Abstract
Background and Aims
This study aimed at clarifying how the water potential gradient (ΔΨ) is maintained in the shoots of evergreen trees with expanding leaves, whose leaf water potentials at the turgor loss point (Ψtlp) are generally high.
Materials
The water relations were examined in current-year expanding (CEX) and 1-year-old (OLD) leaves on the same shoots in temperate (Osaka, Japan) and tropical (Bogor, Indonesia) areas. A temperate evergreen species, Quercus glauca growing in both sites, was compared with a temperate deciduous species, Q. serrata, in Osaka, and two tropical evergreen species, Q. gemelliflora and Q. subsericea, in Bogor.
Key Results
(1) In Osaka, the midday leaf water potential (Ψmidday) was slightly higher in OLD (−0·5 MPa) than in CEX leaves (−0·6 MPa), whereas Ψtlp was significantly lower in OLD (−2·9 MPa) than in CEX leaves (−1·0 MPa). In Bogor, Ψmidday was also higher in OLD leaves (−1·0 MPa) despite the low Ψtlp (−1·9 MPa), although stomatal conductance was not always low in OLD leaves. In the branch bearing CEX and OLD leaves, most of the hydraulic resistance (86 %) exists in the current-year branch, leading to differences in water supply between CEX and OLD leaves. The removal of buds just before breaking did not affect the high Ψmidday in OLD leaves after 1 month. Ψmidday in OLD leaves thus appears to be independent of that in CEX leaves.
Conclusions
The moderate decrease in Ψmidday in OLD leaves would contribute to maintenance of ΔΨ in the shoots during leaf expansion.
Key words: Cohesion–tension theory, hydraulic resistance, leaf expansion, leaf water potential, pressure–volume curve, Quercus, turgor maintenance, water potential gradient
INTRODUCTION
The leaf water potential at the point where leaf cells incipiently lose their turgor (i.e. the turgor loss point, Ψtlp) is high (less negative) in expanding leaves. Ψtlp of leaves early in their development was approx. −1 MPa in Acer saccharum and Populus spp. (Tyree et al., 1978). Ψtlp gradually decreases with leaf maturation, and Ψtlp of mature leaves ranges from − 1·5 to − 3 MPa in many broad-leaved tree species (Hinckley et al., 1978; Roberts et al., 1980; Parker et al., 1982; Saito et al., 2003). The high Ψtlp of expanding leaves potentially causes problems in water flow in the pathway which continues from the soil, through the plant, and into the surrounding atmosphere (the soil–plant–atmosphere continuum).
Water flow in the soil–plant–atmosphere continuum has been explained by the cohesion–tension (C–T) theory (Dixon and Joly, 1894). The C–T theory proposes that when water is transpired from a leaf, a negative gradient of hydrostatic pressure develops in the continuous column of water inside the xylem conduits. This is because hydrogen bonds hold water molecules together and there are resistances to water flow in the xylem conduits that extend from the roots to the leaves. The gradient of water potential thus formed causes water to flow in the tree. The C–T theory has been widely accepted since the introduction of the pressure-chamber technique, which has been used successfully to measure the negative pressure that develops in the xylem of leaves and branches (Scholander et al., 1965). According to the C–T theory, water potential of the leaves at the end of the water transport pathway must be lower than those in other parts of the plant. In other words, the low leaf water potential (Ψleaf) ensures stable water flow through the plant.
However, cells of expanding leaves cannot maintain turgor if the leaf water potential at midday (Ψmidday) decreases to the level that is usually experienced by mature leaves. This is the result of the higher Ψtlp in expanding leaves than that in mature leaves. Ψmidday usually does not decrease below Ψtlp (Tyree and Jarvis, 1982; Saito et al., 2003; Saito and Terashima, 2004). Indeed, turgor loss inhibits physiological activities in plant cells (Hayashi and Takagi, 2003; Heidecker et al., 2003). Ψleaf of expanding leaves will remain high and this indicates that large water potential gradients do not develop in the plant during this period. Thus, water flow within the soil–plant–atmosphere continuum may be unstable when new leaves are expanding.
The shoots of evergreen tree species are particularly interesting because new leaves, which are usually located at the tips of shoots, expand in the presence of mature leaves on the same shoots. This situation does not occur in common deciduous trees which have only a single flush of leaves per season (Kikuzawa, 1982). In the shoots of evergreen trees, water from the roots must pass through branches that bear mature leaves to reach the expanding leaves. If Ψleaf in the mature leaves decreased below the Ψtlp of the expanding leaves, cell turgor could potentially be lost in the expanding leaves because water would move preferentially along the steeper pressure gradient into the mature leaves. Thus, it was hypothesized that the water potential gradient (ΔΨ) within the shoot is maintained despite the high Ψtlp of expanding leaves. The Ψleaf of 1-year-old (OLD) leaves would play an important role in the water flow in the plants.
The aim of the present study was to clarify whether ΔΨ was maintained in the shoots of evergreen trees while new leaves are expanding. We conducted three experiments in a temperate climate (Osaka, Japan) and a tropical climate (Bogor, Indonesia) using Quercus species. Firstly, changes in Ψleaf and Ψtlp in current-year and OLD leaves was followed throughout leaf expansion in Osaka (expt 1). Secondly, the relationship between Ψleaf in current-year expanding (CEX) leaves and in OLD leaves was examined again in Bogor, because the Ψleaf of OLD leaves could be lower due to the drier atmosphere there (expt 2). Thirdly, buds were removed just before breaking and the effect on Ψleaf of OLD leaves was examined in Osaka to test whether the expanding current-year shoot controls Ψleaf of OLD leaves (expt 3). These experiments clarified the role of OLD leaves in turgor maintenance in expanding leaves.
MATERIALS AND METHODS
Experiment 1: leaf development in Osaka
The study site in Osaka was in a secondary forest on the Toyonaka Campus of Osaka University (central Honshu Island, Japan; 34 °N, 135 °E, 55 m a.s.l.). In 2002, the mean annual temperature was 16·6 °C, with the highest mean monthly temperature (28·8 °C) in July and the lowest (6 °C) in January. The annual precipitation was 819 mm (Toyonaka Meteorological Observatory). During the period of measurement in 2002, the mean monthly temperatures were 16·1, 19·5 and 23·4 °C in April, May and June, respectively. Monthly precipitation totalled 61, 114 and 103 mm in the corresponding months.
Quercus glauca, an evergreen, and Q. serrata, a deciduous broad-leaved tree species (both Fagaceae), were used. These species are common in the warm temperate forests of Japan. Healthy mature trees of Q. glauca (10·8 m tall) and of Q. serrata (16·8 m tall) were used. Several shoots at the middle of the canopy in these trees were used for leaf sampling. The photosynthetic photon flux density (PPFD) relative to that in the open site for these shoots was approx. 25 %.
