Abstract
Endothelin-1 (ET-1) is implicated in the development of endothelial dysfunction through the generation of reactive oxygen species by NADPH oxidase activation. Interleukin-10 (IL-10) is an antiinflammatory cytokine that stimulates nitric oxide production, decreases superoxide production, and restores endothelial integrity after vascular injury. In this study, we tested whether IL-10 attenuates ET-1-induced endothelial dysfunction by improving acetylcholine (ACh)-induced relaxation of cultured murine aortic rings. Aortic rings (2 mm long) of C57BL/6 mice were incubated in 2 mL DMEM containing 120 U/mL penicillin and 120 µg/mL streptomycin in the presence of one of 4 treatments: vehicle (deionized water), ET-1 (100 nmol/L), recombinant mouse IL-10 (300 ng/mL), or a combination of both ET-1 and IL-10. After incubation at 37 °C for either 1 or 6 h (short-term exposure) or 22 h (overnight exposure), rings were mounted in a wire myograph and stretched to a passive force of 5 mN. Endothelium-dependent vasorelaxation was assessed by constructing cumulative concentration–response curves to ACh (0.001–10 µmol/L) during 10 µmol/L phenylephrine (PE)-induced contraction. Short-term exposure of ET-1 did not result in an impairment of ACh-induced relaxation. Overnight exposure of aortic rings to ET-1 resulted in a statistically significant endothelial dysfunction characterized by a reduced maximal relaxation response to ACh compared with that of untreated rings (Emax 57% ± 3% versus 82% ± 4%). IL-10 treatment restored ACh-induced relaxation (Emax 77% ± 3%). Western blotting showed decreased eNOS expression in response to ET-1, whereas vessels treated with a combination of ET-1 and IL-10 showed increased expression of eNOS. Immunohistochemical analysis showed decreased eNOS expression in ET-1-treated vessels compared with those treated with both ET-1 and IL-10. We conclude that, in murine aorta, the antiinflammatory cytokine IL-10 prevents impairment in endothelium-dependent relaxation induced in response to long-term incubation with ET-1 via normalization of eNOS expression.
Keywords: interleukin-10, endothelin-1, eNOS, endothelium
Introduction
Nitric oxide (NO) and endothelin-1 (ET-1) are important endothelium-derived mediators that regulate the vascular tone. ET-1 is one of the most potent vasoconstrictors, produced by endothelial cells (Yanagisawa et al. 1988), airway epithelial cells, macrophages, fibroblasts, cardiomyocytes, and various brain neurons (Kedzierski and Yanagisawa 2001; Touyz and Schiffrin 1999). Its primary action is via activation of G protein-coupled receptors (Arai et al. 1990; Simonson and Dunn 1990). ET-1 has been associated with various cardiac (Mallat et al. 1999), renal (Hocher et al. 1997), and pulmonary diseases (Giaid et al. 1993; Hocher et al. 1997; Nishida et al. 2004). Hence, ET-1-receptor blockers are used in the treatment of various cardiac diseases (Rich and McLaughlin 2003). ET-1 also stimulates production of proinflammatory cytokines by leukocytes (Cunningham et al. 1997), and thus impairment of endothelium-dependent relaxation may be due to the action of inflammatory factors.
ET-1 decreases endothelial NO synthase (eNOS) expression and simultaneously increases reactive oxygen species via NADPH oxidase activation (Lund et al. 2005; Wedgwood and Black 2005). NADPH oxidase activity is also regulated by cytokines, hormones, and mechanical forces that are known to be involved in the pathogenesis of vascular diseases (Pollman et al. 1996). antiinflammatory cytokines like transforming growth factor (TGF)-β, interleukin (IL)-10, and IL-1 receptor antagonist exert inhibitory effects on injured vascular cells (Kofler et al. 2005). The antiinflammatory properties of IL-10 include inhibition of nuclear factor κB (leading to suppressed cytokine production), inhibition of matrix-degrading metalloproteinases, and reduction of tissue factor expression (Moore et al. 2001). In the vasculature, IL-10 promotes healing by inhibiting the production of proinflammatory cytokines that stimulate production of reactive oxygen species in endothelial cells (Moore et al. 2001). IL-10 improves eNOS-mediated relaxation of vessels by attenuating increases in superoxide production (Gunnett et al. 2000), which may relate to uncoupling of eNOS (Fortuno et al. 2005). Previously we have shown that IL-10 prevents impairment in endothelium-dependent relaxation via a decrease in NADPH oxidase expression (Zemse et al. 2007).
In this study, our aim was to test the hypothesis that ET-1 causes impairment of acetylcholine (ACh)-induced endothelium-dependent relaxation in murine aortic rings via downregulation of eNOS expression and that IL-10 counteracts the impairment. We addressed this by incubating murine aortic rings with ET-1 for 1, 6, or 22 h, after which rings were mounted in a wire myograph to assess ACh-induced relaxation responses. Furthermore, eNOS expression and localization were determined.
Materials and methods
Animals
Experiments were conducted in 8-week-old male C57BL/6 mice (Jackson Laboratory, Bar Harbor, Me.). All procedures followed the guidelines of, and were approved by, our institutional animal care and use committee.
Isolation of aortic rings and experimental protocols
Mice were euthanized with sodium pentobarbital (50 mg/kg i.v.) (Abbott Laboratories, Abbott Park, Ill.), after which the thoracic aorta was excised, placed in ice-cold physiologic saline solution (PSS), and cleaned of adhering connective and adipose tissue. Each aorta was divided into 4 rings of 2 mm length. The aortic rings were incubated for 22 h at 37 °C in 2 mL Dulbecco’s modified Eagle’s medium (DMEM) containing 120 U/mL penicillin and 120 µg/mL streptomycin and one of 4 treatments: vehicle (deionized water), ET-1 (100 nmol/L), recombinant mouse IL-10 (300 ng/mL), or a combination of both ET-1 and IL-10. Acute studies were also performed using 1-hour and 6-hour incubations in the same 4 treatment media. Pilot studies were performed before the final concentration of IL-10 was selected. At 30 ng/mL, IL-10 had no effect on the impaired endothelium responses to ACh induced by ET-1. Following incubation, the aortic rings were mounted in a wire myograph (Danish Myotech) filled with PSS with the following ionic composition (in mmol/L): 130 NaCl, 4.7 KCl, 1.18 KH2PO4, 1.18 MgSO4·7H2O, 14.9 NaHCO3, 5.6 dextrose, 1.56 CaCl2·2H2O, and 0.026 EDTA. They were maintained at 37 °C and continuously gassed with a mixture of 95% O2 and 5% CO2. A passive force of 5 mN was applied to the aortic rings and they were allowed to equilibrate for at least 60 min. Endothelium-dependent relaxation was measured on 10 µmol/L phenylephrine (PE)-contracted rings, followed by calculation of a cumulative concentration–response curve to ACh from 0.001 to 10 µmol/L. Endothelium-independent relaxation was tested with sodium nitroprusside (SNP) from 0.001 to 100 µmol/L.
