Abstract
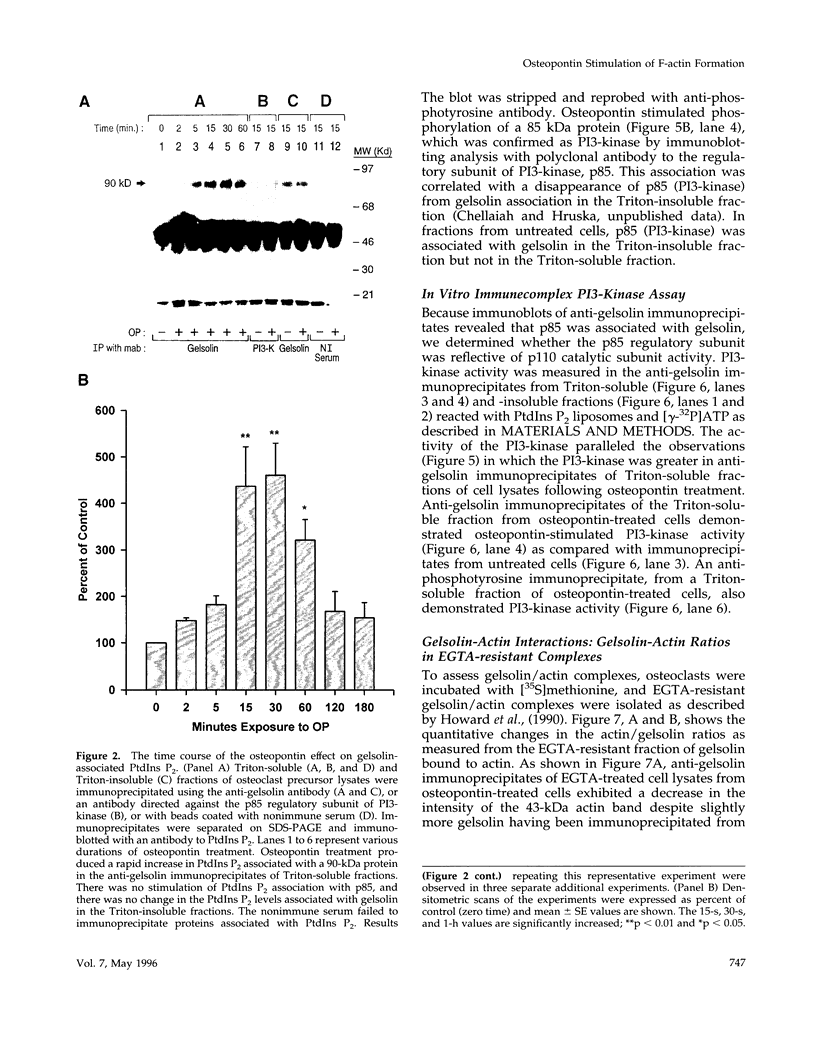
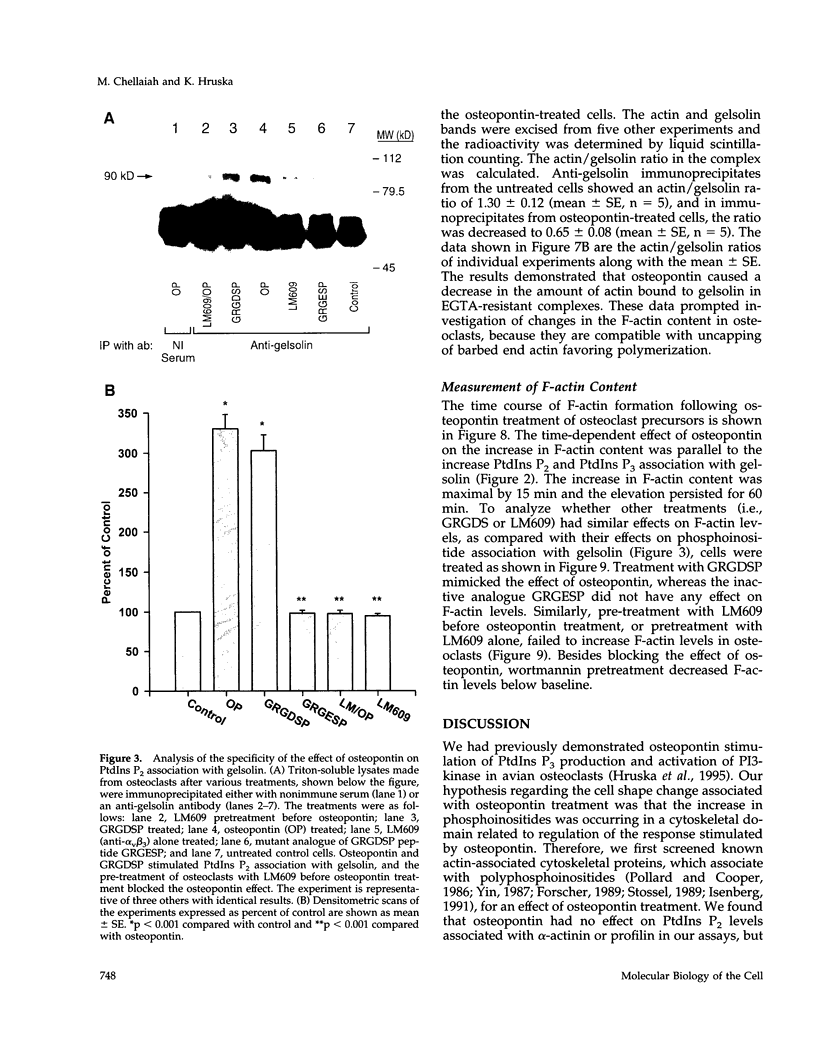
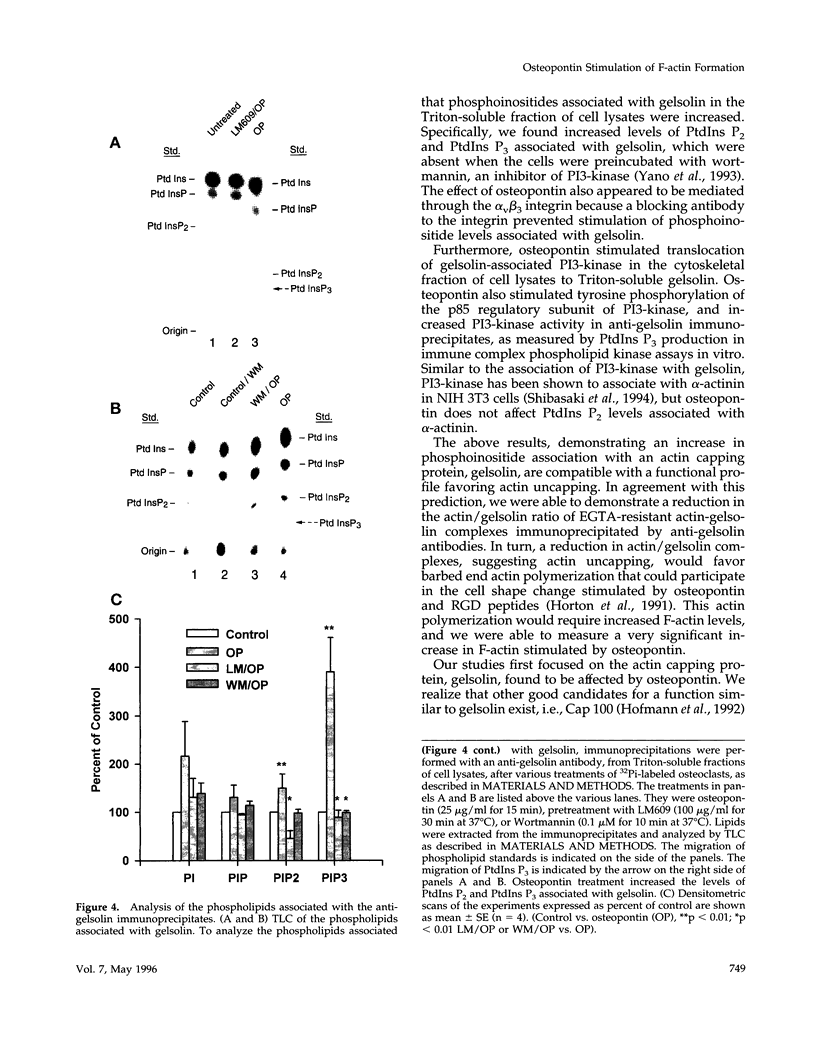
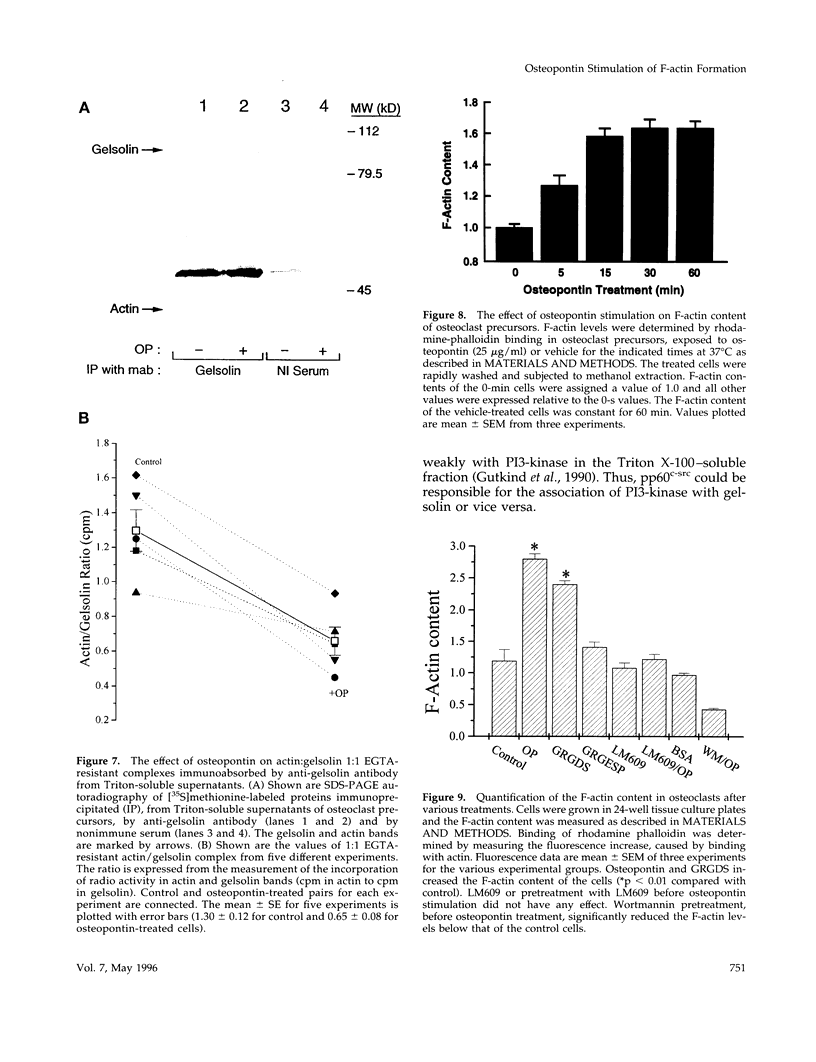
Based on previous studies demonstrating activation of phosphatidylinositol 3-hydroxyl kinase (PI3-kinase) and stimulation of a change in cell shape, we examined the effect of osteopontin on the association of phospholipids with gelsolin, an actin-capping/severing protein. Osteopontin stimulated a rapid increase in phosphatidylinositol bisphosphate and phosphatidylinositol triphosphate levels associated with gelsolin in Triton-soluble fractions of cell lysates. The increased levels of phosphatidylinositol triphosphate associated with gelsolin were due to stimulation of PI3-kinase activity associated with gelsolin in the Triton-soluble fractions, and they were blocked by the PI3-kinase inhibitor wortmannin. Osteopontin stimulated translocation of PI3-kinase from the Triton-insoluble to Triton-soluble gelsolin. Osteopontin also decreased Triton-soluble gelsolin/actin complexes consistent with actin uncapping, and increased F-actin levels, which were also blocked by wortmannin. The osteopontin effects were mediated through binding to the alpha(v)beta 3 integrin. Taken together, our studies indicate that osteopontin/alpha(v)beta 3-mediated changes in gelsolin-associated phosphoinositide levels and PI3-kinase activity are related to stimulation of F-actin formation in osteoclasts.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez J. I., Teitelbaum S. L., Blair H. C., Greenfield E. M., Athanasou N. A., Ross F. P. Generation of avian cells resembling osteoclasts from mononuclear phagocytes. Endocrinology. 1991 May;128(5):2324–2335. doi: 10.1210/endo-128-5-2324. [DOI] [PubMed] [Google Scholar]
