these resistance factors are impaired by another plant factor, in this case cyanide liberation (Lieberei et al., 1989).
Effects of cyanogenesis: importance of threshold concentrations
HCN liberation in plants can impair active defence (rubber tree, Lieberei, 1984, 1986; Lieberei et al., 1989; flax, Lüdtke and Hahn, 1953; Trione, 1960). This finding is hardly accepted by breeders, because it is counter-intuitive to the usual understanding of evolutionary mechanisms. It should be impossible to inherit susceptibility, but both qualitative and quantitative aspects of cyanogenesis are inherited.
A more in-depth look into cyanogenesis indicates that it is a phenomenon in the framework of polyfactorial evolutionary concepts. Cyanogenic plants can be generally divided into obligate cyanogenic plants and facultative cyanogenic species. In obligately cyanogenic plants, the genes for biosynthesis of cyanogenic precursors and their vacuolar storage and the genes for the synthesis of the β-glycosidases specific for each cyanogenic precursor are constitutively expressed. There are many quantitative variations in this phenotypic feature among the cyanogenic plants. Many genotypes contain small amounts of cyanogenic compounds in low micromolecular ranges per tissue dry weight, whereas other genotypes of strongly cyanogenic plants such as Phaseolus lunatus, Manihot esculenta, Hevea brasiliensis, Sorghum bicolor, Passiflora capsularis and many others accumulate large amounts of cyanogenic precursors and the tissue content varies from less than 1 mmol g−1 d. wt to several hundred mmol−1 d. wt of tissue (e.g. Lieberei, 1988a for Hevea species; Busk and Møller, 2002, for Sorghum bicolor; Ballhorn et al., 2005, for Phaseolus lunatus; Amelot et al., 2006, for Passiflora capsularis).
With respect to plant–herbivore interactions, perennial woody plants are highly interesting models for the study of polyfactorial environmental pressure. During the life span of leaves ontogenetic changes in HCN-p and other metabolic patterns occur. This leads to variation of the biochemical scenarios presented to herbivores and leaf-attacking microbes (e.g. Gleadow and Woodrow, 2000a, b, 2002; Miller et al., 2006).
Cyanogenic potential HCN-p (Loyd and Gray, 1970) and the capacity for HCN release per unit time (HCN-c) allow us to quantify cyanide liberation as a reaction to tissue injury. In addition to genotypic variability for HCN-p and HCN-c, tissues often reveal strong variability at different ontogenetic stages. Generally, young leaf stages have higher HCN-p and HCN-c than older leaves (e.g. Gleadow and Woodrow, 2000a, b).
Once liberated, HCN acts as a highly mobile component in the leaf tissue. Within seconds, this compound reaches the cells surrounding the locus of HCN-production. HCN is an inhibitor of many biochemical processes (Solomonson, 1981) and impairs active defence in cells surrounding the lesion (Lieberei, 1988a; Lieberei et al., 1989, 1996).
NEW APPROACHES IN MARKER-ASSISTED BREEDING: HOW TO COMBINE COMPLEX PHYSIOLOGICAL PARAMETERS WITH MOLECULAR MARKERS
Identification of major components that contribute to QTLs permits identification of potentially resistant genotypes. The plants that contain these QTLs generally possess all metabolic systems needed for active defence and, thus, these genotypes are highly interesting for further breeding. When characterized by QTLs for production of large amounts of high quality latex, these plant materials are a primary choice for breeding and selection. The most suitable plants for further development out of this group are those plants with a short ontogenetic transition from complete sink leaves to complete mature source leaves. It is necessary to check how long and to what extent young leaves are devoid of sufficient energy reserves for the processing of active, synthesis-dependent defence reactions. When significant formation of scopoletin and the onset of lignification are necessary for limitation of fungal development, a complete and successful response depends on both the presence of an active phenylalanine ammonia lyase (PAL) and a sizeable pool of aromatic amino acids, especially phenylalanine, for a successful resistance response.