Immunofluorescence
Four aorta isolated from 4 mice were incubated overnight (22 h) with one of 4 treatments, as described in the experimental protocol: vehicle (deionized water), ET-1 (100 nmol/L), IL-10 (300 ng/mL), or the combination of ET-1 and IL-10. Following incubation, aorta were fixed in 4% paraformaldehyde and subsequently washed with phosphate-buffered saline (PBS). Aorta were frozen in Tissue-Tek optical coherence tomography (OCT) medium, cut on a cryostat (model CM3000, Leica) into sections 8 µm wide, and mounted on glass slides. Slides were protected from light and incubated in a blocking buffer (100 mL PBS, 1 mL goat serum, 500 µL of 20% Triton X-100), and then treated with primary antibody to eNOS (BD Biosciences) overnight at 4 °C. Slides were washed with PBS and incubated in Cy3 antibody (Invitrogen) for 1 h. The slides were washed and allowed to dry, then covered with antifade mounting agent and cover slip. Images were obtained with a Zeiss LSM 510 Meta confocal microscope (Thornwood, N.Y.), with an excitation of 488 nm and emission of 574–595 nm or a 560 nm long-pass filter. Time series experiments were performed to quantify the intensity of eNOS expression in terms of numerical values. We selected 8 different endothelial areas of aorta per slide on the slides of all 4 treated groups.
Immunohistochemistry
Isolated aorta were treated as described earlier and then embedded in OCT medium. Sections 8 µm wide were cut from frozen aorta, mounted on Superfrost slides, and treated with blocking serum obtained from Vectastain Elite ABC kit (Vector Laboratories, Burlingame, Calif.). Subsequently slides were treated with primary antibody prepared in the blocking serum overnight at 4 °C. The next day the sections were treated with secondary biotinylated antibody for 30 min. Slides were then treated with ABC reagent for 30 min. At the end of the procedure, slides were stained with DAB (3,3′-diaminobenzidine) solution for 30–45 s (all reagents provided in the Vectastain kit).
Slides were counterstained with hematoxylin and a cover slip was mounted. Slides were observed in a brightfield microscope, and images were captured by Axiom software.
Western blot analysis
Four aorta were isolated from 4 mice and incubated overnight as described earlier. Aortic tissue was lysed by radioimmunoprecipitation assay (RIPA) lysis buffer in the presence of 1 mmol/L phenylmethylsulfonyl fluoride (PMSF), 1 mmol/L sodium orthovanadate, 1 mmol/L sodium fluoride, 1 µg/mL aprotinin, 1 µg/mL leupeptin, and 1 µg/mL pepstatin for 20 min on ice. Whole tissue lysates were centrifuged at 10 000g for 20 min at 4 °C, and the supernatants were collected. The protein concentrations were determined by the Bio-Rad protein assay. Samples of 50 µg were separated by SDS – 10% polyacrylamide gel electrophoresis and transferred by electroblotting onto a Hybond ECL nitro-cellulose membrane (Amersham Biosciences, N.J.). For the immunoassay, the membranes were blocked in 5% (w/v) nonfat dry milk in 1× PBS – 0.2% Tween 20 for 1 h at 4 °C with primary antibodies, polyclonal rabbit anti-eNOS antibody (BD Biosciences, N.J.), and monoclonal anti-β-actin (Sigma-Aldrich, St. Louis, Mo.). Immunocomplexes were detected through horseradish peroxidase-conjugated goat anti-mouse antisera (Amersham Biosciences), followed by enhanced chemiluminescence (ECL) reaction (Pierce Biotechnology, Rockford, Ill.).
Reagents
Phenylephrine, acetylcholine, sodium nitroprusside, and ET-1 were purchased from Sigma Chemical. Mouse recombinant IL-10 was purchased from R&D Systems (Minneapolis, Minn.).
Statistical analysis
Results are presented as means ± SE. Experimental values were calculated relative to the maximal changes from the contraction produced by PE in each segment. The pEC50 values for PE, ACh, and SNP are expressed as the negative logarithm of the molar concentration to produce 50% of the maximal response. Statistical analysis was performed by using two-way analysis of ANOVA followed by Bonferroni post test to compare the concentration–response curves between the groups. The analyses were performed using GraphPad Prism software. Values of p < 0.05 were considered a statistically significant difference.
