Abstract
BACKGROUND
Transplantation of ovarian tissue is, at present, the only clinical option available to restore fertility using cryopreserved ovarian tissue. More than 30 transplantations of cryopreserved tissue have been reported, and six babies have been born, worldwide, following this procedure. Despite these encouraging results, it is essential to optimize the procedure by improving the follicular survival, confirming safety and developing alternatives. Here, we review the different factors affecting follicular survival and growth after grafting.
METHODS
Relevant studies were identified by searching Pubmed up to January 2009 with English language limitation. The following key words were used: (ovarian tissue or whole ovary) AND (transplantation) AND (cryopreservation or pregnancy). Using the literature and personal experience, we examined relevant data on the different exogenous and clinical factors affecting follicular development after grafting.
RESULTS
Clinical factors such as the patient's age and the transplantation sites influenced the lifespan of the graft. A heterotopic transplantation site is not optimal but offers some advantages and it may also promote the hormonal environment after a combined heterotopic and orthotopic transplantation. Exogenous factors such as antioxidants, growth factors or hormones were tested to improve follicular survival; however, their efficiency regarding further follicular development and fertility potential remains to be established.
CONCLUSION
Additional evidence is required to define optimal conditions for ovarian tissue transplantation. Alternatives such as whole ovary or isolated follicles transplantations require further investigation but are likely to be successful in humans in the future.
Keywords: ovarian tissue, transplantation, pregnancy, sites, vascularization
Introduction
Major advances in oncological treatments and diagnosis have resulted in a marked improvement in the survival of children and young adults with cancer over the last decade. Chemotherapy treatments including alkylating agents as well as radiotherapy may unfortunately compromise future fertility (Meirow, 2000; Lobo, 2005). The risk of premature ovarian failure depends on various factors such as the age of the patient, the type and the dose of cytotoxic therapy. Alkylating agents, imposing the highest risk in causing ovarian failure, induce follicular depletion in an exponential proportion to increasing doses. Among premenopausal women treated with alkylating agents for breast cancer, it has been estimated that up to 68% of them faced premature ovarian failure after treatment. Aggressive treatment with cytotoxic chemotherapy and radiotherapy for lymphoma results in ovarian failure in 38–57% of the patients (Meirow and Nugent, 2001). Conditioning regimen for bone marrow transplantation represents the most gonadotoxic regimen, with an ovarian failure rate after treatment exceeding 90% (Meirow, 2000). Radiotherapy is also recognized to cause destruction of the follicular pool, with an LD50 of human oocyte <2 Gy (Wallace et al., 2003). The effective sterilizing dose at which ovarian failure occurs immediately after treatment in almost all the patients is estimated at <20 Gy, when pelvic radiotherapy doses for intra-abdominal tumour, including gynaecological cancer, ranged from 25 to 50 Gy (Meirow and Nugent, 2001; Chemoradiotherapy for cervical cancer Meta-Analysis Collaboration, 2008; Wo and Viswanathan, 2009).
On the other hand, restoration of the ovarian function does not always ensure normal fertility after oncological treatments. The chance of spontaneous pregnancy in women treated after 25 years of age has been estimated to be only 5% (Lobo, 2005).
Because fertility preservation is of great concern for young women diagnosed with cancer, the possibility of treatment-related infertility should systematically be brought up by physicians in collaboration with gynaecologists at the time of diagnosis (Langeveld et al., 2004; Thewes et al., 2005; Lee et al., 2006). According to the type of the disease and the health state of the patient, various options to preserve fertility have been proposed (Donnez et al., 2006; Demeestere et al., 2007). Many young cancer patients desiring fertility preservation may not have access to the technologies used for Assisted Medical Procreation such as embryo or oocytes cryopreservation because of their oncological context and/or personal situation. These options impose a 2–4 week delay before oocyte collection that is often not compatible with the urgency to treat the cancer, and this procedure must be performed before beginning chemotherapy. Oocyte retrieval after even one round of chemotherapy is not practicable because of the dramatic reduction of IVF efficiency, as well as the increased risk of aneuploidy due to this treatment (Dolmans et al., 2005).
