Summary
Dendritic cells (DCs) orchestrate a repertoire of immune responses that endow resistance to infection and tolerance to self. DC plasticity and subsets are prominent determinants of the quality of elicited immune responses. Different DC subsets display different receptors and surface molecules, and express different sets of cytokines/chemokines, all of which lead to distinct immunological outcomes. Recent findings on human DC subsets and their functional specialization have provided insights for the design of novel human vaccines.
The challenges of protective immunity
Generating the right type of immune response can be a matter of life and death. In leprosy, for instance, the tuberculoid form of the disease is characterized by a Type 1 response which keeps the disease in check, while the lepromatous form induces an often fatal Type 2 response.1 Generating the “right” immune response requires the participation of both the innate and the adaptive immune systems. DCs decode and integrate signals obtained from the innate immune system, and ferry this information to the adaptive immune cells, i.e., T and B cells.2, 3 Microbiologists, spearheaded by Louis Pasteur have devised ways to generate vaccines by inactivating pathogens. Most if not all of these vaccines elicit protective humoral immune responses. However, there are still many pathogens for which no efficient vaccines are available, including HIV, Hepatitis C virus, malaria, and Mycobacteria. Most of these cause chronic diseases, where strong cellular immunity in particular cytotoxic T cell response is critical for the clearance of pathogens. Thus, a better understanding of the mechanisms leading to strong cellular immunity is necessary for rational, rather than empirical, design of improved vaccines. A better knowledge of human DCs will be essential to reach this goal.
Biology of Dendritic Cells
The initiation of T-cell immunity faces several challenges which include: low frequency of microbe-specific T cells, limited number of specific peptide-MHC complexes presented by the infected cells (one hundred or less per cell), and lack of co-stimulatory molecules expression on the infected cells. These challenges, however, are overcome by DCs. DCs are viewed as mobile sentinels that collect antigen from peripheral tissues and carry them to secondary lymphoid organs to activate specific T cells. This is facilitated by their activation, i.e., upregulation of co-stimulatory molecules and chemokine receptors in response to microbial components and/or inflammatory cytokines secreted by cells in tissue environment. In addition, as shown recently, soluble antigens can also directly reach lymph node-resident DCs by diffusion through lymphatics and conduits.4 DCs are also important in launching humoral immunity partly due to their capacity to directly activate B cells.5, 6 There, myeloid DCs have been shown to deliver captured antigens into non-degradable compartments and then present unprocessed antigens to B cells.7, 8
In addition to the ability to recognize and eliminate what is foreign or aberrant, the immune system has built-in tolerance mechanisms to ignore components of “self”.9 DCs appear to be essential in maintenance of immunological tolerance both in the thymus and in the periphery.10 Thus, alterations in DC biology trigger the development of autoimmune diseases, such as systemic lupus erythematosus.11
Dendritic cell subsets
DC system consists of two main subsets: the myeloid DCs (mDCs) and the plasmacytoid DCs (pDCs).
Human pDCs circulate in the blood and enter lymphoid organs through high endothelial venules (HEV).12 They can be identified as linnegHLA-DR+ cells expressing high levels of IL-3Rα chain (CD123). pDC also express some specific markers such as BDCA-2 and ILT-7.13 They express a different set of toll-like receptors (TLRs) from mDCs.14 In particular, pDC recognize viral components through TLR7 and TLR9, and secrete large amounts of Type I IFN.12
mDCs consist of subsets with different functions. For example, two mDC subsets present in mouse spleen induce different types of T cell responses. There, CD8α+ DCs induce Type 1 responses, while CD8α− DCs induce Type 2 responses.15, 16 A recent study further demonstrated that CD8α+ DCs preferentially induce antigen-specific CD8+ T cell immunity, while CD8α− DCs preferentially induce antigen-specific CD4+ T cell immunity.17 Accordingly, CD8α+ mDCs and CD8α− mDCs were found to express preferential genes involved in MHC Class I and Class II presentation, respectively.17
Human mDCs can be subcategorized into three components according to their localization: 1) peripheral tissue-resident, 2) secondary lymphoid organ-resident, and 3) circulating blood mDCs. Mouse studies have indicated that lymph node-resident DCs are involved in the maintenance of tolerance in the steady state.4 Upon microbial invasion, they rapidly capture microbial antigens delivered through lymphatics and conduits, and induce the activation and proliferation of antigen-specific T cells.4 Whether these observations hold true to human lymph node-resident DCs remains to be addressed.
