Abstract
Purpose
To determine the association between the characteristics of calf muscle hemoglobin oxygen saturation (StO2) and exercise performance in patients with intermittent claudication.
Methods
Thirty-nine patients with peripheral arterial disease limited by intermittent claudication were studied. Patients were characterized on calf muscle StO2 before, during, and after a graded treadmill test, as well as on demographic and cardiovascular risk factors, ankle/brachial index (ABI), ischemic window, initial claudication distance (ICD), and absolute claudication distance (ACD).
Results
Calf muscle StO2 decreased 72% from rest (55 ± 18% saturation; mean ± SD) to the minimum value (17 ± 19% saturation) attained 459 ± 380 seconds after the initiation of exercise. After exercise, recovery half-time of calf muscle StO2 was attained at 129 ± 98 seconds, whereas full recovery to the resting value was reached at 225 ± 140 seconds. After adjusting for sex, race, and grouping according to the initial decline constant in calf muscle StO2 during exercise, the exercise time to minimum calf muscle StO2 was correlated with the ischemic window (r = -0.493, p = 0.002), ICD (r = 0.339, p = 0.043), and ACD (r = 0.680, p < 0.001). Following treadmill exercise, the recovery half-time of calf muscle StO2 was correlated with the ischemic window (r = 0.531, p < 0.001), ICD (r = -0.598, p < 0.001), and ACD (r = -0.491, p = 0.003).
Conclusion
In patients limited by intermittent claudication, shorter ICD and ACD values are associated with reaching a minimum value in calf muscle StO2 sooner during treadmill exercise, and with having a delayed recovery in calf muscle StO2 following exercise.
Introduction
Peripheral arterial disease (PAD) is prevalent in 16 percent in the US population older than 55 years of age, and the symptom of intermittent claudication is prevalent in 5 percent.1 Intermittent claudication is ischemic muscular leg pain that occurs during ambulation when the peripheral circulation is inadequate to meet the metabolic requirement of the active leg musculature. Consequently, intermittent claudication leads to ambulatory dysfunction2-5 and a decline in daily physical activities.6
The ankle/brachial index (ABI) is a standard outcome measure to quantify vascular insufficiency in PAD patients.1,7 However, ABI is not a measure of exercise-mediated muscular ischemia, and is either modestly4,8,9 or poorly5,10,11 correlated with walking distances to onset and to maximal pain during standardized treadmill tests. The ankle systolic pressure measured following treadmill exercise is another standard outcome measure to assess vascular insufficiency,12-14 but it also does not provide real-time information on muscular ischemia during exercise. In contrast, near Infrared Spectroscopy (NIRS) is a relatively new, non-invasive technique that measures hemoglobin oxygen saturation (StO2) of the calf musculature during exercise.15-22 The measurement of calf muscle StO2 during ambulation provides insight into the balance between oxygen delivery and oxygen demand during exercise.
Calf muscle StO2 decreases more during exercise and recovers more slowly in patients with intermittent claudication than in controls.19,21,23,24 However, less is known about which characteristics of the exercise-mediated changes in calf muscle StO2 are related to walking distances during standardized treadmill exercise and to vascular insufficiency in patients with intermittent claudication. The purpose of this study was to determine the association between calf muscle StO2 characteristics and exercise performance in patients with intermittent claudication.
Methods
Subjects
Recruitment
Subjects between the ages of 50 and 90 years were evaluated in the General Clinical Research Center at the University of Oklahoma Health Sciences Center (HSC). Subjects were recruited by referrals from the HSC vascular clinic, as well as by newspaper advertisements. The procedures used in this study were approved by the Institutional Review Board at the University of Oklahoma HSC. Written informed consent was obtained from each subject prior to investigation.
Screening
Subjects with intermittent claudication secondary to vascular insufficiency were included in this study if they met the following criteria: (a) a history of intermittent claudication, (b) ambulation during a graded treadmill test limited by intermittent claudication,12 and (c) an ABI ≤ 0.90.1 Subjects were excluded from this study for the following conditions: (a) absence of PAD (ABI > 0.90), (b) inability to obtain an ABI measure due to non-compressible vessels, (c) asymptomatic PAD, (d) use of medications indicated for the treatment of intermittent claudication (cilostazol and pentoxifylline) within three months prior to investigation, (e) exercise tolerance limited by factors other than leg pain (e.g., severe coronary artery disease, dyspnea, poorly controlled blood pressure), (g) active cancer, renal disease, or liver disease, (h) calf skin fold > 50 mm because of potential interference with the light path of the NIRS probe from penetrating the subcutaneous tissue, and (i) pulse arterial oxygen saturation of the index finger < 95% because of the potential deleterious effect on calf muscle StO2 from poor pulmonary gas exchange. A total of 39 subjects with intermittent claudication were deemed eligible for this investigation, whereas 14 subjects were ineligible.
