Abstract
Apolipoprotein E (apoE) is the primary recognition signal on triglyceride-rich lipoproteins responsible for interacting with low density lipoprotein (LDL) receptors and LDL receptor-related protein (LRP). It has been shown that lipoprotein lipase (LPL) and hepatic triglyceride lipase (HTGL) promote receptor-mediated uptake and degradation of very low density lipoproteins (VLDL) and remnant particles, possibly by directly binding to lipoprotein receptors. In this study we have investigated the requirement for apoE in lipase-stimulated VLDL degradation. We compared binding and degradation of normal and apoE-depleted human VLDL and apoE knockout mouse VLDL in human foreskin fibroblasts. Surface binding at 37°C of apoE knockout VLDL was greater than that of normal VLDL by 3-and 40-fold, respectively, in the presence of LPL and HTGL. In spite of the greater stimulation of surface binding, lipase-stimulated degradation of apoE knockout mouse VLDL was significantly lower than that of normal VLDL (30, 30, and 80%, respectively, for control, LPL, and HTGL treatments). In the presence of LPL and HTGL, surface binding of apoE-depleted human VLDL was, respectively, 40 and 200% of normal VLDL whereas degradation was, respectively, 25 and 50% of normal VLDL. LPL and HTGL stimulated degradation of normal VLDL in a dose-dependent manner and by a LDL receptor-mediated pathway. Maximum stimulation (4-fold) was seen in the presence LPL (1 µg/ml) or HTGL (3 µg/ml) in lovastatin-treated cells. On the other hand, degradation of apoE-depleted VLDL was not significantly increased by the presence of lipases even in lovastatin-treated cells. Surface binding of apoE-depleted VLDL to metabolically inactive cells at 4°C was higher in control and HTGL-treated cells, but unchanged in the presence of LPL. Degradation of prebound apoE-depleted VLDL was only 35% as efficient as that of normal VLDL. Surface binding of apoE knockout or apoE-depleted VLDL was to heparin sulfate proteoglycans because it was completely abolished by heparinase treatment. However, apoE appears to be a primary determinant for receptor-mediated VLDL degradation.
Supplementary key words: heparan sulfate proteoglycans, LDL receptors, fibroblasts, apoE knockout mice
It is well known that apolipoprotein E (apoE) is a high affinity ligand for all members of the low density lipoprotein (LDL) receptor family (1, 2). Early work by Mahley and associates (3, 4) demonstrated that the affinity of apoE-containing phospholipid disks for LDL receptors was enhanced exponentially by increasing their apoE content. Chemical cross-linking experiments have established that LDL receptor-related protein (LRP) recognizes the apoE component on very low density lipoproteins (VLDL) and remnant particles (5, 6). Takahashi et al. (7) discovered the VLDL receptor as an apoE-specific member of the LDL receptor family. Lipoprotein binding to the VLDL receptor was enhanced by supplementation with exogenous apoE (8). Type III hyperlipoproteinemia, characterized by a marked elevation in plasma β-VLDL concentration, results from homozygosity for apoE isoforms that are receptor binding defective (9, 10). Chylomicron remnants, VLDL, and intermediate density lipoprotein particles accumulate in apoE knockout mice, resulting in hypercholesterolemia (11, 12). Adenovirus-mediated replacement of apoE in these mice corrects thelipoprotein profile (13). Thus there is ample evidence indicating that apoE promotes receptor-mediated lipoprotein catabolism.
The binding and degradation of VLDL and remnant particles by lipoprotein receptors are enhanced several-fold by lipoprotein lipase (LPL) and hepatic triglyceride lipase (HTGL) (6, 14–21). Several investigators have shown that this stimulatory effect of lipases is independent of lipolytic activity. The effect of LPL may at least partially be mediated by the ability of LPL to directly bind to LRP and LDL receptors (15, 22). LPL is internalized and degraded in cultured cells by LDL- as well as LRP-mediated pathways and in cell-free assays it binds purified LRP in a dose-dependent manner (23, 24). We have shown that LPL binds to LDL receptors as well, albeit with a much lower affinity (15). Strickland and associates (19) demonstrated that HTGL directly binds to LRP, and in HepG2 cells, it is internalized and degraded by LRP. Although LDL receptors have been implicated in HTGL-promoted degradation of VLDL particles, the direct binding of HTGL to LDL receptors has not yet been demonstrated (18).
While apoE undoubtedly facilitates lipoprotein uptake and degradation independent of lipases, it is not clear whether lipase-stimulated lipoprotein degradation requires the presence of apoE. As it has been demonstrated that LPL can directly bind to lipoprotein receptors, it is possible that LPL-VLDL complexes undergo receptor-mediated but apoE-independent internalization and degradation. In this study we have investigated the requirement for apoE in lipase-stimulated degradation of VLDL particles. The studies were performed with normal human skin fibroblasts with basal or upregulated LDL receptors. These cells do not synthesize or secrete apoE. ApoE-deficient VLDL was isolated from apoE knockout mice. We also isolated apoE-lacking particles from normal human VLDL by heparin-Sepharose chromatography. Results presented here suggest that apoE was not required for lipase-promoted cell surface binding of VLDL, a large component of which was mediated by heparan sulfate proteoglycans (HSPG). However, even in the presence of lipases, degradation of VLDL particles was inefficient in the absence of apoE.
MATERIALS AND METHODS
Materials
LDL (d = 1.02 to 1.05 g/ml), HDL (d = 1.05 to 1.25 g/ml), and VLDL (d < 1.006 g/ml) particles were isolated by ultracentrifugation of plasma from fasted normolipidemic human subjects with the most common apoE phenotype (E3/3). VLDL were further fractionated by sequential ultracentrifugal flotation to isolate particles with Sf 20–400. Bovine milk LPL was isolated by heparin-Sepharose chromatography as described previously (25). Recombinant human HTGL was produced in Chinese hamster ovary cells and purified from the culture medium (26). Intralipid emulsion (10%) was obtained from the University of Iowa hospital pharmacy. Heparinase was purchased from Sigma (St. Louis, MO). Heparin-Sepharose Fast Flow 6B was from Pharmacia (Uppsala, Sweden). Polyclonal antibody specific against apoCs (IgG Rb23) was developed in rabbits using human apoC-I isolated from VLDL as an antigen. Monoclonal antibodies against apoB-100 (IgG 4G3) and apoE (IgG 1D7) were a gift from R. Milne (Clinical Research Institute of Montreal, Quebec, Canada). Recombinant adenoviral vectors for human LPL (huLPL) and LacZ were produced by cotransfection of 293 cells with pJM17 and the adenovirus shuttle plasmid pAdRSV containing the gene of interest under the control of the Rous sarcoma virus (RSV) promoter. Wild-type C57BL/6 and apoE knockout mice were obtained from Jackson Laboratory (Bar Harbor, ME).
Preparation of protein-free triglyceride emulsions
Protein-free particles with Sf 100–400 were isolated from a 10% Intralipid emulsion (Travenol) by ultracentrifugal flotation, and their triglyceride content was estimated by the GPO-Trinder calorimetric assay (Sigma). They were labeled with [3H]cholesteryl oleyl ether, a nondegradable marker of cellular uptake (17). A glass tube containing 0.5 ml of modified Eagle’s medium, bovine serum albumin (BSA, 4 mg/ml), and 35 µCi of [3H]cholesteryl oleyl ether (Amersham, Arlington Heights, IL) was sonicated for 10 min at room temperature. Intralipid particles with Sf 100–400 containing 3–4 mg of triglycerides were added, and the mixture was incubated at 37°C for 20 min and then returned to room temperature. This treatment resulted in the incorporation of [3H]cholesteryl oleyl ether in the emulsion. The tritiated lipid emulsions were stored at 4°C overnight before use.
Isolation of apoE-deficient VLDL from mouse plasma
ApoE-free VLDL was isolated from apoE knockout mouse plasma (11, 12). Briefly, apoE knockout mice aged 5 to 10 months were fasted for 16–20 h prior to collecting blood by cardiac puncture. After separating blood cells, the plasma from 5 to 10 mice was pooled and centrifuged at 38,000 rpm for 18 h, allowing the VLDL fraction to float to the top of the tube. This was removed and salted to a density of 1.065 with NaCl. The VLDL was then subjected to sequential ultracentrifugal flotation as described earlier to isolate the Sf > 400 and Sf 20–400 subfractions. The Sf 20–400 fraction was characterized for apoprotein composition by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and was found to contain apoB and apoCs. The apoB band was separated on a 5% gel to reveal predominantly apoB-48 and some apoB-100. Using the same protocol, we also isolated Sf 20–400 particles from the plasma of wild-type C57BL/6 mice. However, as normal mice do not accumulate VLDL particles the yield was low and not enough to iodinate.
Preparation of normal and apoE-depleted human VLDL
Human VLDL particles with Sf 20–400 were isolated as described previously by ultracentrifugation of plasma from fasted normolipidemic human subjects with the most common apoE phenotype (E3/3). We isolated apoE-lacking particles from human VLDL by affinity chromatography on a heparin-Sepharose column as described previously (27). Briefly, normal human VLDL (Sf 20–400) particles were adsorbed to 20 ml of heparin-Sepharose (Pharmacia) at 4°C for 16 h in buffer containing 0.05 M NaCl, 5 mM Tris-HCl (pH 7.4), and 25 mM MnCl2 with constant gentle mixing. The Sepharose was then packed into a 2.5-cm-wide column and eluted into five subfractions designated as fractions 1 to 5, respectively, using buffer containing 0.05, 0.12, 0.2, 0.5, and 2 M NaCl. It has been shown that isolated VLDL particles are intact after isolation by this procedure (27). Western blotting with polyclonal antibodies against apoB-100, apoE, and apoCs identified the apolipoprotein content of each fraction. In our separation, apoE was not detected in pools 1 and 2 eluted with 0.05 and 0.12 M NaCl. Only negligible amounts of apoE were present in pool 3 eluted with 0.2 M NaCl (Fig. 4A). However both apoB-100 and apoCs were present in all pools. Pools 1, 2, and 3 were mixed and designated as apoE-depleted VLDL particles and the apolipoprotein content was verified by Western blotting (Fig. 4B). We were able to obtain ~250 µg of apoE-depleted VLDL from 10 mg of starting material.
Fig. 4.
Preparation of apoE-depleted human VLDL. Normal human VLDL with Sf 20–400 was isolated and adsorbed to a heparin-Sepharose column. The column was then subjected to elution with increasing concentrations of NaCl. Pools 1 to 5 were eluted, respectively, with 0.05, 0.12, 0.2, 0.5, and 2 M NaCl. Each of the fractions was resolved by 5 –20% continuous gradient SDS-PAGE, transferred to PVDF membrane, and subjected to Western blotting with antibodies against apoB-100 (IgG 4G3), apoE (IgG 1D7), and apoCs (IgG Rb23). The apolipoprotein pattern for the starting material is shown alongside (A). Pools 1, 2, and 3, which were found lacking in apoE, were mixed and designated as apoE-depleted VLDL. The apolipoprotein content of this mixture is shown in (B). (C) The separation of apoB-100 and apoB-48 in normal human VLDL, apoE-depleted human VLDL, and apoE knockout mouse VLDL on a 5% SDS-polyacrylamide gel by Coo-massie blue staining.
