Abstract
Exposure to a cocaine-paired context increases the propensity for relapse in cocaine users and prompts cocaine-seeking behavior in rats. According to the reconsolidation hypothesis, upon context re-exposure, established cocaine-related associations are retrieved and can become labile. These associations must undergo reconsolidation into long-term memory to effect enduring stimulus control. The dorsal hippocampus (DH), dorsolateral caudate-putamen, and dorsomedial prefrontal cortex are critical for the expression of context-induced cocaine seeking, and these brain regions may also play a role in the reconsolidation of cocaine-related memories that promote this behavior. To test this hypothesis, rats were trained to press a lever for un-signaled cocaine infusions (0.2 mg/infusion, IV) in a distinct environmental context (cocaine-paired context), followed by extinction training in a different context (extinction context). Rats were then re-exposed to the cocaine-paired context for 15 min in order to reactivate cocaine-related memories or received comparable exposure to a novel unpaired context. Immediately thereafter, rats received bilateral microinfusions of the protein synthesis inhibitor anisomycin, the sodium channel blocker tetrodotoxin, or vehicle into one of the above brain regions. After additional extinction training in the extinction context, reinstatement of cocaine-seeking behavior (i.e., non-reinforced lever presses) was assessed in the cocaine-paired context. Tetrodotoxin, but not anisomycin, administered into the DH inhibited drug context-induced cocaine-seeking behavior in a memory reactivation-dependent manner. Other manipulations failed to alter this behavior. These findings suggest that the DH facilitates the reconsolidation of associative memories that maintain context-induced cocaine-seeking behavior, but it is not the site of anisomycin-sensitive memory re-stabilization per se.
Keywords: Cocaine, Context, Reinstatement, Rat
Understanding the neurobiological bases of drug relapse will be essential for the development of successful treatments for cocaine dependence. Exposure to a cocaine-associated context is a major factor that elicits relapse in cocaine users and cocaine-seeking behavior in laboratory animals (Markou et al., 1993; O’Brien et al., 1998). Furthermore, abnormally strong long-term memories of context-response-drug associations may result in intrusive thoughts about drug taking and habitual drug seeking (Tiffany, 1990; Everitt et al., 2001). According to the memory reconsolidation hypothesis, long-term memories become destabilized upon retrieval and must undergo reconsolidation into long-term memory to retain stimulus control (Misanin et al., 1968; Lewis, 1979; Nader et al., 2000b). Thus, the pharmacological inhibition of memory stabilization processes that maintain intrusive cocaine-related memories may be a useful approach to relapse prevention (Lee et al., 2005; Miller & Marshall, 2005; Milekic et al., 2006).
The dorsal hippocampus (DH), dorsolateral caudate-putamen (dlCPu), dorsomedial prefrontal cortex (dmPFC), and basolateral amygdala (BLA), are elements of the neural circuitry that mediate the expression of drug context-induced reinstatement of cocaine-seeking behavior (Fuchs et al., 2005; 2006; 2007), and these brain regions may also play a role in cocaine memory reconsolidation. Consistent with this, administration of the protein synthesis inhibitor anisomycin (ANI), zif268 antisense oligonucleotide treatment, or beta-adrenergic antagonist treatment into the BLA immediately before or after re-exposure to cocaine-paired contextual or explicit conditioned stimuli disrupts subsequent cue-induced cocaine-seeking behavior in a memory reactivation-dependent manner (Lee et al., 2006; Milton et al., 2007; see companion paper, Fuchs et al., submitted). These findings suggest that the BLA is necessary for the re-stabilization of cocaine-related memories that facilitate cue control over cocaine-seeking behavior. However, little is known about the possible contribution of other brain regions, including the DH, dmPFC, and dlCPu, to this phenomenon.
To explore this question, we assessed the effects of ANI and the sodium channel blocker tetrodotoxin (TTX), administered in the DH, dmPFC, and dlCPu following cocaine memory reactivation, on the ability of the cocaine-paired context to subsequently reinstate cocaine-seeking behavior. We predicted that ANI or TTX in the DH or dmPFC would disrupt context-induced cocaine-seeking behavior in a memory reactivation-dependent manner, since the DH has been implicated in memory reconsolidation processes that support Pavlovian contextual fear and morphine-conditioned place preference memories (Debiec et al., 2002; Frankland et al., 2006; Milekic et al., 2006) and the dmPFC may be a component of cortical networks that are theorized to maintain remote associative memories (Amaral et al., 2007). Conversely, we included the dlCPu, a brain region implicated in habit formation (Jog et al., 1999; Everitt et al., 2001; Fuchs et al., 2006), as a negative control region because some evidence suggests that instrumental procedural memories, which are theorized to underlie habitual responding (Kalivas & O’Brien, 2008), do not undergo ANI-sensitive reconsolidation upon retrieval (Hernandez & Kelley, 2004). Furthermore, we included the BLA as a positive control region. In the BLA, TTX treatment administered following cocaine-related memory reactivation was postulated to impair subsequent cocaine-seeking behavior, similar to ANI (Fuchs et al., submitted).
MATERIALS AND METHODS
Animals
Experimentally naïve male Sprague-Dawley rats (Charles-River, N=117), weighing 275-300 g at the start of the experiment, were individually housed in a temperature and humidity controlled vivarium on a reversed light-dark cycle. All rats were maintained on 20-25 g of rat chow per day, with water available ad libitum. Housing and treatment followed the guidelines of the “Guide for the Care and Use of Laboratory Rats” (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, 1996).
Food training
In order to expedite the acquisition of cocaine self-administration, rats were first trained to lever press on a fixed ratio (FR) 1 schedule of food reinforcement (45 mg pellets; Purina, Richmond, IN). All training was conducted in standard sound-attenuated operant conditioning chambers (26 × 27 × 27 cm high; Coulbourn Instruments, Allentown, PA) during a 16-h overnight session. The chambers were equipped with two retractable levers and a food pellet dispenser. During the session, each lever press on the one (active) lever resulted in food pellet delivery only. Lever presses on the other (inactive) lever had no programmed consequences.
Surgery
Forty-eight h after food training, rats were fully anesthetized using ketamine hydrochloride and xylazine (66.6 mg/kg and 1.3 mg/kg, respectively; IP). Chronic indwelling catheters were constructed and implanted into the right jugular vein, as described previously (Fuchs et al., 2006). The catheter ran subcutaneously and exited on the rat’s back, posterior to the shoulder blades. After the catheter surgery, the rats were placed into a stereotaxic instrument (Stoelting, Wood Dale, IL). They received stainless steel guide cannulae (26 gauge, Plastics One), aimed bilaterally at the DH (−3.4 mm AP, ± 3.1 mm ML, −2.1 mm DV), trunk region of the somatosensory cortex just dorsal relative to the DH (SStr; −3.4 mm AP, ± 3.1 mm ML, −0.65 mm DV), dlCPu (+1.2 mm AP, ± 3.6 mm ML, −4.4 mm DV), dmPFC (+3.0 mm AP, ± 0.6 mm ML, −2.2 mm D), or BLA (−2.9 mm AP, ± 5.0 mm ML, −6.7 mm DV, relative to the skull surface at bregma), using standard stereotaxic procedures. Rats were given minimum 5 days for post-operative recovery before the start of the experiment.
To extend catheter patency, catheters were flushed through once daily for 5 days following surgery with 0.1 ml of an antibiotic cefazolin solution (10.0 mg/ml, Schein Pharmaceutical, Florham Park, NJ) dissolved in 70 U/ml heparinized saline (Baxter Healthcare Corp., Deerfield, IL). Thereafter, catheters were flushed with 0.1 ml heparinized saline (10 U/ml) before, and with 0.1 ml of the cefazolin solution and 0.1 ml of heparinized saline (70 U/ml) after, each self-administration session. Catheter patency was periodically verified by infusing 0.1 ml of propofol (10 mg/ml, IV; Abbott Labs., North Chicago, IL), an ultra short-acting barbiturate which produces a rapid loss of muscle tonus only when administered intravenously.
Self-administration
Self-administration training was conducted during 2-h sessions during the rats’ dark cycle, as described before (Fuchs et al., 2007). Rats were trained to lever press on a FR 1 schedule of cocaine reinforcement (cocaine hydrochloride; 0.1 mg/0.05 ml/infusion; NIDA, Research Triangle Park, NC) with a 20-s time out period. Active lever presses resulted in a 2.5-s activation of the infusion pump only. During the subsequent time out period, responses on the active lever had no programmed consequences. Responses on the inactive lever had no programmed consequences. Daily training was continued until a rat reached the acquisition criterion (i.e., ≥ 10 infusions obtained per session on minimum 10 training days).
