Abstract
Understanding the neurobiological underpinnings of putative memory stabilization processes that maintain context-response-cocaine associations in long-term memory and underlie contextual control over addictive behavior is of great interest from an addiction treatment perspective. Using an instrumental animal model of contextual drug relapse, we show that the protein synthesis inhibitor, anisomycin, administered into the basolateral amygdala (BLA) immediately after limited (15- or 60-min) re-exposure to a previously cocaine-paired context subsequently disrupted the ability of the previously cocaine-paired context to reinstate extinguished cocaine-seeking behavior relative to vehicle. Consistent with a BLA-mediated memory reconsolidation deficit, similar impairment in cocaine-seeking behavior was not observed in “no-reactivation” control groups that received anisomycin into the BLA after (re)exposure to either a novel unpaired or an extinction-paired context nor in a neuroanatomical control group that received anisomycin into the posterior caudate-putamen, dorsally adjacent to the BLA, after re-exposure to the cocaine-paired context. Furthermore, anisomycin administered into the BLA after brief (5-min) or extensive (120-min) re-exposure to the cocaine-paired context (which was sufficient to extinguish cocaine-seeking behavior in a vehicle control group) also failed to alter responding. Together, these findings suggest that re-exposure to a cocaine-paired context in the absence of cocaine reinforcement is sufficient to trigger memory reconsolidation processes that support future drug-seeking behavior. The presence and duration of drug-related memory reactivation critically influences and anisomycin-sensitive mechanisms in the BLA selectively control this phenomenon. These findings support the feasibility of novel pharmacotherapeutic approaches that selectively inhibit the reconsolidation of cocaine-related memories in order to prevent drug relapse.
Keywords: cocaine, context, reinstatement, anisomycin, rat
Environmental contexts provide a setting where associations can form between drug-seeking behavior and the motivational effects of cocaine. The resulting long-term memories of context-response-drug associations underlie the ability of drug-associated environmental contexts to reinstate extinguished drug-seeking behavior in laboratory animals (Alleweireldt et al., 2001; Everitt et al., 2001; Crombag et al., 2002) and promote drug relapse in addicts (Ehrman et al., 1992; Foltin & Haney, 2000). It has been theorized that, upon retrieval, these memories can become destabilized and need to be reconsolidated into long-term memory in order to be maintained (Misanin et al., 1968; Lewis, 1979; Nader et al., 2000b). Thus, investigating the neurobiological mechanisms of memory stabilization may provide unique insight into suppressing abnormal learning and memory that contribute to addictive behaviors.
The basolateral amygdala (BLA) plays a critical role in the expression of context-induced reinstatement of cocaine-seeking behavior (Fuchs et al., 2005; 2007) and in memory reconsolidation processes that regulate aversive and appetitive conditioned behaviors (Nader et al., 2000a; Bahar et al., 2004; Dudai & Eisenberg, 2004). Importantly, the BLA mediates memory reconsolidation processes that underlie the motivational effects of drug-associated Pavlovian conditioned stimuli and conditioned reinforcers. For instance, anisomycin (ANI)—induced inhibition of protein synthesis in the BLA after drug-context memory reactivation disrupts morphine-conditioned place preference in a manner consistent with a memory reconsolidation deficit (Milekic et al., 2006). Similarly, zif268 knockdown or NMDA receptor antagonism in the BLA in conjunction with drug-conditioned stimulus (CS) memory reactivation impairs subsequent CS-induced instrumental behaviors, including cocaine-seeking behavior (Lee et al., 2005; 2006a; Milton et al., 2008). Unlike conditioned stimuli that signal imminent reward/reinforcement, drug-associated contexts in instrumental settings act as occasion setters that signal drug availability contingent upon responding (Fuchs et al., 2005). The associative structure that supports contextual control over drug-seeking behavior (i.e. context-response-drug associations) is distinctly different from that maintaining Pavlovian stimulus and CS control over behavior (i.e. CS/context-drug associations). Therefore, it is unclear whether the same or different neural mechanisms control the memory reconsolidation processes that underlie context-induced cocaine-seeking behavior as those that support Pavlovian contextual conditioned and CS-induced instrumental behaviors.
To start investigating this question, the present study evaluated the contribution of ANI-sensitive memory reconsolidation processes within the BLA to context-induced reinstatement of instrumental cocaine-seeking behavior, while the companion paper to this report focused on contributions of the dorsal hippocampus, dorsomedial prefrontal cortex, and dorsolateral caudate-putamen to this phenomenon (Ramirez et al., submitted). ANI or vehicle was administered into the BLA following a non-reinforced session during which drug seeking was permitted to occur in the previously cocaine-paired, extinction-paired, or an unpaired context. The effects of these manipulations on subsequent cocaine-seeking behavior were assessed in the cocaine-paired context. Based on previous studies (Pedreira & Maldonado, 2003; Suzuki et al., 2004; Power et al., 2006), we hypothesized that a brief memory reactivation session in the cocaine-paired context would trigger context-response-drug memory reconsolidation, and the involvement of ANI-sensitive processes in the BLA in this phenomenon would be demonstrated by ANI-induced, memory reactivation-dependent disruption of subsequent cocaine-seeking behavior.
MATERIALS AND METHODS
Animals
Experimentally naïve male Sprague-Dawley rats (Charles River, N=119), weighing 275-300 g at the start of the experiment, were individually housed in a temperature and humidity controlled vivarium on a reversed light-dark cycle. Rats were maintained on 20-25 g of rat chow/day, with water available ad libitum. Rat housing and treatment followed the guidelines of the “Guide for the Care and Use of Laboratory Rats” (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, 1996) and protocols approved by the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee.
Food training
In order to expedite the acquisition of cocaine self-administration, rats were first trained to lever press on a fixed ratio (FR) 1 schedule of food reinforcement (45 mg pellets; Purina, Richmond, IN, USA) in standard sound-attenuated operant conditioning chambers (26 × 27 × 27 cm high; Coulbourn Instruments, Allentown, PA, USA) during a 16-h overnight session. The food training chamber was distinctly different from Contexts 1, 2, and 3 used subsequently in the experiment. It contained a food pellet dispenser located between two retractable levers and an empty plastic tray with no bedding beneath the bar floor (26 cm × 27 cm). Visual, olfactory, and auditory stimuli were not programmed to occur during the training session aside from the sound of the food hopper and electric ventilation fan. During the session, each lever press on the one (active) lever resulted in food pellet delivery only. Lever presses on the other (inactive) lever had no programmed consequences. The contextual stimuli used for subsequent conditioning were not present in the operant conditioning chamber.
Surgery
Forty-eight h after food training, rats were fully anesthetized using ketamine hydrochloride and xylazine (66.6 mg/kg and 1.3 mg/kg, respectively; IP). Chronic indwelling catheters were constructed and implanted into the right jugular vein, as described previously (Fuchs et al., 2006b). The catheter ran subcutaneously and exited on the rat’s back, posterior to the shoulder blades. After the catheter surgery, the rats were placed into a stereotaxic instrument (Stoelting, Wood Dale, IL, USA). They received stainless steel guide cannulae (26 gauge, Plastics One), aimed bilaterally at the BLA or overlying posterior caudate-putamen (pCPu) using standard stereotaxic procedures (BLA: −2.7 mm AP, ± 5.2 mm ML, −6.8 mm DV; pCPu: −2.7 mm AP, ± 5.2 mm ML, −4.8 mm DV, relative to the skull surface at bregma). Rats were given minimum 5 days for post-operative recovery before the start of the experiment.
To extend catheter patency, catheters were flushed through once daily for 5 days following surgery with 0.1 ml of an antibiotic cefazolin solution (10.0 mg/ml, Schein Pharmaceutical, Florham Park, NJ, USA). Thereafter, catheters were flushed with 0.1 ml heparinized saline (10 U/ml; Baxter Healthcare Corp., Deerfield, IL, USA) before, and with 0.1 ml of the cefazolin solution and 0.1 ml of heparinized saline (70 U/ml) after, each self-administration session. Catheter patency was periodically verified by infusing 0.1 ml of propofol (10 mg/ml, IV; Abbott Labs., North Chicago, IL, USA), an ultra short-acting barbiturate which produces a rapid loss of muscle tone only when administered intravenously.
Self-administration
Self-administration training was conducted during 2-h sessions during the rats’ dark cycle, as described before (Fuchs et al., 2007; see experimental timeline in Fig. 2A). Rats were trained to lever press on a FR 1 schedule of cocaine reinforcement (cocaine hydrochloride; 0.10 mg/0.05 ml/infusion; NIDA, Research Triangle Park, NC, USA) with a 20-s time out period. Active (right) lever presses resulted in a 2.5-s activation of the infusion pump only. During the subsequent time out period, responses on the active lever had no consequences. Responses on the inactive (left) lever had no programmed consequences. Daily training was continued until a rat reached the acquisition criterion (i.e., ≥10 infusions self-administered/session on minimum 10 training days).
