Summary
The recognition of viral components by host pattern recognition receptors triggers the induction of the antiviral innate immune response. Toll-like receptor 9 (TLR9) and NALP-3 inflammasome were shown to be the principal specific sensors of viral double-stranded DNA. Here we present evidence that macrophages in vivo activate an innate immune response to a double-stranded DNA virus, adenovirus (Ad), independently of TLR9 or NALP-3 inflammasome. Our studies show that in response to Ad, macrophage-derived IL-1α triggers IL-1RI-dependent production of a defined set of pro-inflammatory cytokines and chemokines. The IL-1α-mediated response required a selective interaction of virus RGD motifs with macrophage β3 integrins. Therefore, these studies identify IL-1α-IL-1RI as a key pathway allowing for the activation of pro-inflammatory responses to the virus, independently of its genomic nucleic acid recognition.
Introduction
Induction of the antiviral innate immune response depends on the recognition of viral components by host pattern recognition receptors (PRRs) (Janeway and Medzhitov, 2002). The natural diversity of virus-associated nucleic acids makes them a legitimate target for recognition by specific cellular sensors that activate antiviral defense. In recent years, considerable evidence has been accumulated that suggests that intracellular sensors of virus-associated nucleic acids play an important role in recognizing virus pathogens and restricting their reproduction in host cells. Endosomal recognition of the viral double-stranded (ds) DNA by TLR9, dsRNA by TLR3, and single-stranded (ss) RNA by TLR7 and TLR8, was shown to be critical for type-I interferon production by plasmocytoid DCs (pDCs) in response to their infection with various virus pathogens (see for review in (Takeuchi and Akira, 2009)).
When virus particles enter the cytoplasm, TLR-independent mechanisms become engaged to ensure pathogen detection and alert the host of ongoing infection. The cytosolic RNA helicases MDA-5 and RIG-I can recognize dsRNA and trigger type-I interferon production (Moore and Ting, 2008; Takeuchi and Akira, 2009). RIG-I also recognizes 5′-triphosphate of a ssRNA, the molecular structure associated with viral infection (Hornung et al., 2006; Pichlmair et al., 2006).
Recent studies also indicate that a supramolecular complex of proteins, NALP3-inflammasome, consisting of caspase-1, ASC, and NALP3, may recognize viral and microbial dsDNA in the cytosole (Muruve et al., 2008). The activation of NALP3-inflammasome by dsDNA leads to the proteolytic processing of pro-IL-1β, resulting in the release of IL-1β and the subsequent activation of a cascade of pro-inflammatory cytokines and chemokines in an IL-1RI-dependent manner. Taken together, these data suggest that an elaborate network of sensors localized in the endosomes and cytoplasm triggers the activation of antiviral innate immune and inflammatory responses following the detection of virus-associated nucleic acids.
Adenovirus (Ad) is a non-enveloped virus with a linear dsDNA genome. Intravenous injection of Ad induces rapid activation of innate immune and inflammatory responses. Although these responses can be severe and even fatal at high doses of the virus (Brunetti-Pierri et al., 2004; Raper et al., 2003), the molecular sensors of Ad and mediators of Ad-induced inflammation in vivo remain poorly defined.
Here we show that in response to Ad, macrophages in vivo activate a cascade of pro-inflammatory cytokines and chemokines independently of TLR9 and NALP-3 inflammasome. Furthermore, we found that Ad triggers an innate immune response via activation of IL-1α, and not IL-1β or type-I interferon. Ad interaction with cell surface receptors and β3 integrins triggers IL-1α activation independently of recognition of the virus-associated nucleic acid by intracellular PRR. Moreover, although IL-1α was earlier implicated in activating inflammation in response to cell damage or stress, our data show the involvement of the IL-1α-IL-1RI pathway in the induction of host antiviral responses.
Results
Phagocytic cells, including MARCO- and CD169-positive splenic marginal zone macrophages, accumulate Ad in vivo
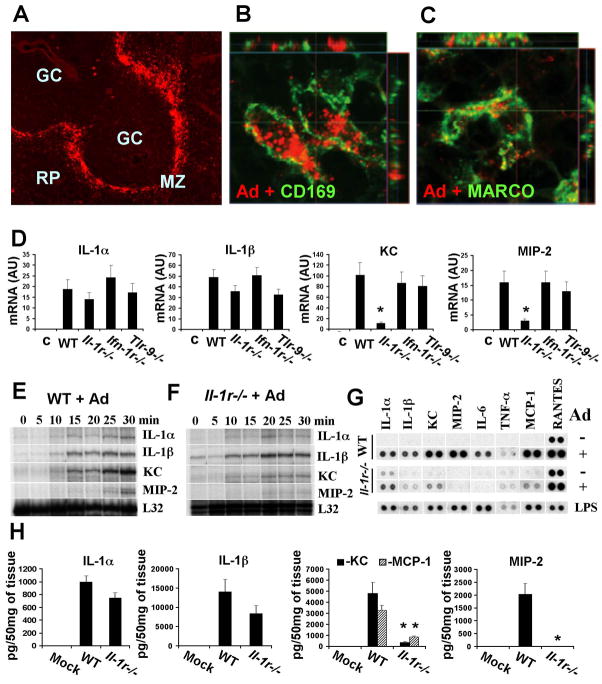
Extensive previous studies have demonstrated that tissue phagocytic cells play a critical role in mounting innate immune response to blood-born Ad in vivo. Although the role of residential macrophages, Kupffer cells, in trapping Ad particles in the liver is well documented, ((Lieber et al., 1997; Worgall et al., 1997), and Figure S1A), the identity of the cells in other tissues that trap blood-born Ad, specifically in the spleen, is poorly defined. We injected mice with human Ad serotype 5, the most commonly used serotype in clinical trials, and analyzed which specific cell types were interacting with the Ad particles in the spleen. A gross evaluation of the Ad distribution in the spleen revealed that the vast majority of virus particles were associated with cells in the marginal zone (Figure 1A). Staining the spleen sections with Ad-specific Ab and Abs for various cell type-specific markers revealed that CD169- and MARCO-positive marginal zone macrophages (MZMφ), but not other cell types, including IgM-positive marginal zone B cells, CD68-positive macrophages, or cells expressing B220, Gr1, CD11b, or CD3 markers, were co-localized with Ad particles after intravascular virus delivery (Figure 1B, C, Supplementary movies 1, 2, and 3, Figure S9, and data not shown). Although we cannot formally exclude that other cell types in the spleen accumulate low amounts of Ad particles, which are undetectable using these immuno-staining approaches, our data is consistent with recent findings implicating CD169-positive macrophages in the sequestering of lymph-born viruses, including Ad, in the lymph nodes (Junt et al., 2007).
Figure 1. CD169- and MARCO-positive splenic marginal zone macrophages trap adenovirus and initiate an IL-1RI-dependent innate immune response in vivo.
(A) Distribution of Ad particles in the spleen 30 min after virus injection. RP- red pulp; GC-germinal center; MZ-marginal zone. Anti-Ad antibody stain is red. Co-localization of Ad particles (red) with (B) CD169- or (C) MARCO-positive macrophages in the splenic MZ. (D) mRNA levels for IL-1α, IL-1β, KC and MIP-2 in the spleen 30 min after Ad injection. N=4. * - P < 0.01. Except for the columns indicated by the star, no statistically significant differences between experimental groups and WT group were identified (P > 0.05). C –mock-infected mice, treated with saline. AU – arbitrary units. (E, F) Kinetics of the transcriptional activation of IL-1α, IL-1β, KC and MIP-2 in wild type (E) or Il-1r−/− (F) mice. Individual mice were injected with Ad and sacrificed at indicated time points. (G) Protein levels for pro-inflammatory cytokines and chemokines in spleen of wild type (WT) and Il-1r−/− mice 1 h after Ad injection. N=4. As a positive control for inflammatory cytokine and chemokine induction, mice were injected with LPS. (H) The amounts of pro-inflammatory cytokines and chemokines in the spleens of mice 1 h after Ad injection. N=3. Mock – negative control mice injected with saline * - P < 0.01.