Self-administration sessions were conducted in operant conditioning chambers that contained one of two distinctly different sets of contextual stimuli in addition to the levers. Context 1 contained a continuous red house light on the wall opposite to the active lever, intermittent pure tone (80 dB, 1 kHz; 2 s on, 2 s off), pine air freshener strip (4.5 × 2 cm, Car Freshener Corp., Watertown, NY), and wire mesh floor (26 cm × 27 cm). Context 2 contained a flashing white stimulus light above the inactive lever (2 s on, 2 s off), continuous pure tone (75 dB, 2.5 kHz), vanilla air freshener strip (4.5 × 2 cm, Sopus Products, Moorpark, CA), and a slanted ceramic tile wall that bisected the bar floor (19 cm × 27 cm). Rats had no exposure to these contextual stimuli prior to self-administration training. The assignment of rats to cocaine self-administration training in Context 1 versus Context 2 was randomized. The contextual stimuli were presented throughout each session independent of responding. The pumps were located outside of the sound-attenuation chambers that housed the operant chambers. Data collection and reinforcer delivery were controlled using Graphic State Notation software version 2.102 (Coulbourn).
Extinction
After the last day of self-administration training, rats underwent 2-h extinction sessions on 7 consecutive days. During the extinction sessions, lever responses had no programmed consequences. The extinction sessions were conducted in Context 2 for rats that had previously self-administered cocaine in Context 1, and vice versa. The rats were adapted to the intracranial microinfusion procedure after their daily extinction training session on post-cocaine day 4. Stainless steel injection cannulae (33 gauge, Plastics One) were inserted into the rat’s guide cannulae 1 (DH, SStr, dmPFC) or 2 mm (dlCPu, BLA) below the tip of the guide cannulae. Rats were held by the experimenter for 4 min while the injection cannulae were left in place but fluid was not infused.
Experiment 1. The role of the DH, dmPFC, dlCPu, & BLA in memory reconsolidation processes that regulate context-induced cocaine seeking
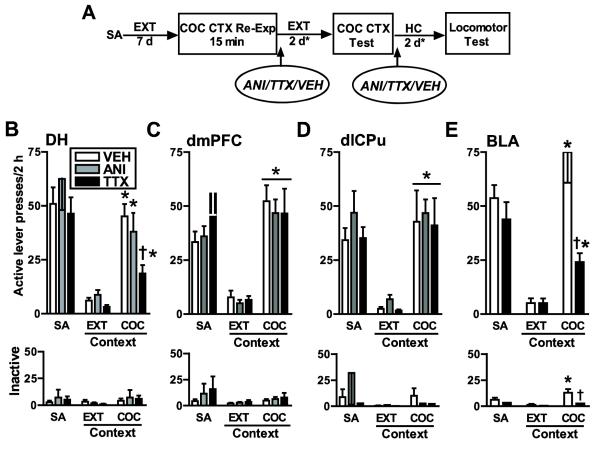
Experiment 1 was designed to assess the effects of ANI, TTX, and phosphate buffered saline vehicle (VEH), administered into the DH, dmPFC, or dlCPu after re-exposure to the cocaine-paired context, on the ability of this context to elicit cocaine-seeking behavior subsequently. The positive control groups received TTX or VEH into the BLA, since we have already demonstrated that ANI administered into the BLA following cocaine memory reactivation is sufficient to disrupt subsequent drug context-induced cocaine-seeking behavior (Fuchs et al., submitted). A schematic depicting the experimental procedure is included in Fig. 2A.
Figure 2.
A: Schematic depicting the experimental timeline in experiment 1. Following completion of cocaine self-administration (SA) training in a distinct context, rats received extinction training (EXT) in a different context. On post-cocaine day 8, rats were re-exposed to the cocaine-paired context (COC CTX) for 15 min without cocaine reinforcement followed immediately by infusion of ANI (62.5 μg/μl; 0.3-1.0 μl/hemisphere), TTX (10 ng/μl; 0.3-0.6 μl/hemisphere) or VEH (0.3-2.0 μl) into the DH, dmPFC, dlCPu, or BLA. The effects of these manipulations were assessed on lever responding in the cocaine-paired context following additional extinction training (*, see extinction criterion in Methods) and on locomotor behavior in a novel context following home cage stay (HC), with an equal treatment-to-testing interval. B - E: Active and inactive lever presses (mean/2 h ± SEM) during SA (mean of last 3 d), in the extinction context (EXT CTX; last session prior to reinstatement testing), and during reinstatement testing in the cocaine-paired context (COC CTX). During testing, lever responding was assessed in the absence of cocaine reinforcement. Asterisks represent a significant difference relative to the EXT CTX (B, E: ANOVA context simple main effect, p = 0.01; C, D: ANOVA main effect, p = 0.001). Dagger represents a significant difference relative to the respective VEH control group (ANOVA treatment simple main effect, p =0.01).
Context Re-exposure
Following self-administration and extinction training, on post-cocaine day 8, rats were placed into the cocaine-paired context for 15 minutes in order to reactivate cocaine-related memories and to assess baseline context-induced motivation for cocaine (Lewis, 1979; Nader et al., 2000b; Tronson & Taylor, 2007). The rats were connected to the infusion apparatus and the levers were extended in order to allow for similar perception of the spatial/tactile elements of the context (e.g., levers, slanted tile) as during cocaine self-administration training. However, fluids were not infused into the catheter upon lever pressing. During the context re-exposure session, lever presses were recorded but had no programmed consequences.
Intracranial Microinfusions
Immediately after the context re-exposure session, rats were removed from the testing room and received bilateral microinfusions of ANI (125 μg/μl concentration; pH ~ 7.0 using 1.0 M NaOH), TTX (10 ng/μl concentration; pH ~ 7.0), or VEH (pH ~ 7.0) into the DH (ANI: 2.0 μl/hemisphere; TTX: 0.5 μl/hemisphere), dmPFC (0.3 μl/hemisphere), dlCPu (0.6 μl/hemisphere); or they received TTX into the BLA (0.5 μl/hemisphere). The concentration of ANI was selected based on previous research demonstrating that intracranial infusions of this concentration impair memory (re)consolidation in other paradigms (Nader et al., 2000a; Wang et al., 2005; Duvarci et al., 2006) and produce robust protein synthesis inhibition (60% and 32% after a 30-min and 60-min post-infusion interval, respectively) as measured using quantitative leucine incorporation autoradiography (Maren et al., 2003; Parsons et al., 2006). TTX was used as part of a secondary approach in order to identify elements of the memory reconsolidation circuitry that facilitate memory reconsolidation but do not serve as the site of ANI-sensitive memory re-stabilization per se. The concentration of TTX was selected based on previous research demonstrating that intracranial infusions of this concentration inhibit sodium conductance, thus the propagation of action potentials, in a tissue radius of ~0.7–0.8 mm, with maximal effects lasting for minimum 120 min then rapidly diminishing within 24 h (Cahill et al., 1987; Zhuravin & Bures, 1991; Ambrogi Lorenzini et al., 1995; Martin & Ghez, 1999). Furthermore, infusion volumes were selected based on previous studies demonstrating that administration of TTX or ANI at these volumes into these target brain regions results in site-specific effects on behavior (Hernandez et al., 2002; Fuchs et al., 2005; Fuchs et al., 2006; Morris et al., 2006). Assignment of rats to the ANI, TTX or VEH treatment groups was counterbalanced based on previous cocaine intake. At the start of the microinfusion procedure, injection cannulae were inserted into the guide cannulae. The injection cannulae were connected to 10-μl Hamilton Syringes (Hamilton Co., Reno, NV) that were mounted on a microdrive pump (KD Scientific, Holliston, MA). The ANI microinfusions were delivered over 8 min for the DH group, 2 min for the dmPFC and dlCPu groups and all TTX microinfusions were delivered over 2 min. The injection cannulae were left in place for 1-2 min before and after the microinfusion, as described previously (Fuchs et al., 2007). Rats were then returned to their home cages.