Results
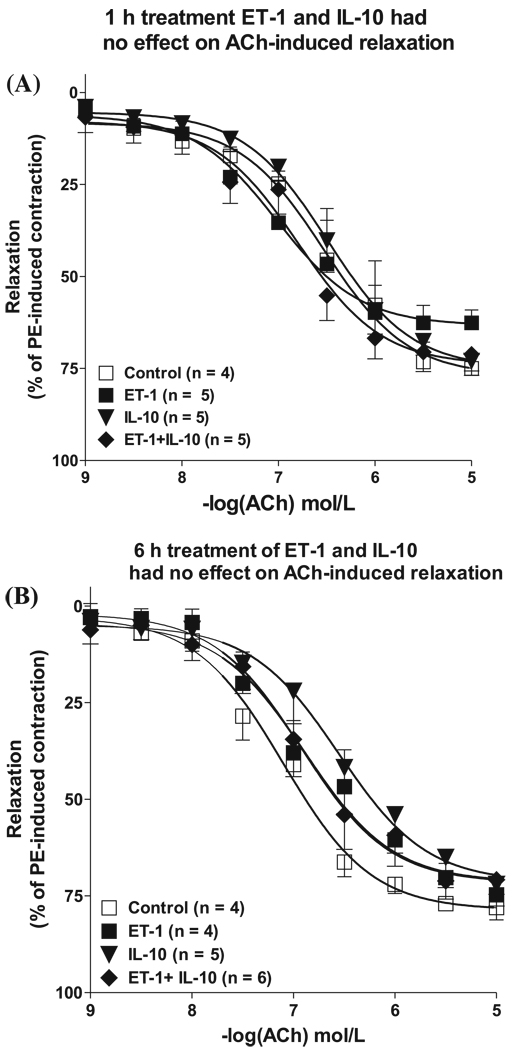
Short-term exposure (1 h and 6 h) to ET-1 and IL-10 had no effect on endothelium-dependent relaxation
Cumulative concentration–response curves to ACh performed 1 h after incubation with ET-1 did not affect sensitivity for ACh compared with that of untreated rings (pEC50 6.53 ± 0.16 versus 7.02 ± 0.19) (Fig. 1 and Table 1). Maximal relaxation to ACh tended to be lower in ET-1-treated rings than in untreated rings but did not differ statistically (Emax 63% ± 4% versus 77% ± 5%) (Fig. 1 and Table 1). We next addressed whether 6 h incubation with ET-1 resulted in an impaired ACh-induced relaxation. Figure 1B shows that relaxing responses to ACh were comparable for untreated rings and ET-1-treated rings. Maximal relaxing responses to ACh were similar in untreated rings and ET-1-treated rings (Emax 79% ± 2% versus 72% ± 4%, respectively) (Fig. 1). Sensitivity for ACh was unchanged for both groups (Table 1).
Fig. 1.
Short-term incubation (1 h or 6 h) with ET-1 and IL-10 had no effect on endothelium-dependent relaxation of aorta in mice. Cumulative concentration–response curves toACh(0.001–10 µmol/L) in PE (10 µmol/L)-contracted aortic rings treated with vehicle (□, deionized H2O), ET-1 (■, 100 nmol/L), IL-10 (▼, 300 ng/mL), or a combination of ET-1 and IL-10 (◆) for either (A) 1 h or (B) 6 h. Vasorelaxation was expressed as a percentage of the contraction induced by PE. Values are means ± SE (n = 4–6). ET, endothelin; IL, interleukin; ACh, acetylcholine; PE, phenylephrine.
Table 1.
pEC50 and Emax values in murine aortic rings after incubation for 1, 6, and 22 h with ET-1 and (or) IL-10.
| 22 h |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 h ACh-induced relaxation |
6 h ACh-induced relaxation |
PE-induced contraction |
ACh-induced relaxation |
SNP-induced relaxation |
||||||
| pEC50, mN |
Emax, % |
pEC50, mN |
Emax, % |
pEC50, mN |
Emax, % |
pEC50, mN |
Emax, % |
pEC50, mN |
Emax, % |
|
| Control | 6.53±0.16 | 77±5 | 7.10±0.07 | 79±2 | 7.12±0.18 | 20±1 | 6.95±0.17 | 82±4 | 8.94±0.09 | 103±2 |
| ET-1 | 7.02±0.19 | 63±4 | 6.92±0.15 | 72±4 | 7.19±0.21 | 19±1 | 6.88±0.12 | 57±3* | 8.67±0.09 | 104±2 |
| IL-10 | 6.49±0.11 | 75±4 | 6.55±0.18 | 72±6 | 7.16±0.22 | 18±1 | 7.16±0.15 | 73±4 | 8.79±0.09 | 99±2 |
| ET-1 + IL-10 | 6.79±0.14 | 74±4 | 6.89±0.16 | 71±4 | 7.12±0.21 | 18±1 | 7.21±0.10 | 77±3 | 8.89±0.10 | 101±2 |
Note: Data are means ± SD; asterisk (*) indicates a statistically significant change from control and ET-1 + IL-10 group (p < 0.05). pEC50, negative logarithm of the molar concentration to produce 50% of the maximal response; Emax, maximal relaxation response; ACh, acetylcholine; PE, phenylephrine; SNP, sodium nitroprusside; IL, interleukin.
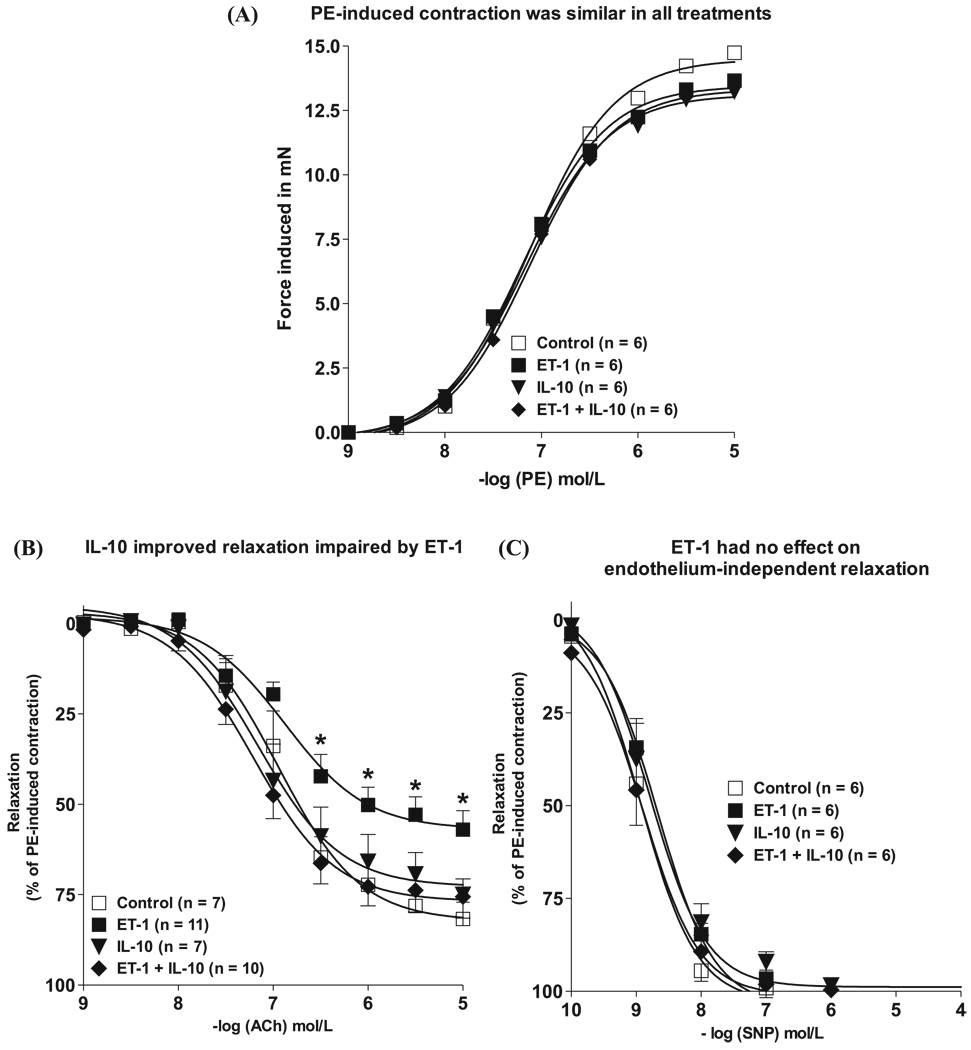
