Abstract
Xanthohumol (XN), a prenylated chalcone present in hops (Humulus lupus L.) and beer, exhibits anti-inflammatory, antioxidant and antiproliferative activity, but has not been studied for effects on T cell-mediated immune responses. Here we demonstrate that XN has profound immunosuppressive effects on T cell proliferation, development of IL-2 activated killer (LAK) cells, cytotoxic T lymphocytes (CTLs), and production of Th1 cytokines (IL-2, IFN-γ and TNF-α). The suppression of these cell-mediated immune responses by XN was at, least in part, due to the inhibition of nuclear factor kappa B (NF-κB) transcription factor through suppression of phosphorylation of IκBα, an inhibitor of NF-κB.
Keywords: Xanthohumol, immunomodulation, cytotoxic T lymphocytes, cytokines, NF-κB
INTRODUCTION
Epidemiological and laboratory studies suggest that diet plays an important role in prevention of human diseases 1,2. Long-term consumption of plant derived foods including vegetables, fruits, beans, and nuts has been linked to a low incidence of cancer, coronary heart disease and inflammatory diseases 3,4. The disease-preventing effects of these foods are attributed in part to the presence of bioflavonoids, a group of naturally occurring polyphenolic compounds with strong antioxidant and anti-inflammatory properties as well as their ability to detoxify carcinogens and modulate a range of cell signaling pathways involved in cellular proliferation, differentiation, survival and apoptosis 5.
Hops or `hop cones', the female inflorescence of the hop plant, Humulus lupus L., are widely used in beer brewing to add bitterness and flavor to beer. Hop extract contains polyphenolic acids, prenylated chalcones, flavonoids, catechins and proanthocyanidins 6,7. Xanthohumol (3-[3,3-dimethyl allyl]-2,4,4-trihydroxychalcone) is the principal prenylated flavonoid found in hop resin (lupulin). Several studies have reported on the potential health benefits of xanthohumol (XN). XN was shown to inhibit cytochrome P-450 CYP enzymes involved in metabolic activation of carcinogens and increase the activity of phase 2 enzymes that detoxify carcinogens 8–10. XN inhibited the growth of a wide variety of human cancer cell lines including breast, colon, prostate, ovarian and blood cancers by inhibiting proliferation and inducing apoptosis 11–15. In other studies, XN inhibited the tumor cell invasion and angiogenesis 16–18 and the activity of diacyltransferase, topoisomerase, and aromatase 19–21.
In contrast to the potent biological effects on cancer cells, very little is known about the effects of XN on cells of the immune system. In two studies, XN was shown to inhibit the expression of proinflammatory iNOS, IL-1β and TNF-α in activated RAW264.7 macrophages 22, 23. Depending on the activation signal, XN inhibited these mediators of inflammation by either inhibiting NF-κB or STAT-1α and IRF-1 activation 23. To the best of our knowledge, the effect of xanthohumol on T cell-mediated immune responses that play critical roles in immunity against tumors and viral infections, pathogenesis of autoimmune diseases, and rejection of transplants has not been studied. Thus, in this study, we investigated the immuno-modulatory effects of XN on the development of T cell-mediated immune responses in mouse splenic T cells. Our results showed for the first time that XN inhibits mitogen/antigen-induced T cell proliferation, development of cell-mediated cytotoxocity and production of Th1 cytokines by inhibiting NF-κB through the suppression of phoshorylation of IκBα, an inhibitor of NF-κB.
MATERIALS AND METHODS
Agents
Xanthohumol was purchased from Alexis Biochemicals (San Diego, CA). Mouse interleukin-2 (mIL-2) (2.5×108 U/mg) and concanavalin A (Con A) were purchased from Sigma Chemical Co. (St. Louis, MO). Anti-NF-κB (p65) and anti-phospho-IκBα antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). A 100 mM stock solution of XN was prepared in DMSO and all test concentrations were prepared by diluting the appropriate amount of stock solution in tissue culture medium.
Mice
Eight to 10-wk-old male C3H (H-2k) and C57 BL/6J (H-2b) mice were purchased from Charles River, NCI (Frederickberg, MD). Mice consumed Breeder Diet (W) 8626 (protein, 20.0%; fat, 10.0%; and fiber, 3.0%) and water ad libitum. Mice were housed for at least one week before experimental use. All animal protocols were approved by the Institutional Animal Care and Use Committee.
Tissue culture medium
All in vitro cell cultures were carried out in RPMI-1640 medium (Grand Island Biological Company, Grand Island, NY), supplemented with 10% fetal calf serum (Hyclone, Logan, UT), 1% penicillin/streptomycin, 25 mmol/L HEPES buffer, and 5×10−5 M 2-mercaptoethanol. Hereafter, this medium will be referred to as complete RPMI-1640 medium.
Preparation of spleen cells
Mice were euthanized by CO2 inhalation and spleens were removed aseptically. Spleens were placed in cold phosphate buffered saline (PBS) and teased apart with a pair of forceps and a needle. Single-cell suspension from the teased tissue was obtained by passing it through a 22 G needle. Cells were washed two times in cold PBS and finally resuspended in complete RPMI-1640 medium.