Iodination of VLDL
Lipoprotein particles including normal and apoE-depleted human VLDL particles and apoE-deficient mouse VLDL were iodinated to specific activities of 300–500 cpm/ng by the iodinemonochloride method (28). SDS-PAGE of iodinated VLDL particles showed that for all species of VLDL the majority of the label was on the apoB component. Even in apoE-containing particles, the label on apoE was insignificant (data not shown).
Cell-binding assays
Normal human foreskin fibroblasts cells were cultured as described (15, 18). LDL receptors were upregulated by incubation prior to the assay for 48 h with medium containing lipoprotein-deficient serum (LPDS, 2 mg/ml), the final 24 h of which was in the presence of lovastatin (1 µg/ml) (29, 30). In control cells (–lovastatin) LDL receptors were downregulated because the incubation medium was supplemented with lipoprotein-containing fetal bovine serum. The cells were washed twice with N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES)/saline/BSA buffer [5 mM HEPES (pH 7.4), 5 mM MgSO4, 0.18 mM CaCl2, 0.54 mM KCl, 13.7 mM NaCl, fatty acid-free BSA (4 mg/ml)]. Surface binding to metabolically inactive cells was studied after incubating cells with 125I-labeled ligands for 3 h at 4°C as previously described (15, 18). Steady state sur face binding and ligand degradation were measured after incubating cells with radiolabeled ligands at 37°C for 5 h (15, 18). At the end of the incubation the culture medium was removed and adjusted to 15% trichloroacetic acid and protein at 5 mg/ml (BSA or LPDS). The soluble fraction was extracted with chloroform to remove lipid-associated radioactivity. Degradation was defined as the trichloroacetic acid-soluble radioactivity in the incubation medium that was not extracted with chloroform. The cell monolayers were washed three times with HEPES/saline/BSA buffer followed by two washes with PBS. They were then incubated in buffer containing tripolyphosphate (10 mg/ml). Sur face binding and internalization were defined, respectively, as radioactivity released and remaining cell-associated after incubating cells at 4°C for 1 h in the tripolyphosphate buffer (31). For surface binding at 4°C, tripolyphosphate dissociated more than 90% of radioactivity from the cells. It is expected that tripolyphosphate displaces ligand from receptor as well as HSPG sites. Nonspecific binding was also determined with a 70-fold excess of unlabeled VLDL and, as with tripolyphosphate displacement, was found to be less than 10%. The cells were solubilized in buffer containing 0.1% SDS. Total cellular protein for each well was determined by the Lowry assay (32) and varied by less than 15% within each experiment. Wells treated with lovastatin or LPDS contained ~60% of the protein amounts present in untreated wells. Thus, results are corrected for protein per well. All experiments were repeated at least three times with similar results. Each figure represents data using the same batch of cells and radioligand. Error bars represent standard deviation from triplicate determinations. Statistical significance is specified in the figure legends. Variability between experiments is inherent to the nature of the cell line as reported by Goldstein et al. (30).
Clearance of 125I-labeled VLDL from mouse plasma
Wild-type C57BL/6 or apoE knockout mice were injected through the tail vein with ~3 × 1011 particles of recombinant adenovirus encoding either huLPL (AdLPL) or LacZ (AdLacZ) under the control of the RSV promoter. Hepatocytes were isolated by collagenase perfusion of the liver from one mouse of each group. Expression of viral proteins was confirmed in control mice by staining hepatocytes isolated from AdLacZ-injected mice for β-galactosidase. Human LPL protein was identified in the culture medium of hepatocytes isolated from mice injected with AdLPL (data not shown). For clearance studies, 4 days after infection, the mice were injected with apoE knockout mouse 125I-labeled VLDL (1 × 106 cpm) via the femoral vein. At various times after the injection blood was collected via a tail clip into heparinized capillary tubes. The tail tip was held tightly between collections to prevent bleeding. The plasma was separated by centrifugation and the radioactivity in 10 µl of sample was determined. The results are presented as a percentage of the radioactivity present in the 1-min sample, which was approximately 2,000 cpm.
RESULTS
We have used normal foreskin fibroblasts for all our studies. These cells do not synthesize apoE but express both LDL receptors and LRP. The LDL receptor levels can be upregulated by maintaining cells in LPDS and by treatment with lovastatin, an inhibitor of endogenous cholesterol biosynthesis (30). Conversely, LDL receptors can be downregulated by supplementing the cell culture medium with cholesterol-containing serum. LRP or VLDL receptor expression is not regulated by treatment with lovastatin or sterols (15, 33). Thus, we and others have frequently used lovastatin treatment to identify a role for LDL receptors in lipoprotein binding and degradation. It has been shown that binding of normal VLDL to LDL receptors is mediated by apoE (2, 3, 31). Lipoproteins enriched with exogenously added apoE can also bind to LRP (5, 34, 35). We and others have shown previously that LPL and HTGL enhance the LDL receptor-mediated binding and degradation of lipoprotein particles (15, 16, 18, 36, 37). However, the role of apoE in this lipase-mediated process in not clear. Here, we have investigated the requirement of apoE in lipase-mediated VLDL catabolism.
LPL and HTGL enhance binding and internalization of protein-free Intralipid emulsions
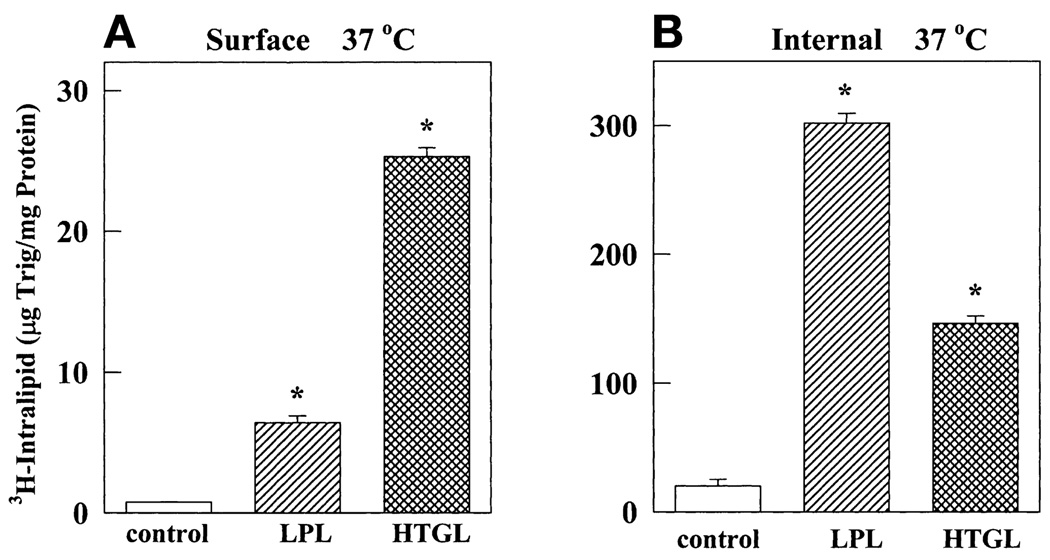
To study the contribution of apoE in lipase-mediated lipoprotein binding and endocytosis, we determined whether LPL and HTGL promote the binding and internalization of apoprotein-free triglyceride/phospholipid emulsion particles (Intralipid). Intralipid was subjected to ultracentrifugal floatation to isolate particles with Sf 100–400. This fraction was labeled with tritiated nondegradable cholesteryl oleyl ether to monitor binding and internalization at 37°C. However, because the radiotag is nondegradable, these particles could not be used to measure degradation. As shown in Fig. 1, in the absence of lipases, normal fibroblasts with upregulated LDL receptors showed negligible amounts of cell surface binding and internalization of Intralipid emulsion. The presence of either LPL or HTGL dramatically increased the binding and internalization of these particles. LPL and HTGL stimulated binding by 8- and 30-fold, respectively, and internalization by 150- and 75-fold, respectively. Because the emulsions are apolipoprotein free, it appears that neither apoE nor other apolipoproteins are required for the surface binding- and endocytosis-promoting functions of lipases. Interestingly, the effect of HTGL on surface binding was greater than that of LPL. On the other hand, internalization was higher in the presence of LPL. It is believed that receptor-mediated endocytosis is more efficient than a receptor-independent internalization pathway (16). We have shown earlier that LPL can directly bind to LDL receptors; the direct binding of HTGL with LDL receptors has not yet been demonstrated (15, 18). In earlier reports we have demonstrated that internalization of Intralipid in the presence of LPL is partially mediated by LDL receptors (15) whereas HTGL-mediated internalization is dependent on HSPG (18).
Fig. 1.
LPL and HTGL promote Intralipid binding and internalization by normal fibroblasts. Normal human foreskin fibroblasts (FSF) were treated with LPDS and lovastatin as described in Materials and Methods. They were then incubated at 37°C in medium containing triglyceride (100 µg/ml) in [3H]cholesterol oleyl ether-labeled emulsions with Sf 100–400 in the presence of LPL (1 µg/ml) or HTGL (3 µg/ml). After 5 h, unbound ligand was removed by washing. Surface binding (A) and internalization (B) were determined, respectively, as the radioactivity that dissociated and remained cell associated after incubating cells for 1 h at 4°C with tripolyphosphate (10 mg/ml). The asterisk (*) represents a P value < 0.0001 compared with control.
ApoE-deficient VLDL shows greater surface binding but reduced degradation
Receptor-mediated endocytosis generally leads to rapid lysosomal degradation. Therefore, we next studied the role of apoE in lipase-stimulated VLDL degradation. To obtain apoE-deficient VLDL particles, we isolated plasma VLDL from apoE knockout mice. The data in Fig. 2 compare binding and degradation of normal human VLDL and apoE knockout mouse VLDL. We found that, in the presence of lipases, surface binding at 37°C of apoE knockout VLDL was significantly higher than that of normal VLDL (Fig. 2A and C). This was particularly striking in the presence of HTGL. LPL and HTGL stimulated surface binding of apoE-deficient mouse VLDL at 37°C by 20- and 100-fold, respectively, compared with a 2- and 1.5-fold stimulation of normal VLDL. Surface binding of apoE knockout VLDL was greater than that of normal VLDL by 3- and 40-fold, respectively, in the presence of LPL and HTGL. The level of surface binding was not dependent on LDL receptor expression as evidenced by the virtually identical binding in lovastatin-treated (Fig. 2A) and -untreated cells (Fig. 2C). On the other hand, degradation was dependent on LDL receptor expression because it was higher in lovastatin-treated cells (Fig. 2B) versus basal cells (Fig. 2D). The most interesting observation was that degradation of apoE-deficient VLDL was lower than that of normal VLDL in spite of the surface binding being so high. Degradation of apoE knockout mouse VLDL was only 30, 30, and 80% that of normal VLDL for control, LPL, and HTGL treatments, respectively. These data led us to hypothesize that in the absence of apoE, LDL receptor-independent surface binding is increased but degradation, which is receptor dependent, is inefficient. Thus apoE may facilitate degradation. In fact, the observed increase in surface binding of apoE-deficient VLDL may be from an inability of the bound ligand to be degraded in the absence of apoE.