Macrophages activate IL-1RI-signaling pathway in response to Ad in vivo
To better define mediators involved in the initiation of this response, we injected C57Bl/6J mice with Ad5 and an Ad that cannot bind its primary cell attachment receptor in mice due to a mutation in its fiber capsid protein (Ad5/35L) (Shayakhmetov et al., 2005b). At 30 minutes, 6 hours, and 24 hours, liver and spleen were harvested and transcriptional activation of pro-inflammatory cytokine and chemokine genes was analyzed using RNAse protection assay. In agreement with earlier findings (Shayakhmetov et al., 2005b), this analysis revealed that genes for IL-1α, IL-1β, TNF-α, and MIP-2, but not for type I interferon (IFN-α4 (Figure S1B)) and IFN-β (data not shown), were transcriptionally activated within 30 min of virus administration. An identical pattern of gene induction was observed after Ad injection in Balb/c, B6.129, and NOD/SCID mice (data not shown). This analysis also showed that the levels of mRNA for these pro-inflammatory genes in the spleen were significantly higher then those in the liver (Figures S1B and C).
Earlier reports suggested that TLR9, IFN-type I receptor, and IL-1RI signaling might be involved in initiating anti-Ad innate immune responses (Muruve et al., 2008; Nociari et al., 2007; Shayakhmetov et al., 2005b; Zhu et al., 2007b). To define if the early expression of pro-inflammaroty cytokines and chemokines depends on the signaling of these receptors, we injected Ad in Il-1r−/−, Ifn-Ir−/− and Tlr9−/− mice (Hemmi et al., 2000; Kolumam et al., 2005). Analysis of the transcriptional activation of IL-1α, IL-1β, KC, MIP-2 and other pro-inflammatory cytokines and chemokines (data not shown) showed that the absence of the type I IFN or TLR9 receptors did not change the levels of mRNA for these cytokines and chemokines in response to Ad administration (Figure 1D). However, the activation of KC and MIP-2, but not IL-1α or IL-1β, genes was dependent on IL-1R signaling (Figure 1D). Because the transcriptional activation of pro-inflammatory cytokine and chemokine genes was dramatic by 30 min after Ad injection (Figure S1B), we analyzed earlier time points to determine the earliest time at which cells respond to a pathogen entry. We found that transcriptional activation of IL-1α, IL-1β and KC genes could be detected within 10 min after virus administration (Figure 1E). However, the full-scale activation of KC and MIP-2 genes required IL-1RI signaling. This IL-1RI-dependent activation of KC and MIP-2 genes was observed as early 15 and 25 min after virus administration, respectively, and did not occur in Il-1r−/− mice (Figure 1F). The analysis of pro-inflammatory proteins in the spleen showed that Ad induced the production of a specific set of cytokines and chemokines with peak levels at 1h to 2h after Ad injection (Figures 1G, and S2). Again, the production of the vast majority of activated pro-inflammatory mediators, including KC, MIP-2, MCP-1, IL-6, and IP-10, but not IL-1α and IL-1β, depended on functional IL-1RI signaling (Figures 1H and S2).
To analyze whether the macrophage response to Ad in vivo was dose-dependent, we administered Ad at varying doses from 109 to 1011 virus particles per mouse. These doses of the virus correspond to actual doses ranging from 10 to 1000 virus particles per one marginal zone MARCO- or CD169-positive cell (see Supplementary Experimental Procedures). This analysis demonstrated that the transcriptional activation of IL-1α, IL-1β, and KC could be detected even at the lowest dose of Ad (Figures S3A and S3B). The dose-dependent activation of pro-inflammatory cytokines and chemokines was also observed at the protein level (Figure S3C). A dose-dependent increase in virus accumulation in macrophages was further confirmed by using immunofluorescent staining of virus particles on spleen sections (Figure S3D).
Collectively, these data demonstrate that CD169- and MARCO-positive MZMφ trap blood-born Ad and within 10 minutes initiate the activation of a specific set of pro-inflammatory cytokines and chemokines. This activation occurs independently of TLR9 or IFN-IR signaling, however, the majority of cytokines and chemokines required functional IL-1RI for their activation.
MZMφ-derived IL-1α is a dominant activator of the Ad-triggered innate immune response
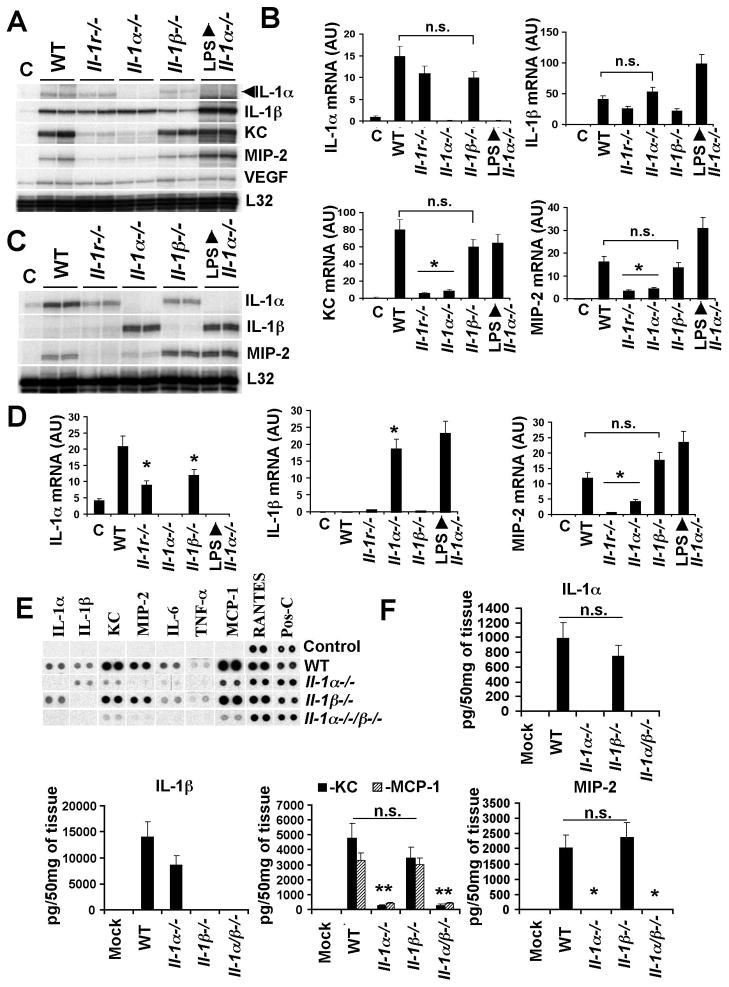
For IL-1RI, both IL-1α and IL-1β can act as functionally active agonists (Dinarello, 1996). Because Ad activates the transcription and synthesis for both of these cytokines within 10 min of intravenous virus injection, we injected virus into control C57Bl/6J mice, or mice knockout for IL-1α (Horai et al., 1998) or IL-1β (Shornick et al., 1996) and analyzed transcription of IL-1α, IL-1β, and KC and MIP-2 (as markers of functional IL-1RI signaling). These analyses revealed that the transcription of IL-1α and IL-1β genes is activated independently of each other (Figure 2A, B). Moreover, we found that the transcription of KC and MIP-2 genes after Ad injection was identical in IL-1β−/− and wild-type mice. In contrast, KC and MIP-2 gene activation was nearly abolished in IL-1α−/− mice, and the levels of these chemokines in IL-1α−/− and Il-1r−/− mice were not statistically different (Figure 2A–D). Administration of IL-1α−/− mice with LPS induced the robust activation of IL-1β, KC and MIP-2, demonstrating that the absence of the IL-1α gene does not render these mice deficient at activating KC and MIP-2 genes by an alternate stimulus. Analysis of the Ad-triggered induction of cytokines and chemokines at the protein level confirmed that the production of KC, MIP-2, and MCP-1 was largely abolished in IL-1α−/− and IL-1α/β−/− mice, but was similar in wild type and IL-1β−/− mice (Figure 2E, F). The analysis of Ad distribution on spleen sections using immunofluorescent staining revealed that MZMφ in both IL-1α−/− and IL-1β−/− mice are capable of trapping Ad particles (Figure S4).