Isolation of T cells
Splenic cells were enriched for T cells by filtering through nylon-wool columns as described elsewhere 24. Briefly, 2–3 ×108 spleen cells were loaded on a column made by packing 3 g acid-washed nylon wool in a 50 ml syringe. Columns were incubated at 37 °C for 45 minutes. After incubation, nonadherent cells were eluted with warm complete RPMI-1640 tissue culture medium. Flow cytometric analysis showed that >95% of these nylon wool nonadherent cells were Thy 1.2 positive, a cell surface marker for murine T lymphocytes.
3H-thymidine incorporation assay
To determine the effect of XN on proliferation of lymphocytes, 2×105 splenic T cells were cultured in 0.2 ml of RPMI-1640 in a 96-well microtiter tissue culture plate in the absence or presence of Con A (1 μg/ml) or mIL-2 (100 ng/ml) or irradiated (20 Gy) allogeneic spleen cells (1:1 ratio) as stimulators. XN was added to the cultures in concentrations as described in the individual experiments. After incubation for 3 or 4 days at 37°C, 95% humidity, and 5% CO2, 0.25 μCi of 3H-thymidine in 20 μl of PBS was added to each well and plates were incubated for additional 18 h. Cultures were harvested with an automatic cell harvester using distilled water. The amount of radioactivity incorporated into DNA was determined by liquid scintillation.
Generation of cytotoxic T lymphocytes (CTLs)
For the generation of alloantigen specific CTLs, 107 splenic T cells of C3H/HeN (responders) mice and an equal number of irradiated (20 Gy) allogeneic spleen cells of C57BL/6 (stimulators) mice were cultured in 10 mL of complete RPMI-1640 tissue culture medium. XN was added to the cultures at concentrations ranging from 2.5 to 40 μM. After incubation for 5 days, cells were harvested and viability determined. Cells were tested for cytotoxicity against 51Cr labeled EL-4 lymphoma cells of C57BL/6 origin as target cells in a 4 h 51Cr release assay.
Generation of lymphokine activated killer (LAK) cells
For the generation of nonspecific cytotoxic LAK cells, C3H spleen cells (5×106 cells/5ml) were cultured in medium alone or in the medium containing IL-2 (150 ng/ml). XN was added to the cultures at concentrations ranging from 2.5 to 40 μM. After incubation for 72 h, cells were harvested, viability determined, and tested for cytotoxicity against YAC-1 target cells in 4 h 51Cr release assay.
Cytotoxicity assay
Target cells were resuspended at 1×107 cells/mL RPMI-1640 and 100 μCi of sodium 51chromate was added to cells. Cells were incubated for 90 min at 37°C. Following incubation, cells were washed three times in PBS to remove unbound radioactivity. The effector and labeled target cells were adjusted to desired cell concentrations and added to wells of a U bottomed 96 well microtiter plate in triplicate to obtain effector:target (E:T) ratios of 100:1 to 12.5:1. For maximum releasable radioactivity, target cells were lysed in 1% SDS solution. For spontaneous release of radioactivity, target cells were incubated in medium alone. Plates were centrifuged at 800 rpm for 2 min and incubated at 37°C for 4 h. 100 μl of the supernatant from each well was removed and the amount of radioactivity released was measured in a gamma scintillation counter. Percent cytotoxicity was determined as: experimental release (cpms) - spontaneous release (cpms)/ maximum release (cpms) - spontaneous release (cpms) × 100.
Cytokine measurement
To determine the effect of XN on cytokine production, splenic T cells (1×106/ml) were pretreated with XN at concentrations of 2.5 to 40 μM for 18 h and then stimulated with Con A (1 μg/ml) for 24 h. Control cells were either not treated at all or treated with Con A only. Culture supernatants were collected by centrifugation and levels of IL-2, IFN-γ and TNF-α were measured using cytokine-specific ELISA kits from eBioscience (San Diego, CA) according to instructions provided by the vendor.
Western blot analysis of NF-κB and IκBα
Preparation of nuclear extract and total cell lysate for Western blot analysis of NF-κB and IκBα, respectively was carried out as described elsewhere 25. For nuclear extract, splenic T cells treated or not with XN for 18 h were stimulated with Con A (1 μg/ ml) for 90 minutes. Cells were then collected and incubated on ice for 15 minutes in hypotonic buffer (10 mM HEPES, pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 0.5 mM PMSF, and 0.6% NP-40). Cells were vortexed gently and nuclei were separated from the cytosol by centrifugation at 12,000 × g for 1 minute. Nuclei were resuspended in buffer containing 20 mM HEPES, pH 7.9, 25% glycerol, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.5 mM PMSF and shaken for 30 minutes at 4°C. Nuclear extract was obtained by centrifugation at 12,000 × g for 10 minutes. Cellular lysates were prepared by detergent lysis (1% Triton-X 100 (v/v), 10 mM Tris-HCl (pH 7.5), 5 mM EDTA, 150 mM NaCl, 10% glycerol, 2 mM sodium vanadate, 5 μg/mL leupeptin, 1 μg/mL aprotinin, 1 μg/mL pepstatinin, and 10 μg/mL 4-2-aminoethyl-benzenesulfinyl fluoride). Lysates were clarified by centrifugation at 14,000 × g for 10 min at 4°C. Protein concentration in extracts was measured by Bradford assay (Bio-Rad, Richmond, CA). Extracts were fractionated on 12% SDS-PAGE gel, transferred to nitrocellulose membrane. Nuclear extract and cell lysate membranes were probed with anti-NF-κB (p65) and anti-IκBα antibody, respectively. Signal bands were visualized using enhanced chemiluminescence detection system.
