Abstract
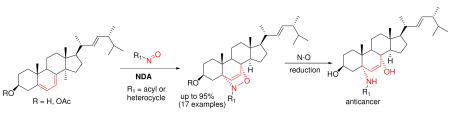
A series of novel sterol analogs was prepared using nitroso Diels-Alder reactions with ergosterol. Most cycloaddition reactions proceeded in an excellent regio- and stereoselective fashion. Further N-O bond cleavage of cycloadducts generated compounds with biological activity in PC-3 and MCF-7 cancer cell lines.
Natural products and their derivatives represent the most prolific source of molecular diversity in drug discovery. 1 Functional-group transformation of natural products constitutes one of the main avenues for generating pharmacologically relevant compounds with altered and sometimes improved biological properties. Typical chemical derivatizations of natural products are often limited to standard modification of nucleophilic or electrophilic functional groups. Since many natural products contain multiple functional groups of the same or similar type, derivatization selectivity is often problematic. Hence, new derivatization methods are still needed. Our effort in this area has involved nitroso Diels-Alder (NDA) reactions as efficient methods for derivatization and functionalization of diene-containing natural products. We and others2 have demonstrated that many complex diene-containing natural products readily undergo nitroso cycloadditions, generating 1-amino-4-hydroxy-2-ene heterocycle scaffolds with high regio- and stereoselectivity. Ergosterol (Figure 1), a natural sterol containing a conjugated diene was chosen for further exploration of this method for modular enhancement of Nature’s diversity (MEND).2a Ergosterol 1a (ergosta-5,7,22-trien-3β-ol) is biologically relevant as a precursor (a provitamin) to Vitamin D2 3 and an integral component of fungal cell membranes. The presence of ergosterol in fungal cell membranes coupled with its absence in animal cell membranes makes it a useful target for antifungal drugs. 4 Several groups have shown that sterols with a hetero-atom substituent at C-24 or C-25 are effective antifungal compounds. 