Figure 2. IL-1α is the dominant mediator of an innate immune response to Ad in vivo.
(A) The mRNA levels for IL-1α, IL-1β, KC and MIP-2 in spleens of mice 30 min after Ad injection. Biological duplicates are shown. C – mock-infected mice, injected with saline. (B) Quantitative representation of mRNA levels from the gel shown in (A) after phosphorimager analysis. N=6. n.s. – not statistically significant. * - P < 0.01. AU – arbitrary units. (C) IL-1α, IL-1β, and MIP-2 mRNA levels in livers of mice 30 min after Ad injection. N=3. (D) Quantitative representation of mRNA levels from the gel shown in (C) after phosphorimager analysis. N=6. n.s. – not statistically significant. * - P < 0.01. (E) Protein levels of inflammatory cytokines and chemokines in spleens 1 h after Ad injection. Pos-C are dots that show the manufacturer’s internal positive control samples on the membrane. Control – the spleen protein sample of a mouse injected with saline. (F) The amounts of pro-inflammatory cytokines and chemokines in the spleens of mice 1 hour after Ad injection. N=4. Mock – negative control mice injected with saline. n.s. – not statistically significant. * - P < 0.01.
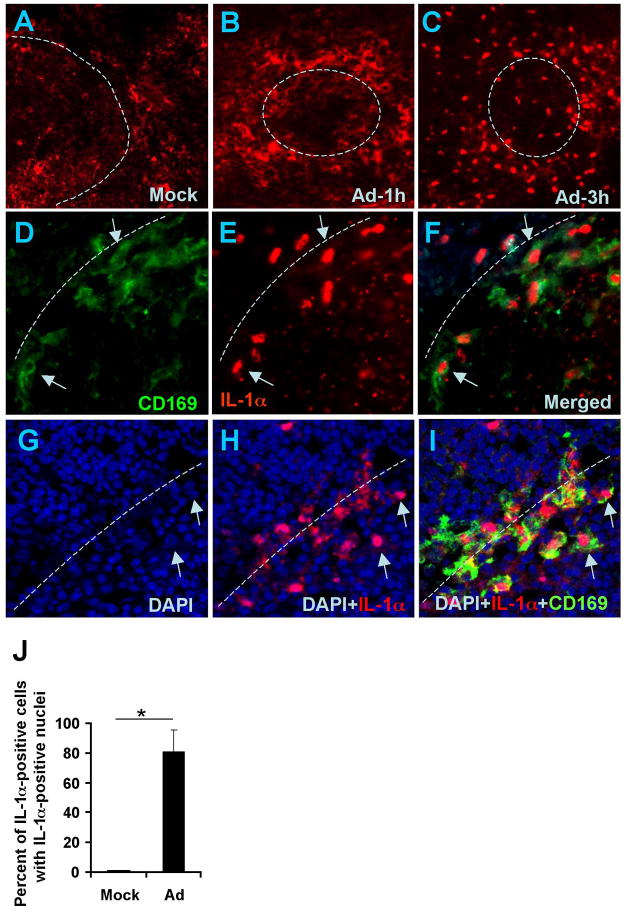
IL-1α is synthesized as a pre-protein, pre-IL-1α (Dinarello, 1996). However, upon cell stimulation with ligands for TLRs, IL-1RI, or TNF-RI, pre-IL-1α is processed in the cytoplasm by neutral proteases, including calpains (Kobayashi et al., 1990), leading to the translocation of the N-terminal IL-1α pro-piece (IL-1α-NTP) to the nucleus, while mature IL-1α is released from the cell (Werman et al., 2004). Staining of spleen sections with IL-1α-specific Ab revealed that by 1 hour after Ad injection, macrophages in marginal zone express IL-1α. However, by 3 hours after virus injection, IL-1α staining was primarily localized to the nuclei of these cells, suggesting IL-1α activation through pre-protein processing (Figure 3A). Semiquantitative analysis of the number of MZMφ with IL-1α positive nuclei revealed that in mice injected with Ad, IL-1α translocated to the nuclei of over 80% of IL-1α-expressing cells (Figure 3B). Collectively, these finding identify IL-1α as a primary cytokine mediating the initiation of an innate immune response to Ad in vivo.
Figure 3. Ad induces expression of IL-1α and its translocation to the nuclei in marginal zone macrophages.
Immunofluorescent and confocal microscopy analysis of IL-1α expression on spleen sections of mock-injected (A) or Ad-injected mice at 1 h (B) or 3 h (C) post injection. The physical border of the germinal centers are depicted by punctuate lines. Confocal microscopy analysis confirms co-localization of IL-1α positive staining with marginal zone macrophage staining (D-F). Arrows indicate CD169-positive cells in the marginal zone, co-localized with IL-1α-positive staining. Representative pictures are shown. N=5. Confocal microscopy analysis shows the co-localization of nuclei of splenic marginal zone cells stained with DAPI (blue, G), IL-1α (red, H), and CD169 macrophage marker (I). Macrophage cells with co-localized DAPI and IL-1α staining are shown by arrows. (J). Semi-quantitative presentation of the proportion of IL-1α-expressing marginal zone cells with IL-1α-positive nuclei in mock-injected mice and Ad injected mice. The nuclear IL-1α-positive staining was analyzed in two hundred IL-1α-positive cells on spleen sections of the Ad-injected group. No IL-1α-positive nuclei were found on spleen sections of mock-injected animals. N=6. * - P < 0.01.