Extinction and Reinstatement Testing
Twenty-four h after the memory reactivation session, rats were returned to the extinction context for additional daily 2-h extinction training sessions until they reached the extinction criterion (≤ 25 active lever responses per session on minimum 2 consecutive days). After this, the ability of the cocaine-paired context to elicit cocaine-seeking behavior was assessed during a single reinstatement test session. The procedure for this test session was identical to that of the context re-exposure session except that the session length was 2 h.
Locomotor Activity Test
Although unlikely due to the lengthy treatment-to-testing interval, the nonspecific motor effects of intracranial treatments can alter lever pressing behavior. To assess this, the effects of ANI, TTX or VEH infused into the target brain regions on locomotor activity were assessed in a novel environment. The exact treatment-to-testing interval was the same for each rat as in the preceding reinstatement experiment (72-94 h). Locomotor activity was measured in novel Plexiglas chambers (42 × 20 × 20 cm high) using a computerized activity system (San Diego Instruments, San Diego, CA) described previously (Fuchs et al., 2007). The system recorded the number of times eight photobeams were broken by a rat moving in the chamber during the 2-h test session.
Experiment 2. No-reactivation control experiment
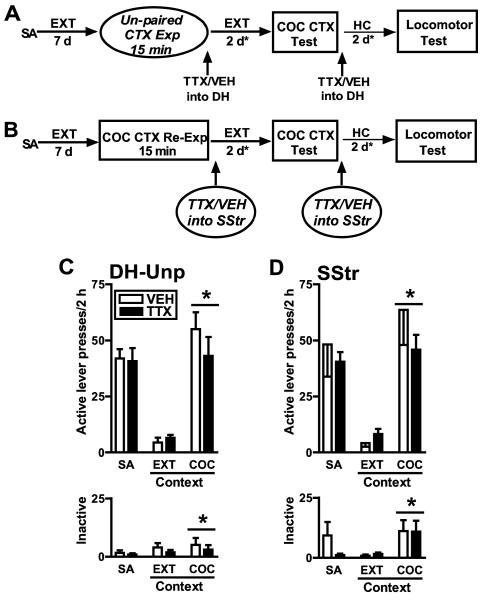
Memory reconsolidation is theorized to depend on memory reactivation (Lewis, 1979; Nader et al., 2000b). Experiment 2 was designed to evaluate whether the intra-DH TTX effect seen in experiment 1 would be observed in the absence of cocaine memory reactivation. The experimental protocol was identical to that used in experiment 1 except that all rats received cannula implants into the DH and were placed into a novel, unpaired context, Context 3, for 15 min on the context re-exposure day prior to receiving bilateral microinfusions of TTX or VEH into the DH (see experimental timeline in Fig. 3A). Context 3 contained a continuous white houselight on the wall opposite to the active lever, continuous white stimulus lights above both the active and inactive levers, a continuous complex tone (80 dB; alternating between 1, 1.5 and 2.5 kHz at 1 s intervals), citrus-scented air freshener strip (4.5 × 2 cm, Locasmarts LLC., Ormond Beach, FL), and a ceramic tile floor (26 cm × 27 cm).
Figure 3.
A: Schematic depicting the experimental design in experiment 2. All procedures were the same as those used in experiment 1 except that rats in experiment 2 were exposed to a novel, unpaired context, rather than the cocaine-paired context, prior to infusion of either TTX or VEH into the DH on post-cocaine day 8. B: Schematic depicting the experimental design in experiment 3. All procedures were the same as those used in experiment 1 except that rats in experiment 3 received infusions of TTX or VEH into the SStr on post-cocaine day 8 after a 15-min exposure to the cocaine-paired context. C: Active and inactive lever presses (mean/2 h ± SEM) in experiment 2 during self-administration (SA; mean of last 3 d), in the extinction context (EXT CTX; last session prior to reinstatement testing), and during reinstatement testing in the cocaine-paired context (COC CTX). D: Active and inactive lever presses (mean/2 h ± SEM) in experiment 3. During testing, lever responding was assessed in the absence of cocaine reinforcement. Asterisks represent a significant difference relative to the EXT CTX (ANOVA context main effect, p=0.04-0.001).
Experiment 3. Anatomical control experiment
Experiment 3 was designed to evaluate the anatomical selectivity of the intra-DH TTX effect obtained in experiment 1. The experimental protocol was identical to that used in experiment 1 except that all rats received cannula implants into the SStr, a structure dorsally adjacent to the DH (see experimental timeline in Fig. 3B). The SStr was selected because unintended spread of TTX was expected to be disproportional in the dorsal direction (Baker et al., 1996; Neisewander et al., 1998). Similar to the DH-cannulated rats in experiment 1, the anatomical control groups received bilateral microinfusions of TTX (10 ng/ul; 0.5 μl/hemisphere) or VEH (0.5 μl) into the SStr immediately after a 15-min re-exposure to the cocaine-paired context and 72-94 h prior to the locomotor activity test.
Histological and Data Analysis
Rats in experiments 1-3 were fully anesthetized with sodium pentobarbital (Sigma, 100 mg/kg, IP) and perfused transcardially. Brains were extracted and sectioned on a vibratome at a thickness of 75 μm. Cannula placements were determined on cresyl violet-stained brain sections based on the rat brain atlas of Paxinos & Watson (1997).
Cocaine-reinforced active lever presses, inactive lever presses, and cocaine intake were analyzed separately for each brain region using one-way analyses of variance (ANOVA) or t-tests, where appropriate (e.g., BLA, SStr), to assess potential pre-existing group differences. Similarly, non-reinforced active and inactive lever presses during the context re-exposure session were analyzed separately using one-way ANOVAs or t-tests, where appropriate. Non-reinforced active and inactive lever presses during the reinstatement test session and immediately preceding extinction session were analyzed separately using 2 × 3 mixed factorial ANOVAs with test context (extinction, cocaine-paired) as the within-subjects factor and treatment (ANI, TTX, and VEH) as the between-subjects factor, where appropriate. To further localize treatment effects, separate 2 × 2 mixed factorial ANOVAs were conducted with test context (extinction, cocaine-paired) as the within-subjects factor and treatment (either ANI vs. VEH or TTX vs. VEH) as the between-subjects factor. For these analyses, only statistically significant effects are reported. To assess the effects of unpaired context exposure on subsequent extinction responding, non-reinforced active lever presses were analyzed using a mixed factorial ANOVA with day (post-cocaine day 7, post-cocaine day 9) as the within-subjects factor and treatment (TTX, VEH) as the between-subjects factor. Locomotor activity counts were analyzed using mixed factorial ANOVAs with treatment as the between-subjects factor and time (20-min intervals) as the within-subjects factor. Significant ANOVA main and interaction effects were followed up by Tukey tests, where appropriate. Alpha was set at 0.05.
RESULTS
Histology
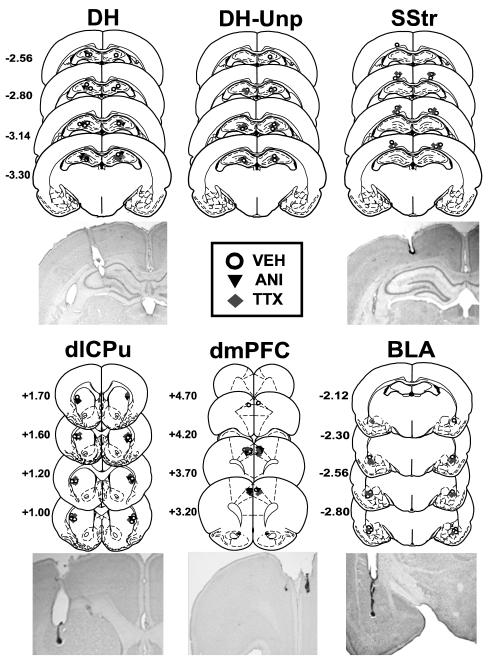
Schematic representations of injection cannula placements and representative photomicrographs are shown in Fig. 1. The target regions were defined as follows: dmPFC, the Cg1 region and the Cg1/Cg2 transition region of the medial prefrontal cortex; dlCPu, the dorsolateral region of the caudate putamen; DH, the dorsal hippocampus; SStr, the trunk region of the somatosensory cortex overlying the dorsal hippocampus; BLA, the lateral/basolateral nuclei of the amygdaloid complex. The data of rats with misplaced cannulae were excluded from data analysis. The most ventral point of the injection cannula tracks were located bilaterally within a target brain region for the following number of rats per group in experiment 1: [DH ANI (N=9), DH TTX (N=8), DH VEH (N=9), dmPFC ANI (N=7), dmPFC TTX (N=8), dmPFC VEH (N=8), dlCPu ANI (N=8), dlCPu TTX (N=8), dlCPu VEH (N=7), BLA TTX (N=7), BLA VEH (N=7)], experiment 2: [DH unpaired context TTX (N=9) and DH unpaired context VEH (N=7)], and experiment 3: [SStr TTX (N=7), SStr VEH (N=8)]. Because experimental groups received microinfusions of either TTX or ANI in experiment 1, VEH control groups received microinfusions using the infusion protocols that matched those used for ANI (50% of rats) or TTX (50% of rats) infusions. Inspection of the cannula placements with high-power microscopy did not reveal irregularities in neuronal integrity (i.e., cell loss or gliosis).