5 Nitroso Diels-Alder reactions with ergosterol acetate 1b were reported by Kirby et. al.;2d–e however, this method was never exploited as a tool for derivatization of sterol compounds and the dienophiles used were often limited to transient aroyl nitroso species. Herein we report the syntheses of novel C5 and C8 disubstituted sterol analogs using nitroso Diels-Alder reactions of ergosterol, and its acetate, with acyl- and iminonitroso agents, and wish to demonstrate the utility of this methodology in natrual product derivatization by evaluation of their biological activity.
Figure 1.
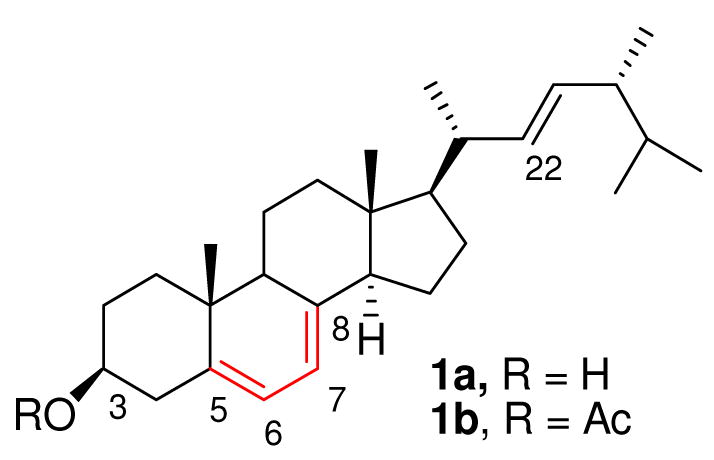
Ergosterol (1a) and ergosterol acetate (1b)
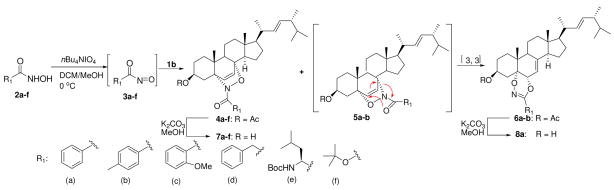
A series of hydroxamic acids 2a–e was obtained by reaction of the corresponding methyl esters with hydroxylamine; 6 N-hydroxycarbamate 2f was prepared from direct acylation of hydroxylamine with di-tert-butyl dicarbonate. Oxidation of compounds 2a-f to acyl nitroso dienophiles 3a–f with tetrabutylammonium periodate in the presence of ergosterol acetate 1b gave the 5αN-8αO-adducts 4a–f in various yields (Scheme 1, Table 1). The stereoisomeric configuration of 4a–f was assigned by 1H NMR studies based on literature analysis of 4a.2d In the case of benzohydroxamic acid 2a (entry 1, Table 1), similar to Kirby’s observation,2d dioxazine 6a was isolated as the major product in 54% yield, with adduct 4a as the minor product in 29% yield. Compound 6a was formed as a result of [3, 3] sigmatropic rearrangement of 5αO-8αN-adduct 5a, a much more sterically congested isomer compared to adduct 4a.2d Reaction with 4-methylbenzohydroxamic acid 2b gave a similar result, although dioxazine 6b was only isolated as a mixture with adduct 5b (entry 2). In contrast, oxidation of 2-methoxybenzohydroxamic acid 2c, phenylacetohydroxamic acid 2d, L-leucine-derived hydroxamic acid 2e and tert-butyl N-hydroxycarbamate 2f gave 4c–f as the sole products in good to excellent yields (entry 3–6). Further K2CO3 mediated deacetylation of 4a–f, 6a gave compounds 7a–f and 8a in 90% average isolated yields.
Scheme 1.
Table 1.
Results of acylnitroso Diels-Alder reactions
| entry | compd | adduct | yield (%) | dioxazine | yield (%) |
|---|---|---|---|---|---|
| 1 | 2a | 4a | 29 | 6a | 54 |
| 2 | 2b | 4b | 12 | 6b | 63a |
| 3 | 2c | 4c | 74 | 6c | 0b |
| 4 | 2d | 4d | 95 | 6d | 0b |
