SUMMARY
Understanding the physiopathology of affective disorders and their treatment relies on the availability of experimental models that accurately mimic aspects of the disease. Here we describe a mouse model of an anxiety/depressive-like state induced by chronic corticosterone treatment. Furthermore, chronic antidepressant treatment reversed the behavioral dysfunctions and the inhibition of hippocampal neurogenesis induced by corticosterone treatment. In corticosterone-treated mice where hippocampal neurogenesis is abolished by X-irradiation, the efficacy of fluoxetine is blocked in some but not all behavioral paradigms, suggesting both neurogenesis-dependent and independent mechanisms of antidepressant actions. Finally, we identified a number of candidate genes, the expression of which is decreased by chronic corticosterone and normalized by chronic fluoxetine treatment selectively in the hypothalamus. Importantly, mice deficient in one of these genes, β-arrestin 2, displayed a reduced response to fluoxetine in multiple tasks, suggesting that β-arrestin signaling is necessary for the antidepressant effects of fluoxetine.
INTRODUCTION
Depression and anxiety are distinct psychiatric disorders with a high comorbidity. Selective serotonin reuptake inhibitors (SSRIs) are the most commonly prescribed drugs for the treatment of depression and several anxiety disorders. However, the actions of SSRIs at the molecular and cellular level still remain poorly understood. Furthermore, successful development of animal models displaying features of depression/anxiety disorders that are responsive to treatment remain in their infancy. Recently, compelling work has suggested that SSRIs exert their behavioral activity in rodents through cellular and molecular changes in the hippocampus as well as other brain structures (Santarelli et al., 2003; Airan et al., 2007; Surget et al., 2008, Wang et al., 2008; David et al., 2007).
The hypothalamo-pituitary-adrenal (HPA) axis, a crossroad between central and peripheral pathways, is also known to play a key role in the pathogenesis of mood disorders (de Kloet et al., 2005). Similarities between features of depression/anxiety and disorders associated with elevated glucocorticoid levels have been reported (Sheline et al., 1996; Gould et al., 1998; McEwen et al., 1999; Airan et al., 2007; Grippo et al., 2005; Popa et al., 2008). Based on these findings, long-term exposure to exogenous corticosterone in rodents has been used to induce anxiety/depression-like changes in behavior, neurochemistry and brain morphology (Ardayfio et al., 2006; Murray et al., 2008; Gourley et al. 2008). Recent results demonstrated that behavioral deficits and decreased cell proliferation in the dentate gyrus of adult mice induced by elevation of glucocorticoid levels are reversed by chronic monoaminergic antidepressant treatment (Murray et al., 2008). In addition, in a chronic stress paradigm, the behavioral effects of some but not all antidepressants are blocked by the ablation of hippocampal neurogenesis (Surget et al., 2008).
In this study we model an anxiety/depressive-like state in mice by studying the consequences of excess glucocorticoids in an attempt to investigate both neurogenesis-dependent and independent mechanisms required for the functions of monoaminergic antidepressants. To this end, we show that chronic treatment with fluoxetine and imipramine in mice reverses the behavioral dysfunction induced by long-term exposure to corticosterone in the Open Field paradigm (OF), Novelty Suppressed Feeding test (NSF), Forced Swim test (FST) and splash test of grooming behavior.
Chronic antidepressant treatment also stimulates the proliferation, differentiation and survival of neural progenitors in the dentate gyrus. Focal X-irradiation that ablates neurogenesis in the hippocampus while leaving other brain areas intact (Santarelli et al., 2003; David et al., 2007) coupled with behavioral tests indicates that there are neurogenesis-dependent and independent mechanisms mediated by chronic fluoxetine in our model of anxiety/depression-like state.
The neurogenesis-independent mechanisms underlying antidepressant efficacy may be linked to changes in signaling in brain areas other than the hippocampus, as we show that three genes related to G protein receptor coupling, β-arrestin 1, β-arrestin 2 and Giα2 proteins, have decreased expression in the hypothalamus that is reversed by fluoxetine. Genetic ablation of β-arrestin 2 blocked several effects of fluoxetine on behavior, suggesting that β-arrestins are necessary for the anxiolytic/antidepressant activity of this drug.
RESULTS
A complete statistical summary is included in supplemental tables 2–4.
Effects of a 3-week antidepressant treatment in a novel stress-related model of anxiety/depression
Recently, multiple studies have confirmed that long-term exposure to glucocorticoids induces anxiety and depressive-like states in rodents (Stone and Lin, 2008; Gourley et al., 2008; Murray et al., 2008). Using a low dose of corticosterone (35 ug/ml/day or 5 mg/kg/day), we found that C57BL/6Ntac and CD1 mice treated for 4 weeks developed an anxiety-like phenotype in both the Open Field (OF) and the Novelty Suppressed Feeding (NSF) test (Figure 1, supplemental figure 2 and 5).
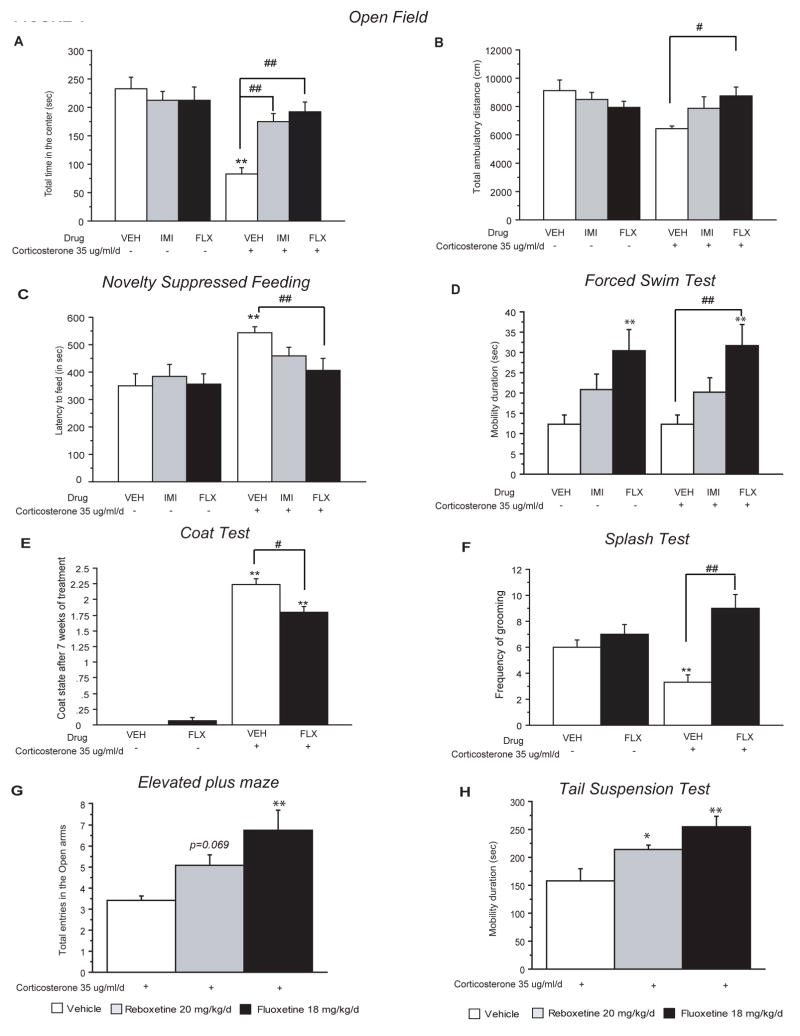
Figure 1. Chronic antidepressant treatment following corticosterone-induced behavioral changes.
(A–B) Effects of 3 weeks of antidepressant treatment (IMI: imipramine; FLX: fluoxetine), started after 4 weeks of corticosterone (35 ug/ml/day), on anxiety behaviors in the Open-Field. Anxiety is measured as mean of the total time spent in the center in seconds (A). Locomotor activity measured as total ambulatory distance traveled (B). Values plotted are mean±SEM (n=10–12 per group). **p<0.01; #p<0.05, ##p<0.01, versus control group and corticosterone/vehicle group respectively. (C) Effects of chronic antidepressant treatment after 7 weeks of corticosterone, on anxiety- and depression-like behaviors in the Novelty Suppressed Feeding paradigm. Results are expressed as mean of latency to feed in seconds. Values plotted are mean±SEM (n=10–12 per group). **p<0.01, ##p<0.01, versus control group and corticosterone/vehicle group respectively. (D) Effects of chronic antidepressant treatment after 7 weeks of corticosterone in the mouse Forced Swim Test. Results are expressed as mean of mobility duration in seconds. Values plotted are mean±SEM (n=10–12 per group). **p<0.01, ##p<0.01, versus control group and corticosterone/vehicle group respectively. (E) Effects of chronic antidepressant treatment on corticosterone induced deterioration of the coat state. Results are expressed as the total resulting from the sum of the score of five different body parts. Values plotted are mean±SEM (n=10–12 per group). **p<0.01; #p<0.05 versus vehicle group and corticosterone/vehicle group respectively. (F) Effects of chronic antidepressant treatment on corticosterone induced anxiety- and depression related behaviors in the splash test. Results are expressed as mean frequency of grooming after squirting a 10% sucrose solution on the mouse’s snout. Values plotted are mean±SEM (n=10–12 per group). **p<0.01, ##p<0.01, versus control group and corticosterone/vehicle group respectively. (G) The effects of 3 weeks of antidepressant treatment (reboxetine 20 mg/kg/day; fluoxetine, 18 mg/kg/day), started after 4-weeks of corticosterone (35 ug/ml/day), on anxiety behaviors in the Elevated plus. Anxiety is expressed as mean total entries in the open arms. Values plotted are mean±SEM (n=12–15 per group). **p<0.01, versus corticosterone/vehicle group. (H) Effects of chronic antidepressant treatment on corticosterone induced behavior in the Tail Suspension test. Results are expressed as mean of mobility duration in seconds. Values plotted are mean±SEM (n=12–15 per group). *p<0.05, **p<0.01, versus corticosterone/vehicle group.