The resistance-impairing effects of cyanogenesis have to be considered independently from the leaf development stage. Cyanide inhibits active defence by inhibition of photosynthetic CO2-fixation and by inhibition of a number of other enzymes (Solomonson, 1981), among them the key enzyme for cinnamic acid production, PAL. Hanson and Havir (1972) reported in detail on the inhibition kinetics for PAL. Körner (1994) repeated these experiments and evaluated the same inhibitory response for PAL extracted from leaves of H. brasiliensis. This finding also explains the report of Lieberei et al. (1989), who showed better resistance when cyanide concentrations were experimentally lowered in inoculated leaves. Furthermore, unpredictable test responses for clones GT1, IAN 873 and other genotypes that are known to be strongly cyanogenic (García et al., 1999) can be explained in this manner. Cyanide inhibition may also explain the discrepancy with some plants of greater susceptibility under in vitro conditions in comparison with field conditions (Lespinasse et al., 2000a).
In general, this new approach for making selections of plant material for breeding studies can be simplified by avoiding use of strongly cyanogenic genotypes in experiments. Furthermore, only genotypes with short flushing periods and fast leaf maturation patterns should be used. Additionally, plants should be included that have irregular leaf shedding instead of regular and complete change of the canopy. The most promising genotypes are those with genes from H. benthamiana (Junqueira et al., 1990).
MOLECULAR METHODS CAN BE USED TO LOWER PLANT CYANOGENESIS
Techniques for application of antisense technology to produce lower cyanogenesis levels have been successfully accomplished with respect to cassava. Both the groups of Møller (Kristensen et al., 2005) and Sayre (Siritunga et al., 2004) offer excellent molecular approaches to this field. Whenever weakly cyanogenic plants are selected, it should be verified to what extent a certain threshold level of cyanogenesis confers on a plant protection from pests and pathogens. Pathogens and pests employ different strategies to respond to cyanogenesis and other plant defensive factors. Evolution is dynamic, and so are our problems.
ACKNOWLEDGEMENTS
The technical, linguistic and idealistic support of Volker Nölting, Dirk Selmar, Karin Puttfarken, David Seigler, Christoph Reisdorff, Daniel Kadow and Gerald Koch are gratefully acknowledged. Parts of these studies have been carried out in close collaboration with many colleagues and friends of the Brazilian Agricultural Research Organization EMBRAPA in Central Amazonia, Manaus, especially Nilton T.V. Junqueira, Luadir Gasparotto and Vincente H.F. Moraes. The research projects have been substantially supported by DFG Li 238/1 and /2.
LITERATURE CITED
- Allen PW. Fresh germplasm for natural rubber. Span. 1984;27:7–8. [Google Scholar]
- Amelot MEA, Núñez JLA, Duarte L, Oliveras-Bastidas A. Hydrogen cyanide release during feeding of generalist and specialist lepidopteran larvae on a cyanogenic plant, Passiflora capsularis. Physiological Entomology. 2006;31:307–315. [Google Scholar]
- Bahia DB, Pinheira E, Gomes ARS, Valois ACC, Goncalves PS, Melho JRV, Pereira JP. Ilheus, Brazil: CEPLAC; 1985. Clones de Seringueira (Hevea sp. (HBK) Muell. Arg.): origem e ancestralidade. p. 428. [Google Scholar]