DC subsets in human skin
At least three different mDC subsets localize in human skin. Langerhans cells (LCs) reside in epidermis, while dermis displays CD1a+ DCs and CD14+ DCs (Figure 1).18 Epidermal LCs and dermal CD14+ DCs express different sets of molecules. In particular, CD14+ DCs express a large number of surface C-type lectins including DC-SIGN, DEC-205, LOX-1, CLEC-6, Dectin-1 and DCIR. In contrast, LCs express the lectins Langerin and DCIR. Furthermore, dermal CD14+ DCs express variable TLRs recognizing bacterial PAMPs, such as TLR2, 4, 5, 6, 8, and 10.19, 20 Therefore, dermal CD14+ DC may represent a DC subset specialized for bacterial recognition in the skin. LCs have been reported to express TLR1, 2, 3, 6, and 10.19, 21 However, our own data using microarray of highly purified LCs failed to show much TLR expression.22 Thus, additional studies will be necessary.
Figure 1. Human skin mDC subsets.
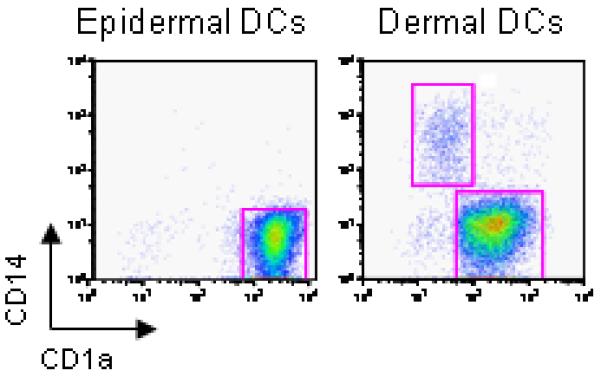
Epidermal- and dermal-resident DCs were allowed to migrate from their respective tissues and were harvested after 2 days. The cells were enriched with a Ficoll-diatrizoate gradient, stained with CD1a and CD14 mAbs and analyzed by flow cytometry. Epidermal sheets yielded CD1ahiCD14− cells. Dermis yielded two distinct populations: CD1a−CD14+ cells (dermal CD14+ DCs) and CD1adimCD14− cells (dermal CD1a+ DCs).
The two mDC subsets can also be generated in vitro by culturing CD34+-hematopoietic progenitor cells (HPCs), with GM-CSF and TNF-α̣. 23 While CD14+ DCs, both in vitro-generated and ex vivo-isolated, produce a large set of soluble factors including IL-1β, IL-6, IL-8, IL-10, IL-12, GM-CSF, MCP and TGF-β in response to stimulation via CD40, LCs produce only a few cytokines with a notable exception being the CD8+ T cell- and NK cell-enhancing cytokine IL-15 (Figure 2). The functional differences of these DC subsets in the regulation of immune responses might be largely due to the difference in their cytokine production profiles as discussed hereunder.
Figure 2. Skin DC subsets different sets of cytokines.
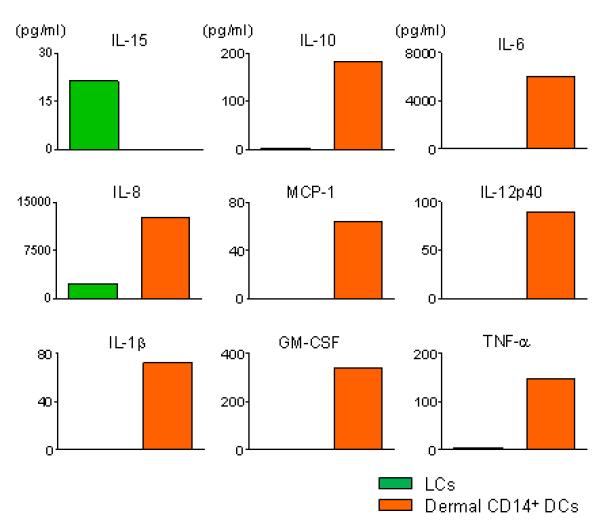
Isolated skin DC subsets were stimulated with CD40L for 24 h, and the secreted cytokine/chemokines were measured.