Fig. 2.
LPL- and HTGL-stimulated surface binding of apoE knockout mouse VLDL is much greater than that of normal human VLDL but degradation is not proportionately enhanced. Fibroblasts were treated with either LPDS and lovastatin (A and B) or maintained in lipoprotein-containing medium (C and D) as described in Materials and Methods. They were then incubated for 5 h at 37°C in medium containing 125I-labeled VLDL (5 µg/ml) alone or in the presence of LPL (1 µg/ml) or HTGL (3 µg/ml). The VLDL used was isolated either from normal human plasma (normal, open bars) or from apoE knockout mice (apoE-KO, shaded bars). After washing unbound ligand, surface-bound radioactivity was dissociated by incubating cells for 30 min at 4°C in buffer containing polyphosphate at 10 mg/ml (A and C). Degradation was measured as the radioactivity in the incubation medium that was soluble in 15% trichloroacetic acid (B and D). Results are averages of triplicate measurements. Symbols on the figures represent statistical significance. P values versus the corresponding value for normal VLDL are shown, with * P < 0.0001, % P < 0.001, and @ P < 0.01. P values versus corresponding minus-lipase control are also shown, with # P < 0.0001, $ P < 0.001, and & P < 0.01.
It is clear from Fig. 2 that upregulation of LDL receptors did not influence surface binding at 37°C but increased degradation of both normal and apoE-deficient VLDL. This observation supports the idea that cell surface HSPG may be the initial binding site responsible for sequestration of VLDL particles and that this process may be LDL receptor and apoE independent (1). The subsequent degradation may be LDL receptor mediated. Lovastatin treatment increased lipase-stimulated degradation by about 4-fold for normal VLDL but by less than 2-fold for apoE knockout VLDL. The substantially smaller increase increase in apoE knockout VLDL degradation (compared with normal VLDL) on upregulation of LDL receptors suggests that even though some degradation of apoE knockout VLDL may proceed by an LDL receptor-dependent mechanism in the presence of lipases, apoE greatly enhances receptor-mediated degradation.
Cell surface heparin sulfate proteoglycans mediate binding and degradation of apoE-deficient VLDL
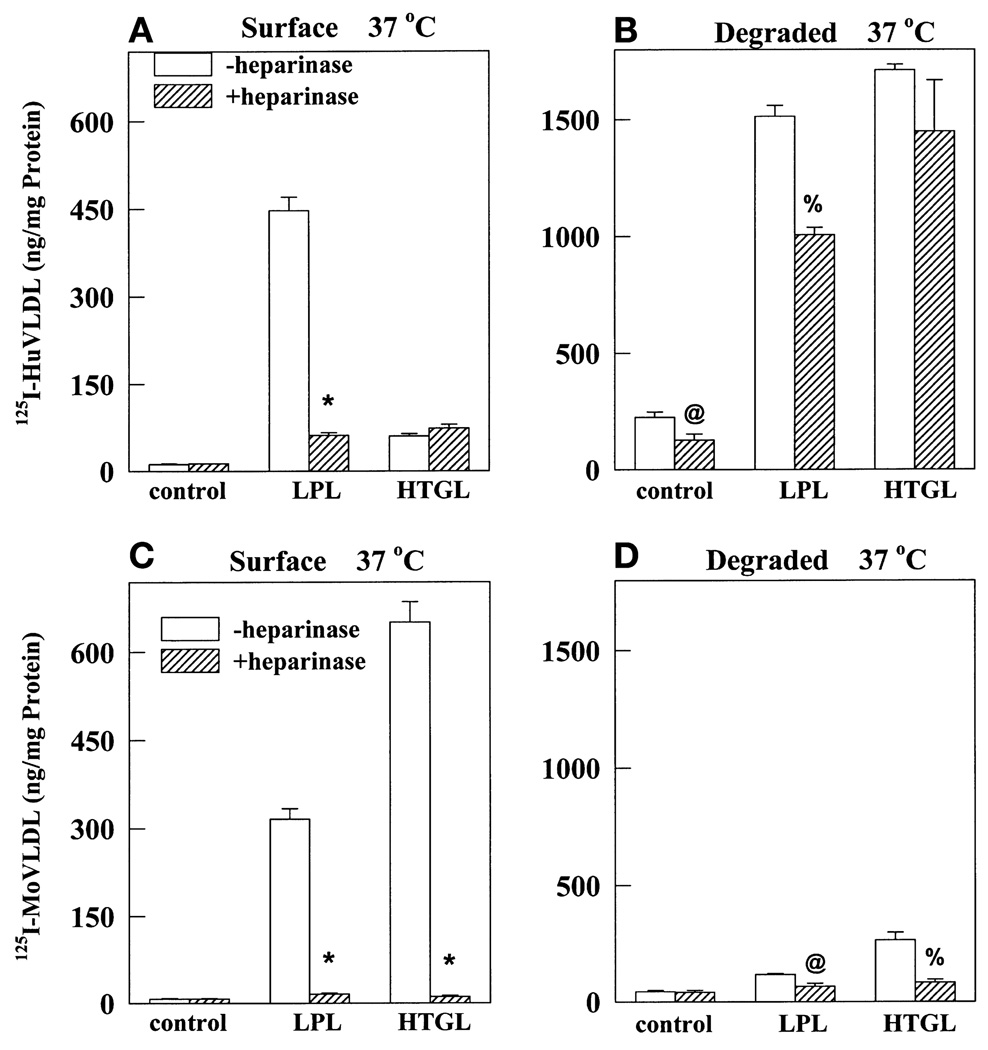
In addition to a receptor-mediated pathway, endocytosis and degradation may also proceed via HSPG. To determine the contribution of HSPG in binding and degradation of apoE-deficient VLDL, we preincubated cells with heparinase prior to the binding assay. Heparinase is known to digest cell surface HSPG (38). In addition, ligand binding to HSPG sites was competitively inhibited by the presence of heparin at 100 µg/ml. At this low concentration heparin competes with VLDL binding to HSPGs. Displacement of LDL receptor-bound VLDL requires heparin at a concentration of 10 mg/ml (30). As shown in Fig. 3, heparinase/heparin treatment completely abolished lipase-promoted binding and degradation of apoE-deficient VLDL (Fig. 3C and D). On the other hand, heparinase treatment had no effect on surface binding of normal VLDL in the presence of HTGL and it reduced LPL-stimulated binding to 16% (Fig. 3A). Even so, definite lipase-dependent and HSPG-independent binding was evident for normal VLDL but not for apoE knockout VLDL (hatched bars, Fig. 3A and C). Control or lipase-stimulated degradation of normal VLDL was not significantly affected by heparinase treatment (Fig. 3B) whereas degradation of apoE-deficient VLDL was relatively minuscule even in the absence of heparinase and presence of lipases (open bars, Fig. 3D). This experiment suggests that the presence of apoE allows direct binding of lipase-VLDL complexes to non-HSPG sites, probably LDL receptors, resulting in VLDL degradation. On the other hand, in the absence of apoE, even lipase-promoted binding is almost completely to HSPG, leading to negligible amounts of degradation.
Fig. 3.
Heparinase treatment completely inhibits LPL- and HTGL-stimulated binding and degradation of apoE knockout mouse VLDL but not of normal humanVLDL. LDL receptors on normal fibroblasts were upregulated with LPDS and lovastatin as described. The cells were preincubated at 37°C for 30 min in the presence (shaded bars) or absence (open bars) of heparinase (0.01 unit/ml). After washing, cells were incubated with a 5-µg/ml concentration of normal human 125I-labeled VLDL (A and B) or apoE knockout mouse 125I-labeled VLDL (C and D) alone or in the presence of LPL (1 µg/ml) or HTGL (3 µg/ml). During this incubation, heparin (10 µg/ml) was added to wells treated with heparinase. After 5 h at 37°C, cells were washed and surface-bound (A and C) and degraded (B and D) ligand was measured as described in Fig. 2. Results are averages of triplicate measurements. P values versus the corresponding value in the absence of heparinase treatment are represented, with * P < 0.0001, % P < 0.001, and @ P < 0.01.
Preparation and characterization of apoE-depleted human VLDL
In the experiments described thus far, we have compared the binding characteristics of normal human VLDL and apoE knockout mouse VLDL. We investigated whether the differences in binding/degradation may be due to differences in human versus mouse VLDL composition. As has been described earlier (11, 12), we found that mouse VLDL particles consist predominantly of apoB-48 rather than apoB-100. However, we have shown earlier that 75 to 90% of VLDL binding to LDL receptors is mediated by apoE and is independent of apoB-100 (31). The composition of the core lipids in the two species may also be different. We determined the cholesterol and triglyceride content of apoE knockout mouse VLDL and normal human VLDL. Although their cholesterol concentrations were comparable (human VLDL: 2.57 ± 0.3 mmol/g protein vs. mouse VLDL: 2.83 ± 0.22 mmol/g protein, n = 6), apoE knockout mouse VLDL was much more cholesterol-rich (human VLDL: 2.92 ± 0.21 mmol/g protein vs. mouse VLDL: 26.3 ± 1.05 mmol/g protein, n = 6). To ascertain that the observed differences were not due to species-specific variations in the composition of human and mouse VLDL but a function of apoE, we isolated a fraction of apoE-depleted human VLDL. For this we used a procedure published by Trezzi et al. (27). The method involves separation of VLDL particles into different subfractions on the basis of their affinity for heparin-Sepharose. Because apoE has a strong affinity for heparin, higher salt concentrations are required to dissociate apoE-rich particles from a heparin-Sepharose column whereas apoE-lacking particles elute at lower concentrations of NaCl. Figure 4A is a Western blot analysis showing apolipoprotein composition of the starting material loaded on a heparin-Sepharose column and the subfractions (pools 1 to 5) eluted with increasing salt concentrations. The presence of apoB-100, apoE, and apoCs in each sample was determined using, respectively, IgG 4G3, IgG 1D7, and IgG Rb23. The starting material was normal human VLDL with Sf 20–400 and pools 1 to 5 were eluted sequentially with 0.05, 0.12, 0.2, 0.5, and 2 M NaCl, respectively. Pool 4 was the most protein-rich fraction. This pool was diluted 100-fold prior to loading on the gel. Ten microliters of each sample was loaded. As shown in Fig. 4A, the starting material contained apoB-100, apoE, and apoCs. In pools 1 and 2 there was no detectable apoE whereas in pool 3 there were negligible amounts of apoE, relative to the abundant amounts of apoB-100 and apoCs. Pools 4 and 5 contained significant amounts of apoE. Pool 1 (68 µg of protein), pool 2 (53 µg of protein), and pool 3 (142 µg of protein) were combined and the mixture was designated as apoE-depleted human VLDL. Figure 4B compares the lipoprotein composition of the starting material (Sf 20–400) and apoE-depleted VLDL. Although the concentrations of apoB and apoCs in the two VLDL are comparable, apoE-depleted VLDL is completely lacking in apoE, because it was not detected even after prolonged exposure of the blot. We also ascertained the absence of apoE in the preparation by silver staining. Further analysis to separate apoB-100 and apoB-48 showed that while normal human VLDL contains mostly apoB-100, apoE-depleted VLDL has a roughly equal distribution of the two apoBs (Fig. 4C). Trezzi et al. have shown that the VLDL particles are intact after isolation. They determined that particle size as well as triglyceride content decrease whereas the protein content increases from fraction 1 to fraction 5. The cholesterol (3.55 ± 0.12 mmol/g protein, n = 6) and triglyceride (3.33 ± 0.08 mmol/g protein, n = 6) content of apoE-depleted VLDL was similar to that of normal human VLDL (Table 1) with a cholesterol-to-triglyceride ratio of 1.07 (compared with 1.13 for normal human VLDL).