Statistical analysis
Data are expressed as mean ± SD. The difference between control and treatment groups was determined using Dunnett multiple comparison test. Differences with p<0.05 were considered statistically significant.
RESULTS
Antiproliferative effects of xantohumol
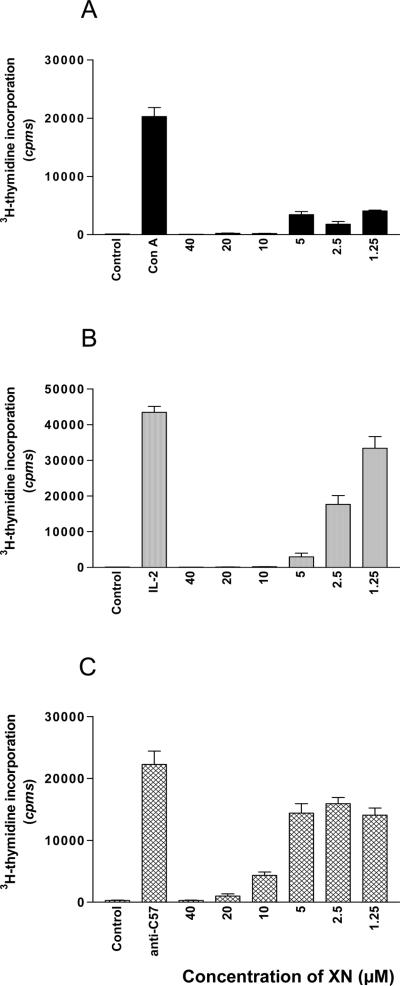
The antiproliferative activity of XN for splenic T lymphocytes was measured by 3H-thymidine incorporation. XN ranging in concentration from 1.25 to 40 μM was added at the initiation of cultures. The results presented in Fig. 1 demonstrate the effect XN on Con A (A), IL-2 (B), and alloantigen (C) induced proliferation of splenic T lymphocytes. Con A-induced proliferative response was completely blocked by XN at 10, 20 and 40 μM and remained significantly suppressed at1.25 to 5 μM of XN (Fig. 1A). XN also inhibited the IL-2 induced proliferation of splenic T cells (Fig. 1B). The antiproliferative effect was most pronounced at 5 to 40 μM XN with 30 to 70% recovery of proliferation at concentrations of 2.5 and 1.25 μM, respectively compared to IL-2 induced proliferation in the absence of XN. XN effectively inhibited the alloantigen induced proliferation of splenic T cells (C3H anti C57BL/6) at 10, 20 and 40 μM; however it only modestly reduced the proliferation at concentrations of 1.25 to 5 μM. These data demonstrated that XN is a potent inhibitor of mitogen/cytokine (Con A and IL-2) induced proliferation of T cells at a low concentration of 2.5 μM but a higher concentration of at least 10 μM XN was required for significant suppression of alloantigen-induced T cell proliferation.
Figure 1. Effect of XN on proliferation of splenic T lymphocytes.
C3H/HeN splenic T cells (2×105 cells/well) were stimulated with Con A (1 μg/mL) (A) or IL-2 (100 ng/mL) (B) or 2×105 C57BL/6 spleen cells (irradiated) in triplicate in 96-well microtiter tissue culture plates for 72 h in the absence or the presence of XN (1.251– 40 μM). Cultures were pulsed with 3H-thymidine (0.25 μCi/well) for 18 h and 3H-thymidine incorporation was determined by liquid scintillation spectrometry. Data are presented as mean (cpm) ± S.D. of 3 to 4 experiments.
The inhibitory effect of XN on proliferation of T cells was not due to DMSO used for dissolving XN, since equivalent concentrations of DMSO alone had no effect on T cell proliferation (not shown).
