Abstract
Aim
The region on chromosome 6p21 (IDDM1) confers the largest part of genetic susceptibility to type 1 diabetes (T1D) with particular human leucocyte antigen (HLA) alleles predisposing and others protecting from it. As T1D is primarily a “sporadic” disease, the pathophysiology must involve gene–environment interactions. We searched for indirect evidence for such major histocompatibility complex (MHC)–environment interactions by asking two questions: (i) can the degree of an HLA association vary over time periods? and (ii) if a prenatal event like an intrauterine infection – that might cluster in seasons – leads to differences of HLA associations in patients with particular birth months?
Methods
We screened the Type 1 Diabetes Genetics Consortium (T1DGC) database (in addition our own database and the original UK, US and SCAND databases) for MHC DR-DQ and CTLA4 associations. First, we separated the groups of patients with onset of disease before 1980 in comparison with onset after 1980. Second, we analysed the data according to dates of birth (grouped in months). Not all patients’ dates of birth or manifestation periods were available, leading to different group sizes. There were 282 patients analysed for manifestation periods and 329 for birth month.
Results
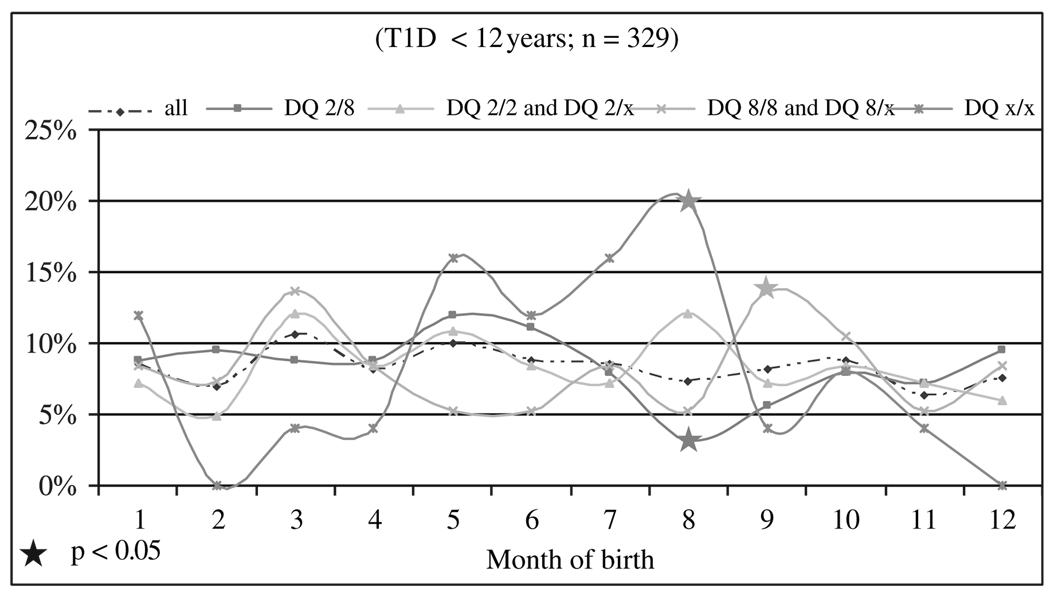
The cohorts of manifestation before 1980 demonstrated a significantly lower frequency of DQ2/X (2 vs. 14.2%; p = 0.03). There was a trend for DQ8/x to be more frequent for manifestations before 1980 (34 vs. 21.6%; p < 0.10). Other alleles did not differ significantly. The months of birth were not evenly distributed. Significant deviations from the whole group were seen in August (DQ2/8 trough and DQx/x high), whereas birth in September was more frequent in DQ8/x or DQ8/8 carriers. This pattern was significantly different from the expected distribution of months at birth (13.9 vs. 7.6%; p < 0.04).
Conclusions
We demonstrate the feasibility of an analysis that searches for indirect evidence of gene–environment interactions. These preliminary data need to be confirmed in larger data sets.