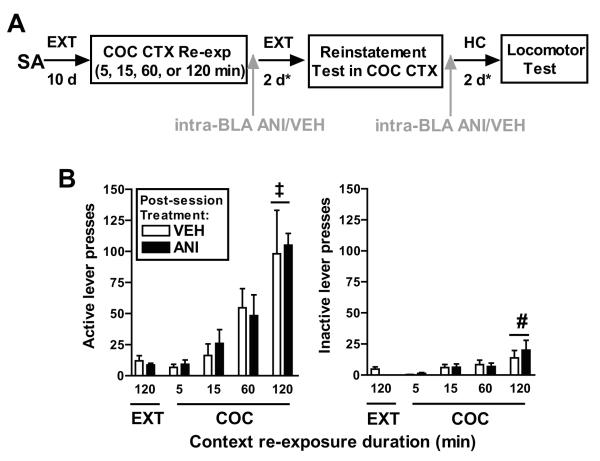
Figure 2.
A: Schematic representation of the experimental timeline for experiment 1. After cocaine self-administration (SA) training in a distinct context, rats underwent extinction (EXT) training in a different context. Rats were then re-exposed to the cocaine-paired context (COC CTX Re-exp) for 5, 15, 60, or 120 min followed immediately by microinfusion of ANI (62.5 μg/0.5 μl) or VEH (0.5 μl) into the BLA. The effects of these manipulations on cocaine-seeking behavior (responding on the previously cocaine-paired lever in the cocaine-paired context) were assessed after the rats reached the extinction criterion (*, ≤25 active lever responses/session on minimum two consecutive days). After an identical treatment-to-testing period, during which rats remained in their home cages (HC), the effects of ANI and VEH were also assessed on general locomotor activity in a novel context. B: Responses on the active and inactive levers (mean/2 h ± SEM) during re-exposure to the cocaine-paired context (COC), and during the preceding extinction session in the EXT context, by the groups that subsequently received ANI or VEH treatment into the BLA. Lever pressing was assessed in the absence of cocaine reinforcement or response-contingent stimulus presentation. The context re-exposure session was designed to reactivate context-cocaine associations and/or promote extinction learning, and it also provided an index of baseline context-induced motivation for cocaine. Symbol represents significant difference in lever responding during the 120-min re-exposure relative to all shorter sessions (‡, Tukey test, p=0.03–0.0001) and relative to the 5-min session (#, Tukey test, p=0.002).
Self-administration training was conducted in operant conditioning chambers that contained one of two distinctly different sets of contextual stimuli in addition to the levers. Context 1 contained a continuous red houselight on the wall opposite to the active lever, beeping pure tone (80 dB, 1 kHz; 2 s on, 2 s off), pine-scented air freshener strip (4.5 × 2 cm, Car Freshener Corp., Watertown, NY, USA), and corn cob bedding beneath a wire mesh floor (26 cm × 27 cm). Context 2 contained a blinking white stimulus light above the inactive lever (2 s on, 2 s off), continuous pure tone (75 dB, 2.5 kHz), vanilla-scented air freshener strip (4.5 × 2 cm, Sopus Products, Moorpark, CA, USA), a slanted ceramic tile wall that bisected chamber, and corn cob bedding beneath a bar floor (19 cm × 27 cm). Rats had no exposure to the self-administration context prior to self-administration training. Assignment of rats to cocaine self-administration training in Context 1 versus Context 2 was random. The contextual stimuli were presented throughout each session independent of responding. The pumps were located outside of the sound-attenuation chambers. Data collection and reinforcer delivery were controlled using Graphic State Notation software version 2.102 (Coulbourn).
Extinction
After the last day of self-administration training, rats underwent 2-h extinction sessions on 10 consecutive days, during which lever responses had no programmed consequences. Extinction sessions were conducted in Context 2 for rats that had previously self-administered cocaine in Context 1, and vice versa. On extinction day 7, the rats were adapted to the intracranial microinfusion procedure prior to placement into the chamber. Stainless steel injection cannulae (33 gauge, Plastics One) were inserted into the rat’s guide cannulae, 2 mm below the tip of the guide cannulae. Rats were held by the experimenter for 4 min while the injection cannulae were left in place but fluid was not infused.
Context Re-exposure Manipulation
On post-cocaine day 11, rats were placed into the cocaine-paired context for 5, 15, 60 or 120 minutes to reactivate cocaine-related memories and/or permit extinction learning (Tronson & Taylor, 2007). The levers were extended and the rats exposed to the cocaine-paired context were connected to the infusion apparatus in order to allow for similar perception of the spatial/tactile elements of the context (e.g., levers, slanted tile) as during cocaine self-administration training. Thus, this session also provided an assessment of baseline drug context-induced motivation for cocaine. Control groups were placed into the extinction context (“extinction control group”) or a novel unpaired context, Context 3 (“no reactivation control group”) for 15 min. Context 3 contained a continuous white houselight on the wall opposite to the active lever, continuous white stimulus lights above the active and inactive levers, a continuous complex tone (80 dB; alternating between 1, 1.5, and 2.5 kHz at 1 s intervals), citrus-scented air freshener strip (4.5 × 2 cm, Locasmarts LLC., Ormond Beach, FL, USA), and a ceramic tile floor (26 cm × 27 cm). For all groups, lever presses were recorded but had no programmed consequences. Fluids were not infused into the catheter upon lever pressing.
Intracranial Microinfusions
Immediately after the context re-exposure session, rats were removed from the testing room and received bilateral microinfusions of ANI (62.5 μg/0.5 μl, pH adjusted to pH ~7.0 using 1.0 M NaOH) or phosphate buffered saline vehicle (VEH, 0.5 μl) into the BLA or pCPu. The dose of ANI was selected based on previous research demonstrating that microinfusions of this dose into the BLA impair memory consolidation and reconsolidation in other paradigms (Nader et al., 2000a; Wang et al., 2005; Duvarci et al., 2006) and produce robust protein synthesis inhibition (60% and 32% after a 30-min and 60-min post-infusion interval, respectively) as measured using quantitative leucine incorporation autoradiography (Maren et al., 2003; Parsons et al., 2006). The pCPu was selected as a control infusion site to provide information about the anatomical selectivity of BLA manipulations, because unintended spread of ANI was expected to be disproportional in the dorsal direction (Baker et al., 1996; Neisewander et al., 1998). The microinfusions were delivered over 2 min, and the injection cannulae were left in place for 1 min prior to and after the microinfusion, as described previously (Fuchs et al., 2007). Assignment to the ANI versus VEH treatment groups was counterbalanced based on previous cocaine intake.
Reinstatement Test
To assess the ability of the cocaine-paired context to elicit cocaine-seeking behavior, rats were placed into the cocaine-paired context. The procedure for this test was identical to that of the context re-exposure session except that all rats were exposed to the cocaine-paired context and the session length was uniformly 2 h. The reinstatement test occurred after rats underwent additional daily extinction training following the context re-exposure session and reached the extinction criterion (i.e., ≤25 responses/session on 2 consecutive days).
Locomotor Activity Test
While it is unlikely, protracted motor side-effects of intracranial treatments can attenuate lever pressing behavior. To assess this, effects of ANI or VEH infused into the BLA or pCPu on locomotor activity were assessed in a novel environment 72-96 h after intracranial treatment. The exact treatment-to-testing interval was the same for each rat as in the preceding reinstatement experiment. Horizontal locomotor activity was measured in novel Plexiglas chambers (42 × 20 × 20 cm high) using a computerized activity system (San Diego Instruments, San Diego, CA, USA) described previously (Fuchs et al., 2007). The system recorded the number of times eight photobeams were broken by a rat moving in the chamber during the 2-h test session.
Histological and Data Analysis
Rats were fully anesthetized with sodium pentobarbital (Sigma, 100 mg/kg, IP) and perfused transcardially. Brains were extracted and sectioned on a vibratome at a thickness of 75 μm. Cannula placements were determined on cresyl violet-stained brain sections based on the rat brain atlas (Paxinos & Watson, 1997). The data of rats with misplaced cannulae were excluded from data analysis.
To test for potential pre-existing differences in cocaine history, cocaine-reinforced active lever presses, inactive lever presses, and cocaine intake (mean of last three sessions) were analyzed using separate one-way analyses of variance (ANOVA) with group (eight groups in experiment 1) as the between subjects factor or using t tests, where appropriate (experiments 2, 3). To test for potential pre-existing differences in baseline context-induced motivation to seek cocaine, non-reinforced active and inactive lever presses during the cocaine context re-exposure session were analyzed using separate 4 × 2 between-subjects factorial ANOVAs with context re-exposure session duration (5, 15, 60, or 120 min) and group (ANI, VEH) as factors (experiment 1) or using separate t tests (experiments 2, 3), where appropriate. To assess the effects of extinction context re-exposure on subsequent extinction responding, non-reinforced active lever presses were analyzed using a 2 × 2 mixed factorial ANOVA with test day (post-cocaine day 10, post-cocaine day 12) as a within-subjects factor and group (ANI, VEH) as a between-subjects factor. Because extinction learning during the context re-exposure session was expected to alter reinstatement responding, only qualitative comparisons were made across experiments with differing context re-exposure durations. Context re-exposure duration was not included as a factor in the analysis of post-manipulation instrumental responding. Accordingly, to assess the effects of the intracranial manipulations on reinstatement of cocaine-seeking behavior, non-reinforced active and inactive lever presses during the reinstatement test session and preceding extinction session were analyzed separately using 2 × 2 mixed factorial ANOVAs with test context (extinction, cocaine-paired) as the within-subjects factor and treatment (VEH, ANI) as the between-subjects factor. Locomotor activity counts were analyzed separately for each brain region using 2 × 6 mixed factorial ANOVAs with treatment as the between-subjects factor and time (20-min intervals) as the within-subjects factor. Significant ANOVA main and interaction effects were followed up by Tukey LSD post hoc tests, when appropriate. Alpha was set at 0.05.