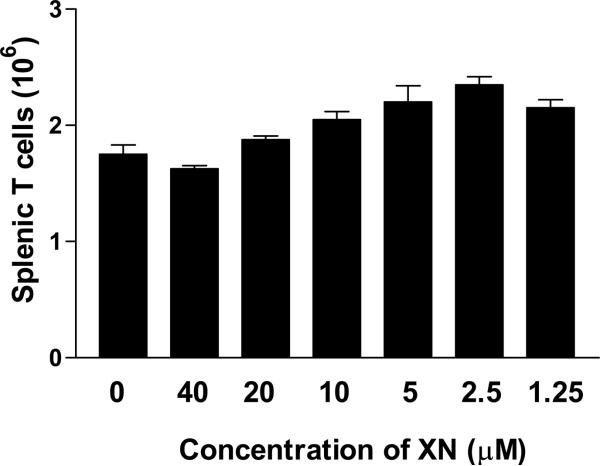
Effect of XN on T cell viability
In order to determine that the antiproliferative activity of XN was not due the cytotoxic effect on T cells, we evaluated the viability of T cells treated with XN. For this purpose, 2.5×106 splenic T cells in one milliliter of complete RPMI-1640 were cultured with or without XN (1.25–40 μM). After incubation for 72 h, the viable cell count of cultures was determined using trypan blue dye exclusion method. As shown in Figure 2, there was about 30% reduction in the number of viable T cells cultured in medium alone compared to the number of cells at the initiation of cultures (1.75×106 versus 2.5×106 cells). The viable cell count in cultures treated with 40 and 20 μM XN was comparable to untreated cultures (e.g., 1.62×106 and 1.87×106 versus 1.75×106 cells). There was slight increase in viable T cells in cultures treated at 10, 5, 2.5 or 1.25 μM XN (e.g., 2.05×106, 2.20×106, 2.35×106 and 2.15×106 cells, respectively). This result demonstrated that XN in concentration range of 1.25 to 40 μM is not toxic to T cells.
Figure 2. Effect of XN on the viability of T lymphocytes.
2.5×106 splenic T cells were cultured in one milliliter of complete RPMI-1640 in the absence or presence of XN (1.25–40 μM). After incubation for 72 h, cell viability was determined by trypan blue dye exclusion using a hemocytometer. Data presented are mean ± S.D. of viable cells in two experiments.
XN inhibit the development of cell-mediated cytotoxicity
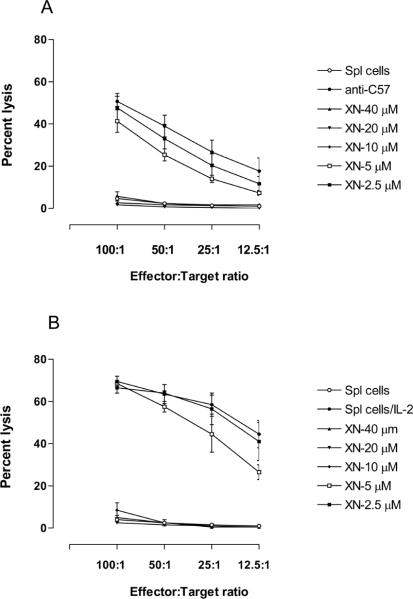
The suppression of mitogen/alloantigen induced proliferation of splenic lymphocytes suggested that XN might also affect the development of cell-mediated cytotoxic responses. In order to test this, we examined the effect of XN on generation of broadly non-specific IL-2 induced cytotoxic cells (LAK cells) and alloantigen specific cytotoxic T lymphocytes (CTLs). For effect on the generation of LAK cells, spleen cells were stimulated with IL-2 for three days in the presence or the absence of XN and the cytotoxic activity LAK cells was determined against YAC-1 target cells. As shown in Fig. 3A, the generation of LAK cell-mediated cytotoxicity was drastically reduced at concentrations of 10 to 40 μM (2% to 8% cytotoxicity of XN treated cells versus 69% cytotoxicity of control LAK cells), but was not affected at 2.5 and 5 μM XN. For effect on the generation of CTLs, splenic T cells from C3H/HeN mice (H-2k) were incubated with spleen cells (irradiated) of C57BL/6 (H-2b) mice for five days in the absence or the presence of XN. The cytolytic activity of effector cells against EL-4 lymphoma cells of C57BL/6 origin as target cells. CTL generation was completely abrogated by XN at concentrations of 10 to 40 μM (2% to 6% cytotoxicity versus 51% control cytotoxicity), but was only modestly reduced at 5 μM (41% cytotoxicity) and insignificantly affected at 2.5 μM XN (Fig. 3B). These data demonstrated that the generation of both broadly non-specific LAK cells and alloantigen specific CTLs is significantly inhibited by XN at concentrations of 10 μM and above; however, these immune responses were only modestly affected at lower concentrations of XN.
Figure 3. Effect of XN on the development of LAK and CTLs.
A. For effect on LAK cells, C3H/HeN spleen cells (5×106/ml) were incubated with IL-2 (150 ng/ml) in the absence or presence of XN (2.5–40 μM) for 72 h to generate LAK cells. Cytotoxicity of LAK cells against YAC-1 target cells was measured in a 4 h 51Cr-release assay at different effector: target (E:T) ratios. Each panel represents mean ± S.D. percent cytotoxicity of 3 experiments.
B. For the effect on the generation of CTLs, 1× 107 C3H/HeN splenic T cells were co-cultured with an equal number of irradiated C57BL/6 spleen cells in 10 ml of RPMI-1640 culture medium in the absence or presence of XN (2.5–40 μM). After incubation for 5 days, cells were harvested and tested for cytotoxicity against EL-4 lymphoma cells of C57BL/6 origin in a 4 h 51Cr-release assay at different effector: target (E:T) ratios. Each panel represents mean ± S.D. percent cytotoxicity of 3 experiments.