Figure 1.
Schematic representation of cannula placements and photomicrographs of cresyl violet-stained brain sections. Rats received bilateral microinfusions of anisomycin (ANI), tetrodotoxin (TTX), or phosphate buffered saline vehicle (VEH) into the dorsal hippocampus (DH), dorsomedial prefrontal cortex (dmPFC), dorsolateral caudate putamen (dlCPu), basolateral amygdala (BLA), or somatosensory cortex - trunk region (SStr) after re-exposure to the previously cocaine-paired context or a novel unpaired context (Unp). The symbols represent the most ventral point of the infusion cannula tract for each rat. The numbers indicate the distance from bregma in millimeters according to the rat brain atlas (Paxinos & Watson, 1997).
Self-administration and Extinction
All groups in experiments 1-3 exhibited stable responding on the active lever during the last 3 self-administration days (< 10% variability in daily cocaine intake; time main effects, F<1.0). Furthermore, there was no pre-existing difference between the groups that subsequently received ANI, TTX, or VEH into the DH, dmPFC, or dlCPu in active lever responding (3 × 3 ANOVA group main and interaction effects: F(1-4, 14-46)=0.02-2.52, p=0.11-0.98), inactive lever responding (DH, dmPFC, dlCPu: F(1-4, 14-46)=0.49-1.95, p=0.18-0.62), or cocaine intake (DH, dmPFC, dlCPu: F(1-4, 14-46)=0.10-3.22, p=0.06-0.83) during self-administration training. There was also no pre-existing difference between groups that received TTX or VEH into the BLA or SStr on active lever responding (ANOVA group main and interaction effects: F(1,11-13)=0.43-1.75, p=0.21-0.66), inactive lever responding (F(1,11-13)=0.35-1.80, p=0.20-0.71), or cocaine intake (F(1,11-13)=0.02-2.02, p=0.15-0.90) during self-administration training. Collapsed across groups, the mean daily cocaine intake (± SEM) was approx. 4.61 ± 0.13 mg/kg/session (23.05 ± 0.63 infusions).
Responding declined upon removal of cocaine reinforcement on extinction day 1 (39.05 ± 2.73 responses/session), and the microinfusion adaptation procedure did not alter responding on post -cocaine day 4 (data not shown). Subsequently, responding gradually extinguished to a mean of 8.71 ± 1.27 active lever presses per session by post-cocaine day 7, the day preceding the context re-exposure session.
Experiment 1. The role of the DH, dmPFC, dlCPu, and BLA in memory reconsolidation processes that regulate context-induced cocaine seeking
Cocaine-paired context Re-exposure
After self-administration and extinction training, the groups were re-exposed to the cocaine-paired context for 15 min on post-cocaine day 8 (see experimental timeline in Fig. 2A). During this session (data not shown), there was no pre-existing difference between the groups that subsequently received ANI, TTX or VEH into the DH, dmPFC, or dlCPu in active lever responding (F(2, 22-25)=0.19-0.39, p=0.89-0.83) or inactive lever responding (F(2, 22-25)=0.38-0.99, p=0.39-0.69). Further, there was no pre-existing difference between groups that received TTX or VEH into the BLA on active lever responding (t(12)=1.84, p=0.09) or inactive lever responding (t(12)=1.79, p=0.10). The mean active and inactive lever presses (± SEM) during this session were 22.72 ± 2.22 and 4.02 ± 0.83 responses/session, respectively. After this manipulation, there was no difference between the groups in the level of extinction responding on post-cocaine day 9 (data not shown) or in the number of days needed to reach the extinction criterion (2.26 ± 0.86 days). Thus, ANI or TTX treatment administered into the target brain regions did not have nonspecific effects on extinction learning or instrumental behavior in general.
Cocaine-seeking Behavior
After reaching the extinction criterion, exposure to the cocaine-paired context produced a robust increase in active lever pressing in VEH-pretreated groups (see Fig. 2). ANI failed to alter reinstatement, whereas the effects of TTX differed depending on the site of infusion.
DH
TTX, but not ANI, administered into the DH following re-exposure to the cocaine-paired context subsequently attenuated drug context-induced reinstatement, relative to VEH. The ANOVA of active lever responding during the reinstatement test and preceding extinction session indicated significant context main (F(1,23)=44.20, p=0.001) and treatment main effects (F(2,23)=5.32, p=0.013), but no context × treatment interaction effect (F(2,23)=2.55, p=0.10). The lack of an interaction effect was due to the influence of a negative ANI effect on the omnibus ANOVA, since a separate ANOVA comparing the TTX and VEH groups only revealed a significant context × treatment interaction effect (F(1,15)=10.71, p=0.005) in addition to context (F(1,15)=55.16, p=0.001) and treatment (F(1,15)=12.91, p=0.003) main effects (Fig. 2B). In contrast, a similar ANOVA comparing the ANI and VEH groups only revealed a significant context main effect (F(1,16)=36.31, p=0.001). Thus, all groups exhibited increased active lever responding in the cocaine -paired context, relative to the extinction context. However, the TTX-infused group responded less on the active lever relative to the VEH group in the cocaine-paired context (Tukey test, p<0.05), but not in the extinction context. The ANOVA of inactive lever responding indicated no significant context or treatment main or interaction effects (all F’s <1). Thus, neither TTX nor ANI altered inactive lever responding in either the cocaine-paired or extinction context, relative to VEH.
dmPFC and dlCPu
TTX or ANI administered into the dmPFC or dlCPu following re-exposure to the cocaine-paired context subsequently failed to alter cocaine context-induced reinstatement, relative to VEH (Fig. 2C, D). Consistent with this, the ANOVA of active lever responding in the dmPFC or dlCPu-cannulated groups during the reinstatement test session and preceding extinction session revealed a significant context main effect (dmPFC: F(1,19)=61.11, p=0.001; dlCPu: F(1,20)=36.84, p=0.001), but no context × treatment interaction (dmPFC: F(2,19)=0.19, p=0.83; dlCPu: F(2,20)=0.002, p=0.99) or treatment main effects (dmPFC: F(2,19)=0.46, p=0.64; dlCPu: F2,20)=0.25, p=0.78). Similarly, separate ANOVAs comparing the ANI vs. VEH or TTX vs. VEH groups indicated context main effects only (F(1,13-14)=28.06-78.59, p=0.001). Thus, active lever responding increased upon exposure to the cocaine -paired context as compared to the extinction context, irrespective of treatment. The ANOVA of inactive lever responding indicated no significant context or treatment main or interaction effects (all F’s < 1). Thus, neither TTX nor ANI altered inactive lever responding in either the cocaine-paired or extinction context, relative to VEH.
BLA
Previous findings from our laboratory revealed that ANI administered into the BLA immediately following re-exposure to the cocaine-paired context subsequently attenuated cocaine context-induced reinstatement, relative to VEH (Fuchs et al., Submitted). Similarly, in the present study, TTX administered into the BLA attenuated cocaine-context-induced reinstatement, relative to VEH (see Fig. 2E). The ANOVA of active lever responding in the BLA-cannulated groups during the reinstatement test session and preceding extinction session revealed a significant context × treatment interaction (F(1,12)=9.24, p=0.01), and context (F(1,12)=27.32, p=0.001) and treatment (F(1,12)=8.68, p=0.012) main effects. Both groups exhibited increased active lever responding in the cocaine-paired context relative to the extinction context. However, the TTX-infused group responded less on the active lever relative to VEH in the cocaine-paired context (Tukey test, p<0.05), but not in the extinction context. Infusion of TTX into the BLA also decreased inactive lever responding during the reinstatement test, relative to VEH. The ANOVA of inactive lever responding indicated a significant context × treatment interaction effect (F(1,12)=14.44, p=0.003), and significant context main (F(1,12)= 18.47, p=0.001) and treatment main effects (F(1,12)=27.25, p<0.001). The group that received VEH after cocaine memory reactivation exhibited increased inactive lever responding in the cocaine-paired context relative to the extinction context (Tukey test, p<0.05), whereas the TTX-infused group did not. Thus, the TTX-infused group responded less on the inactive lever relative to the VEH-infused group in the cocaine-paired context (Tukey test, p<0.05), but not in the extinction context.