We first tested the effects of 3-week treatment of two distinct antidepressants, a tricyclic (imipramine 40 mg/kg/day) and a SSRI (fluoxetine 18 mg/kg/day), in our model of corticosterone induced anxiety/depression-like behavior in C57BL/6Ntac mice (see experimental design supplemental figure 1). In the OF, chronic exogenous corticosterone had a marked effect on all anxiety parameters, resulting in decreased time spent in the center (figure 1A) and decreased number of entries to the center (data not shown). Interestingly, this anxiety phenotype was reversed by chronic antidepressant treatment [two-way ANOVA, **p<0.01, figure 1A, supplemental figure 7, significant effects of pretreatment, treatment factors and sampling pre-treatment × treatment interactions during the open field sessions (**p<0.01)]. Regarding the total ambulatory distance, chronic corticosterone treatment showed a non-significant trend that was abolished by chronic fluoxetine treatment (figure 1B). Since this trend may affect interpretation of results, we also checked the ratio of total distance in center divided by total distance (or percent path in the center). We found that corticosterone still induced an anxiety-like phenotype as it decreased this measure (Supplemental Figure 7B). Both fluoxetine and imipramine significantly reversed this phenotype. These data suggest that chronic corticosterone treatment can model an anxious-like state that is responsive to treatment with distinct classes of antidepressants.
In the NSF test, we found that chronic corticosterone treatment led to a significant increase in latency to feed (Figure 1C). We then explored whether antidepressants were able to reverse this anxiety/depressive-like state observed in the NSF. Similar to the OF, the change (+36%) in latency to feed induced by chronic corticosterone was reversed by chronic fluoxetine (18 mg/kg/day) and imipramine (40 mg/kg/day), respectively (figure 1C, Kaplan–Meier survival analysis, Mantel–Cox log-rank test **p<0.01, supplemental figure 7C), without affecting the home food consumption (supplemental figure 7). This data further suggests that chronic corticosterone models a state of anxiety/depression that is responsive to antidepressant treatment.
In the mouse Forced Swim Test (FST), two-way ANOVA revealed that chronic corticosterone had no effect, while both fluoxetine and imipramine treatment decreased the duration of mobility during the last four minutes of the test [figure 1D; significant treatment factor effect (**p<0.01)]. The increase in mobility duration with both antidepressants was observed in corticosterone (from 12.2s±2.3 in corticosterone group to 31.7s±5.1 and 20.3s±3.3 in corticosterone/fluoxetine and corticosterone/imipramine group respectively) and non-corticosterone treated animals (from 12.2s±2.4 in vehicle to 30.3s±5.3 and 20.7s±3.6 in fluoxetine and imipramine group respectively).
We next assessed the coat state of the animals. This measure has been described as a reliable and well-validated index of a “depressed-like” state (Griebel et al., 2002; Santarelli et al., 2003). Long-term glucocorticoid exposure, similar to chronic stress (Surget et al., 2008), induced physical changes including deterioration of coat state (figure 1E, supplemental figure 3A) and altered body weight (supplemental figure 3B). Importantly, a 3 week fluoxetine regimen significantly reversed the deterioration of the coat state (figure 1E) induced by chronic corticosterone (from 2.23±0.09 to 1.80±0.08) [two-way ANOVA with significant effect of pre-treatment, treatment factors and sampling pre-treatment × treatment interactions (**p<0.01)].
We then investigated whether the deterioration of the coat state was linked to changes in grooming behavior (figure 1F). We observed that after squirting a 10% sucrose solution on the mouse’s snout, the decreased grooming frequency (−55%, figure 1F) induced by corticosterone treatment was reversed with 3 weeks of fluoxetine treatment (18 mg/kg/day) (from 3.3±0.5 to 9±1) [two-way ANOVA with significant treatment and pretreatment factors (*p<0.05 and **p<0.01)]. Taken together, these results suggest through multiple behavioral readouts that chronic antidepressant treatment is effective in reversing an anxiety/depression-like phenotype induced by excess glucocorticoids.
To further validate our model, we next tested the effects of fluoxetine and a NRI (reboxetine 20 mg/kg/day) in chronic corticosterone treated animals using two additional behavioral measures. In the elevated plus maze, a test associated with anxiety, we found that chronic fluoxetine increased entries into the open arms, while mice treated with reboxetine displayed a strong trend in this measure [(figure 1G; [one-way ANOVA, significant effect of treatment (**p<0.01)]. Furthermore, in the Tail Suspension Test (TST), a test of response to antidepressants, both chronic fluoxetine and reboxetine significantly increased mobility (figure 1H; [one-way ANOVA, significant effect of treatment (**p<0.01, *p<0.05)]).
We also looked at the effects of chronic corticosterone treatment on the response of the HPA axis to an acute stress. The increase of corticosterone elicited by stress in the control mice was markedly attenuated in corticosterone-treated animals (supplemental figure 3E) [two-way ANOVA with significant effect of pre-treatment, treatment factor and pre-treatment × treatment interaction for corticosterone levels (**p<0.01)]. Fluoxetine and imipramine had no effect on stress induced corticosterone levels, both in baseline conditions and after chronic corticosterone treatment.
Chronic fluoxetine treatment after long-term corticosterone exposure affects all stages of adult hippocampal neurogenesis
To investigate the potential cellular mechanisms underlying the behavioral effects of fluoxetine, we next evaluated changes in adult hippocampal neurogenesis hypothesized to be relevant for antidepressant action (Santarelli et al., 2003; Airan et al., 2007).
In agreement with previous observations (Murray et al., 2008; Qiu et al., 2007), chronic corticosterone exposure mimicked the effect of chronic stress on cell proliferation (Surget et al., 2008), decreasing BrdU-positive cells in the dentate gyrus of the adult mouse hippoc0ampus (−25%) (figure 2A) [Two-way ANOVA with significant effect of treatment factor and sampling pre-treatment × treatment interactions (**p<0.01)]. This change in cell proliferation induced by corticosterone was completely reversed by 3-weeks of fluoxetine treatment (18 mg/kg/day). Interestingly, fluoxetine induced a very large and significant effect on proliferation in corticosterone treated mice but not in non-corticosterone treated animals (BrdU-positive cells: from 1335±98.3 in corticosterone treated animals to 3570±733.1 in corticosterone/fluoxetine group).
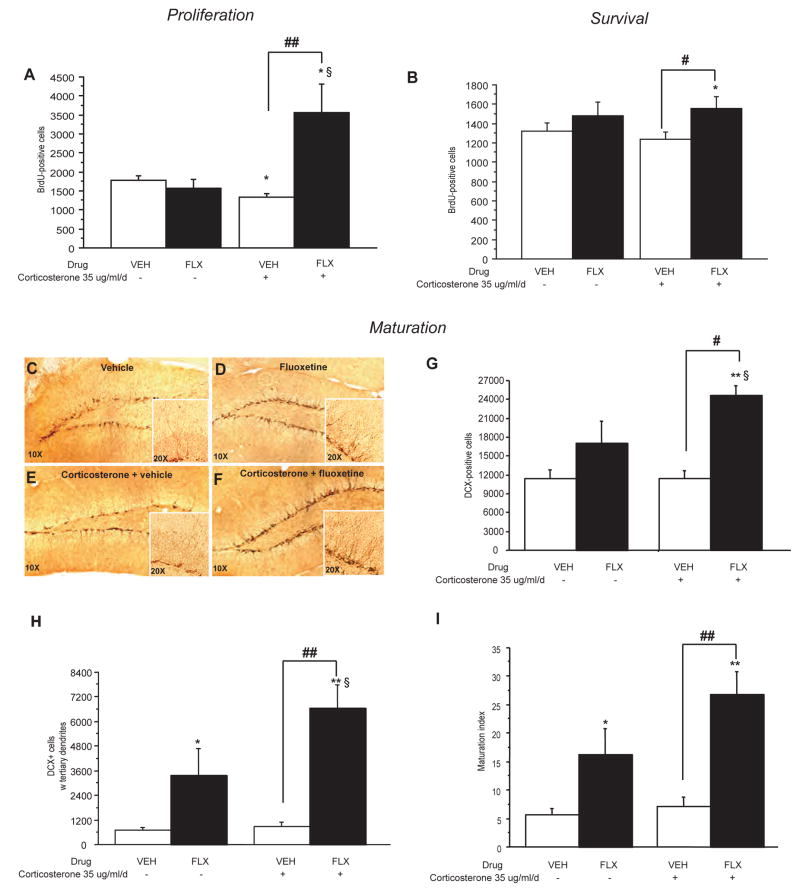
Figure 2. Fluoxetine stimulates cell proliferation, survival and dendritic maturation of young neurons in the dentate gyrus of the adult hippocampus.