In these experiments also the suppression of cytotoxic responses by XN was not due to DMSO used for dissolving XN, since equivalent amounts of DMSO alone showed no effect on the generation of these responses.
XN inhibits Th1 cytokines
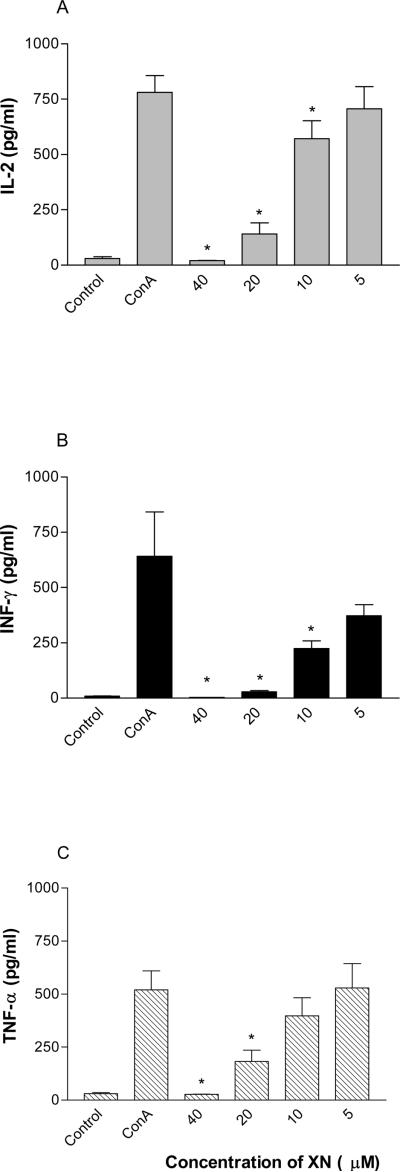
Type 1 cytokines (IL-2, IFN-γ and TNF-α) produced by Th1 helper cells play a critical role in the development of cell-mediated immune responses including T cell proliferation and the generation of CTLs. The suppression of these T cell immune responses by XN suggested that it might also impair the production type 1 cytokines. To evaluate the effect of XN on production of IL-2, IFN-γ and TNF-α, splenic T cells were treated with XN for 18 h before stimulating them with Con A for 24 h. The amounts of these cytokines in culture supernatants were measured by cytokine-specific ELISA. As shown in Fig. 4 A, B and C, stimulation of T lymphocytes with Con A significantly induced the production of IL-2, IFN-γ and TNF-α compared to unstimulated cells (IL-2: 37±3 Vs 780±74 pg/ml; IFN-γ: 33±5 Vs 827±129 pg/ml; TNF-α: 28±4 Vs 629±19 pg/ml). IL-2 production (Fig. 4 A) was significantly inhibited at XN concentrations of 40, 20 and 10 μM (19±2, 191±11 and 571±80 pg/ml, respectively), but was only slightly reduced at 5 μM XN (706±100 pg/ml). IFN-γ production (Fig. 4 B) was significantly inhibited at all concentrations of XN tested, i.e., 40, 20, 10 and 5 μM (27±3, 33±6, 220±7 and 392±43 pg/ml, respectively). The effect of XN on TNF-α production (Fig. 4 C) was similar to that on IL-2 production, e.g., significantly reduced at 40, 20 and 10 μM (28±2, 107±15 and 424±56 pg/ml, respectively) with no change in production at 5 μM (616±14 pg/ml). Thus, XN is an effective inhibitor of type 1 cytokine production.
Figure 4. Effect of XN on cytokine production.
C3H/HeN splenic T cells (1×106/mL) were pretreated with XN (5–40 μM) for 18 h and then stimulated with Con A (1 μg/mL) for 24 h. Control cells were either not treated or treated with Con A alone. Culture supernatants were collected and the amounts of IL-2, IFN-γ, TNF-α were measured using commercially available cytokine specific ELISA kits. Data are presented as mean (cpm) ± S.D. of 3 experiments.
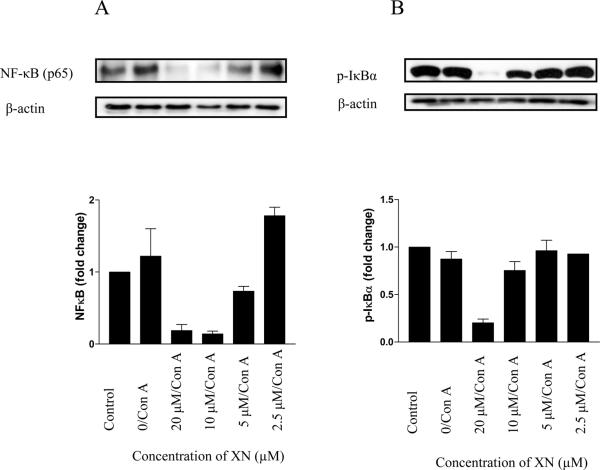
XN inhibits NF-κB through suppression of IkBα phosphorylation
Because transcription factor NF-κB has been shown to control the expression of a number of genes related to cell proliferation and cytokine production, we examined the effect of XN on nuclear NF-κB levels and phosphorylation of IκBα, an inhibitor of NF-κB. For this purpose, splenic T lymphocytes were treated or not with XN for 18 h before stimulation with Con A for 90 min. Nuclear extract and total cellular lysate were analyzed by immunoblotting for NF-κB and IκBα, respectively. As shown in Figure 5 A, treatment with Con A increased the level of NF-κB in the nucleus. On the other hand, both the basal and induced levels of NF-κB were dramatically reduced by XN at concentrations of 5 μM and completely abolished at 10 and 20 μM XN. Compared to normal cells, the level of p-IκBα was not altered in cells treated with Con A; however, p-IκBα was significantly to completely inhibited in cells treated with 10 and 20 μM XN. It was only slightly reduced at 5 μM XN. This result demonstrated that the inhibition of T cell responses by XN is attributable at least in part to the inhibition of NF-κB through suppression of IκBα phosphorylation and its degradation.