Measurements
Medical History, Physical Examination, and Anthropometry
Demographic information, height, weight, cardiovascular risk factors, co-morbid conditions, claudication history, blood samples, and a list of current medications were obtained from a medical history and physical examination at the beginning of the study. During the physical examination, arterial oxygen saturation was measured from the index finger using a standard pulse oximeter. Afterwards, subcutaneous fat over the medial gastrocnemius muscle was measured from a skin fold obtained by a trained technician using a Lange skin fold caliper according to standard guidelines,25 and waist and hip circumferences were recorded.25
Gardner Treadmill Test
Patients performed a progressive, graded treadmill protocol (2 mph, 0% grade with 2% increase every 2 minutes) until maximal claudication pain as previously described.12 The initial claudication distance (ICD), defined as the walking distance at which the patient first experienced pain, and the absolute claudication distance (ACD), defined as the walking distance at which ambulation could not continue due to maximal pain, were both recorded to quantify the severity of claudication. Exercise capacity was measured by oxygen uptake at peak exercise with a Medical Graphics VO2000 metabolic system. Using these procedures, the test-retest intraclass reliability coefficient is R = 0.89 for ICD,12 R = 0.93 for ACD12, and R = 0.88 for peak oxygen uptake.26 Additionally, ABI measures were obtained from the more severely diseased lower extremity before and 1, 3, 5, and 7 minutes after each treadmill test as previously described.12,13 The reduction in ankle systolic blood pressure following treadmill exercise from the resting baseline value was quantified by calculating the area under the curve (AUC), referred to as the ischemic window.27 Because the ischemic window is a function of both PAD severity as well as the amount of exercise performed, the ischemic window was divided by ACD to normalize the ischemic window per meter walked.
Hemoglobin Oxygen Saturation (StO2) of the Calf Musculature
Instrumentation
Calf muscle StO2 was measured before, during, and after exercise using a continuous-wave, NIRS spectrometer (InSpectra model 325; Hutchinson Technology, Inc, Hutchinson, MN), an optical cable attached to a 25-mm probe, InSpectra software (version 2.0), and a dedicated laptop computer. The non-invasive technique of NIRS uses specific, calibrated wavelengths of near infrared light to quantify the percentage of hemoglobin oxygen saturation in the microvasculature of the tissue below the NIRS probe, as well as a small contribution of intracellular myoglobin. The degree of light absorption is dependent on the amount of oxygen attached to hemoglobin in the arterioles, venules, and capillaries. Non-absorbed light is returned as an optical signal and analyzed to produce a ratio of oxygenated hemoglobin to total hemoglobin, expressed as percentage of StO2 saturation continuously displayed on the spectrometer interfaced to a laptop computer. Consequently, the NIRS technique is a measure of the balance between local oxygen delivery and oxygen demand.
Calibration and Measurements
Prior to the treadmill test, the InSpectra machine was calibrated against low (38%) and high (88%) reference standards of hemoglobin oxygen saturation by placing the NIRS probe inside low and high reference ports in an InSpectra System Check device. After successful calibration of the unit, the 25-mm NIRS probe was attached to the skin over the medial gastrocnemius muscle of the more severely affected leg using a double-sided adhesive light-excluding patch.16 The more severely affected leg was defined as the more symptomatic leg having the lower ABI value. Calf muscle StO2 values were obtained every 3.5 seconds throughout the testing procedures, and the data file was then saved for subsequent analyses to characterize the pattern of change in calf muscle StO2 during and after treadmill exercise.