MZMφ activate IL-1α independently of inflammasome, MyD88, TRIF, and TRAF6 signaling
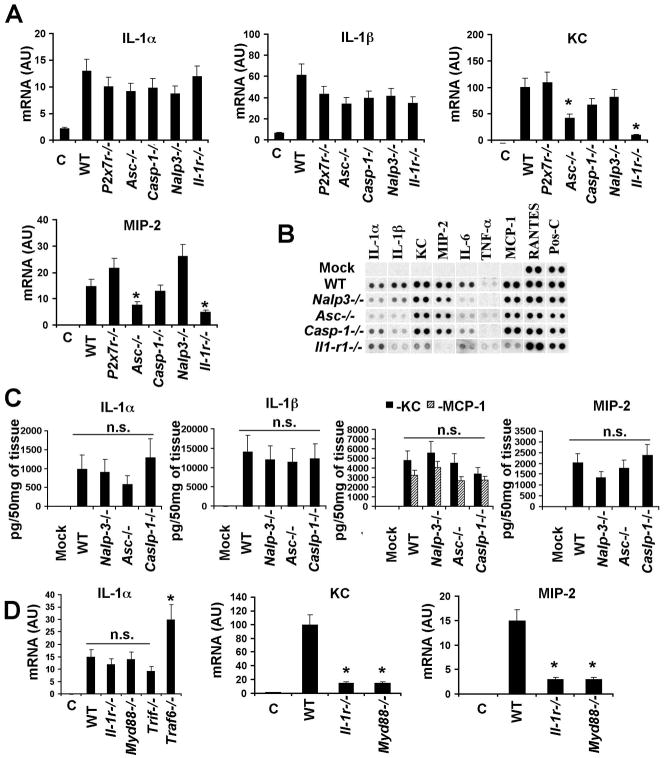
To evaluate whether NALP-3 inflammasome mediates the activation of IL-1α in MZMφ in vivo in response to Ad, we administered Ad into mice knockout for inflammasome components caspase-1, ASC, NALP-3, as well as other proteins involved in inflammasome activation (P2X7R)(Kanneganti et al., 2006; Kuida et al., 1995; Mariathasan et al., 2006). Comparative analysis of IL-1α mRNA levels in these mice after Ad injection showed no statistically significant differences in all analyzed strains (Figure 4A). Moreover, we found that KC and MIP-2 genes were also activated in caspase-1−/− and Nalp3−/− mice to levels comparable to those observed in wild type mice. The analysis of Ad-induced cytokine production at the protein level showed no statistically significant differences in production of IL-1α, IL-1β, KC, MIP-2, and MCP-1 between wild type mice and inlammasome component knockout mice (Figure 4B, C). The immunofluorescent staining of Ad particles on spleen sections of Nalp3−/−, Asc−/−, and Caspase-1−/− mice confirmed that MZMφ are capable of trapping blood-born Ad (Figure S5). Analysis of the transcriptional activation of IL-1α in Myd88−/− and Trif−/− mice (Kawai et al., 1999; Yamamoto et al., 2003) 30 min after Ad injection also showed no statistically significant differences, compared to wild type animals (Figure 4D). After Ad injection, we observed a significant increase in IL-1α mRNA in mice knockout for TRAF6 (Naito et al., 1999), compared to control mice. This analysis also showed a lack of MIP-2 and KC activation in Myd88−/− mice, confirming MyD88’s role as an adaptor for IL-1RI. Collectively, these data demonstrate that the IL-1α-mediated activation of an innate immune response to Ad does not depend on virus sensing by NALP-3 inflammasome components. Furthermore, the IL-1α gene is activated independently of MyD88- or TRIF-dependent TLRs or TRAF6.
Figure 4. Activation of an IL-1α-mediated innate response to Ad does not depend on NALP3-inflammasome, MyD88, TRIF, or TRAF6.
(A) Mice were injected with Ad and mRNA levels for IL-1α, IL-1β, KC and MIP-2 were analyzed 30 min p.i. N=4. Except for the columns indicated by the star, no statistically significant differences between experimental groups and WT group were identified (P > 0.05). * - P < 0.01. C – mock-infected mice, treated with saline. AU – arbitrary units. (B) Analysis of proteins in spleens of mice 1 hour after Ad injection. N=4. Mock – negative control mice injected with saline. Pos-C are dots that show the manufacturer’s internal positive control samples on the membrane. (C) The amounts of pro-inflammatory cytokines and chemokines in the spleens of mice 1 hour after Ad injection. N=3. Mock – negative control mice injected with saline. n.s. – no statistically significant difference were identified between WT and gene knockout animals (P > 0.05). (D) IL-1α, MIP-2 and KC mRNA levels in spleens of mice 30 min after Ad injection. N=4. * - P < 0.01. C – mock-infected mice, treated with saline. n.s. - not statistically significant.
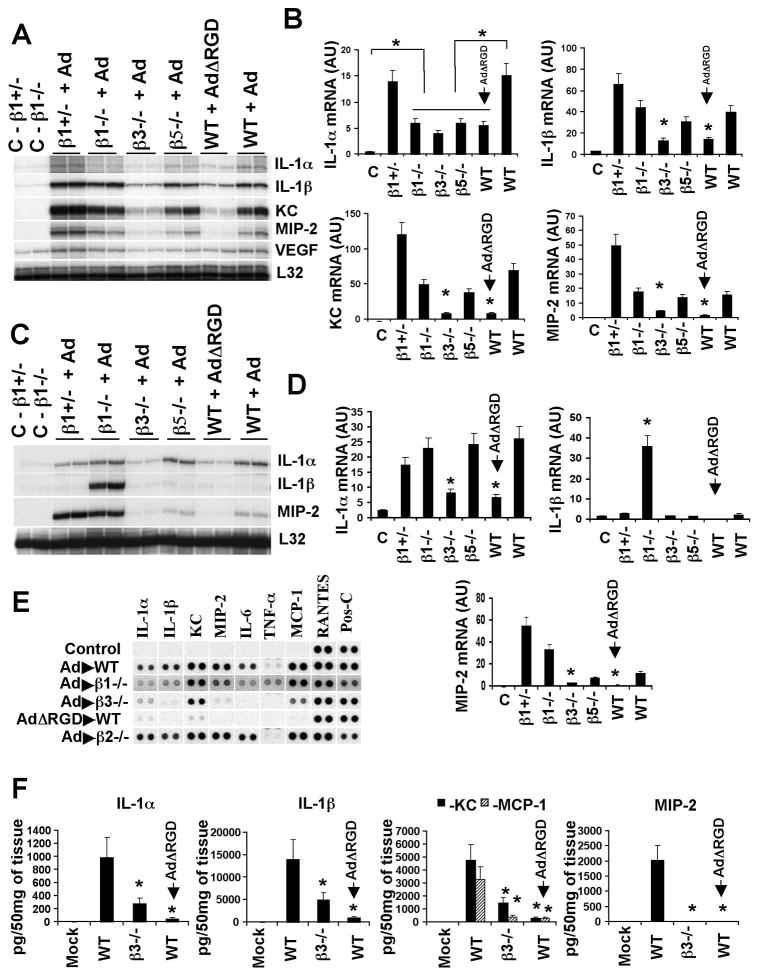
The IL-1α-mediated response to Ad is triggered by virus interaction with β3-integrins
The extremely early transcriptional activation of IL-1α and IL-1β after Ad injection (Figure 1E) suggests that virus sensing occurs either at the cell surface or shortly after virus internalization into the macrophage. Ad entry into a cell is a step-wise process in which the initial binding of the virus to the cell is mediated by a fiber protein, followed by a homo-pentameric penton base protein (pIII) interaction with cellular integrins via RGD amino acid motifs (Wickham et al., 1993). RGD motif-interacting integrins, β1-integrins in particular, are known to activate alarm cytokines genes (Attur et al., 2000; Yurochko et al., 1992). To evaluate the contribution of RGD motif-interacting integrins in the induction of anti-Ad innate immune responses, we administered Ad into mice knockout for either β3- or β5-integrins, or mice where β1-integrin was knocked out in hematopoietic cells. In addition we injected wild type mice with an Ad mutant, AdΔRGD (Shayakhmetov et al., 2005a), that possesses an RGD motif three amino acid deletion within its penton protein, and analyzed the transcriptional activation of IL-1α, IL-1β, KC and MIP-2. This analysis revealed that IL-1α mRNA levels were significantly elevated only in virus-injected groups, compared to mock-injected mice (Figure 5A, B), implying that the transcriptional activation of IL-1α gene does not require virus-integrin interactions. However, absolute levels of IL-1α mRNA were two- to three-fold lower in virus-injected integrin knockout mice compared to their levels in virus-injected wild type (WT) or β1-integrin+/− mice. Similarly, reduced levels of IL-1α mRNA were also observed in wild type mice injected with AdΔRGD virus, compared to those of wild type mice injected with unmodified Ad.
Figure 5. The engagement of β3 integrins by Ad is critical for initiation of the innate immune response.