- Ballhorn DJ, Lieberei R, Ganzhorn JU. Plant cyanogenesis of Phaseolus lunatus and its relevance concerning herbivore plant interactions: the importance of quantitative data. Journal of Chemical Ecology. 2005;31:1445–1473. doi: 10.1007/s10886-005-5791-2. [DOI] [PubMed] [Google Scholar]
- Belgrave WNC. Notes on the South American leaf disease of rubber. Journal of the Board of Agriculture of British Guiana. 1922;15:132–138. [Google Scholar]
- Besse P, Seguin M, Lebrun P, Chevallier M.H, Nicolas D. Genetic diversity among wild and cultivated populations of Hevea brasiliensis assessed by nuclear RFLP analysis. Theoretical and Applied Genetics. 1994;88:199–207. doi: 10.1007/BF00225898. [DOI] [PubMed] [Google Scholar]
- Blasquez CH, Owen JH. Physiological studies of Dothidella ulei. Phytopathology. 1957;47:727–732. [Google Scholar]
- Blasquez CH, Owen JH. Histological studies of Dothidella ulei on susceptible and resistant Hevea clones. Phytopatholoy. 1963;53:58–65. [Google Scholar]
- Bos H, McIndoe KG. Breeding of Hevea for resistance against Dothidella ulei. Journal of the Rubber Research Institute of Malaya. 1965;19:98–107. [Google Scholar]
- Breton F, Sanier C, D'Auzac J. Scopoletin production and degradation in relation to resistance of Hevea brasiliensis to Corynespora cassiicola. Journal of Plant Physiology. 1997;151:595–602. [Google Scholar]
- Busk PK, Møller BL. Dhurrin synthesis in Sorghum is regulated at the transcriptional level and induced nitrogen fertilization in older plants. Plant Physiology. 2002;129::1222–1231. doi: 10.1104/pp.000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee KH. Assessing susceptibility of Hevea clones to Microcyclus ulei. Annals of Applied Biology. 1976;84:135–145. [Google Scholar]
- Chee KH. South American leaf blight of Hevea brasiliensis: Culture of Microcyclus ulei. Transactions of the British Mycological Society. 1978;70:341–344. [Google Scholar]
- Chee KH. Planter. Vol. 56. Kuala Lumpur: 1980. Management of South American leaf blight; pp. 314–325. [Google Scholar]
- Chee KH, Holliday P. South American leaf blight of Hevea Rubber. Malaysian Rubber Research and Development Board. 1986:50. Malaysian Rubber Research and Development Board Monograph No. 13. [Google Scholar]
- Chong J, Baltz RL, Fritig B, Saindrenan P. An early salicylic acid-, pathogen-, and elicitor-inducible tobacco glucosyltransferase: role in compartmentalization of phenolics and H2O2 metabolism. FEBS letters. 1999;458:204–208. doi: 10.1016/s0014-5793(99)01154-0. [DOI] [PubMed] [Google Scholar]
- CIRAD. Le Cirad. Plant Genomics. Rubber tree genetic transformation. 2005.
- Dean W. Brazil and the struggle for Rubber. Cambridge: Cambridge University Press; 1987. [Google Scholar]
- Degen B. Dendrobase User's Manual. France D.O.M: Guyane; 1999. INRA Station de Recherchee Forestieres Couru Cedex. [Google Scholar]
- Dijkman MJ. Coral Gables, FL: University of Miami Press; 1951. Hevea: thirty years of research in the Far East; p. 329. [Google Scholar]
- Dornelas MC, Rodríguez APM. The rubber tree (Hevea brasiliensis Muell. Arg.) homologue of the LEAFY/FLORICAULA gene is preferentially expressed in both male and female floral meristems. Journal of Experimental Botany. 2005;56:1965–1974. doi: 10.1093/jxb/eri194. [DOI] [PubMed] [Google Scholar]
- Eschrich W, Currier HB. Identification of callose by its diachrome and fluorochrome reactions. Stain Technology. 1964;39:303–307. [Google Scholar]
- FAO. 2006. http://faostat.fao.org/site/339/default.aspx .