TABLE 1.
Cholesterol and triglyceride content of VLDL preparationsa
| Cholesterol (n = 6) | Triglyceride (n = 6) | Cholesterol: Triglyceride Ratio | |
|---|---|---|---|
| mmol/g protein | |||
| Normal huVLDL | 2.92 ± 0.21 | 2.57 ± 0.3 | 1.13 |
| ApoE-depleted huVLDL | 3.55 ± 0.12 | 3.33 ± 0.08 | 1.07 |
| ApoE knockout moVLDL | 26.3 ± 1.05 | 2.83 ± 0.22 | 9.4 |
VLDL preparations were analyzed for cholesterol (cholesterol oxidase method) and triglyceride (GPO-Trinder method) content, using diagnostic kits from Sigma.
ApoE is required for lipase-promoted VLDL degradation
In subsequent experiments we have compared the binding properties of normal and apoE-depleted human VLDL. Figure 5 shows surface binding and degradation of normal and apoE-depleted human VLDL by basal and lovastatin-treated fibroblasts. The results obtained for apoE-depleted human VLDL (Fig. 5) were generally similar to those obtained with apoE knockout mouse VLDL (Fig. 2). In lovastatin-treated cells, in the presence of LPL and HTGL, surface binding of apoE-depleted human VLDL was, respectively, 40 and 200% of normal VLDL (Fig. 5A). Surface binding was not to LDL receptors because it was not increased by lovastatin treatment. Similar to results in Fig. 3, a majority of surface binding of apoE-depleted VLDL was mediated by HSPG (data not shown). Surface binding of apoE-depleted VLDL was higher than that of normal VLDL in the presence or absence of HTGL in both basal (Fig. 5C) and lovastatin-treated (Fig. 5A) normal fibroblasts. However, in the presence of LPL, binding of normal VLDL was greater than that of apoE-depleted VLDL (Fig. 5A and C). The reason for this difference between the two lipases is not clear. Degradation of apoE-depleted VLDL was significantly lower than that of normal VLDL in spite of the presence of lipases (Fig. 5B and D). In fibroblasts with upregulated LDL receptors, degradation in the absence of lipases was similar for normal and apoE-depleted VLDL (Fig. 5B), in spite of much higher surface binding of the latter. In the presence of LPL and HTGL, degradation of apoE-depleted VLDL was, respectively, 25 and 50% of normal VLDL (Fig. 5B). LPL and HTGL increased degradation of normal VLDL by 7.5-and 4-fold, respectively, whereas degradation of apoE-depleted VLDL was increased by less than 2-fold each. When LDL receptors were not induced, degradation of apoE-depleted VLDL was totally absent (Fig. 5D). This result suggests that in lovastatin-treated fibroblasts, apoE-depleted VLDL undergoes LDL receptor-mediated degradation either via apoB-100, a known ligand for LDL receptors, or via small amounts of apoE that may be present but were not detected by Western blot analysis. However, the degradation of apoE-depleted VLDL is small and is not significantly induced by lipases. This experiment reinforces the hypothesis that apoE is required for lipase-promoted VLDL degradation by the LDL receptor pathway.
Fig. 5.
Depletion of apoE from human VLDL increases surface binding but decreases lipase-stimulated degradation by fibroblasts. Fibroblasts were treated with either LPDS and lovastatin (A and B) or maintained in lipoprotein-containing medium (C and D) as described in Materials and Methods. They were then incubated for 5 h at 37°C in medium containing a 5-µg/ml concentration of normal (open bars) or apoE-depleted (shaded bars) human 125I-labeled VLDL alone or in the presence of LPL (1 µg/ml) or HTGL (3 µg/ml). Surface binding (A and C) and degradation (B and D) were estimated as described in Fig. 2. Results are averages of triplicate measurements. Symbols represent statistical significance. P values versus corresponding value for normal VLDL: *P < 0.0001, % P < 0.001, and @ P < 0.01. P values versus value for corresponding minus-lipase control: # P < 0.0001, $ P < 0.001, and & P < 0.01.
We further investigated the cooperation between lipases and apoE in LDL receptor-mediated VLDL degradation (Fig. 6). Here we compared the degradation of normal and apoE-depleted VLDL in the presence of increasing concentrations of LPL (Fig. 6A) or HTGL (Fig. 6B) by basal or lovastatin-treated fibroblasts. As expected, degradation of normal VLDL (closed symbols) was stimulated by LPL and HTGL in a dose-dependent manner. Maximum stimulation was seen in lovastatin-treated cells in the presence of LPL (1 µg/ml) (~8-fold stimulation) or HTGL (3 µg/ml) (4-fold stimulation). The increase was also evident in cells without LDL receptor upregulation, albeit it was only 25% or lower than in upregulated cells. The dose-response curves for degradation of apoE-depleted VLDL were relatively flat, with maximum degradation being only a fourth of that of normal VLDL. Although degradation of apoE-depleted VLDL was higher in lovastatin-treated cells, it was clearly not a lipase-dependent increase. Thus, in the absence of apoE, neither LPL nor HTGL substantially promote VLDL degradation.
Fig. 6.
LPL and HTGL do not promote degradation of apoE-depleted VLDL. Fibroblasts were treated with either LPDS and lovastatin (closed symbols) or maintained in lipoprotein-containing medium (open symbols) as described in Materials and Methods. They were then incubated for 5 h at 37°C in medium containing a 5-µg/ml concentration of normal (circles) or apoE-depleted (squares) human 125I-labeled VLDL in the presence of increasing concentrations of LPL (A) or HTGL (B). Degradation was estimated as described in Fig. 2. Results are averages of triplicate measurements.
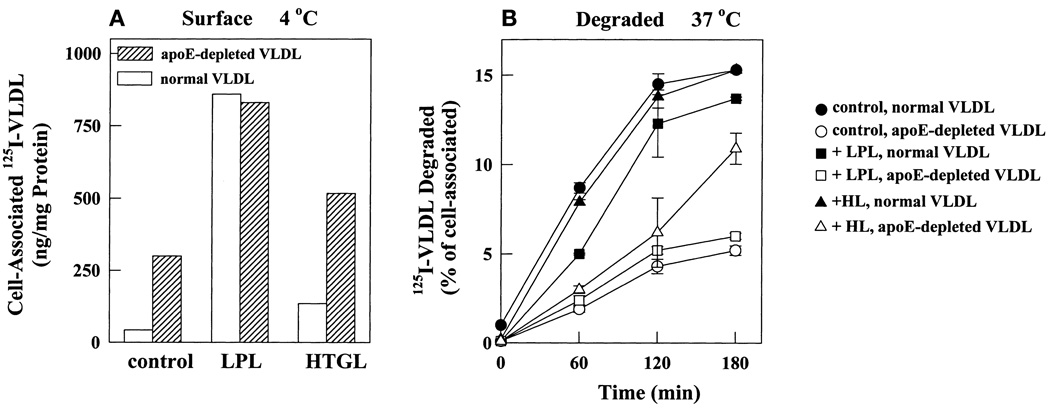
Depletion of apoE inhibits degradation of surface-bound VLDL
We compared cell surface binding of normal and apoE-depleted VLDL to metabolically inactive cells at 4°C (Fig. 7A). Similar to observations at 37°C, surface binding of apoE-depleted VLDL at 4°C was significantly higher than that of normal VLDL; 6.5- and 4-fold, respectively, in the absence and presence of HTGL. However, there was no difference in surface binding of normal and apoE-depleted VLDL in the presence of LPL. As shown in Fig. 3 for apoE-deficient mouse VLDL, HSPG play a major role in surface binding of apoE-depleted human VLDL as well (data not shown). Here we investigated whether HSPG-bound apoE-depleted VLDL was efficiently internalized and degraded by cells with upregulated LDL receptors. Cells with surface-bound ligand were transferred to 37°C and the kinetics of degradation were monitored (Fig. 7B). For consistency between conditions, results are expressed as a percentage of cell-associated ligand and are averages of duplicate determinations. When presented in this manner, degradation time course curves in the presence and absence of lipases were overlapping. The efficiency of degradation was three times lower for apoE-depleted VLDL than for normal VLDL irrespective of the presence or absence of lipases. By 3 h degradation of normal VLDL had leveled off and 15% of cell-associated ligand had degraded. Only 5% of cell-associated apoE-depleted VLDL was degraded in that time. Thus even in the presence of LPL and HTGL, which may bind directly to LDL receptors, surface binding of apoE-depleted VLDL was normal or high but degradation was significantly inhibited. This experiment clearly indicates that degradation of apoE-depleted VLDL proceeds by a mechanism much slower than LDL receptor-mediated degradation of normal VLDL.
Fig. 7.
Depletion of apoE from human VLDL retards degradation of prebound ligand. Fibroblasts with upregulated LDL receptors were incubated at 4°C with medium containing a 5-µg/ml concentration of 125I-labeled labeled normal or apoE-depleted VLDL alone or in the presence of LPL (1 µg/ml) or HTGL (3 µg/ml). After 2 h unbound ligand was removed by washing and cell monolayers were either solubilized to determine total cell-associated radioactivity (A) or were transferred to 37°C for 0 to 180 min and degradation was measured as in Fig. 2 (B). Degradation in (B) is represented as a percentage of total cell-associated ligand shown in (A).