Phenological events and measurements are summarized in Table 1. In 2002, changes in leaf area were monitored by measuring width and length of leaves every second or third day after bud break. The bud break occurred around 4 April for Q. glauca and 6 April for Q. serrata (Saito et al., 2006). The leaf area attained 99 % of the final size on 24 April in Q. glauca and on 26 April in Q. serrata. These dates were designated as the dates of full leaf expansion (FLE) for these species. Leaf ages in the text preceded by a − or + sign denote the number of days before or after FLE, respectively.
Table 1.
Phenological events and measurements
| Date | Month | Year | Events and measurments |
|---|---|---|---|
| Leaf development in Osaka | |||
| 4 | April | 2002 | Bud break in Q. glauca |
| 6 | Bud break in Q. serrata | ||
| 15 April to 19 July | Ψmidday, Ψpredawn, P–V curve, leaf area | ||
| 24 | April | Full leaf expansion in Q. glauca | |
| 26 | Full leaf expansion in Q. serrata | ||
| Leaf development in Bogor | |||
| 6–31 | October | 2003 | P–V curve |
| 14 | Installing a weather station | ||
| 19 | Ψpredawn, Ψmidday, gs in CEX and OLD | ||
| 27 | Ψmidday, gs in CEX and OLD | ||
| 28 | Ψpredawn, Ψmidday, gs in CEX and OLD | ||
| 4 October to 3 November | 2004 | P–V curve, hydraulic resistance | |
| 7 | October | Installing a weather station | |
| 22 | Ψpredawn, Ψmidday, gs in CMT and OLD | ||
| 25 | Ψmidday, gs in CMT and OLD | ||
| Debudding experiment in Osaka | |||
| 6 | April | 2005 | Ψpredawn, Ψmidday, gs |
| 7 | Removing buds from treatment shoots | ||
| 8–9 | P–V curve | ||
| 10 | Bud break in control shoots | ||
| 18 | Leaf area | ||
| 28–29 | Ψmidday, gs | ||
| 30 | Full leaf expansion | ||
| 30 April to 1 May | P–V curve | ||
Pre-dawn and midday leaf water potential
Changes in leaf water potential (Ψleaf) were followed from 15 April to 19 July throughout leaf development using a pressure chamber (Soilmoisture Equipment, Santa Barbara, CA, USA). Ψleaf was measured before dawn (Ψpredawn) and at midday (Ψmidday, 1200–1400 h). For Q. glauca, the measurements were conducted separately on current-year and OLD leaves. For each set of measurements, at least six leaves from three trees were used for each species. One of the three trees was the tree used for the measurements of leaf area expansion and pressure–volume curves, and others were growing adjacent to the tree.
Pressure–volume measurements
Shoots about 300 mm in length were cut from the trees, and the bases of the shoots were cut again under water. Each cut end of the shoots was submerged in water in a flask. The shoot was covered with a plastic bag and kept in a dark room. The next day, water drops were carefully removed from each sample leaf. Immediately after cutting, Ψleaf was measured with the pressure chamber. Leaf weight was measured before and after the measurement of Ψleaf, and mean values were recorded. Then, decreases in Ψleaf and leaf weight were followed as the leaf dried on the laboratory bench. Data were plotted with the aid of a calculation program working on spreadsheet software and a pressure–volume (P–V) curve was constructed (Scholander et al., 1965; Maruyama and Morikawa, 1983; Turner, 1988; Saito et al., 2006). For each leaf, more than 12 data points (in a few cases, seven points) were obtained in an initial curve, and four to six points were obtained in the liner part. From the P–V curves, the leaf water potential when leaf cells incipiently lose their turgor (Ψtlp; MPa) and the ratio of leaf dry weight to leaf fresh weight at water saturation (DW/SW) were obtained.
Experiment 2: leaf development in Bogor
The study site was in the Bogor Botanical Garden (6 °S, 106 °E, 235–250 m a.s.l.), about 60 km south of Jakarta (western Java, Indonesia). The seasonal change in temperature is small, but precipitation varies widely. The mean annual temperature in 2002 was 27·4 °C, with the highest mean monthly temperature (28·5 °C) in October and the lowest (25·7 °C) in February (Center for Soil and Agro-Climate in Bogor). The rainy season usually lasts October to April, and the short dry period from June to August, although the monthly precipitation varies widely from year to year (Hatta et al., 2005). The highest monthly precipitation (779 mm) was recorded in January and the lowest (134 mm) was in August in 2002· The annual precipitation was as much as 4551 mm in 2002, so that Bogor city is called ‘Kota Hujan’ (rainy city). In October 2002, the mean temperature was 28·5 °C and the precipitation was 199·8 mm. The midday vapour-pressure deficit (VPD) on sunny days was usually > 0·04 Pa Pa−1.
On 14 October in 2003 and 7 October 2004, a custom-built weather station was installed at the nursery of Treub Laboratory, which is located about 0·75 km from the sample trees. PPFD, air temperature and relative humidity were recorded at 10-min intervals on a data logger (Thermic, 2300A, Eto-denki, Tokyo, Japan) until the end of October.
Two saplings of Q. glauca (8 m tall), a temperate species native to Japan, were used for the experiment in Bogor. The saplings had been planted > 5 years ago and were growing healthily. Also, one sapling of Q. gemelliflora (8 m tall) and one mature tree of Q. subsericea (30 m tall) were used. Both are tropical species from west Malesia. All the trees were growing adjacently with about 20 m in distance with other neighbouring trees in an open location, and were not irrigated or pruned by gardeners. New leaves expanded, over a range of dates, on the shoots of both Q. gemelliflora and Q. glauca. In contrast, new leaves expanded simultaneously in mid-October in most shoots of Q. subsericea.
A phenological study conducted in the garden at Bogor (Hatta et al., 2005) reported that leaf longevity was > 14 months in Q. gemelliflora, from 13 to 18 months in Q. glauca, and 10 months or > 24 months in Q. subsericea. Thus, the actual age of the mature old leaves could not be identified. Accordingly, the leaves that had expanded one-cycle before the latest expansion were called ‘one-cycle-old leaves’.
Leaf water relations
In 2003, diurnal changes in Ψleaf and stomatal conductance (gs) were monitored from 0500 to 1600 h on 19 and 28 October and from 1100 to 1300 h on 27 October (Table 1) at intervals of approx. 1 h. These values were obtained from the CEX leaves which were at around FLE and the OLD leaves on the same shoot, simultaneously. In 2004, measurements were conducted from 0500 to 1600 h on 22 October and from 1100 to 1400 h on 25 October. Ψleaf and gs were obtained from the current-year mature (CMT) leaves and OLD leaves on the same shoot, simultaneously. Ψpredawn was measured between 0500 and 0600 h.