- Feder N, O'Brien TP. Plant microtechnique: some principles and new methods. American Journal of Botany. 1968;55:123–142. [Google Scholar]
- Figari A. Sustancias fenolicas toxicas al hongo Dothidella ulei en hojas de clones de Hevea brasiliensis. Turrialba. 1965;15:103–110. [Google Scholar]
- Gachon MMC, Langlois-Meurinne M, Saindrenan P. Plant secondary metabolism glycoyltransferases: the emerging fuctional analysis. Trends in Plant Science. 2005;10:542–549. doi: 10.1016/j.tplants.2005.09.007. [DOI] [PubMed] [Google Scholar]
- García D, Cazaux E, Rivano F, D'Auzac J. Chemical and structural barriers to Microcyclus ulei, the agent of South American leaf blight, in Hevea spp. European Journal of Forest Pathology. (a) 1995;25:282–292. [Google Scholar]
- García D, Sanier C, Macheix JJ, D'Auzac J. Accumulation of scopoletin in Hevea brasiliensis infected by Microcyclus ulei (P. Henn.) v. Arx and evaluation of its fungitoxocity for three leaf pathogens of rubber tree. Physiological and Molecular Plant Pathology. (b) 1995;47:213–223. [Google Scholar]
- García D, Troispoux V, Grange N, Rivano R, D'Auzac J. Evaluation of the resistance of 36 Hevea clones to Microcyclus ulei and relation to their capacity to accumulate scopoletin and lignins. European Journal of Forest Pathology. 1999;29:323–338. [Google Scholar]
- Gasparotto L, Zambolim Ll, Maffia L, Ribeiro do Vale FX, Junqueira NTV. Efeito da temperatura e da Unidade sobre a infecção da seringueira por Microcyclus ulei. Fitopatologia Brasileira. (a) 1989;14:38–41. [Google Scholar]
- Gasparotto L, Zambolim L, Ribero do Vale FX, Maffia LA, Jungqueira NTV. Epidemiologia do mal das folhas da seringueira. I. Ponte Nova-MG. Fitopatologia Brasileira. (b) 1989;14:65–70. [Google Scholar]
- Gasparotto L, Zambolim L, Ventura JA, Costa H, Ribeiro do Vale FX, Maffia LA. Epidemiologia do mal das folhas da seringueira no Estado do Epírito Santo. Fitopatologia Brasileira. 1991;16:180–184. [Google Scholar]
- Gasparotto L, dos Santos AF, Pereira JCR, Ferreira FA. Brasilia: EMBRAPA; 1997. Doenças da Seringueira no Brazil; p. 168. [Google Scholar]
- Giesemann A. Resistenzbedingende Faktoren in der Wirt-Pathogen-Beziehung Hevea sp./Microcyclus ulei. Germany: Technical University of Braunschweig; 1986. PhD thesis. [Google Scholar]
- Giesemann A, Biehl B, Lieberei R. Identification of scopoletin as a phytoalexin of the rubber tree Hevea brasiliensis. Journal of Phytopathology. 1986;117:373–376. [Google Scholar]
- Gleadow RM, Woodrow IE. Temporal and spatial variation in cyanogenic glycosides in Eucalyptus cladocalyx. Tree Physiology. (a) 2000;20:591–598. doi: 10.1093/treephys/20.9.591. [DOI] [PubMed] [Google Scholar]
- Gleadow RM, Woodrow IE. Polymorphism in cyanogenic glycoside content and cyanogenic β-glucosidase activity in natural populations of Eucalyptus cladocalyx. Australian Journal of Plant Physiology. (b) 2000;27:693–699. [Google Scholar]
- Gleadow RM, Woodrow IE. Constraints on effectiveness of cyanogenic glycosides in herbivore defense. Journal of Chemical Ecology. 2002;28:1301–1313. doi: 10.1023/a:1016298100201. [DOI] [PubMed] [Google Scholar]
- Gonçalves P de S. The resistance of Fx and IAN rubber clones to leaf disease in Brazil. Tropical Agriculture. 1968;45:331–336. [Google Scholar]
- Gonçalves P de S. Simpósio sobre a Cultura de seringueira no Estado de Sao Paulo. Piracicaba, Brazil: Anais. Campinas: FundaçãoCargill: 1986. Melhoramento genético de seringueira (Hevea spp.) pp. 99–123. [Google Scholar]
- Gonçalves P de S, Paiva JR, Souza RA. Retrospectiva e atualidade do melhoramento genético da seringueira (Hevea spp.) no Brasil e em paises asiáticos. Manaus, Brazil: EMBRAPA-CNPSD; 1983. p. 69. [Google Scholar]
