Summary
The Pseudomonas aeruginosa PsrA autorepressor has dual roles as a repressor of the fadBA5 β-oxidation-operon and an activator of the stationary-phase sigma factor rpoS and exsCEBA-operon of the type III secretion system (TTSS). Previously, we demonstrated that the repression of the fadBA5-operon by PsrA is relieved by long-chain fatty acids (LCFA). However, the signal affecting the activation of rpoS and exsC via PsrA is unknown. In this study, microarray and gene-fusion data suggested that LCFA (e.g. oleate) affected the expression of rpoS and exsC. DNA binding studies confirmed that PsrA binds to the rpoS and exsC promoter regions. This binding was inhibited by LCFA, indicating that LCFA directly affects the activation of these two genes through PsrA. LCFA decreased rpoS and exsC expression, resulting in increased N-(butyryl)-l-homoserine-lactone quorum-sensing signal and decreased ExoS/T production, respectively. Based on the crystal structure of PsrA, site-directed mutagenesis of amino acid residues, within the hydrophobic channel thought to accommodate LCFA, created two LCFA-nonresponsive PsrA mutants. The binding and activation of rpoS and exsC by these PsrA mutants was no longer inhibited by LCFA. These data support a mechanistic model where LCFA influence PsrA regulation to control LCFA metabolism and some virulence genes in P. aeruginosa.
Introduction
Pseudomonas aeruginosa is a saprophytic opportunistic pathogen, exceptionally diverse in its spectrum of human infections. This Gram-negative bacterium causes chronic lung infections in the majority of cystic fibrosis (CF) patients (Doring, 1997; Govan and Nelson, 1992) and is one of the primary causative agents of hospital-acquired pneumonia (Bowton, 1999; Lode et al., 2000; Richards et al., 1999). A large repertoire of virulence determinants (LasA/LasB protease, alkaline protease, phospholipases, lipases, exotoxin A, type III section exoenzymes S/T/U/Y, rhamnolipid, alginate and hydrogen cyanide synthesis, and others), which likely evolved outside the human host, aid P. aeruginosa in infecting plants, animals, and other microbes (Abd et al., 2008; Matz et al., 2008; Rahme et al., 1995). It is generally accepted that virulence determinants that damage the host are expressed as a bacterial response to nutrient deprivation, illustrating the interconnection between virulence expression and nutrient acquisition (e.g. carbon sources) as a survival mechanism. One well-documented example of this nutrient deprivation mechanism relates the expression of virulence factors to iron-depleted conditions (Bollinger et al., 2001; Lamont et al., 2002). Other studies indicate the relationship between nutrient availability and virulence in P. aeruginosa (Son et al., 2007; Weir et al., 2008). While phenotypic studies have implicated various factors relating nutrient acquisition to virulence expression (Lindsey et al., 2008; O'Toole et al., 2000; Sonnleitner et al., 2003), very few specific molecular mechanisms regulating both virulence and carbon source metabolism have been elucidated in P. aeruginosa to date.
PsrA controls genes of the type III secretion system (TTSS), the stationary phase sigma factor rpoS, and type IV pilus in P. aeruginosa. PsrA, a TetR family regulator and a demonstrated autorepressor, activates expression of the TTSS exsCEBA operon in P. aeruginosa (Shen et al., 2006) (Fig. 1). Despite these findings, two recent studies have published disagreeing microarray data on psrA mutants suggesting that PsrA might repress genes in the TTSS (Gooderham et al., 2008) or that no TTSS gene regulation by PsrA was observed (Kang et al., 2008). The TTSS in P. aeruginosa is important for the secretion of exotoxins, including ExoS (Yahr et al., 1996), ExoT (Shafikhani and Engel, 2006; Yahr et al., 1997), ExoU (Finck-Barbancon et al., 1997), and ExoY (Yahr et al., 1998), directly into eukaryotic cells upon contact. As a result, the unidentified signal(s) recognized by PsrA for TTSS regulation have been proposed to play an important role during infection (Shen et al., 2006). PsrA was also shown to be an activator of rpoS (Kojic and Venturi, 2001; Kojic et al., 2002) (Fig. 1), encoding for a stationary phase sigma factor evidenced to regulate quorum sensing, virulence, and several hundred stationary phase genes (Schuster et al., 2004). Moreover, a model for the regulation of rpoS and psrA involving an unidentified signal in late-log or early-stationary phase was proposed (Kojic et al., 2002). Additional microarray data indicated that type IV pili genes (PA4297-PA4306) could be activated by PsrA (Gooderham et al., 2008; Kang et al., 2008), potentially contributing to swarming motility and virulence in the CF lung (Havasi et al., 2008; Köler et al., 2000). Besides controlling virulence expression, PsrA and long-chain fatty acid (LCFA) signals have recently been shown to act as repressor-inducer partners to regulate genes for fatty acid degradation (Fad) (Kang et al., 2008). Previous in vivo microarray data revealed that psrA and fad genes were expressed in the CF lung (Son et al., 2007), suggesting that P. aeruginosa may concomitantly sense and utilize LCFAs as signal molecules and nutrient sources in CF infections. The ability of PsrA to regulate genes encoding virulence factors, motility, and nutrient metabolism, potentially in response to environmental signals such as LCFA, begs further investigation into the interconnection of pathogenic and metabolic processes in P. aeruginosa.
Fig. 1.
Model of the PsrA regulation of fadBA5, psrA, rpoS and exsC. PsrA acts as both a repressor and activator by repressing itself and the fadBA5 β-oxidation operon while activating genes for the stationary-phase sigma factor rpoS and the type III secretion system exsC. LCFA has been shown to reverse this mechanism by derepressing the fadBA5-operon, yet LCFA influence in modulating the expression of psrA, rpoS, and exsC genes remains enigmatic.
In an earlier study, we demonstrated that PsrA directly repressed specific Fad genes (fadBA5), while the binding of LCFAs to PsrA inhibited repression. In this study, we investigated whether LCFA is the environmental signal sensed by PsrA which regulates psrA, rpoS, and exsC expression (Fig. 1). The microarray data, gene-fusion experiments, in vitro DNA-binding studies, and site-directed mutants of the PsrA effector binding site all supported this notion. By switching the regulation mechanism of PsrA, LCFA was shown here to control the metabolism of this nutrient and modulate some virulence determinants.
Results
The LCFA, oleate (C18:1Δ9 ), down regulates rpoS and TTSS genes shown by microarray analyses
Conflicting data in the literature regarding PsrA regulation of TTSS genes (Gooderham et al., 2008; Shen et al., 2006) and rpoS (Kang et al., 2008; Kojic and Venturi, 2001; Kojic et al., 2002), and the recent identification of LCFAs as inducer molecules (Kang et al., 2008) demands further investigation. To initially identify genes induced by LCFA, we performed microarray experiments via reciprocal comparisons of PAO1 strain grown in two conditions with identical growth rates, LB and LB + oleate (C18:1Δ9 ; an unsaturated LCFA). The complete microarray data of PAO1 grown in LB compared to LB + oleate, and that of the reverse comparison (LB + oleate versus LB), can be found online as supplementary materials (Tables S1 and S2). Table S1 indicates genes induced by LCFA. It is interesting that some genes marking anaerobic metabolism were induced in the presence of LCFA such as the nar-operon (PA3872-3879), nir-operon (PA0510-0522), nor-operon (PA0523-0525), and arc-operon (PA5170-5173). Many of these were also induced in CF sputum (Son et al., 2007). We also observed the induction of other types of virulence factors by LCFA such as the psl-operon for biofilm synthesis (PA2231-2242) and the pvd-operons for pyoverdine synthesis (PA2385-2386, 2394-2397, and PA2424-2426). Relevantly, fadB5 (PA3014), fadA5 (PA3013), and psrA (PA3006) were induced by LCFA 7.5-, 9.4-, and 2.9-fold, respectively. Supplementary Table S2 indicated several hundred genes that are down-regulated by the LCFA, oleate, including some virulence genes such as lipA (lipase) and genes of the TTSS. Table 3 summarizes genes of the TTSS that were downregulated in the presence of oleate from Table S2 online. Although not all genes involved in TTSS had expression levels ≥2-fold in our microarray data, certain genes from all five clusters of the TTSS e.g. exsC, pscE, popB, pcr1, and pscQ; Yahr and Wolfgang, 2006) were observed to have expression ≥2-fold in LB compared to LB + oleate. Importantly, we saw down-regulation of exsC, exsE, exsB, and the effector molecule exoS (Table 3). However, we suspected that the other TTSS genes had significant but less than 2-fold down-regulation. Indeed, several other TTSS genes (e.g. exoenzyme T) and rpoS exhibited slightly less than a 2-fold decrease in expression in the presence of oleate (Table 3). Overall, the microarray data indicated that the presence of LCFA decreased the expression of TTSS genes and rpoS. The exact regulatory mechanism of rpoS and TTSS genes involving LCFA is unknown, although PsrA was shown to activate rpoS and the exsCEBA-operon (Shen et al., 2006) and, more recently, that direct binding of LCFA to PsrA was demonstrated (Kang et al., 2008).
Table 3.