Experiment 2. No Reactivation Control Experiment
To verify that the intra-DH TTX induced decrease in cocaine-seeking behavior in experiment 1 reflected a memory reconsolidation deficit, experiment 2 assessed whether this effect was dependent on cocaine context re-exposure (see experimental timeline in Fig. 3A). To this end, DH-cannulated groups were exposed to a novel, unpaired context for 15 min prior to TTX or VEH infusion into the DH. The self-administration history of the groups in experiment 2 did not differ from that in experiment 1.
Unpaired Context Exposure
During the 15-min unpaired context exposure session, there was no pre-existing difference between groups that subsequently received TTX or VEH into the DH in active lever responding (t(14)=0.31, p=0.77) and inactive lever responding (t(14)=1.56, p=0.14). The mean active and inactive lever presses (± SEM) during this session were 5.44 ±1.57 and 2.06 ± 0.80 responses/session, respectively. A separate ANOVA of active lever responses during the 120 min extinction sessions immediately preceding and following the unpaired context exposure session indicated no day or treatment main or interaction effects (F(1,14)=0.43-1.68, p=0.22-0.58). Similarly, the ANOVA of inactive lever responses indicated no day or treatment main or interaction effects (F1,14)=0.004-2.97, p=0.11-0.95). Thus, TTX administered into the DH did not alter extinction responding or instrumental behavior in general, relative to VEH.
Cocaine-seeking Behavior
TTX administered into the DH after re-exposure to the unpaired context had no effect on the subsequent ability of the cocaine-paired context to produce reinstatement (see Fig. 3C). The ANOVA of active lever responses during the reinstatement test session and preceding extinction session indicated a significant context main effect (F(1,14)=62.22, p=0.001), but no context × treatment interaction (F(1,14)=1.61, p=0.23) or treatment main effects (F(1,14)=0.59, p=0.46). Thus, both groups exhibited more active lever responding in the cocaine-paired context than in the extinction context, irrespective of treatment. The ANOVA of inactive lever responses indicated no day or treatment main or interaction effects (F1,14)=0.001-6.49, p=0.49-0.98; see Fig. 3C). Thus, TTX did not alter inactive lever responding in either the cocaine-paired or extinction context, relative to VEH.
Experiment 3. Anatomical Control Experiment
To verify that the intra-DH TTX-induced effects on cocaine-seeking behavior were specific to the DH in experiment 1, experiment 3 examined whether this effect was anatomically specific. The SStr was included as a control region because it is dorsally adjacent to the DH. SStr-cannulated groups in experiment 3 were re-exposed to the cocaine-paired context for 15 min prior to TTX or VEH infusion (see experimental timeline in Fig. 3B).
Cocaine-paired context Re-exposure
During the 15-min re-exposure to the cocaine-paired context, there was no pre-existing difference between groups that subsequently received TTX or VEH into the SStr in inactive lever responding (t(13)=0.09, p=0.28), but the subsequently VEH-treated group exhibited more active lever responding than the subsequently TTX-treated group (t(13)=2.90, p=0.012). After TTX or VEH administration, there was no difference between the groups in the level of extinction responding on post-cocaine day 9 (t(13)=0.85, p=0.41) or in the number of days needed to reach the extinction criterion (mean 2.13 ± 0.13 days; t(13)=0.93, p=0.37). Thus, TTX administered into the SStr did not have nonspecific effects on extinction learning or instrumental behavior in general, relative to VEH.
Cocaine-seeking Behavior
TTX administered into the SStr following 15 min of cocaine context re-exposure did not alter subsequent cocaine context-induced reinstatement, relative to VEH (see Fig. 3D). The ANOVA of active lever responses in the SStr-cannulated groups during the reinstatement test session and preceding extinction session revealed a significant context main effect (F(1,13)=32.90, p=0.001), but no context × treatment interaction (F(1,13)=2.70, p=0.12) or treatment main effect (F(1,13)=0.98, p=0.34). Similarly, the ANOVA of inactive lever responding during the reinstatement test session and preceding extinction session indicated a significant context main effect (F(1,13)=5.72, p=0.03), but no context × treatment interaction (F(1,13)=0.27, p=0.61) or treatment main effects (F(1,13)=0.13, p=0.73). Thus, both groups exhibited more active, and to a lesser extent inactive, lever responding in the cocaine -paired context than in the extinction context, irrespective of treatment.
Effects of Intracranial Manipulations on Locomotor Activity
Infusion of either ANI or TTX into the DH, dmPFC, dlCPu, BLA, or SStr failed to alter locomotor activity in a novel context, relative to VEH. Separate 3 × 6 or 2 × 6 ANOVAs of photobeam breaks revealed significant time main effects (F(5,60-115)=2.71-73.37, p=0.0001-0.027), but no time × treatment interaction (F(5-10,60-115)=0.36-2.92, p=0.06-0.96) or treatment main effects (F(1-2,12-23)=0.04-1.26, p=0.07-0.84). An additional 2 × 6 ANOVA for the groups that received TTX or VEH into the DH also indicated a significant time main effect only (F(5,115)=71.94, p=0.0001). Thus, locomotor activity declined across time (interval 1 > intervals 2-6; Tukey test, p<0.05), irrespective of treatment, and the intracranial manipulations did not alter general activity.
DISCUSSION
The present study represents the first demonstration that the DH is critical for the post-reactivation processing of cocaine-related memories, which in turn maintain the ability of a cocaine-paired context to elicit drug-seeking behavior 72-94 h later. Briefly, TTX, but not ANI, treatment administered into the DH immediately after brief re-exposure to the cocaine-paired context inhibited subsequent drug context-induced reinstatement of cocaine-seeking behavior relative to VEH, in a memory reactivation-dependent fashion (Fig. 2B). Conversely, TTX or ANI treatment administered into the dmPFC (Fig. 2C), dlCPu (Fig. 2D), or SStr (Fig. 3D) following re-exposure to the cocaine-paired context failed to alter subsequent context-induced cocaine-seeking behavior relative to VEH. Furthermore, TTX treatment administered into the BLA after re-exposure to the cocaine-paired context did impair subsequent cocaine-seeking behavior relative to VEH (Fig. 2E), similar to the effects of ANI observed previously (see companion manuscript, Fuchs et al., submitted).
Involvement of the DH in the Reconsolidation of Context-related Memories that Guide Drug Context-induced Cocaine-Seeking Behavior
The above effect of TTX in the DH likely reflects that functional integrity of the DH is critical for the post-reactivation reconsolidation of memories that facilitate context-induced cocaine-seeking behavior. Thus, the present findings expand upon our earlier studies which indicate that the DH plays a requisite role in the expression of drug context-induced cocaine-seeking behavior (Fuchs et al., 2005; 2007). According to the memory reconsolidation hypothesis, upon reactivation, long-term memories become labile and must be re-stabilized into long-term memory in order to be preserved (Misanin et al., 1968; Lewis, 1979; Nader et al., 2000b; Tronson & Taylor, 2007). Similarly, in the present study, deficits in cocaine-seeking behavior were observed when TTX was infused into the DH after memory reactivation (i.e., cocaine-paired context re-exposure), but not in the absence of memory reactivation (i.e., after unpaired context exposure; Fig. 3C). This impairment was also anatomically specific and not due to motor suppression. Specifically, TTX had no effect on cocaine-seeking behavior after administration into the SStr, the brain region situated just dorsally relative to the DH, thus in the most likely path of drug diffusion following DH microinfusion. TTX also failed to alter locomotor activity in a novel context or inactive lever responding in the cocaine-paired context during the reinstatement test session. Together these findings support the interpretation that TTX-induced neuronal inactivation in the DH likely disrupted of the motivational effects of the cocaine-paired context in a manner consistent with a memory reconsolidation deficit.