(A) The mRNA levels for IL-1α, IL-1β, KC and MIP-2 in spleens of wild type mice (WT) and mice knockout for β 3-, β5-, or conditionally knockout for β1-integrin in hematopoietic cells (β1−/−), as well as WT mice injected with Ad mutant lacking an RGD motif within its penton protein (AdΔRGD) 30 min after virus injection. N=3. C – mock-infected mice, injected with saline. (B) Quantitative representation mRNA levels from the gel shown in (A) after phosphorimager analysis. N=6. Statistically significant differences between experimental groups and mock-injected controls [C] or WT injected with Ad are indicated by the star. * - P < 0.01. WT mice injected with AdΔRGD are indicated by the arrow. AU – arbitrary units. (C) IL-1α, IL-1β, and MIP-2 mRNA levels in livers of mice shown in (A). N=3. (D) Quantitative representation mRNA levels from the gel shown in (C) after phosphorimager analysis. N=6. Statistically significant differences between experimental groups and WT mice injected with Ad are indicated by the star. * - P < 0.01. (E) Protein levels of cytokines and chemokines in spleens of mice knockout for integrins β1, β2, β3, or WT mice injected with Ad or AdΔRGD 1 hour after virus injection. N=4. Pos-C are dots that show the manufacturer’s internal positive control samples on the membrane. Control – the spleen protein sample of a mouse injected with saline. (F) The amounts of cytokines and chemokines in the spleens of mice 1 hour after Ad injection. N=4. Mock – negative control mice injected with saline. Statistically significant differences between experimental groups and WT mice injected with Ad are indicated by the star. * - P < 0.01.
In contrast to IL-1α gene activation after Ad injection, mRNA levels for IL-1β, KC, and MIP-2 were significantly reduced in the β3-integrin knockout mice, but not in other experimental groups, suggesting a non-redundant role of β3 integrins in the activation of IL-1β and IL-1RI-dependent KC and MIP-2 chemokines (Figure 5B). Administration of wild type mice with AdΔRGD also resulted in reduced IL-1β, KC, and MIP-2 mRNA levels, implicating the importance of direct interaction between viral RGD motifs and cellular β3 integrins for activation of these cytokines and chemokines. Consistent with these findings, the analysis of IL-1α, IL-1β and MIP-2 mRNA levels in the liver also revealed that for all of these genes, their levels were significantly lower in β3-integrin knockout mice, or wild type mice injected with AdΔRGD, compared to control animals (Figure 5C, D). We further confirmed at the protein level that the production of all analyzed cytokines and chemokines was significantly lower in β3-integrin knockout mice and mice injected with AdΔRGD, than in control wild type mice (Figure 5E, F), mice with hematopoietic cells knockout for β1 integrin, or mice knockout for non-RGD-interacting β2 integrin (Figure 5E).
Immunofluorescent staining of Ad particles confirmed that MZMφ in β3-integrin-KO mice can trap Ad after its intravenous injection (Figure S6). However, similar analysis revealed that when wild type mice were injected with AdΔRGD, there was a marked reduction in Ad-specific staining associated with cells in the marginal zone (Figure S6). To quantify the total amount of Ad genomic DNA associated with spleen tissue after intravenous virus injection, we used quantitative real-time PCR. This analysis showed that the highest levels of Ad genomes were present in spleens of wild type mice, compared to other analyzed groups (Figure S7A). Moreover, we found a statistically significant fivefold reduction in the amount of AdΔRGD DNA associated with spleen tissue, compared to the amount of Ad DNA in wild type animals. To compensate for the reduced amounts of AdΔRGD in the spleen, we injected mice with a 10-fold higher dose of the virus (1011 virus particles per mouse) and found that at this high dose, co-localization of AdΔRGD particles with MZMφ was restored (Figure S6, AdΔRGD, 1011 virus dose). We next analyzed the innate immune response to a high dose AdΔRGD at the protein level. This analysis revealed that there was a three-fold reduction in the amounts of IL-1α found in the spleens of mice injected with a high dose of AdΔRGD, compared to IL-1α amounts in control mice, injected with a standard (1010 virus particles per mouse) dose of Ad. However, we found no activation of IL-1RI-dependent cytokines and chemokines in mice after an injection of a high dose of AdΔRGD, implying that IL-1α protein was not activated to induce IL-1RI signaling (Figures S7B,C).
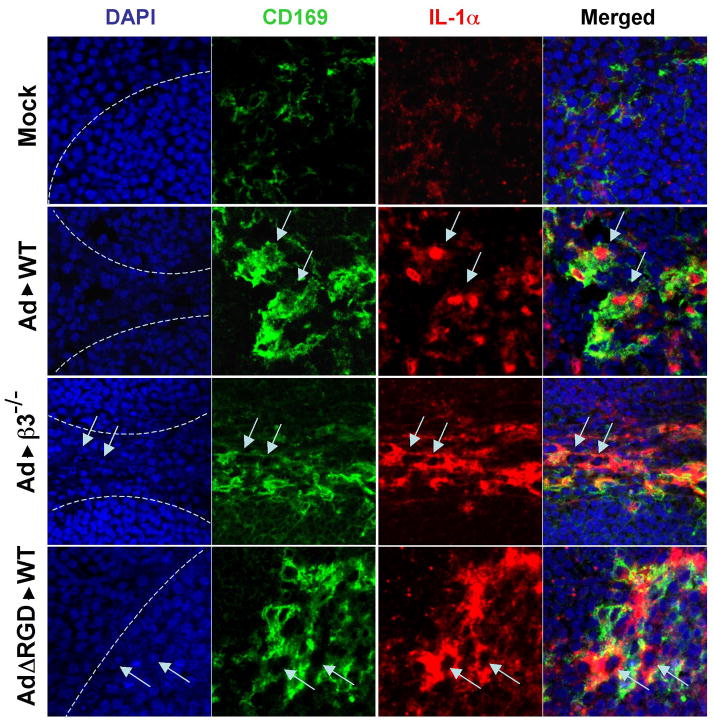
Ad injection into mice showed IL-1α activation and translocation of N-terminal IL-1α pro-piece (IL-1α-NTP) into the macrophage nuclei (Figure 3). To analyze if the injection of wild type mice with AdΔRGD or β3-integrin-KO mice with unmodified Ad would induce translocation of IL-1α-NTP into the nuclei of MZMφ, we injected mice with high doses of viruses and stained spleen sections with an IL-1α-specific antibody, as well as an antibody for MZMφ. Confocal microscopy analysis showed high level of IL-1α-specific staining in MZMφ of wild type and β3-integrin-KO mice injected with Ad as well as in wild type mice injected with AdΔRGD (Figure 6). However, the nuclei of IL-1α-expressing cells in Ad-injected β3-integrin-KO mice or Ad5ΔRGD-injected wild type mice were spared of IL-1α staining, demonstrating the lack of IL-1α processing and translocation of IL-1α-NTP into the nuclei of infected cells (Figure 6 and S7D). Collectively, these data demonstrate that Ad RGD motif binding to β3-integrins on MZMφ is a critical event that leads to the IL-1α-mediated activation of the innate immune and inflammatory macrophage responses to Ad in vivo.
Figure 6. Confocal microscopy analysis of IL-1α translocation into the nuclei of marginal zone macrophages in WT mice and β3 integrin knockout mice injected with Ad, or WT mice injected with AdΔRGD mutant.
Mice were injected intravenously with a high dose of the indicated viruses (1011 virus particles per mouse), and 3 hours later spleens were harvested and sections were prepared and stained with DAPI (blue) to detect nuclei of splenocytes, as well as Abs specific for CD169 (green) or IL-1α (red). Confocal images were obtained using a Zeiss 510 Meta Confocal microscope. The physical border of splenic germinal centers are indicated by punctuate lines. Marginal zone macrophages expressing IL-1α are indicated by arrows. Representative pictures are shown. N=4.