| 5 | 2e | 4e | 93 | 6e | 0b |
| 6 | 2f | 4f | 93 | 6f | 0b |
as a nonseparable mixture of dioxazine 6b and 5αO-8αN-adduct 5b.
not detected.
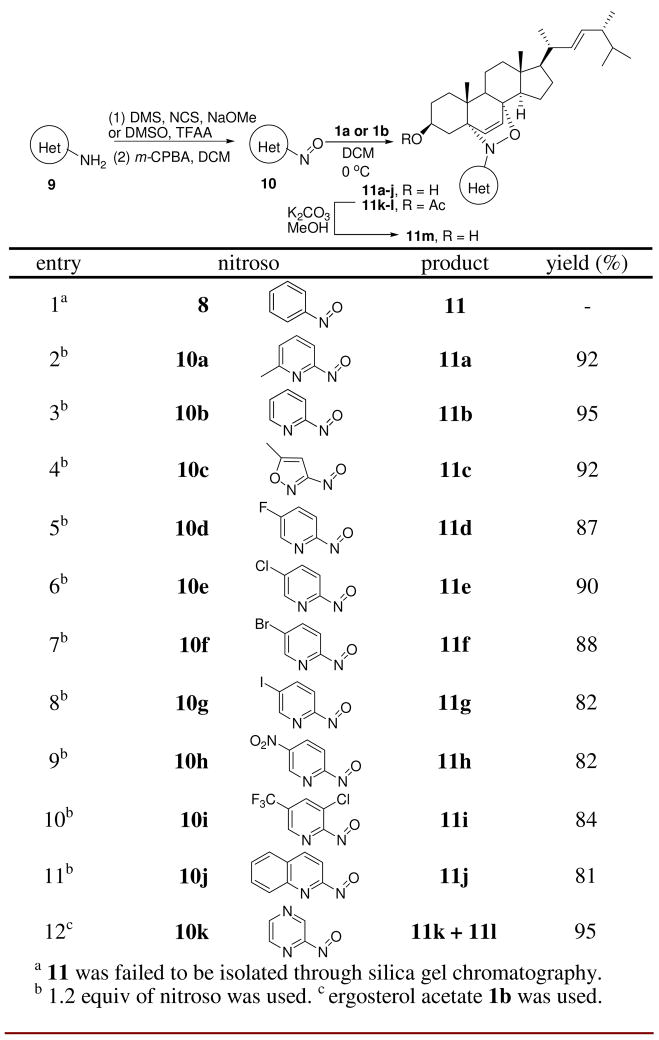
A nitroso Diels-Alder reaction with ergosterol 1a was also investigated using nitrosobenzene 8 as a representative aryl nitroso dienophile (entry 1, Scheme 2). The NDA reaction occured based on TLC and 1H NMR analysis; however, adduct 11 was not able to be isolated in pure form by chromatography. Additionally, cycloadditions with various iminonitroso agents 10, mainly 2-nitrosopyridine derivatives, were examined (Scheme 2). Those nitroso compounds were prepared from aminoheterocyclic precursors 9 in a two-step sequence (N, N-dimethyl sulfilimine intermediate formation, followed by oxidation using m-CPBA).7 Most of the iminonitroso compounds could be isolated and stored in pure form, except for 10k. The NDA reactions between ergosterol 1a and iminonitroso species 10a–j usually were complete within 30 min at 0 °C. In each case, the cycloadducts 11a–j were obtained as single 5αO-8αN-adducts in good to excellent yields after column chromatography (Scheme 2). The regio- and stereochemistry of the adducts was assigned based on our previous report and NMR studies.2a The instability of nitroso agent 10k required its immediate use, so it was trapped in situ with ergosterol acetate 1b. Interestingly, both regioisomers 11k and 11l were obtained in a 8.5:1 ratio in 95% combined yield (entry 12, Scheme 2). The major adduct 11k has the same stereochemistry as cycloadducts 11a–j, based on X-ray crystallography (Figure 2). Further deacetylation of 11k generated compound 11m. Clearly, 2-nitrosopyridines 10, as stabilized forms of iminonitroso reagents, constitute an ideal combination of reactivity and stability for NDA reactions with ergosterol, relative to benzenenitroso 8 and acylnitroso moieties 3, respectively.
Scheme 2.
Figure 2.
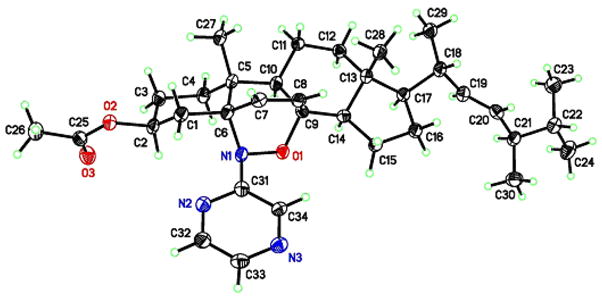
X-ray of cycloadduct 11k with ergosterol acetate 1b
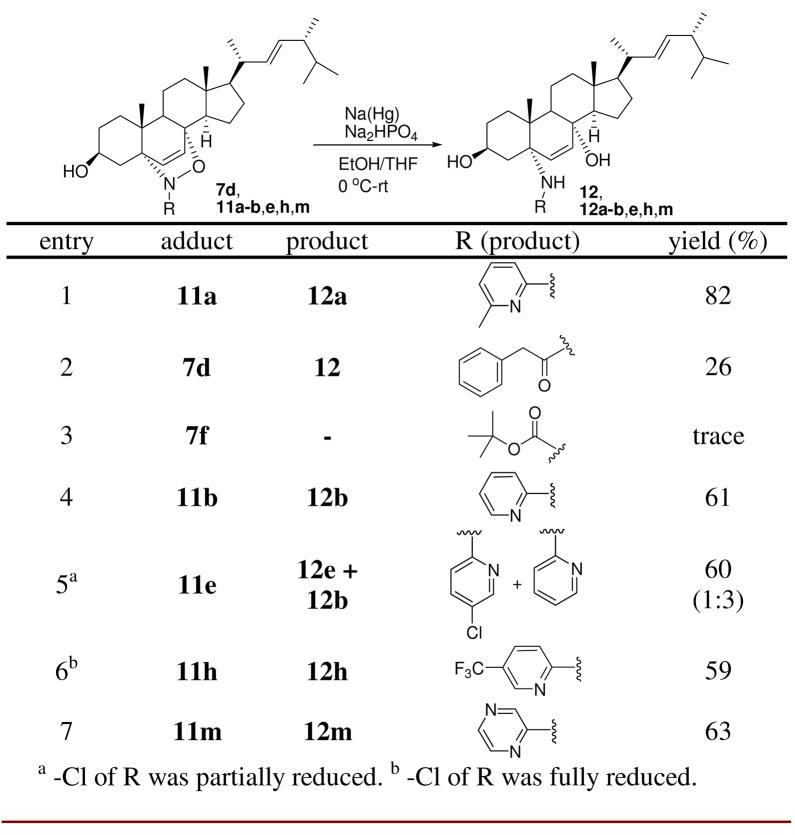