P. aeruginosa rpoS and type III secretion genes expressed when grown to mid-log phase in LB versus LB + oleate identified using microarrays
| Accession Number |
Gene Name |
Fold Change1 |
Description |
|---|---|---|---|
| Fold-Change ≥ 2-fold | |||
| PA1694 | pscQ | 2.1 | Yersinia YscQ homolog translocation protein in type III secretion |
| PA1699 | pcr1 | 2.5 | Yersinia TyeA conserved hypothetical protein in type III secretion |
| PA1702 | pcr4 | 2.1 | Type III secretion conserved hypothetical protein |
| PA1707 | pcrH | 2.9 | PcrH regulatory protein |
| PA1708 | popB | 2.4 | PepB translocator protein |
| PA1709 | popD | 2.4 | PopD translocator protein |
| PA1710 | exsC | 3.3 | Exoenzyme S synthesis protein C precursor |
| PA1711 | exsE | 2.7 | ExsC interacting protein |
| PA1712 | exsB | 2.7 | Exoenzyme S synthesis protein B |
| PA1718 | pscE | 2.1 | Type III export protein PscE |
| PA1722 | pscI | 2.1 | Type III export protein PscI |
| PA3841 | exoS | 2.4 | Exoenzyme S |
| Fold-change 1.2- to 1.9-fold | |||
| PA0044 | exoT | 1.4 | Exoenzyme T/ADP ribosyltransferase |
| PA1691 | pscT | 1.4 | Type III translocation protein |
| PA1692 | pscS | 1.5 | Yersinia YscS homolog Type III translocation protein |
| PA1693 | pscR | 1.3 | Type III translocation protein |
| PA1695 | pscP | 1.5 | Type III translocation protein |
| PA1696 | pscO | 1.9 | PscO translocation protein in type III secretion |
| PA1697 | pscN | 1.8 | Type III secretion system ATP synthase |
| PA1698 | popN | 1.9 | Outer membrane protein PopN |
| PA1700 | pcr2 | 1.7 | Type III secretion conserved hypothetical protein |
| PA1701 | pcr3 | 1.6 | Type III secretion conserved hypothetical protein |
| PA1703 | pcrD | 1.7 | Type III secretory apparatus protein PcrD |
| PA1704 | pcrR | 1.2 | Transcriptional regulator protein PcrR |
| PA1705 | pcrG | 1.4 | Regulator in type III secretion |
| PA1706 | pcrV | 1.7 | Type III secretion protein PcrV |
| PA1713 | exsA | 1.7 | Transcriptional activator |
| PA1715 | pscB | 1.5 | Type III export apparatus protein |
| PA1716 | pcsC | 1.7 | Type III secretion protein PscC |
| PA1717 | pscD | 1.7 | Type III export protein PscD |
| PA1719 | pscF | 1.6 | Type III export protein PscF |
| PA1720 | pscG | 1.7 | Type III export protein (Yersinia YscG homolog) |
| PA1721 | pscH | 1.6 | Type III export protein PscH |
| PA1724 | pscK | 1.9 | Type III export protein PscK |
| PA3622 | rpoS | 1.9 | Stationary-phase sigma factor |
| PA3842 | spcS | 1.9 | Exoenzyme S chaperone |
Fold change values were averaged over three different GeneChips® for each condition (LB and LB+0.2% oleate) with P-values ≤ 0.05.
Gene-fusion studies indicate LCFA induces the expression of psrA and decreases rpoS and exsC expression
To further characterize how the expression of rpoS and exsC correlates with the expression of their activator (PsrA) in the presence of LCFA and to confirm our microarray data, we monitored the expression of all three genes by gene-fusion experiments over the entire log- and early-stationary phases in P. aeruginosa strain PAO1. Since PsrA has been shown to bind to and repress its own promoter and activate the promoter of rpoS and exsC (Kojic et al., 2002; Shen et al., 2006), we constructed three fusion strains (PpsrA-lacZ, PrpoS-lacZ, and PexsC-lacZ) as a single copy on the PAO1 chromosome using the mini-CTX2 integration system (Hoang et al., 2000). While monitoring the expression of psrA, rpoS, and exsC, we observed that LCFA induces psrA and decreases rpoS and exsC expression (Fig. 2). All three fusion strains had identical growth rates in LB and LB + oleate when compared to PAO1, but strains grown in the presence of the oleate had a longer stationary-phase (Fig. 2A). Although each fusion strain had identical growth rates during the entire log-phase in both media, the gene expression profile was significantly different. PsrA expression was significantly induced by oleate, even in early-log, (Fig. 2B) and remained high throughout log-phase. This has never been observed with any environmental signal. Since PsrA has been shown to activate rpoS and exsC promoters, we expected to see increased expression of these two genes as a result of increased PsrA expression; however, the opposite scenario was observed. In the presence of LCFA, exsC expression immediately decreased and, shortly after, rpoS followed (Fig. 2C and 2D). We reasoned that the expression of autoregulator, PsrA, was induced by binding to LCFA to derepress itself (Fig. 2B), similar to how the binding of PsrA to LCFA to derepress fadBA5 in our earlier study (Kang et al., 2008). This increase in the PsrA activator alone should bind to the promoters of rpoS and exsC to activate these genes, but this was not the case. Although there was an increase in PsrA expression in the presence of oleate, the presence of excess oleate may also inhibit the activation of rpoS and exsC by PsrA. Therefore, when grown in the presence of LCFA, we predict that the PsrA-LCFA complex dissociates from the promoter regions of these two genes preventing their activation. We next performed experiments to test this hypothesis.
Fig. 2.
Growth-curve (A) and expression analysis of three PAO1 fusion strains: PpsrA-lacZ (B), PrpoS-lacZ (C), and PexsC-lacZ (D). The growth rates were identical for all fusion strains and comparable to wildtype PAO1 strain. The expression of PsrA was induced (B) and the expression of ExsC was downregulated (D) by oleate in early-log and remained so for the entire log-phase. (C) The expression of rpoS decreased by the LCFA oleate as early as mid-log and lasted until stationary-phase, at which time the expression of rpoS in both media increased significantly.
Overexpression of psrA without LCFA increases rpoS and exsC expression
To test our prediction above, we uncoupled the expression of psrA from the presence of the LCFA signal. Since PsrA represses itself and cannot be induced without LCFA, we cloned the coding region of psrA without its promoter into a low-copy P. aeruginosa vector, pUCP-Nde (Cronin and McIntire, 1999), for inducible expression with isopropyl-β-d-thiogalactopyranoside (IPTG). We introduced this psrA inducible plasmid into strain PAO1-psrA::Tn-Gmr carrying either the miniCTX2-PrpoS-lacZ or miniCTX2-PexsC-lacZ chromosomal fusion. This allowed uncoupling of i) the IPTG inducible expression of psrA in the absence of LCFA and ii) the addition of LCFA without influencing the expression of psrA. Using these two fusion strains (PAO1/psrA::Tn-Gmr/attB::miniCTX2-PrpoS-lacZ/pUCP-Nde-psrA and PAO1/psrA::Tn-Gmr/attB::miniCTX2-PexsC-lacZ/pUCP-Nde-psrA), we showed that the expression of PsrA alone in the absence of LCFA activated both rpoS and exsC, whereas the expression of PsrA in the presence of LCFA inhibits the activation of rpoS and exsC (Fig. 3A and 3B). Without psrA induction (no IPTG), leaky and low-level expression of PsrA is bound by excess LCFA added, giving an even lower basal expression level of rpoS and exsC (Fig. 3A and 3B). To further confirm this basal level, these two fusion strains (PAO1/psrA::Tn-Gmr /attB::miniCTX2-PrpoS-lacZ and PAO1/psrA::Tn-Gmr /attB::miniCTX2-PexsC-lacZ) with pUCP-Nde vector alone were grown in LB ± oleate and the basal expression levels of rpoS and exsC were similar (Fig. 3C compare to 3A and 3B). These data also suggest that LCFA only influences the expression of rpoS and exsC through PsrA. This LCFA modulation of rpoS and exsC expression occurs through PsrA in the entire log-phase. These data (Fig. 2 and 3) suggest that the extent of PsrA activation of rpoS and exsC depends on the concentration of cellular LCFA. It also suggests that LCFA binding to PsrA may force the PsrA-LCFA complex to be displaced from the promoter regions of rpoS and exsC down-regulating their expression, which is tested below.
Fig. 3.
Growth curves and the expression of rpoS and exsC in strain PAO1-psrA::Tn-Gmr carrying chromosomal fusions with either miniCTX2-PrpoS-lacZ or miniCTX2-PexsC-lacZ. (A) Growth rates for strain PAO1-psrA::Tn-Gmr/attB::miniCTX2-PrpoS-lacZ/pUCP-Nde-psrA were identical for all conditions. The oleate alone condition indicated that any activation of rpoS due to the leaky expression of PsrA was presumably inhibited by excess oleate. The addition of IPTG alone induced overexpression of PsrA to highly activated the expression of rpoS. This activation was inhibited by the presence of LCFA (IPTG + oleate). (B) Similar results were observed for strain PAO1/psrA::Tn-Gmr/attB::miniCTX2-PexsC-lacZ/pUCP-Nde-psrA. The data indicated that the modulation of rpoS and exsC expression in the entire log-phase was only affected by LCFA and was not due to growth rate differences. (C) Without psrA complementation using plasmid alone as a control, PAO1-psrA::Tn-Gmr/attB::miniCTX2-PrpoS-lacZ/pUCP-Nde and PAO1-psrA::Tn-Gmr/attB::miniCTX2-PexsC-lacZ/pUCP-Nde showed similar basal level expressions of rpoS and exsC, as in (A) and (B). β-Galactosidase activities were measured in triplicates and shown as the mean + s.e.m.