- Boulikas T., Kong C. F. Multitude of inverted repeats characterizes a class of anchorage sites of chromatin loops to the nuclear matrix. J Cell Biochem. 1993 Sep;53(1):1–12. doi: 10.1002/jcb.240530802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaponnier C., Yin H. L., Stossel T. P. Reversibility of gelsolin/actin interaction in macrophages. Evidence of Ca2+-dependent and Ca2+-independent pathways. J Exp Med. 1987 Jan 1;165(1):97–106. doi: 10.1084/jem.165.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong L. D., Traynor-Kaplan A., Bokoch G. M., Schwartz M. A. The small GTP-binding protein Rho regulates a phosphatidylinositol 4-phosphate 5-kinase in mammalian cells. Cell. 1994 Nov 4;79(3):507–513. doi: 10.1016/0092-8674(94)90259-3. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Carter A. N. Phosphoinositide 3-kinase: a new effector in signal transduction? Cell Signal. 1991;3(6):501–513. doi: 10.1016/0898-6568(91)90027-r. [DOI] [PubMed] [Google Scholar]
- Forscher P. Calcium and polyphosphoinositide control of cytoskeletal dynamics. Trends Neurosci. 1989 Nov;12(11):468–474. doi: 10.1016/0166-2236(89)90098-2. [DOI] [PubMed] [Google Scholar]
- Grondin P., Plantavid M., Sultan C., Breton M., Mauco G., Chap H. Interaction of pp60c-src, phospholipase C, inositol-lipid, and diacyglycerol kinases with the cytoskeletons of thrombin-stimulated platelets. J Biol Chem. 1991 Aug 25;266(24):15705–15709. [PubMed] [Google Scholar]
- Gutkind J. S., Lacal P. M., Robbins K. C. Thrombin-dependent association of phosphatidylinositol-3 kinase with p60c-src and p59fyn in human platelets. Mol Cell Biol. 1990 Jul;10(7):3806–3809. doi: 10.1128/mcb.10.7.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss S. G., Cooper J. A. Regulation of CapZ, an actin capping protein of chicken muscle, by anionic phospholipids. Biochemistry. 1991 Sep 10;30(36):8753–8758. doi: 10.1021/bi00100a006. [DOI] [PubMed] [Google Scholar]