- Hallé F, Martin R. Etude de la croissance rhythmique chez l'hévéa (Hevea brasiliensis Müll.-Arg. Euphorbiacées-Crotonïdées) Adansonia. 1968;2:475–503. [Google Scholar]
- Hamzah S, Chan JL, Yeang HY. Pollen tube growth and fruit set success in Hevea brasiliensis hand pollination influenced by the choice of clone and female flower. Euphytica. 2002;123:1–8. [Google Scholar]
- Hanson KR, Havir EA. Mechanism and properties of phenylalanine ammonia-lyase from higher plants. Recent Advances in Phytochemistry. 1972;4:46–85. [Google Scholar]
- Hashim I, de Almeida LCC. Identification of races and in vitro sporulation of Microcyclus ulei. Journal of Natural Rubber Research. 1987;2:111–117. [Google Scholar]
- Hashim I, Pereira JCR. Lesion size, latent period and sporulation of leaf disc as indicators of resistance of Hevea to Microcyclus ulei. Journal of Natural Rubber Research. 1989;4:56–65. [Google Scholar]
- Hashim I, Chee KH, Duncan EJ. Reaction of Hevea leaves to infection with Microcyclus ulei. Journal of the Rubber Research Institute of Malaysia. 1978;26:67–72. [Google Scholar]
- Hashim I, Chee KH, Wilson LA. The relationship of phenols and oxidative enzymes with resistance of Hevea to South American leaf blight. Phytopathologische Zeitschrift. 1980;97:332–345. [Google Scholar]
- Holliday P. Phytopathological Papers 12. Kew, UK: Commonwealth Mycological Institute; 1970. South American leaf blight (Microcyclus ulei) of Hevea brasiliensis; p. 31. [Google Scholar]
- Hughes JC, Swain T. Scopoletin production in potato tubers infected with Phytophthora infestans. Phytopathology. 1960;50:389–401. [Google Scholar]
- Junqueira NTV. Brazil: Universidade Fédéral de Viçosa; 1985. Variabilidade fisiológica de Microcyclus ulei (P. Henn.) v. Arx. Doctoral Thesis. [Google Scholar]
- Junqueira NTV, Chaves GM, Zambolim L, Alfenas AC, Gasparotto L. Reação de clones de seringueira a varios isolados de. Microcyclus ulei. Pesquisa Agropecuaria Brasileira. 1988;23:877–893. [Google Scholar]
- Junqueira NTV, Lieberei R, Kalil Filho AN, Lima MIPM. Components of partial resistance in Hevea clones to rubber tree leaf blight, caused by Microcyclus ulei. Fitopatologia Brasileira. 1990;15:211–214. [Google Scholar]
- Körner R. Germany: Univesity of Hamburg; 1994. Resistenzkomponenten von Kautschukbäumen (Hevea spp.) gegen Blattschadpilze. PhD thesis. [Google Scholar]
- Kristensen C, Morant M, Olsen CE, Ekström Galbraith DW, Möller BK, Bak S. Metabolic engeneering of dhurrin in transgenic Arabidopsis plants with marginal inadvertent effects on the metabolome and transcriptome. Proceedings of the National Academy of Sciences of the USA. 2005;102:1779–1784. doi: 10.1073/pnas.0409233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois-Meurinne M, Gachon CMM, Saindrenan P. Pathogen responsive expression of glycosltransferase genes UGT 73B3 and UGT 73 B5 is necessary for resistance to Pseudomonas syringae pv tomato in Arabidopsis. Plant Physiology. 2005;139:1890–1901. doi: 10.1104/pp.105.067223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Guen V, Lespinasse D, Lover G, Rodier-Goud M, Pinard F, Seguin M. Molecular mapping of genes conferring field resistance to South American Leaf Blight (Microcyclus ulei) in trubber tree. Theoretical and Applied Genetics. 2003;108:160–167. doi: 10.1007/s00122-003-1407-9. [DOI] [PubMed] [Google Scholar]
- Le Guen V, Rodier-Goud M, Troispoux V, Xiong T-C, Brottier P, Billot P, Seguin M. Characterization of polymorphic microsatellite markers for Microcyclus ulei, causal agent of South American leaf blight of trubber tree. Molecular Ecology Notes. 2004;4:122–124. [Google Scholar]
- Lespinasse D, Grivet L, Troispoux V, Rodier-Goud M, Pinard F, Sequin M. Identification of QTLs involved in the resistance to South American leaf blight (Microcyclus ulei) in the rubber tree. Theoretical and Applied Genetics. (a) 2000;100:875–884. [Google Scholar]