Interestingly, intra-DH TTX resulted in only a partial suppression of cocaine-seeking behavior, similar to the effects of intra-BLA ANI or TTX treatment (Fuchs et al., submitted; present study). In contrast, both ANI and the protein synthesis inhibitor cycloheximide completely suppress Pavlovian conditioned responses (Nader et al., 2000b; Milekic et al., 2006). Partial TTX-effects observed in the present study may stem from differences between instrumental and Pavlovian memories in terms of resistance to memory reconsolidation inhibitor treatment upon reactivation. In particular, food-motivated instrumental memories appear to be resistant to systemic ANI treatment (Hernandez & Kelley, 2004). Similarly, TTX-resistant context-response and/or response-reward associations may be sufficient to maintain instrumental cocaine-seeking behavior after the disruption of context-reward associations in the present study. Alternatively, instrumental and Pavlovian memories may differ in resistance to memory reconsolidation inhibition due to differences in the extent of training and memory age (Tronson & Taylor, 2007; Brown et al., 2008), as is discussed in more detail in the companion paper (Fuchs et al., submitted).
It is unlikely that TTX in the DH attenuated cocaine seeking by promoting extinction learning in the cocaine-paired context. Consistent with this, muscimol-induced neural inactivation of, or c-Jun N-terminal kinase or p38 mitogen-activated protein kinase inhibition in, the DH impairs — as opposed to facilitates — contextual extinction memory in other paradigms (Corcoran et al, 2005; Bevilaqua et al, 2007; Rossato et al, 2006). Furthermore, TTX-induced inactivation of the DH in the present study failed to alter extinction responding, an index of response-no drug associations, in the extinction context 24 h after the manipulation. In some studies, a reinforced memory reactivation session has been used to rule out facilitation of extinction learning (Duvarci & Nader, 2004). However, the same approach could not be used here because cocaine activates intracellular signaling molecules that mediate memory consolidation and reconsolidation processes (e.g., protein kinase A, mitogen-activated protein kinase, extracellular-regulated kinase, zif268; for review see Tronson & Taylor, 2007; Rodriguez et al., 1993; Blaiss & Janak, 2007). These unconditioned effects of cocaine would have likely competed against and potentially diminished the effects of TTX on memory reconsolidation. In other studies, facilitation of extinction learning was assessed through a test of spontaneous recovery (Duvarci & Nader, 2004; Lin et al., 2006), the well-documented return of an extinguished conditioned response after time away from testing (DiCiano & Everitt, 2002). The underlying assumption for this test is that lack of spontaneous recovery indicates permanent loss of a memory trace thus a deficit in memory reconsolidation. However, findings suggestive of spontaneous recovery from amnesia can be obtained despite a genuine memory reconsolidation deficit if multiple memory trances exist. In particular, it has been theorized that memory traces encoded in cortical assemblies are more slowly assembled than DH-dependent memory traces and are not recruited to control the behavior until their consolidation is fully completed (Eichenbaum et al., 1994; Squire & Alvarez, 1995; Anagnostaras et al., 2001; Amaral et al., 2007; Amaral et al., 2008). Thus, the delayed availability of cortical, or perhaps other DH-independent, memory traces may account for apparent recovery from amnesia after the impairment of the DH-dependent memory trace. The time-limited involvement of the DH in memory storage appears to be supported by the finding that zif268 expression in the DH is only apparent after the retrieval of 24-h, but 28-d-old, memories of context-fear associations (Hall et al., 2001; McCleery & Harvey, 2004), and only recent contextual fear memories are susceptible to ANI-induced disruption of memory reconsolidation (Frankland et al., 2006, but see Debiec et al., 2002). It is currently not known whether DH involvement in context-induced cocaine-seeking behavior is similarly restricted by memory age or other factors. If so, this suggests that potential treatments for relapse prevention will need to target other, more permanent neural substrates of memory reconsolidation. One such candidate may be the BLA, which appears to play a role in the reconsolidation of cocaine-CS memories that regulate cocaine-seeking behavior for up to 30 d after initial learning (Lee et al., 2006).
Empirical evidence suggests that memory re-stabilization per se requires protein synthesis, the post-translational modification of proteins, and/or other ANI-sensitive neurochemical mechanism (Debiec et al., 2002; Routtenberg & Rekart, 2005; Frankland et al., 2006; Milekic et al., 2006; Tronson & Taylor, 2007; Qi & Gold, 2009). ANI administered at the dose used in the present study disrupts protein synthesis and, similar to other protein synthesis inhibitors, likely impairs the synthesis and post-translational modification of signaling molecules and transcription factors (Maren et al., 2003; Routtenberg & Rekart, 2005; Parsons et al., 2006). Intra-DH administration of ANI has been shown to inhibit memories that underlie morphine-conditioned place preference in a memory reactivation-dependent manner (Milekic et al., 2006). Thus, it was somewhat unexpected that post-memory reactivation ANI treatment administered into the DH failed to alter context-induced cocaine-seeking behavior in the present study. The training-to-memory reactivation interval (i.e., memory age) and ANI dose were comparable in these studies. Thus, the discrepancy between these findings may be due to the differential involvement of ANI-sensitive processes in the re-stabilization of morphine-related versus cocaine-related associations or of Pavlovian versus instrumental associations. Regarding the latter possibility, instrumental conditioning is typically more extensive than Pavlovian conditioning in the addiction research literature, and extensively-trained memories are more resistant to disruption by ANI (for review, see Alberini et al., 2006; Tronson & Taylor, 2007). Furthermore, in Pavlovian paradigms (e.g., CPP), context exposure is explicitly paired with drug exposure, and the context is established as a CS that signals imminent drug effects. Conversely, in instrumental paradigms, the context is a background stimulus that acts as an occasion setter or discriminative stimulus which signals drug availability contingent upon responding (Fuchs et al., in press). Occasion setter-response-drug associations may differ in their sensitivity to ANI in the DH relative to CS-drug associations. In fact, empirical evidence supports that there is a complex relationship between the function of the context in a particular setting and the recruitment of the DH in memory reconsolidation. Thus, while intra-DH ANI treatment disrupts Pavlovian contextual fear memory reconsolidation (Debiec et al., 2002), context pre-exposure prior to conditioning inhibits this effect of ANI, or of NMDA antagonist treatment, perhaps by establishing the context as a background stimulus as opposed to an explicit CS (Biedenkapp & Rudy, 2004; Matus-Amat et al., 2007).
One possible explanation for the differential effects of TTX versus ANI in the DH in the present study is that the DH may be an element of the memory circuitry that facilitates context-induced cocaine-seeking behavior, but it is not the site where ANI-sensitive memory re-stabilization occurs. For instance, memory restabilization may occur in the BLA, since administration of ANI or TTX into the BLA inhibits context-induced cocaine-seeking behavior in a memory reactivation-dependent fashion (present study, Fuchs et al., submitted). Theta synchronicity occurs between the BLA and DH during the reconsolidation of contextual fear memories (Narayanan et al., 2007). Thus, the DH may facilitate memory reconsolidation, perhaps by maintaining the destabilized associations in short-term and in intermediate-term memory (Kesner et al., 2004) while they undergo reconsolidation in the BLA. Alternatively, a modulatory relationship may exist between the BLA and DH, with both regions influencing memory reconsolidation independently. Accordingly, future functional disconnection studies will be needed in order to distinguish between these possibilities by directly investigating whether functional interaction between the BLA and DH is necessary for memory reconsolidation processes that are relevant for context-induced cocaine-seeking behavior per se.