Keywords: β-cell destruction, antigen presentation, immunogenetics, prediction, thymic development
Introduction
Type 1 diabetes (T1D) results from the immune-mediated targeted destruction of pancreatic β-cells after a triggering event in genetically predisposed individuals. Although genes play an important role, this disease is by far a spontaneous disorder with only few T1D patients that have first-degree relatives also affected. Therefore gene–environment interactions are thought to be critical for risk enhancement.
The human leucocyte antigen (HLA) molecules DR and DQ encoded by their genes on chromosome 6 are the most relevant out of all T1D susceptibility genes and account for at least 30–40% of all heritable risk [1]. Little is known with regard to HLA-DR, HLA-DQ genes and their interaction with environmental factors to enhance T1D susceptibility. Two well-known epidemiological facts have not been conclusively linked to genetic factors. An increase of T1D cases has been reported from several regions, pointing to possible driving external forces. Furthermore, a well-known seasonal variation of incidence has been also been observed for clusters of dates of birth [2–4].
Individuals born in different seasons of the year have a variable intrauterine environment with higher rates of viral infections during a gestational period in autumn/winter seasons compared with spring/summer [5]. Likewise vitamin D levels are significantly lower in winter and early spring rendering differences in immunity of both the pregnant mother and the foetus. We therefore wondered whether HLA susceptibility genes are found in different proportions of patients either born in different seasons of the year or having manifested their disease in different historical periods over time.
Materials and Methods
Subjects
The Type 1 Diabetes Genetics Consortium (T1DGC) database has been described in detail elsewhere [6,7]. In addition to data from the T1DGC, we screened the original UK, US, SCAND databases and our own database of T1D patients [8]. We filtered for those with HLA-DR, DQ and CTLA4 genotypes available and then separated groups of patients with onset of disease before 1980 in comparison with onset after 1980, which represented the larger group. Separation before and after 1980 allowed to detect a significant difference. We also analysed in relation to dates of birth grouped in months. T1D diagnosis was based on WHO criteria.
Genotyping
HLA-DR, DQ and CTLA4 genotyping was performed according to the protocols of the T1DGC [7].
Results
Historical Periods of T1D Manifestation: HLA-DQ Combinations Before and After 1980
Separation before and after 1980 exhibited a significant difference by group. There were 50 T1D patients with manifestations before 1980 and 232 after 1980. Among those with T1D manifested before 1980, there were significantly less with HLA-DQ2/x (2 vs. 14.2%; p < 0.03). In addition, we observed a trend for DQ8/x being more frequent in this group (34 vs. 21.6%; p < 0.10). All other alleles and their combinations showed no significant difference (table 1) by group.
Table 1.
Genetic susceptibility to type 1 diabetes over time
| Manifestation year (T1D) | |||
|---|---|---|---|
| <1980, n (%) | ≥1980, n (%) | p value | |
| Genotype | |||
| DQ2/8 | 15 (30.0) | 82 (35.3) | 0.5772 |
| DQ2/2 | 6 (12.0) | 15 (6.5) | 0.2914 |
| DQ8/8 | 8 (16.0) | 26 (11.2) | 0.4810 |
| DQ2/x | 1 (2.0) | 33 (14.2) | 0.0301 |
| DQ8/x | 17 (34.0) | 50 (21.6) | 0.0905 |
| DQx/x | 3 (6.0) | 26 (11.2) | 0.3993 |
| Total | 50 | 232 | |
| Allele | |||
| DQ2 | 28 (28.0) | 145 (31.3) | 0.6033 |
| DQ8 | 48 (48.0) | 184 (39.7) | 0.1538 |
| DQx | 24 (24.0) | 135 (29.1) | 0.3657 |
| Total | 100 | 464 | |
Dates of Birth and HLA-DQ Alleles
There was no even distribution for all dates of birth. There was a small increase of those born in March and May (figure 1). There were fluctuations in those subgroups with the following HLA-DQ combinations: DQ2/8, DQ2/2 and DQ2/x, DQ8/8 and DQ8/x and DQx/x. A significant deviation from the expected distribution was found for DQx/x. This group had higher rates of birth in August where a trough was found for DQ2/8 heterozygotes. Another high rate of birth was observed for DQ8/x or DQ8/8 carriers in September (13.9 vs. 7.6%; p < 0.04; figure 1). There were no significant differences observed for CTLA4 alleles for birth months or manifestation periods (data not shown).