- Lespinasse D, Rodier-Goud M, Grivet L, Leconte A, Legnate H, Sequin M. A saturated genetic linkage map of the rubber tree (Hevea spp.) based on RFLD, AFLP, microsatellite, and isozyme markers. Theoretical and Applied Genetics. (a) 2000;100:127–138. [Google Scholar]
- Lieberei R. Cyanogenese und Resistenz. Germany: Technische Universität Braunschweig; 1984. p. 265. Habilitationsschrift, Naturwissenschaftliche Fakultät. [Google Scholar]
- Lieberei R. Cyanogenesis of Hevea brasiliensis during infection with Microcyclus ulei. Journal of Phytopathology. 1986;115:134–146. [Google Scholar]
- Lieberei R. Relationship of cyanogenic capacity (HCN-c) of the rubber tree Hevea brasiliensis to susceptibility to Microcyclus ulei, the agent causing South American leaf blight. Journal of Phytopathology. (a) 1988;122:54–67. [Google Scholar]
- Lieberei R. The role of cyanide and cyanogenesis in plant pathogen interactions. In: Evered D, Garnety SF, editors. No 140 CIBA-Foundation Symposium Cyanide compounds in biology. b. Chichester, UK: Wiley; 1988. pp. 195–200. [Google Scholar]
- Lieberei R. Physiological characteristics of Microcyclus ulei (P.Henn) V. Arx.–A fungal pathogen of the cyanogenic host Hevea brasiliensis. Journal of Applied Botany and Food Quality. 2006;80:63–68. [Google Scholar]
- Lieberei R, Schrader A, Biehl B, Chee KH. Effect of cyanide on Microcyclus ulei cultures. Journal of the Rubber Research Institute of Malaysia. 1983;31:227–235. [Google Scholar]
- Lieberei R, Selmar D, Biehl B. Metabolization of cyanogenic glucosides in Hevea brasiliensis. Plant Systematics and Evolution. 1985;150:49–63. [Google Scholar]
- Lieberei R, Biehl B, Giesemann A, Junqueira NTV. Cyanogenesis inhibits active defense reactions in plants. Plant Physiology. 1989;90:33–36. doi: 10.1104/pp.90.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberei R, Fock HP, Biehl B. Cyanogenesis inhibits active pathogen defense in plants: Inhibition by gaseous HCN of photosynthetic CO2-fixation and respiration in intact leaves. Angewandte Botanik. 1996;70::230–238. [Google Scholar]
- Loyd RC, Gray E. Amount and distribution of hydrocyanic acid potential during the life cycle of plants of three Sorghum cultivars. Agronomy Journal. 1970;62::394–397. [Google Scholar]
- Lüdtke M, Hahn H. Über den Linamaringehalt gesunder und von Colletotrichum befallener junger Leinpflanzen. Biochemische Zeitschrift. 1953;324:433–442. [PubMed] [Google Scholar]
- Mattos RRC, Garcia D, Pinard F, Le Guen V. Variabilidade de isolados de Microcyclus ulei no Sudeste da Bahia. Fitopatologia Brasileira. 2003;28:502–507. [Google Scholar]
- Mevenkamp G. Die Periconia-Blattfleckkrankheit des Kautschukbaumes (Hevea brasiliensis Muell. Arg., Hevea benthamiana Muell.Arg.). –Morphologische und biochemische Studien zu pflanzlichen Resistenzfaktoren gegen Blattschadpilze und zur induzierten Resistenz. Germany: Technical University of Braunschweig; 1992. PhD thesis. [Google Scholar]
- Miller RE, Jensen R, Woodrow IE. Frequency of cyanogenesis in tropical rainforest of far north Queensland, Australia. Annals of Botany. 2006;97:1017–1044. doi: 10.1093/aob/mcl048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlevliet JE. Partial resistance of barley to leaf rust, Puccinia hordei. I. Effect of cultivar and development stage on latent period. Euphytica. 1975;24:21–27. [Google Scholar]
- Rivano F. La maladie sud-américaine des feuilles de l'hévéa. Etude, en conditions naturelles et contrôlées, des composants de la résistance partielle à Microcyclus ulei (P. Herm. Arx.) Paris: Université de Paris-Sud, Centre d'Orsay; 1992. PhD Thesis. [Google Scholar]