StO2 Variables
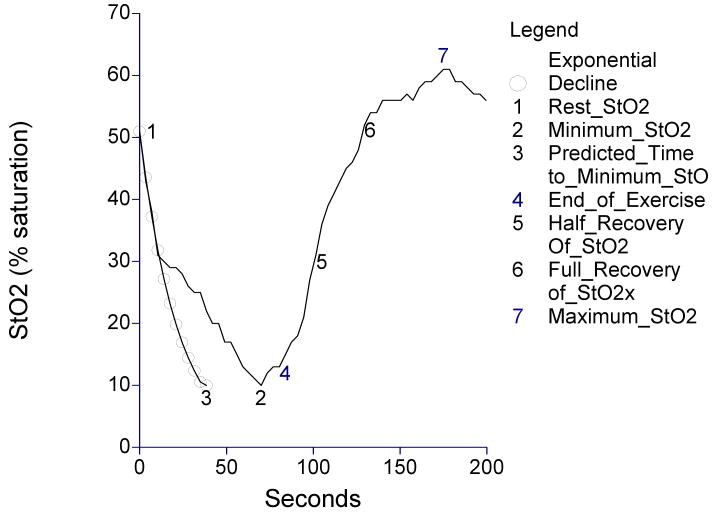
A number of calf muscle StO2 variables were obtained before, during, and after treadmill exercise, and are shown as different points on the curve in Figure 1. Prior to the treadmill test, a baseline measure of calf muscle StO2 was obtained at rest as patients stood on the treadmill for two minutes to allow for equilibration (point 1 shown on Figure 1). During treadmill exercise, the minimum StO2 value (point 2), the time taken to reach the minimum value (time between point 1 and point 2), the absolute and percentage drops in calf muscle StO2 from rest (point 1) to the minimum exercise value (point 2), and the average rate of decline from rest (point 1) to the minimum exercise value (point 2) were obtained.
Figure 1.
Calf muscle hemoglobin oxygen saturation (StO2) obtained before, during, and after treadmill exercise in a patient with intermittent claudication.
Additionally, an exponential of the form ekt was fitted to the data from the initial decline of StO2 from the resting value by estimating k as the slope of ln(StO2) regressed on time in seconds. Data for at least 15 seconds were included in the model for each subject with additional adjacent times included until a departure from linearity was indicated by an increase in root mean square error. The value obtained for k for each subject is referenced as the Initial Decline Constant (IDC). The resulting distribution of IDC's was distinctly bimodal with 34 (87%) subjects having IDC values between -0.0990 and -0.0035 sec-1, and 5 (13%) having values less than -0.1856 sec-1. For analysis purposes the larger group was referenced as IDC Group I and the smaller referenced as IDC Group II.
The time to reach minimum StO2 if the fitted IDC had been maintained throughout exercise (time between point 1 and point 3) was recorded as the predicted time to reach the minimum StO2. StO2 exercise time delay was calculated as the difference between the observed and predicted times to reach the minimum exercise StO2 value (time between point 2 and point 3). Thus, StO2 exercise time delay represents the time in which StO2 declined at a slower rate than the IDC until the minimum StO2 value was attained. The end of treadmill exercise was recorded (point 4), as well as the StO2 values during recovery that were one half of the resting value (point 5), the full resting value (point 6), and the maximal value (point 7). The recovery times for StO2 to reach one half of the resting StO2 value (recovery half-time, measured as the time between point 4 and point 5), the full resting StO2 value (full recovery time, measured as the time between point 4 and point 6), and the maximum StO2 value (time between point 4 and point 7) were calculated.
Statistical Analyses
Preliminary examination of data revealed that many of the variables of interest had appreciably different mean values for IDC Groups, for gender groups, and for Caucasian and African-American groups. To guard against these differences distorting correlation estimates, partial correlation coefficients were calculated by adjusting for these three dichotomous variables. Also, it was noted that the distributions for some of the variables were markedly asymmetric. To minimize the possible distorting effect of extreme values, both Pearson and Spearman partial correlation coefficients were computed for each pair of variables of interest, with Spearman used only if the two estimates differed by more than 0.1. All analyses were performed using the NCSS statistical package. Statistical significance was set at p < 0.05. Measurements are presented as means ± standard deviations.