RESULTS
Histology
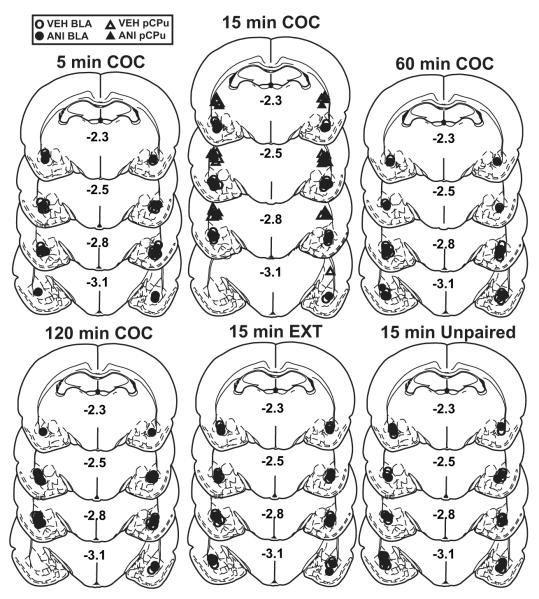
The target regions were defined as the lateral/basolateral nuclei of the amygdala (BLA) and dorsally adjacent posterior caudate-putamen (pCPu). The most ventral point of each injection cannula track was located bilaterally within the target brain region for the following number of rats per group (Fig. 1): BLA 5 min VEH (N=9), BLA 5 min ANI (N=7), BLA 15 min VEH (N=7), BLA 15 min ANI (N=9), BLA 15 min VEH-extinction control (N=8), BLA 15 min ANI-extinction control (N=10), BLA 15 min VEH-No Reactivation control (N=8), BLA 15 min ANI-No Reactivation control (N=8), BLA 60 min VEH (N=7), BLA 60 min ANI (N=9), BLA 120 min VEH (N=10), BLA 120 min ANI (N=8), pCPu 15 min VEH (N=10), pCPu 15 min ANI (N=9). ANI did not produce more gliosis or cell loss visible at 25X magnification than VEH treatment in either the BLA or pCPu.
Figure 1.
Microinfusion cannula placement as verified on cresyl violet-stained sections. The symbols represent the most ventral point of the infusion cannula tract for each rat on coronal sections based on the atlas of Paxinos and Watson (1997). Rats received microinfusions of anisomycin (ANI, 62.5 μg/0.5 μl/hemisphere) or vehicle (VEH, 0.5 μl/hemisphere) into the basolateral amygdala (BLA) or overlying posterior caudate-putamen (pCPu) immediately after 5, 15, 60, or 120 min of re-exposure to the cocaine paired context (COC), after 15 min of re-exposure to the extinction context (EXT), or after 15 min of exposure to a novel, unpaired context (Unpaired; No Reactivation control groups) and 72-96 h before a locomotor test session. The numbers indicate the distance from bregma in mm.
Experiment 1. Effects of ANI administered into the BLA after re-exposure to the cocaine-paired context on subsequent drug context-induced reinstatement of cocaine seeking
Self-administration and Extinction
Rats exhibited stable responding on the active lever during the last 3 self-administration days (< 10% variability in daily cocaine intake). There was no pre-existing difference in active lever responding (F(7,58)=0.57, p=0.78), inactive lever responding (F(7,58)=1.02, p=0.43), or cocaine intake (F(7,58)=0.18, p=0.99) between the eight groups that later in the experiment received VEH or ANI into the BLA after a 5, 15, 60, or 120 min cocaine context re-exposure session. Collapsed across these groups, the mean daily cocaine intake (± SEM) was 33.30 ± 2.19 infusions (approx. 11.1 ± 0.73 mg/kg/session). Responding declined in all groups upon removal of cocaine reinforcement on post-cocaine day 1 (48.09 ± 8.10 lever presses/session). The microinfusion adaptation procedure did not alter responding on post-cocaine day 7 (data not shown). Subsequently, responding gradually extinguished to a mean of 10.09 ± 1.72 active lever presses/session by post-cocaine day 10, the day preceding the context re-exposure manipulation (see experimental timeline in Fig. 2A).
Cocaine Context Re-exposure
On post-cocaine day 11, re-exposure to the cocaine-paired context in the absence of cocaine reinforcement elicited an increase in responding on the active lever, as a function of session duration (Fig. 2B). The ANOVA of active lever presses revealed a significant context re-exposure duration main effect (F(3,58)=10.60, p=0.0001), but no group × context re-exposure duration interaction effect (F(3,58)=0.07, p=0.98) or group main effect (F(1,58)=0.06, p=0.79). Similarly, the ANOVA of inactive lever presses revealed a significant context re-exposure duration main effect (F(3,58)=5.19, p=0.003), but no group × context re-exposure duration interaction effect (F(3,58)=0.37, p=0.78) or group main effect (F(1,58)=0.29, p=0.59). Subsequent post-hoc tests indicated that active lever responding was greater during the 120-min context re-exposure session relative to all shorter sessions (Tukey tests, p=0.03-0.0001), and inactive lever responding was greater during the 120-min context re-exposure session than during the 5-min session (Tukey test, p=0.002). Importantly, there was no difference in active or inactive lever responding between the groups that subsequently received ANI or VEH, indicating no pre-existing difference in baseline context-induced motivation for cocaine.
After ANI or VEH treatment, which was administered immediately following the context re-exposure session on post-cocaine day 11, there was no difference between the groups in the magnitude of extinction responding on post-cocaine day 12 on the active lever (mean = 10.68 + 1.77 responses; all group and context re-exposure duration main and interaction effects, F(1-3,58)=0.02-1.29, p=0.28-0.88) or inactive lever (mean = 2.58 + 0.43 responses; all group and context re-exposure duration main and interaction effects, F(1-3,58)=0.58-0.98, p=0.33-0.63). Similarly, there was no difference between the groups in the number of additional extinction sessions needed to reach the extinction criterion (mean = 2.50 + 0.16 days; all group and context re-exposure duration main and interaction effects, F(1-3,58)=0.30-1.69, p=0.17-0.82). This indicates that intra-BLA ANI treatment did not have a nonspecific effect on instrumental responding or extinction learning in the extinction context.
Cocaine-seeking Behavior
The effects of intra-BLA ANI treatment on responding during the context-induced reinstatement test varied depending on the duration of the preceding context re-exposure session (Fig. 3).
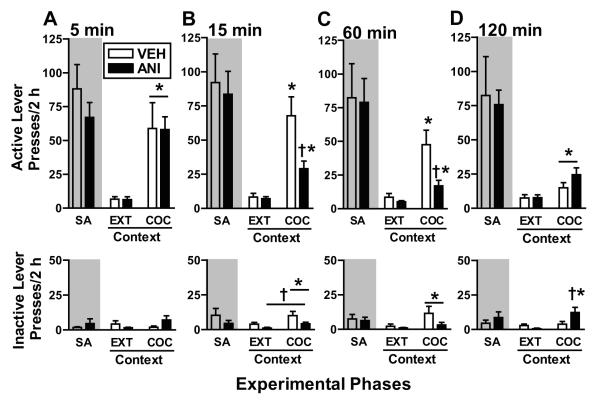
Figure 3.
Responses on the active and inactive levers (mean/2 h ± SEM) during self-administration (SA, mean of last 3 days), in the EXT context (last extinction session before the reinstatement test), and in the previously COC-paired context (reinstatement test). SA history (shaded area) is included as a reference point. Rats received treatment with ANI (62.5 μg/0.5 μl/hemisphere) or VEH (0.5 μl/hemisphere) into the BLA immediately after a 5- (A), 15- (B), 60- (C), or 120-min (D) re-exposure to the cocaine-paired context on post-cocaine day 11 (see Fig. 2), 72-94 h prior to reinstatement testing. During the EXT and reinstatement test sessions, lever pressing was assessed in the absence of cocaine reinforcement or response-contingent stimulus presentation in the EXT and COC-paired contexts, respectively. Symbols represent significant difference relative to responding in the EXT context (*, ANOVA context main or simple main effect, p<0.05) or relative to the respective VEH control group (†, ANOVA treatment main or simple main effect, p<0.05).