Figure 5. Effect of XN on NF-κB activation and p-IκBα.
107 C3H/HeN splenic T cells were pretreated with XN (2.5–20 μM) for 18 h before stimulating with Con A (1 μg/mL) for 90 min. Control cells were either not treated or stimulated with Con A without pretreatment with XN. Nuclear and cellular lysates were prepared from control and treated cells and analyzed for NF-κB and p-IκBα, respectively by immunoblotting using anti-NF-κB (p65) (A) or anti-p-IκBα antibody (B) probes. Histograms depict fold-change in expression of NF-κB (p65) and p-IκBα. Similar results were obtained in 3 independent experiments.
DISCUSSION
Natural products have been used in traditional medicine for centuries to treat a variety of human diseases. Hop cones are used as sedatives, antispasmodic, bitter stomachic and microbicidal in folk medicine. Recent laboratory studies have shown that flavonones and chalcones with prenyl and geranyl groups present in hops have numerous biological effects, including chemopreventive, antiproliferative, antioxidant and anti-inflammatory activities 8,11,12,22,23,26. However, the immunomodulatory effects of the active principals of hops are less well known. Since most of the biological effects of hops are attributed to xanthohumol, we investigated the effects of XN on the development of T cell-mediated immune responses, such as mitogen/antigen induced T cell proliferation, cell-mediated cytolysis and production of cytokines that together play a crucial role in the pathogenesis of chronic inflammatory disorders, viral infections and rejection of transplants and tumors. XN inhibited the Con A, IL-2 and alloantigen induced proliferation of splenic T lymphocytes. Although the antiproliferative activity of XN for T cells has not been reported before, the inhibition of T cell proliferation by XN corroborates the results of other studies in which XN was shown to inhibit the proliferation of cancer cells 11,12. Ribonucleotide reductase and DNA polymerase are two key enzymes involved in DNA synthesis as well as processes that are essential to allow cells to progress through the S phase of the cell cycle. In MDA-MB-435 breast cancer cell, XN suppressed DNA synthesis by inhibiting DNA polymerase α and arrested cells in the S phase 8. Whether XN targets ribonucleotide reductase or DNA polymerase in suppressing T cell proliferation has not been established yet.
Cytotoxic T lymphocytes play an important role in protection against viral infections and cancer and rejection of organ transplants. The development of cytotoxic T cells is a complex process involving antigen induced activation of Th1 helper cells and production of essential cytokines needed for the differentiation and maturation of precursors of cytotoxic T cells 27. We investigated the effect of XN on both the generation of cell-mediated cytotoxicity and production of cytokines. The generation of IL-2 induced non-specific LAK cells as well as alloantigen specific CTLs was markedly reduced by XN. These results demonstrate for the first time the ability of XN to inhibit the development of cytotoxic cells. The CTL inhibitory effect of XN correlated with the inhibition of Th1-type cytokine production. IL-2, IFN-γ and TNF-α produced by Th1 cells are required for the induction of T cell responses. Pretreatment of splenic T cells with XN inhibited the production of IL-2, IFN-γ and TNF-α. Thus, the inhibition of these cytokines by XN provides an insight into the mechanism by which XN inhibits the generation of cytotoxic T cells and other T cell-mediated immune responses.
The NF-κB family of transcription factors is composed of homodimers or heteodimers of Rel proteins that regulate a large number of genes related to immune responses, inflammation, cell proliferation, survival and apoptosis 28. Among several distinct signaling cascades, the recognition of MHC-antigen peptide complex by T-cell receptor/CD3 complex in conjunction with costimulatory receptors initiates activation of NF-κB signaling pathway, which is essential for antigen-induced T cell proliferation, cytokine production and the development of T cell-mediated immune responses 29. Others have shown that XN inhibits NF-κB and Akt signaling pathways in cancer cells 13,17. We considered the possibility that the suppression of lymphocyte proliferation, CTL development, and cytokine production by XN might also result from inhibition of NF-κB. XN inhibited the nuclear levels of NF-κB in splenic T cells stimulated with Con A. In resting cells, NF-κB remains sequestered in the cytoplasm bound to IκBα 28. Uponstimulation of cells with mitogens, antigens, or cytokines, IκBα is phosphorylated and degraded, allowing NF-κB to translocate to the nucleus where it binds to κB motifs in promoter region of the responsive genes. XN reduced the levels of phosphorylated IκBα, suggesting that NF-κB is retained in the cytoplasm in cells treated with XN. The inhibition of NF-κB through suppression of the phosphorylation of IκBα in splenic T lymphocytes is consistent with the results of a previous report in which XN was shown to inhibit the phosphorylation of IκBα 30. Thus, suppression of proliferation of T cells, generation of cytotoxic effector cells, and production of cytokines by XN may result from inhibition of NF-κB responsive genes involved in the development of these immune responses.