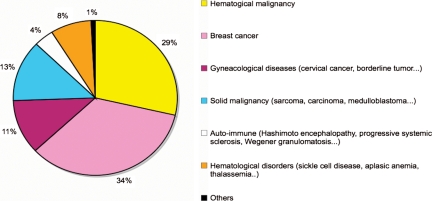
Cryopreservation of ovarian tissue is a promising experimental technology, presenting with several advantages. It allows the storage of a large number of primordial and primary follicles, can be rapidly performed at any time of the menstrual cycle and is the only available option to preserve fertility in children (Nugent et al., 1997; Donnez and Bassil, 1998; Oktay, 2001; Poirot et al., 2002, 2007; Demeestere et al., 2003; Dudzinski, 2004; Oktay and Sonmezer, 2004; Oktay et al., 2004; Donnez et al., 2006; Lee et al., 2006; Meirow et al., 2007a; Moffa et al., 2007; Weintraub et al., 2007). The procedure is proposed as a fertility preservation option for various indications in a growing number of centres around the world. In our institution, breast and haematological cancers represent two-thirds of the indications (Fig. 1, unpublished data). The ovarian tissue cryopreservation procedure benefits those not only with oncological diseases, but also with benign diseases such as drepanocytosis or thalassaemia that require conditioning regimen for bone marrow transplantation (Sonmezer et al., 2005). Patients affected by autoimmune diseases such as lupus nephritis, genetic disorders associated with premature ovarian failure, or benign ovarian diseases requiring oophorectomy may also be concerned with fertility preservation (Hreinsson et al., 2002; Demeestere et al., 2003; Gidoni et al., 2008; Huang et al., 2008a; Oktay and Oktem, 2008). These non-oncological conditions represent nearly 20% of the indications in our population of patients requiring fertility preservation (Fig. 1). Finally, healthy women who chose to delay childbearing for professional or personal reasons may also wish to retain their fertility (Tao and Del Valle, 2008).
Figure 1.
Indications for ovarian tissue cyopreservation in Erasme Hospital from 1999 to 2008 (n = 133).
Although the freezing-thawing procedure of ovarian tissue is now relatively well established, the use of the cryopreserved ovarian cortex in order to restore fertility remains a challenge. Orthotopic or heterotopic transplantation is currently the only available option to restore fertility using cryopreserved ovarian tissue. Alternatives such as in vitro follicular culture require additional research before becoming available for humans (Hovatta, 2000; Picton et al., 2008). To date, 43 women who underwent cryopreserved or fresh ovarian tissue transplantations have been reported in the literature, leading to the restoration of spontaneous cycles for several months in almost all cases (Bedaiwy et al., 2008). Restoring fertility after auto-transplantation of cryopreserved ovarian tissue has been recently achieved, and five healthy babies were born following this procedure (Donnez et al., 2004; Meirow et al., 2005; Demeestere et al., 2007; Andersen et al., 2008). Pregnancies have also been obtained after heterologous transplantation of fresh or cryopreserved cortical tissue between twins discordant for premature ovarian failure (Silber et al., 2005, 2008a; Silber and Gosden, 2007). Despite these encouraging results, some important concerns still limit the application of the procedure and its success. Besides the age of the patient at tissue collection, a key factor is the ischemic injury occurring during the time necessary for the revascularization of the transplanted tissue from the support vessels. This affects follicular survival as well as the life span of the ovarian tissue after transplantation, which are both correlated with the fertility restoration potential.
This review will provide insight into these different factors that affect follicular development and fertility restoration after ovarian tissue transplantation.
Methods
A MEDLINE search was performed to identify articles published in the English language dealing with ovarian tissue and whole ovary transplantation in both animals and humans. Relevant articles up to January 2009 were selected and checked for previously unidentified articles. The following keywords were used: (ovarian tissue or whole ovary) AND (transplantation) AND (cryopreservation or pregnancy).
Selection criteria and outcomes of interest: We reviewed all literature focused on ovarian tissue and whole ovary transplantation and selected relevant articles based on their originality and innovating characteristics. Articles and recent reviews were classified by human and animals experiments. Outcomes of interest were pregnancies, vascularization and factors affecting further follicular development after transplantation.
Ischemic injuries after ovarian tissue transplantation without vascular anastomosis
Because transplantation of fragments of ovarian cortex is performed without vascular reanastomosis, perfusion of the tissue depends on the growth invasion of new blood vessels. The time needed to achieve an adequate perfusion of the transplanted tissue is critical for the follicular survival and the functional longevity of the graft. In mice, initial perfusion of the autograft (revealed with Evan's blue dye injection) is observed 3 days post-transplantation (Nugent et al., 1998). The first stage of neovascularization is detected within 48 h in autologous immature transplanted rat ovaries and the tissue is revascularized and functional after 1 week (Dissen et al., 1994). Using MRI and histology, functional vessels have been detected within ectopic xenotransplanted rat ovarian tissue after only 7 days (Israely et al., 2004). In humans, the neovascularization process was observed after only 3 days following ovarian tissue transplantation onto a chick chorioallantoic membrane (Martinez-Madrid et al., 2009).