In vivo clearance of apoE-deficient mouse VLDL is augmented in the presence of endogenous apoE
We next studied the in vivo effect of lipase overexpression on clearance of apoE knockout mouse VLDL from the plasma. We injected wild-type C57BL/6 mice and apoE knockout mice with LPL-expressing adenovirus (AdLPL) or a control virus expressing LacZ (AdLacZ). Adenovirus-mediated gene expression was driven by the RSV promoter and is targeted to the liver (39). From control mice we isolated hepatocytes 4 days after injection of AdLacZ and ascertained expression of adenoviral gene by staining cells for β-galactosidase (39) (data not shown). Similarly, hepatocytes isolated from AdLPL-injected mice secreted human LPL in the culture medium as determined by immunoprecipitation and Western blotting (data not shown). As expected, we were not able to detect the presence of human LPL in the plasma of AdLPL-injected mice, suggesting that the level of LPL expression was low and the secreted LPL remained anchored to sinusoidal endothelial cells.
We compared binding and degradation of human VLDL and apoE knockout mouse VLDL by hepatocytes isolated from apoE knockout mice 4 days after injection with AdLPL or AdlacZ (Fig. 8A). Hepatocytes isolated by collagenase perfusion of the livers were plated onto Primaria plates (Falcon; Becton Dickinson Labware, Lincoln Park, NJ) and assayed 24 h later. Hepatocytes from AdLPL-injected mice bound and degraded, respectively, four and seven times more normal human VLDL than did hepatocytes from AdlacZ-injected mice (Fig. 8A). These results confirmed the expression of huLPL in AdLPL-infected mice. Contrary to normal VLDL, binding and degradation of apoE knockout mouse VLDL were nonexistent in both AdLPL- and AdlacZ-injected mice (Fig. 8A). The absence of cell surface binding is different from the results obtained in skin fibroblasts, but may be a function of the different cell type.
Fig. 8.
Clearance of apoE-deficient mouse VLDL is impaired in apoE knockout mice but not in normal C57BL/6 mice. Wild-type C57BL/6 and apoE knockout mice were injected into the tail vein with ~3 3 × 1011 particles of adenovirus AdLPL or AdLacZ. (A) Hepatocytes were isolated from C57BL/6 mice by collagenase perfusion 4 days after injection of the adenovirus. Monolayers of hepatocytes from AdLPL- or AdLacZ-injected mice were incubated with 125I-labeled huVLDL or 125I-labeled mouse VLDL for 5 h at 37°C. Degradation was measured as described in Materials and Methods. Symbols over bars indicate statistical significance. Compared with corresponding value for huVLDL: * P < 0.0001 and % P < 0.001. Compared with corresponding value for AdLacZ hepatocytes: # P < 0.0001 and $ P < 0.001. (B) Four days after adenovirus injection, mice were injected via the femoral vein with iodinated apoE knockout VLDL. At different times after injection, blood samples were collected into heparinized capillary tubes by tail clips. The amount of radioactivity in 10 µl of the plasma was determined and is represented as a percentage of that present 1 min after injection. Each line represents a separate animal. Results are representative of two separate experiments.
We next studied the kinetics of clearance of 125I-labeled labeled apoE knockout mouse VLDL injected into the femoral vein of these mice (4 days after injection of adenovirus). We found that apoE knockout mouse VLDL clearance was a little more rapid in AdLPL-injected mice than in AdlacZ-injected mice. However, in both groups clearance was slow, with more than 75% still remaining in the plasma 1 h after injection (Fig. 8B). Thus, moderate overexpression of LPL did not significantly improve clearance of VLDL in the complete absence of apoE. On the other hand, when injected in wild-type C57BL/6 mice, the removal of apoE-deficient mouse VLDL was relatively rapid, with 50% being cleared by 30 min and only about 35% still circulating after 1 h (Fig. 8B). The rates of clearance were identical in AdLPL- and AdLacZ-injected wild-type mice. This result supports the secretion-recapture mechanism for apoE function. Accordingly, endogenous apoE secreted by wild-type liver cells may be incorporated into injected apoE-deficient VLDL, thereby modifying the particles into a high affinity ligand for hepatic lipoprotein receptors (40, 41). On the other hand, in apoE knockout mice, clearance of injected apoE-deficient VLDL remains impaired because of the lack of hepatic apoE.
Lipases do not significantly stimulate degradation of apoE-poor LDL and HDL particles
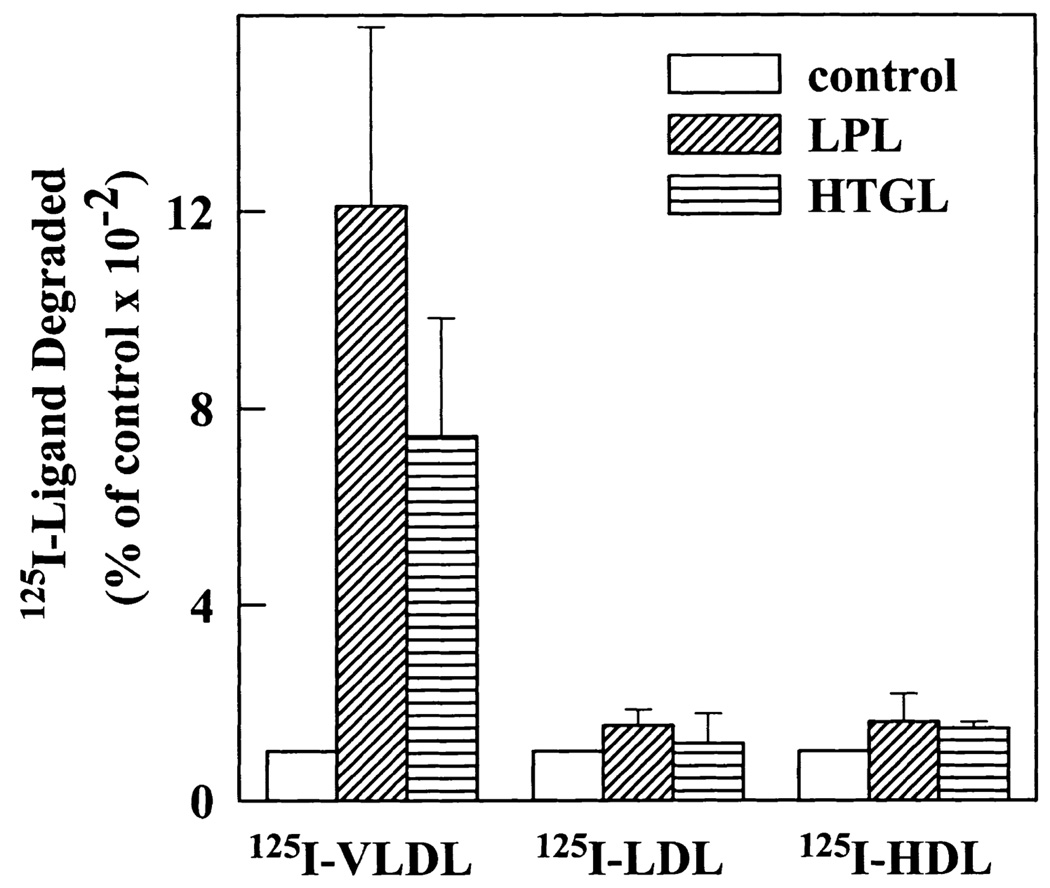
The apoE content of the different lipoproteins varies widely. Normal VLDL particles are apoE rich whereas LDL and HDL particles are apoE poor. We compared the stimulatory effect of LPL and HTGL on degradation of these three classes of lipoproteins. In the presence of LPL and HTGL, degradation of VLDL particles by upregulated fibroblasts was stimulated 12- and 7-fold, respectively (Fig. 9). However, both LPL and HTGL failed to significantly stimulate degradation of LDL and HDL. VLDL is considered a better substrate for LPL than LDL and HDL (42) and that may partly explain the higher stimulation of VLDL degradation by LPL. However, HTGL also failed to stimulate LDL and HDL degradation in spite of these smaller lipoproteins being good substrates of HTGL (43). This may be due to the low apoE content of LDL and HDL, supporting an essential role for apoE in lipase-stimulated receptor-mediated lipoprotein degradation.
Fig. 9.
LPL and HTGL stimulate degradation by fibroblasts of apoE-rich VLDL particles but not of apoE-poor LDL and HDL particles. LPDS- and lovastatin-treated fibroblasts were incubated at 37°C with medium containing either 125I-labeled VLDL (5 µg/ml), 125I-labeled LDL (1 µg/ml), or 125I-labeled HDL (1 µg/ml) in the absence or presence of LPL (1 µg/ml) or HTGL (3 µg/ml). After 5 h, cells were washed and the amount of degraded ligand in the incubation medium was measured as described. Results are averages of three different experiments and are presented as a percentage of degradation under control conditions (in the absence of LPL or HTGL).
DISCUSSION
ApoE is a 34-kDa protein constituent of triglyceride-rich plasma lipoproteins (2, 44) and a high affinity ligand for LDL receptors and LRP (1, 45). ApoE plays a pivotal role in facilitating the rapid clearance of remnant particles by the liver. Binding studies have demonstrated that apoE promotes receptor-mediated lipoprotein uptake and accumulation of cholesteryl esters by cultured cells (5, 35, 46). ApoE is believed to assist remnant clearance in vivo by a “secretion-recapture” mechanism (40, 41). ApoE is secreted by hepatocytes into the space of Disse, where it is thought to remain anchored to hepatocyte cell surface proteoglycans. The secreted apoE is integrated into lipoproteins, increasing their affinity for and recapture by hepatic lipoprotein receptors. Of the three common apoE isoforms, apoE2 demonstrates impaired binding to lipoprotein receptors (47). The apoE2/2 phenotype predisposes to type III hyperlipoproteinemia and may be associated with remnant accumulation (48).