Dermal CD14+ DCs: potent inducers of Tfh cells
More than a decade ago, CD34+ HPCs-derived CD14+ DCs were found to induce CD40-activated naïve B cells to produce large amounts of IgM via the secretion of IL-6 and IL-12,24 while LCs lacked this capacity. We have recently shown that CD14+ DCs induce naïve T cells to differentiate into cells with properties of T follicular helper cells (Tfh).25 Thus, CD4+ T cells primed by CD14+ DCs, both in vitro-generated and ex vivo-isolated, are able to induce naïve B cells to produce much larger amounts of IgM than those primed with LCs. Most remarkably, only CD4+ T cells primed by CD14+ DCs induce naïve B cells to switch isotypes towards IgG and IgA. Furthermore, CD4+ T cells primed by CD14+ DCs secrete the chemokine CXCL13, a typical chemokine secreted by Tfh cells. Taken together, we have proposed human dermal CD14+ DCs as a DC subset specialized for the development of humoral responses.10, 25
We have recently found that activated human DCs promote the differentiation of IL-21-producing Tfh-like cells through IL-12.26 DCs activated with ligands of TLR 4, 5, and 7/8, heat-inactivated bacteria, or CD40 ligand, efficiently induce naïve CD4+ T cells to become IL-21-producers. Among DC-derived cytokines, IL-12 and to a minor extent IL-23 induced activated naïve CD4+ T cells to secrete IL-21. IL-12 is much more potent than IL-23 in this context. Interestingly, these two cytokines induce IL-21-producing CD4+ T cells through different pathways. While signal transducers and activator of transcription (STAT)4 is the critical transcriptional factor in the development of IL-21-producers by IL-12, STAT3 is more critical in their development by IL-23 and IL-21.
Importantly, naïve CD4+ T cells polarized with IL-12 induce B cells to produce Ig in a manner dependent on IL-21 and inducible costimulator (ICOS).26 Conversely, blocking IL-12 with anti IL-12p70 mAb during DC-T coculture potently inhibited naïve CD4+ T cells from becoming IL-21-producing CD4+ T cells that provide B cell help. Furthermore, IL-12 also regulates IL-21 secretion by memory CD4+ T cells. Thus, taken together with the involvement in the direct DC and B cell interaction,24 IL-12 produced by activated DCs appears to be a key cytokine for the optimal antibody responses (Figure 3).
Figure 3. IL-12, a key cytokine for the development of human humoral responses.
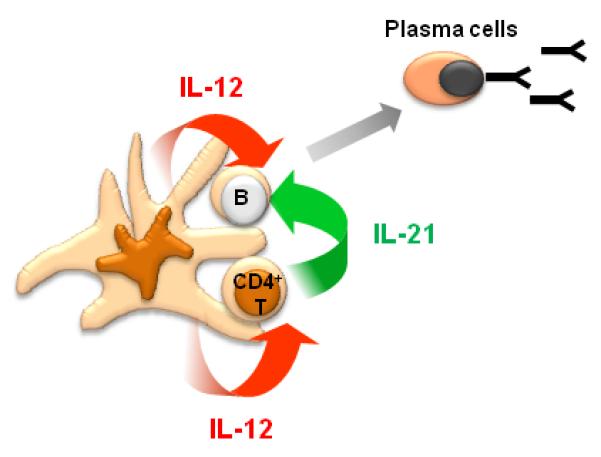
When DCs form the “ménage à trois” complex with T cells and B cells, IL-12 derived from activated DCs directly acts on naïve B cells to induce their differentiation into plasma cells, while IL-12 from DCs also induces CD4+ T cells to secrete IL-21 that promotes plasma cell differentiation.