(A) BrdU (150 mg/kg) was given 2 hours before sacrifice to examine the effects of 7 weeks of corticosterone (35 ug/ml/day) ± fluoxetine (FLX, 18 mg/kg/day) during the last 3 weeks. Data are the mean±SEM of the BrdU-positive cell counts from 3–4 animals per treatment group for the SGZ and adjacent zone defined as a two-cell body wide zone along the hilar border (40X magnification). *p<0.05; ##p<0.01; §p<0.05 versus vehicle group, corticosterone/vehicle group and fluoxetine/vehicle group respectively. (B) BrdU was given twice a day for 3 days prior to drug treatment to examine the effects of 7 weeks of corticosterone ± fluoxetine during the last 3 weeks. Data are the mean±SEM of the BrdU-positive cells from 5–6 animals per treatment group. *p<0.05; #p<0.05 versus vehicle group and corticosterone/vehicle group respectively. (C–F) Images of doublecortin staining following corticosterone for 7 weeks ± chronic fluoxetine treatment for the last 3 weeks. 10x magnification and 20x for the inset. Left panels (D, F) are vehicle and right panels (E, G) are fluoxetine treated groups. (G) Effects of fluoxetine treatment on total number of DCX+ cells; mean±SEM (n=4 per group) were measured after 7 weeks of corticosterone. **p<0.01; #p<0.05; p<0.05§ versus vehicle group; corticosterone/vehicle group and fluoxetine group respectively. (H–I) DCX+ cells were categorized as to whether or not they exhibited tertiary dendrites. Effects of fluoxetine treatment on the DCX+ cells with tertiary dendrites (H) and maturation (I) of newborn granule cells were measured after 7 weeks of corticosterone. Values are mean±SEM (n=5 per group). **p<0.01; ##p<0.01; p<0.05§ versus vehicle group, corticosterone/vehicle group, fluoxetine/vehicle group respectively.
Although chronic corticosterone treatment alone altered cell proliferation, it did not affect the survival of newborn neurons (figure 2B) or the number of dendrites and dendritic morphology in doublecortin positive cells (figure 2C–F, H, I). A similar lack of effect on cell survival has been observed after chronic mild stress in rats (Heine et al., 2004; Airan et al., 2007). Furthermore, as we previously described, chronic fluoxetine increased the number of doublecortin positive cells with tertiary dendrites and the maturation index in control animals (figure 2H–I) (Wang et al., 2008). However, the effect of fluoxetine is more pronounced in the presence of corticosterone when assessing survival (figure 2B, two-way ANOVA with significant effect of treatment factor, *p<0.05) as well as when counting the number of doublecortin positive cells and assessing their dendritic morphology [figure 2G; significant effect of treatment factor, (**p<0.01); [figure 2H; two-way ANOVA with significant effect of treatment factor (**p<0.01)]. These results indicate that antidepressants stimulate all stages of adult neurogenesis in an animal model of an anxiety/depression-like phenotype.
The behavioral effects of fluoxetine in the chronic corticosterone model are mediated by both neurogenesis-dependent and neurogenesis-independent mechanisms
To assess whether adult neurogenesis is required for the antidepressant-mediated reversal of chronic corticosterone treatment in several behavioral tasks, we next submitted animals to focal hippocampal X-irradiation prior to a chronic corticosterone regimen alone or in combination with fluoxetine (see timeline, supplemental figure 1).
In the Open Field paradigm, the complete loss of hippocampal neurogenesis did not impact the anxiety/depression-like effects of chronic corticosterone. Moreover, the efficacy of fluoxetine was not modified in irradiated mice for all the OF parameters tested (figures 3A–D). Thus, the total decrease in the time spent in the center (sham, 144.7s±16.2 and X-ray, 143.2s±18.4 in corticosterone-treated animals), the total number of entries (sham, 285s±45.1 and X-ray, 275.2s±40.1 in corticosterone-treated animals) and the ratio center/total distance traveled (sham, 17.9s±4.4 and X-ray, 13.2s±3.2 in corticosterone-treated animals) for all sessions after 7 weeks of corticosterone treatment, were reversed by chronic fluoxetine treatment regardless of whether the mice were exposed to X-irradiation [figure 3A–3D; two-way ANOVA with significant treatment factor (*p<0.05)].
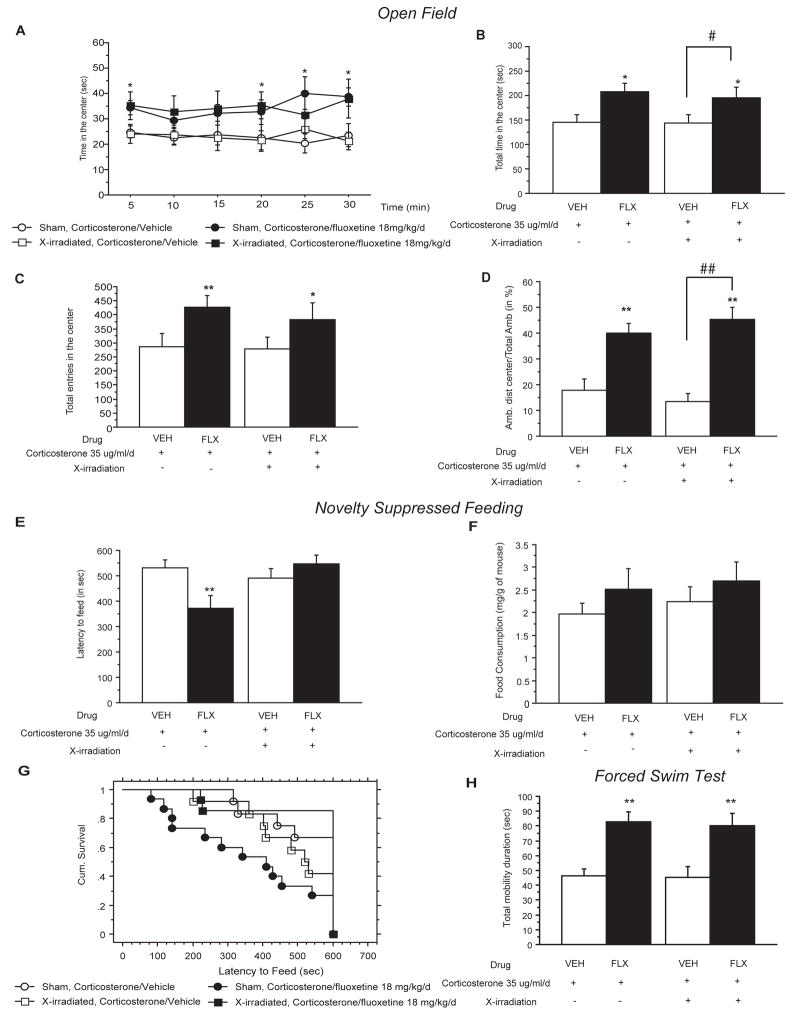
Figure 3. Neurogenesis-dependent and independent effects of chronic fluoxetine on corticosterone-induced behavioral changes.
(A–D) The effects of fluoxetine (FLX, 18 mg/kg/day) treatment after focal X-irradiation of the mouse hippocampus on corticosterone (35 ug/ml/day) induced anxiety-like behaviors in the Open-Field. Anxiety is expressed as mean total of the time spent in seconds for each 5 min period (A), for the entire session (B) and also for the number of entries (C). Locomotor activity is reported as percentage ambulatory distance in the center over total ambulatory distance traveled (D). Values are mean±SEM (n=10–12 per group). *p<0.05, **p<0.01; #p<0.05, versus control group and corticosterone/vehicle group respectively. (EG) Effects of fluoxetine treatment after focal X-irradiation on corticosterone induced anxiety- and depression related behaviors in the Novelty Suppressed Feeding paradigm. Results are mean of latency to feed in seconds (E) or cumulative survival of animals that have not eaten over 10 minutes (G). Feeding drive was assessed by returning the animals to their home cage and measuring food consumed over a period of 5 min (mg/g of mouse) (F). Values are mean±SEM (n=10–12 per group). **p<0.01 versus SHAM corticosterone/vehicle group. (H) Effects of 3 weeks of fluoxetine treatment in 7 weeks corticosterone treated animals after X-irradiation on behavior in the Forced Swim Test. Results are mean of mobility duration in seconds. Values are mean±SEM (n=10–12 per group). **p<0.01 versus control group.
In contrast, the effects of fluoxetine to reverse the anxiety/depressive-like state induced by chronic corticosterone in the NSF paradigm was completely abolished with hippocampal irradation (from 371.3s±50.29 in sham corticosterone/fluoxetine group to 546.2s±36.5 in irradiated corticosterone/fluoxetine group) [figure 3E, 3G; two-way ANOVA with significant interaction between irradiation and treatment, **p<0.01], suggesting a dependence on adult hippocampal neurogenesis. Home cage food consumption was not affected by fluoxetine or irradiation (Figure 3F).
In the mouse FST, the fluoxetine-induced decrease in immobility duration in corticosterone treated animals was not affected by focal irradiation (Figure 3H).