Fig. 1.
Months of birth in patients with type 1 diabetes and their human leucocyte antigen DQ genotypes.
Discussion
We observed a higher proportion of HLA-DQ8/x and a lower proportion for DQ2/x among T1D patients who had manifested their disease before 1980 in comparison with the period thereafter. As this observation is based on a limited number of patients, an analysis of larger numbers is mandatory before drawing final conclusions. These findings, however, are in accordance with other groups who found a higher proportion of high-risk individuals from the UK who had presented their diabetes in the period between 1922 and 1946, compared with cohorts diagnosed after 1985 [9]. Thus, stronger environmental forces may lead to higher incidence of T1D over time and one could hypothesize that the impact of certain HLA risk alleles may diminish. This is also reflected in a continuing decrease of T1D manifestation age in Belgian boys over a 15-year period [10], illustrating the particular environmental force acting in subgroups who may be more vulnerable to diabetogenic factors because of genetic, endocrine or developmental backgrounds.
In many Western societies, there is a rising incidence of T1D in childhood that is thought to result from an increase of environmental factors interacting with backgrounds of genetic susceptibility in vulnerable time windows [11]. Such a developmental milieu would also be the time period of gestation. Pregnancies during winter periods undergo different environmental adverse events than those during spring and summer [12]. These environmental factors include ambient temperatures through climate conditions as well as UV exposure and resulting vitamin D levels, viral and other infections that prevail in late winter. Furthermore, nutrition differs in seasons because of the availability of certain foods that may harbour infectious or potentially toxic agents. The potential influence of gestational or birth-related factors on the risk to contract T1D is illustrated by the recent meta-analysis demonstrating an increased rate of T1D after caesarean sections [13].
During gestation, the main part of the immune repertoire of the thymus with regard to tolerance induction is shaped. In the murine system, thymic development is completed by week 8, whereas in humans, thymic development continues into early childhood [14].
Within the thymus, there are parts essential in the development of normal immunity: self-tolerance and competence are acquired first in the cortex where positive selection occurs, whereas the thymic medulla deletes self-reactive T-lymphocytes. Surviving T-cells travel to lymph nodes where first primary and then memory immunity is generated. This process is under constant surveillance and depends on several cofactors for optimal outcome [14].
The genes in the major histocompatibility complex (MHC) are divided into MHC class I (HLA-A, HLA-B and HLA-C) and MHC class II (HLA-DP, HLA-DQ, HLA-DR) loci. Not only MHC class II but also MHC class I loci harbour strong susceptibility as detected recently [15]. As the islet infiltrating lymphocytes are predominantly MHC class I–restricted CD8+ T cells, their thymic priming might be vulnerable in certain time windows [16]. Therefore, time windows before disease onset as well as the critical periods of pregnancy and early childhood may contain both the environmental or developmental risk factors operative at the amino acid level of HLA susceptibility [17].
Seasonality of birth months in children and adolescents with T1D has been observed in ethnically homogeneous, but not heterogeneous, populations [18]. These populations were from Israel (Ashkenazy Jews, Israeli Arabs, Sardinians, UK-Canterbury English, New Zealanders and Afro-Americans) [19–22]. In addition, other autoimmune endocrine disorders have been studied for variation in birth months. Graves’ disease diagnosed in Greece has been found to cluster for certain months of birth, pointing to prenatal risk factors [23]. Taken together, these data illustrate the feasibility of an interaction analysis taking into account both environmental, developmental and genetic data for risk factor analysis in T1D.