CONCLUSION
Our data demonstrate that xanthohumol is a potent inhibitor of mitogen and alloantigen induced T cell proliferation, generation of cytotoxic cells (LAK and CTLs) and production of Th1 type cytokines. NF-κB appears to be part of the mechanism by which XN suppresses these T cell responses. Thus, XN may be a useful agent for preventing the rejection of organ transplants and treatment of T cell-mediated inflammatory conditions.
ACKNOWLEDGEMENTS
This work was supported by NIH grant CA130948-01 to S.C.G.
REFERENCES
- 1.Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr. Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 2.Willett WC. Diet and health: what should we eat? Science. 1994;264:532–537. doi: 10.1126/science.8160011. [DOI] [PubMed] [Google Scholar]
- 3.Kuo SM. Dietary flavonoids and cancer prevention: Evidence and potential mechanism. Crit Rev Oncol. 1997;8:47–69. doi: 10.1615/critrevoncog.v8.i1.30. [DOI] [PubMed] [Google Scholar]
- 4.Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Rosner BA, Hennekens CH, Willett WC. Dietary fat intake and the risk of coronary heart disease in women. N. Engl. J. Med. 1997;337:1491–1499. doi: 10.1056/NEJM199711203372102. [DOI] [PubMed] [Google Scholar]
- 5.Surh Y. Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat. Res. 1999;428:305–327. doi: 10.1016/s1383-5742(99)00057-5. [DOI] [PubMed] [Google Scholar]
- 6.Stevens JF, Taylor AW, Deinzer ML. Quantitative analysis of xanthohumol and related prenylflavonoids in hops and beer by liquid chromatography-tandem mass spectrometry. J. Chromatogr A. 1999;832:97–107. doi: 10.1016/s0021-9673(98)01001-2. [DOI] [PubMed] [Google Scholar]
- 7.Stevens JF, Page JE. Xanthohumol and related prenylflavonoids from hops and beer: to your good health! Phytochemistry. 2004;65:1317–1330. doi: 10.1016/j.phytochem.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 8.Gerhauser C, Alt A, Heiss E, Gamal-Eldeen A, Klimo K, Knauft J, Neumann I, Scherf HR, Frank N, Bartsch H, Becker H. Cancer chemopreventive activity of Xanthohumol, a natural product derived from hop. Mol. Cancer Ther. 2002;1:959–969. [PubMed] [Google Scholar]
- 9.Miranda CL, Aponso GL, Stevens JF, Deinzer ML, Buhler DR. Prenylated chalcones and flavanones as inducers of quinone reductase in mouse Hepa 1c1c7 cells. Cancer Lett. 2000;149:21–29. doi: 10.1016/s0304-3835(99)00328-6. [DOI] [PubMed] [Google Scholar]
- 10.Dietz BM, Kang YH, Liu G, Eggler AL, Yao P, Chadwick LR, Pauli GF, Farnsworth NR, Mesecar AD, van Breemen RB, Bolton JL. Xanthohumol isolated from Humulus lupulus inhibits menadione-induced DNA damage through induction of quinone reductase. Chem Res Toxicol. 2005;18:1296–1305. doi: 10.1021/tx050058x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miranda CL, Stevens JF, Helmrich A, Henderson MC, Rodriguez RJ, Yang YH, Deinzer ML, Barnes DW, Buhler DR. Antiproliferative and cytotoxic effects of prenylated flavonoids from hops (Humulus lupulus) in human cancer cell lines. Food Chem Toxicol. 1999;37:271–285. doi: 10.1016/s0278-6915(99)00019-8. [DOI] [PubMed] [Google Scholar]
- 12.Delmulle L, Bellahcene A, Dhooge W, Comhaire F, Roelens F, Huvaere K, Heyerick A, Castronovo V, De Keukeleire D. Anti-proliferative properties of prenylated flavonoids from hops (Humulus lupulus L.) in human prostate cancer cell lines. Phytomedicine. 2006;13:732–734. doi: 10.1016/j.phymed.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Colgate EC, Miranda CL, Stevens JF, Bray TM, Ho E. Xanthohumol, a prenylflavonoid derived from hops induces apoptosis and inhibits NF-kappaB activation in prostate epithelial cells. Cancer Lett. 2006;246:201–209. doi: 10.1016/j.canlet.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Lust S, Vanhoecke B, Janssens A, Philippe J, Bracke M, Offner F. Xanthohumol kills B-chronic lymphocytic leukemia cells by an apoptotic mechanism. Mol. Nutr. Food Res. 2005;49:844–850. doi: 10.1002/mnfr.200500045. [DOI] [PubMed] [Google Scholar]