IL-10 restored endothelium-dependent relaxation impaired by ET-1 after 22-hour incubation
Maximal force induced in response to 10 µmol/L PE was not influenced by the different treatments: vehicle (19 ± 0.9 mN), ET-1 (18 ± 1.0 mN), IL-10 (18 ± 1.0 mN), or combined ET-1 and IL-10 (18 ± 1.0 mN) (Fig. 2A). For untreated rings, sensitivity and maximal relaxation with 10 µmol/L ACh were 6.95 ± 0.11 mN and 82% ± 4%, respectively (Fig. 2B and Table 1). Overnight (22 h) exposure with ET-1 (100 nmol/L) did not affect pEC50, but significantly reduced Emax values (57% ± 3%) (Fig. 2B and Table 1). This effect of ET-1 appears to be endothelium-dependent, since relaxation to the NO donor SNP was similar in aortic rings treated with vehicle or ET-1 (Fig. 2C).
Fig. 2.
IL-10 restored endothelium-dependent relaxation impaired by ET-1 after 22-hour incubation. Cumulative concentration–response curves to 0.001–10 µmol/L PE (A), ACh (B), and SNP (C) in PE (10 µmol/L)-contracted aortic rings treated for 22 h with □, vehicle (deionized H2O); ■, ET-1 (100 nmol/L); ▼, IL-10 (300 ng/mL); or ◆, a combination of ET-1 and IL-10. Vasorelaxation induced by increasing concentrations of ACh was expressed as a percentage of the relaxation to PE-contracted vessels. Values are means ± SE (n = 6–10). Significant at *, p < 0.05 vs. all other groups. IL, interleukin; PE, phenylephrine; ACh, acetylcholine; SNP, sodium nitroprusside.
We next examined the effect of IL-10 on ACh-induced relaxation in aortic rings treated with or without ET-1. Figure 2B shows that aortic rings treated with both ET-1 (100 nmol/L) and recombinant IL-10 (300 ng/mL) had completely restored maximal relaxation responses to ACh (77% ± 3%), and increased sensitivity for ACh compared with ET-1-treated rings (7.21 ± 0.10 mN versus 6.88 ± 0.12, respectively) (Table 1). Rings treated with IL-10 alone showed responses similar to those of untreated vessels (Emax 73% ± 4%) (Table 1). Relaxation to SNP in aortic rings treated with either IL-10 or ET-1 in combination with IL-10 was not different from control values, as depicted in Fig. 2C.
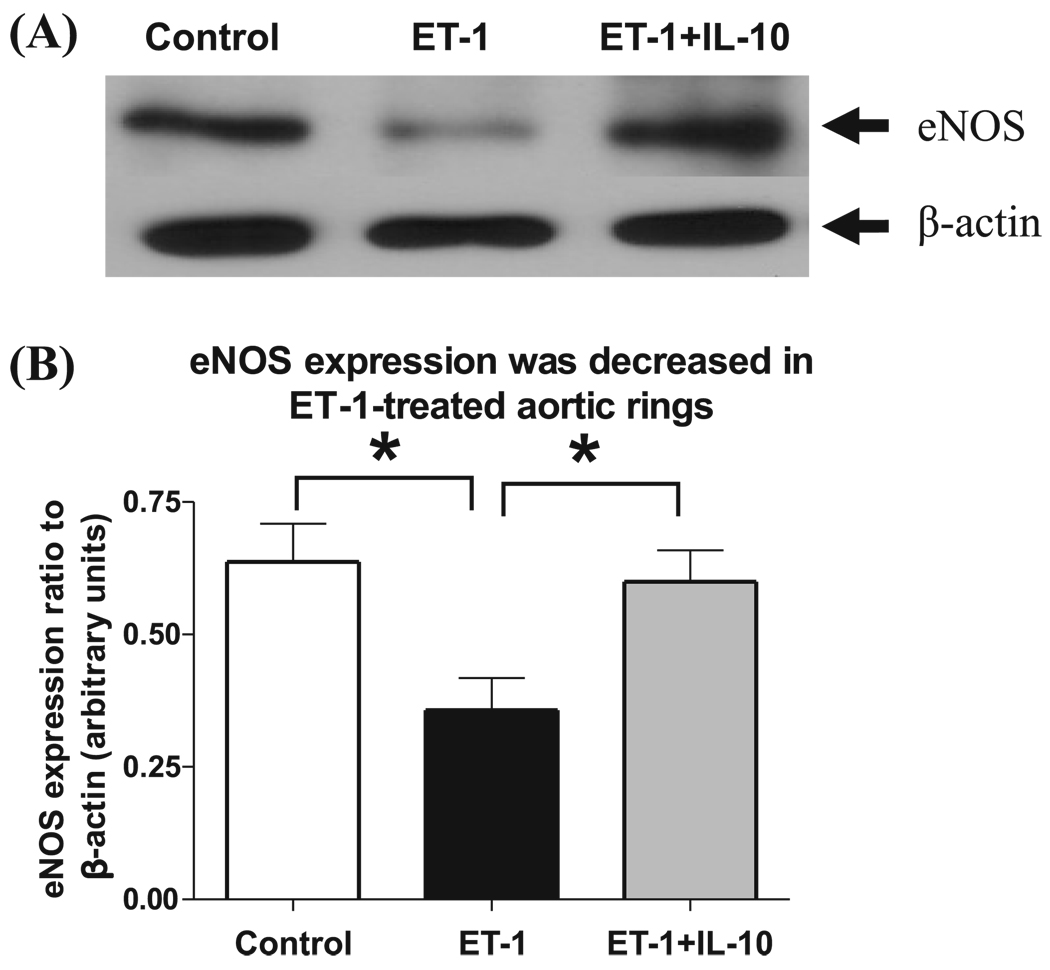
IL-10 restored eNOS expression in ET-1-treated aortic rings
Representative Western blotting images are shown in Fig. 3A for aortic rings treated for 22 h in the presence of vehicle, or ET-1, or the combination of ET-1 and IL-10. Protein expression level of eNOS was significantly decreased in aortic rings treated with ET-1 compared with aortic rings treated with the vehicle (Fig. 3A). Treatment with IL-10 in the presence of ET-1 restored the eNOS protein expression level to that of aortic rings treated with vehicle alone (Fig. 3A). eNOS staining quantified via densitometry analysis was significantly lower in aortic rings treated with ET-1 than in those treated with the combination of ET-1 and IL-10 (Fig. 3B).