Binding of His6-PsrA to PpsrA, PrpoS, and PexsC is inhibited by the LCFA oleate
We demonstrate here that purified His6-PsrA binds to the promoter regions of psrA, rpoS, and exsC (i.e. PpsrA, PrpoS, and PexsC) and that this binding is inhibited by the LCFA, oleate. We initially titrated the amount of purified His6-PsrA required to completely shift 100 ng of each of the three promoter regions (PpsrA, PrpoS, and PexsC). Compared to radioactive labeled DNA for EMSA studies, this larger amount of DNA was recommended by the manufacturer of EMSA kit E33075 (Invitrogen/Molecular Probes). However, the protein to DNA molar ratios of 30:1 (124 bp PpsrA), 62:1 (127 bp PrpoS), and 125:1 (341 bp PexsC) to completely shift 100 ng of DNA are most likely physiological, considering the amount of PsrA inside the cell should be well in excess of the single copy DNA. Because of the larger amount of DNA (100 ng) required using this approach, the amount of PsrA required to shift 50% of the DNA cannot be used to estimate the DNA-protein dissociation constant (Kd; Hall and Stump, 1992).
Using purified His6-PsrA in electrophoretic mobility shift assays (EMSA) with PpsrA, PrpoS, and PexsC DNA, respectively, we observed that the binding of His6-PsrA to each promoter could be inhibited by oleate concentrations as low as 5 µM (Fig. 4). Complete inhibition of binding was achieved at 50 µM of oleate, which is an appropriate concentration as published previously (Kang et al., 2008). In Escherichia coli, FadR binds to the first intermediate of β-oxidation, oleoyl-CoA, to derepress fad genes (DiRusso et al., 1992); however, there was no observable inhibition even at 50 µM of oleoyl-CoA for all three promoters (data not shown), indicating that LCFA is the specific molecule that binds to PsrA in P. aeruginosa. Our results suggest that LCFA influences the conformation of PsrA upon binding, such that the PsrA-LCFA complex is unable to act as a repressor for PpsrA nor as an activator for PrpoS and PexsC.
Fig. 4.
EMSA indicates that direct binding of PsrA to the psrA, rpoS and exsC promoter regions (PpsrA, PrpoS, and PexsC) is specifically inhibited by LCFA. Binding of PsrA to PpsrA (A), PrpoS (B), and PexsC (C) was inhibited by increasing concentrations of oleate in a gradient fashion. In each reaction, 100 ng of PpsrA, PrpoS, and PexsC DNA was used while 1, 2 and 1.5 µg of His6-PsrA was utilized for the respective promoter. Lanes 3 and 4 of each gel were with or without 0.01% Brij-58 control (added to help solubilize oleate), respectively, illustrating that there is no affect on binding in the presence of the Brij-58 non-ionic detergent. Lanes 5–9 of each gel contain increasing concentrations of oleate as labeled above the images (µM).
LCFA affects the expression of the N-(butyryl)-l-homoserine-lactone ( BHL) quorum-sensing system, ExoS, and ExoT
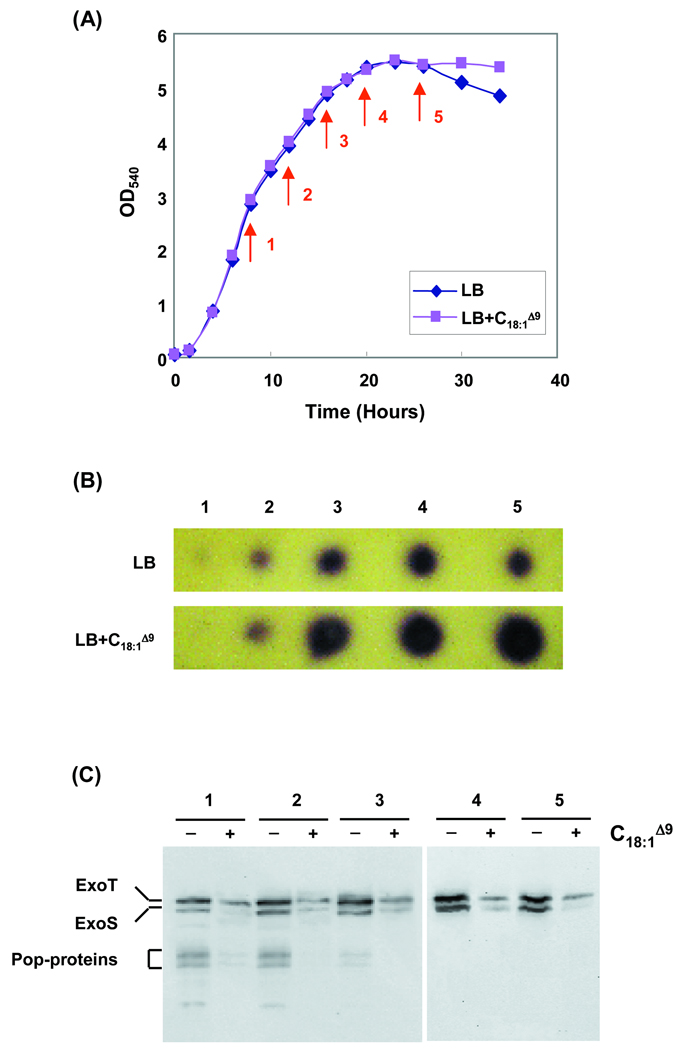
We wanted to next determine whether the expression of rpoS and TTSS, influenced by LCFA, in microarray and gene-fusion experiments would affect the level of BHL, ExoS, and ExoT produced. It was previously shown that a P. aeruginosa rpoS mutant of strain PAO1 elevated the expression of rhlI (encoding the BHL synthase), which approximately doubled the BHL level from mid-log (ML) to early-stationary (ES) growth phases (Whiteley et al., 2000). This resulted in greatly increased expression of virulence genes controlled by this quorum-sensing system (e.g. pyocyanin and hydrogen cyanide synthases) (Whiteley et al., 2000). To determine if LCFA-decreased rpoS expression would also influence BHL production, we monitored the BHL production of cultures from ML to ES growth phases in the presence or absence of LCFA (Fig. 5). In agreement with the earlier rpoS mutant study (Whiteley et al., 2000), decreased rpoS expression in the presence of LCFA also increased BHL production from ML to ES growth phases (Fig. 5B), possibly resulting from LCFA binding to PsrA, inhibiting rpoS activation (Fig. 2). We next wanted to see if the decrease in TTSS gene expression would translate to decrease in TTSS protein production. Using antibodies to TTSS proteins (Cisz et al., 2008), LCFA decreased the expression of ExoS and ExoT in all growth phases from ML to ES when the same volume of culture supernatant was tested (Fig. 5C). Clearly, down-regulation of rpoS and TTSS (e.g. exoS and exoT) in the presence of LCFA significantly affects virulence behavior of P. aeruginosa.
Fig. 5.
Production of N-(butyryl)-l-homoserine-lactone (BHL) and ExoS/ExoT influenced by LCFA. (A) PAO1 strain was grown in LB supplemented with C18:1Δ9 (oleate). At various time points indicated by arrows (A), ethyl acetate extracts of these culture-supernatants were analyzed for the presence of BHL (B). In the presence of LCFA oleate, higher production levels of BHL were observed. (C) In agreement with the microarray data, the expression of ExoS and ExoT was suppressed by LCFA observed in cell-free supernatants from ML to ES phases.
Structural analysis of PsrA
It has previously been shown that adding LCFA (C12:0 to C18:1Δ9) to PsrA inhibits the binding of PsrA to the promoter region of the fadBA5-operon (Kang et al., 2008), suggesting that LCFA are effector molecules for PsrA. As other members of the TetR transcriptional regulator protein family, the binding of an effector molecule is expected to be localized at the PsrA C-terminal domain, resulting in a conformational change affecting the N-terminal helix-turn-helix DNA binding motif. To gain molecular insight into the interactions of PsrA with its effector, we decided to deduce the structure of PsrA.
The crystal structure of PsrA was determined at 1.80 Å resolution by single wavelength anomalous dispersion (SAD) using selenomethionine labeled protein crystals. The phase information from the SAD experiment with the Se peak data was used to build the initial model which was then iteratively refined and rebuilt. The final model of PsrA consists of 213 residues and displays excellent overall stereochemistry, judged by Ramachandran plot, which shows that 100% of the residues are in the allowed region. The final refinement of PsrA yielded a protein model that has an R-value of 0.183 (Rfree 0.230). Data collection and refinement statistics are summarized in Table 4.
Table 4.