The IL-12-IL-21 axis in human cells does not appear to be shared by mouse cells, inasmuch as several studies showed that IL-12 does not induce mouse naïve CD4+ T cells to secrete IL-21. In mice, the cytokines IL-6 and IL-21, as well as STAT3, have been reported as critical factors in the generation of IL-21-producing CD4+ T cells both in vitro and in vivo.27 Thus, the distinct mechanism in the development of IL-21-producing CD4+ T cells adds to the list of differences between the human and mouse immune systems.28
LCs: potent activators of CD8+ T cells
LCs, both in vitro-generated and ex vivo-isolated from human skin, induce more robust proliferation of naïve allogeneic CD4+ and CD8+ T cells compared to CD14+ DCs.25 Furthermore, tumor (MART-1, gp100, tyrosinase) or viral (HIV gag)-specific primary CD8+ T cell response are more efficiently induced by LCs than by CD14+ DCs loaded with peptides. LCs are also more efficient in cross-presenting peptides from protein antigens to CD8+ T cells. CD8+ T cells primed by different DC subsets differ in their quality as well. LCs prime high avidity CD8+ T cells when compared to CD14+ DCs. Furthermore, naive CD8+ T cells primed by LCs express higher levels of cytotoxic molecules, such as granzymes and perforin, than those primed by CD14+ DCs. Collectively, CD8+ T cells primed by LCs acquire more potent cytotoxicity than those primed by CD14+ DCs, and are able to efficiently kill target cells, including tumor cell lines which express peptide/HLA complex only at low levels.25
While CD14+ DCs educate naïve CD4+ T cells to become Tfh-like cells, LCs polarize naïve CD4+ T cells into cells secreting Type 2 cytokines such as IL-4, IL-5 and IL-13. This is consistent with mouse studies showing the preferential induction of Th2 responses upon delivery of an antigen to the LC-rich epidermis.29 IFN-γ-secreting CD4+ T cells are induced by human LCs and CD14+DCs at a similar level. However, further studies are necessary in order to determine whether these IFN-γ-secreting CD4+ T cells share similar characteristics. CD4+ T cells induced by LCs might be powerful helpers for the development of CTLs.
Our attempts to identify the molecular mechanisms by which LCs induce potent CTL responses have thus far only been partly conclusive. LCs, but not CD14+ DCs, express IL-15, which is known to enhance CD8+ T cell responses.30 However, none of the tested IL-15 mAbs could convincingly inhibit LCs to prime CD8+ T cells, although some of the antibodies did inhibit the function of recombinant IL-15 (Klechevsky et al. unpublished data). IL-15 added to the cultures, however, could enhance the ability of CD14+ DCs to prime naive CD8+ T cells, suggesting the possible involvement of IL-15 in potent priming by LCs.
Alternatively, CD14+ DCs may express molecules inhibiting effective CD8+ T cell priming. As discussed earlier, CD14+ DCs produce IL-10 in response to CD40-ligation. Addition of IL-10 to coculture of LC naïve CD8+ T cell, inhibited induction of effector cytotoxic granules by the primed T cells (Klechevsky; unpublished data). However, blocking IL-10 appears to be insufficient for CD14+ DCs to induce potent effector CD8+ T cells.
For many years, LCs have been viewed as a paradigm population in DC biology. Induction of potent CTL response by LCs is observed in mouse studies by subcutaneous injections of peptide-loaded epidermal LCs.31 Another recent mouse study showed that LCs actually cross present antigens to CD8+ T cells in vivo.32 In contrast, several mouse studies, for example models using herpes simplex virus (HSV), have questioned the contribution of LCs to the induction of antigen-specific responses in vivo. These studies attribute the HSV-specific immunity to CD8α+ DCs, rather than to LCs.33, 34 However, this observation might be due to the apoptosis of LCs exposed to HSV.35 Whether the differences with regard to the function of LCs between mice and humans derive from the differences in the immune systems remains to be concluded.