- Hofmann A., Eichinger L., André E., Rieger D., Schleicher M. Cap100, a novel phosphatidylinositol 4,5-bisphosphate-regulated protein that caps actin filaments but does not nucleate actin assembly. Cell Motil Cytoskeleton. 1992;23(2):133–144. doi: 10.1002/cm.970230206. [DOI] [PubMed] [Google Scholar]
- Holtrop M. E., King G. J. The ultrastructure of the osteoclast and its functional implications. Clin Orthop Relat Res. 1977 Mar-Apr;(123):177–196. [PubMed] [Google Scholar]
- Horton M. A., Taylor M. L., Arnett T. R., Helfrich M. H. Arg-Gly-Asp (RGD) peptides and the anti-vitronectin receptor antibody 23C6 inhibit dentine resorption and cell spreading by osteoclasts. Exp Cell Res. 1991 Aug;195(2):368–375. doi: 10.1016/0014-4827(91)90386-9. [DOI] [PubMed] [Google Scholar]
- Howard T., Chaponnier C., Yin H., Stossel T. Gelsolin-actin interaction and actin polymerization in human neutrophils. J Cell Biol. 1990 Jun;110(6):1983–1991. doi: 10.1083/jcb.110.6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska K. A., Rolnick F., Huskey M., Alvarez U., Cheresh D. Engagement of the osteoclast integrin alpha v beta 3 by osteopontin stimulates phosphatidylinositol 3-hydroxyl kinase activity. Endocrinology. 1995 Jul;136(7):2984–2992. doi: 10.1210/endo.136.7.7540546. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Nomura S., Yamaguchi A., Suda T., Yoshiki S. In situ hybridization of bone matrix proteins in undecalcified adult rat bone sections. J Histochem Cytochem. 1992 Aug;40(8):1079–1088. doi: 10.1177/40.8.1619274. [DOI] [PubMed] [Google Scholar]
- Isenberg G. Actin binding proteins--lipid interactions. J Muscle Res Cell Motil. 1991 Apr;12(2):136–144. doi: 10.1007/BF01774032. [DOI] [PubMed] [Google Scholar]