X-ray data collection and refinement statistics for PsrA.
| X-ray data | Peak |
|---|---|
| Space group | C 2 (C 1 2 1) |
| Unit cell (Å3) | a= 82.92, b= 70.14, c = 47.85 |
| α = 90.0 , β = 111.23, γ = 90.0 | |
| Resolution (Å) | 1.80 |
| Wavelength (l) | 0.9793983 |
| Intensity (I/<s>I) | 36.7 |
| Completeness (%) | 98.8 |
| R/Rfree | 0.183/0.230 |
| Protein atoms (no.) | 1754 |
| Water molecules (no.) | 242 |
| RMSD bond length (Å) | 0.021 |
| RMSD bond angles (°) | 1.658 |
| Average protein atoms B-factor (Å2) | 28.1 |
| Average water molecules B-factor (Å2) | 44.1 |
Expectedly, the PsrA molecule structure demonstrated the general architecture typical of TetR regulators. It featured the N-terminal helix-turn-helix motif and a distinct C-terminal domain composed of eight α-helices (Fig. 6A). In crystal lattice, the two adjacent PsrA molecules form a tight dimer with a molecular interface of 2163 Å (calculated by PISA server) involving α9 to α11 as well as α6 from each monomer. A cone-like tunnel with a wider opening on one side is found in the central part of the PsrA C-terminal domain. Notably, α10 of each monomer in the PsrA dimer is extended to partially cover the entrance into this tunnel belonging to the other monomer (Fig. 6A).
Fig. 6.
PsrA structure. (A) Ribbon diagram of the PsrA homodimer. The two PsrA monomers are colored in grey and blue, with the molecular surface shown for the grey-colored molecule. The secondary structure elements are numbered. An arrow indicates the location of the effector728 binding tunnel. (B) Close-view on PsrA effector-binding tunnel. The secondary structure elements are represented in grey ribbons. The Fo−Fc electron density map calculated at 3σ is shown in green. The side chains of amino acids forming the surface of the tunnel and selected for site-directed mutagenesis are shown and named. This figure was prepared using PyMOL.
In the PsrA structure, this tunnel contained additional elongated density not attributed to the polypeptide chain (Fig. 6B). This density did not match with the compounds used in the PsrA crystallization solution (see Experimental procedures for details), suggesting that the corresponding molecule was acquired during recombinant expression and purification of PsrA from E. coli cell extract. The shape of the density and hydrophobic nature of surrounding amino acids (Fig. 6B) indicated that this molecule might be a lipid but the identification of its exact chemical composition was not possible, owing to the low occupancy in PsrA structure. The location of the tunnel and the ability to bind a molecule chemically similar to LCFA suggested that this tunnel might correspond to a PsrA effector-binding site.
LCFA-nonresponsive PsrA mutants identify potential effector binding-site
To determine whether LCFA binds in the tunnel identified in the PsrA C-terminal domain structure, we designed several site-directed (SD) mutants with altered tunnel residues and tested them for the ability to bind to various promoter regions in the presence and absence of LCFA. Specifically, we created different PsrA mutants with a Phe138Arg (mPsrA-1) single mutation, Phe138Arg/Leu165Glu (mPsrA-2) double mutations, and Leu93Glu/Leu165Arg (mPsrA-5) double mutations introducing charged residues at the entrance of the tunnel (Fig. 6B). We also introduced Phe64Arg/Val100Glu (mPsrA-3) and Phe111Arg/Leu61Glu (mPsrA-4) double mutations, substituting charged side chains inside the tunnel (Fig. 6B). All SD mutants were sequenced to confirm the mutations (data not shown). We transformed these mutant plasmids individually into a P. aeruginosa PAO1-psrA::Gmr strain carrying a well characterized fadBA5-promoter lacZ-fusion (PfadBA5-lacZ) previously described (Kang et al., 2008). The wildtype PsrA repressed the fadBA5-promoter in LB media and was derepressed by LCFA in LB as observed previously (Kang et al., 2008) (Fig. 7). Entrance tunnel SD mutants (mPsrA- 1, mPsrA-2 and mPsrA-5) did not abolish the response to LCFA, however, mutants in the hydrophobic channel (mPsrA-3 and mPsrA-4) were nonresponsive to the LCFA effector molecule, oleate (Fig. 7). We tested all mutants for the in vitro binding ability to the fadBA5-promoter. These mutants bound to the PfadBA5 as well as the wildtype PsrA protein in the EMSA study (not shown). However, only mPsrA-3 and mPsrA-4 mutants lost the ability to respond to LCFA in vitro, even at the highest concentration of LCFA (50 µM) that inhibited the binding of wildtype PsrA to PfadBA5 described previously (Kang et al., 2008) (data not shown). Similar results were observed for the binding of mPsrA-3 and mPsrA-4 mutants to the promoter of psrA (data not shown). Therefore, mutations in the hydrophobic channel of the predicted LCFA effector binding-site generated PsrA ‘-super repressors’ of the fadBA5-operon and the psrA gene itself, corroborating the importance of LCFA as a signal which derepresses both psrA and fadBA5.
Fig. 7.
Repression profile of the well-characterized fadBA5-promoter (PfadBA5-lacZ) by the five engineered PsrA mutants. Of these five mutants, the mPsrA-3 and mPsrA-4 demonstrated super-repression of PfadBA5-lacZ in the presence of the oleate. The other three mutants (mPsrA-1, mPsrA-2, and mPsrA-5) still responded to oleate and behaved identically to the wildtype PsrA.
Effector binding-site mutants, mPsrA-3 and mPsrA-4, activate rpoS and exsC in a LCFA-nonresponsive manner
Since wildtype PsrA (wtPsrA) can activate rpoS and exsC and this activation is responsive to the LCFA signal (Fig. 3), we hypothesized that mPsrA-3 and mPsrA-4 mutants should ‘super-activate’ rpoS and exsC independent of the LCFA signal. Induction of the mPsrA-3 or mPsrA-4 mutants activates rpoS (Fig. 8A and 8B) and exsC (Fig. 8C and 8D). However, unlike the LCFA inhibiting the activation of rpoS and exsC by wtPsrA (Fig. 3), the presence of LCFA did not diminish the activation of rpoS and exsC by mPsrA-3 or mPsrA-4. In vitro DNA binding studies of purified mPsrA-3 or mPsrA-4 confirmed that wtPsrA binding to the promoter region of rpoS and exsC was completely inhibited at 50 µM of the LCFA oleate (Fig. 9A and 9D), while an oleate concentration as high as 800 µM was required to completely inhibit the binding of mPsrA-3 and mPsrA-4 to rpoS (Fig. 9B and 9C) or exsC (Fig. 9E and 9F). These data confirmed the importance of LCFA as the cellular effector molecule that signals PsrA to down-regulate rpoS and exsC.
Fig. 8.
Super-activation of rpoS and exsC by mPsrA-3 and mPsrA-4 in strain PAO1-psrA::Tn-Gmr carrying chromosomal fusions with either miniCTX2-PrpoS-lacZ or miniCTX2-PexsC-lacZ and the IPTG-inducible plasmid pUCP-Nde-mpsrA-3 or pUCP-Nde-mpsrA-4. Strain PAO1-psrA::Tn-Gmr/attB::miniCTX2-PrpoS-lacZ/pUCP-Nde-mpsrA-3 (A) or PAO1-psrA::Tn-Gmr/attB::miniCTX2-PrpoS-lacZ/pUCP-Nde-mpsrA-4 (B) were grown in LB ± IPTG ± LCFA. Addition of IPTG induced the expression of mutant PsrAs, which activated the expression of rpoS. The expression of rpoS gene was super-activated by mPsrA-3 and mPsrA-4 and was non-responsive to LCFA (IPTG + oleate condition). The higher basal expression level of rpoS with oleate alone represents the activation of rpoS due to the leaky expression of mutated PsrA super-activators, which were no longer inhibited by oleate. Similar results were observed for the exsC fusion in strain PAO1-psrA::Tn-Gmr/attB::miniCTX2-PexsC-lacZ/pUCP-Nde-mpsrA-3 (C) or PAO1-psrA::Tn-Gmr/attB:: miniCTX2-PexsC-lacZ/pUCP-Nde-mpsrA-4 (D).
Fig. 9.
EMSA indicates that mutant PsrAs, mPsrA-3 and mPsrA-4, do not respond to LCFA in vitro. One µg of purified wildtype PsrA (A), mPsrA-3 (B), or mPsrA-4 (C) was used for each reaction with 100 ng of PrpoS DNA. Similarly, one µg of purified wildtype PsrA (D), mPsrA-3 (E), or mPsrA-4 (F) was used for each reaction with 100 ng of PexsC DNA. Lanes 3–9 of each gel contain 0.01% Brij-58 without and with increasing concentrations of oleate as labeled above the images (in µM). LCFA at 50 µM completely inhibited the binding of wild-type PsrA to the promoters of rpoS and exsC, while mutant PsrAs remained bound to PrpoS and PexsC. Complete inhibition of binding was only observed at 800 µM of oleate.
Discussion
PsrA has previously been shown to activate rpoS and genes of the TTSS (Kojic and Venturi, 2001; Kojic et al., 2002; Shen et al., 2006), however, more recent microarray data indicated that PsrA may not be the activator of TTSS genes (Gooderham et al., 2008; Kang et al., 2008). In addition, another important issue that complicates the regulation mechanism of rpoS and genes of the TTSS is the unknown signal(s) controlling their regulation (Kojic et al., 2002; Shen et al., 2006). The goal of this study, based on our earlier results in which LCFA induced the fadBA5-operon by inhibiting PsrA repression of PfadBA5 (Kang et al., 2008), was to determine if LCFA could also influence PsrA to alter the expression of itself, rpoS, and exsC in P. aeruginosa strain PAO1. Initial microarray data indicated that hundreds of genes were affected by LCFA (Supplementary Tables S1 and S2). Perhaps through PsrA, LCFA serves as one of the previously proposed unidentified signals (Kojic and Venturi, 2001; Kojic et al., 2002; Shen et al., 2006) that inactivate the expression of rpoS and TTSS genes (Table 3). This microarray data suggests that LCFA affects the expression of many genes (Supplementary Tables S1 and S2), some of which by PsrA as previously described (Gooderham et al., 2008; Kang et al., 2008). Other genes, however, may be influenced indirectly by PsrA and LCFA (e.g. by rpoS), which could explain the vast number of genes affected by LCFA. It will also be interesting to determine whether other genes from the microarray data are directly or indirectly regulated by LCFA and PsrA in future studies.