Stomatal conductance (gs) was measured using a porometer (AP4; Delta-T Devices, Cambridge, UK). Then, each of the leaves was severed, placed in a humidified plastic bag, and brought to the Treub Laboratory within 30 min by motorbike. Ψleaf was measured with the pressure chamber inside the laboratory. At each set of the measurements, four to five leaves were measured. After sorting Ψleaf values of diurnal change, daily minimum leaf water potential (Ψmidday) was obtained by averaging the six lowest points from the minimum value. Similarly, midday gs was calculated. The leaves used for the calculation of Ψmidday were not always identical to those used for the midday gs. Two erroneous points of Ψleaf in OLD leaves below − 2 MPa which were obtained on 28 October 2003 were omitted, because the other six points measured at the same time were all greater than − 1·29 MPa. P–V measurement was conducted in the Treub Laboratory. Shoots were sampled in the early morning and were re-hydrated for > 2 h. P–V curves of CEX and CMT leaves were measured on the sampling day and that of OLD leaves on the next day.
Hydraulic resistance in the shoots
To describe the difference in water status for the leaves in different positions, the hydraulic resistances of the component parts of a whole one-cycle-old shoot were examined. The values were not standardized on the length and cross-sectional area of the xylem in the branch.
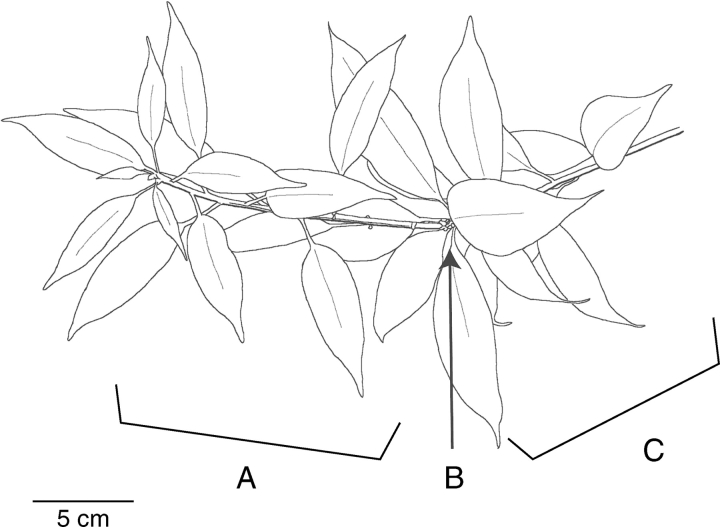
The whole 1-cycle-old shoots, each of which contained only one current-year shoot, were selected (approx. 350 mm in total length; see Fig. 1). At around 0800 h, the shoots were cut at the bud-scale scars at the base of one-cycle-old shoots, and immediately placed in a humidified plastic bag. In the Treub Laboratory, the shoots were re-cut at 10 mm from the cut end under water, and covered with a plastic bag for at least 30 min for absorbing water.
Fig. 1.
Morphology of a typical shoot of Quercus subsericea in Bogor. (A) A current-year shoot with current-year expanding (CEX) leaves; (B) the junction with bud-scale scars between the current-year and one-cycle-old shoots; (C) a one-cycle-old shoot with one-cycle-old (OLD) leaves. ‘one-cycle-old’ leaves and shoots denote the mature old leaves and shoots which had expanded one-cycle before the latest expansion when measurements were conducted.
The shoot was supported horizontally by a clamp, and exposed to the air of the laboratory at around 22 °C, with a VPD of 0·013 Pa Pa−1 and a PPFD of 6 µmol m−2 s−1. A plastic tube which was 5 mm in diameter and 1500 mm in length was filled with distilled water. One end of the tube was connected tightly to the distal end of the shoot. Bubbles in the tube were carefully removed. Then, the other end was submerged in distilled water in a plastic bag placed inside the pressure chamber.
A pressure of 0·1 MPa was applied to the water column in the tube using the pressure chamber. Water leaked neither from the connection between the tube and the shoot, nor from any injuries on the shoot. After about 10 min, the top part of the current-year shoot bearing two or three leaves (about 15 mm in length) was cut from the shoot. Water exuded from the cut end was collected using pink tissue paper held in small plastic tubes (20 mm long). The pink colour made it easier to watch wetting of the paper. The wet tissue paper and tube were weighed at 5-min intervals for 45 min and the amount of water exuded (W, mg) was obtained. The total hydraulic conductance of the shoot (Gt; mg s−1 MPa−1) was calculated as follows:
| 1 |
where T is time (s) and P is the pressure applied (MPa). In practice, W was plotted against T, and the slope of the regression line was designated as Gt. The total hydraulic resistance (Rt) was calculated as the reciprocal of Gt. No apparent reduction in Gt was detected during the observation for 45 min. The effect of xylem embolisms was minimized by sampling the shoots early in the morning and by applying sufficient hydrostatic pressure to the shoot.
After this measurement, a pressure of 0·01 MPa was applied to the shoot using a water column 1 m in height created using a plastic bottle (1·5 L) and the same type of tubing. After connection of the tube to the shoot, the current-year shoot was cut at about 10 mm above the bud-scale scars. The hydraulic conductance of the remaining branch was measured in the same manner to obtain the sum of the hydraulic resistance in the junction and that in the one-cycle-old shoot (Rj + Ro). The resistance of the current-year shoot (Rc) was calculated for each shoot as follows:
| 2 |
Then, the junction was cut just below the bud-scale scars. Ro was measured and Rj was calculated. These procedures were repeated using more than five shoots for each species.
Experiment 3: removal of the current-year shoot
On the Toyonaka Campus in Osaka, one mature Q. glauca tree was selected just before bud break in March 2005· The tree was growing adjacent to the trees used in the experiment in 2002· Ten healthy shoots were randomly selected from a large branch (70 mm in diameter) and divided into the control shoots and the ‘treatment’ shoots to be debudded. Ψpredawn was measured between 0530 and 600 h on 6 April (Table 1) using ten OLD leaves on the shoots adjacent to the sample shoots. Then, Ψmidday and gs were measured from 1000 to 1300 h using five OLD leaves on the control and treatment shoots, respectively. Ψleaf was measured with the pressure chamber and gs was measured with a steady-state porometer (Li-1600, LiCor, Lincoln, NE, USA). On 7 April, all buds on the treatment shoots were removed with a razor blade. On 8 and 9 April, Ψtlp values were obtained from the P–V curves using eight leaves from four shoots similar to the sample shoots.
The date of FLE of the control shoots in 2005 was 30 April. The FLE was estimated from leaf area measured on 18 April 2005 with the detailed time course of expansion in 2002· On 28 and 29 April, Ψmidday and gs were measured between 1000 and 1200 h using ten OLD leaves each on the control and debudded shoots. On 30 April and 1 May, these shoots were sampled to measure P–V curves for OLD leaves. Ψtlp and DW/SW were obtained.