Taken together, these results demonstrate that hippocampal neurogenesis is required for the behavioral activity of fluoxetine in the NSF test but not in the OF and FST, suggesting distinct underlying mechanisms. Interestingly, this is the first report of fluoxetine mediating its effects through distinct neurogenesis-dependent and –independent mechanisms. A recent report has suggested that antidepressants utilize both mechanisms, but fluoxetine was suggested to be neurogenesis-dependent while distinct compounds that are V1B and CRF1 antagonists were suggested to be neurogenesis-independent (Surget et al., 2008).
Chronic fluoxetine treatment restored normal levels of β-arrestin 1 and 2, and Giα2 mRNA in the hypothalamus but not in the amygdala and the hippocampus of corticosterone-treated animals
We next wanted to further explore the distinct neurogenesis-dependent and -independent mechanisms responsible for the anxiolytic/antidepressant-like activity of fluoxetine. To this end, we used a candidate based approach to assess whether there were changes in the expression of genes previously linked to mood disorders (Avissar et al., 2004; Schreiber and Avissar, 2007; Perlis et al., 2007; de Kloet et al., 2005) in different brain regions. Amongst a panel of more than 20 genes involved in mood disorders, we only found three that were changed in our corticosterone model.
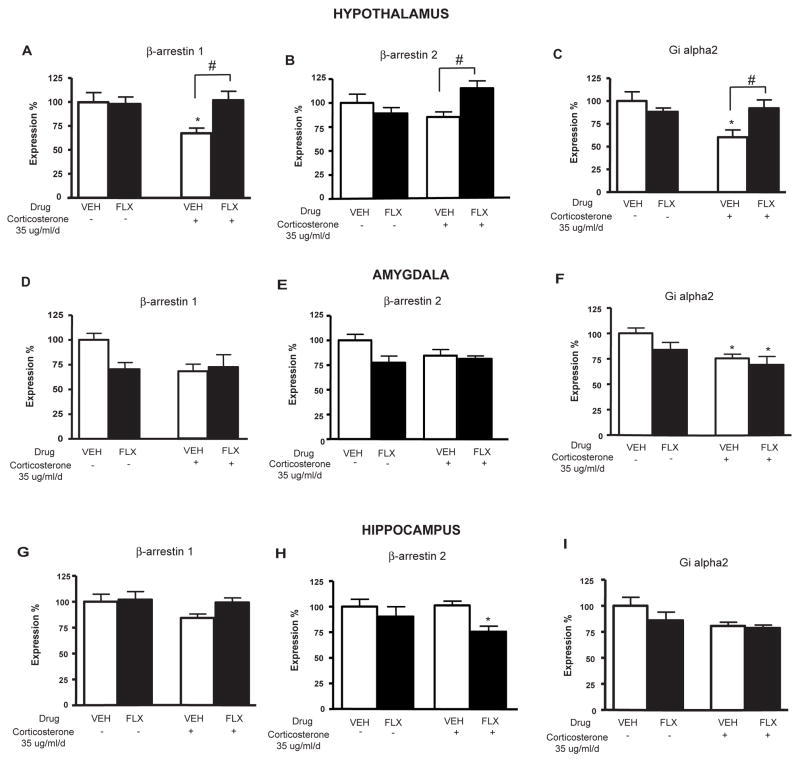
Long-term exposure to corticosterone (35 ug/ml/day) significantly decreased β-arrestin 1 expression in the hypothalamus and there was a similar trend in the amygdala (figure 4A, 4D), but did not effect expression in the hippocampus (figure 4G) (one-way ANOVA for gene expression in the hypothalamus, **p<0.01). Expression of Giα2 expression is also significantly decreased with chronic corticosterone treatment in the hypothalamus and the amygdala (figure 4C, 4F) (one-way ANOVA for gene expression in the hypothalamus and the amygdala, **p<0.01). Interestingly, the decrease of β-arrestin 1 (figure 4A) and Giα2 (figure 4C) gene expression after 7 weeks of corticosterone treatment was totally reversed by chronic fluoxetine treatment only in the hypothalamus but not in the amygdala and the hippocampus (figure 4D, 4F, 4G, 4I) (one-way ANOVA for gene expression in the hypothalamus, **p<0.01). We also found that with β-arrestin 2 expression, a trend of decreased expression (−16%) was reversed with fluoxetine treatment in the hypothalamus but not in the amygdala (figure 4B, 4E, 4H) (corticosterone/Vehicle group versus corticosterone/fluoxetine group in the hypothalamus, p<0.05). Interestingly, in the hippocampus, fluoxetine had an opposite effect on β-arrestin 2 levels (Figure 4H).
Figure 4. Effects of chronic fluoxetine treatment on corticosterone-induced changes in β-arrestin 1, β-arrestin 2 and Giα2 gene expression in mouse hypothalamus, amygdala and hippocampus.
(A–C) Effects of fluoxetine (FLX, 18 mg/kg/day) treatment in corticosterone (35 ug/ml/day) treated animals on the mean β-arrestin 1, β-arrestin 2 and Giα2 gene expression (in % normalized to cyclophilin and GAPDH gene expression)±SEM (n=10–12 per group) in the mouse hypothalamus. *p<0.05; #p<0.05 versus control group and corticosterone/vehicle group respectively. (D–F) Same as above performed for the mouse amygdala. *p<0.05 versus control group. (G–I) Same as above performed for the mouse hippocampus. *p<0.05 versus control group.
From these three genes, we were particularly interested in β-arrestin 2 because the gene expression profile was affected differentially in the hippocampus and hypothalamus, which may indicate an involvement in neurogenesis-dependent and –independent effects of fluoxetine. Interestingly, β-arrestin 2 has been implicated in pathways associated with responsiveness to the mood stabilizer lithium (Beaulieu et al., 2008). There is also evidence in humans implicating β-arrestins in depression and in response to stress, and that these changes are reversible by antidepressant treatment (Dwivedi et al., 2002, Avissar et al., 2004).
β-arrestin 2 is necessary for the anxiolytic/antidepressant effects of chronic fluoxetine
We next proceeded to investigate the contribution of β-arrestin 2 to the behavioral effects of a 3 week treatment with fluoxetine (18 mg/kg/day). We started with the OF, where we found that β-arrestin 2 deficient mice (mixed background 129/Sv × C57BL/6J) in the control group display an anxious-like phenotype evidenced by a decreased of the time spent in the center (figure 5A) as well as a decreased number of entries in the center relative to the untreated wild-type mice (data not shown). Similar to a previous report (Beaulieu et al, 2008), and like the corticosterone treated C57BL/6Ntac mice, we found a non-significant trend toward decreased ambulatory activity in the β-arrestin 2 deficient mice. We therefore checked the percent path in the center of the open field for these mice, and using this normalized data found that the β-arrestin 2 mice were indeed more anxious-like than their wild-type littermates (supplemental figure 7F).
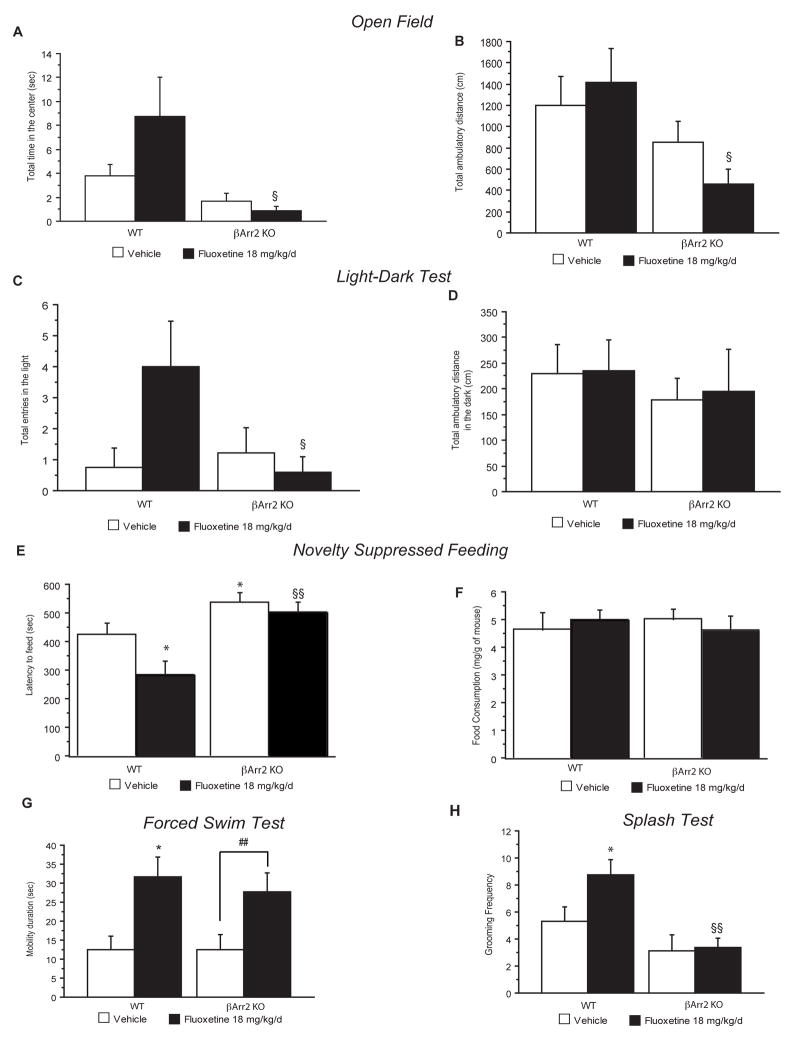
Figure 5. The role of β-arrestin 2 in mediating the behavioral effects of chronic fluoxetine.