Acknowledgements
This research utilizes resources provided by the Type 1 Diabetes Genetics Consortium, a collaborative clinical study sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Allergy and Infectious Diseases (NIAID), National Human Genome Research Institute (NHGRI), National Institute of Child Health and Human Development (NICHD) and Juvenile Diabetes Research Foundation International (JDRF) and supported by U01 DK062418. This study was also supported by the German foundation ‘Das zuckerkranke Kind’.
Footnotes
Conflict of interest:
The authors declare that they have no conflicts of interest in publishing this article.
References
- 1.Erlich H, Valdes AM, Noble J, et al. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes. 2008;57:1084–1092. doi: 10.2337/db07-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Songini M, Casu A, Ashkenazi I, Laron Z. Seasonality of birth in children (0–14 years) and young adults (0–29 years) with type 1 diabetes mellitus in Sardinia differs from that in the general population. The Sardinian Collaborative Group for Epidemiology of IDDM. J Pediatr Endocrinol Metab. 2001;14:781–783. doi: 10.1515/jpem.2001.14.6.781. [DOI] [PubMed] [Google Scholar]
- 3.Kordonouri O, Shuga N, Lewy H, Ashkenazi I, Laron Z. Seasonality of month of birth of children and adolescents with type 1 diabetes mellitus in Berlin differs from the general population. Eur J Pediatr. 2002;161:291–292. doi: 10.1007/s00431-002-0941-9. [DOI] [PubMed] [Google Scholar]
- 4.Muntoni S, Karvonen M, Muntoni S, Tuomilehto J. Seasonality of birth in patients with type 1 diabetes. Lancet. 2002;359:1246–1248. doi: 10.1016/S0140-6736(02)08227-2. [DOI] [PubMed] [Google Scholar]
- 5.Wu P, Dupont WD, Griffin MR, et al. Evidence of a causal role of winter virus infection during infancy on early childhood asthma. Am J Respir Crit Care Med. 2008;178:1123–1129. doi: 10.1164/rccm.200804-579OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Concannon P, Erlich HA, Julier C, et al. Type 1 diabetes: evidence for susceptibility loci from four genome-wide linkage scans in 1,435 multiplex families. Diabetes. 2005;54:2995–3001. doi: 10.2337/diabetes.54.10.2995. [DOI] [PubMed] [Google Scholar]
- 7.Rich SS, Concannon P, Erlich H, et al. The Type 1 Diabetes Genetics Consortium. Ann N Y Acad Sci. 2006;1079:1–8. doi: 10.1196/annals.1375.001. [DOI] [PubMed] [Google Scholar]
- 8.Ramos-Lopez E, Bruck P, Jansen T, Herwig J, Badenhoop K. CYP2R1 (vitamin D 25-hydroxylase) gene is associated with susceptibility to type 1 diabetes and vitamin D levels in Germans. Diabetes Metab Res Rev. 2007;23:631–636. doi: 10.1002/dmrr.719. [DOI] [PubMed] [Google Scholar]
- 9.Gillespie KM, Bain SC, Barnett AH, et al. The rising incidence of childhood type 1 diabetes and reduced contribution of high-risk HLA haplotypes. Lancet. 2004;364:1699–1700. doi: 10.1016/S0140-6736(04)17357-1. [DOI] [PubMed] [Google Scholar]
- 10.Weets I, Rooman R, Coeckelberghs M, et al. The age at diagnosis of type 1 diabetes continues to decrease in Belgian boys but not in girls: a 15-year survey. Diabetes Metab Res Rev. 2007;23:637–643. doi: 10.1002/dmrr.758. [DOI] [PubMed] [Google Scholar]