- 15.Pan L, Becker H, Gerhauser C. Xanthohumol induces apoptosis in cultured 40–16 human colon cancer cells by activation of the death receptor- and mitochondrial pathway. Mol. Nutr. Food Res. 2005;49:837–843. doi: 10.1002/mnfr.200500065. [DOI] [PubMed] [Google Scholar]
- 16.Vanhoecke B, Derycke L, Van Marck V, Depypere H, De Keukeleire D, Bracke M. Antiinvasive effect of xanthohumol, a prenylated chalcone present in hops (Humulus lupulus L.) and beer. Int. J. Cancer. 2005;117:889–895. doi: 10.1002/ijc.21249. [DOI] [PubMed] [Google Scholar]
- 17.Albini A, Dell'Eva R, Vene R, Ferrari N, Buhler DR, Noonan DM, Fassina G. Mechanisms of the antiangiogenic activity by the hop flavonoid xanthohumol: NFkappaB and Akt as targets. FASEB J. 2006;20:527–529. doi: 10.1096/fj.05-5128fje. [DOI] [PubMed] [Google Scholar]
- 18.Monteiro R, Calchu R, Silva AO, Pinheiro-Silva S, Guerreiro S, Gartner F, Azevedo I, Soares R. Xanthohumol inhibits inflammatory factor production and angiogenesis in breast cancer xenografts. J. Cell Biochem. 2008;104:1699–1707. doi: 10.1002/jcb.21738. [DOI] [PubMed] [Google Scholar]
- 19.Tabata N, Ito M, Tomoda H, Omura S. Xanthohumols, diacylglycerol acyltransferase inhibitors, from Humulus lupulus. Phytochemistry. 1997;46:683–687. doi: 10.1016/s0031-9422(97)00157-x. [DOI] [PubMed] [Google Scholar]
- 20.Lee SH, Kim HJ, Lee JS, Lee IS, Kang BY. Inhibition of topoisomerase 1 activity and efflux drug transporter's expression by xanthohumol from hops. Arch. Pharm. Res. 2007;30:1435–1439. doi: 10.1007/BF02977368. [DOI] [PubMed] [Google Scholar]
- 21.Monteiro R, Faria A, Azevedo I, Calhau C. Modulation of breast cancer cell survival by aromatase inhibiting hop (Humulus lupus L.) flavonoids. J. Steroid Bioche. Mol. Biol. 2007;105:124–130. doi: 10.1016/j.jsbmb.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 22.Zhao F, Nozawa H, Daikonnya A, Kondo K, Kitanaka S. Inhibitors of nitric oxide production from hops (Humulus lupulus L.) Biol Pharm Bull. 2003;26:61–65. doi: 10.1248/bpb.26.61. [DOI] [PubMed] [Google Scholar]
- 23.Cho YC, Kim HJ, Kim YJ, Lee KY, Choi HJ, Lee IS, Kang BY. Differential anti-inflammatory pathway by xanthohumol in IFN-gamma and LPS-activated macrophages. Int. Immunopharmacol. 2008;8:567–73. doi: 10.1016/j.intimp.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 24.Julius MH, Simpson E, Herzenberg LZ. A rapid method for the isolation of functional thymus derived murine lymphocytes. Eur. J. Immunol. 1973;3:645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- 25.Xu YX, Pindolia KR, Janakiraman N, Chapman RA, Gautam SC. Curcumin inhibits IL1α and TNF-α induction of AP-1 and NF-κB DNA-binding activity in bone marrow stromal cells. Hematopath. Mol. Hematol. 1997;11:49–62. [PubMed] [Google Scholar]
- 26.Miranda CL, Stevens JF, Ivanov V, McCall M, Frei B, Deinzer ML, Buhler DR. Antioxidant and prooxidant actions of prenylated and nonprenylated chalcones and flavanones in vitro. J. Agric. Food Chem. 2000;48:3876–3884. doi: 10.1021/jf0002995. [DOI] [PubMed] [Google Scholar]
- 27.Husmann LA, Bevan MJ. Cooperation between helper T cells and cytotoxic T lymphocytes precursors. Ann. NY. Acad. Sci. 1988;532:158–169. doi: 10.1111/j.1749-6632.1988.tb36335.x. [DOI] [PubMed] [Google Scholar]
- 28.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 29.Kane LP, Lin J, Weiss A. It's all Rel-ative: NF-κB and CD28 costimulation of T-cell activation. Trends Immunol. 2002;23:413–420. doi: 10.1016/s1471-4906(02)02264-0. [DOI] [PubMed] [Google Scholar]
- 30.Harikumar KB, Kunnumakkara AB, Ahn KS, Anand P, Krishnan S, Guha S, Aggarwal BB. Modification of the cysteine residues in I{kappa}B {alpha} kinase and NF-{kappa}B (p65) by xanthohumol leads to suppression of NF-{kappa}B-regulated gene products and potentiation of apoptosis in leukemia cells. Blood. 2008 doi: 10.1182/blood-2008-04-151944. (epub) [DOI] [PMC free article] [PubMed] [Google Scholar]