Results
The clinical characteristics of the patients with intermittent claudication are shown in Table I. The group consisted of a similar proportion of men and women, as well as Caucasians and African-Americans. The ABI, ICD, ACD, and cardiovascular risk factors are typical for those with intermittent claudication. The calf muscle StO2 measures of the group are shown in Table II. On average, calf muscle StO2 decreased 72% from rest to the minimum value attained 459 seconds after the initiation of exercise. The predicted time to reach the minimum StO2 value was 126 seconds, and the StO2 exercise time delay was 333 seconds. At the completion of exercise, the time for calf muscle StO2 to increase to half of the resting value was 129 seconds, whereas full recovery was reached at 225 seconds. Calf muscle StO2 continued to increase to an average maximum value of 82% saturation 665 seconds after completion of exercise. The two IDC groups were similar on all variables shown in Tables I and II except that IDC group II (n = 5) had lower ABI (0.47 ± 0.11 vs. 0.66 ± 0.17, p = 0.015), higher ischemic window (0.87 ± 0.41 vs. 0.36 ± 0.31, p = 0.009), and shorter time delay in reaching the minimum exercise StO2 (0.14 ± 0.46 sec vs. 382.13 ± 328.07 sec, p = 0.002) than ICD group I (n = 34).
Table I.
Clinical characteristics of 39 peripheral arterial disease patients with intermittent claudication. Values are means (SD) and percentages.
| Variables | Values |
|---|---|
| Age (years) | 68 (10) |
| Weight (kg) | 84.6 (18.5) |
| Body Mass Index | 30.2 (7.4) |
| Ankle/Brachial Index | 0.64 (0.18) |
| Ischemic Window (min × mmHg / meter) | 0.48 (0.41) |
| ICD (meters) | 274 (178) |
| ACD (meters) | 480 (273) |
| Peak Oxygen Uptake (ml.kg-1.min-1) | 12.6 (4.3) |
| Sex (% Men) | 54 |
| Race (% Caucasian) | 49 |
| Current Smoking (% yes) | 38 |
| Diabetes (% yes) | 38 |
| Hypertension (% yes) | 87 |
| Dyslipidemia (% yes) | 87 |
| Obesity (% yes) | 44 |
ACD = absolute claudication distance, AUC = area under the curve, ICD = initial claudication distance,. Obesity was defined as having a body mass index ≥ 30 kg / m2.
Table II.
Measures of calf muscle hemoglobin oxygen saturation (StO2) in 39 peripheral arterial disease patients with intermittent claudication. Values are means (SD).
| Variables | Values |
|---|---|
| StO2 at rest (% saturation) | 55 (18) |
| Initial Decline Constant (sec-1) | - 0.052 (0.088) |
| Minimum exercise StO2 (% saturation) | 17 (19) |
| Predicted Time to minimum exercise StO2 (sec) | 126 (104) |
| Measured Time to minimum exercise StO2 (sec) | 459 (380) |
| Time Delay in reaching minimum exercise StO2 (sec) | 333 (332) |
| Absolute Drop in StO2 during exercise (% saturation) | 38 (18) |
| Percentage Drop in StO2 during exercise (%) | 72 (28) |
| Average Rate of Decline in StO2 from rest to minimum exercise value (% saturation/sec) | 0.306 (0.519) |
| Recovery Half-Time of StO2 (sec) | 129 (98) |
| Recovery Time of StO2 (sec) | 225 (140) |
| Recovery Time to Maximal StO2 (sec) | 665 (705) |
| Maximum recovery StO2 (% saturation) | 82 (20) |
The partial correlation coefficients between calf muscle StO2 measures and exercise performance measures are shown in Table III. After adjusting for sex, race, and IDC Groups, the measured exercise time to minimum calf muscle StO2 was negatively correlated with the ischemic window (p = 0.002), and was positively correlated with ICD (p = 0.043), and ACD (p < 0.001). The StO2 exercise time delay was negatively correlated with ischemic window (p = 0.004), and positively correlated with ABI (p = 0.024) and ACD (p < 0.001). Additionally, the minimum StO2 value attained during exercise was positively correlated with ICD (p = 0.015), the IDC was positively associated with ACD (p = 0.048), and the average rate of decline in calf muscle StO2 during exercise was positively correlated with the ischemic window (p = 0.004) and negatively associated with ACD (p < 0.001).
Table III.