Fig. 3.
IL-10 restored eNOS expression in ET-1-treated aortic rings. (A) Representative Western blot shows expression of eNOS in untreated (control), ET-1-treated (100 nmol/L), and ET-1- and IL-10-treated aortic rings. (B) Densitometric analysis was performed on untreated (control) (white bar), ET-1-treated (100 nmol/L, black bar), and ET-1- and IL-10-treated (grey bar) aortic rings. Values are expressed as the normalized ratio of the intensities of eNOS to β-actin (n = 7–9). Significant at *, p < 0.05.
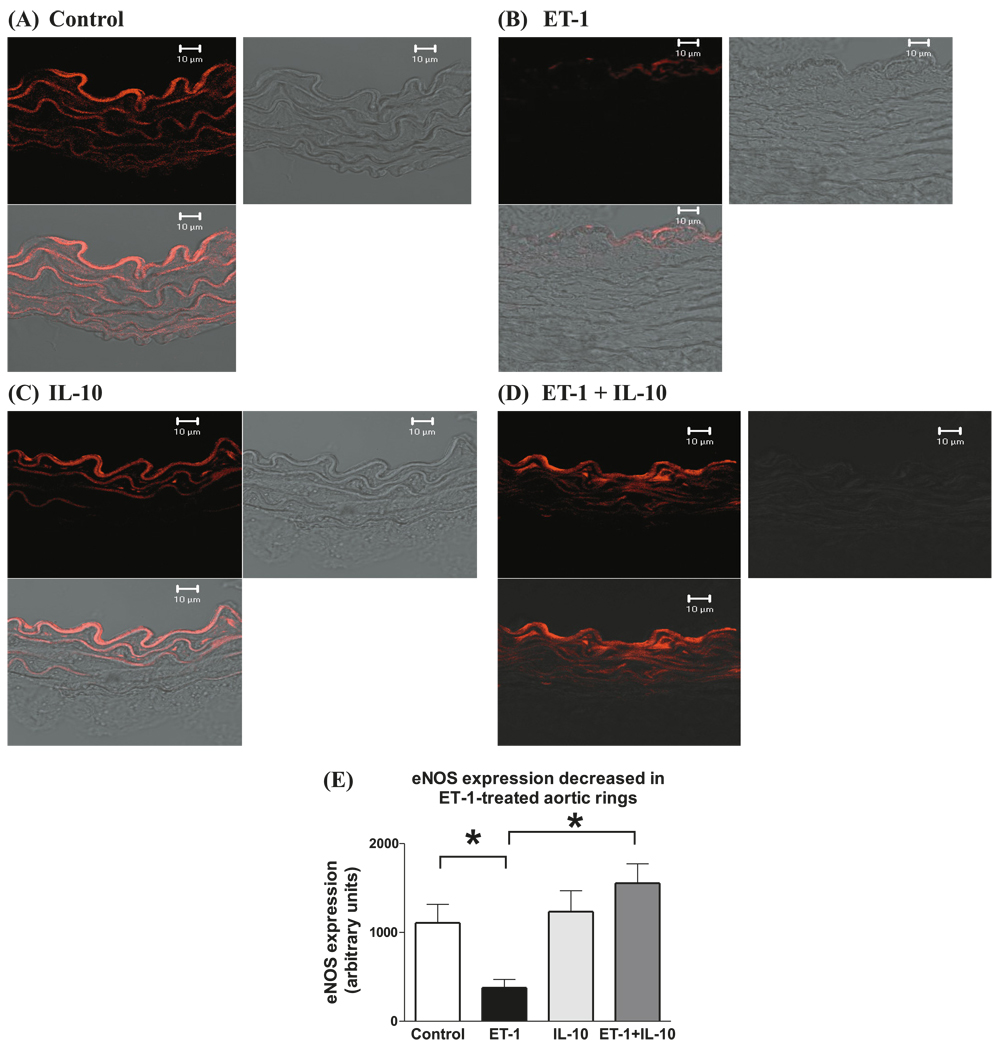
ET-1-treated vessels showed decreased immunofluorescence staining to eNOS
Immunofluorescence of cross-sections of ET-1-treated aortic rings showed decreased eNOS expression (Fig. 4B) compared with that of untreated rings (Fig. 4A). IL-10 in combination with ET-1 restored eNOS expression to levels comparable with that of untreated rings (Fig. 4D and 4E). eNOS staining quantified via densitometry analysis was significantly greater in aortic rings treated with ET-1 than in rings treated with the combination of ET-1 and IL-10 (arbitrary units; 1554 ± 217 versus 377 ± 94) (Fig. 4E). eNOS staining in control rings (Fig. 4A) was similar to that of rings treated with IL-10 (Fig. 4C).