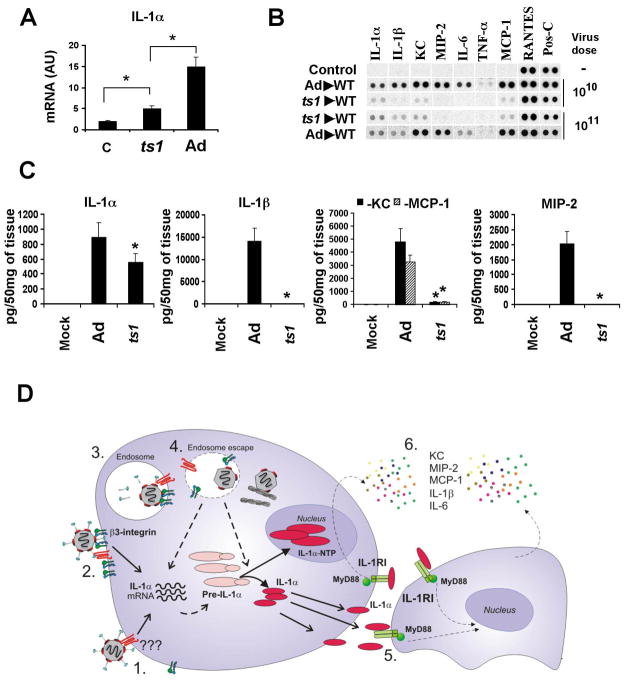
Activation of IL-1α mRNA and IL-1R signaling is amplified by the virus-induced endosome rupture
Ad RGD motif-mediated interaction with cellular integrins triggers the signaling that facilitates virus internalization into the cell, and also initiates a virus disassembly program, promoting to the release of the viral protein pVI, which exhibits endosomolytic activity (Wiethoff et al., 2005). To better determine whether Ad-mediated endosome rupture is required for IL-1α activation at the transcriptional or protein processing levels, we analyzed the spleens of the mice that were injected with a mutant virus ts1. Ts1 is a thermo-sensitive Ad mutant that possesses a single point mutation in the viral protease p23, and when grown at a non-permissive temperature, ts1 is deficient at the endosome rupture step of infection (Greber et al., 1996). Intravenous injection of wild type mice with ts1 resulted in a highly efficient virus co-localization with splenic MZMφ, suggesting that ts1 is not deficient at the internalization step of infection (Figure S8). Moreover, ts1 injection induced significant elevation in IL-1α gene transcription and protein synthesis, compared to mock-injected mice, suggesting that virus recognition by macrophage receptors occurs prior to the endosome rupture step of infection (Figure 7A–C). However, the absolute amounts of IL-1α mRNA and protein were lower after ts1 injection, compared to those observed in Ad-injected mice. Also, we did not observe an activation of the IL-1RI-dependent chemokines KC, MIP-2, and MCP-1 in response to ts1, suggesting that the produced IL-1α protein did not mature and failed to activate IL-1R signaling (Figure 7B, C). Collectively, this data shows that IL-1α gene activation and protein synthesis occur prior to and independently of the endosome rupture step of Ad infection. However, both IL-1α gene transcription and the functional maturation of IL-1α are greatly amplified and facilitated by virus-induced endosome rupture.
Figure 7. Transcriptional and functional activation of IL-1α in response to ts1 mutant virus and the model of IL-1α-mediated activation of the innate immune response to Ad by macrophages in vivo.
(A) Mice were injected with Ad or ts1 mutant and mRNA levels for IL-1α were analyzed 30 min p.i. N=4. * - P < 0.01. C – mock-infected mice, treated with saline. AU – arbitrary units. (B) Analysis of proteins in spleens of mice 1 hour after Ad or ts1 injection at indicated doses. N=4. Mock – negative control mice injected with saline. Pos-C are dots that show the manufacturer’s internal positive control samples on the membrane. (C) The amounts of pro-inflammatory cytokines and chemokines in the spleens of mice 1 hour after Ad or ts1 injection. N=3. Mock – negative control mice injected with saline.* - P < 0.01. (D) Model of Ad induction of IL-1α that triggers the activation of macrophage innate immune and inflammatory responses in vivo. 1. Ad interaction with a macrophage receptor induces transcription and synthesis of pre-IL-1α. 2–4. β3 integrin interaction with virus RGD motifs triggers intracellular signaling that promotes virus internalization into the cell and endosome rupture, thus, leading to the amplification of IL-1α gene transcription, pre-IL-1α processing, translocation of IL-1α -NTP to the nucleus, and enabling mature IL-1α to initiate IL-1RI signaling. 5. IL-1α -mediated IL-1RI signaling leads to the activation and production of pro-inflammatory cytokines and chemokines, including KC, MIP-2, MCP-1, and IL-6.
Discussion
In this report we provide evidence for a novel mechanism of a viral pathogen-mediated activation of innate immunity that suggests virus sensing at the cell surface and does not require virus-associated nucleic acid recognition by intracellular PRRs. Using a set of mice knockout for critical mediators of innate immunity and inflammation, we demonstrated that macrophage-derived IL-1α is the principal activator of the innate immune response to a dsDNA virus, Ad, in vivo. Activation of IL-1α does not require MyD88-, TRIF-, or TRAF6-signaling, and occurs in mice knockout for IL-1β, IFN-IR, or inflammasome components caspase-1, ASC, and NALP3. Collectively, these findings suggest that signaling pathways that were earlier implicated in the activation of an innate antiviral response and leading to type-I IFN production, are not involved in triggering inflammatory and innate immune responses to Ad. The IL-1α-mediated response critically depends on viral RGD motif-mediated binding to macrophage β3 integrins, which occurs prior to the internalization of the virus into the cell (Greber et al., 1993; Wickham et al., 1993) and is further amplified by the virus-mediated endosome rupture. These findings define a unique innate immune pathway and implicate β3-integrin as a sensor of pathogen-associated molecular patterns and IL-1α as a mediator of innate antiviral response (Figure 7D).
Using various in vitro systems, several groups reported earlier that type I IFN is a key mediator of innate immune and inflammatory responses to Ad (Nociari et al., 2007; Zhu et al., 2007a). However, our in vivo studies do not support this conclusion because we did not observe the activation of type I IFN transcription shortly after Ad injection (Figure S1B), and the abrogation of secondary cytokine and chemokine gene transcription occurs only in IL-1RI-KO mice and not in type I IFN-KO mice. The discrepancy between our in vivo data and in vitro data obtained by these groups could be explained by the differential pathways of Ad entry into cells in vitro and in vivo. Using mice knockout for TLR9 we also excluded the involvement of this sensor in activation of innate immunity to Ad in vivo, since the expression levels of all analyzed early cytokines and chemokines were comparable in wild type and TLR9-KO mice.
Recently, Muruve et al. proposed that the NALP3 inflammasome may recognize cytosolic dsDNA, including the Ad genome, and trigger the activation of host innate immune responses (Muruve et al., 2008). In contrast to this model, our data suggest that NALP3, ASC, and caspase-1 are not involved in the sensing of Ad entry into macrophages in vivo. First, the transcriptional activation of the primary cytokines, IL-1α and IL-1β in response to Ad occurs up to similar levels in NALP3-KO, ASC-KO, caspase-1-KO and IL-1RI-KO mice (Figure 4). Second, the activation of secondary chemokines KC and MIP-2 occurred in NALP3-KO and caspase-1-KO mice at significantly higher levels than in the IL-1RI-KO mice. Finally, the major reduction of the anti-Ad response in mice knockout for IL-1α and the high level of response in IL-1β-KO mice clearly show the non-essential role of IL-1β, NALP-3, and other inflammasome components in triggering innate immune and inflammatory responses to Ad in vivo. The discrepancy between the conclusions drawn from Muruve et al. and our present study can be explained by the differences in virus delivery routes, the experimental readouts, and the times of analyses, all of which differed between our study and those described in (Muruve et al., 2008).