The weather station was placed in the open place beside the sample tree during the measurements. PPFD, air temperature and relative humidity were recorded at 10-min intervals using the data logger. The gs data were recalculated using the flow rate of dry air provided by the porometer and the ambient air temperature and relative humidity from the weather station as described in Saito et al. (2003).
Statistical analysis
All statistical tests were conducted according to the methods of Sokal and Rohlf (1995). Regression lines were calculated using the least-squares method. Significant differences between means were detected using the t-test.
RESULTS
Experiment 1: leaf development in Osaka
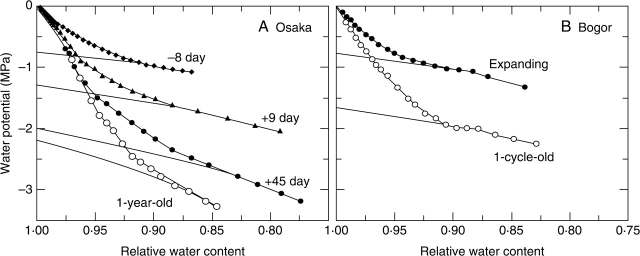
Figure 2A shows representative P–V curves for Q. glauca leaves in Osaka. As leaves aged, the turgor loss point (Ψtlp) became more negative, changing from about − 1·0 MPa on − 8 day to a value of about − 2·5 MPa on + 45 day. The relative water content when turgor loss occurs decreased as leaves aged, though this tendency did not apply to OLD leaves. Similar changes were observed in P–V curves of Q. glauca in Bogor (Fig. 2B), but the turgor of OLD leaves was lost when < 10 % water was lost from the leaves and Ψleaf was − 2 MPa.
Fig. 2.
Representative pressure–volume curves for Q. glauca leaves measured in (A) Osaka and (B) Bogor. Data points represent the measured leaf water potential and the curves extrapolated denote the estimated osmotic potential. Leaf age in (A) represents the number of days before (−) and after (+) full leaf expansion.
The measurement of Ψleaf was conducted on mostly sunny days. The predawn leaf water potential (Ψpredawn) was around − 0·1 MPa in both species throughout the experiment (Fig. 3A and B). The stable and high value for Ψpredawn indicates that the sample leaves did not experience long-term water stress during leaf development.
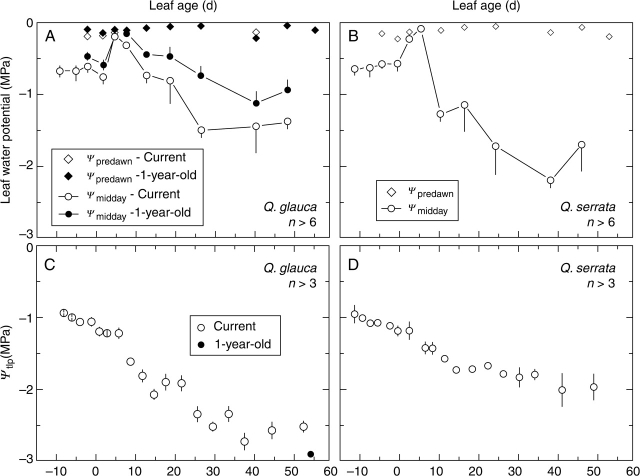
Fig. 3.
Predawn (Ψpredawn) and midday (Ψmidday) leaf water potentials in (A) evergreen Q. glauca and (B) deciduous Q. serrata, and leaf water potential at the turgor loss point (Ψtlp) in (C) Q. glauca and (D) Q. serrata. Measurements were conducted in Osaka. Values are means±s.d.
Ψmidday of the current-year leaves before FLE stayed constant at approx. − 0·6 MPa in both species. The abrupt increase in Ψmidday in + 3 day and + 6 day in both species resulted from cloudy weather. Subsequently, Ψmidday of current-year leaves in Q. glauca decreased gradually to a value of − 1·5 MPa on + 27 day and Ψmidday of Q. serrata decreased to approx. − 2·2 MPa on + 39 day. In Q. glauca, Ψmidday of OLD leaves was approx. −0·5 MPa before FLE and decreased gradually, in parallel with that of the current-year leaves. Ψmidday of OLD leaves was always greater (less negative) than that of the current-year leaves, by approx. 0·15 MPa before FLE and by > 0·30 MPa afterwards.
The leaf water potential at the turgor loss point (Ψtlp) decreased gradually as the leaves matured in both species (Fig. 3C and D). Before FLE, Ψtlp was approx. − 1·0 MPa in both species, but decreased slowly. After FLE, Ψtlp in Q. glauca decreased to a minimum value of approx. −2·5 MPa by + 40 day. The corresponding value in Q. serrata was approx. −1·8 MPa by + 40 day. In Q. glauca, Ψtlp in OLD leaves was − 2·91 MPa. Ψmidday of current-year leaves appeared to parallel the gradual decrease in Ψtlp of these leaves. In addition, a lower PPFD on 1-year-old shoots was reported for solitary grown Q. glauca trees (Miyazawa et al., 2004), but the shoots of Q. glauca were arranged sparsely in the sample trees in this study. Thus, PPFD relative to that in the open site would be around 25 % in both CEX and OLD leaves.
Experiment 2: three Quercus species in Bogor
Leaf area and leaf mass per area
The actual age of the current-year leaves could not be identified in Bogor due to the limited study period. Instead, LMA and DW/SW were used as an indicator of leaf age. The LMA of CEX leaves ranged from 35 to 50 g m−2 and was approximately half that of OLD leaves in the three species (Table 2). Also, DW/SW obtained from P–V curves was approximately 50 % and 80 % of the values for OLD leaves in CEX and CMT leaves, respectively. Based on the detailed time courses for changes in LMA and DW/SW with leaf age in Osaka in 2002 (Saito et al., 2006), CEX leaves were around FLE and CMT leaves were more than + 20 days. The leaf area ranged from 27 to 40 cm2 and neither of the differences between CEX and OLD leaves was significant in the three species (Table 2).
Table 2.
Leaf mass per area (LMA) and leaf area in the three Quercus species
| LMA (g m–2) |
Leaf area (cm2) |
|||
|---|---|---|---|---|
| Expanding (CEX) | One-cycle-old (OLD) | Expanding (CEX) | One-cycle-old (OLD) | |
| Q. gemelliflora | 48·6 ± 3·3 | 96·7 ± 13·5** | 35·3 ± 10·6 | 30·7 ± 9·2 n.s. |
| Q. glauca | 50·3 ± 12·0 | 92·3 ± 9·8** | 35·7 ± 10·0 | 40·4 ± 9·4 n.s. |
| Q. subsericea | 35·4 ± 7·2 | 79·1 ± 6·1** | 27·2 ± 8·9 | 32·5 ± 10·8 n.s. |
Measurements were conducted in Bogor using 16 leaves from six leaves in 2003 and ten leaves in 2004. (mean ± s.d.)