In rats that had received 5 min of re-exposure to the cocaine-paired context previously (Fig. 3A), the ANOVA of active lever presses revealed a significant context main effect (F(1,14)=19.41, p=0.001), but no treatment main effect (F(1,14)=0.003, p=0.96) or a context × treatment interaction effect (F(1,14)=0.001, p=0.98). Furthermore the ANOVA of inactive lever presses revealed a significant context × treatment interaction effect (F(1,14)=5.49, p=0.03), but no context main effect (F(1,14)=1.02, p =0.33) or treatment main effect (F(1,14)=0.30, p=0.59). Thus, upon exposure to the cocaine-paired context, both the previously VEH and ANI-treated groups exhibited similar increases in active lever responding, relative to responding in the extinction context. Inactive lever responding remained low and pair-wise comparisons indicated no treatment or context simple main effects, suggesting that the interaction effect was spurious.
In rats that had received 15 min of re-exposure to the cocaine-paired context previously (Fig. 3B), the ANOVA of active lever presses revealed a significant context × treatment interaction effect (F(1,14)=7.44, p=0.02) and significant context main (F(1,14)=35.05, p=0.0001) and treatment main effects (F(1,14)=8.27, p=0.01). Furthermore, the ANOVA of inactive lever presses revealed a significant context main (F(1,14)=12.56, p=0.003) and treatment main effects (F(1,14)=5.09, p=0.04), but no context × treatment interaction effect (F(1,14)=1.34, p=0.26). Thus, both groups reinstated (ANOVA simple main effect tests, p=0.003-0.006). However, the ANI-treated group exhibited less active lever responding upon exposure to the cocaine-paired context (p=0.012), but not the extinction context, relative to the VEH-treated group. Inactive lever responding remained negligible, but the ANI-treated group exhibited less inactive lever responding than the VEH-treated group in the cocaine-paired and extinction contexts.
In rats that had received 60 min of re-exposure to the cocaine-paired context previously (Fig. 3C), the ANOVA of active lever presses revealed a significant context × treatment interaction effect (F(1,14)=7.48, p=0.02) and significant context (F(1,14)=26.03, p=0.0001) and treatment main effects (F(1,14)=7.96, p=0.01). The ANOVA of inactive lever presses revealed a significant context main effect (F(1,14)=4.47, p=0.05), but no context × treatment interaction effect (F(1,14)=1.68, p=0.22) or treatment main effect (F(1,14)=3.93, p=0.06). Thus, both groups reinstated on the active lever (Tukey tests, p=0.03-0.008). However, the ANI-treated group exhibited less active lever responding upon exposure to the cocaine-paired context (Tukey test, p=0.013), but not the extinction context, relative to the VEH-treated group. There was no difference between the ANI- and VEH-treated groups in inactive lever responding, but both groups exhibited a small increase in inactive lever responding upon exposure to the cocaine-paired context relative to the extinction context.
In rats that had received 120 min of re-exposure to the cocaine-paired context previously (Fig. 3D), the ANOVA of active lever presses revealed a significant context main effect (F(1,16)=14.28, p=0.002), but no context × treatment interaction effect (F(1,16)=2.14, p=0.16) or treatment main effect (F(1,16)=1.62, p=0.22). The ANOVA of inactive lever presses revealed a significant context × treatment interaction effect (F(1,16)=8.46, p=0.01) and context main effect (F(1,16)=11.89, p=0.003), but no treatment main effect (F(1,16)=1.98, p=0.18). Furthermore, a separate analysis indicated that active lever responding in the VEH-treated control group in the cocaine-paired context was significantly less during the reinstatement test than during self-administration training (t(9)=2.29, p=0.04, unlike for all other re-exposure durations t(6-8)=1.05-1.42, p=0.20-0.33), indicating that the 120-min cocaine context re-exposure session alone resulted in significant extinction learning. Nevertheless, upon exposure to the cocaine-paired context, both groups exhibited a slight increase in active lever responding. There was no difference between the ANI- and VEH-treated groups in active lever responding, but the ANI-treated group exhibited more inactive lever responding in the cocaine-paired context than in the extinction context (Tukey test, p=0.042) and relative to the VEH-treated group (Tukey test, p=0.015).
Experiment 2. Effects of ANI administered into the BLA after re-exposure to the extinction context on subsequent drug context-induced reinstatement of cocaine seeking
Experiment 2 evaluated whether the effects of ANI observed in experiment 1 depended on cocaine memory reactivation per se, as is expected from a memory reconsolidation deficit (see experimental timeline in Fig. 4A). The experimental procedure was identical to that in experiment 1 except that rats received 15-min re-exposure to the extinction context instead of the cocaine-paired context, prior to ANI or VEH treatment. In terms of experimental history, the BLA-cannulated rats in experiment 2 exhibited stable and similar cocaine-reinforced responding (Fig. 4C), mean daily cocaine intake (± SEM; approx. 11.0 ± 1.44 mg/kg/session), and extinction responding prior to the context re-exposure session (8.28 ± 1.97 active lever presses/session on post-cocaine day 10) as rats in experiment 1.
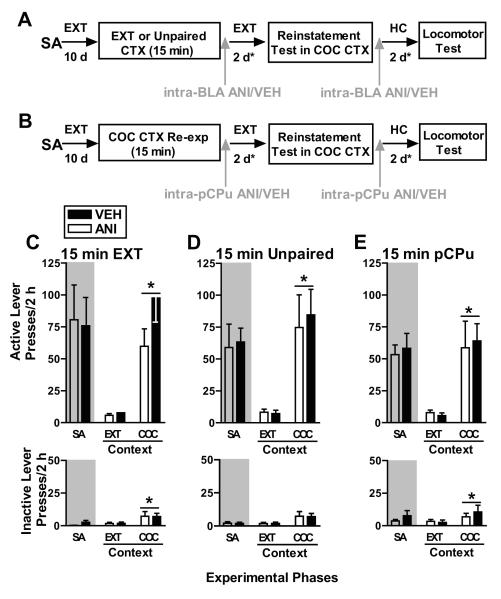
Figure 4.
A: Schematic representation of the experimental timeline in experiments 2-3. The procedure was identical to that used in experiment 1 except that groups were re-exposed to the extinction context (Exp. 2) or a novel unpaired context (Exp. 3) for 15 min prior to ANI (62.5 μg/0.5 μl/hemisphere) or VEH (0.5 μl/hemisphere) treatment administered into the BLA. B: Schematic representation of the experimental timeline in experiment 4. The procedure was identical to that used in experiment 1 except that all groups received ANI or VEH treatment into the pCPu, rather than the BLA, after re-exposure to the cocaine context for 15 min. C: Responses on the active and inactive levers (mean/2 h ± SEM) during self-administration (SA, mean of last 3 days), in the EXT context (last extinction session before the reinstatement test), and in the COC-paired context (reinstatement test) in experiment 2. D: Responses on the active and inactive levers (mean/2 h ± SEM) in experiment 3. E: Responses on the active and inactive levers (mean/2 h ± SEM) in experiment 4. Symbols represent significant difference relative to responding in the EXT context (*, ANOVA context main effect, p<0.05).
Extinction Context Re-exposure
On post-cocaine day 11, there was no difference between the subsequently ANI- versus VEH-treated extinction control groups in active (t(16)=0.44, p=0.67; mean=3.22 ± 0.80 responses/session) and inactive (t(16)=1.01, p=0.33; mean=0.61 ± 0.34 responses/session) lever responding during the 15-min extinction context re-exposure session. Furthermore, ANI or VEH treatment administered after this session failed to alter active lever responding during the extinction session on post-cocaine day 12 (meanVEH=11.14 ± 5.03; meanANI=7.70 ± 2.00) relative to responding on post-cocaine day 10 (meanVEH= 8.63 ± 1.79; meanANI=8.00 ± 3.36), the last extinction session before the context re-exposure session and ANI or VEH treatment. Consistent with this, separate ANOVAs indicated no significant session or group main or interaction effects on active and inactive lever responding (F(1,15)=0.12-0.40, p=0.53-0.73 and F(1,15)=0.48-0.93, p=0.35-0.49, respectively). Thus, ANI- and VEH-treated groups did not differ in active lever responding during the first extinction session following treatment, nor did they differ in the number of additional extinction sessions needed to reach the extinction criterion (2.13 ± 0.11 days, data not shown) prior to the reinstatement test. Thus, ANI failed to disrupt extinction. Furthermore, the memory age at the time of testing was similar in experiments 1 and 2.
Cocaine-Seeking Behavior
Intra-BLA ANI treatment immediately after 15 min of re-exposure to the extinction-paired context failed to alter responding during the cocaine context-induced reinstatement test relative to VEH treatment (Fig. 4C). The ANOVA of active lever presses revealed a significant context main effect (F(1,16)=32.25, p=0.0001), but no context × treatment interaction effect (F(1,16)=1.99, p=0.18) or a treatment main effect (F(1,16)=2.51, p=0.13). Similarly, the ANOVA of inactive lever presses revealed a significant context main effect (F(1,16)=5.16, p=0.04), but no context × treatment interaction effect (F(1,16)=0.008, p=0.93) or a treatment main effect (F(1,16)=0.001, p=0.98). Thus, upon re-exposure to the cocaine-paired context, both VEH and ANI-treated groups exhibited an increase in responding on the active lever and, to a lesser extent on the inactive lever, relative to responding in the extinction context. Furthermore, there was no difference between the ANI-treated and VEH-treated groups in responding on either lever. Thus, ANI failed to disrupt cocaine-seeking behavior in the absence of memory reactivation.