Fig. 4.
ET-1-treated aorta of mice showed decreased immunofluorescence staining to eNOS. Immunohistochemical staining of eNOS on cross-sections (8 µm thick) of aortic rings treated for 22 h at 37 °C with (A) vehicle, (B) ET-1 (100 nmol/L), (C) IL-10 (300 ng/mL), or (D) ET-1 and IL-10. Each figure is subdivided into darkfield staining, brightfield staining (to visualize the tissue), and a merged figure of darkfield and brightfield. Fluorescence intensity of immunohistochemical staining for eNOS in aortic rings of vehicle-treated (white bar), ET-1-treated (black bar), IL-10-treated (light grey bar), and combined ET-1- and IL-10-treated (dark grey bar). Values are arbitrary units (n = 5). Significant at *, p < 0.01.
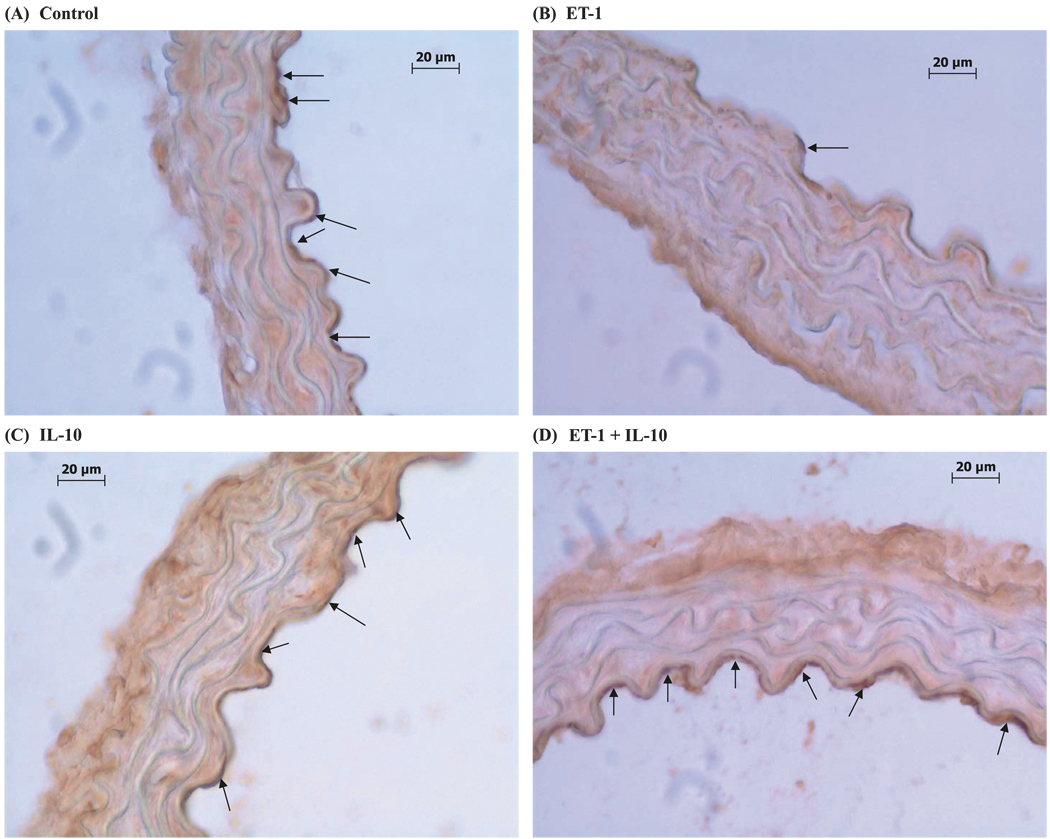
DAB staining was decreased in ET-1-treated vessels and reversed by IL-10 treatment
Immunohistochemistry revealed a reduction in eNOS staining in ET-1-treated rings (Fig. 5B) compared with that of untreated rings (Fig. 5A). eNOS staining in control rings (Fig. 5A) was similar to that of rings treated with the combination of ET-1 and IL-10 (Fig. 5D).
Fig. 5.
DAB staining was decreased in ET-1-treated vessels and reversed by IL-10 treatment. DAB staining of eNOS on cross-sections (8 µm thick) of aortic rings treated for 22 h at 37 °C with (A) vehicle, (B) ET-1 (100 nmol/L), (C) IL-10 (300 ng/mL), or (D) ET-1 and IL-10. eNOS staining is shown by the arrows.
Discussion
Here we demonstrated that overnight (22 h) treatment, but not short-term (1 or 6 h) incubation, with ET-1 lead to impairment in ACh-induced endothelium-dependent relaxation in murine aortic rings. The primary cause for this endothelial dysfunction is most likely a decrease in eNOS expression due to treatment with ET-1. Our next observation was that combined treatment of ET-1 with IL-10 restored this impairment in relaxation, suggesting that exogenously added antiinflammatory IL-10 inhibits ET-1-induced endothelial dysfunction, probably via a normalization of eNOS expression.
ET-1 acts via two receptors, ETA and ETB, on the smooth muscle and endothelial cells. The ETA receptors on endothelial cells and smooth muscle cells cause vasoconstriction, whereas ETB receptors on endothelial cells promotes vasodilation. The primary action of ET-1, however, is vasoconstriction via its ETA receptors and ETB receptors on smooth muscle cells (Kedzierski and Yanagisawa 2001; Touyz and Schiffrin 1999). ET-1 also causes a decrease in eNOS expression and an increase in the reactive oxygen species under certain conditions (Groeneweg et al. 2006; Wedgwood and Black 2005). There is limited knowledge about the effect of ET-1 on production of cytokines in vascular function (Cunningham et al. 1997; Kuga et al. 1996; Tanowitz et al. 2005). Here we hypothesized that IL-10 would improve the impairment in endothelium-dependent ACh-induced relaxation induced by ET-1, presumably by normalizing the balance of pro- and antiinflammatory cytokines. Serum levels of IL-10 are relatively low, ranging around 200–500 pg/mL (Sablotzki et al. 1997; Torres et al. 2005). Clinical studies suggest that serum levels of IL-10 are elevated in patients with acute coronary syndromes (Bossowska et al. 2003) and preeclampsia (Orange et al. 2005). Here we used higher (300 ng/mL) than the reported levels of IL-10 to enable sufficient levels of IL-10 to counterbalance the actions of ET-1. Our previous experiments using 30 ng/mL IL-10 did not improve ACh-induced relaxations in the presence of ET-1 (data not shown).