** and n.s. denote P < 0·01 and not significant for statistical significances in the differences between leaf ages for a species.
Micrometeorology
All field measurements were conducted on sunny days. During the day (1000 to 1400 h) on 19, 27 and 28 October 2003, PPFD, air temperature and VPD was generally more than 1000 µmol m−2 s−1, 30 °C and 0·03 Pa Pa−1, respectively. On 22 and 25 October 2004, those values were more than 1000 µmol m−2 s−1, 32 °C and 0·035 Pa Pa−1, respectively. Thus, the measurements would not have been biased by the difference between 2003 and 2004·
Leaf water relations
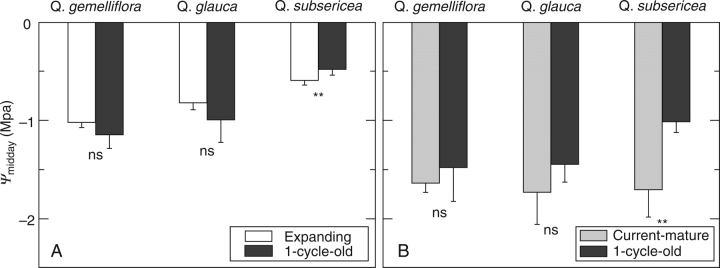
In all the species, Ψpredawn was approx. −0·2 MPa irrespective of the leaf ages (data not shown). Ψmidday of CEX leaves was high (more than − 1·0 MPa; Fig. 4A) as was observed in Osaka (Fig. 4A). Ψmidday in OLD leaves was also generally high (more than − 1·1 MPa) and was similar to that in CEX leaves on the same shoot. For example, Ψmidday values were − 1·02 and − 1·14 MPa, respectively, in CEX and OLD leaves on the same shoot of Q. gemelliflora. Although the value of CEX leaves was slightly higher than that of OLD leaves, the inverse water flow from CEX toward OLD leaves would not be significant. On the shoot with CMT leaves, Ψmidday of CMT leaves decreased to less than − 1·5 MPa in the three species (Fig. 4B). Ψmidday of OLD leaves also decreased to the level of CMT leaves. For example, the Ψmidday values were − 1·64 and − 1·49 MPa in CMT and OLD leaves on the same shoot in Q. gemelliflora. A significant difference inΨmidday between leaf ages was observed only in Q. subsericea in Fig. 4A and B.
Fig. 4.
Midday leaf water potential (Ψmidday) in the three Quercus species in Bogor. Data were collected from (A) expanding leaves and one-cycle-old leaves on the same shoot, and (B) current-year mature leaves and one-cycle-old leaves on the same shoot. Values are means ± s.d. (n=6). ** and ns denote P < 0·01 and not significant for statistical significances in the difference between leaf ages for a species.
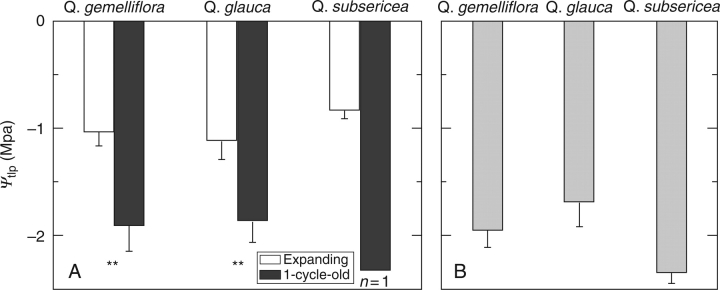
The leaf water potential at the turgor loss point (Ψtlp) was significantly lower in OLD leaves than in CEX leaves on the same shoot in all the three species (Fig. 5A). For example, Ψtlp were − 1·05 and − 1·92 MPa, respectively, in CEX and OLD leaves of Q. gemelliflora. After maturation of the leaves, Ψtlp of CMT leaves (Fig. 5B) was similar to the Ψtlp values of OLD leaves measured with CEX leaves. Ψtlp of CMT leaves (Fig. 5B) was lower than Ψmidday of CMT leaves in Q. gemelliflora and Q. subsericea (Fig. 4B), although slightly higher in Q. glauca (−1·69 MPa and − 1·73 MPa, respectively). In addition, Ψtlp of OLD leaves in Q. glauca in Bogor (−1·86 MPa; Fig. 5A) was much higher than that in Osaka (−2·91 MPa; Fig. 3C).
Fig. 5.
Leaf water potential at the turgor loss point (Ψtlp) in the three Quercus species in Bogor. Data was collected from (A) expanding leaves and the one-cycle-old leaves on the same shoot, and (B) current-year mature leaves. Values are means ± s.d. (n > 5). ** and ns denote P < 0·01 and not significant for statistical significances in the difference between leaf ages for a species.
Stomatal conductance
In the expanding shoots, gs in CEX leaves was similar in the three species and was around 100 mmol m−2 s−1 (Table 3). But, gs in OLD leaves varied and was highest in Q. gemelliflora, followed by Q. glauca and Q. subsericea. In the mature shoots, gs in CMT leaves was 3–7 times higher than CEX leaves and was highest in Q. gemelliflora followed by Q. glauca and Q. subsericea. Also, gs values in CMT leaves were twice that in OLD leaves on the same shoot. gs of OLD leaves in mature shoots was lower than that in OLD leaves in expanding shoots.
Table 3.
Midday stomatal conductance (gs, mmol m–2 s−1) in the three Quercus species in Bogor
| Expanding shoot |
Mature shoot |
|||
|---|---|---|---|---|
| Expanding leaves (CEX) | One-cycle-old leaves (OLD) | Current-year mature leaves (CMT) | One-cycle-old leaves (OLD) | |
| Q. gemelliflora | 165·5 ± 66·0 | 482·5 ± 89·3** | 556·7 ± 98·5 | 309·5 ± 34·5** |
| Q. glauca | 71·0 ± 15·9 | 276·5 ± 61·3** | 471·7 ± 31·4 | 221·5 ± 34·9** |
| Q. subsericea | 135·5 ± 62·5 | 115·8 ± 28·3 n.s. | 340·8 ± 64·6 | 107·0 ± 9·7** |
Data were collected from expanding leaves (CEX) and one-cycle-old (OLD) leaves on the same shoots, or from current-year mature leaves (CMT) and OLD on the same shoots.
Values were mean ± standard deviations of six largest points in the data of diurnal measurements.
** and n.s. denote P < 0·01 and not significant for statistical significance in the difference between leaf ages.
PPFD on the sample leaves varied from 22 to 50 µmol m−2 s−1 in 2003, and from 68 to 397 µmol m−2 s−1 in 2004, with large standard deviations due to sunflecks. Between the years, the effects of different PPFD on gs would not be large. Among the species, PPFD values on Q. subsericea leaves were below 68 µmol m−2 s−1 and tended to be lower than those of other two species. Between the leaves with different ages, a clear difference in PPFD was not found in any of the three species. Thus, the values of gs would reflect the capacity of the leaves.
gs of CMT leaves of Q. glauca in Bogor was approx. seven times the value measured in Osaka (Miyazawa et al., 2004). The much higher precipitation in October in Bogor (200 mm) than Osaka (50 mm) may have ensured an abundant water supply to the leaves. In addition, it may be worth mentioning that different instruments were used in these two studies.