PsrA has been shown to be an autoregulator that positively activates the expression of the stationary-phase sigma-factor rpoS (Kojic et al., 2002) and the exsCEBA-operon of the type III secretion system in P. aeruginosa (Shen et al., 2006). Our microarray data from an earlier study indicated that PsrA was induced (5.5-fold) in vivo during P. aeruginosa infection of the cystic fibrosis lung versus in vitro growth (Son et al., 2007), suggesting the presence of sufficient LCFA in vivo for PsrA induction. It has been previously noted that TetR family regulators, including PsrA (Kojic et al., 2002), autoregulate their own synthesis by a feedback control mechanism to maintain an appropriate cellular level of these regulators (Ramos et al., 2005). We have shown here that LCFA induces psrA by binding directly to PsrA, preventing autorepression of itself (Fig. 2B). The increased LCFA-induced expression of psrA resulted in a decrease in rpoS and exsC expression rather than an increase (Fig. 2C and 2D). In the presence of LCFA, PsrA modulated rpoS expression from ML to ES growth phases (Fig. 2C), and the greater increase in rpoS expression in later stationary phase suggests other regulatory mechanisms of rpoS were involved. Nevertheless, this decrease of rpoS from ML to ES growth phases significantly altered the level of BHL (Fig. 5B). Although it has previously been confirmed that doubling the production of BHL in an rpoS mutant during these growth phases resulted in the expression of virulence genes (e.g. pyocyanin and hydrogen cyanide synthases) (Whiteley et al., 2000), we did not observe the expression of these genes due to the limitation of microarray analysis at a single time point (ML). Similar regulation by PsrA and LCFA was observed for exsC, where significant induction was observed in LB media and repression in LB + LCFA (Fig. 2D). Many other genes (eg. exoS and exoT) were similarly influenced by LCFA during ML phase, according to the microarray data (Table 3). Specifically, the down regulation of ExoS and ExoT was confirmed by western blot analysis from ML to ES growth phases in the presence of LCFA.
Although PsrA is an activator of rpoS and exsC, this activation was only observed in the absence of the LCFA signal (Fig. 3), suggesting that LCFA complexes with PsrA to inactivate it. Hence, we proposed a working model for the multiple regulatory roles of PsrA in response to LCFA (Fig. 10) where LCFA causes derepression of psrA and fadBA5 allowing their expression. The accumulation of PsrA will then activate exsC and rpoS only if the level of LCFA does not exceed the PsrA level (Fig. 3 and 10B). However, if available LCFA is sufficient, exceeding the level of PsrA, then there will be little to no activation of exsC and rpoS (Fig. 2 and 10A). His6-PsrA binding to PpsrA, PrpoS, and PexsC was inhibited by the LCFA (Fig. 4) and effector binding sites mutants abolished LCFA response (Fig. 7, 8, and 9), confirming that LCFA is a environmental signal regulating these genes.
Fig. 10.
Model of environmental LCFA signal influencing the transcription of fadBA5, psrA, rpoS, and exsC. LCFA enters the cell by an unknown mechanism in P. aeruginosa. In the cell, LCFA derepresses or induces the expression of psrA and fadBA5. When PsrA accumulates, the activation of rpoS and exsC by PsrA depends on the level of cellular LCFA. (A) We posit that, if the LCFA level is in excess, the excess LCFA will ‘titrate-out’ the activator form of PsrA into an inactive PsrA-LCFA complex, such that there is no activation or recruitment of RNA polymerase (blue) to the promoter regions of rpoS and exsC for increased transcription. However, if LCFA is shuffled through other metabolic processes (e.g. β-oxidation) and intracellular LCFA is limited, the rpoS and exsC genes are activated (B).
The availability of nutrients (e.g. LCFA) within the site of infection likely affects the behavior of P. aeruginosa. In the absence of potentially rich and highly reduced nutrients (e.g. LCFA), harming the host to release nutrients by modulating expression of stationary-phase genes and toxins most likely contributes to host damage. Since PsrA regulates exsCEBA and rpoS and RpoS has been shown to be a global regulator (including virulence associated quorum-sensing) (Schuster et al., 2004), it is intriguing that a single environmental signal could have an influence on P. aeruginosa virulence without affecting its growth rate. Besides TTSS and rpoS, PsrA has been shown to control lipase and type IV pili as noted in previous microarray studies (Kang et al. 2008; Gooderham et al., 2008). We also observed PsrA-mediated induction of other virulence factors by LCFA such as the psl-operon for biofilm synthesis (PA2231-2242) and the pvd-operons for pyoverdine synthesis (PA2385-2386, 2394-2397, and PA2424-2426; supplementary Table S1). This study helps elucidate how the absence or presence of a specific environmental signal (LCFA) may mechanistically play an important role in P. aeruginosa pathogenesis.
Previous studies proposed an unidentified signal that allows PsrA to regulate rpoS and genes of the TTSS (Kojic et al., 2002; Shen et al., 2006). It has also been recently noted that the most rewarding and most difficult task for future studies will be to identify the host and environmental signals that induce or repress the expression of TTSS (Yahr and Wolfgang, 2006). We have found one such signal to be LCFA and have deciphered a small part of the complex regulatory mechanism of TTSS. The PsrA regulation mechanism, involving environmental LCFA signals, should be added to the TTSS regulatory network.
Finally, the crystal structure of the P. aeruginosa PsrA (PA3006), without its identifiable signal, complicates the understanding of its true biochemical functions. Site-directed mutagenesis of the proposed binding site for LCFA within a hydrophobic channel generated two ‘super-repressor’/‘super-activator’ mutants that maintained the regulatory function but no longer bound or responded to the LCFA effector molecule. Hence, the results of this study will aid future researches to further define the effector binding site of PsrA, a member of the TetR family of regulators.
Experimental procedures
Bacterial strains, media, and culture conditions
Strains and plasmids utilized in this study are shown in Table 1. E. coli EPMax10B (BioRad) was routinely used as a strain for cloning and was cultured in Luria-Bertani (LB) medium (Difco). P. aeruginosa strain PAO1 (Holloway et al., 1994; Stover et al., 2000) and derivatives were cultured in Pseudomonas Isolation Agar (PIA; Difco) or LB medium. All chemicals were purchased from Sigma. Stock solution of fatty acid (FA) (oleic acid, C18:1Δ9) at 3% (w/v) was made with equimolar KOH and 1% of the non-ionic detergent Brij-58 (Kang et al., 2008). Growth curves were performed in LB + 1% Brij-58 ± 0.2% C18:1Δ9 (Fig. 2 and 5) or LB + 350 µg/ml carbenicillin (Cb) + 1% Brij-58 ± 0.2% C18:1Δ9 (Fig. 7). Bacteria were also cultured in LB + 350 µg/ml Cb + 1% Brij-58 ± 1 mM IPTG ± 0.2% C18:1Δ9 (Fig. 3A, 3B and 8), or LB + 350 µg/ml Cb + 1% Brij-58 ± 0.2% C18:1Δ9 (Fig. 3C). Unless indicated otherwise, cultures were grown at 37°C with a shaking speed of 200 r.p.m.
Table 1.
Bacterial strains and plasmids*
| Strains/plasmids | Lab ID** | Relevant properties | Reference |
|---|---|---|---|
| Strains | |||
| E. coli | |||
| EPMax10B | E1231 |
F-mcrA Δ(mrr-hsdRMS-mcrBC) φ80dlacZM15 ΔlacX74 deoR recA1 endA1 araD139 Δ(ara, leu)7697 galU galK rpsL nupG λ- |
BioRad |
| C. violaceum | |||
| CV026 | E0070 | cvil:mini-Tn5 | (Winson et al., 1995) |
| P. aeruginosa | |||
| PAO1 | P007 | prototroph | (Holloway et al., 1994) |
| PAO1-psrA::Tn-Gmr/PfadBA5-lacZ | P358 | Gmr; psrA transposon mutant with PfadBA5-lacZ fusion | (Kang et al., 2008) |
| PAO1-PpsrA-lacZ | P366 | PAO1 integrated with PpsrA-lacZ fusion | This study |
| PAO1-PrpoS-lacZ | P372 | PAO1 integrated with PrpoS-lacZ fusion | This study |
| PAO1-PexsC-lacZ | P374 | PAO1 integrated with PexsC-lacZ fusion | This study |
| PAO1-psrA::Tn-Gmr/ attB::miniCTX2-PpsrA-lacZ |
P394 | Tetr, Gmr; psrA mutant strain integrated with PrpoS-lacZ fusion |
This study |
| PAO1-psrA::Tn-Gmr/ attB::miniCTX2-PrpoS-lacZ |
P396 | Tetr, Gmr; psrA mutant strain integrated with PrpoS-lacZ fusion |
This study |
| PAO1-psrA::Tn-Gmr/ attB:miniCTX2-PexsC-lacZ |
P398 | Tetr, Gmr; psrA mutant strain integrated with PexsC-lacZ fusion |
This study |
| Plasmids | |||
| pUCP-Nde | E0016 | Apr; broad-host-range cloning vector | (Cronin and McIntire, 1999) |
| pUCP-Nde-psrA | E1256 | Apr; pUCP-Nde with wildtype psrA cloned into it | This study |
| pUCP-Nde-mpsrA-1 | E1401 | Apr; pUCP-Nde with mutant mpsrA-1 cloned into it | This study |
| pUCP-Nde-mpsrA-2 | E1403 | Apr; pUCP-Nde with mutant mpsrA-2 cloned into it | This study |
| pUCP-Nde-mpsrA-3 | E1430 | Apr; pUCP-Nde with mutant mpsrA-3 cloned into it | This study |
| pUCP-Nde-mpsrA-4 | E1431 | Apr; pUCP-Nde with mutant mpsrA-4 cloned into it | This study |
| pUCP-Nde-mpsrA-5 | E1433 | Apr; pUCP-Nde with mutant mpsrA-5 cloned into it | This study |
For plasmids and strains constructed in this study, please see text for further details.