After treating the aortic rings with ET-1 for either 1 or 6 h, we found no significant impairment in relaxation induced, which suggests that the effect of ET-1 is not acute (Fig. 1 and Table 1). Hence, we performed longer (22 h) treatment with ET-1, and, similar to the results of our previous work using angiotensin II, this overnight exposure to ET-1 caused impairment in endothelium-dependent, ACh-induced relaxation in murine aorta (Zemse et al. 2007). The impaired relaxation was accompanied by a reduction in eNOS protein. In large arteries such as the aorta, NO derived from eNOS is the main vasorelaxing compound (Gryglewski et al. 1986; Lee et al. 2006). Indeed, downregulation of eNOS would be the most likely primary cause of this impaired endothelium-dependent relaxation. We observed that coincubation of IL-10 along with ET-1 not only restored the endothelium-dependent relaxation responses to ACh, but also normalized eNOS expression levels. Hence IL-10 may play a beneficial role in maintaining eNOS expression in endothelial cells. Studies have shown that IL-10 plays an important role in vascular protection in atherosclerosis (Mallat et al. 1999; Pinderski et al. 2002), possibly by inhibiting the production of pro-inflammatory cytokines that stimulate production of reactive oxygen species in endothelial cells (Moore et al. 2001). IL-10 also protects eNOS-mediated relaxation of vessels by attenuating increases in superoxide production (Gunnett et al. 2002). Our previous data showed that IL-10 inhibited the angiotensin II-mediated impairment in relaxation by normalizing NADPH oxidase expression (Zemse et al. 2007). These data suggest that IL-10 plays an important role in maintaining the protective state of the endothelium by inhibiting the inflammatory effects of such vasoconstrictors as angiotensin II and ET-1 (Moore et al. 2001). This inflammation is also inhibited by antioxidant vitamins and superoxide dismutase (SOD) (Heistad 2003). Further studies need to be performed to determine the precise action of ET-1, including whether it occurs through upregulation of ETA receptor or downregulation of ETB receptors.
In conclusion, our studies show that 22-hour treatment with ET-1 leads to impairment in endothelium-dependent relaxation to ACh via downregulation of eNOS expression. IL-10 normalizes this impaired relaxation by normalization of eNOS expression.
Footnotes
This article is one of a selection of papers published in the special issue (part 2 of 2) on Forefronts in Endothelin.
Contributor Information
Saiprasad M. Zemse, Department of Physiology, Medical College of Georgia, 1120 Fifteenth Street, Augusta, GA 30912-3000, USA..
Rob H.P. Hilgers, Cardiovascular Research Intstitute Maastricht, Department of Pharmacology & Toxicology, University of Maastricht, the Netherlands.
G. Bryan Simkins, Department of Pharmacology, Medical College of Georgia, Augusta, Georgia, USA..
R. Daniel Rudic, Department of Pharmacology, Medical College of Georgia, Augusta, Georgia, USA..
R. Clinton Webb, Department of Physiology, Medical College of Georgia, 1120 Fifteenth Street, Augusta, GA 30912-3000, USA..
References
- Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348:730–732. doi: 10.1038/348730a0. PMID:2175396. [DOI] [PubMed] [Google Scholar]
- Bossowska A, Kiersnowska-Rogowska B, Bossowski A, Galar B, Sowinski P. Cytokines in patients with ischaemic heart disease or myocardial infarction. Kardiol. Pol. 2003;59:105–114. PMID:14560325. [PubMed] [Google Scholar]
- Cunningham ME, Huribal M, Bala RJ, McMillen MA. Endothelin-1 and endothelin-4 stimulate monocyte production of cytokines. Crit. Care Med. 1997;25:958–964. doi: 10.1097/00003246-199706000-00011. PMID:9201047. [DOI] [PubMed] [Google Scholar]
- Fortuno A, Jose GS, Moreno MU, Diez J, Zalba G. Oxidative stress and vascular remodelling. Exp. Physiol. 2005;90:457–462. doi: 10.1113/expphysiol.2005.030098. PMID:15890797. [DOI] [PubMed] [Google Scholar]
- Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N. Engl. J. Med. 1993;328:1732–1739. doi: 10.1056/NEJM199306173282402. PMID:8497283. [DOI] [PubMed] [Google Scholar]
- Groeneweg JG, Huygen F, Heijmans-Antonissen C, Niehof S, Zijlstra F. Increased endothelin-1 and diminished nitric oxide levels in blister fluids of patients with intermediate cold type complex regional pain syndrome type 1. BMC Musculoskelet. Disord. 2006;7:91. doi: 10.1186/1471-2474-7-91. PMID:17137491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski RJ, Palmer RMJ, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–456. doi: 10.1038/320454a0. PMID:3007998. [DOI] [PubMed] [Google Scholar]
- Gunnett CA, Heistad DD, Berg DJ, Faraci FM. IL-10 deficiency increases superoxide and endothelial dysfunction during inflammation. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H1555–H1562. doi: 10.1152/ajpheart.2000.279.4.H1555. PMID:11009441. [DOI] [PubMed] [Google Scholar]
- Gunnett CA, Heistad DD, Faraci FM. Interleukin-10 protects nitric oxide-dependent relaxation during diabetes: role of superoxide. Diabetes. 2002;51:1931–1937. doi: 10.2337/diabetes.51.6.1931. PMID:12031983. [DOI] [PubMed] [Google Scholar]
- Heistad DD. A radical review of the superfamily of cardiovascular risk factors. Circ. J. 2003;67:805–809. doi: 10.1253/circj.67.805. [DOI] [PubMed] [Google Scholar]