Association between measures of calf muscle hemoglobin oxygen saturation (StO2) and exercise performance in 39 peripheral arterial disease patients with intermittent claudication.
| Variables | ABI | IW | ICD | ACD |
|---|---|---|---|---|
| StO2 at rest | 0.059 | 0.005 | 0.294 | 0.043 |
| Initial Decline Constant (IDC) | -0.052 | -0.287 | 0.172 | 0.331 * |
| Minimum exercise StO2 | 0.167 | -0.004 | 0.403 * | 0.136 |
| Predicted Time to minimum exercise StO2 | -0.206 | -0.070 | -0.205 | 0.030 |
| Measured Time to minimum exercise StO2 | 0.289 | -0.493 ** | 0.339 * | 0.680 *** |
| Time Delay in reaching minimum exercise StO2 | 0.376 * | -0.464 ** | 0.261 | 0.659 *** |
| Absolute Drop in StO2 during exercise | 0.105 | 0.066 | -0.150 | -0.010 |
| Relative Drop in StO2 during exercise | -0.202 | 0.016 | -0.475 ** | -0.157 |
| Average Rate of Decline in StO2 from rest to minimum exercise value | -0.258 | 0.471 ** | -0.172 | -0.583 *** |
| Recovery Half-Time of StO2 | -0.316 | 0.531 *** | -0.598 *** | -0.491 ** |
| Recovery Time of StO2 | -0.280 | 0.461 ** | -0.540 *** | -0.475 ** |
| Recovery Time to Maximal StO2 | -0.161 | 0.275 | -0.454 ** | -0.261 |
| Maximum recovery StO2 | 0.229 | 0.029 | 0.448 ** | 0.145 |
Values are either Pearson partial correlation coefficients or Spearman partial correlation coefficients (indicated in italicized print) adjusted for sex, race, and IDC Group.
p < 0.05,
p < 0.01,
p < 0.001.
Following treadmill exercise, the recovery half-time of calf muscle StO2 was positively correlated with the ischemic window (p < 0.001), and negatively correlated with ICD (p < 0.001), and ACD (p = 0.003). Similarly, the recovery time of calf muscle StO2 to reach the resting value was positively correlated with the ischemic window (p = 0.007), and negatively associated with ICD (p < 0.001), and ACD (p = 0.005). The recovery time to maximal calf muscle StO2 was negatively correlated with ICD (p = 0.006), and the maximal calf muscle StO2 value was positively correlated with ICD (p = 0.008, respectively).
The partial correlation coefficients between measures of ABI and ischemic window with exercise performance are shown in Table IV. ABI measurements obtained at rest and after treadmill exercise were positively associated with ICD and ACD (p < 0.05), and the ischemic window was negatively correlated with ICD (p < 0.05) and ACD (p < 0.001).
Table IV.
Association between measures of ankle/brachial index and ischemic window with exercise performance in 39 peripheral arterial disease patients with intermittent claudication.
| Variables | ICD | ACD |
|---|---|---|
| Ankle/Brachial Index: Rest | 0.402 * | 0.438 ** |
| Ischemic Window | -0.451 ** | -0.673 *** |
| Ankle/Brachial Index: 1 min Recovery | 0.391 * | 0.425 ** |
| Ankle/Brachial Index: 5 minute Recovery | 0.450 ** | 0.442 ** |
| Ankle/Brachial Index: 15 minute Recovery | 0.410 * | 0.464 ** |
Values are either Pearson partial correlation coefficients or Spearman partial correlation coefficients (indicated in italicized print) adjusted for sex, race, and IDC Group.
p < 0.05,
p < 0.01,
p < 0.001.
Discussion
The major findings of this investigation are that (1) shorter exercise time for calf muscle StO2 to reach a minimum value during treadmill walking is associated with shorter ICD and ACD, and greater ischemic window after exercise, and (2) slower recovery in calf muscle StO2 following exercise is associated with shorter ICD and ACD, and greater ischemic window after exercise.
Change in Calf Muscle StO2 During Exercise
Although calf muscle StO2 declines during treadmill exercise in patients with intermittent claudication, eventually reaching a minimum plateau prior to the end of exercise,15,20 less attention has been given to the characteristics of the exercise-mediated decline. A key measure of calf muscle StO2 during exercise is the observed time to reach the minimum StO2 value. The time to minimum calf StO2 during exercise was positively associated with ICD and ACD, and negatively associated with the ischemic window, suggesting that patients with greater impairment in exercise performance have faster de-oxygenation of the active ischemic musculature during standardized treadmill walking. This observation supports previous reports that PAD patients have greater absolute and percentage declines in calf muscle StO2 during treadmill walking than controls,19,21,23,24 and that the decline in calf muscle StO2 occurs early in exercise and does not increase until cessation of exercise in which there are two different recovery patterns.15,20 The current study extends these findings by demonstrating that the key variable in the decline in calf muscle StO2 during exercise is the time taken to reach the minimum value, as patiens who reach the minimum calf muscle StO2 sooner experience ICD and ACD sooner as well.