- 11.Rewers M, Zimmet P. The rising tide of childhood type 1 diabetes – what is the elusive environmental trigger? Lancet. 2004;364:1645–1647. doi: 10.1016/S0140-6736(04)17368-6. [DOI] [PubMed] [Google Scholar]
- 12.Jongbloet PH, Groenewoud HM, Huber S, Fieder M, Roeleveld N. Month of birth related to fecundity and childlessness among contemporary women. Hum Biol. 2007;79:479–490. doi: 10.1353/hub.2008.0006. [DOI] [PubMed] [Google Scholar]
- 13.Cardwell CR, Stene LC, Joner G, et al. Caesarean section is associated with an increased risk of childhood-onset type 1 diabetes mellitus: a meta-analysis of observational studies. Diabetologia. 2008;51:726–735. doi: 10.1007/s00125-008-0941-z. [DOI] [PubMed] [Google Scholar]
- 14.Anderson G, Lane PJ, Jenkinson EJ. Generating intra-thymic microenvironments to establish T-cell tolerance. Nat Rev Immunol. 2007;7:954–963. doi: 10.1038/nri2187. [DOI] [PubMed] [Google Scholar]
- 15.Nejentsev S, Howson JM, Walker NM, et al. Localization of type 1 diabetes susceptibility to the MHC class I genes HLA-B and HLA-A. Nature. 2007;450:887–892. doi: 10.1038/nature06406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roep BO. Diabetes: missing links. Nature. 2007;450:799–800. doi: 10.1038/450799a. [DOI] [PubMed] [Google Scholar]
- 17.Thomson G, Valdes AM, Noble JA, et al. Relative predispositional effects of HLA class II DRB1-DQB1 haplotypes and genotypes on type 1 diabetes: a meta-analysis. Tissue Antigens. 2007;70:110–127. doi: 10.1111/j.1399-0039.2007.00867.x. [DOI] [PubMed] [Google Scholar]
- 18.Laron Z, Lewy H, Wilderman I, et al. Seasonality of month of birth of children and adolescents with type 1 diabetes mellitus in homogenous and heterogeneous populations. Isr Med Assoc J. 2005;7:381–384. [PubMed] [Google Scholar]
- 19.Neu A, Kehrer M, Ashkenazi I, Laron Z. Seasonality of birth in children (0–14 years) with diabetes mellitus type 1 in Baden-Wuerttemberg, Germany. J Pediatr Endocrinol Metab. 2000;13:1081–1085. doi: 10.1515/jpem.2000.13.8.1081. [DOI] [PubMed] [Google Scholar]
- 20.Roche EF, Lewy H, Hoey HM, Laron Z. Differences between males and females in the seasonality of birth and month of clinical onset of disease in children with type 1 diabetes mellitus in Ireland. J Pediatr Endocrinol Metab. 2003;16:779–782. doi: 10.1515/jpem.2003.16.5.779. [DOI] [PubMed] [Google Scholar]
- 21.Laron Z. Interplay between heredity and environment in the recent explosion of type 1 childhood diabetes mellitus. Am J Med Genet. 2002;115:4–7. doi: 10.1002/ajmg.10338. [DOI] [PubMed] [Google Scholar]
- 22.Willis JA, Scott RS, Darlow BA, Lewy H, Ashkenazi I, Laron Z. Seasonality of birth and onset of clinical disease in children and adolescents (0–19 years) with type 1 diabetes mellitus in Canterbury, New Zealand. J Pediatr Endocrinol Metab. 2002;15:645–647. doi: 10.1515/jpem.2002.15.5.645. [DOI] [PubMed] [Google Scholar]
- 23.Krassas GE, Tziomalos K, Pontikides N, Lewy H, Laron Z. Seasonality of month of birth of patients with Graves’ and Hashimoto’s diseases differ from that in the general population. Eur J Endocrinol. 2007;156:631–636. doi: 10.1530/EJE-07-0015. [DOI] [PubMed] [Google Scholar]