We next set out to demonstrate the synthetic utility of ergosterol nitroso cycloadducts through N-O bond cleavage, a method for generating 1,4-amino alcohols suitable for futher diversification. Adduct 11a was chosen as model for optimizing reaction conditions. Reactions with SmI2/THF, 8 Zn/CH3COOH, Mo(CO)6, 9 etc. were attempted; however, all of these conditions failed to yield desired product 12a (supporting information). Detailed screenings showed that Na amalgam 10 successfully reduced the N-O bond of 11a to give 12a in 82% yield (entry 1, Scheme 3). Then, a series of ergosterol nitroso adducts was subjected to the optimized Na(Hg) conditions. While low transformation was found with acylnitroso adducts 7d and 7f (entries 2–3), moderate yields were achieved for pyridinylnitroso adducts. Not surprisingly, these conditions also reduced the –Cl substituent (entries 5–6).
Scheme 3.
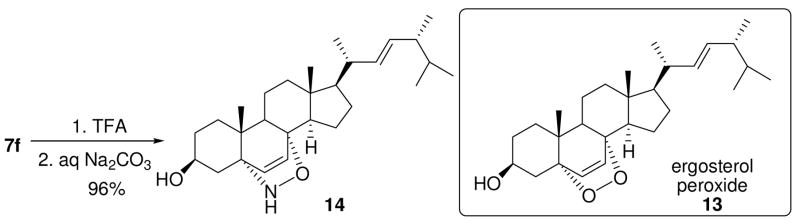
Ergosterol peroxide 13 11 is a natural sterol derivative that possesses a variety of biological properties including immunosuppressive and antitumor activity. Simply removing the N-Boc group of cycloadduct 7f afforded a quick access to compound 14 as an isoelectronic analog of ergosterol peroxide 13 (Scheme 4).
Scheme 4.
Broad antibacterial testing of these novel sterol analogs using the argar diffusion method revealed no significant activity against Gram-positive or Gram-negative organisms. However, N-O cleaved analogs had their largest zone of inhibition against the fungal strain, Sporobolomyces salmonicolor, whereas ergosterol, 1a, and adducts did not (supporting information). More interestingly, N-O reduced compounds 12, 12a–b,e,h,j, along with ergosterol acylnitroso adducts 7a–f, showed growth inhibitory activity in MCF-7 (breast cancer) and PC-3 (prostate cancer) tumor cell assays, while parent erogsterol, 1a, and most pyridinylnitroso adducts were relatively inactive (entries 1, 9, 10, 12, 14–16, Table 2). All N-O reduced analogs reached the low micromolar range of inhibition against both PC-3 and MCF-7 cells (entries 17–22). In general, all active compounds showed significantly improved inhibitory activity against the MCF-7 cell line. These biological assays clearly suggest that the nitroso heterocycle changed the biological activity profile of its parent natural product.