Please use lab ID for requesting strains and plasmids.
Molecular methods and reagents
Restriction enzymes, dNTPs, T4 DNA polymerase, and ligase were purchased from New England Biolabs and used as recommended by the supplier. Plasmids and DNA gel bands were isolated using the Zyppy Plasmid Miniprep Kit I and Zymoclean Gel DNA Recovery kit, respectively, from Zymo Research Corporation. Chromosomal DNA was isolated with the IsoQuick nucleic acid extraction kit (Orca Research). E. coli competent cell preparations, blunt-ending of DNA fragments with T4 DNA polymerase, and other molecular techniques were performed according to Sambrook & Russell (Sambrook and Russell, 2001). Oligonucleotide primers (Table 2) were synthesized by Integrated DNA Technologies (IDT). P. aeruginosa competent cells were prepared as described elsewhere (Choi et al., 2006). Generally, we performed the various PCRs by initial denaturation for 1 min at 94°C and 34 cycles of 45 sec at 94°C, 30 sec at 58°C, and 1 min kb−1 at 72°C, and a final extension step of 10 min at 72°C was included. Pfu DNA polymerase was purchased from Stratagene.
Table 2.
Oligonucleotide primers utilized in this study*
| Primer umber/Name | Sequences** |
|---|---|
| #727-PpsrA-up-HindIII | 5'-TCTTAAGCTTCCGTTTTTCGGGACTTC-3' |
| #728-PpsrA-down | 5'-ATGGTTTCTCCGCCTGACAA-3' |
| #736-PrpoS-up-HindIII | 5'-GTGGAAGCTTGGCCTGCGAGCGGTA-3' |
| #737-PrpoS-down | 5'-AACGGTCCCACCAGACGCAG-3' |
| #738-PexsC-up-HindIII | 5'-CCAAGCTTAACGCCTCGGTCCA-3' |
| #739-PexsC-down | 5'-CATGGGGGCGCCTCCTAAA-3' |
| #782-psrA-L61E | 5'-GTTCTCGCGCTTCGAAGGGCCATTCTGC-3' |
| #783-psrA-F64R | 5'-TCCTCGGGCCACGCTGCGCCAGCCT-3' |
| #784-psrA-L93E-ΔBsgI | 5'-CTGGAGGACCTCCTGCATCTGGAGGTGTCCCAGGCGATG-3' |
| #785-psrA-V100E-reverse | 5'-GCGCGGCTTCTCCGCCATCGC-3' |
| #786-psrA-F111R-reverse | 5'-GAGCAAGCGCATGCGGATCGACAGGTCG-3' |
| #787-psrA-F138R-ΔBbsI | 5'-CTACGGCAAGGTCCGCCGGCGCTACA-3' |
| #788-psrA-L165E-ΔNotI | 5'-GCGCGTGCACTTCATGGAGGGCGCAGCCGCCTTCAGCATG-3' |
| #789-psrA-L165R-ΔNotI | 5'-CGCGTGCACTTCATGCGCGGCGCAGCCGCCTTCAGCATG-3' |
Oligonucleotides were synthesized by Integrated DNA Technologies.
Restriction enzyme sites are underlined.
Microarray study of PAO1 grown on LB ± LCFA
PAO1 starter culture was grown overnight in PIA, washed twice with LB, and 1:100 dilutions were inoculated into 20 ml LB + 1% Brij-58 ± 0.2% C18:1Δ9. Both cultures were then grown to mid-log phase, cells were harvested, and total mRNA was isolated as previously described (Kang et al., 2008). cDNA syntheses and labeling, GeneChip processing, and microarray analysis were performed as described previously (Son et al., 2007). Analysis was done by conducting pair-wise comparisons between three replicates of PAO1 grown in either LB or LB + C18:1Δ9. Genes that exhibited a two-fold or greater increase in expression in LB + C18:1Δ9 relative to LB are listed in supplementary Table S2 (online) while genes that showed a two-fold or greater increase in expression in LB relative to LB + C18:1Δ9 are listed in supplementary Table S1 (online). Gene assignment was assisted using COG predictions in the Pseudomonas Genome Database (http://www.pseudomonas.com/index.jsp).
The array data have been deposited in the NCBI Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO series accession number GSE13248.
Expression of psrA, rpoS and exsC fusions in LB ± LCFA
Three different transcriptional lacZ fusion strains, PAO1-PpsrA-lacZ, PAO1-PrpoS-lacZ, and PAO1-PexsC-lacZ, were engineered as outlined below. The promoter region of psrA was amplified by PCR from PAO1 chromosomal DNA using oligos #727 + #728. The resulting 124 bp promoter fragment was digested with HindIII and cloned into a miniCTX2-PfadE-lacZ plasmid (laboratory collection) that was digested with HindIII + SmaI, yielding miniCTX2-PpsrA-lacZ. Similarly, the promoter regions of rpoS or exsC were amplified using oligos #736 + #737 or #738 + #739. The 127 bp PrpoS or 341 bp PexsC fragments were digested with HindIII and cloned into the miniCTX2-PfadE-lacZ plasmid following HindIII + SmaI digestion, yielding miniCTX2-PrpoS-lacZ and miniCTX2-PexsC-lacZ, respectively. The chromosomal integration of these three miniCTX2–lacZ fusion vectors into P. aeruginosa, excision of unwanted plasmid sequences, and verification of insertion at the chromosomal attB locus were performed as described previously (Hoang et al., 2000).
Growth curve studies were performed and β-galactosidase activities were measured on these three PpsrA-lacZ, PrpoS-lacZ, and PexsC-lacZ PAO1 fusion strains grown in LB ± LCFA (Fig. 2). These fusion strains were grown overnight in PIA medium. Overnight cultures were washed twice with one volume of LB and resuspended in an equal volume of LB medium. Resuspended cultures were then diluted 100-fold into fresh LB + 1% Brij-58 supplemented with or without 0.2% C18:1Δ9. Growth curves were determined for each culture by diluting them four-fold in prewarmed 4% Brij-58 (42°C) and measuring the OD540. At various time points during log and early stationary phases, one ml cell culture aliquots were taken and β-galactosidase assays were performed. β-Galactosidase assays were done in triplicate and Miller Units (mean ± s.e.m.) were determined (Miller, 1992).
Expression of rpoS and exsC in PAO1-psrA::Tn-Gmr/pUCP-Nde-psrA
The psrA mutant strain PAO1-psrA::Tn-Gmr was previously engineered (Kang et al., 2008). PrpoS-lacZ or PexsC-lacZ fusions and pUCP-Nde-psrA were introduced into this psrA mutant strain as described above, resulting in PAO1-psrA::Tn-Gmr /attB::miniCTX2-PrpoS-lacZ/pUCP-Nde-psrA and PAO1-psrA::Tn-Gmr /attB::miniCTX2-PexsC-lacZ/pUCP-Nde-psrA. Growth curve studies were performed and β-galactosidase activities were measured on these newly engineered fusion strains grown in LB ± IPTG ± oleate (Fig. 3A and 3B) similar to those described above. For Fig. 3C, PrpoS-lacZ or PexsC-lacZ fusions and the empty vector pUCP-Nde were introduced into the psrA mutant strain, resulting in PAO1-psrA::Tn-Gmr/attB::miniCTX2-PrpoS-lacZ/pUCP-Nde and PAO1-psrA::Tn-Gmr /attB::miniCTX2-PexsC-lacZ/pUCP-Nde, respectively. Growth curve studies and β-galactosidase assays were performed on these two fusion strains in LB ± oleate.
Site-directed mutagenesis of rpoS and exsC and gene-fusion analyses
Site-directed mutagenesis was performed using QuikChange Multi Site-Directed Mutagenesis Kit (Stratagene) as recommended by the manufacturer. Five PsrA mutants were engineered using pUCP-Nde-psrA as a template and the site-directed mutagenesis primers as follow: mPsrA-1, oligo #787; mPsrA-2, oligo #787 + #788; mPsrA-3, oligo #783 + #785; mPsrA-4, oligo #782 + #786; mPsrA-5, oligo #784 + #789. All five psrA mutants were sequenced to confirm that the correct mutations were introduced and no unwanted mutations were created during PCR. Next, the pUCP-Nde vectors containing these individual psrA mutants were introduced into the PAO1-psrA::Tn-Gmr/PfadBA5-lacZ fusion strain, yielding PAO1-psrA::Tn-Gmr/PfadBA5-lacZ/pUCP-Nde-mpsrA1-5. These resulting strains were grown in LB ± oleate (as mentioned above) to mid-log phase and β-galactosidase activities were measured (Fig. 7). Plasmids pUCP-Nde-mpsrA3 and pUCP-Nde-mpsrA4 were individually transformed into PAO1-psrA::Tn-Gmr/attB::miniCTX2-PrpoS-lacZ or PAO1-psrA::Tn-Gmr /attB::miniCTX2-PrpoS-lacZ. The resulting strains were grown in LB ± IPTG ± oleate and β-galactosidase activities were measured as described above.