Humoral vs. Cellular immunity regulated by two mDC subsets
Collectively, we hypothesize that two different adaptive immunity arms, i.e., humoral and cellular arm, are preferentially regulated by different mDC subsets. Thus, while humoral immunity is preferentially regulated by CD14+ dermal DCs, cellular immunity is preferentially regulated by LCs (Figure 4). Our hypothesis is also supported by mouse study showing that dermal DCs upon activation migrate into the outer paracortex just beneath the B cell follicles, whereas LCs migrate into the T cell rich inner paracortex.36 Another human skin DC subset, dermal CD1a+ DCs are functionally intermediate between LCs and CD14+ DCs in our hands. Whether this DC subset shows a unique asset in the regulation of immune responses remains to be addressed.
Figure 4. Understanding human myeloid dendritic cell subsets for the rational design of novel DC-targeting vaccines.
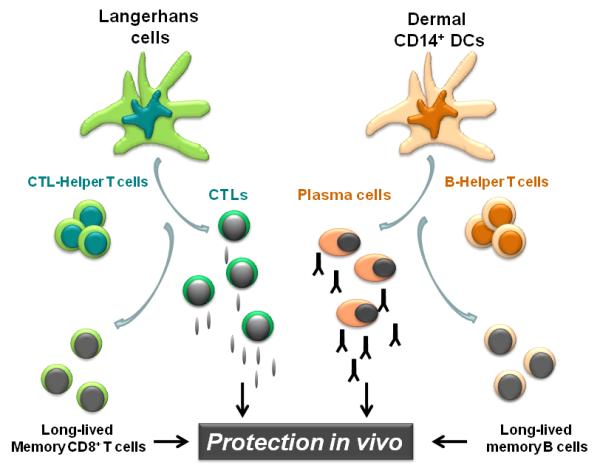
Novel vaccines that will prevent and treat chronic diseases such as HIV rely on rational immunological approaches and aim at activating both the cellular and the humoral arm. We envision that targeting antigens and activation of distinct mDC subsets, with different specializations, will result in the generation of a broad and long lived immune protection. Thus, the most efficient vaccines might be those that will target both LCs and dermal CD14+ DCs thereby allowing the maximal stimulation of cellular and humoral immune responses and the generation of long-term memory protection.
Functional specializations of Dendritic cell subsets - from bench to bedside
Translating the accumulating knowledge on DC subsets and their unique functional specializations into designs of novel vaccines is an emerging topic in the vaccine field. DC-based vaccines include two main approaches: ex-vivo generated DC vaccines, and DC targeting. Clinical trials with ex-vivo generated DC vaccines have been performed primarily in cancer. The discussion about this approach is omitted in this review, as this topic is discussed in detail in another review in the same issue (Palucka et al). Another approach is delivering antigens directly to in vivo DCs using a chimeric proteins composed of an anti-DC receptor antibody and an antigen (DC targeting).37 Our studies with human skin DC subsets 25 suggest that targeting LCs for antigen delivery will be optimal for the induction of potent antigen-specific CTL response. There, LC-specific molecule, such as Langerin, can be used as a target DC receptor.38 Dermal CD14+ DCs might be a good target for the induction of potent humoral response. There, LOX-1 or DC-SIGN expressed by the subset will serve as target DC receptors. In a DC-targeting approach, selection of a right adjuvant is also a critical parameter for the induction of the immunity of the desired type. For example, although TLR-ligands are widely considered to promote an immunity against infectious agents, a recent report has shown that TLR2-ligand indeed promotes the induction of Tregs rather than Th1 or Th17 cells.39
DCs originating from a specific tissue have the unique capacity to instruct T cells to home back to that tissue.40 Similarly, different DC subsets might also guide T cells to migrate to different sites. Furthermore, DCs activated by different adjuvants might induce T cells with different migration property. Addressing this aspect is critical for the design of vaccines, where the desired T cell migration sites are different according to diseases. For example, while vaccines against melanoma are expected to induce T cells that migrate into tumor sites including skin, vaccines against influenza virus are desired to induce T cells to migrate into airway mucosal surfaces.