- Rivano F. La maladie sud-américaine des feuilles de l'hévéa. I. Variabilité du pouvoir pathogène de Microcyclus ulei. Plantations: Recherche et Developpement. 1997;4:104–110. [Google Scholar]
- Samsuddin Z, Khir MAR, Impens I. The development of leaf blade class concept for the characterisation of Hevea brasiliensis Muell. Arg. leaf stage. Journal of the Rubber Research Institute of Malaysia. 1978;26:1–5. [Google Scholar]
- Seguin M, Besse P, Lespinasse D, Lebrun P, Rodier-Goud M. Hevea molecular genetics. Plantations: Recherche et Developpement. 1996;3:77–88. [Google Scholar]
- Seigler DS. Cyanogenic glycosides and cyanolipids. In: Seigler DS,, editor. Plant secondary metabolism. Boston, MA: Kluwer Academic; 1998. pp. 273–299. [Google Scholar]
- Selmar D, Lieberei R, Biehl B. Mobilisation and utilisation of cyanogenic glycosides. Plant Physiology. 1988;86:711–716. doi: 10.1104/pp.86.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmar D, Lieberei R, Biehl B, Conn EE. α-Hydroxynitrile lyase in Hevea brasiliensis and its significance for rapid cyanogenesis. Physiologia Plantarum. 1989;75:97–101. [Google Scholar]
- Sequeira L. Synthesis of scopolin and scopoletin in tobacco plants infected by Pseudomonas solanacearum. Phytopathology. 1969;52:36–49. [Google Scholar]
- Simmonds NNW. Breeding horizontal resistance to South America Leaf Blight of rubber. Journal of Natural Rubber Research. 1989;5:102–113. [Google Scholar]
- Siritunga D, Arias-Garzon D, White W, Sayre RT. Over-expression of hydroxynitrile lyase in transgenic cassava roots accelerates cyanogenesis and food detoxification. Plant Biotechnology Journal. 2004;2:37–43. doi: 10.1046/j.1467-7652.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- Smith NJH, Alvim P de T, Serrão EAS, Falesi IC. Amazonia. In: Kasperson JX, Kasperson RE, Turner BL,, editors. Regions at risk: comparisons of threatened environments. Tokyo, New York, Paris: United Nations University Press; 1995. [Google Scholar]
- Solomonson LP. Cyanide as a metabolic inhibitor. In: Vennesland B, Conn EE, Knowles C, Westley J, Wissing F,, editors. Cyanide in biology. London: Academic Press; 1981. pp. 11–28. [Google Scholar]
- Tal B, Robeson DJ. The metabolism of sunflower phytoalexins ayapin and scopoletin. Plant Physiology. 1986;82:167–172. doi: 10.1104/pp.82.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AM, Low FC. Phytoalexin production by Hevea brasiliensis in response to infection by Colletotrichum gloeosporioides and its effect on other fungi. International Rubber Conference (1976). Proceedings, International Rubber Conference, 1975.; Kuala Lumpur: Kuala Lumpur, Malaysia; 1975. Malaysia. Kuala Lumbur: Rubber Research Institute of Malaysia. [Google Scholar]
- Trione EJ. The HCN-content of flax in relation to flax wilt resistance. Phytopathology. 1960;50:482–486. [Google Scholar]
- Tattersal DB, Bak S, Jones RP, Olsen CE, Nielsen JK, Hansen ML, Hoj OB, Møller Resistance to a herbivore through engineered glucoside synthesis. Science. 2001;293:1826–1828. doi: 10.1126/science.1062249. [DOI] [PubMed] [Google Scholar]
- Ule E. Kautschukgewinnung und Kautschukhandel am Amazonen-Strome. Tropenpflanzer. 1905;6:1–71. [Google Scholar]
- Vance CP, Kirk TK, Sherwood RT. Lignification as a mechanism of disease resistance. Annual Review of Plant Pathology. 1980;18:259–288. [Google Scholar]
- Voβ K. Biologische Bedeutung und Aktivierbarkeit der β-D-Glycosidase in Blättern von Hevea brasiliensis (Willd.) Muell. Arg. (1865) Germany: University of Hamburg; 2001. PhD thesis. [Google Scholar]
- Weir JR. The South American leaf blight and disease resistant rubber. Quarterly Journal of the Rubber Researsch Institute of Malaya. 1929;1:91–97. [Google Scholar]