(A–B) Effects of 4 weeks of fluoxetine treatment (18 mg/kg/day) in β-arrestin 2 knockout mice (βArr2-KO) and littermates on anxiety behaviors in the Open Field. Anxiety is expressed as mean time in the center (A). Locomotor activity is reported as ambulatory distance traveled for the entire session (B). Values are mean±SEM (n=15–18 per group). §p<0.05, versus fluoxetine treated wild-type mice. (C–D) Effects of chronic fluoxetine in β-arrestin 2 knockout mice and littermates in the light/dark paradigm. Results are mean total entries into the light (C). Locomotor activity is reported as ambulatory distance traveled in the dark (D). Values plotted are mean±SEM (n=9–10 per group). §p<0.05, versus fluoxetine treated wild-type mice. (E–F) Effects of chronic fluoxetine in β-arrestin 2 knockout mice and littermates in Novelty Suppressed Feeding. Results are mean of latency to feed in seconds (E) Feeding drive was assessed by returning the animals to their home cage after the test and measuring food consumed over 5 min (mg/g of mouse) (F). Values are mean±SEM (n=15–18 per group). *p<0.05, versus control group and fluoxetine treated wild-type mice respectively. (G) Effects of chronic fluoxetine in β-arrestin 2 knockout mice and littermates in the Forced Swim Test. Results are mean of mobility duration in seconds. Values are mean±SEM (n=15–18 per group). *p<0.05; #p<0.05, significant difference versus vehicle wild-type or β-arrestin 2 knockout animals respectively. (H) Effects of chronic fluoxetine in the splash test. Results are mean frequency of grooming after squirting a 10% sucrose solution on the mouse’s snout. Values are mean±SEM (n=9–10 per group). *p<0.05; §§p<0.01, versus vehicle wild-type group or fluoxetine treated wild-type respectively.
Chronic fluoxetine treatment had an effect on all anxiety parameters in wild-type animals, resulting in a trend toward increased time spent in the center (figure 5A) and total number of entries in the center (data not shown). Interestingly, planned comparisons unveiled that this effect of fluoxetine treatment is abolished in β-arrestin 2 knockout mice [two-way ANOVA, **p<0.01, figure 5A, significant effects of pretreatment (**p<0.01)]. This absence of effects of fluoxetine in β-arrestin 2 knockout mice is also observed with the total number of entries in the center (data not shown) and the total ambulatory distance [figure 5B, significant effect of pre-treatment (**p<0.01)]. Therefore, similar to the chronic corticosterone model, β-arrestin 2 knockout mice display an anxiety phenotype in the OF. However, unlike the chronic corticosterone treated mice, β-arrestin 2 knockout mice do not respond to fluoxetine treatment in the OF.
We next tested the β-arrestin 2 deficient mice in the Light-Dark test, a behavioral paradigm also associated with anxiety. Unlike the OF, vehicle treated β-arrestin 2 knockout mice did not display an anxious-like phenotype as assessed by entries in the light (figure 5C). This is similar to a previous report, where the β-arrestin 2 knockout mice did not display a phenotype in latency to cross using this test (Beaulieu et al, 2008). However, we found a trend for fluoxetine to increase entries into the light in control mice that was absent in the β-arrestin 2 knockout mice. Planned comparisons unveiled that the two groups of mice were indeed responding differently to fluoxetine (figure 5C, [two-way ANOVA, significant interaction pretreatment × treatment, p=0.04]). Importantly, there was no significant difference observed in ambulatory distance in the dark amongst any of the groups (Figure 5D, [two-way ANOVA, no effect for pretreatment or treatment]). This data further demonstrates a behavioral measure in which β-arrestin 2 knockout mice are not responsive to fluoxetine.
We next tested the effects of fluoxetine in β-arrestin 2 knockout mice using the NSF paradigm. Importantly, untreated β-arrestin 2 knockout mice display an anxious/depressive phenotype evidenced by an increased latency to feed relative to the untreated wild-type mice. Furthermore, while in wild-type mice fluoxetine significantly decreased the latency to feed in the novel environment, fluoxetine had no effect in mutant mice (figure 5E, supplemental figure 7G: Kaplan-Meier survival analysis, Mantel-Cox log-rank test **p<0.01). Food consumption in the home cage was not altered (figure 5F; two-way ANOVA, p>0.4). Taken together, these data indicate that β-arrestin 2 is required for the behavioral effects of fluoxetine in the OF, Light/Dark and NSF.
To further understand the effects of fluoxetine in β-arrestin 2 knockout mice, we assessed behavior in the FST. Interestingly, we found that β-arrestin 2 knockout mice treated with fluoxetine behaved similarly to wild-type mice in that they displayed an increase in mobility relative to the control group. Therefore, in contrast to the Open Field, Light/Dark and NSF results, β-arrestin 2 is not necessary for the behavioral effects of chronic fluoxetine in the mouse FST [two-way ANOVA, figure 5G, significant effects of treatment (**p<0.01)].
Finally, we tested whether fluoxetine was effective in β-arrestin 2 knockout mice using the sucrose splash test of grooming. While fluoxetine significantly increased grooming in control littermates, β-arrestin 2 knockout mice did not respond (figure 5H).
Gene expression profiles in β-arrestin 2-deficient mice indicate a lack of response to fluoxetine
We next assessed gene expression profiles in β-arrestin 2 knockout mice and wild-type littermates treated with vehicle or fluoxetine. We did not detect significant differences in β-arrestin 1 levels, suggesting that there is not compensation amongst the arrestin proteins in the areas that we studied (hypothalamus, amygdala, hippocampus) (Supplemental figure 8A, F, K). Interestingly, we did find that fluoxetine increased CREB1 levels in the hippocampus in wild-type mice, but not in β-arrestin 2 knockouts (Supplemental Figure 8M). Likewise, fluoxetine increased Erk-1 levels in the hypothalamus of wild-type mice but not β-arrestin 2 knockouts (supplemental figure 8E). Taken together, this data suggests a differential response to fluoxetine in the β-arrestin 2-deficient mice.
DISCUSSION
Our data indicate that the behavioral activity of antidepressants such as fluoxetine requires both neurogenesis-dependent and –independent mechanisms. We also provide evidence that some of the effects of fluoxetine are mediated by a β-arrestin signaling pathway.
Elevation of glucocorticoids levels induce an anxiety/depressive state in mice that is reversed by chronic antidepressants
Enhanced activity of the HPA axis involving elevated glucocorticoid levels is considered a key neurobiological alteration in major depression (for review see Antonijevic et al., 2006). In depressed patients, many studies have shown that successful antidepressant therapies are associated with normalization of impairments in the HPA axis negative feedback (Greden et al., 1983; Linkowski et al 1987; Heuser et al 1996; Holsboer-Trachsler et al., 1991). This elevation of glucocorticoid levels in human has been modeled in rodent to reproduce an anxiety and depressive-like state (Ardayfio and Kim, 2006; Murray et al., 2008; Zhao et al., 2008; Gourley et al., 2008). Our model of elevated glucocortocoids was able to blunt the response of the HPA axis as shown by the markedly attenuated stress-induced corticosterone levels observed in these mice (Supplemental figure 3E). This is probably a consequence of the negative feedback exerted by corticosterone on the HPA axis. Consistent with previous findings, our results demonstrate that an elevation of glucocorticoid levels is sufficient to induce anxiety in C57BL/6Ntac and CD1 mice as measured by the decrease in center measures in the OF paradigm as well as with the increase in latency to feed in the NSF (figure 1, supplemental figure 2, supplemental figure 5). A depressive-like state in the C57BL/6J corticosterone–treated animals was also observed as measured by a deterioration of the coat state, a decreased grooming behavior and a flattened circadian rhythm with reduction in home cage activity (figure 1, supplemental figure 4). These symptoms are similar to those elicited by chronic stress (Surget et al., 2008). Similarly, a subset of depressed patients with elevated cortisol display anhedonia, cognitive dysfunctions/distortions and personal neglect (Morgan et al., 2005). Therefore, chronic corticosterone treatment appears to model an anxious and depressed-like state in mice.
When using the C57BL/6Ntac mice, in marked contrast to the OF and NSF, the FST was the only behavioral model in which antidepressants exerted effects in untreated “non-anxious/depressed” mice. The absence of antidepressant effect in both the NSF and OF suggests that different neurobiological mechanisms are recruited by antidepressants when animals are examined in pathological conditions rather than standard homecage conditions. Therefore, when pretreated with corticosterone, mice that are normally nonresponsive to fluoxetine are rendered responsive. Interestingly, when a more anxious strain is used such as the 129SvEv mice, it is possible to detect effects of chronic antidepressants in standard homecage conditions (Santarelli et al., 2003). This is also evident in the β-arrestin 2 mice, which are on a mixed background of C57BL/6J × 129SvEv and are responsive to fluoxetine in standard homecage conditions (Figure 5).