Experiment 3. Effects of ANI administered into the BLA after re-exposure to an unpaired context on subsequent drug context-induced reinstatement of cocaine seeking
Experiment 3 was a no-reactivation control experiment that further examined whether the effects of ANI observed in experiment 1 depended on cocaine memory reactivation per se, as is expected from a memory reconsolidation deficit (see experimental timeline in Fig. 4A). The experimental procedure was identical to that in experiment 2 except that rats received 15-min re-exposure to a novel unpaired context instead of the extinction context, prior to ANI or VEH treatment. In terms of experimental history, the BLA-cannulated rats in experiment 3 exhibited stable and similar cocaine-reinforced responding (Fig. 4D), mean daily cocaine intake (± SEM; approx. 8.45 ± 0.65 mg/kg/session), and extinction responding prior to the context re-exposure session (11.37 ± 2.84 active lever presses/session on post-cocaine day 10) as rats in experiments 1-2.
Extinction Context Re-exposure
On post-cocaine day 11, exposure to the novel unpaired context elicited negligible lever responding. There was no difference between the subsequently ANI- versus VEH-treated no reactivation control groups in active (t(14)=0.21, p=0.83; mean=10.75 ± 2.84 responses/session) and inactive (t(14)=0.60, p=0.77; mean=3.87 ± 1.79 responses/session) lever responding during the 15-min unpaired context re-exposure session. Furthermore, ANI or VEH treatment administered after this session failed to alter active lever responding in the extinction context on post-cocaine day 12 (meanVEH=9.71 ± 3.25; meanANI=9.25 ± 2.65) relative to responding on post-cocaine day 10 (meanVEH= 10.75 ± 5.27; meanANI=12.00 ± 2.55), the last extinction session before the manipulation. Consistent with this, separate ANOVAs indicated no significant session or group main or interaction effects on active and inactive lever responding (F(1,14)=0.10-0.22, p=0.64-0.92 and F(1,14)=0.04-0.70, p=0.42-0.84, respectively). Thus, ANI- and VEH-treated groups did not differ in active lever responding during the first extinction session following treatment, nor did they differ in the number of additional extinction sessions needed to reach the extinction criterion (2.11 ± 0.08 days, data not shown) prior to the reinstatement test. Thus, ANI did not have a nonspecific effect on instrumental performance in the extinction context. Furthermore, the memory age at the time of testing was similar in experiments 1-3.
Cocaine-Seeking Behavior
Intra-BLA ANI treatment immediately after 15 min of re-exposure to the unpaired context failed to alter responding during the cocaine context-induced reinstatement test relative to VEH treatment (Fig. 4D). The ANOVA of active lever presses revealed a significant context main effect (F(1,14)=20.78, p=0.0001), but no context × treatment interaction effect (F(1,14)=0.12, p=0.74) or a treatment main effect (F(1,14)=0.70, p=0.79). In contrast, the ANOVA of inactive lever presses did not indicate a context or treatment main or interaction effect (F(1,14)=0.12-3.38, p=0.08-0.75). Thus, upon re-exposure to the cocaine-paired context, both VEH and ANI-treated groups exhibited an increase in responding on the active lever and, to a lesser extent on the inactive lever, relative to responding in the extinction context. Furthermore, there was no difference between the ANI-treated and VEH-treated groups in responding on either lever. Thus, ANI failed to disrupt cocaine-seeking behavior in the absence of cocaine memory reactivation and did not have a nonspecific effect on instrumental responding in the cocaine context.
Experiment 4. Effects of ANI administered into the pCPu after re-exposure to the cocaine-paired context on subsequent context-induced reinstatement of cocaine seeking
As a follow-up to experiment 1, experiment 4 examined whether the effects of ANI following 15-min re-exposure to the cocaine-paired context were specific to the BLA (see experimental timeline in Fig. 4B). The pCPu-cannulated rats in experiment 4 exhibited stable and similar cocaine-reinforced responding (Fig. 4E) and mean daily cocaine intake (approx. 9.43 ± 0.86 mg/kg/session) as rats in experiment 1.
Cocaine Context Re-exposure
On post-cocaine day 11, there was no difference between the subsequently ANI- versus VEH-treated groups in active (t(17)=0.58, p=0.56; mean = 19.51 ± 9.66 lever responses/session) and inactive (t(17)=0.43, p=0.67; mean = 4.00 ± 1.73 lever responses/session) lever responding during the 15-min cocaine context re-exposure session (data not shown). After ANI or VEH treatment, which was administered immediately following the context re-exposure session, there was no difference between the groups in the magnitude of extinction responding or in the number of additional extinction sessions needed to reach the extinction criterion (2.53 ± 0.19 days, data not shown). Thus, intra-pCPu ANI treatment did not have a nonspecific effect on operant responding or extinction learning.
Cocaine-Seeking Behavior
Intra-pCPu ANI treatment immediately after 15 min of re-exposure to the cocaine-paired context failed to alter responding during the context-induced reinstatement test (Fig. 4E). The ANOVA of active lever presses revealed a significant context main effect (F(1,17)=18.30, p=0.001), but no context × treatment interaction effect (F(1,17)=0.09, p=0.76) or a treatment main effect (F(1,17)=0.01, p=0.91). The ANOVA of inactive lever presses revealed a significant context main effect (F(1,17)=4.95, p=0.04), but no context × treatment interaction effect (F(1,17)=0.78, p=0.38) or a treatment main effect (F(1,17)=0.17, p=0.69). Upon exposure to the cocaine-paired context, both VEH and ANI-treated groups exhibited increases in responding on the active lever, and to a lesser extent on the inactive lever, relative to responding in the extinction context. Furthermore, there was no difference between the ANI-treated and VEH-treated groups in responding on either lever.
Locomotor Activity
ANI pretreatment administered into the BLA or pCPu failed to alter locomotor activity in a novel context after a post-treatment interval identical to that in experiments 1 and 4, respectively (Fig. 5). For the BLA-cannulated groups, the ANOVA of photobeam breaks across the 2-h locomotor test indicated a significant time main effect (F(5,320)=79.67, p=0.0001), but no treatment × time interaction (F(5,320)=1.22, p=0.30) or a treatment main effect (F(1,64)=2.27, p=0.14). Similarly, for the pCPu-cannulated groups, the ANOVA indicated a significant time main effect (F(5,85)=9.15, p=0.0001), but no treatment × time interaction (F(5,85)=1.21, p=0.31) or a treatment main effect (F(1,17)=1.2, p=0.29). Thus, locomotor activity was highest during the first 20-min interval (interval 1> intervals 2-6; Tukey test, p<0.05) gradually decreased across time at similar rates in the ANI-treated and VEH-treated groups. Furthermore, ANI treatment failed to alter locomotor activity regardless of the site of administration relative to VEH treatment.
Figure 5.
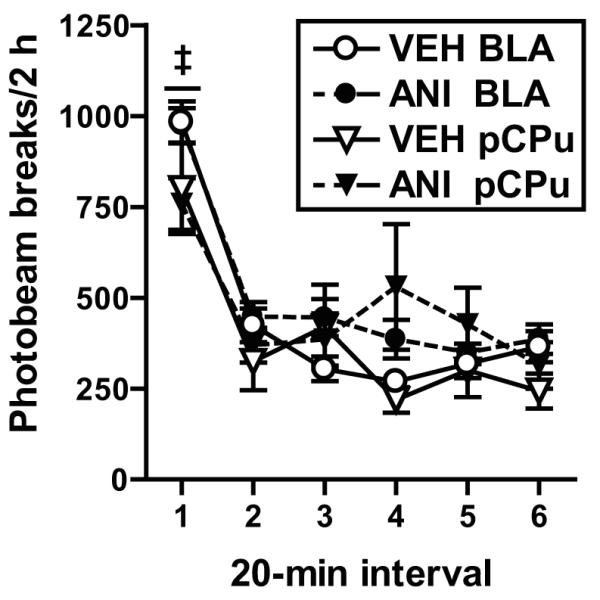
Locomotor activity (mean photobeam breaks/2 h ± SEM) in a novel context. Microinfusions of ANI (62.5 μg/0.5 μl/hemisphere) or VEH (0.5 μl/hemisphere) were administered into the BLA or overlying pCPu 48-72 h prior testing. The time that elapsed from intracranial microinfusion to locomotor testing was adjusted for each rat to be the same as in the preceding reinstatement experiment. An automated photocell system recorded the number of times photobeams were broken by an animal moving in the chamber. Symbol represents significant difference relative to intervals 2-6 (‡, Tukey test, p<0.05).
DISCUSSION
The existence of a distinct memory reconsolidation process has been the source of debate, and it has been considered to represent a mechanism for memory storage, retrieval link formation, or lingering consolidation (Dudai & Eisenberg, 2004; Tronson & Taylor, 2007). According to the memory reconsolidation hypothesis, it is a process that stabilizes already established memories into long-term memory stores after reactivation-induced destabilization (Misanin et al., 1968; Lewis, 1979). Memory reconsolidation appears to vary in duration (i.e., lasts for ~2-4 h after memory reactivation) as a function of memory complexity, strength, age, and the brain region and signaling molecule examined (Dudai & Eisenberg, 2004; Alberini et al., 2006; Tronson & Taylor, 2007). While previous studies focused on Pavlovian contextual conditioned and CS-induced instrumental behaviors, the present study represents the first demonstration that memory reconsolidation processes regulate contextual control over instrumental behavior, specifically drug context-induced instrumental cocaine-seeking behavior.