Contributions of the dlCPu and dmPFC to Context-induced Cocaine-Seeking Behavior
Infusion of ANI or TTX into either the dlCPu or dmPFC following cocaine-context memory reactivation failed to alter subsequent context-induced cocaine seeking, suggesting that these brain regions are not critical for processes that maintain this behavior under the current experimental conditions. These negative effects were not likely due to insufficient dosing, since ANI and TTX at the doses administered produce robust inhibition of protein synthesis (Maren et al., 2003; Parsons et al., 2006) and the propagation of action potentials, respectively (Cahill et al., 1987; Zhuravin & Bures, 1991; Ambrogi Lorenzini et al., 1995; Martin & Ghez, 1999; Hernandez et al., 2002). Also, TTX or GABA agonist treatment impairs the expression of context-induced cocaine-seeking behavior when administered into the dmPFC or dlCPu immediately prior to reinstatement testing (Fuchs et al., 2005; Fuchs, et al., 2006). In particular, it was unexpected that intra-dmPFC ANI or TTX treatment failed to alter context-induced cocaine-seeking behavior, given that mature memories are thought to be maintained by cortical assemblies (McClelland et al., 1995; Squire & Alvarez, 1995; Frankland & Bontempi, 2005). Furthermore, the anterior cingulate subregion of the dmPFC exhibits increased zif268 expression following the retrieval of contextual fear memories (Thomas et al., 2002; Thomas et al., 2003). In contrast, a negative finding was expected after ANI administration into the dlCPu, since dlCPU-mediated habitual responding is theorized to rely on the instrumental procedural memory (Kalivas & O’Brian, 2008), which is not vulnerable to systemic ANI treatment following memory reactivation (Hernandez & Kelley, 2004). The present findings appear to corroborate and extend the latter finding by indicating that neither ANI-sensitive processes nor TTX-sensitive neural activity in the dlCPu are required for memory reconsolidation that maintains drug context-induced cocaine-seeking behavior. However, negative findings in the dmPFC as well as the dlCPu may simply reflect that our study parameters, including memory age, strength (i.e., extent of training), and complexity, as well as the extent of memory reactivation, were not conducive for the recruitment of the these brain regions (Dudai & Eisenburg, 2004; Alberini et al., 2006; Bernardi et al., 2007; Tronson & Taylor, 2007; Abrari et al., 2008). Therefore, future studies that systematically vary study parameters that correspond to the boundary conditions of memory reconsolidation will need to be conducted in order to conclusively determine the role of the dmPFC and dlCPu in this phenomenon.
Conclusions
In combination with our previous study (Fuchs et al., submitted), the present findings support the involvement of the DH and BLA in memory reconsolidation processes that facilitate drug context-induced cocaine seeking and dissociate the roles of these brain regions in this process. TTX-sensitive mechanisms in the DH are necessary for the reconsolidation of cocaine-related memories, but not their ANI-sensitive restabilization per se. In contrast, based our previous findings (Fuchs et al., submitted), ANI-sensitive processes in the BLA are likely required for the re-stabilization of cocaine-related memories, perhaps with input from the DH via TTX-sensitive anatomical connections. Cocaine-related memories provide powerful impetus for drug relapse. Accordingly, future studies will need to increase our understanding of the neuroanatomical circuitry and molecular mechanisms that reactivate, stabilize, and modify these memories. This research endeavor has the potential to provide critical information for the development of new treatments for cocaine dependence.
Acknowlegdements
The authors are grateful to Dr. Ann E. Kelley and Dr. Pepe Hernandez for information about the preparation of the anisomycin solution. The authors would like to also thank Andrew Hamlet, Stephanie Kaszycki, KaiCee Ponds, Daniel Reich, Jennifer Shurney, Chandler Sours, and Zu-In Su and for their excellent technical assistance. Portions of this work were presented previously at the 46th annual meeting of ACNP in 2007, the 69th annual meeting of CPDD in 2007, and the 37th annual meeting for SfN in 2007. This work was supported by NIDA grant R01 DA017673 (R.A.F.), a NIDA R01 grant supplement to promote diversity in health-related research (DA017673-S1; D.R.R.), a University of North Carolina at Chapel Hill Junior Faculty Development Award (R.A.F), and the Mason and Linda Stephenson Faculty Award (R.A.F.).
Abbreviations
- ANI
Anisomycin
- BLA
basolateral amygdala
- DH
dorsal hippocampus
- dlCPu
dorsolateral caudate-putamen
- dmPFC
dorsomedial prefrontal cortex
- SStr
somatosensory cortex, trunk region
- TTX
tetrodotoxin
- VEH
vehicle
REFERENCES
- Abrari K, Rashidy-Pour A, Semnanian S, Fathollahi Y. Administration of corticosterone after memory reactivation disrupts subsequent retrieval of a contextual conditioned fear memory: Dependence upon training intensity. Neurobiol Learn Mem. 2008;89:178–184. doi: 10.1016/j.nlm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Alberini CM, Milekic MH, Tronel S. Mechanisms of memory stabilization and de-stabilization. Cell Mol Life Sci. 2006;63:999–1008. doi: 10.1007/s00018-006-6025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral OB, Luft T, Cammarota M, Izquierdo I, Roesler R. Temporary inactivation of the dorsal hippocampus induces a transient impairment in retrieval of aversive memory. Behav Brain Res. 2007;180:113–118. doi: 10.1016/j.bbr.2007.02.031. [DOI] [PubMed] [Google Scholar]
- Amaral OB, Osan R, Roesler R, Tort AB. A synaptic reinforcement-based model for transient amnesia following disruptions of memory consolidation and reconsolidation. Hippocampus. 2008;18:584–601. doi: 10.1002/hipo.20420. [DOI] [PubMed] [Google Scholar]
- Ambrogi Lorenzini CG, Baldi E, Bucherelli C, Tassoni G. Time-dependent deficits of rat’s memory consolidation induced by tetrodotoxin injections into the caudate-putamen, nucleus accumbens and globus palladus. Neurobio Learn Mem. 1995;63:87–93. doi: 10.1006/nlme.1995.1008. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, Faneslow MS. Hippocampus and contextual fear conditioning: Recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Baker DA, Khroyan TV, O’Dell LE, Fuchs RA, Neisewander JL. Differential effects of intraaccumbens sulpiride on cocaine-induced locomotion and conditioned place preference. J Pharmacol Exp Ther. 1996;279:392–401. [PubMed] [Google Scholar]
- Bernardi RE, Lattal KM, Berger SP. Anisomycin disrupts a contextual memory following reactivation in a cocaine-induced locomotor paradigm. Behav Neurosci. 2007;121:156–163. doi: 10.1037/0735-7044.121.1.156. [DOI] [PubMed] [Google Scholar]
- Bevilaqua LR, Rossato JI, Clarke JH, Medina JH, Izguierdo I, Cammarota M. Inhibition of c-Jun N-terminal kinase in the CA1 region of the dorsal hippocampus blocks extinction of inhibitory avoidance memory. Behav Pharmacol. 2007;18:483–489. doi: 10.1097/FBP.0b013e3282ee7436. [DOI] [PubMed] [Google Scholar]
- Biedenkapp JC, Rudy JW. Context memories and reactivation: Constraints on the reconsolidation hypothesis. Behav Neurosci. 2004;118:956–964. doi: 10.1037/0735-7044.118.5.956. [DOI] [PubMed] [Google Scholar]
- Blaiss CA, Janak PH. Post-training, but not post-reactivation, administration of amphetamine and anisomycin modulates Pavlovian conditioned approach. Neurobiol Learn Mem. 2007;87:644–658. doi: 10.1016/j.nlm.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, Lee BR, Sorg BA. The NMDA antagonist MK-801 disrupts reconsolidation of a cocaine-associated memory for conditioned place preference but not for self-administration in rats. Learn Mem. 2008;15:857–65. doi: 10.1101/lm.1152808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Coopersmith RM, Leon M, McGaugh JL. Local infusion of tetrodotoxin decreases metabolic activity in discrete brain regions: A 2-deoxyglucose autoradiography analysis. Society for Neuroscience Abstracts. 1987;13:1414. [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci. 2005;25:8978–87. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, Ledoux JE, Nader K. Cellular and systems reconsolidation in the hippocampus. Neuron. 2002;36:527–538. doi: 10.1016/s0896-6273(02)01001-2. [DOI] [PubMed] [Google Scholar]
- DiCiano P, Everitt BJ. Reinstatement and spontaneous recovery of cocaine-seeking following extinction and different durations of withdrawal. Behav Pharmacol. 2002;13:397–405. doi: 10.1097/00008877-200209000-00013. [DOI] [PubMed] [Google Scholar]