Importantly, we found high levels of mobility during the first two minutes of the FST in all groups. Therefore, we only assessed the last four minutes of the six minute test for our analysis. It is believed that this is the critical time to detect potential effects of antidepressants (Porsolt et al., 1977).
It is also noteworthy that neither fluoxetine or imipramine restored normal levels of corticosterone after an acute stressor, which suggests that their mechanism of action may be independent of the HPA axis.
Enhanced effects of fluoxetine treatment on neurogenesis in corticosterone-treated mice
Glucocorticoids and antidepressants have been shown to modulate adult neurogenesis in opposite directions and hippocampal neurogenesis is required for some of the effects of antidepressants (Gould et al., 1992; McEwen, 1999; Duman et al., 2000; Malberg et al., 2000; McEwen, 2001; Santarelli et al., 2003; Airan et al., 2007; Surget et al., 2008; Murray et al., 2008; Qui et al., 2007, Conrad et al., 2007). Since we previously demonstrated that antidepressants increase all stages of neurogenesis including proliferation, maturation and survival in normal mice, we sought to understand the effects of fluoxetine on neurogenesis in mice that were in an anxious and depressed-like state.
In agreement with previous findings (Murray et al., 2008; Qui et al., 2007), a reduction in the proliferation of progenitor cells after chronic corticosterone treatment was observed (figure 2), demonstrating a role for glucocorticoids in the regulation of the proliferation stage of the neurogenic process. Indeed, it had been reported that ablation of the adrenal glands abolishes stress-induced decreases of cell proliferation (Tanapat et al., 2001). Interestingly, the effects of corticosterone on neurogenesis are limited to the proliferation stage and not the survival or maturation of newborn neurons. Similar results were observed in rat (Heine et al., 2004) and it has been proposed that a decrease in apoptosis counteracts the reduction in neurogenesis elicited by stress and explains the absence of change in number of newborn neurons after chronic stress.
Surprisingly, chronic fluoxetine treatment did not affect hippocampal cell proliferation in non-corticosterone treated C57BL/6Ntac mice. Strain differences in hippocampal adult proliferation have been reported (Schauwecker, 2006, Navailles et al., 2008) and C57BL/6 strain exhibit one of the highest numbers of proliferating cells within the subgranular zone, as compared to other strain of mice.
Interestingly, the effects of fluoxetine on all stages of neurogenesis (proliferation, differentiation and survival) were more pronounced in corticosterone treated mice than in controls. It is possible that our model of corticosterone-induced stress may increase the dynamic range in which fluoxetine exerts effects on different stages of neurogenesis. These enhanced effects may be due to change in the serotonin system elicited by chronic stress. In fact we and others have shown that chronic stress results in a desensitization of 5-HT1A autoreceptors (Hensler et al., 2007; and our data not shown) which is likely to result in an increase in serotonin release and therefore possibly in a stronger effect of fluoxetine. There is also an interesting parallel between these enhanced effects of fluoxetine on neurogenesis and the fact that fluoxetine is more active behaviorally in the corticosterone-treated mice.
Neurogenesis-dependent and independent mechanisms
We had shown earlier that some of the effects of antidepressants in the NSF test require hippocampal neurogenesis (Santarelli et al., 2003). Therefore, we hypothesized that the effect of fluoxetine on the anxiogenic/depressive-like state in corticosterone-treated mice may also require neurogenesis. Indeed, in the corticosterone model, the effects of fluoxetine in the NSF test were blocked by X-irradiation. However, in the same animals, in the OF and the FST, ablation of hippocampal neurogenesis did not modify the anxiolytic/antidepressant-like activity of fluoxetine (figure 4). These behavioral effects are therefore likely to recruit different pathways. To our knowledge, this is the first study, using a model of anxiety/depression in mice, showing that neurogenesis dependent and independent mechanisms are both necessary for the effects of fluoxetine. Overall, these studies suggest that the hippocampal neurogenesis plays an important role in the behavioral effects of fluoxetine. However, there is accumulating evidence that other brain regions are also involved in antidepressant-like activity including amygdala, nucleus accumbens or cingulate cortex. It is also possible that adult neurogenesis outside of the hippocampus may play a role in the effects of fluoxetine (Kokoeva et al., 2005; 2007).
To explore the mechanism underlying the neurogenesis-independent effects of fluoxetine, we analyzed gene expression profiles in the hypothalamus, amygdala and hippocampus, three brains structures involved in the stress response (Nemeroff and Owens, 2004; McEwen et al., 2004; Mayberg et al., 2005; Joels, 2008). We explored the variations in mRNA levels encoding candidate genes selected for their implication in mood disorders including G protein-coupled receptors (GPCR), transcription factors and genes involved in the stress response (Koch et al., 2002; Calfa et al., 2003; Avissar et al., 2004; de Kloet et al., 2005; Matuzany-Ruban et al., 2005; Schreiber and Avissar, 2007; Perlis et al., 2007; Holsboer, 2008). Among these genes, only 3 displayed a change in mRNA levels in the chronic corticosterone group that was reversed by fluoxetine treatment. Furthermore this bidirectional change was only observed in the hypothalamus. Interestingly all 3 genes are involved in GPCR signaling (β-arrestin 1 and 2, and Giα2; figure 4 and supplemental figure 6). The present data are consistent with previous findings in animal and human studies showing decreases in β-arrestin 1 and 2 or Giα2 in depression or after stress and reversal of these changes by various antidepressant treatment (Dwivedi et al., 2002; Avissar et al., 2004). Interestingly, corticotropin-releasing factor type 1 (CRF(1)) receptor, a potential target for the treatment of depression/anxiety and other stress-related disorders, has been shown to recruit β-arrestin 2 (Oakley et al., 2007). Moreover, Beaulieu and colleagues (2008) have recently shown that lithium, a drug used in the management of mood disorders, exerts some of its biochemical and behavioral effects via a β-arrestin signaling complex.
β-arrestin 2 is required for both neurogenesis-dependent and independent effects of fluoxetine
Interestingly, the effects of chronic corticosterone on behavior were similar to those of the β-arrestin 2 ablation (figure 5, supplemental figure 7E–7H). Given that chronic corticosterone treatment decreases β-arrestin levels (particularly in the hypothalamus), it is possible that β-arrestin 2 (figure 5), at least in part, is responsible for mediating the effects of corticosterone on behavior. Furthermore, β-arrestin 2 knockout mice displayed a reduced response to fluoxetine in the Open Field and Novelty Suppressed Feeding paradigms. This suggests that β-arrestin 2 modulates the behavioral response to fluoxetine in both neurogenesis-independent and –dependent tasks.
To further understand how β-arrestin 2 may regulate multiple effects of chronic corticosterone and fluoxetine treatments on behavior, future work will require the usage of tissue-specific knockouts. Classical β-arrestin functions include desensitization of G-protein coupled receptors (Gainetdinov et al., 2004), so it is possible that β-arrestin 2 may be important for desensitization of 5-HT1A receptors in the Raphe Nucleus, a process that has been hypothesized as necessary for the effects of fluoxetine (Artigas et al., 1996). However, our preliminary results suggest that 5-HT1A autoreceptor desensitization in response to chronic fluoxetine is normal in β-arrestin 2 knockout mice. Alternatively, other cell signaling functions of β-arrestins have also been uncovered (Pierce and Lefkowitz, 2001, Beaulieu et al., 2005, Lefkowitz et al., 2006; Beaulieu et al., 2008) and some of lithium’s behavioral effects appear to be mediated by a β-arrestin 2/Akt/Gsk3β signaling pathway.
When compared to corticosterone treated mice, the β-arrestin 2 deficient mice display many similar phenotypes (Table 1). However, while the corticosterone treated mice respond to fluoxetine, in most behavioral readouts the β-arrestin 2 deficient mice do not, suggesting that β-arrestin 2 may be an essential mediator of the fluoxetine induced reversal of an anxious/depressed state.
Table 1. Behavioral effects of a chronic fluoxetine treatment in the chronic corticosterone paradigm and the β-arrestin 2 KO mice.
Summary of effects seen in multiple behavioral tests throughout the study. ↓ decrease parameter; ↑ increase parameter; 0 no effect; Vehicle1: versus vehicle-treated group; Chronic Fluoxetine2: versus chronic corticosterone-treated group; Vehicle3: versus vehicle-treated wild type littermate; Chronic Fluoxetine4: versus vehicle-treated β-arrestin KO mice
| Open Field | Light Dark | Elevated plus Maze |
Novelty Suppressed Feeding |
Splash Test |
Forced Swim Test |
Tail Suspension Test |
||
|---|---|---|---|---|---|---|---|---|
| Time in the center |
Entries into the light |
Time in Open arms |
Latency to Feed |
Grooming frequency |
Mobility duration |
Mobility duration |
||
| Chronic Corticosterone paradigm | Vehicle1 | ↓ | Not tested | Not tested | ↑ | ↓ | 0 | Not tested |
| Chronic Fluoxetine2 | ↑ | Not tested | ↑ | ↓ | ↑ | ↑ | ↑ | |
| βarrestin 2 KO mice | Vehicle3 | ↓ | 0 | Not tested | ↑ | 0 | 0 | ↑ |
| Chronic Fluoxetine4 | 0 | 0 | Not tested | 0 | 0 | ↑ | 0 |
Legend: ↓decrease parameter; ↑ increase parameter; 0 no effect; Vehicle1: versus vehicle-treated group; Chronic Fluoxetine2: versus chronic corticosterone-treated group; Vehicle3: versus vehicle-treated wild type littermate; Chronic Fluoxetine4: versus vehicle-treated β-arrestin KO mice
Conclusion
We have developed an anxiety/depression-like model based on elevation of glucocorticoid levels that offers an easy and reliable alternative to existing models such as the various chronic stress paradigms. It is also the first model that allows the simultaneous study of multiple effects of antidepressant treatment in the same animal, some of which are neurogenesis-dependent while others are not.