Electrophoretic mobility shift assay (EMSA) studies
His6-PsrA proteins were purified and concentrations were determined as previously described (Kang et al., 2008). EMSA was performed as described previously (Kang et al., 2008) using the wild type or mutant His6-PsrA proteins and the DNA promoter fragments of psrA, rpoS, and exsC. The amount of His6-PsrA used to shift 100 ng of PpsrA, PrpoS, or PexsC DNA was 1 µg, 2 µg, or 1.5 µg, respectively.
Detection of N-(butyryl)-l-homoserine-lactone
N-(butyryl)-l-homoserine-lactone (BHL) was detected following previously described procedures (Hoang et al., 1999) with slight modifications. PAO1 was grown in LB + 1% Brij-58 with or without 0.2% C18:1Δ9. At various time points, one ml of each culture was taken to pellet the cells and obtain the clarified supernatant. BHL was extracted three times from the clarified supernatant with 250 µl of ethyl acetate. The ethyl acetate extract (750 µl of each sample) was then completely vacuum-dried and BHL samples were dissolved in 250 µl of acetonitrile. Five µl of the acetonitrile resuspended samples were spotted on C18 reverse-phase TLC plates (Whatman) to dry. The Chromobacterium violaceum BHL detection strain, CV026, was grown overnight at room temperature in LB broth and diluted 1:100 in 200 ml of warm (42°C) LB medium + 0.3% agar. This mixture was used immediately to overlay the TLC plates. The presence of BHLs was evident by the appearance of purple spots after overnight incubation at room temperature.
Western Blot Analysis of ExoS/ExoT Secretion
The cell-free supernatant of PAO1 grown in LB ± C18:1Δ9 for the BHL detection experiment above was also used for the ExoS/ExoT analysis. After centrifugation, the clarified supernatant was immediately mixed with equal volume of 2× SDS-PAGE loading buffer and boiled for 5 minutes. Total protein from the supernatant was then resolved on 10% SDS-PAGE gels, blot to PVDF membrane and analyzed by WesternBreeze Chemiluminescent Kit-Anti-Rabbit (Invitrogen) as directed by the manufacturer, utilizing antibodies to ExoS/ExoT (Cisz et al., 2008).
PsrA purification and crystallization
For structural analysis, the PsrA was expressed and purified as previously described (Zhang et al., 2001). The expression plasmid was transformed into E. coli BL21-Gold (DE3) (Stratagene), which harbors an extra plasmid (pMgk) encoding three rare tRNAs (AGG and AGA for Arg, ATA for Ile). To produce selenomethionine-enriched protein these E. coli cells were cultured in SeMET high-yield media (Shanghai Medicilon). Protein was purified using Ni-NTA affinity chromatography, and the crystallization experiment was set up after its affinity tag was cleaved by digestion with TEV protease followed by dialysis in a crystallization buffer containing 10 mM HEPES (pH 7.5) and 500 mM NaCl.
PsrA protein was crystallized by hanging-drop vapor diffusion by mixing 2 µl of the protein solution (10 mg/ml) with 2 µl of a precipitant solution containing 25% PEG 5K MME, 0.2 M CaCl2, 0.1 M Bis-Tris pH 6.5 and 2 mM L-Cysteine. Prior to data collection, crystals were cryo-protected by transferring to a mixture of 30% PEG 5K MME, 0.2 M CaCl2, 0.1 M Bis-Tris pH 6.5, 5% Glycerol, 5% Sucrose, 5% Ethylene Glycol and flash frozen in liquid nitrogen. X-ray diffraction data were collected at the beamline 19ID of Advanced Photon Source at Argonne National Laboratories.
X-ray diffraction and structure determination
The diffraction data were processed and scaled with the HKL2000 suite of programs (DENZO/SCALEPACK) (Otwinowski, 1997). Initial phases were obtained with BnP (Weeks et al., 2002) using the single wavelength anomalous dispersion (SAD) phasing method with the selenium (Se) peak diffraction data. Six out of eight selenium sites were identified using BnP. Subsequent electron density modification followed by initial model building were done using RESOLVE (Terwilliger, 2000). Additional regions of the protein model were built with ARP/wARP (Perrakis et al., 1999). The model then was iteratively refined using REFMAC (Murshudov et al., 1997) and rebuilt using COOT (Emsley and Cowtan, 2004). Figure 6 was produced with PyMol (DeLano, 2002).
Supplementary Material
Acknowledgements
This work was supported by a National Institutes of Health Grant (NIH) grant R21-AI073816 and in parts by an NIH grant GM62414-01, the Ontario Research and Development Challenge Fund, and the Canadian Institutes of Health Research Grant. We thank Dr. Arne Rietsch (Case Western Reserve Univ.) for the generous gift of ExoS/ExoT antibody. We wish to thank all members of the SBC (particularly Youngchang Kim) at ANL for their help in conducting experiments.
Footnotes
Accession number
The atomic coordinates and structure factors for PsrA (PDB ID 2FBQ) have been deposited to the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org).
References
- Abd H, Wretlind B, Saeed A, Idsund E, Hultenby K, Sandström G. Pseudomonas aeruginosa utilizes its type III secretion system to kill the free-living amoeba Acanthamoeba castellanii. J. Eukaryot. Microbiol. 2008;55:235–243. doi: 10.1111/j.1550-7408.2008.00311.x. [DOI] [PubMed] [Google Scholar]
- Barrett AR, Kang Y, Inamasu KS, Son MS, Vukovich JM, Hoang TT. Genetic tools for allelic replacement in Burkholderia species. Appl. Environ. Microbiol. 2008;74:4498–4508. doi: 10.1128/AEM.00531-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger N, Hassett DJ, Iglewski BH, Costerton JW, McDermott TR. Gene expression in Pseudomonas aeruginosa: evidence of iron override effects on quorum sensing and biofilm-specific gene regulation. J. Bacteriol. 2001;183:1990–1996. doi: 10.1128/JB.183.6.1990-1996.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowton DL. Nosocomial Pneumonia in the ICU-Year 2000 and Beyond. Chest. 1999;115:28S–33S. doi: 10.1378/chest.115.suppl_1.28s. [DOI] [PubMed] [Google Scholar]
- Choi K-H, A K, Schweizer HP. A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: application for the DNA fragment transfer between chromosomes and plasmid transformation. J. Microbiol. Meth. 2006;64:391–397. doi: 10.1016/j.mimet.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Cisz M, Lee P-C, Rietsch A. ExoS Controls the Cell Contact-Mediated Switch to Effector Secretion in Pseudomonas aeruginosa. J. Bacteriol. 2008;190:2726–2738. doi: 10.1128/JB.01553-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin CN, McIntire WS. pUCP-Nco and pUCP-Nde: Escherichia-Pseudomonas shuttle vectors for recombinant protein expression in Pseudomonas. Anal. Biochem. 1999;272:112–115. doi: 10.1006/abio.1999.4160. [DOI] [PubMed] [Google Scholar]
- DeLano WL. The PyMOL Molecular Graphics System. [WWW document] 2002 URL http://www.pymol.org.