Therefore, multiple parameters need to be considered for the development of DC targeting vaccines. These include: 1) biological function of target DC subsets, 2) effect of activation signals on DCs delivered through the targeted DC receptor, and 3) local and systemic effect of adjuvant. Thus, establishing biological aspects of human DC subsets will be a key to the success of the generation of novel DC-based vaccines.
Concluding remarks
Studies performed in the last decade have highlighted the commonalities and uniqueness of the various DC subsets. This new knowledge represents a fertile ground to work on to design better strategies for intervening in numerous clinical situations. The capacity of LCs and CD14+ DCs to preferentially prime cellular immunity and humoral immunity respectively has significant implications, most particularly in the context of novel human vaccines. The effective vaccines developed against a variety of infectious agents, including polio, measles and Hepatitis B, certainly represent major achievements in medicine. Yet these vaccines are all specific for acute infections and their protective capacity arises largely from their induction of humoral immune responses.41 Given both the methods by which these vaccines are delivered and the data discussed here, it is likely that they principally deliver antigen to and activate CD14+ DCs and possibly CD1a+ DCs but not LCs. Therefore, targeting LCs will be important for the design of vaccines that aim at eliciting strong cellular immunity. Such vaccines might be particularly useful at preventing, and perhaps even treating, chronic diseases including viral (HIV, Hepatitis C Virus), bacterial (mycobacteria) and parasitic (malaria) diseases, as well as cancer.42 The most efficient vaccines might actually be those that will target both CD14+ DCs and LCs, thereby allowing the maximal stimulation of both humoral and cellular immune responses (Figure 4). In this regard it is intriguing to consider that one of the most effective vaccines, smallpox vaccine,43 acts through a combination of strong cellular and humoral immunity and requires scarification of the skin, a procedure that injures both epidermis and dermis and that is likely to mobilize and activate LCs as well as dermal DCs. Likewise, one of the most potent vaccines ever generated against Yellow Fever (YF17D) activate multiple dendritic cell subsets 44 and leads to integrated immune response that include both humoral and cellular immunity.45
We foresee that the translation of this new knowledge on how DCs regulate the immune system into medicine will result in a wealth of new treatments that target DCs to improve the quality of life of many patients. We foresee that the improved vaccines that target DCs will permit us to treat and prevent many chronic diseases, and likewise, manipulation of DCs will also permit to dampen overly enhanced immune responses as occurs in allergy and autoimmunity possibly by turning on regulatory mechanisms.
References
- 1.Yamamura M, et al. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science (New York, N.Y. 1991;254:277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 4.Itano AA, et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 5.Jego G, Pascual V, Palucka AK, Banchereau J. Dendritic cells control B cell growth and differentiation. Curr Dir Autoimmun. 2005;8:124–139. doi: 10.1159/000082101. [DOI] [PubMed] [Google Scholar]
- 6.Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science (New York, N.Y. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- 7.Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol. 2009;9:15–27. doi: 10.1038/nri2454. [DOI] [PubMed] [Google Scholar]
- 8.Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23:503–514. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annual review of immunology. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 10.Ueno H, et al. Dendritic cell subsets in health and disease. Immunol Rev. 2007;219:118–142. doi: 10.1111/j.1600-065X.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- 11.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science (New York, N.Y. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 12.Siegal FP, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science (New York, N.Y. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 13.Cao W, et al. Plasmacytoid dendritic cell-specific receptor ILT7-Fc epsilonRI gamma inhibits Toll-like receptor-induced interferon production. J Exp Med. 2006;203:1399–1405. doi: 10.1084/jem.20052454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadowaki N, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maldonado-Lopez R, et al. CD8alpha+ and CD8alpha− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pulendran B, et al. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci U S A. 1999;96:1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dudziak D, et al. Differential antigen processing by dendritic cell subsets in vivo. Science (New York, N.Y. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 18.Valladeau J, Saeland S. Cutaneous dendritic cells. Semin Immunol. 2005;17:273–283. doi: 10.1016/j.smim.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 19.van der Aar AM, et al. Loss of TLR2, TLR4, and TLR5 on Langerhans cells abolishes bacterial recognition. J Immunol. 2007;178:1986–1990. doi: 10.4049/jimmunol.178.4.1986. [DOI] [PubMed] [Google Scholar]