The form of memory reconsolidation identified in this study is mediated at least in part by ANI-sensitive processes in the BLA, depending on factors related to the intensity of memory reactivation. Consistent with this, intra-BLA ANI treatment administered after 15-min or 60-min re-exposure to the cocaine-paired context subsequently impaired cocaine context-induced drug-seeking behavior (Fig. 3B, 3C), whereas the same treatment administered after a brief (5 min) re-exposure to the cocaine-paired context failed to alter subsequent drug-seeking behavior (Fig. 3A). The effect of ANI was also dependent on cocaine-related memory reactivation per se since intra-BLA ANI treatment administered following 15 min re-exposure to the extinction context (Fig. 4C) or exposure to an unpaired context (Fig. 4D) also failed to alter subsequent cocaine context-induced drug-seeking behavior. The effects of ANI on cocaine-seeking behavior were specific to the BLA as ANI microinfusion into the overlying pCPu, the brain region in the most likely path of unintended ANI spread, after 15-min re-exposure to the cocaine-paired context failed to alter context-induced cocaine-seeking behavior (Fig. 4E). Similarly, previous studies have shown that microinfusions of reconsolidation inhibitors into the central amygdala (CeA) fail to alter established conditioned behaviors in other paradigms (Bahar et al., 2004; Hellemans et al., 2006). ANI also failed to alter locomotor activity in a novel context (Fig. 5) and did not have a consistent effect on inactive lever responding during the reinstatement test, suggesting that it did not produce a non-specific performance deficit. Instead, increased inactive lever responding during the reinstatement test likely represented a widening of the response repertoire in an attempt to procure drug reinforcement using a new response strategy (Domjan, 1980; Fuchs et al., 1998; Fuchs et al., 2004). Accordingly, sporadic ANI effects on the inactive lever probably reflected attenuation in an alternate form of context-induced cocaine-seeking behavior in the present study. Thus, overall, the present findings are consistent with the idea that context retrieval elicits memory destabilization, after which contextual control over cocaine-seeking behavior becomes dependent on ANI-sensitive processes within the BLA. Importantly, these findings demonstrate for the first time that principles of the memory reconsolidation hypothesis apply to context-induced instrumental goal-directed behaviors, similar to other conditioned behaviors (Nader et al., 2000a; Wang et al., 2005; Lee et al., 2006a; Milekic et al., 2006). Thus, at the level of the BLA, there may be some overlap in the memory stabilization mechanisms that facilitate Pavlovian responses, CS-induced instrumental behaviors, and context-induced instrumental behaviors even though these behaviors are theorized to rely on distinctly different types of associative memories (i.e., context/CS-drug, CS-drug, and context-response-drug associations, respectively).
ANI is a potent protein synthesis inhibitor with a relatively short half-life (~30 min; Maren et al., 2003; Dudai & Eisenberg, 2004; Parsons et al., 2006), and it has been used to argue that some memory reconsolidation processes are dependent on de novo protein synthesis (Tauscher et al., 1999; Nader et al., 2000a; Inda et al., 2005). However, ANI may act via several other mechanisms. First, recent studies indicate that ANI triggers robust norepinephrine, dopamine, serotonin, and acetyl choline release followed by transient norepinephrine and dopamine depletion (Canal et al., 2007; Qi & Gold, 2009). In particular, beta-adrenoceptor antagonist and agonist treatments, timed to counteract the biphasic effect of ANI on norepinephrine release in the amygdala or ventral hippocampus inhibit ANI-induced memory reconsolidation deficits in an inhibitory avoidance paradigm (Canal et al., 2007; Qi & Gold, 2009). Thus, ANI-induced abnormalities in neurotransmitter responses may produce amnesia by disrupting the post-translational modification of proteins critical for memory stabilization (Qi & Gold, 2009). However, the possibility that ANI directly impaired the expression of cocaine-seeking behavior in the present study by altering monoamine release is mitigated by the fact that testing occurred minimum 72 h after ANI administration, thus at least 24 h after monoamine levels were reported to normalize (i.e., 48 h post ANI, Canal et al., 2007). Second, ANI can also act as a ribotoxin at high doses (Rudy et al. 2006). However, it is unlikely that ANI-induced cell death elicited the attenuation in reinstatement because this effect depended on the presence and duration of cocaine context re-exposure. Furthermore, overt brain damage was not present in brain tissue. Finally, ANI has been hypothesized to prompt delayed superinduction of gene expression and disrupt memory reconsolidation by impairing the synthesis or post-translational modification of transcription inhibitors (Routtenberg & Rekart, 2005). Since ANI has multiple potential mechanisms of action, the most parsimonious explanation is that ANI impaired cocaine-seeking behavior in the present study by inhibiting protein synthesis or post-translational modification or by altering gene transcription in the BLA following memory reactivation. Based on the results of the present study, future studies are warranted to determine the contribution of particular signaling molecules to memory stabilization processes that regulate context-induced cocaine-seeking behavior.
Interestingly, intra-BLA ANI failed to completely inhibit subsequent cocaine-seeking behavior; even though, the same dose of ANI or the protein synthesis inhibitor cycloheximide has been sufficient to fully inhibit Pavlovian conditioned responses in other paradigms (Nader et al., 2000a; Milekic et al., 2006). Associative memories that underlie instrumental goal-directed behaviors may be more resistant to reconsolidation inhibition than the associative memories that underlie Pavlovian conditioned responses, perhaps in part due to differences in the extent of training and memory age (Tronson & Taylor, 2007; Brown et al., 2008). For instance, experimental subjects in drug self-administration studies are typically overtrained in order to increase the face validity of the procedure and to provide the subjects with extensive drug exposure over an extended training period. Overtraining may, in turn, increase the resistance of drug memories to destabilization. Indeed, studies indicate that old and strong memories, much like the cocaine memories in the present study, require extensive memory reactivation to become labile (Flood et al., 1973; Suzuki et al., 2004; Alberini et al., 2006). Alternatively, partial ANI effects may reflect the contribution of multiple parallel mechanisms of memory stabilization. ANI-sensitive and ANI-insensitive mechanisms of memory reconsolidation may operate within the BLA and outside of the BLA, the latter of which we explore in the companion paper to this report (Ramirez et al. submitted). Furthermore, one study suggests that the long-term memories of context-response and response-reward associations that support food-reinforced instrumental responses do not undergo ANI-sensitive reconsolidation upon retrieval (Hernandez & Kelley, 2004), and such ANI-insensitive instrumental memories may be sufficient to elicit residual cocaine-seeking behavior in the present study.
Contrary to the effects of ANI administered into the BLA after 15- or 60-min re-exposure to the cocaine-paired context, ANI administered after a very brief (5-min) context re-exposure period failed to alter subsequent cocaine-seeking behavior (Fig. 3A). This negative effect was not due to insufficient ANI dosing, as the 62.5 μg/0.5 μl dose has strong inhibitory effects on protein synthesis in the amygdala (Maren et al., 2003) and on contextual memory reconsolidation in other experimental settings (Parsons et al., 2006; Yim et al., 2006). Most likely, brief context re-exposure was either (A) insufficient to trigger memory destabilization, hence reconsolidation, or (B) it initiated ANI-insensitive memory reconsolidation processes within the BLA. In support of the former, very brief memory reactivation sessions lead to incomplete or no memory reconsolidation in other paradigms (Suzuki et al., 2004; Diergaarde et al., 2006). Interestingly, the minimum duration of memory reactivation necessary for memory reconsolidation exceeds 10 min in this and other instrumental models (Diergaarde et al., 2006; present study), whereas it is as brief as 2 min in some Pavlovian models (Pedreira & Maldonado, 2003; Pedreira et al., 2004; Power et al., 2006; Rudy et al., 2006). As discussed above, differences in the minimum duration of cue re-exposure necessary for memory destabilization in instrumental versus Pavlovian models may reflect dissimilarities in the type of associations, corresponding memory stabilization processes, and/or training parameters (Tronson & Taylor, 2007; Brown et al., 2008).
Based on the trace dominance hypothesis (Eisenberg et al., 2003; Eisenberg & Dudai, 2004) and other work (Falls et al., 1992; Lu et al., 2001; Wang et al., 2005; Lee et al., 2006b; Brown et al., 2007), we predicted that extinction learning would be dominant at the end of the extended (120-min) context re-exposure session and this would initiate new extinction memory consolidation. Indeed, re-exposure to the cocaine-paired context for 120-min was sufficient to extinguish cocaine-seeking behavior in the present study, as indicated by diminished reinstatement responding in the VEH control group (Fig. 3D). However, ANI treatment administered into the BLA failed to restore reinstatement responding to levels seen during the 120-min context re-exposure session, consistent with the results of a recent auditory fear conditioning study (Duvarci et al., 2006). This negative finding in the present study suggests either that (A) ANI-sensitive processes in the BLA are not critical for new extinction memory consolidation in the reinstatement model or that (B) extinction memory consolidation is completed before the end of the 120-min session and the onset of ANI action. Tetrodotoxin-induced functional inactivation of the BLA induced after a similar 120-min session is sufficient to disrupt extinction memory consolidation in the CS-induced reinstatement model (Fuchs et al., 2006b), somewhat mitigating the latter possibility. However, future studies will be needed in order to systematically examine this question since the rate of extinction memory consolidation may vary as a function of cue type.