Although earlier studies showed that IL-1α is produced by epithelial cells infected with respiratory syncytial virus or human Ad serotype 37 (Ad37) (Chang et al., 2003; Chang et al., 2002), IL-1α-IL-RI pathway was recently defined as a key pathway of inflammatory host response to necrotic cells (Chen et al., 2007). One of the functions of intracellular IL-1α is to lower the threshold of NF-κB and AP-1-dependent gene expression to subpicomolar concentrations of inflammatory stimuli (Werman et al., 2004). Our finding that IL-1α is activated and translocated into the macrophage nuclei in response to Ad in vivo (Figure 3) may indicate the host attempt to establish a higher state of alert facilitating the activation of a downstream cascade of inflammatory mediators. Although the Ad receptor(s) on macrophages activating IL-1α transcription is as yet unknown, the short time required for IL-1α gene induction implies that the virus sensing event occurs either at the cell surface or shortly after the internalization of virus particle into the cell. This is consistent with our observation that the ts1 mutant virus, which cannot rupture the endosomes, is still capable of activating the transcription and synthesis of IL-1α (Figure 7A–C).
Ad internalization into a cell is mediated by cellular integrins (Wickham et al., 1993). Several classes of integrins, such as α5β1, αvβ3, and αvβ5, bind proteins, containing RGD amino acid motif. We demonstrate that the lack of β3 integrins, but not β1- or β5-integrins, ablates IL-1α-mediated activation of an innate immune response to Ad (Figure 5). We also found that although Ad induces IL-1α transcription and synthesis in β3-integrin-KO mice, IL-1α is not translocated to the nuclei of infected cells, suggesting the lack of IL-1α activation. Importantly, the same phenotype of macrophage responses was observed in wild type mice, injected with an Ad mutant lacking an RGD motif within the virus capsid (Zubieta et al., 2005) and ts1 virus mutant, which cannot rupture cellular endosomes. This data suggests that β3 integrins may promote both the virus internalization and the virus-induced endosome rupture in macrophages, leading to the amplification of IL-1α transcription and functional activation of IL-1α protein. Earlier studies of Ad-induced inflammatory responses in vitro and in vivo showed that the induction of pro-inflammatory mediators such as TNFα, RANTES, IP-10, MIP-1α, and MIP-1β was significantly reduced if cells or animals were infected with an Ad mutant lacking the RGD amino acid motif (Koizumi et al., 2006; Schoggins and Falck-Pedersen, 2006; Tibbles et al., 2002). Our findings may provide a mechanistic link between Ad-integrin interactions and the activation of IL-1α, that triggers innate immune and inflammatory responses.
Viruses are obligate intracellular parasites that cannot reproduce outside their host cell. To ensure their evolutionary survival, viruses developed elaborate evasion mechanisms that prevent recognition of the virus infection within the cell and downregulate activation of antiviral responses. However, the detection of pathogens at the cell surface may be more beneficial and effective for the host than their intracellular detection because the finely-tuned very first steps of infection, such as virus attachment and internalization into the cell, are unlikely targets for evasion due to the severe evolutionary pressure. In fact, the minor variations in amino acid composition of the hemagglutinin, a receptor binding protein of the avian influenza H5N1 virus, may modulate the virulence of H5N1 viruses and restrict the cross-species infection transmission (Yen et al., 2009). Epidemiological studies in humans have revealed that the lack of the chemokine receptor and HIV co-receptor, CCR5, on the surface of human leukocytes leads to an abortive virus infection and resistance to HIV (Mosier, 2000). In this study we demonstrate a novel pathway of activation of innate immunity that is triggered by the virus binding to a macrophage plasma membrane receptor and IL-1α as a principal mediator of this response. Further characterization of this pathway may provide valuable insights into the mechanisms of pathogen recognition and the activation of host responses to invading pathogens.
Experimental procedures
Viruses
The replication-defective Ad5-based vectors, Ad5 (Ad5L), Ad5/35L, Ad mutant lacking the RGD motifs in the penton protein, AdΔRGD, and ts1 Ad mutant were previously constructed and described in detail elsewhere.
Gene knocked-out mice
C57BL/6 mice were purchased from Charles River, Wilmington, MA. Il-1r−/− and P2X7R−/−, β1-integrinflox/flox, β3-integrin−/− and β5-integrin−/− mice were purchased from Jackson Laboratory. Caspase-1−/− mice were described in (Kuida et al., 1995); Asc−/− and Nalp3−/− mice were provided by Vishva Dixit (Genentech) (Mariathasan et al., 2004). Tlr9−/−, Trif−/− and Myd88−/− mice were provided by Shizuo Akira (Osaka University). Traf6−/− mice were provided by Jun-Ichiro Inoue (University of Tokyo). IFN-IR0 mice were described in (Kolumam et al., 2005). IL-1α−/−, IL-1α/β−/− mice were described in (Horai et al., 1998). IL-1β−/− mice were described in (Shornick et al., 1996). In these mice, IL-1β gene possesses a neo gene insertion within exon 4, disrupting IL-1β protein production. However, mutated IL-1β mRNA is detectable by IL-1β exon 1-specific RNA probe, supplied by BD Biosciences for RNAse protection assay. All mice were on C57BL/6 genetic background, matched by age and housed in specific-pathogen-free facilities.
Adenovirus delivery in vivo
All experimental procedures involving animals were conducted in accordance with the institutional guidelines set forth by the University of Washington. Unless otherwise specified, mice were injected with 1010 virus particles in 200 μl of phosphate buffered saline (PBS) via tail vein infusion. At indicated times, mice were sacrificed and organs were harvested for further analyzes.
Protein Immuno-arrays
A “Mouse Proteome Array” (#ARY006, R&D System) was used, according to the manufacturer’s instructions. Each spleen was homogenized in 2 ml of sample solution, and 1 ml (1/2 spleen) was used to incubate with each membrane on a rocking platform overnight. Membranes were developed with ImmunoStar HRP-sustrate (BioRad, #1705041).
Antibodies and other materials
Propidium iodide was purchased from Sigma, (Cat. #81845), antibodies from Abcam: biotinylated anti-Ad-Hexon (#ab34374, final dilution 1/100), anti-Ad5 (#ab6982, final dilution 1/50), anti-IL-1α (#ab9724, final dilution 5 ug/ml), Antibodies from BMA: anti-Marco (BMA, #T2026, 2 ug/ml), anti-Moma-1 (or CD-169)(BMA, #T2011, 2 ug/ml), Antibodies from BD: anti-IgM (#553405, 1 ug/ml), FITC-labeled anti-GR-1 (#553127). Secondary antibodies and reagents were from Jackson Immunoresearch: Cy2 or Cy3-labeled streptavidin, or donkey anti-rat or rabbit antibodies, Cy2-, Cy3- or HRP-labeled.
Statistical analyses
Statistical analysis in each independent experiment was performed with an unpaired, two-tailed Student’s t-test. Data are reported as mean +/− standard deviation. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We are thankful to Christopher Wilson (University of Washington, Seattle, WA) for stimulating discussions. We are grateful to Shizuo Akira (Osaka University, Japan) and Jun-Ichiro Inoue (University of Tokyo, Japan) for providing knockout mice. We are thankful to Andrew Byrnes (US FDA) for providing ts1 virus. We are grateful to Michael Barry and Sean Hoffher (May Clinic, Rochester, MN) for assisting with real-time PCR analyses. R. A. F. is a Howard Hughes Medical Institute investigator. This study was supported by US NIH grants AI062853, AI064882, and AI065429 to D.M.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental data. Supplemental data include Supplemental Experimental Procedures, nine figures, and three movies.