The big unanswered question is which of these behavioral, cellular and molecular readouts is most relevant to antidepressant action in human. In other words would a compound that produces just neurogenesis-dependent effects or just some of the neurogenesis independent effects reported here be as effective as SSRIs or tricyclics. To begin to answer this question we are currently testing in this paradigm a series of compounds, which may stimulate neurogenesis more directly than SSRIs such as agomelatine or compounds that target more directly the HPA axis such as CRF antagonists. Ultimately, the success of these new compounds in the clinic will inform us about the predictive value of the biomarkers that we have indentified in this report.
METHODS
Subjects
Adult male C57BL/6Ntac mice were purchased from Taconic Farms (Germantown, NY, USA; Lille Skensved, Denmark). Male heterozygous β-arrestin 2 +/− and heterozygous female mutant β-arrestin 2 +/− mice (age 4–6 months, 25–30 g body weight) were bred on a mixed S129/Sv × C57BL/6 genetic background at Columbia University (New York, USA). Resulting pups were genotyped by polymerase chain reaction (Beaulieu et al., 2008). All corticosterone treated mice were 7–8 weeks old and weighed 23–35g at the beginning of the treatment, were maintained on a 12L:12 D schedule, and were housed five per cage. β-arrestin 2 mice began receiving fluoxetine at 3 months. Food and water were provided ad libitum. Behavioral testing occurred during the light phase for the OF, NSF and FST, splash test. All testing was conducted in compliance with the NIH laboratory animal care guidelines and with protocols approved by the Institutional Animal Care and Use Committee (Council directive # 87–848, October 19, 1987, Ministère de l’Agriculture et de la Forêt, Service Vétérinaire de la Santé et de la Protection Animale, permissions # 92–256 to DJD).
Drugs
Corticosterone (4-pregnen-11b-DIOL-3 20-DIONE 21-hemisuccinate from Sigma, St Louis, MO) was dissolved in vehicle (0.45% hydroxypropyl-β-cyclodextrin (β-CD), Sigma, St Louis, MO). Imipramine hydrochloride (40 mg/kg per day) and fluoxetine hydrochloride (18 mg/kg per day) were purchased from Sigma (St Louis, MO, USA) and Anawa Trading (Zurich, Switzerland) respectively. Reboxetine hydrochloride (Lundbeck Inc.) (20 mg/kg per day) was also used for behavior testing. Corticosterone (7 ug/ml or 35 ug/ml, equivalent to 1 and 5 mg/kg/day) was delivered alone or in presence of antidepressant in opaque bottles to protect them from light, available ad libitum in the drinking water. CORT treatment did not modify levels of antidepressant in the brain (data not shown). Control mice received β-CD. For β-arrestin-2 mice, fluoxetine was delivered by a standard gavage protocol (18 mg/kg/day).
Behavioral testing
Open field
Performed as described (Dulawa et al., 2004). Briefly, motor activity was quantified in four Plexiglas open field boxes 43×43 cm2 (MED Associates, Georgia, VT). Two sets of 16 pulse-modulated infrared photobeams on opposite walls 2.5-cm apart recorded x–y ambulatory movements. Activity chambers were computer interfaced for data sampling at 100-ms resolution. The computer defined grid lines that dividing center and surround regions, with the center square consisting of four lines 11 cm from the wall.
Novelty suppressed feeding
Novelty suppressed feeding (NSF) is a conflict test that elicits competing motivations: the drive to eat and the fear of venturing into the center of brightly lit arena. The NSF test was carried out during a 10-min period as previously described (Santarelli et al., 2003; David et al., 2007). For more detail please see Supplemental Data.
Forced swim test
A modified forced swim test procedure consisting of an increase in water depth was used to enhance sensitivity for detecting putative antidepressant activity of drugs (Porsolt et al., 1977, Dulawa et al., 2004). Mice were placed into plastic buckets (19 cm diameter, 23 cm deep, filled with 23–25°C water) and videotaped for the entire session. As described previously by Porsolt (1977), only the last 4 min were scored for mobility duration.
Data analysis and statistics
Results from data analyses were expressed as mean ± SEM. Data were analyzed using StatView 5.0 software (SAS Institute, Cary, NC). For all experiments one-way, two-way or three way ANOVA with repeated measure were applied to the data as appropriate. Significant main effects and/or interactions were followed by Fisher’s PLSD post hoc analysis, unpaired t tests, or Newman-Keuls as appropriate. In the NSF test, we used the Kaplan–Meier survival analysis due to the lack of normal distribution of the data. Animals that did not eat during the 10 min testing period were censored. Mantel–Cox log-rank test was used to evaluate differences between experimental groups.
More experimental procedures available online in Supplemental Data.
Supplementary Material
Acknowledgments
This work has been supported by the technical assistance of the Animal care facility of the Institut Fédératif de recherche-IFR141 of the Paris XI University and Columbia University. We thank Marc Caron for helpful discussions and, with Bob Lefkowitz, providing β-arrestin 2 mice. This work was supported by NARSAD (R.H.), NIMH Grant R01 MH068542 (R.H.), NICHD Training Grant 5-T32HD55165-02 (B.A.S.), Columbia Lundbeck Translational Fellowship (B.A.S.), Ministère de l’Education Nationale, de l’Enseignement Supérieur et de la Recherche (MENESR, Paris, France) Fellowship (Q.R.), NIMH Grant 1K99MH083943-01 (M.D.), NIMH Grant 5K08MH076083 (E.D.L.), Lundbeck Research USA and AstraZeneca.
Footnotes
The Supplemental Data for this article is online.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317:819–823. doi: 10.1126/science.1144400. [DOI] [PubMed] [Google Scholar]
- Antonijevic IA. Depressive disorders - is it time to endorse different pathophysiologies? Psychoneuroendocrinology. 2006;31:1–15. doi: 10.1016/j.psyneuen.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Ardayfio P, Kim KS. Anxiogenic-like effect of chronic corticosterone in the light-dark emergence task in mice. Behav Neurosci. 2006;120:249–256. doi: 10.1037/0735-7044.120.2.249. [DOI] [PubMed] [Google Scholar]
- Artigas F, Romero L, de Montigny C, Blier P. Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci. 1996;19:378–383. doi: 10.1016/S0166-2236(96)10037-0. [DOI] [PubMed] [Google Scholar]
- Avissar S, Matuzany-Ruban A, Tzukert K, Schreiber G. Beta-arrestin-1 levels: reduced in leukocytes of patients with depression and elevated by antidepressants in rat brain. Am J Psychiatry. 2004;161:2066–2072. doi: 10.1176/appi.ajp.161.11.2066. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Marion S, Rodriguiz RM, Medvedev IO, Sotnikova TD, Ghisi V, Wetsel WC, Lefkowitz RJ, Gainetdinov RR, Caron MG. A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell. 2008;132:125–136. doi: 10.1016/j.cell.2007.11.041. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Calfa G, Kademian S, Ceschin D, Vega G, Rabinovich GA, Volosin M. Characterization and functional significance of glucocorticoid receptors in patients with major depression: modulation by antidepressant treatment. Psychoneuroendocrinology. 2003;28:687–701. doi: 10.1016/s0306-4530(02)00051-3. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McEwen BS, Gould E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci. 1995;15:4687–4692. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, McLaughlin KJ, Harman JS, Foltz C, Wieczorek L, Lightner E, Wright RL. Chronic glucocorticoids increase hippocampal vulnerability to neurotoxicity under conditions that produce CA3 dendritic retraction but fail to impair spatial recognition memory. J Neurosci. 2007;27:8278–8285. doi: 10.1523/JNEUROSCI.2121-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DJ, Klemenhagen KC, Holick KA, Saxe MD, Mendez I, Santarelli L, Craig DA, Zhong H, Swanson CJ, Hegde LG, et al. Efficacy of the MCHR1 antagonist N-[3-(1-{[4-(3,4-difluorophenoxy)phenyl]methyl}(4-piperidyl))-4-methylphen yl]-2-methylpropanamide (SNAP 94847) in mouse models of anxiety and depression following acute and chronic administration is independent of hippocampal neurogenesis. J Pharmacol Exp Ther. 2007;321:237–248. doi: 10.1124/jpet.106.109678. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Nakagawa S, D’Sa C. Neuronal plasticity and survival in mood disorders. Biological psychiatry. 2000;48:732–739. doi: 10.1016/s0006-3223(00)00935-5. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. mRNA and protein expression of selective alpha subunits of G proteins are abnormal in prefrontal cortex of suicide victims. Neuropsychopharmacology. 2002;27:499–517. doi: 10.1016/S0893-133X(02)00335-4. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- Gould E, Cameron HA, Daniels DC, Woolley CS, McEwen BS. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J Neurosci. 