- Jackson T. R., Stephens L. R., Hawkins P. T. Receptor specificity of growth factor-stimulated synthesis of 3-phosphorylated inositol lipids in Swiss 3T3 cells. J Biol Chem. 1992 Aug 15;267(23):16627–16636. [PubMed] [Google Scholar]
- Janmey P. A., Stossel T. P. Modulation of gelsolin function by phosphatidylinositol 4,5-bisphosphate. Nature. 1987 Jan 22;325(6102):362–364. doi: 10.1038/325362a0. [DOI] [PubMed] [Google Scholar]
- Kouns W. C., Fox C. F., Lamoreaux W. J., Coons L. B., Jennings L. K. The effect of glycoprotein IIb-IIIa receptor occupancy on the cytoskeleton of resting and activated platelets. J Biol Chem. 1991 Jul 25;266(21):13891–13900. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lassing I., Lindberg U. Specific interaction between phosphatidylinositol 4,5-bisphosphate and profilactin. Nature. 1985 Apr 4;314(6010):472–474. doi: 10.1038/314472a0. [DOI] [PubMed] [Google Scholar]
- Lassing I., Lindberg U. Specificity of the interaction between phosphatidylinositol 4,5-bisphosphate and the profilin:actin complex. J Cell Biochem. 1988 Jul;37(3):255–267. doi: 10.1002/jcb.240370302. [DOI] [PubMed] [Google Scholar]
- Marchisio P. C., Cirillo D., Naldini L., Primavera M. V., Teti A., Zambonin-Zallone A. Cell-substratum interaction of cultured avian osteoclasts is mediated by specific adhesion structures. J Cell Biol. 1984 Nov;99(5):1696–1705. doi: 10.1083/jcb.99.5.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchisio P. C., Cirillo D., Teti A., Zambonin-Zallone A., Tarone G. Rous sarcoma virus-transformed fibroblasts and cells of monocytic origin display a peculiar dot-like organization of cytoskeletal proteins involved in microfilament-membrane interactions. Exp Cell Res. 1987 Mar;169(1):202–214. doi: 10.1016/0014-4827(87)90238-2. [DOI] [PubMed] [Google Scholar]