LPL and HTGL are equally important for normal remnant catabolism (43, 49–51). Humans lacking LPL or its activator, apoC-II, develop massive hypertriglyceridemia due to the accumulation of both chylomicrons and large VLDL (52, 53). Familial HTGL deficiency results in typical type III hyperlipoproteinemia with impaired clearance of chylomicron remnants (54). Lipases regulate lipoprotein catabolism by two mechanisms, as lipolytic enzymes and as ligands for lipoprotein receptors (49–51). Felts, Itakura, and Crane (55) first suggested that by associating with remnant particles, LPL may provide the recognition signal for uptake by hepatic receptors. It has now been demonstrated that LPL directly binds to all members of the LDL receptor family (1 and references therein). HTGL is known to directly bind LRP, and we have demonstrated that it promotes VLDL catabolism by LDL receptors as well (18, 19). But it is not clear whether HTGL is a ligand for LDL receptors. In cultured cells LPL and HTGL stimulate receptor-mediated uptake and degradation of chylomicron remnants and VLDL particles independently of lipolytic activity (15, 18, 20, 22, 56). Although not necessary, lipolysis clearly stimulates lipoprotein catabolism even further (1 and references therein). It has been suggested that the lipolysis-induced increase in VLDL catabolism results from an increase in accessibility of apoE to receptors due to hydrolysis of obscuring lipid components (57, 58). Thus stimulation of VLDL degradation may be a cooperative effort between lipases and apoE. In control experiments we determined that incubation of LPL or HTGL with VLDL at 37°C results in hydrolysis of triglycerides and the release of free fatty acids. Also, we were able to coimmunoprecipitate VLDL particles (apoB) from a lipase-lipoprotein mixture, using antibodies against LPL or HTGL. Radiolabeled VLDL particles specifically bound to HTGL or LPL immobilized to microtiter wells (data not shown). These observations suggest that lipases may exert their stimulatory effect on VLDL catabolism by both lipolytic and adapter-like functions. A discussion of the relative contribution of each can be found in earlier publications. In this study we have focused on the requirement for apoE in VLDL degradation.
We demonstrate here that lipases stimulate cell surface binding of VLDL independently of apoE but that stimulation of VLDL degradation is apoE dependent. We have used three approaches to generate apoE-free triglyceride-rich particles. These include apolipoprotein-free triglyceride-phosholipid emulsions (Intralipid particles), VLDL isolated from apoE knockout mice, and human VLDL depleted of apoE-rich particles by fractionation on heparin-Sepharose. Our conclusion that apoE is not required for cell surface binding is based on in vitro studies in cultured normal human skin fibroblasts. Consistent with earlier reports (15, 18), both LPL and HTGL enhanced the binding and uptake of apolipoprotein-free Intralipid particles at 37°C. In the absence of apoE, this stimulation must be mediated by the direct binding of lipase to the cell surface. Similarly, LPL and HTGL greatly increased cell surface binding at 37°C of VLDL isolated from apoE knockout mice and apoE-depleted human VLDL.
LDL receptors were not responsible for lipase-stimulated in vitro binding of Intralipid or apoE-deficient VLDL because the increase was not significantly dependent on the level of LDL receptor expression, which was modulated by lovastatin treatment (15, 18). Lipase-stimulated binding may be to LRP because both LPL and HTGL are ligands for LRP. However, we determined that lipase-stimulated cell surface binding of Intralipid (18) as well as apoE-deficient VLDL was to HSPG because it returned to control levels (–lipase) with heparinase treatment. Control and HTGL-stimulated surface binding of normal VLDL at 37°C was not significantly affected by heparinase, indicating HSPG-independent mechanisms. Consistent with earlier reports (16, 59, 60), a significant component of LPL-stimulated surface binding was to HSPG.
The efficient cell surface binding of apoE-deficient VLDL is not surprising because apoE, apoB-100, LPL, and HTGL are all known to be heparin-binding proteins and either one can mediate lipoprotein binding to HSPG. In fact, it is believed that in vivo remnant clearance is initiated by their rapid sequestration to hepatocyte cell surface HSPG (1). Using remnants containing mutant variants of apoE, Ji, Fazio, and Mahley (61) showed that in the absence of lipase, clearance correlates directly with the ability of apoE to bind HSPG. The initial clearance of remnants from plasma and sequestration by hepatocytes is inhibited by heparinase and is independent of LDL receptors and LRP (62). Thus it is clear that even in the absence of apoE, lipase-VLDL complexes bind to HSPG with high affinity and apoE is not required for lipase-stimulated binding.
Our results indicate an inhibitory effect of apoE on lipase-mediated VLDL binding to the cell surface. We found that surface binding of apoE-depleted VLDL at 4°C as well as at 37°C was 5- to 10-fold greater than that of normal VLDL even in the absence of lipases. The basis of this interaction in the absence of both apoE and lipase is not clear. It was not due to apoB-100 binding to LDL receptors because it was not increased by upregulation of LDL receptors. However, apoB-100 may bind to HSPG via its amino-terminal domain, which is hydrophilic and is known to interact with heparin (63). Because apoE is also a heparin-binding protein, it may inhibit the apoB-100-HSPG interaction, thus explaining the higher binding of apoE-depleted VLDL. It is interesting that apoE-deficient VLDL from apoE knockout mice did not demonstrate higher cell surface binding (in the absence of lipases) than normal VLDL. This may be due to the higher lipid-to-protein ratio in VLDL particles from these markedly hyperlipidemic mice. On the other hand, our preparation of apoE-depleted VLDL has the same lipid-to-protein ratio as normal VLDL.
LPL- and HTGL-stimulated binding of apoE-deficient mouse VLDL and HTGL-stimulated binding of apoE-depleted human VLDL was significantly higher than that of normal VLDL. Thus apoE may inhibit lipase-HSPG interactions as well. We observed dramatically higher effects of HTGL than LPL on surface binding at 37°C of all three kinds of apoE-free particles. On the other hand, LPL was more potent stimulator of normal VLDL binding. The reason for this is not clear; it is possible that apoE is a better inhibitor of HTGL-HSPG than LPL-HSPG interactions.
Our results are consistent with earlier investigations of the effect of apoE on LPL-mediated hydrolysis and binding. In one report, Jong et al. (64) suggest that apoE may inhibit the lipolytic activity of LPL. They demonstrated that hydrolysis of VLDL triacylglycerol by LPL is inversely related to the apoE content of VLDL particles. Similarly, Rensen and van Berkel (65) reported an apoE concentration-dependent inhibition of in vitro and in vivo LPL-mediated lipolysis of triglyceride emulsions. Thus apoE-poor VLDL particles are better substrates of LPL. This may be due to an inhibitory effect of apoE on the binding interaction between LPL and VLDL. Saxena et al. (66) demonstrated that apoE inhibits the interaction of LPL with HSPG as well as apoB-100. In their studies, the addition of apoE alone or in phospholipid liposomes reduced LPL-stimulated binding of LDL to HSPG in an apoE dose-dependent manner.
We found that while apoE is not required for cell surface binding, it greatly enhances VLDL degradation even in the presence of lipases. Although binding is independent of lipoprotein receptors, subsequent endocytosis and degradation are believed to be receptor-mediated. Herz et al. (67) provided evidence of this by reporting that plasma clearance of injected chylomicrons was normal but that their appearance in endosomes and hydrolysis of cholesteryl esters was greatly reduced in LDL receptor knockout mice. A minor back-up role for LRP was also demonstrated. We investigated whether the ability of lipases to bind lipoprotein receptors could eliminate the need for apoE in VLDL degradation in the presence of LPL or HTGL. Our conclusion that apoE is required even for lipase-stimulated VLDL degradation is based on the following observations: i) Degradation of apoE-deficient mouse VLDL as well as apoE-depleted human VLDL is significantly lower than that of normal VLDL in spite of higher surface binding; ii) degradation of normal VLDL is stimulated up to 8- and 4-fold, respectively, by LPL and HTGL in a dose-dependent manner but degradation of apoE-depleted VLDL is not significantly increased over control levels even in the presence of LPL (1 µg/ml) or 10 µg/ml HTGL; iii) less than 5% of prebound apoE-depleted VLDL is degraded by 3 h at 37°C whereas degradation of normal VLDL is three times more efficient. Results presented here indicate that a low level of degradation of apoE-poor VLDL is possible in the presence of LPL and HTGL. However, relative to normal VLDL, this is negligible and apoE is required for efficient receptor-mediated catabolism. We ascertained that degradation in the absence of apoE is mediated by LDL receptors because it is dependent on upregulation of LDL receptor expression by lovastatin treatment. It is well known that lovastatin treatment induces LDL receptor expression but does not influence expression of other lipoprotein receptors, including LRP or VLDL receptors. An effect of lovastatin on other unknown factors that may influence VLDL catabolism is not being ruled out. Interestingly, HTGL was more effective than LPL in promoting apoE-poor VLDL degradation. The reason for this is not clear. It may just be a reflection of the tremendously higher stimulation by HTGL of apoE-poor VLDL surface binding. As reported earlier, HTGL was less potent than LPL in stimulating degradation of normal VLDL (18).
Our results are consistent with those of Hendriks et al. (68). They reported significantly lower binding and degradation of apoE-deficient mouse VLDL than normal VLDL in J774 macrophages. In the presence of LPL, surface binding of apoE-deficient VLDL and normal VLDL was comparable. Degradation of apoE-deficient VLDL, although stimulated by LPL, remained considerably lower than that of normal VLDL. They suggest that degradation proceeds via a distinct macrophage-specific receptor for triglyceride-rich lipoproteins described earlier. Mann et al. (69) also reported distinct roles for apoE and LPL in VLDL catabolism. They demonstrated that whereas individually apoE and LPL promote chylomicron uptake by hepatocytes, the addition of both together has an additive effect. Zsigmond et al. (70) showed that overexpression of LPL in apoE knockout mice and LDL receptor knockout mice normalizes their plasma lipoprotein profile concurrent with an increase in plasma LPL activity. The greatest reduction was seen in VLDL cholesterol. They propose that increased lipolysis of VLDL by LPL gene therapy may be a primary mechanism for the virtual elimination of VLDL cholesterol in these mice. It is unlikely that LPL corrects the lipoprotein profile by substituting as a ligand for LDL receptors because the cholesterol-lowering effects are seen in both apoE−/− and LDLR−/− mice. The adenovirus-mediated expression in their mice is much greater than what we obtained in Fig. 8. In our studies, the plasma LPL levels or lipolytic activities were not increased. The lower LPL expression allowed us to investigate the lipolysis-independent effect of LPL in receptor-mediated VLDL catabolism of apoE-deficient VLDL. The in vivo VLDL clearance data clearly indicate that apoE knockout VLDL is removed from the circulation only when apoE is available for incorporation into the particles. This reinforces the essential role for apoE in VLDL catabolism.
These studies provide interesting insight into the protective effects of apoE against atherosclerosis. Overexpression of apoE in the vascular wall is believed to prevent and reverse atherosclerosis by promoting reverse cholesterol transport. Our studies suggest that in the absence of apoE, lipase-stimulated surface binding of VLDL to HSPG is greatly increased. Thus, apoE appears to inhibit VLDL binding to HSPG. We have generated transgenic mice with macrophage-specific overexpression of LPL (J. D. Medh, G. L. Fry, K. M. Wilson, and D. A. Chappell, unpublished data). Ongoing investigations with these mice are aimed at understanding the in vivo interactions between apoE and LPL in the vascular wall. Further studies with catalytically inactive LPL and HTGL will be required to differentiate between the lipolytic and receptor-binding functions of lipases.