Another important measure of calf muscle StO2 during exercise is the StO2 exercise time delay between the predicted and observed times to reach the minimum StO2 value. This measure is determined by first calculating the predicted time to minimum StO2 based on the exponential decline in calf muscle StO2 at the onset of exercise. The StO2 exercise time delay may reflect an increase in capillary blood volume during exercise,24 thereby changing the exponential decline in StO2 occurring at the onset of exercise to a slower rate of decline during the remainder of exercise. The StO2 exercise time delay was positively associated with ABI and ACD, and was negatively associated with the ischemic window, suggesting that the patients with greater PAD severity and impairment in exercise performance have faster de-oxygenation of the calf muscle perhaps due to smaller increases in capillary blood volume during standardized treadmill exercise.
It is interesting to note that the StO2 exercise time delay is associated with ABI, ACD, and ischemic window primarily due to differences in the observed time to minimum calf StO2 rather than the predicted time. We found that neither the IDC nor the predicted time to minimum StO2 during exercise were associated with any of the vascular and exercise performance measures, except that IDC was positively associated with ACD. These findings suggest that the exponential decline in StO2 at the onset of exercise either is not predictive or is positively associated with claudication distances (i.e., a more negative IDC is associated with shorter ACD). This does not support a previous report which found that PAD patients have a slower rate of decline in StO2 (i.e., less negative) during the onset of treadmill walking than controls,17 and during isometric contraction performed at very low intensities of 5% and 10% maximal voluntary contraction.18 We calculated the StO2 decline differently to best fit the exponential drop at the onset of exercise, thus making comparisons difficult. However, when the decrease in calf muscle StO2 is expressed as an average rate of decline during exercise, our finding that worse exercise performance is associated with an accelerated average drop in StO2 supports previous work.23
Change in Calf Muscle StO2 During Recovery
Calf muscle StO2 increases following treadmill exercise in patients with intermittent claudication, eventually reaching a maximum value. The increase in calf muscle StO2 is a reflection of oxygen delivery exceeding oxygen extraction. Key measures of calf muscle StO2 during recovery include the time for StO2 to reach one half of the resting StO2 value, and the time to reach the full resting StO2 value. The recovery times to one-half and to full recovery of calf muscle StO2 were negatively associated with ICD and ACD, and positively associated with ischemic window, suggesting that patients with worse exercise performance and vascular insufficiency have delayed StO2 recovery times. Our findings support previous reports that PAD patients have longer StO2 recovery times than controls,19,21,23,24 and extends this observation by showing that prolonged recovery times of calf muscle StO2 following treadmill exercise occurs in PAD patients with greater exercise limitations.
There are limitations to this study that are associated with the measurement of calf muscle StO2. Although the calf muscle StO2 primarily reflects the relative balance between oxygen delivery and oxygen utilization of the local tissue, the contribution of myoglobin to the StO2 measurement cannot be excluded, especially during the onset on exercise.17 However, any contribution that local myoglobin may have on the calf muscle StO2 should be minimal beyond the initial phase of exercise. Another limitation with the StO2 measure is that the redistribution of oxyhemoglobin and de-oxyhemoglobin in the local tissue represents a mixture of capillary and venular blood that have different oxygen saturations.17 The subcutaneous fat thickness also may interfere with the calf muscle StO2 measurement. However, it is unlikely that this affected the results because there is no relationship between calf skin fold and calf muscle StO2 in PAD patients,16 and because only patients having calf skin folds less than 50 mm participated. Since subcutaneous tissue is only half the thickness of the skin fold measurement, the penetration of the light path of the NIRS probe to a depth of 25 mm should go beyond the subcutaneous tissue into the calf musculature. Finally, the results of this study are only applicable to PAD patients who are limited by intermittent claudication, and thus may not be generalizable to patients with different symptomatology. Despite these potential limitations, the measurement of calf muscle StO2 provides real-time information during exercise into the balance between oxygen delivery and oxygen demand of the calf musculature. Thus, this technique may be a useful research tool to evaluate the efficacy of interventions designed to improve claudication and peripheral circulation.