Table 2.
Results of anticancer screeningsa
| % inhibition at 20 μM |
IC50 (μM) |
||||
|---|---|---|---|---|---|
| entry | compd | PC-3 | MCF-7 | PC-3 | MCF-7 |
| 1 | 1a | 10 | 15 | - | - |
| 2 | 7a | 64 | 97 | - | 14 |
| 3 | 7b | 40 | 97 | 16 | 14 |
| 4 | 7c | 94 | 97 | 11 | 6 |
| 5 | 7d | 76 | 96 | 12 | 14 |
| 6 | 7e | 57 | 95 | 12 | 8 |
| 7 | 7f | 73 | 89 | 17 | 20 |
| 8 | 14 | 48 | 82 | >20 | >20 |
| 9 | 11a | <10 | 15 | - | - |
| 10 | 11b | 15 | 29 | - | - |
| 11 | 11c | 76 | 100 | - | 15 |
| 12 | 11d–e, g–h | <10 | <10 | - | - |
| 13 | 11i | 22 | 90 | - | 17 |
| 14 | 11j | 15 | <10 | - | - |
| 15 | 11k | 15 | 29 | - | - |
| 16 | 11m | 8 | 20 | - | - |
| 17 | 12 | 94 | 94 | 9 | 6.5 |
| 18 | 12a | 100 | 100 | 6 | 2 |
| 19 | 12b | 100 | 100 | 6 | 3.5 |
| 20 | 12e | 100 | 100 | 7 | 3 |
| 21 | 12h | 100 | 100 | 10 | 2.5 |
| 22 | 12j | 100 | 100 | 4 | 2.5 |
Trichostatin A was used as the positive control (MCF-7 IC50 = 16 nM, PC-3 IC50 = 160 nM).
In summary, we have reported that nitroso Diels-Alder reactions between ergosterol (or ergostrol acetate) and 2-nitrosopyrdines as well as acylnitroso agents proceeded with excellent regio- and stereoselectivity. A new class of sterol analogs that show encouraging anticancer activity was generated through N-O bond cleavage of the cycloadducts. The method presented herein provides a novel and efficient way to derivatize diene-containing natural products. Efforts to determine the exact mechanism of anticancer action and further diversification of ergosterol adducts are in progress.
Supplementary Material
Acknowledgments
We acknowledge NIH (GM075885) for support of this research. We also thank Timothy Wencewicz (UND) and Uta Wohlfeld (HKI) for performing antibacterial assays, the NMR facility at Notre Dame, Dr. Allen Oliver (UND) for X-ray crystallography, Nonka Sevova (UND) for mass spectroscopic analyses. B.Y.Y acknowledges a Reilly Graduate Fellowship (2008–2009, UND).