ANI treatment, administered after re-exposure to the extinction context and the putative reactivation of previously acquired extinction memories, did not restore cocaine-seeking behavior in the extinction context 24 h later relative to cocaine-seeking behavior seen in the same context at earlier stages of extinction training. One possible explanation for the negative effect is that ANI-sensitive processes in the BLA are not critical for extinction memory reconsolidation that inhibits context-induced cocaine-seeking behavior. Similarly, tetrodotoxin-induced functional inactivation of the BLA fails to disrupt extinction memory reconsolidation in the CS-induced reinstatement model (Fuchs et al., 2006b). However, extensive extinction training history and the lack of opportunity to incorporate new information may cause extinction memory reconsolidation to occur more time efficiently, concluding prior to the end of the memory reactivation session and the onset of ANI effects. The latter explanation is less likely due to the shortness of the re-exposure session (15-min). Furthermore, at least in the contextual memory model of the crab Chasmagnathus, reconsolidation appears to commence at the end of the context re-exposure session regardless of session duration (Pedreira et al., 2004). To be noted, ANI treatment produced a cocaine memory reconsolidation deficit after a memory reactivation session of comparable duration. This suggests that the reconsolidation of inhibitory versus excitatory memories that regulate context-induced cocaine-seeking behavior may be mediated by different neural mechanisms within the BLA.
Context-Cocaine Memory Reconsolidation, Extinction, and Drug Relapse Prevention
Several elements of the brain circuitry that mediate the expression of context-induced drug-seeking behavior, including the BLA, dorsal hippocampus, and nucleus accumbens (Weiss et al., 2001; Crombag et al., 2002; Bossert et al., 2006; Fuchs et al., 2005; Di Pietro et al., 2006), are reportedly also involved in memory reconsolidation (Miller & Marshall, 2005; Alberini et al., 2006; Milekic et al., 2006; Tronson & Taylor, 2007). Drug-induced and experience-based adaptations in these brain regions are theorized to be critical for increased stimulus control over addictive behavior and the transition from casual drug use to drug dependence (Onaivi et al., 1996; Nestler & Aghajanian, 1997; Goldstein & Volkow, 2002; Kalivas & McFarland, 2003; Sutton et al., 2003; Wolf et al., 2003; Fuchs et al., 2006a; Lu et al., 2006). Some of these neuroadaptations may occur in memory, as opposed to motivational, subcircuits within these structures (Grant et al., 1996; Kilts et al., 2004; Fuchs et al., 2006b; Lu et al., 2006). Thus, treatments that selectively inhibit the re-stabilization of drug-related memories are of special interest for addiction treatment development. These treatments are likely feasible since the results from the present study suggest that the neural mechanisms of drug-related memory reconsolidation and extinction memory (re)consolidation processes that control context-induced drug-seeking behavior are at least partially distinct within the BLA. Similarly, recent studies have indicated that cannabinoid 1 receptors and L-type voltage-gated calcium channels selectively mediate extinction, but not memory reconsolidation (Suzuki et al., 2004), whereas protein kinase A activity in the BLA mediates auditory fear memory reconsolidation but not extinction (Tronson et al., 2006). Future studies will need to investigate the longevity of these effects in order to evaluate the feasibility of reconsolidation inhibition as an approach to relapse prevention, since post-reactivation manipulations only transiently disrupt certain conditioned behaviors (Eisenberg & Dudai, 2004; Amaral et al., 2007). It is encouraging in this respect that antisense oligonucleotide-induced disruption of zif268 expression after CS-cocaine memory reactivation produces deficits in CS-induced cocaine-seeking behavior that endure for up to 30 d (Lee et al., 2006a).
Acknowledgments
The authors are grateful to Dr. Ann E. Kelley and Dr. Pepe Hernandez for information about the preparation of the anisomycin solution. The authors would like to also thank Julian Duda, Heather Lasseter, and Stephanie Traina for excellent technical assistance. Portions of this work were first presented in poster form at the 45th annual meeting of ACNP in Hollywood, FL, in December, 2006. This work was supported by NIH grant C06 RR015455 (pilot grant, R.A.F.), NIDA R01 grant DA017673 (R.A.F.), and a supplement to promote diversity in health-related research (DA017673-S1; D.R.R.).
Abbreviations
- Anisomycin
ANI
- vehicle
VEH
- fixed ratio
FR
- BLA
basolateral amygdala
- CeA
central nucleus of the amygdala
- pCPu
posterior caudate-putamen
REFERENCES
- Alberini CM, Milekic MH, Tronel S. Mechanisms of memory stabilization and de-stabilization. Cell Mol Life Sci. 2006;63:999–1008. doi: 10.1007/s00018-006-6025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleweireldt AT, Weber SM, Neisewander JL. Passive exposure to a contextual discriminative stimulus reinstates cocaine-seeking behavior in rats. Pharmacol Biochem Behav. 2001;69:555–560. doi: 10.1016/s0091-3057(01)00573-1. [DOI] [PubMed] [Google Scholar]
- Amaral OB, Luft T, Cammarota M, Izquierdo I, Roesler R. Temporary inactivation of the dorsal hippocampus induces a transient impairment in retrieval of aversive memory. Behav Brain Res. 2007;180:113–118. doi: 10.1016/j.bbr.2007.02.031. [DOI] [PubMed] [Google Scholar]
- Bahar A, Dorfman N, Dudai Y. Amygdalar circuits required for either consolidation or extinction of taste aversion memory are not required for reconsolidation. Eur J Neurosci. 2004;19:1115–1118. doi: 10.1111/j.0953-816x.2004.03215.x. [DOI] [PubMed] [Google Scholar]
- Baker DA, Khroyan TV, O’Dell LE, Fuchs RA, Neisewander JL. Differential effects of intraaccumbens sulpiride on cocaine-induced locomotion and conditioned place preference. J Pharmacol Exp Ther. 1996;279:392–401. [PubMed] [Google Scholar]
- Bossert JM, Liu SY, Shaham Y. The mGluR2/3 agonist, Ly379268, attenuates reinstatement of heroin and sucrose seeking induced by re-exposure to drug-associated contextual cues in rats. Neuropsychopharmacology. 2006;31:2197–209. [Google Scholar]
- Brown TE, Forquer MR, Cocking DL, Jansen HT, Harding JW, Sorg BA. Role of matrix metalloproteinases in the acquisition and reconsolidation of cocaine-induced conditioned place preference. Learn Mem. 2007;14:214–223. doi: 10.1101/lm.476207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, Lee BR, Sorg BA. The NMDA antagonist MK-801 disrupts reconsolidation of a cocaine-associated memory for conditioned place preference but not for self-administration in rats. Learn Mem. 2008;15:857–65. doi: 10.1101/lm.1152808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE, Chang Q, Gold PE. Amnesia produced by altered release of neurotransmitters after intraamygdala injections of a protein synthesis inhibitor. Proc Natl Acad Sci USA. 2007;104:12500–5. doi: 10.1073/pnas.0705195104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Siracusano A, Calabresi P, Bernardi G. Removing pathogenic memories: a neurobiology of psychotherapy. Mol Neurobiol. 2005;32:123–132. doi: 10.1385/MN:32:2:123. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Grimm JW, Shaham Y. Effect of dopamine receptor antagonists on renewal of cocaine seeking by reexposure to drug-associated contextual cues. Neuropsychopharmacology. 2002;27:1006–1015. doi: 10.1016/S0893-133X(02)00356-1. [DOI] [PubMed] [Google Scholar]
- Di Pietro NC, Black YD, Kantak KM. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. Eur J Neurosci. 2006;24:3285–3298. doi: 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, Schoffelmeer AN, De Vries TJ. Beta-adrenoceptor mediated inhibition of long-term reward-related memory reconsolidation. Behav Brain Res. 2006;170:333–336. doi: 10.1016/j.bbr.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Domjan M. The principles of learning and behavior. 4th edition Brooks/Cole Publishing Company; Pacific Grove: 1980. [Google Scholar]