Acknowledgments
We thank Mary Lou Booth and Gregory Aylsworth for assistance with tissue culture. This work was supported by grant HL49264 from the National Institutes of Health and a grant-in-aid award from the American Heart Association.
Abbreviations
- apoE
apolipoprotein E
- BSA
bovine serum albumin
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid
- HSPG
heparan sulfate proteoglycans
- HTGL
hepatic triglyceride lipase
- LDL
low density lipoproteins
- LPDS
lipoprotein-deficient serum
- LPL
lipoprotein lipase
- LRP
LDL receptor-related protein
- RSV
Rous sarcoma virus
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- VLDL
very low density lipoproteins
REFERENCES
- 1.Chappell DA, Medh JD. Receptor-mediated mechanisms of lipoprotein remnant catabolism. Prog. Lipid Res. 1998;37:393–422. doi: 10.1016/s0163-7827(98)00017-4. [DOI] [PubMed] [Google Scholar]
- 2.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 3.Innerarity TL, Mahley RW. Enhanced binding by cultured human fibroblasts of apo-E-containing lipoproteins as compared with low density lipoproteins. Biochemistry. 1978;17:1440–1447. doi: 10.1021/bi00601a013. [DOI] [PubMed] [Google Scholar]
- 4.Innerarity TL, Pitas RE, Mahley RW. Binding of arginine-rich (E) apoprotein after recombination with phospholipids vesicles to the low density lipoprotein receptors of fibroblasts. J. Biol. Chem. 1979;254:4186–4190. [PubMed] [Google Scholar]
- 5.Beisiegel U, Weber W, Ihrke G, Herz J, Stanley KK. The LDL-receptor-related protein, LRP, is an apolipoprotein E-binding protein. Nature. 1989;341:162–164. doi: 10.1038/341162a0. [DOI] [PubMed] [Google Scholar]
- 6.Beisiegel U, Weber W, Bengtsson-Olivecrona G. Lipoprotein lipase enhances the binding of chylomicrons to low density lipoprotein receptor-related protein. Proc. Natl. Acad. Sci. USA. 1991;88:8342–8346. doi: 10.1073/pnas.88.19.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi S, Kawarabayasi Y, Nakai T, Sakai J, Yamamoto T. Rabbit very low density lipoprotein receptor: a low density lipoprotein receptor-like protein with distinct ligand specificity. Proc. Natl. Acad. Sci. USA. 1992;89:9252–9256. doi: 10.1073/pnas.89.19.9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi S, Suzuki J, Kohno M, Oida K, Tamai T, Miyabo S, Yamamoto T, Nakai T. Enhancement of the binding of triglyceride-rich lipoproteins to the very low density lipoprotein receptor by apolipoprotein E and lipoprotein lipase. J. Biol. Chem. 1995;270:15747–15754. doi: 10.1074/jbc.270.26.15747. [DOI] [PubMed] [Google Scholar]
- 9.Gabelli C, Gregg RE, Zech LA, Manzato E, Brewer HB., Jr. Abnormal low density lipoprotein metabolism in apolipoprotein E deficiency. J. Lipid Res. 1986;27:326–333. [PubMed] [Google Scholar]
- 10.Hui DY, Innerarity TL, Mahley RW. Defective hepatic lipoprotein receptor binding of beta-very low density lipoproteins from type III hyperlipoproteinemic patients. Importance of apolipoprotein E. J. Biol. Chem. 1984;259:860–869. [PubMed] [Google Scholar]
- 11.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 12.Plump AS, Smith JD, Hayek T, Aalto-Setala K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 13.Kashyap VS, Santamarina-Fojo S, Brown DR, Parrott CL, Applebaum-Bowden D, Meyn S, Talley G, Paigen B, Maeda N, Brewer HB., Jr. Apolipoprotein E deficiency in mice: gene replacement and prevention of atherosclerosis using adenovirus vectors. J. Clin. Invest. 1995;96:1612–1620. doi: 10.1172/JCI118200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishibashi S, Yamada N, Shimano H, Mori N, Mokuno H, Gotohda T, Kawakami M, Murase T, Takaku F. Apolipoprotein E and lipoprotein lipase secreted from human monocyte-derived macrophages modulate very low density lipoprotein uptake. J. Biol. Chem. 1990;265:3040–3047. [PubMed] [Google Scholar]
- 15.Medh JD, Bowen SL, Fry GL, Ruben S, Andracki M, Inoue I, Lalouel JM, Strickland DK, Chappell DA. Lipoprotein lipase binds to low density lipoprotein receptors and induces receptor-mediated catabolism of very low density lipoproteins in vitro. J. Biol. Chem. 1996;271:17073–17080. doi: 10.1074/jbc.271.29.17073. [DOI] [PubMed] [Google Scholar]
- 16.Mulder M, Lombardi P, Jansen H, van Berkel TJ, Frants RR, Havekes LM. Low density lipoprotein receptor internalizes low density and very low density lipoproteins that are bound to heparan sulfate proteoglycans via lipoprotein lipase. J. Biol. Chem. 1993;268:9369–9375. [PubMed] [Google Scholar]
- 17.Rumsey SC, Obunike JC, Arad Y, Deckelbaum RJ, Goldberg IJ. Lipoprotein lipase-mediated uptake and degradation of low density lipoproteins by fibroblasts and macrophages. J. Clin. Invest. 1992;90:1504–1512. doi: 10.1172/JCI116018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medh JD, Bowen SL, Fry GL, Ruben S, Hill J, Wong H, Chappell DA. Hepatic triglyceride lipase promotes low density lipoprotein receptor-mediated catabolism of very low density lipoproteins in vitro. J. Lipid Res. 1999 In press. [PubMed] [Google Scholar]
- 19.Kounnas MZ, Chappell DA, Wong H, Argraves WS, Strickland DK. The cellular internalization and degradation of hepatic lipase is mediated by low density lipoprotein receptor-related protein and requires cell surface proteoglycans. J. Biol. Chem. 1995;270:9307–9312. doi: 10.1074/jbc.270.16.9307. [DOI] [PubMed] [Google Scholar]
- 20.Krapp A, Ahle S, Kersting S, Hua Y, Kneser K, Nielsen M, Gliemann J, Beisiegel U. Hepatic lipase mediates the uptake of chylomicrons and beta-VLDL into cells via the LDL receptor-related protein (LRP) J. Lipid Res. 1996;37:926–936. [PubMed] [Google Scholar]
- 21.Ji ZS, Lauer SJ, Fazio S, Bensadoun A, Taylor JM, Mahley RW. Enhanced binding and uptake of remnant lipoproteins by hepatic lipase-secreting hepatoma cells in culture. J. Biol. Chem. 1994;269:13429–13436. [PubMed] [Google Scholar]
- 22.Williams SE, Inoue I, Tran H, Fry GL, Pladet MW, Iverius PH, Lalouel JM, Chappell DA, Strickland DK. The carboxyl-terminal domain of lipoprotein lipase binds to the low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor (LRP) and mediates binding of normal very low density lipoproteins to LRP. J. Biol. Chem. 1994;269:8653–8658. [PubMed] [Google Scholar]
- 23.Chappell DA, Fry GL, Waknitz MA, Iverius PH, Williams SE, Strickland DK. The low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor binds and mediates catabolism of bovine milk lipoprotein lipase. J. Biol. Chem. 1992;267:25764–25767. [PubMed] [Google Scholar]
- 24.Nykjaer A, Bengtsson-Olivecrona G, Lookene A, Moestrup SK, Petersen CM, Weber W, Beisiegel U, Gliemann J. The alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein binds lipoprotein lipase and beta-migrating very low density lipoprotein associated with the lipase. J. Biol. Chem. 1993;268:15048–15055. [PubMed] [Google Scholar]
- 25.Iverius PH, Ostlund-Lindqvist AM. Lipoprotein lipase from bovine milk. Isolation procedure, chemical characterization, and molecular weight analysis. J. Biol. Chem. 1976;251:7791–7795. [PubMed] [Google Scholar]
- 26.Hill JS, Davis RC, Yang D, Wen J, Philo JS, Poon PH, Phillips ML, Kempner ES, Wong H. Human hepatic lipase subunit structure determination. J. Biol. Chem. 1996;271:22931–22936. doi: 10.1074/jbc.271.37.22931. [DOI] [PubMed] [Google Scholar]
- 27.Trezzi E, Calvi C, Roma P, Catapano AL. Subfractionation of human very low density lipoproteins by heparinsepharose affinity chromatography. J. Lipid Res. 1983;24:790–795. [PubMed] [Google Scholar]
- 28.Bilheimer DW, Eisenberg S, Levy R. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim. Biophys. Acta. 1972;260:212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- 29.Chappell DA, Fry GL, Waknitz MA, Berns JJ. Ligand size as a determinant for catabolism by the low density lipoprotein (LDL) receptor pathway. A lattice model for LDL binding. J. Biol. Chem. 1991;266:19296–19302. [PubMed] [Google Scholar]
- 30.Goldstein JL, Basu SK, Brown MS. Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol. 1983;98:241–260. doi: 10.1016/0076-6879(83)98152-1. [DOI] [PubMed] [Google Scholar]
- 31.Chappell DA, Fry GL, Waknitz MA, Muhonen LE, Pladet MW. Low density lipoprotein receptors bind and mediate cellular catabolism of normal very low density lipoproteins in vitro. J. Biol. Chem. 1993;268:25487–25493. [PubMed] [Google Scholar]
- 32.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–276. [PubMed] [Google Scholar]
- 33.Kutt H, Herz J, Stanley KK. Structure of the low-density lipoprotein receptor-related protein (LRP) promoter. Biochim. Biophys. Acta. 1989;1009:229–236. doi: 10.1016/0167-4781(89)90107-3. [DOI] [PubMed] [Google Scholar]
- 34.Lund H, Takahashi K, Hamilton RL, Havel RJ. Lipoprotein binding and endosomal itinerary of the low density lipoprotein receptor-related protein in rat liver. Proc. Natl. Acad. Sci. USA. 1989;86:9318–9322. doi: 10.1073/pnas.86.23.9318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kowal RC, Herz J, Goldstein JL, Esser V, Brown MS. Low density lipoprotein receptor-related protein mediates uptake of cholesteryl esters derived from apoprotein E-enriched lipoproteins. Proc. Natl. Acad. Sci. USA. 1989;86:5810–5814. doi: 10.1073/pnas.86.15.5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aviram M, Bierman EL, Chait A. Modification of low density lipoprotein by lipoprotein lipase or hepatic lipase induces enhanced uptake and cholesterol accumulation in cells. J. Biol. Chem. 1988;263:15416–15422. [PubMed] [Google Scholar]