- DiRusso CC, Heimert TL, Metzger AK. Characterization of FadR, a global transcription regulator of fatty acid metabolism in Escherichia coli. J. Biol. Chem. 1992;267:8685–8691. [PubMed] [Google Scholar]
- Doring G. Cystic fibrosis respiratory infections: interactions between bacteria and host defense. Monaldi Arch. Chest Dis. 1997;52:363–366. [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta. Cryst. 2004;D60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Finck-Barbancon V, Goranson J, Zhu L, Sawa T, Wiener-Kronish JP, Fleiszig SM, Wu C, Mende-Mueller L, Frank DW. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol. Microbiol. 1997;25:547–557. doi: 10.1046/j.1365-2958.1997.4891851.x. [DOI] [PubMed] [Google Scholar]
- Gooderham WJ, Bains M, McPhee JB, Wiegand I, Hancock REW. Induction by cationic antimicrobial peptides and involvement in intrinsic polymyxin and antimicrobial peptide resistance, bioflm formation, and swarming motility of PsrA in Pseudomonas aeruginosa. J. Bacteriol. 2008;190:5624–5634. doi: 10.1128/JB.00594-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govan JR, Nelson JW. Microbiology of lung infection in cystic fibrosis. Br. Med. Bull. 1992;48:912–930. doi: 10.1093/oxfordjournals.bmb.a072585. [DOI] [PubMed] [Google Scholar]
- Hall KB, Stump WT. Interaction of N-terminal domain of U1A protein with an RNA stem/loop. Nucleic Acids Res. 1992;20:4283–4290. doi: 10.1093/nar/20.16.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havasi V, Hurst CO, Briles TC, Yang F, Bains DG, Hassett DJ, Sorscher E. Inhibitory effects of hypertonic saline on P. aeruignosa motility. J. Cyst. Fibros. 2008;7:267–269. doi: 10.1016/j.jcf.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang TT, Ma Y, Stern RJ, McNeil MR, Schweizer HP. Construction and use of low-copy number T7 expression vectors for purification of problem proteins: purification of Mycobacterium tuberculosis RmlD and Pseudomonas aeruginosa LasI and RhlI proteins, and functional analysis of RhlI. Gene. 1999;237:361–371. doi: 10.1016/s0378-1119(99)00331-5. [DOI] [PubMed] [Google Scholar]
- Hoang TT, Kutchma AJ, Becher A, Schweizer HP. Integration-proficient plasmids for Pseudomonas aeruginosa: site-specific integration and use for engineering of reporter and expression strains. Plasmid. 2000;43:59–72. doi: 10.1006/plas.1999.1441. [DOI] [PubMed] [Google Scholar]
- Kang Y, Nguyen DT, Son MS, Hoang TT. The Pseudomonas aeruginosa PsrA responds to long-chain fatty acid signals to regulate the fadBA5 β-oxidation operon. Microbiology. 2008;154:1584–1598. doi: 10.1099/mic.0.2008/018135-0. [DOI] [PubMed] [Google Scholar]
- Köhler T, Curty LK, Barja F, van Delden C, Pechée JC. Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J. Bacteriol. 2000;182:5990–5996. doi: 10.1128/jb.182.21.5990-5996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojic M, Venturi V. Regulation of rpoS gene expression in Pseudomonas: involvement if a TetR family regulator. J. Bacteriol. 2001;183:3712–3720. doi: 10.1128/JB.183.12.3712-3720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojic M, Aguilar C, Venturi V. TetR family member PsrA dirtectly binds the Pseudomonas rpoS and psrA promoter. J. Bacteriol. 2002;184:2324–2330. doi: 10.1128/JB.184.8.2324-2330.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont IL, Beare PA, Ochsner U, Vasil AI, Vasil ML. Siderophore-mediated signaling regulates virulence factor production in Pseudomonas aeruginosa. PNAS. 2002;99:7072–7077. doi: 10.1073/pnas.092016999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey TL, Hagins JM, Sokol PA, Silo-Suh LA. Virulence determinants from a cystic fibrosis isolate of Pseudomonas aeruginosa include isocitrate lyase. Microbiology. 2008;154:1616–1627. doi: 10.1099/mic.0.2007/014506-0. [DOI] [PubMed] [Google Scholar]
- Lode H, Raffenberg M, Erbes R, Geerdes-Fenge H, Mauch H. Nosocomial pneumonia: epidemiology, pathogenesis, diagnosis, treatment and prevention. Curr. Opin. Infect. Dis. 2000;13:377–384. doi: 10.1097/00001432-200008000-00009. [DOI] [PubMed] [Google Scholar]
- Matz C, Moreno AM, Alhede M, Manefield M, Hauser AR, Givskov M, Kjelleberg S. Pseudomonas aeruginosa uses type III secretion secretion system to kill biofilm-associated ameobae. ISME J. 2008;2:843–852. doi: 10.1038/ismej.2008.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JH. A Short Course in Bacterial Genetics. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- Murshudov GN, Vagin AA, Dodson EJ. Refinement of Macromolecular Structures by the Maximum-Likelihood Method. Acta. Cryst. 1997;D53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- O'Toole GA, Gibbs KA, Hager PW, Phibbs PV, Jr, Kolter R. The global carbon metabolism regulator Crc is a component of a signal transduction pathway required for biofilm development by Pseudomonas aeruginosa. J. Bacteriol. 2000;182:425–431. doi: 10.1128/jb.182.2.425-431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski ZM, W . Macromolecular Crystallography, Part A. In: Carter CWJ, Sweet RM, editors. Methods in Enzymology. Vol. 276. New York: Academic Press; 1997. pp. 307–326. [Google Scholar]
- Perrakis A, Morris RJ, Lamzin VS. Automated protein model building combined with iterative structure refinement. Nat. Struct. Biol. 1999;6:458–463. doi: 10.1038/8263. [DOI] [PubMed] [Google Scholar]
- Rahme LG, Stevens EJ, Wolfort SF, Shao J, Tompkins RG, Ausubel FM. Common virulence factors for bacterial pathogenicity in plants and animals. Science. 1995;268:1899–1902. doi: 10.1126/science.7604262. [DOI] [PubMed] [Google Scholar]
- Ramos JL, Martínez-Bueno M, Molina-Henares AJ, Terán W, Watanabe K, Zhang X, Gallegos MT, Brennan R, Tobes R. The TetR family of transcriptional repressors. Microbiol. Mol. Biol. Rev. 2005;69:326–356. doi: 10.1128/MMBR.69.2.326-356.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in medical intensive care units in the United States. Crit. Care Med. 1999;27:887–892. doi: 10.1097/00003246-199905000-00020. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Schuster M, Hawkins AC, Harwood CS, Greenberg EP. The Pseudomonas aeruginosa RpoS regulon and its relactionship to quorum sensing. Mol. Microbiol. 2004;51:973–985. doi: 10.1046/j.1365-2958.2003.03886.x. [DOI] [PubMed] [Google Scholar]
- Shafikhani SH, Engel J. Pseudomonas aeruginosa type III-secreted toxin ExoT inhibits host-cell division by targeting cytokinesis at multiple steps. Proc. Natl. Acad. Sci. U.S.A. 2006;103:15605–15610. doi: 10.1073/pnas.0605949103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen DK, Filopon D, Kuhn L, Polack B, Toussaint B. PsrA is a positive transcriptional regulator of the type III secretion system in Pseudomonas aeruginosa. Infect. Immun. 2006;74:1121–1129. doi: 10.1128/IAI.74.2.1121-1129.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son MS, Matthews WJJ, Kang Y, Nguyen DT, Hoang TT. In vivo evidence of Pseudomonas aeruginosa nutrient acquisition and pathogenesis in the lungs of cystic fibrosis patients. Infect. Immun. 2007;75:5313–5324. doi: 10.1128/IAI.01807-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnleitner E, Hagensa S, Rosenaub F, Wilhelmb S, Habelc A, Jager K-E, Blasi U. Reduced virulence of a hfq mutant of Pseudomonas aeruginosa 01. Microb. Pathog. 2003;35:217–228. doi: 10.1016/s0882-4010(03)00149-9. [DOI] [PubMed] [Google Scholar]
- Terwilliger TC. Maximum likelihood density modification. Acta. Cryst. 2000;D56:965–972. doi: 10.1107/S0907444900005072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks CM, Blessing RH, Miller R, Mungee R, Potter SA, Rappleye J, Smith GD, Xu H, Furey W. Towards automated protein structure determination: BnP, the SnB-PHASES interface. Z. Kristallogr. 2002;217:686–693. [Google Scholar]
- Weir TL, Stull VJ, Badri D, Trunck LA, Schweizer HP, Vivanco J. Global gene expression profiles suggest an important role for nutrient acquisition in early pathogenesis in a plant model of Pseudomonas aeruginosa infection. Appl. Environ. Microbiol. 2008;74:5784–5791. doi: 10.1128/AEM.00860-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley M, Parsek MR, Greenberg EP. Regulation of quorum sensing by RpoS in Pseudomonas aeruginosa. J. Bacteriol. 2000;182:4356–4360. doi: 10.1128/jb.182.15.4356-4360.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winson MK, Camara M, Latifi A, Foglino M, Chhabbra SR, Daykin M, Bally M, Chapon V, Salmond GPC, Bycroft BW, Lazdunski A, Stewart GSAB, Williams P. Multiple N-acyl-l-homoserine lactone signal molecules regulate production of virulence determinants and secondary metabolites in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U.S.A. 1995;92:9427–9431. doi: 10.1073/pnas.92.20.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahr TL, Goranson J, Frank DW. Exoenzyme S of Pseudomonas aeruginosa is secreted by a type III pathway. Mol. Microbiol. 1996;22:991–1003. doi: 10.1046/j.1365-2958.1996.01554.x. [DOI] [PubMed] [Google Scholar]
- Yahr TL, Mende-Mueller LM, Friese MB, Frank DW. Identification of type III secreted products of the Pseudomonas aeruginosa Exoenzyme S regulon. J. Bacteriol. 1997;179:7165–7168. doi: 10.1128/jb.179.22.7165-7168.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahr TL, Vallis AJ, Hancock MK, Barbieri JT, Frank DW. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III secretion system. Proc. Natl. Acad. Sci. U.S.A. 1998;95:13899–13904. doi: 10.1073/pnas.95.23.13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahr TL, Wolfgang MC. Transcriptional regulation of the Pseudomonas aeruginosa type III secretion system. Mol. Microbiol. 2006;62:631–640. doi: 10.1111/j.1365-2958.2006.05412.x. [DOI] [PubMed] [Google Scholar]
- Zhang RG, Skarina T, Katz JE, Beasley S, Khachatryan A, Vyas S, Arrowsmith CH, Clarke S, Edwards A, Joachimiak A, Savchenko A. Structure of Thermotoga maritima stationary phase survival protein SurE: a novel acid phosphatase. Structure. 2001;9:1095–1106. doi: 10.1016/s0969-2126(01)00675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.