- Dudai Y, Eisenberg M. Rites of passage of the engram: reconsolidation and the lingering consolidation hypothesis. Neuron. 2004;44:93–100. doi: 10.1016/j.neuron.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Mamou CB, Nader K. Extinction is not a sufficient condition to prevent fear memories from undergoing reconsolidation in the basolateral amygdala. Eur J Neurosci. 2006;24:249–260. doi: 10.1111/j.1460-9568.2006.04907.x. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O’Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology. 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Eisenberg M, Dudai Y. Reconsolidation of fresh, remote, and extinguished fear memory in Medaka: old fears don’t die. Eur J Neurosci. 2004;20:3397–3403. doi: 10.1111/j.1460-9568.2004.03818.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg M, Kobilo T, Berman DE, Dudai Y. Stability of retrieved memory: inverse correlation with trace dominance. Science. 2003 Aug 22;301(5636):1102–4. doi: 10.1126/science.1086881. 2003. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. NMDA receptor blockade in the basolateral amygdala disrupts consolidation of stimulus-reward memory and extinction learning during reinstatement of cocaine-seeking in an animal model of relapse. Neurobiol Learn Mem. 2007;88:435–444. doi: 10.1016/j.nlm.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood JF, Rosenzweig MR, Bennett EL, Orme AE. The influence of duration of protein synthesis inhibition on memory. Physiol Behav. 1973;10:555–562. doi: 10.1016/0031-9384(73)90221-7. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Haney M. Conditioned effects of environmental stimuli paired with smoked cocaine in humans. Psychopharmacology (Berl) 2000;149:24–33. doi: 10.1007/s002139900340. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006a;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seekign in rats. Eur J Neurosci. 2007;26:487–98. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004;24:6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Feltenstein MW, See RE. The role of the basolateral amygdala in stimulus-reward memory and extinction memory consolidation and in subsequent conditioned cued reinstatement of cocaine seeking. Eur J Neurosci. 2006b;23:2809–2813. doi: 10.1111/j.1460-9568.2006.04806.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Tran-Nguyen LT, Specio SE, Groff RS, Neisewander JL. Predictive validity of the extinction/reinstatement model of drug craving. Psychopharmacology (Berl) 1998;135:151–160. doi: 10.1007/s002130050496. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans KG, Everitt BJ, Lee JL. Disrupting reconsolidation of conditioned withdrawal memories in the basolateral amygdala reduces suppression of heroin seeking in rats. J Neurosci. 2006;26:12694–12699. doi: 10.1523/JNEUROSCI.3101-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez PJ, Kelley AE. Long-term memory for instrumental responses does not undergo protein synthesis-dependent reconsolidation upon retrieval. Learn Mem. 2004;11:748–754. doi: 10.1101/lm.84904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inda MC, Delgado-Garcia JM, Carrion AM. Acquisition, consolidation, reconsolidation, and extinction of eyelid conditioning responses require de novo protein synthesis. J Neurosci. 2005;25:2070–2080. doi: 10.1523/JNEUROSCI.4163-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo I, Bevilaqua LR, Rossato JI, Bonini JS, Medina JH, Cammarota M. Different molecular cascades in different sites of the brain control memory consolidation. Trends Neurosci. 2006;29:496–505. doi: 10.1016/j.tins.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Gross RE, Ely TD, Drexler KP. The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatry. 2004;161:233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- Lee JL, Di Ciano P, Thomas KL, Everitt BJ. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron. 2005;47:795–801. doi: 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci. 2006a;26:5881–5887. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Reconsolidation and extinction of conditioned fear: inhibition and potentiation. J Neurosci. 2006b;26:10051–10056. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DJ. Psychobiology of active and inactive memory. Psychol Bull. 1979;86:1054–1083. [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Lu HY, Gean PW. The similarities and diversities of signal pathways leading to consolidation of conditioning and consolidation of extinction of fear memory. J Neurosci. 2003;23:8310–8317. doi: 10.1523/JNEUROSCI.23-23-08310.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu KT, Walker DL, Davis M. Mitogen-activated protein kinase cascade in the basolateral nucleus of amygdala is involved in extinction of fear-potentiated startle. J Neurosci. 2001;21:RC162. doi: 10.1523/JNEUROSCI.21-16-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Maren S, Ferrario CR, Corcoran KA, Desmond TJ, Frey KA. Protein synthesis in the amygdala, but not the auditory thalamus, is required for consolidation of Pavlovian fear conditioning in rats. Eur J Neurosci. 2003;18:3080–3088. doi: 10.1111/j.1460-9568.2003.03063.x. [DOI] [PubMed] [Google Scholar]
- Milekic MH, Brown SD, Castellini C, Alberini CM. Persistent disruption of an established morphine conditioned place preference. J Neurosci. 2006;26:3010–3020. doi: 10.1523/JNEUROSCI.4818-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Milton AL, Lee JL, Butler VJ, Gardner R, Everitt BJ. Intra-amygdala and systemic antagonism of NMDA receptors prevents the reconsolidation of drug-associated memory and impairs subsequently both novel and previoulsy acquired drug-seeking behavior. J Neurosci. 2008;28:8230–7. doi: 10.1523/JNEUROSCI.1723-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misanin JR, Miller RR, Lewis DJ. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science. 1968;160:554–555. doi: 10.1126/science.160.3827.554. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000a;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, LeDoux JE. The labile nature of consolidation theory. Nat Rev Neurosci. 2000b;1:216–219. doi: 10.1038/35044580. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Fuchs RA, O’Dell LE, Khroyan TV. Effects of SCH-23390 on dopamine D1 receptor occupancy and locomotion produced by intraaccumbens cocaine infusion. Synapse. 1998;30:194–204. doi: 10.1002/(SICI)1098-2396(199810)30:2<194::AID-SYN9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Bishop-Robinson C, Motley ED, Chakrabarti A, Chirwa SS. Neurobiological actions of cocaine in the hippocampus. Ann N Y Acad Sci. 1996;801:76–94. doi: 10.1111/j.1749-6632.1996.tb17433.x. [DOI] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Baruch DE, Riedner BA, Helmstetter FJ. Long-term stability of fear memory depends on the synthesis of protein but not mRNA in the amygdala. Eur J Neurosci. 2006;23:1853–1859. doi: 10.1111/j.1460-9568.2006.04723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; 1997. [DOI] [PubMed] [Google Scholar]
- Pedreira ME, Maldonado H. Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron. 2003;38:863–869. doi: 10.1016/s0896-6273(03)00352-0. [DOI] [PubMed] [Google Scholar]
- Pedreira ME, Perez-Cuesta LM, Maldonado H. Mismatch between what is expected and what actually occurs triggers memory reconsolidation or extinction. Learn Mem. 2004;11:579–585. doi: 10.1101/lm.76904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power AE, Berlau DJ, McGaugh JL, Steward O. Anisomycin infused into the hippocampus fails to block “reconsolidation” but impairs extinction: the role of re-exposure duration. Learn Mem. 2006;13:27–34. doi: 10.1101/lm.91206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z, Gold PE. Intrahippocampal infusion of anisomycin produces amnesia: contribution of increased release of norepinephrine, dopamine, and acetylcholine. Learn Mem. 2009;16:308–314. doi: 10.1101/lm.1333409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez DR, Bell GH, Lasseter HC, Xie X, Traina SA, Fuchs RA. Dorsal hippocampal regulation of memory reconsolidation processes that facilitate drug context-induced cocaine-seeking behavior in rats. Companion maniscript, submitted. [DOI] [PMC free article] [PubMed]
- Routtenberg A, Rekart JL. Post-translational protein modification as the substrate for long-lasting memory. Trends Neurosci. 2005;28:12–19. doi: 10.1016/j.tins.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Biedenkapp JC, Moineau J, Bolding K. Anisomycin and the reconsolidation hypothesis. Learn Mem. 2006;13:1–3. doi: 10.1101/lm.157806. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421:70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauscher J, Kufferle B, Asenbaum S, Fischer P, Pezawas L, Barnas C, Tauscher-Wisniewski S, Brucke T, Kasper S. In vivo 123I IBZM SPECT imaging of striatal dopamine-2 receptor occupancy in schizophrenic patients treated with olanzapine in comparison to clozapine and haloperidol. Psychopharmacology (Berl) 1999;141:175–181. doi: 10.1007/s002130050822. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Wiseman SL, Olausson P, Taylor JR. Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat Neurosci. 2006;9:167–169. doi: 10.1038/nn1628. [DOI] [PubMed] [Google Scholar]
- Vianna MR, Coitinho AS, Izquierdo I. Role of the hippocampus and amygdala in the extinction of fear-motivated learning. Curr Neurovasc Res. 2004;1:55–60. doi: 10.2174/1567202043480170. [DOI] [PubMed] [Google Scholar]
- Wang SH, Ostlund SB, Nader K, Balleine BW. Consolidation and reconsolidation of incentive learning in the amygdala. J Neurosci. 2005;25:830–835. doi: 10.1523/JNEUROSCI.4716-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Martin-Fardon R, Ciccocioppo R, Kerr TM, Smith DL, Ben-Shahar O. Enduring resistance to extinction of cocaine-seeking behavior induced by drug-related cues. Neuropsychopharmacology. 2001;25:361–72. doi: 10.1016/S0893-133X(01)00238-X. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Mangiavacchi S, Sun X. Mechanisms by which dopamine receptors may influence synaptic plasticity. Ann N Y Acad Sci. 2003;1003:241–249. doi: 10.1196/annals.1300.015. [DOI] [PubMed] [Google Scholar]
- Yim AJ, Morales CR, Ferreira TL, Oliveira MG. Protain synthesis inhibition in the basolateral amygdala following retrieval does not impair expression of morphine-associated conditioned place preference. Behav Brain Res. 2006;171:162–169. doi: 10.1016/j.bbr.2006.03.031. [DOI] [PubMed] [Google Scholar]