- 20.Klechevsky E, et al. Understanding human myeloid dendritic cell subsets for the rational design of novel vaccines. Human immunology. 2009;70:281–288. doi: 10.1016/j.humimm.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flacher V, et al. Human Langerhans cells express a specific TLR profile and differentially respond to viruses and Gram-positive bacteria. J Immunol. 2006;177:7959–7967. doi: 10.4049/jimmunol.177.11.7959. [DOI] [PubMed] [Google Scholar]
- 22.Klechevsky E, et al. Understanding human myeloid dendritic cell subsets for the rational design of novel vaccines. Human immunology. 2009 doi: 10.1016/j.humimm.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caux C, et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF alpha. J Exp Med. 1996;184:695–706. doi: 10.1084/jem.184.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caux C, et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor alpha: II. Functional analysis. Blood. 1997;90:1458–1470. [PubMed] [Google Scholar]
- 25.Klechevsky E, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmitt N, et al. Human Dendritic Cells Induce the Differentiation of Interleukin-21-producing T Follicular Helper-like Cells through Interleukin-12. Immunity. 2009 doi: 10.1016/j.immuni.2009.04.016. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou L, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nature immunology. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 28.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 29.Alvarez D, et al. Cutaneous antigen priming via gene gun leads to skin-selective Th2 immune-inflammatory responses. J Immunol. 2005;174:1664–1674. doi: 10.4049/jimmunol.174.3.1664. [DOI] [PubMed] [Google Scholar]
- 30.Oh S, Perera LP, Burke DS, Waldmann TA, Berzofsky JA. IL-15/IL-15Ralpha-mediated avidity maturation of memory CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:15154–15159. doi: 10.1073/pnas.0406649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Celluzzi CM, Falo LD., Jr. Epidermal dendritic cells induce potent antigen-specific CTL-mediated immunity. J Invest Dermatol. 1997;108:716–720. doi: 10.1111/1523-1747.ep12292095. [DOI] [PubMed] [Google Scholar]
- 32.Stoitzner P, et al. Langerhans cells cross-present antigen derived from skin. Proc Natl Acad Sci U S A. 2006;103:7783–7788. doi: 10.1073/pnas.0509307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao X, et al. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J Exp Med. 2003;197:153–162. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allan RS, et al. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science (New York, N.Y. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 35.Bosnjak L, et al. Herpes simplex virus infection of human dendritic cells induces apoptosis and allows cross-presentation via uninfected dendritic cells. J Immunol. 2005;174:2220–2227. doi: 10.4049/jimmunol.174.4.2220. [DOI] [PubMed] [Google Scholar]
- 36.Kissenpfennig A, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Caminschi I, Lahoud MH, Shortman K. Enhancing immune responses by targeting antigen to DC. Eur J Immunol. 2009;39:931–938. doi: 10.1002/eji.200839035. [DOI] [PubMed] [Google Scholar]
- 38.Idoyaga J, et al. Cutting Edge: Langerin/CD207 Receptor on Dendritic Cells Mediates Efficient Antigen Presentation on MHC I and II Products In Vivo. J Immunol. 2008;180:3647–3650. doi: 10.4049/jimmunol.180.6.3647. [DOI] [PubMed] [Google Scholar]
- 39.Manicassamy S, et al. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nature medicine. 2009;15:401–409. doi: 10.1038/nm.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mora JR, et al. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 41.Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006;124:849–863. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 42.Letvin Correlates of Immune protection and the Development of Human Immunodeficiency Virus Vaccine. Immunity. 2007;27:366–369. doi: 10.1016/j.immuni.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Frey SE, et al. Dose-related effects of smallpox vaccine. The New England journal of medicine. 2002;346:1275–1280. doi: 10.1056/NEJMoa013431. [DOI] [PubMed] [Google Scholar]
- 44.Querec T, et al. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med. 2006;203:413–424. doi: 10.1084/jem.20051720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaucher D, et al. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med. 2008;205:3119–3131. doi: 10.1084/jem.20082292. [DOI] [PMC free article] [PubMed] [Google Scholar]