1992;12:3642–3650. doi: 10.1523/JNEUROSCI.12-09-03642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci U S A. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Wu FJ, Kiraly DD, Ploski JE, Kedves AT, Duman RS, Taylor JR. Regionally specific regulation of ERK MAP kinase in a model of antidepressant-sensitive chronic depression. Biological psychiatry. 2008;63:353–359. doi: 10.1016/j.biopsych.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greden JF, Gardner R, King D, Grunhaus L, Carroll BJ, Kronfol Z. Dexamethasone suppression tests in antidepressant treatment of melancholia. The process of normalization and test-retest reproducibility. Arch Gen Psychiatry. 1983;40:493–500. doi: 10.1001/archpsyc.1983.01790050019002. [DOI] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Serradeil-Le Gal C, Wagnon J, Pascal M, Scatton B, Maffrand JP, Soubrie P. Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proc Natl Acad Sci USA. 2002;99:6370–6375. doi: 10.1073/pnas.092012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Sullivan NR, Damjanoska KJ, Crane JW, Carrasco GA, Shi J, Chen Z, Garcia F, Muma NA, Van de Kar LD. Chronic mild stress induces behavioral and physiological changes, and may alter serotonin 1A receptor function, in male and cycling female rats. Psychopharmacology. 2005;179:769–780. doi: 10.1007/s00213-004-2103-4. [DOI] [PubMed] [Google Scholar]
- Heine VM, Maslam S, Joels M, Lucassen PJ. Prominent decline of newborn cell proliferation, differentiation, and apoptosis in the aging dentate gyrus, in absence of an age-related hypothalamus-pituitary-adrenal axis activation. Neurobiol Aging. 2004;25:361–375. doi: 10.1016/S0197-4580(03)00090-3. [DOI] [PubMed] [Google Scholar]
- Hensler JG, Advani T, Monteggia LM. Regulation of serotonin-1A receptor function in inducible brain-derived neurotrophic factor knockout mice after administration of corticosterone. Biological psychiatry. 2007;62:521–529. doi: 10.1016/j.biopsych.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Heuser IJ, Schweiger U, Gotthardt U, Schmider J, Lammers CH, Dettling M, Yassouridis A, Holsboer F. Pituitary-adrenal-system regulation and psychopathology during amitriptyline treatment in elderly depressed patients and normal comparison subjects. Am J Psychiatry. 1996;153:93–99. doi: 10.1176/ajp.153.1.93. [DOI] [PubMed] [Google Scholar]
- Holsboer F. How can we realize the promise of personalized antidepressant medicines? Nat Rev Neurosci. 2008;9:638–646. doi: 10.1038/nrn2453. [DOI] [PubMed] [Google Scholar]
- Holsboer-Trachsler E, Stohler R, Hatzinger M. Repeated administration of the combined dexamethasone-human corticotropin releasing hormone stimulation test during treatment of depression. Psychiatry Res. 1991;38:163–171. doi: 10.1016/0165-1781(91)90041-m. [DOI] [PubMed] [Google Scholar]
- Joels M. Functional actions of corticosteroids in the hippocampus. Eur J Pharmacol. 2008;583:312–321. doi: 10.1016/j.ejphar.2007.11.064. [DOI] [PubMed] [Google Scholar]
- Koch JM, Kell S, Hinze-Selch D, Aldenhoff JB. Changes in CREB-phosphorylation during recovery from major depression. J Psychiatr Res. 2002;36:369–375. doi: 10.1016/s0022-3956(02)00056-0. [DOI] [PubMed] [Google Scholar]
- Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science. 2005;310:679–683. doi: 10.1126/science.1115360. [DOI] [PubMed] [Google Scholar]
- Kokoeva MV, Yin H, Flier JS. Evidence for constitutive neural cell proliferation in the adult murine hypothalamus. The Journal of comparative neurology. 2007;505:209–220. doi: 10.1002/cne.21492. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Rajagopal K, Whalen EJ. New roles for beta-arrestins in cell signaling: not just for seven-transmembrane receptors. Mol Cell. 2006;24:643–652. doi: 10.1016/j.molcel.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Linkowski P, Mendlewicz J, Kerkhofs M, Leclercq R, Golstein J, Brasseur M, Copinschi G, Van Cauter E. 24-hour profiles of adrenocorticotropin, cortisol, and growth hormone in major depressive illness: effect of antidepressant treatment. J Clin Endocrinol Metab. 1987;65:141–152. doi: 10.1210/jcem-65-1-141. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuzany-Ruban A, Avissar S, Schreiber G. Dynamics of beta-arrestin1 protein and mRNA levels elevation by antidepressants in mononuclear leukocytes of patients with depression. J Affect Disord. 2005;88:307–312. doi: 10.1016/j.jad.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Magarinos AM. Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Hum Psychopharmacol. 2001;16:S7–19. doi: 10.1002/hup.266. [DOI] [PubMed] [Google Scholar]
- Morgan VA, Mitchell PB, Jablensky AV. The epidemiology of bipolar disorder: sociodemographic, disability and service utilization data from the Australian National Study of Low Prevalence (Psychotic) Disorders. Bipolar Disord. 2005;7:326–337. doi: 10.1111/j.1399-5618.2005.00229.x. [DOI] [PubMed] [Google Scholar]
- Murray F, Smith DW, Hutson PH. Chronic low dose corticosterone exposure decreased hippocampal cell proliferation, volume and induced anxiety and depression like behaviours in mice. Eur J Pharmacol. 2008;583:115–127. doi: 10.1016/j.ejphar.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Navailles S, Hof PR, Schmauss C. Antidepressant drug-induced stimulation of mouse hippocampal neurogenesis is age-dependent and altered by early life stress. The Journal of comparative neurology. 2008;509:372–381. doi: 10.1002/cne.21775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB, Owens MJ. Pharmacologic differences among the SSRIs: focus on monoamine transporters and the HPA axis. CNS Spectr. 2004;9:23–31. doi: 10.1017/s1092852900025475. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Olivares-Reyes JA, Hudson CC, Flores-Vega F, Dautzenberg FM, Hauger RL. Carboxyl-terminal and intracellular loop sites for CRF1 receptor phosphorylation and beta-arrestin-2 recruitment: a mechanism regulating stress and anxiety responses. Am J Physiol Regul Integr Comp Physiol. 2007;293:R209–222. doi: 10.1152/ajpregu.00099.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis RH, Purcell S, Fava M, Fagerness J, Rush AJ, Trivedi MH, Smoller JW. Association between treatment-emergent suicidal ideation with citalopram and polymorphisms near cyclic adenosine monophosphate response element binding protein in the STAR*D study. Arch Gen Psychiatry. 2007;64:689–697. doi: 10.1001/archpsyc.64.6.689. [DOI] [PubMed] [Google Scholar]
- Pierce KL, Lefkowitz RJ. Classical and new roles of beta-arrestins in the regulation of G-protein-coupled receptors. Nat Rev Neurosci. 2001;2:727–733. doi: 10.1038/35094577. [DOI] [PubMed] [Google Scholar]
- Popa D, Lena C, Alexandre C, Adrien J. Lasting syndrome of depression produced by reduction in serotonin uptake during postnatal development: evidence from sleep, stress, and behavior. J Neurosci. 2008;28:3546–3554. doi: 10.1523/JNEUROSCI.4006-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- Qiu G, Helmeste DM, Samaranayake AN, Lau WM, Lee TM, Tang SW, So KF. Modulation of the suppressive effect of corticosterone on adult rat hippocampal cell proliferation by paroxetine. Neurosci Bull. 2007;23:131–136. doi: 10.1007/s12264-007-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Schauwecker PE. Genetic influence on neurogenesis in the dentate gyrus of two strains of adult mice. Brain Res. 2006;1120:83–92. doi: 10.1016/j.brainres.2006.08.086. [DOI] [PubMed] [Google Scholar]
- Schreiber G, Avissar S. Regulators of G-protein-coupled receptor-G-protein coupling: antidepressants mechanism of action. Expert Rev Neurother. 2007;7:75–84. doi: 10.1586/14737175.7.1.75. [DOI] [PubMed] [Google Scholar]
- Sheline YI. Hippocampal atrophy in major depression: a result of depression-induced neurotoxicity? Mol Psychiatry. 1996;1:298–299. [PubMed] [Google Scholar]
- Stone EA, Lin Y. An anti-immobility effect of exogenous corticosterone in mice. Eur J Pharmacol. 2008;580:135–142. doi: 10.1016/j.ejphar.2007.10.045. [DOI] [PubMed] [Google Scholar]
- Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, Hen R, Belzung C. Drug-Dependent Requirement of Hippocampal Neurogenesis in a Model of Depression and of Antidepressant Reversal. Biological psychiatry. 2008;64:293–301. doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Rydel TA, Galea LA, Gould E. Exposure to fox odor inhibits cell proliferation in the hippocampus of adult rats via an adrenal hormone-dependent mechanism. The Journal of comparative neurology. 2001;437:496–504. doi: 10.1002/cne.1297. [DOI] [PubMed] [Google Scholar]
- Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28:1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Ma R, Shen J, Su H, Xing D, Du L. A mouse model of depression induced by repeated corticosterone injections. Eur J Pharmacol. 2008;581:113–120. doi: 10.1016/j.ejphar.2007.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.