Footnotes
Supporting Information Available: Full experimental procedures, characterization data and copies of 1H NMR and 13C NMR spectra, protocols of antibacterial and anticancer assay. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.(a) Butler MS. Nat Prod Rep. 2005;22:162. doi: 10.1039/b402985m. [DOI] [PubMed] [Google Scholar]; (b) Newman DJ, Cragg GM, Snader KM. J Nat Prod. 2003;66:1022. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 2.(a) Li FZ, Yang BY, Miller MJ, Zajicek J, Noll BC, Mollmann U, Dahse HM, Miller P. Org Lett. 2007;9:2923. doi: 10.1021/ol071322b. [DOI] [PubMed] [Google Scholar]; (b) Krchnak V, Waring KR, Noll BC, Moellmann U, Dahse HM, Miller MJ. J Org Chem. 2008;73:4559. doi: 10.1021/jo8004827. [DOI] [PubMed] [Google Scholar]; (c) Ruan BF, Pong K, Jow F, Bowlby M, Crozier RA, Liu D, Liang S, Chen Y, Mercado ML, Feng XD, Bennett F, Schack DV, McDonald L, Zaleska MM, Wood A, Reinhart PH, Magolda RL, Skotnicki J, Pangalos MN, Koehn FE, Carter GT, Abou-Gharbia M, Graziani EI. Proc Natl Acad Sci USA. 2008;105:33. doi: 10.1073/pnas.0710424105. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Kirby GW, Mackinnon JWM. J Chem Soc, Perkin Trans. 1;1985:887. [Google Scholar]; (e) Kirby GW, Mackinnon JWM. J C S Chem Commun. 1977;1:23. [Google Scholar]; (f) Kirby GW, Bentley KW, Horsewood P, Singh S. J Chem Soc, Perkin Trans. 1;1979:3064. doi: 10.1039/p19720000302. [DOI] [PubMed] [Google Scholar]; (g) Kirby GW, Sweeny JG. Chem Commun. 1973:704. [Google Scholar]
- 3.Rajakumar K, Greenspan SL, Thomas SB, Holick MF. Am J Public Health. 2007;97:1746. doi: 10.2105/AJPH.2006.091736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Keith BB, Graham D. Acta Biochimica Polonica. 1995;42:465. [PubMed] [Google Scholar]; (b) Walsh TJ, Viviani MA, Arathoon E, Chiou C, Ghannoum M, Groll AH, Odds FC. Med Mycology. 2000;38:335. [PubMed] [Google Scholar]
- 5.(a) Nes WD, Guo D, Zhou W. Arch Biochem Biophys. 1997;342:68. doi: 10.1006/abbi.1997.9984. [DOI] [PubMed] [Google Scholar]; (b) Ator MA, Schmidt SJ, Adams JL, Dolle JM, Kruse RE, Frey LI, Barone CL. J Med Chem. 1992;35:100. doi: 10.1021/jm00079a012. [DOI] [PubMed] [Google Scholar]; (c) Acuna-Johnson AP, Oehlschlager C, Pierce AM, Pierce HD, Jr, Czyzewska EK. Bioorg Med Chem. 1997;5:821. doi: 10.1016/s0968-0896(97)00010-2. [DOI] [PubMed] [Google Scholar]; (d) Beuchet P, Dherbomez M, Elkiel L, Charles G, Letourneux Y. Bioorg Med Chem Lett. 1999;9:1599. doi: 10.1016/s0960-894x(99)00233-4. [DOI] [PubMed] [Google Scholar]
- 6.Miller MJ, Biswas A, Krook MA. Tetrahedron. 1983;39:2571. [Google Scholar]
- 7.(a) Taylor EC, Tseng CP, Rampal JB. J Org Chem. 1982;47:552. [Google Scholar]; (b) Sharma AK, Swern D. Tetrahedron Lett. 1974;16:1503. [Google Scholar]
- 8.Revuelta J, Cicchi S, Brandi A. Tetrahedron Lett. 2004;45:8375. [Google Scholar]
- 9.Cicchi S, Goti A, Brandi A, Guarna A, De Sarlos FD. Tetrahedron Lett. 1990;31:3351. [Google Scholar]
- 10.Keck GE, Fleming S, Nickell D, Weider P. Syn Commun. 1979;9:281. [Google Scholar]
- 11.(a) Bok JW, Lermer L, Chilton J, Klingeman HG, Towers GHN. Phytochem. 1999;51:891. doi: 10.1016/s0031-9422(99)00128-4. [DOI] [PubMed] [Google Scholar]; (b) Kim DS, Baek NI, Oh SR, Jung KY, Lee IS, Kin JK, Lee HK. Arch Pharm Res. 1997;20:201. doi: 10.1007/BF02976145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.