- Medhora M. M., Teitelbaum S., Chappel J., Alvarez J., Mimura H., Ross F. P., Hruska K. 1 alpha,25-dihydroxyvitamin D3 up-regulates expression of the osteoclast integrin alpha v beta 3. J Biol Chem. 1993 Jan 15;268(2):1456–1461. [PubMed] [Google Scholar]
- Moore M. A., Gotoh Y., Rafidi K., Gerstenfeld L. C. Characterization of a cDNA for chicken osteopontin: expression during bone development, osteoblast differentiation, and tissue distribution. Biochemistry. 1991 Mar 5;30(9):2501–2508. doi: 10.1021/bi00223a029. [DOI] [PubMed] [Google Scholar]
- Oldberg A., Franzén A., Heinegård D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8819–8823. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter R. G., Ginsberg M. Concanavalin A induces interactions between surface glycoproteins and the platelet cytoskeleton. J Cell Biol. 1982 Feb;92(2):565–573. doi: 10.1083/jcb.92.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payrastre B., van Bergen en Henegouwen P. M., Breton M., den Hartigh J. C., Plantavid M., Verkleij A. J., Boonstra J. Phosphoinositide kinase, diacylglycerol kinase, and phospholipase C activities associated to the cytoskeleton: effect of epidermal growth factor. J Cell Biol. 1991 Oct;115(1):121–128. doi: 10.1083/jcb.115.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Cooper J. A. Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu Rev Biochem. 1986;55:987–1035. doi: 10.1146/annurev.bi.55.070186.005011. [DOI] [PubMed] [Google Scholar]
- Reinholt F. P., Hultenby K., Oldberg A., Heinegård D. Osteopontin--a possible anchor of osteoclasts to bone. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4473–4475. doi: 10.1073/pnas.87.12.