We conclude that in patients limited by intermittent claudication, shorter ICD and ACD values are associated with reaching a minimum value in calf muscle StO2 sooner during treadmill exercise, and with having a delayed recovery in calf muscle StO2 following exercise. The implication is that calf muscle StO2 quantifies the ischemic response to exercise in patients with claudication. Further work is needed to establish whether calf muscle StO2 changes in response to various interventions targeted to improve exercise performance in patients with intermittent claudication.
Acknowledgments
This research was supported by grants from the National Institute on Aging (NIA) (R01-AG-24296; AWG), by a Oklahoma Center for the Advancement of Science and Technology grant (HR04-113S; AWG), and by the University of Oklahoma Health Sciences Center General Clinical Research Center grant (M01-RR-14467), sponsored by the National Center for Research Resources from the National Institutes of Health.
Reference List
- 1.Weitz JI, Byrne J, Clagett GP, et al. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: a critical review. Circulation. 1996;94:3026–3049. doi: 10.1161/01.cir.94.11.3026. [DOI] [PubMed] [Google Scholar]
- 2.Bonde-Petersen F. Physical performance capacity in patients with dysbasia arteriosclerotica. II. Bicycle ergometry, walking tolerance and arteriographic diagnosis. Scand J Rehabil Med. 1974;6:26–30. [PubMed] [Google Scholar]
- 3.Gardner AW. Claudication pain and hemodynamic responses to exercise in younger and older peripheral arterial disease patients. J Gerontol. 1993;48:M231–M236. doi: 10.1093/geronj/48.5.m231. [DOI] [PubMed] [Google Scholar]
- 4.Gardner AW, Ricci MA, Case TD, Pilcher DB. Practical equations to predict claudication pain distances from a graded treadmill test. Vasc Med. 1996;1:91–96. doi: 10.1177/1358863X9600100201. [DOI] [PubMed] [Google Scholar]
- 5.Hiatt WR, N D, R J, H KR. The evaluation of exercise performance in patients with peripheral vascular disease. J Cardiopulmonary Rehabil. 1988;12:525–532. [Google Scholar]
- 6.Sieminski DJ, Gardner AW. The relationship between free-living daily physical activity and the severity of peripheral arterial occlusive disease. Vasc Med. 1997;2:286–291. doi: 10.1177/1358863X9700200402. [DOI] [PubMed] [Google Scholar]
- 7.Hiatt WR, Hirsch AT, Regensteiner JG, Brass EP. Clinical trials for claudication. Assessment of exercise performance, functional status, and clinical end points. Vascular Clinical Trialists. Circulation. 1995;92:614–621. doi: 10.1161/01.cir.92.3.614. [DOI] [PubMed] [Google Scholar]
- 8.Gardner AW, Skinner JS, Cantwell BW, Smith LK. Prediction of claudication pain from clinical measurements obtained at rest. Med Sci Sports Exerc. 1992;24:163–170. [PubMed] [Google Scholar]
- 9.Yao ST, Needham TN, Gourmoos C, Irvine WT. A comparative study of strain-gauge plethysmography and Doppler ultrasound in the assessment of occlusive arterial disease of the lower extremities. Surgery. 1972;71:4–9. [PubMed] [Google Scholar]
- 10.Ouriel K, McDonnell AE, Metz CE, Zarins CK. Critical evaluation of stress testing in the diagnosis of peripheral vascular disease. Surgery. 1982;91:686–693. [PubMed] [Google Scholar]
- 11.Wilkinson D, Vowden P, Parkin A, Wiggins PA, Robinson PJ, Kester RC. A reliable and readily available method of measuring limb blood flow in intermittent claudication. Br J Surg. 1987;74:516–519. doi: 10.1002/bjs.1800740633. [DOI] [PubMed] [Google Scholar]
- 12.Gardner AW, Skinner JS, Cantwell BW, Smith LK. Progressive vs single-stage treadmill tests for evaluation of claudication. Med Sci Sports Exerc. 1991;23:402–408. [PubMed] [Google Scholar]