Hydraulic resistance in the shoots
The resistances in the respective parts of the shoot (Table 4) are expressed as proportions to Rt because of large differences in Rt (from 0·56 to 5·42 MPa s mg−1). Rc was > 86 % of Rt when new leaves were at FLE. Rj was < 10 % and Ro was only about 3 % of Rt in the three species.
Table 4.
Hydraulic resistances of the parts of shoots
| Relative hydraulic resistance |
||||
|---|---|---|---|---|
| n | Current-year branch (Rc) | Junction (Rj) | One-cycle-old branch (Ro) | |
| Q. gemelliflora | 6 | 0·868 ± 0·077 | 0·098 ± 0·050 | 0·034 ± 0·029 |
| Q. glauca | 7 | 0·859 ± 0·086 | 0·106 ± 0·061 | 0·034 ± 0·033 |
| Q. subsericea | 5 | 0·953 ± 0·040 | 0·026 ± 0·018 | 0·021 ± 0·024 |
The one-cycle-old branches with one current-year branch were examined for the three Quercus species in Bogor.
Values are relative to the total hydraulic resistance of the whole shoot (mean ± standard deviation).
The parts of the shoots are current-year branch, the junction between current and one-cycle-old branch, and the one-cycle-old branch.
Experiment 3: removal of the current-year shoot
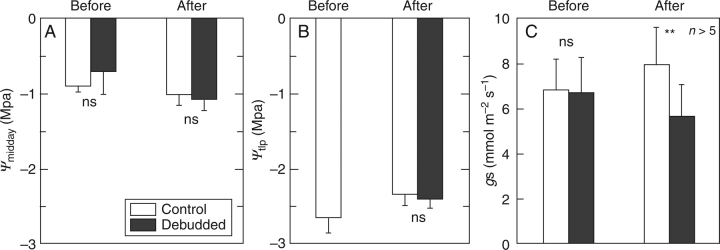
Ψmidday in OLD leaves did not change much from bud break to FLE in both the control and debudded shoots (from around −0·8 MPa to around − 1·0 MPa; Fig. 6A). Also, Ψtlp in OLD leaves did not change much during the corresponding period in both shoots (from −2·64 MPa to around − 2·4 MPa; Fig. 6B). The difference between the control and debudded shoots was not significant in both Ψmidday and Ψtlp (P > 0·05). There were large differences between Ψmidday and Ψtlp in OLD leaves both before and after FLE, so that the daily minimum turgor of leaf cells was about 1·5 MPa.
Fig. 6.
(A) Leaf water potential at midday (Ψmidday), (B) leaf water potential at the turgor loss point (Ψtlp), and (C) stomatal conductance at midday (gs) in 1-year-old leaves of Q. glauca. Debudded shoots had the buds that would generate the current-year shoots removed before bud break. Measurements were conducted before bud break (Before) and after full leaf expansion (After) on the control shoots in Osaka. Values are means ±s.d. (n > 5). ** and ns denote P < 0·01 and not significant for statistical significances in the difference between leaf ages for a species.
gs of OLD leaves was around 6·8 mmol m−2 s−1 in both shoots before bud break (Fig. 6C). At FLE, gs increased in the control shoot (8·0 mmol m−2 s−1), but decreased in the debudded shoot (5·7 mmol m−2 s−1), so that the difference in gs was significant between the shoots (P < 0·01). In addition, Ψpredawn was −0·14 ± 0·07 (average ± standard deviation) before bud break, suggesting that the plants were not experiencing serious water stress. Ψmidday values at FLE in 2005 were slightly lower than in 2002 (Fig. 3A).
All measurements were conducted on sunny days. Both before bud break and at FLE in April 2005, air temperature and VPD during the day (1000–1400 h) were generally above 20 °C and 0·02 Pa Pa−1, respectively. PPFD at FLE was 1300 µmol m−2 s−1 and higher by 300 µmol m−2 s−1 than that before bud break. This may partly explain the larger gs after FLE in the control shoots. The higher gs in control shoots than debudded shoots at FLE indicates that the development of current-year shoots enhances hydraulic conductance of 1-year-old shoots. Probably, basipetal transport of IAA (indole-3-acetic acid) from expanding leaves and shoots promoted cambial activity of the 1-year old stem (Funada et al., 2001).
DISCUSSION
The results support the hypothesis that the gradient in water potential in the shoot is maintained even when new leaves are expanding. Despite the low Ψtlp below − 1·8 MPa in OLD leaves (Figs 3C and 5A), Ψmidday was usually greater than − 1 MPa in these leaves (Figs 3A and 4A). This value was higher than Ψmidday and Ψtlp in CEX leaves. Thus, the moderate decrease in Ψmidday in OLD leaves played a central role in maintaining the water potential gradient both in temperate and tropical areas.
Leaf development in Osaka
From the data measured in Osaka, changes in cell turgor in CEX leaves were examined. The minimum level of turgor at midday can be estimated using the Ψmidday value and the P–V curves (Pavlik, 1984; Saito et al., 2003). From the results, a minimum turgor pressure of approx. 0·3 MPa was maintained before FLE in both species. After FLE, a minimum turgor of approx. 0·6 MPa was consistently maintained in Q. glauca. On the other hand, zero turgor probably occurred in Q. serrata on + 25 and + 39 days due to decreases in Ψmidday (Fig. 3B and D). This would result from the optimistic stomatal opening in deciduous species in comparison to evergreen species (Sobrado, 1986). However, Ψpredawn remained sufficiently high even on these days (Fig. 3B). Thus, cell turgor was almost consistently maintained in CEX leaves in both species.
The high Ψmidday of CEX leaves (about − 0·7 MPa) could potentially disturb the water potential gradient in the shoots of Q. glauca. But in reality, Ψmidday in OLD leaves (about − 0·5 MPa) was slightly higher than that in CEX leaves (Fig. 3A). Thus, the water potential gradient would be maintained in the shoot. Moreover, Ψtlp in OLD leaves (–2·9 MPa) was considerably lower than Ψmidday (Fig. 3C). Consequently, the daily minimum turgor in OLD leaves was as much as 1·7 MPa. This suggests that the high Ψmidday in OLD leaves is not for maintaining the critical level of turgor of the leaves, which is around 0·5 MPa in the various plants (discussed in Saito and Terashima, 2004). Alternatively, the conservative decrease in Ψleaf in OLD leaves ensures a water potential gradient within the shoot during leaf expansion and consequently maintains turgor in CEX leaves.