- 37.Komaromy M, Azhar S, Cooper AD. Chinese hamster ovary cells expressing a cell surface-anchored form of hepatic lipase. Characterization of low density lipoprotein and chylomicron remnant uptake and selective uptake of high density lipoprotein-cholesteryl ester. J. Biol. Chem. 1996;271:16906–16914. doi: 10.1074/jbc.271.28.16906. [DOI] [PubMed] [Google Scholar]
- 38.Chappell DA, Fry GL, Waknitz MA, Muhonen LE, Pladet MW, Iverius PH, Strickland DK. Lipoprotein lipase induces catabolism of normal triglyceride-rich lipoproteins via the low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor in vitro. A process facilitated by cell-surface proteoglycans. J. Biol. Chem. 1993;268:14168–14175. [PubMed] [Google Scholar]
- 39.Kozarsky KF, McKinley DR, Austin LL, Raper SE, Stratford-Perricaudet LD, Wilson JM. In vivo correction of low density lipoprotein receptor deficiency in the Watanabe heritable hyperlipidemic rabbit with recombinant adenoviruses. J. Biol. Chem. 1994;269:13695–13702. [PubMed] [Google Scholar]
- 40.Ji ZS, Fazio S, Lee YL, Mahley RW. Secretion-capture role for apolipoprotein E in remnant lipoprotein metabolism involving cell surface heparan sulfate proteoglycans. J. Biol. Chem. 1994;269:2764–2772. [PubMed] [Google Scholar]
- 41.Shimano H, Namba Y, Ohsuga J, Kawamura M, Yamamoto K, Shimada M, Gotoda T, Harada K, Yazaki Y, Yamada N. Secretion-recapture process of apolipoprotein E in hepatic uptake of chylomicron remnants in transgenic mice. J. Clin. Invest. 1994;93:2215–2223. doi: 10.1172/JCI117218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Musliner TA, Herbert PN, Kingston MJ. Lipoprotein substrates of lipoprotein lipase and hepatic triacylglycerol lipase from human post-heparin plasma. Biochim. Biophys. Acta. 1979;575:277–288. doi: 10.1016/0005-2760(79)90029-8. [DOI] [PubMed] [Google Scholar]
- 43.Jansen H, Hulsmann WC. Enzymology and physiological role of hepatic lipase. Biochem. Soc. Trans. 1985;13:24–26. doi: 10.1042/bst0130024. [DOI] [PubMed] [Google Scholar]
- 44.Havel RJ, Yamada N, Shames DM. Role of apolipoprotein E in lipoprotein metabolism. Am. Heart J. 1987;113:470–474. doi: 10.1016/0002-8703(87)90616-8. [DOI] [PubMed] [Google Scholar]
- 45.Cooper AD. Hepatic uptake of chylomicron remnants. J. Lipid Res. 1997;38:2173–2192. [PubMed] [Google Scholar]
- 46.Innerarity TL, Arnold KS, Weisgraber KH, Mahley RW. Apolipoprotein E is the determinant that mediates the receptor uptake of beta-very low density lipoproteins by mouse macrophages. Arteriosclerosis. 1986;6:114–122. doi: 10.1161/01.atv.6.1.114. [DOI] [PubMed] [Google Scholar]
- 47.Havel RJ, Chao Y, Windler EE, Kotite L, Guo LS. Isoprotein specificity in the hepatic uptake of apolipoprotein E and the pathogenesis of familial dysbetalipoproteinemia. Proc. Natl. Acad. Sci. USA. 1980;77:4349–4353. doi: 10.1073/pnas.77.7.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rall SC, Jr., Weisgraber KH, Innerarity TL, Mahley RW. Structural basis for receptor binding heterogeneity of apolipoprotein E from type III hyperlipoproteinemic subjects. Proc. Natl. Acad. Sci. USA. 1982;79:4696–4700. doi: 10.1073/pnas.79.15.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olivecrona G, Olivecrona T. Triglyceride lipases and atherosclerosis. Curr. Opin. Lipidol. 1995;6:291–305. doi: 10.1097/00041433-199510000-00009. [DOI] [PubMed] [Google Scholar]
- 50.Santamarina-Fojo S, Dugi KA. Structure, function and role of lipoprotein lipase in lipoprotein metabolism. Curr. Opin. Lipidol. 1994;5:117–125. doi: 10.1097/00041433-199404000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Santamarinafojo S, Haudenschild C, Amar M. The role of hepatic lipase in lipoprotein metabolism and atherosclerosis. Curr. Opin. Lipidol. 1998;9:211–219. doi: 10.1097/00041433-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 52.Breckenridge WC, Alaupovic P, Cox DW, Little JA. Apolipoprotein and lipoprotein concentrations in familial apolipoprotein C-II deficiency. Atherosclerosis. 1982;44:223–235. doi: 10.1016/0021-9150(82)90116-2. [DOI] [PubMed] [Google Scholar]
- 53.Santamarina-Fojo S, Brewer HB., Jr. The familial hyperchylomicronemia syndrome. New insights into underlying genetic defects. J. Am. Med. Assoc. 1991;265:904–908. [PubMed] [Google Scholar]
- 54.Breckenridge WC, Little JA, Alaupovic P, Wang CS, Kuksis A, Kakis G, Lindgren F, Gardiner G. Lipoprotein abnormalities assoicated with a familial deficiency of hepatic lipase. Atherosclerosis. 1982;45:161–179. doi: 10.1016/0021-9150(82)90136-8. [DOI] [PubMed] [Google Scholar]
- 55.Felts JM, Itakura H, Crane RT. The mechanism of assimilation of constituents of chylomicrons, very low density lipoproteins and remnants—a new theory. Biochem. Biophys. Res. Commun. 1975;66:1467–1475. doi: 10.1016/0006-291x(75)90524-0. [DOI] [PubMed] [Google Scholar]
- 56.Chappell DA, Inoue I, Fry GL, Pladet MW, Iverius PH, Lalouel JM, Strickland DK. The carboxy-terminal domain of lipoprotein lipase induces cellular catabolism of normal very low density lipoproteins via the low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. Ann. N.Y. Acad. Sci. 1994;737:434–438. doi: 10.1111/j.1749-6632.1994.tb44333.x. [DOI] [PubMed] [Google Scholar]
- 57.Brasaemle DL, Cornely-Moss K, Bensadoun A. Hepatic lipase treatment of chylomicron remnants increases exposure of apolipoprotein E. J. Lipid Res. 1993;34:455–465. [PubMed] [Google Scholar]
- 58.Sehayek E, Lewin-Velvert U, Chajek-Shaul T, Eisenberg S. Lipolysis exposes unreactive endogenous apolipoprotein E-3 in human and rat plasma very low density lipoprotein. J. Clin. Invest. 1991;88:553–560. doi: 10.1172/JCI115339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Barlingen HH, de Jong H, Erkelens DW, de Bruin TW. Lipoprotein lipase-enhanced binding of human triglyceride-rich lipoproteins to heparan sulfate: modulation by apolipoprotein E and apolipoprotein C. J. Lipid Res. 1996;37:754–763. [PubMed] [Google Scholar]
- 60.Fernandez-Borja M, Bellido D, Vilella E, Olivecrona G, Vilaro S. Lipoprotein lipase-mediated uptake of lipoprotein in human fibroblasts: evidence for an LDL receptor-independent internalization pathway. J. Lipid Res. 1996;37:464–481. [PubMed] [Google Scholar]
- 61.Ji ZS, Fazio S, Mahley RW. Variable heparan sulfate proteoglycan binding of apolipoprotein E variants may modulate the expression of type III hyperlipoproteinemia. J. Biol. Chem. 1994;269:13421–13428. [PubMed] [Google Scholar]
- 62.Ji ZS, Sanan DA, Mahley RW. Intravenous heparinase inhibits remnant lipoprotein clearance from the plasma and uptake by the liver: in vivo role of heparan sulfate proteoglycans. J. Lipid Res. 1995;36:583–592. [PubMed] [Google Scholar]
- 63.Sivaram P, Choi SY, Curtiss LK, Goldberg IJ. An amino-terminal fragment of apolipoprotein B binds to lipoprotein lipase and may facilitate its binding to endothelial cells. J. Biol. Chem. 1994;269:9409–9412. [PubMed] [Google Scholar]
- 64.Jong MC, Dahlmans VE, Hofker MH, Havekes LM. Nascent very-low-density lipoprotein triacylglycerol hydrolysis by lipoprotein lipase is inhibited by apolipoprotein E in a dose-dependent manner. Biochem. J. 1997;328:745–750. doi: 10.1042/bj3280745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rensen PCN, van Berkel TJC. Apolipoprotein E effectively inhibits lipoprotein lipase-mediated lipolysis of chylomicron-like triglyceride-rich lipid emulsions in vitro and in vivo. J. Biol. Chem. 1996;271:14791–14799. doi: 10.1074/jbc.271.25.14791. [DOI] [PubMed] [Google Scholar]
- 66.Saxena U, Auerbach BJ, Ferguson E, Wolle J, Marcel YL, Weisgraber KH, Hegele RA, Bisgaier CL. Apolipoprotein B and E basic amino acid clusters influence low-density lipoprotein association with lipoprotein lipase anchored to the subendothelial matrix. Arterioscler. Thromb. Vasc. Biol. 1995;15:1240–1247. doi: 10.1161/01.atv.15.8.1240. [DOI] [PubMed] [Google Scholar]
- 67.Herz J, Qiu SQ, Oesterle A, DeSilva HV, Shafi S, Havel RJ. Initial hepatic removal of chylomicron remnants is unaffected but endocytosis is delayed in mice lacking the low density lipoprotein receptor. Proc. Natl. Acad. Sci. USA. 1995;92:4611–4615. doi: 10.1073/pnas.92.10.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hendriks WL, van der Sman-de Beer F, van Vlijmen BJ, van Vark LC, Hofker MH, Havekes LM. Uptake by J774 macrophages of very-low-density lipoproteins isolated from apoE-deficient mice is mediated by a distinct receptor and stimulated by lipoprotein lipase. Arterioscler. Thromb. Vasc. Biol. 1997;17:498–504. doi: 10.1161/01.atv.17.3.498. [DOI] [PubMed] [Google Scholar]
- 69.Mann WA, Meyer N, Weber W, Rinninger F, Greten H, Beisiegel U. Apolipoprotein E and lipoprotein lipase coordinately enhance binding and uptake of chylomicrons by human hepatocytes. Eur. J. Clin. Invest. 1995;25:880–882. doi: 10.1111/j.1365-2362.1995.tb01700.x. [DOI] [PubMed] [Google Scholar]
- 70.Zsigmond E, Kobayashi K, Tzung KW, Li L, Fuke Y, Chan L. Adenovirus-mediated gene transfer of human lipoprotein lipase ameliorates the hyperlipidemias associated with apolipoprotein E and LDL receptor deficiencies in mice. Hum. Gene Ther. 1997;8:1921–1933. doi: 10.1089/hum.1997.8.16-1921. [DOI] [PubMed] [Google Scholar]