- 13.Gardner AW, Skinner JS, Smith LK. Effects of handrail support on claudication and hemodynamic responses to single-stage and progressive treadmill protocols in peripheral vascular occlusive disease. Am J Cardiol. 1991;68:99–105. doi: 10.1016/0002-9149(91)90719-2. [DOI] [PubMed] [Google Scholar]
- 14.Strandness DE, Jr, BELL JW. An Evaluation of the Hemodynamic Response of the Claudicating Extremity to Exercise. Surg Gynecol Obstet. 1964;119:1237–1242. [PubMed] [Google Scholar]
- 15.Komiyama T, Shigematsu H, Yasuhara H, Muto T. Near-infrared spectroscopy grades the severity of intermittent claudication in diabetics more accurately than ankle pressure measurement. Br J Surg. 2000;87:459–466. doi: 10.1046/j.1365-2168.2000.01381.x. [DOI] [PubMed] [Google Scholar]
- 16.Afaq A, Montgomery PS, Scott KJ, Blevins SM, Whitsett TL, Gardner AW. The effect of current cigarette smoking on calf muscle hemoglobin oxygen saturation in patients with intermittent claudication. Vasc Med. 2007;12:167–173. doi: 10.1177/1358863X07081317. [DOI] [PubMed] [Google Scholar]
- 17.Bauer TA, Brass EP, Hiatt WR. Impaired muscle oxygen use at onset of exercise in peripheral arterial disease. J Vasc Surg. 2004;40:488–493. doi: 10.1016/j.jvs.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 18.Bauer TA, Brass EP, Barstow TJ, Hiatt WR. Skeletal muscle StO2 kinetics are slowed during low work rate calf exercise in peripheral arterial disease. Eur J Appl Physiol. 2007;100:143–151. doi: 10.1007/s00421-007-0412-0. [DOI] [PubMed] [Google Scholar]
- 19.Comerota AJ, Throm RC, Kelly P, Jaff M. Tissue (muscle) oxygen saturation (StO2): a new measure of symptomatic lower-extremity arterial disease. J Vasc Surg. 2003;38:724–729. doi: 10.1016/s0741-5214(03)01032-2. [DOI] [PubMed] [Google Scholar]
- 20.Komiyama T, Shigematsu H, Yasuhara H, Muto T. An objective assessment of intermittent claudication by near-infrared spectroscopy. Eur J Vasc Surg. 1994;8:294–296. doi: 10.1016/s0950-821x(05)80144-6. [DOI] [PubMed] [Google Scholar]
- 21.McCully KK, Halber C, Posner JD. Exercise-induced changes in oxygen saturation in the calf muscles of elderly subjects with peripheral vascular disease. J Gerontol. 1994;49:B128–B134. doi: 10.1093/geronj/49.3.b128. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe T, Matsushita M, Nishikimi N, Sakurai T, Komori K, Nimura Y. Near-infrared spectroscopy with treadmill exercise to assess lower limb ischemia in patients with atherosclerotic occlusive disease. Surg Today. 2004;34:849–854. doi: 10.1007/s00595-004-2833-2. [DOI] [PubMed] [Google Scholar]
- 23.Kemp GJ, Roberts N, Bimson WE, et al. Mitochondrial function and oxygen supply in normal and in chronically ischemic muscle: a combined 31P magnetic resonance spectroscopy and near infrared spectroscopy study in vivo. J Vasc Surg. 2001;34:1103–1110. doi: 10.1067/mva.2001.117152. [DOI] [PubMed] [Google Scholar]
- 24.Mohler ER, III, Lech G, Supple GE, Wang H, Chance B. Impaired exercise-induced blood volume in type 2 diabetes with or without peripheral arterial disease measured by continuous-wave near-infrared spectroscopy. Diabetes Care. 2006;29:1856–1859. doi: 10.2337/dc06-0182. [DOI] [PubMed] [Google Scholar]
- 25.Lohman TC, R A, M R. Anthropometric standardization reference manual. Human Kinetics Books; 1988. pp. 39–70. [Google Scholar]
- 26.Gardner AW. Reliability of transcutaneous oximeter electrode heating power during exercise in patients with intermittent claudication. Angiology. 1997;48:229–235. doi: 10.1177/000331979704800305. [DOI] [PubMed] [Google Scholar]
- 27.Feinberg RL, Gregory RT, Wheeler JR, et al. The ischemic window: a method for the objective quantitation of the training effect in exercise therapy for intermittent claudication. J Vasc Surg. 1992;16:244–250. doi: 10.1067/mva.1992.36947. [DOI] [PubMed] [Google Scholar]