- Dudai Y, Eisenberg M. Rites of passage of the engram: reconsolidation and the lingering consolidation hypothesis. Neuron. 2004;44:93–100. doi: 10.1016/j.neuron.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Nader K. Characterization of fear memory reconsolidation. J Neurosci. 2004;24:9269–9275. doi: 10.1523/JNEUROSCI.2971-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Mamou CB, Nader K. Extinction is not a sufficient condition to prevent fear memories from undergoing reconsolidation in the basolateral amygdala. Eur J Neurosci. 2006;24:249–260. doi: 10.1111/j.1460-9568.2006.04907.x. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Otto T, Cohen NJ. Two functional components of the hippocampal memory system. Behav Brain Sci. 1994;17:449–518. [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Ding HK, Takahashi E, Suzuki A, Kida S, Silva AJ. Stability of recent and remote contextual fear memory. Learn Mem. 2006;13:451–457. doi: 10.1101/lm.183406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Bell GH, Ramirez DR, Eaddy JL, Su Z-I. Basolateral amygdala involvement in memory reconsolidation processes that facilitate drug context-induced cocaine seeking. Submitted. [DOI] [PMC free article] [PubMed]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: A critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci. 2007;26:487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology (Berl) 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Lasseter HC, Ramirez DR, Xie X. Relapse to drug seeking following prolonged abstinence: the role of environmental stimuli. Drug Discovery Today: Disease Models. doi: 10.1016/j.ddmod.2009.03.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Cellular imaging of zif268 expression in the hippocampus and amyghala during contextual and cued fear memory retrieval: selective activation of hippocampal CA1 neurons during the recall of contextual memories. J Neurosci. 2001;21:2186–2193. doi: 10.1523/JNEUROSCI.21-06-02186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez PJ, Kelley AE. Long-term memory for instrumental responses does not undergo protein synthesis-dependent reconsolidation upon retrieval. Learn Mem. 2004;11:748–754. doi: 10.1101/lm.84904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez PJ, Sadeghian K, Kelley AE. Early consolidation of instrumental learning requires protein synthesis in the nucleus accumbens. Nat Neurosci. 2002;5:1327–1331. doi: 10.1038/nn973. [DOI] [PubMed] [Google Scholar]
- Jog MS, Kubota Y, Connolly CI, Hillegaart, Graybiel AM. Building neural representations of habits. Science. 1999;286:1745–1749. doi: 10.1126/science.286.5445.1745. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2009;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Lee I, Gilbert P. A behavioral assessment of hippocampal function based on a subregional analysis. Rev Neurosci. 2004;15:333–51. doi: 10.1515/revneuro.2004.15.5.333. [DOI] [PubMed] [Google Scholar]
- Lee JL, Di Ciano P, Thomas KL, Everitt BJ. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron. 2005;47:795–801. doi: 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci. 2006;26:5881–5887. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DJ. Psychobiology of active and inactive memory. Psychol Bull. 1979;86:1054–1083. [PubMed] [Google Scholar]
- Lin HC, Mao SC, Gean PW. Effects of intra-amygdala infusion of CB1 receptor agonists on the reconsolidation of fear-potentiated startle. Learn Mem. 2006;13:316–321. doi: 10.1101/lm.217006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Ferrario CR, Corcoran KA, Desmond TJ, Frey KA. Protein synthesis in the amygdala, but not the auditory thalamus, is required for consolidation of Pavlovian fear conditioning in rats. Eur J Neurosci. 2003;18:3080–3088. doi: 10.1111/j.1460-9568.2003.03063.x. [DOI] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology. 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- Martin JH, Ghez C. Pharmacological inactivation in the analysis of the central control of movement. J Neurosci Methods. 1999;86:145–159. doi: 10.1016/s0165-0270(98)00163-0. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Sprunger D, Wright-Hardesty K, Rudy J. The role of the dorsal hippocampus and basolateral amygdala NMDA receptors in the acquisition and retrieval of context and contextual fear memories. Behav Neurosci. 2007;121:721–731. doi: 10.1037/0735-7044.121.4.721. [DOI] [PubMed] [Google Scholar]
- McCleery JM, Harvey AG. Integration of psychological and biological approaches to trauma memory: implications for pharmacological prevention of PTSD. J Trauma Stress. 2004;17:485–496. doi: 10.1007/s10960-004-5797-5. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O’Reilly RC. Why are there complimentaty learning systems in the hippocampus and neocortex: insight form the successes and failures of connectionist models of learning and memory. Psychol. Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- Milekic MH, Brown SD, Castellini C, Alberini CM. Persistent disruption of an established morphine conditioned place preference. J Neurosci. 2006;26:3010–3020. doi: 10.1523/JNEUROSCI.4818-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Milton AL, Lee JL, Everitt BJ. 2007 Neuroscience Meeting Planner. Society for Neuroscience; San Diego, CA: 2007. The role of glutamate receptors and adrenergic receptors in CS-US memory reconsolidation: implications for the treatment of psychiatric disorders. Program No. 308.1. 2007. Online. [Google Scholar]
- Misanin JR, Miller RR, Lewis DJ. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science. 1968;160:554–555. doi: 10.1126/science.160.3827.554. [DOI] [PubMed] [Google Scholar]
- Morris RG, Inglis J, Ainge JA, Olverman HJ, Tulloch J, Dudai Y, Kelly PA. Memory reconsolidation: sensitivity of spatial memory to inhibition of protein synthesis in dorsal hippocampus during encoding and retrieval. Neuron. 2006;50:479–489. doi: 10.1016/j.neuron.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, LeDoux JE. The labile nature of consolidation theory. Nat Neurosci. 2000a;1:216–219. doi: 10.1038/35044580. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, LeDoux JE. Fear memories require protein synthesis in the amygdala for refconsolidation after retrieval. Nature. 2000b;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Narayanan RT, Seidenbecher T, Sangha S, Stork O, Page HC. Theta resynchronization during reconsolidation of remote contextual fear memory. Neuroreport. 2007;18:1107–11. doi: 10.1097/WNR.0b013e3282004992. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Fuchs RA, O’Dell LE, Khroyan TV. Effects of SCH-23390 on dopamine D1 receptor occupancy and locomotion produced by intraaccumbens cocaine i-infusion. Synapse. 1998;30:194–204. doi: 10.1002/(SICI)1098-2396(199810)30:2<194::AID-SYN9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Baruch DE, Riedner BA, Helmstetter FJ. Long-term stability of fear memory depends on the synthesis of protein but not mRNA in the amygdala. Eur J Neurosci. 2006;23:1853–1859. doi: 10.1111/j.1460-9568.2006.04723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Compact 3 Edition. Academic Press; 1997. [DOI] [PubMed] [Google Scholar]
- Qi Z, Gold PE. Intrahippocampal infusion of anisomycin produces amnesia: contribution of increased release of norepinephrine, dopamine, and acetylcholine. Learn Mem. 2009;16:308–314. doi: 10.1101/lm.1333409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez WA, Rodriguez SB, Phillips MY, Martinez JL., Jr. Post-reactivation cocaine administration facilitates later acquisition of an avoidance response in rats. Behav Brain Res. 1993;59:125–129. doi: 10.1016/0166-4328(93)90158-m. [DOI] [PubMed] [Google Scholar]
- Rossato JI, Bevilaqua LR, Lima RH, Medina JH, Izguierdo I, Cammarota M. On the participation of hippocampal p38 mitogen-activated protein kinase in extinction and reacquisition of inhibitory avoidance memory. Neuroscience. 2006;143:15–23. doi: 10.1016/j.neuroscience.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Routtenberg A, Rekart JL. Post-translational protein modification as the substrate for long-lasting memory. Trends Neurosci. 2005;28:12–19. doi: 10.1016/j.tins.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Squire LR, Alvarez P. Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr Opin Neurobiol. 1995;5:169–177. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Thomas KL, Hall J, Everitt BJ. Cellular imaging with zif268 expression in the rat nucleus accumbens and frontal cortex further dissociates the neural pathways activated following the retrieval of contextual and cued fear memory. Eur J Neurosci. 2002;16:1789–1796. doi: 10.1046/j.1460-9568.2002.02247.x. [DOI] [PubMed] [Google Scholar]
- Thomas KL, Arroyo M, Everitt BJ. Induction of the learning and plasticity-associated gene Zif268 following exposure to a discrete cocaine-associated stimulus. Eur J Neurosci. 2003;17:1964–1972. doi: 10.1046/j.1460-9568.2003.02617.x. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- Wang SH, Ostlund SB, Nader K, Balleine BW. Consolidation and reconsolidation of incentive learning in the amygdala. J Neurosci. 2005;25:830–835. doi: 10.1523/JNEUROSCI.4716-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuravin IA, Bures J. Extent of the tetrodotoxin blockade examined by papillary paralysis elicited by intracerebral infection of the drug. Exp Brain Res. 1991;83:687–690. doi: 10.1007/BF00229849. [DOI] [PubMed] [Google Scholar]