- Hocher B, Thone-Reineke C, Rohmeiss P, Schmager F, Slowinski T, Burst V, et al. Endothelin-1 transgenic mice develop glomerulosclerosis, interstitial fibrosis, and renal cysts but not hypertension. J. Clin. Invest. 1997;99:1380–1389. doi: 10.1172/JCI119297. PMID:9077548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedzierski RM, Yanagisawa M. Endothelin system: the double-edged sword in health and disease. Annu. Rev. Pharmacol. Toxicol. 2001;41:851–876. doi: 10.1146/annurev.pharmtox.41.1.851. PMID:11264479. [DOI] [PubMed] [Google Scholar]
- Kofler S, Nickel T, Weis M. Role of cytokines in cardiovascular diseases: a focus on endothelial responses to inflammation. Clin. Sci. (Lond.) 2005;108:205–213. doi: 10.1042/CS20040174. PMID:15540988. [DOI] [PubMed] [Google Scholar]
- Kuga S, Otsuka T, Niiro H, Nunoi H, Nemoto Y, Nakano T, et al. Suppression of superoxide anion production by interleukin-10 is accompanied by a downregulation of the genes for subunit proteins of NADPH oxidase. Exp. Hematol. 1996;24:151–157. PMID:8641336. [PubMed] [Google Scholar]
- Lee DL, Sturgis LC, Labazi H, Osborne JB, Jr, Fleming C, Pollock JS, et al. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H935–H940. doi: 10.1152/ajpheart.00708.2005. PMID:16284237. [DOI] [PubMed] [Google Scholar]
- Lund AK, Peterson SL, Timmins GS, Walker MK. Endothelin-1-mediated increase in reactive oxygen species and NADPH oxidase activity in hearts of Aryl hydrocarbon Receptor (AhR) null mice. Toxicol. Sci. 2005;88:265–273. doi: 10.1093/toxsci/kfi284. PMID:16107552. [DOI] [PubMed] [Google Scholar]
- Mallat Z, Besnard S, Duriez M, Deleuze V, Emmanuel F, Bureau MF, et al. Protective role of interleukin-10 in atherosclerosis. Circ. Res. 1999;85:e17–e24. doi: 10.1161/01.res.85.8.e17. PMID:10521249. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. PMID:11244051. [DOI] [PubMed] [Google Scholar]
- Nishida M, Eshiro K, Okada Y, Takaoka M, Matsumura Y. Roles of endothelin ETA and ETB receptors in the pathogenesis of monocrotaline-induced pulmonary hypertension. J. Cardiovasc. Pharmacol. 2004;44:187–191. doi: 10.1097/00005344-200408000-00007. PMID:15243299. [DOI] [PubMed] [Google Scholar]
- Orange S, Rasko JE, Thompson JF, Vaughan J, Olive E, Pedler M, et al. Interleukin-10 regulates arterial pressure in early primate pregnancy. Cytokine. 2005;29:176–185. doi: 10.1016/j.cyto.2004.10.011. PMID:15652450. [DOI] [PubMed] [Google Scholar]
- Pinderski LJ, Fischbein MP, Subbanagounder G, Fishbein MC, Kubo N, Cheroutre H, et al. Overexpression of interleukin-10 by activated T lymphocytes inhibits atherosclerosis in LDL receptor-deficient mice by altering lymphocyte and macrophage phenotypes. Circ. Res. 2002;90:1064–1071. doi: 10.1161/01.res.0000018941.10726.fa. PMID:12039795. [DOI] [PubMed] [Google Scholar]
- Pollman MJ, Yamada T, Horiuchi M, Gibbons GH. Vasoactive substances regulate vascular smooth muscle cell apoptosis: countervailing influences of nitric oxide and angiotensin II. Circ. Res. 1996;79:748–756. doi: 10.1161/01.res.79.4.748. PMID:8831498. [DOI] [PubMed] [Google Scholar]
- Rich S, McLaughlin VV. Endothelin receptor blockers in cardiovascular disease. Circulation. 2003;108:2184–2190. doi: 10.1161/01.CIR.0000094397.19932.78. PMID:14597580. [DOI] [PubMed] [Google Scholar]
- Sablotzki A, Welters I, Lehmann N, Menges T, Gorlach G, Dehne M, et al. Plasma levels of immunoinhibitory cytokines interleukin-10 and transforming growth factor-[beta] in patients undergoing coronary artery bypass grafting. Eur. J. Cardiothorac. Surg. 1997;11:763–768. doi: 10.1016/s1010-7940(97)01154-8. PMID:9151050. [DOI] [PubMed] [Google Scholar]
- Simonson MS, Dunn MJ. Cellular signalling by peptides of the endothelin gene family. FASEB J. 1990;4:2989–3000. doi: 10.1096/fasebj.4.12.2168326. PMID:2168326. [DOI] [PubMed] [Google Scholar]
- Tanowitz HB, Huang H, Jelicks LA, Chandra M, Loredo ML, Weiss LM, et al. Role of endothelin 1 in the pathogenesis of chronic chagasic heart disease. Infect. Immun. 2005;73:2496–2503. doi: 10.1128/IAI.73.4.2496-2503.2005. PMID:15784596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MB, Vega VL, Bedri M, Saad D, Trentzsch H, Reeves RH, et al. IL-10 plasma levels are elevated after LPS injection in splenectomized A/J mice. J. Surg. Res. 2005;129:101–106. doi: 10.1016/j.jss.2005.06.008. PMID:16087192. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Schiffrin EL. Ang II-stimulated superoxide production is mediated via phospholipase D in human vascular smooth muscle cells. Hypertension. 1999;34:976–982. doi: 10.1161/01.hyp.34.4.976. PMID:10523394. [DOI] [PubMed] [Google Scholar]
- Wedgwood S, Black SM. Endothelin-1 decreases endothelial NOS expression and activity through ETA receptor-mediated generation of hydrogen peroxide. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;288:L480–L487. doi: 10.1152/ajplung.00283.2004. PMID:15531748. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. PMID:2451132. [DOI] [PubMed] [Google Scholar]
- Zemse SM, Hilgers RH, Webb RC. Interleukin-10 counteracts impaired endothelium-dependent relaxation induced by angiotensin II in murine aortic rings. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H3103–H3108. doi: 10.1152/ajpheart.00456.2006. PMID:17322422. [DOI] [PubMed] [Google Scholar]