Expanding leaves and one-cycle-old leaves in Bogor
The moderate decrease in Ψleaf in OLD leaves would play a more critical role in expansion of new leaves in Bogor. The present results show that Ψmidday in OLD leaves was again high (more than − 1·2 MPa) and comparable to that in CEX leaves (Fig. 4A). In contrast, Ψtlp of OLD leaves was below − 1·8 MPa (Fig. 5A). Thus, the daily minimum turgor of OLD leaves was approx. 0·5 MPa in Q. gemelliflora, 0·8 MPa in Q. glauca, and would be 1·3 MPa in Q. subsericea. This high Ψmidday in OLD leaves was maintained in spite of high gs during leaf expansion (Table 3). Values of gs in OLD leaves were comparable to those of deciduous species in temperate areas (Saito et al., 2003). After maturation of the current-year leaves, Ψmidday of OLD leaves also decreased following the decrease in Ψmidday of CMT leaves (Fig. 4B), as was observed in Osaka (Fig. 3A).
Stomatal conductance (gs) of CEX leaves was generally smaller than that of OLD leaves (Table 3), but Ψmidday values of CEX leaves were similar to those of OLD leaves (Fig. 4A). These results indicate that there is a large resistance to water flow between OLD leaves and CEX leaves. In fact, > 85 % of the total resistance of the branch existed in the current-year branch in all three species (Table 4). Ikeda and Suzaki (1984) reported that the hydraulic conductivity of current-year shoots was small relative to other parts of trees of Lithocarpus edulis Makino (Fagaceae) as well. These results suggest that the large hydraulic resistance of current-year shoots and their small gs contribute to maintaining stable water conditions in the CEX leaves.
The high Ψmidday in OLD leaves suggests that the stem on the pathway of water flow to CEX leaves should have higher water potential than CEX leaves. Indeed, shoots were re-hydrated before leaf or flower buds began to expand during a severe dry season in savanna in Costa Rica (Reich and Borchert, 1984; Borchert, 1994). In contrast, the inverse gradient of water potential is observed between developing flowers or fruits and subsidiary leaves growing on the same shoot (Trolinder et al., 1993; Nobel et al., 1994; Chapotin et al., 2003). Even backflows of water from fruit to stem via the xylem were observed (Lang and Thorpe, 1989; Lang, 1990). With the inverse gradient, water would be transported by the phloem along with photosynthates (Nobel et al., 1994). A similar situation is assumed at the start of the leaf expansion. However, this study dealt with the leaves at a later stage of expansion.
Removal of current-year shoots
As the current-year leaves matured, Ψmidday of OLD leaves decreased following the decreasing Ψmidday of CMT leaves (Figs 3A and 4B). This result suggests that CEX leaves control Ψleaf of OLD leaves by sending a hormonal signal such as ABA to OLD leaves. The ABA could cause stomatal closure (Davies et al., 1994), resulting in low gs and high Ψmidday in OLD leaves. However, the Ψmidday of OLD leaves was not significantly affected by the removal of buds (Fig. 6A). In addition, gs of OLD leaves in the debudded shoots was not high, but significantly lower than in the control shoots at FLE (Fig. 6C). This rejects the assumption that Ψmidday of OLD leaves is controlled by a signal from the expanding current-year shoot. The hydration of OLD leaves by root pressure at the whole-tree level is not likely, because leaf expansion did not always occur simultaneously in all the shoots in Bogor. It is likely that the charging of water occurs in each shoot that will produce CEX leaves, but the mechanism is not clear at present.
This apparently autonomous behaviour of water relations in OLD leaves contrasts with the previous reports that gs was tightly regulated by hydraulic signals propagated along the water transport pathway within an individual. For example, when the covering of a large part of the crown was removed suddenly, the gs of other foliage decreased within 1 min in some conifer trees (Whitehead et al., 1996; Pepin et al., 2002).
Turgor maintenance in leaf cells is usually examined in individual leaves. In contrast, this study showed for the first time that turgor of CEX leaves is maintained by physiological adjustment at the shoot level. In this process, adjusting ΔΨt is indispensable for ensuring an adequate water supply to the shoot tip. In further examinations into ΔΨ, the water in the stem that could behave as a buffer for water use in CEX and OLD leaves should be considered. If the buffering effect is large, water transport to CEX leaves will occur even when the Ψleaf of OLD leaves is lower than that in CEX leaves. Thus, it is not necessary for the Ψleaf of OLD leaves to be high for water flow to CEX leaves. However, in the shoots of Quercus in this study, Ψleaf of OLD leaves was higher or similar to that of CEX leaves. Thus, water buffer is not required for water supply to CEX leaves. Also, it is possible that CEX and OLD leaves use a different xylem conduit in the branch. Farther studies including a mathematical modelling are required.
CONCLUSIONS
Ψmidday of OLD leaves did not decrease below Ψtlp of CEX leaves despite the much lower Ψtlp in OLD leaves in both temperate and tropical areas. This small decrease in Ψmidday in OLD leaves did not result from stomatal closure and was not affected by removal of the current-year shoot. The results suggest that the conservative decrease in Ψmidday in OLD leaves contributes to the maintenance of a water potential gradient in the shoots of evergreen trees.
ACKNOWLEDGEMENTS
We thank Mr Budianjo and Mr Sugiri for their assist with our measurements at the Treub Laboratory. We also thank Dr H. Simblon, Dr S. Yuni and Mr M. Mansur in the herbarium of Indonesian Institute of Science (LIPI) in Bogor, Indonesia, Prof. T. Kohyama and the members of his laboratory of Hokkaido University, and the members of the Plant Ecophysiology Laboratory of Osaka University. We thank to Prof. Nancy Dengler and two anonymous reviewers for evaluating our new insight into the behavior of OLD leaves. This study was funded by the Ministry of Education, Culture, Sports, Science and Technology, Japan (No. 14255003).