References
- Attur MG, Dave MN, Clancy RR, Patel IR, Abramson SB, Amin AR. Functional genomic analysis in arthritis-affected cartilage: Yin-yang regulation of inflammatory mediators by alpha(5)beta(1) and alpha(v)beta(3) integrins. Journal of Immunology. 2000;164:2684–2691. doi: 10.4049/jimmunol.164.5.2684. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Palmer DJ, Beaudet AL, Carey KD, Finegold M, Ng P. Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Hum Gene Ther. 2004;15:35–46. doi: 10.1089/10430340460732445. [DOI] [PubMed] [Google Scholar]
- Chang CH, Huang Y, Anderson R. Activation of vascular endothelial cells by IL-1 alpha released by epithelial cells infected with respiratory syncytial virus. Cellular Immunology. 2003;221:37–41. doi: 10.1016/s0008-8749(03)00058-3. [DOI] [PubMed] [Google Scholar]
- Chang CH, Huang Y, Issekutz AC, Griffith M, Lin KH, Anderson R. Interleukin-1 alpha released from epithelial cells after adenovirus type 37 infection activates intercellular adhesion molecule 1 expression on human vascular endothelial cells. Journal of Virology. 2002;76:427–431. doi: 10.1128/JVI.76.1.427-431.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nature Medicine. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- Greber UF, Webster P, Weber J, Helenius A. The role of the adenovirus protease on virus entry into cells. Embo J. 1996;15:1766–1777. [PMC free article] [PubMed] [Google Scholar]
- Greber UF, Willetts M, Webster P, Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;75:477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- Horai R, Asano M, Sudo K, Kanuka H, Suzuki M, Nishihara M, Takahashi M, Iwakura Y. Production of mice deficient in genes for interleukin (IL)-1 alpha, IL-1 beta, IL-1 alpha/beta, and IL-1 receptor antagonist shows that IL-1 beta is crucial in turpentine-induced fever development and glucocorticoid secretion. Journal of Experimental Medicine. 1998;187:1463–1475. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Ellegast J, Kim S, Brzozka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Schlee M, et al. 5′-triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- Janeway CA, Medzhitov R. Innate immune recognition. Annual Review of Immunology. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88- deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Yamamoto K, Saido T, Kawasaki H, Oppenheim JJ, Matsushima K. Identification of Calcium-Activated Neutral Protease as a Processing Enzyme of Human Interleukin-1-Alpha. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:5548–5552. doi: 10.1073/pnas.87.14.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi N, Kawabata K, Sakurai F, Watanabe Y, Hayakawa T, Mizuguchi H. Modified adenoviral vectors ablated for coxsackievirus-adenovirus receptor, alpha(v) integrin, and heparan sulfate binding reduce in vivo tissue transduction and toxicity. Human Gene Therapy. 2006;17:264–279. doi: 10.1089/hum.2006.17.264. [DOI] [PubMed] [Google Scholar]
- Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. Journal of Experimental Medicine. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MSS, Flavell RA. Altered Cytokine Export and Apoptosis in Mice Deficient in Interleukin-1-Beta Converting-Enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- Lieber A, He CY, Meuse L, Schowalter D, Kirillova I, Winther B, Kay MA. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J Virol. 1997;71:8798–8807. doi: 10.1128/jvi.71.11.8798-8807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- Moore CB, Ting JPY. Regulation of mitochondrial antiviral signaling pathways. Immunity. 2008;28:735–739. doi: 10.1016/j.immuni.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Mosier DE. Virus and target cell evolution in human immunodeficiency virus type 1 infection. Immunologic Research. 2000;21:253–258. doi: 10.1385/IR:21:2-3:253. [DOI] [PubMed] [Google Scholar]
- Muruve DA, Petrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, Parks RJ, Tschopp J. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–111. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- Naito A, Azuma S, Tanaka S, Miyazaki T, Takaki S, Takatsu K, Nakao K, Nakamura K, Katsuki M, Yamamoto T, Inoue J. Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes to Cells. 1999;4:353–362. doi: 10.1046/j.1365-2443.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- Nociari M, Ocheretina O, Schoggins JW, Falck-Pedersen E. Sensing infection by adenovirus: Toll-like receptor-independent viral DNA recognition signals activation of the interferon regulatory factor 3 master regulator. Journal of Virology. 2007;81:4145–4157. doi: 10.1128/JVI.02685-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichlmair A, Schulz O, Tan CP, Naslund TI, Liljestrom P, Weber F, Sousa CRE. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science. 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, Wilson JM, Batshaw ML. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Schoggins JW, Falck-Pedersen E. Fiber and penton base capsid modifications yield diminished adenovirus type 5 transduction and proinflammatory gene expression with retention of antigen-specific humoral immunity. Journal of Virology. 2006;80:10634–10644. doi: 10.1128/JVI.01359-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayakhmetov DM, Eberly AL, Li ZY, Lieber A. Deletion of penton RGD motifs affects the efficiency of both the internalization and the endosorne escape of viral particles containing adenovirus serotype 5 or 35 fiber knobs. Journal of Virology. 2005a;79:4553–4553. doi: 10.1128/JVI.79.2.1053-1061.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayakhmetov DM, Li ZY, Ni SH, Lieber A. Interference with the IL-1-signaling pathway improves the toxicity profile of systemically applied adenovirus vectors. Journal of Immunology. 2005b;174:7310–7319. doi: 10.4049/jimmunol.174.11.7310. [DOI] [PubMed] [Google Scholar]
- Shornick LP, DeTogni P, Mariathasan S, Goellner J, StraussSchoenberger J, Karr RW, Ferguson TA, Chaplin DD. Mice deficient in IL-1 beta manifest impaired contact hypersensitivity to trinitrochlorobenzene. Journal of Experimental Medicine. 1996;183:1427–1436. doi: 10.1084/jem.183.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Innate immunity to virus infection. Immunological Reviews. 2009;227:75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbles LA, Spurrell JC, Bowen GP, Liu Q, Lam M, Zaiss AK, Robbins SM, Hollenberg MD, Wickham TJ, Muruve DA. Activation of p38 and ERK signaling during adenovirus vector cell entry lead to expression of the C-X-C chemokine IP-10. J Virol. 2002;76:1559–1568. doi: 10.1128/JVI.76.4.1559-1568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werman A, Werman-Venkert R, White R, Lee JK, Werman B, Krelin Y, Voronov E, Dinarello CA, Apte RN. The precursor form of IL-1 alpha is an intracrine proinflammatory activator of transcription. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2434–2439. doi: 10.1073/pnas.0308705101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- Wiethoff CM, Wodrich H, Gerace L, Nemerow GR. Adenovirus protein VI mediates membrane disruption following capsid disassembly. Journal of Virology. 2005;79:1992–2000. doi: 10.1128/JVI.79.4.1992-2000.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worgall S, Wolff G, Falck-Pedersen E, Crystal RG. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum Gene Ther. 1997;8:37–44. doi: 10.1089/hum.1997.8.1-37. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- Yen HL, Aldridge JR, Boon ACM, Ilyushina NA, Salomon R, Hulse-Post DJ, Marjuki H, Franks J, Boltz DA, Bush D, et al. Changes in H5N1 influenza virus hemagglutinin receptor binding domain affect systemic spread. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:286–291. doi: 10.1073/pnas.0811052106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurochko AD, Liu DY, Eierman D, Haskill S. Integrins as a primary signal transduction molecule regulating monocyte immediate-early gene induction. Proc Natl Acad Sci U S A. 1992;89:9034–9038. doi: 10.1073/pnas.89.19.9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JG, Huang XP, Yang YP. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. Journal of Virology. 2007a;81:3170–3180. doi: 10.1128/JVI.02192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JG, Huang XP, Yang YP. Type IIFN signaling on both B and CD4 T cells is required for protective antibody response to adenovirus. Journal of Immunology. 2007b;178:3505–3510. doi: 10.4049/jimmunol.178.6.3505. [DOI] [PubMed] [Google Scholar]
- Zubieta C, Schoehn G, Chroboczek J, Cusack S. The structure of the human adenovirus 2 penton (vol 17, pg 121, 2005) Molecular Cell. 2005;17:319–320. doi: 10.1016/j.molcel.2004.11.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.