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki F., Fukami K., Fukui Y., Takenawa T. Phosphatidylinositol 3-kinase binds to alpha-actinin through the p85 subunit. Biochem J. 1994 Sep 1;302(Pt 2):551–557. doi: 10.1042/bj3020551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P. From signal to pseudopod. How cells control cytoplasmic actin assembly. J Biol Chem. 1989 Nov 5;264(31):18261–18264. [PubMed] [Google Scholar]
- Stossel T. P. On the crawling of animal cells. Science. 1993 May 21;260(5111):1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- Weber G. F., Ashkar S., Glimcher M. J., Cantor H. Receptor-ligand interaction between CD44 and osteopontin (Eta-1). Science. 1996 Jan 26;271(5248):509–512. doi: 10.1126/science.271.5248.509. [DOI] [PubMed] [Google Scholar]
- Whitman M., Kaplan D. R., Schaffhausen B., Cantley L., Roberts T. M. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985 May 16;315(6016):239–242. doi: 10.1038/315239a0. [DOI] [PubMed] [Google Scholar]
- Yano H., Nakanishi S., Kimura K., Hanai N., Saitoh Y., Fukui Y., Nonomura Y., Matsuda Y. Inhibition of histamine secretion by wortmannin through the blockade of phosphatidylinositol 3-kinase in RBL-2H3 cells. J Biol Chem. 1993 Dec 5;268(34):25846–25856. [PubMed] [Google Scholar]
- Yin H. L. Gelsolin: calcium- and polyphosphoinositide-regulated actin-modulating protein. Bioessays. 1987 Oct;7(4):176–179. doi: 10.1002/bies.950070409. [DOI] [PubMed] [Google Scholar]
- Zambonin-Zallone A., Teti A., Carano A., Marchisio P. C. The distribution of podosomes in osteoclasts cultured on bone laminae: effect of retinol. J Bone Miner Res. 1988 Oct;3(5):517–523. doi: 10.1002/jbmr.5650030507. [DOI] [PubMed] [Google Scholar]
- Zhang J., Fry M. J., Waterfield M. D., Jaken S., Liao L., Fox J. E., Rittenhouse S. E. Activated phosphoinositide 3-kinase associates with membrane skeleton in thrombin-exposed platelets. J Biol Chem. 1992 Mar 5;267(7):4686–4692. [PubMed] [Google Scholar]