LITERATURE CITED
- Borchert R. Water status and development of tropical trees during seasonal drought. Trees. 1994;8:115–125. doi: 10.1093/treephys/14.3.299. [DOI] [PubMed] [Google Scholar]
- Chapotin SM, Holbrook NM, Morse SR, Gutiérrez MV. Water relations of tropical dry forest flowers: pathways for water entry and the role of extracellular polysaccharides. Plant, Cell and Environment. 2003;26:623–630. [Google Scholar]
- Davies WJ, Tardieu F, Trejo CL. How do chemical signals work in plants that grow in drying soil? Plant Physiology. 1994;104:309–314. doi: 10.1104/pp.104.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon HH, Joly J. On the ascent of sap. Annals of Botany. 1894;8:468–470. [Google Scholar]
- Funada R, Kubo T, Tabuchi M, Sugiyama T, Fushitani M. Seasonal variations in endogenous indole-3-acetic acid and abscisic acid in the cambial region of Pinus densiflora Sieb. et Zucc. stems in relation to early wood–latewood transition and cessation of tracheid production. Holzforschung. 2001;55:128–134. [Google Scholar]
- Hatta H, Mujahidin, Gumilang AR, Fijridiyanto IA, Hashiba K, Darnaedi D. Phenology and growth habits of tropical trees: long-term observations in the Bogor and Cibodas Botanic Gardens, Indonesia. In: Hatta H, Darnaedi D, editors. Phenology and growth habits of tropical trees. No. 30. National Science Museum Monographs; 2005. pp. 15–59. [Google Scholar]
- Hayashi T, Takagi S. Ca2+ dependent cessation of cytoplasmic streaming induced by hypertonic treatment in Vallisneria mesophyll cells: possible role of cell wall–plasma membrane adhesion. Plant and Cell Physiology. 2003;44:1027–1036. doi: 10.1093/pcp/pcg123. [DOI] [PubMed] [Google Scholar]
- Heidecker M, Wegner LH, Binder K-A, Zimmermann U. Turgor pressure changes trigger characteristic changes in the electrical conductance of the tonoplast and the plasmalemma of the marine alga Valonia utricularis. Plant, Cell and Environment. 2003;26:1035–1051. [Google Scholar]
- Hinckley TM, Lassoie JP, Running SW. Temporal and spatial variations in the water status of forest trees. Forest Science Monograph. 1978;20:1–72. [Google Scholar]
- Ikeda T, Suzaki T. Distribution of xylem resistance to water flow in stems and branches of hardwood species. Journal of the Japanese Forestry Society. 1984;66:229–236. [Google Scholar]
- Kikuzawa K. Leaf survival of woody plants in deciduous broad-leaved forests. 1· Tall trees. Canadian Journal of Botany. 1982;61:2133–2139. [Google Scholar]
- Lang A. Xylem, phloem and transpiration flows in developing apple fruits. Journal of Experimental Botany. 1990;41:645–651. [Google Scholar]
- Lang A, Thorpe MR. Xylem, phloem and transpiration flows in a grape: application of a technique for measuring the volume of attached fruits to high resolution using Archimedes' principle. Journal of Experimental Botany. 1989;40:1069–1078. [Google Scholar]
- Maruyama Y, Morikawa Y. Measurement of leaf water relations using the pressure-volume technique. Journal of the Japanese Forestry Society. 1983;65:23–28. [Google Scholar]
- Miyazawa S-I, Suzuki AA, Sone K, Terashima I. Relationships between light, leaf nitrogen and nitrogen remobilization in the crowns of mature evergreen Quercus glauca trees. Tree Physiology. 2004;24:1157–1164. doi: 10.1093/treephys/24.10.1157. [DOI] [PubMed] [Google Scholar]
- Nobel PS, Andrade JL, Wang N, North GB. Water potentials for developing cladodes and fruits of a succulent plant, including xylem-versus-phloem implications for water movement. Journal of Experimental Botany. 1994;45:1801–1807. [Google Scholar]
- Parker WM, Pallardy SG, Hinckley TM, Teskey RO. Seasonal changes in tissue water relations of three woody species of the Quercus–Carya forest type. Ecology. 1982;63:1259–1267. [Google Scholar]
- Pavlik BM. Seasonal changes of osmotic pressure, symplasmic water content and tissue elasticity in the blades of dune grasses growing in situ along the coast of Oregon. Plant, Cell and Environment. 1984;7:531–539. [Google Scholar]
- Pepin S, Livingston NJ, Whitehead D. Responses of transpiration and photosynthesis to reversible changes in photosynthetic foliage area in western red cedar (Thuja plicata) seedlings. Tree Physiology. 2002;22:363–371. doi: 10.1093/treephys/22.6.363. [DOI] [PubMed] [Google Scholar]
- Reich PB, Borchert R. Water stress and tree phenology in a tropical dry forest in the lowland of Costa Rica. Journal of Ecology. 1984;72:61–74. [Google Scholar]
- Roberts SW, Strain BR, Knoerr KR. Seasonal patterns of leaf water relations in four co-occurring forest tree species: parameters from pressure–volume curves. Oecologia. 1980;46:330–337. doi: 10.1007/BF00346260. [DOI] [PubMed] [Google Scholar]
- Saito T, Tanaka T, Tanabe H, Matsumoto Y, Morikawa Y. Variations in transpiration rate and leaf cell turgor maintenance in saplings of deciduous broad-leaved tree species common in cool temperate forests in Japan. Tree Physiology. 2003;23:59–66. doi: 10.1093/treephys/23.1.59. [DOI] [PubMed] [Google Scholar]
- Saito T, Terashima I. Reversible decreases in the bulk elastic modulus of mature leaves of deciduous Quercus species subjected to two drought treatments. Plant, Cell and Environment. 2004;27:863–875. [Google Scholar]
- Saito T, Soga K, Hoson T, Terashima I. The bulk elastic modulus and the reversible properties of cell walls in developing Quercus leaves. Plant and Cell Physiology. 2006;47:715–725. doi: 10.1093/pcp/pcj042. [DOI] [PubMed] [Google Scholar]
- Scholander PF, Hammel HT, Bradstreet ED, Hemmingsen EA. Sap pressure in vascular plants. Science. 1965;148:339–346. doi: 10.1126/science.148.3668.339. [DOI] [PubMed] [Google Scholar]
- Sobrado MA. Aspects of tissue water relations and seasonal changes of leaf water potential components of evergreen and deciduous species coexisting in tropical dry forests. Oecologia. 1986;68:413–416. doi: 10.1007/BF01036748. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. 3rd edn. New York, NY: W. H. Freeman and Co.; 1995. [Google Scholar]
- Trolinder NL, McMichael BL, Upchurch DR. Water relations of cotton flower petals and fruit. Plant, Cell and Environment. 1993;16:755–760. [Google Scholar]
- Turner NC. Measurement of plant water status by the pressure chamber technique. Irrigation Science. 1988;9:289–308. [Google Scholar]
- Tyree MT, Cheung YNS, Macgregor ME, Talbot AJB. The characteristics of seasonal and ontogenetic changes in the tissue-water relations of Acer, Populus, Tsuga, and Picea. Canadian Journal of Botany. 1978;56:635–647. [Google Scholar]
- Tyree MT, Jarvis PG. Water in tissues and cells. In: Lange OL, Novel PS, Osmond CB, Ziegler H, editors. Encyclopedia of plant physiology. Vol. 12. Berlin: Springer-Verlag; 1982. pp. 36–77. (New Series). Part B. [Google Scholar]
- Whitehead D, Livingston NJ, Kelliher FM, Hogan KP, Pepin S, Mcseveny TM, et al. Responses of transpiration and photosynthesis to a transient change in illuminated foliage for a Pinus radiata D. Don tree. Plant, Cell and Environment. 1996;19:949–957. [Google Scholar]