The integrity of the stroma is also essential for the neovascularization process and follicular survival after graft. Primordial follicles can tolerate ischemia for at least 4 h during tissue transport (Schmidt et al., 2003), whereas stromal cells surrounding the follicles appeared to be more sensitive to ischemia compared with primordial follicles (Kim et al., 2004a).
Consequences on the follicular pool
The ischemic injury occurring directly after transplantation without vascular anastomosis is involved in the dramatic follicular depletion observed in grafted ovarian tissue. At least 25% of the primordial follicles are lost as a result of cryopreserved xenografts of human ovarian tissue into mice (Newton et al., 1996; Nisolle et al., 2000). Others estimated that ischemic injury during autograft processes induces the depletion of 60–95% of the follicular reserve, including the loss of virtually the entire population of growing follicles (Candy et al., 1997; Aubard et al., 1999; Baird et al., 1999; Aubard, 2003; Liu et al., 2008). This phenomenon is associated with a dramatic reduction of the graft size and a significant fibrosis in most grafts (Kim et al., 2002). This follicular depletion observed after ovarian tissue transplantation is a main concern, especially in humans and large animal species that have a dense ovarian cortex, as it may affect the follicular growth dynamic, the hormonal environment and the fertility restoration potential.
Consequences on graft function
In sheep, oestrus cycles were maintained until 22 months after ovarian tissue transplantation (Baird et al., 1999). Salle et al. (2003) observed gestation for more than 2 years after hemi-ovary autograft in ewes. Experiments in sheep, however, have shown that the autograft resulted in a 3- to 4-fold increase in FSH during the oestrus cycle, possibly due to a deficiency in inhibin A production by the growing follicles (Campbell et al., 2000). An inhibin deficiency, associated with an elevation of FSH level, could explain the granulosa cell hyperplasia observed in the grafted tissue during the re-establishment of follicular development (Callejo et al., 2003). Low anti-Müllerian hormone levels, normally produced by the pool of developing follicles in intact ovaries, also promote massive follicular recruitment after ovarian tissue transplantation (Visser and Themmen, 2005).
This hormonal environment reflects a poor ovarian reserve that could affect the natural fertility capacity and the response to gonadotrophin stimulation. In humans, follicular depletion and cortical injury lead to a ‘poor responder’ status after transplantation. Both hormonal profiles and follicular dynamics observed after transplantation in humans are indeed in agreement with experiments in large mammals. It is established that an optimal hormonal environment is associated with a higher response rate during the IVF cycle (Broekmans et al., 2006).
Follicular development and restoration of ovarian function usually occurs 4–5 months after a transplantation procedure (Donnez et al., 2006), as more than 120 days are necessary to initiate follicular growth and approximately 85 days to reach final maturation stage from a pre-antral follicle (Gougeon, 1996). Ovarian function after transplantation remains for a few months to more than 5 years (Oktay and Karlikaya, 2000; Callejo et al., 2001; Radford et al., 2001; Schmidt et al., 2005; Donnez et al., 2006; Demeestere et al., 2007; Oktay and Oktem, 2008). Despite the restoration of regular menstruation cycles, high basal FSH levels are usually observed after ovarian tissue transplantation in women, reflecting the poor ovarian reserve (Donnez et al., 2005). Persistence of high FSH concentrations most likely contributes to poor oocyte quality and an inadequate maturation stage (Tryde Schmidt et al., 2004). Recently, a large prospective study showed that a basal FSH level greater than 8 IU/l was a strong negative predictor of spontaneous pregnancy in a general subfertile population, even after taking into consideration the age and the cycle length (Van der Steeg et al., 2007). Scarce pregnancies described after ovarian transplantation were obtained during an adequate menstrual cycle (Donnez et al., 2004; Meirow et al., 2005; Demeestere et al., 2006, 2007; Silber et al., 2008b). These case reports well illustrate the importance of achieving an optimal hormonal environment by improving the vascularization process and by grafting sufficient amounts of ovarian tissue.
Consequences on fertility restoration
Although restoration of long-term fertility after ovarian tissue grafts and normal reproductive performance have been reported in mice (Candy et al., 2000), most authors describe a lower fertility rate after ovarian transplantation compared with non-grafted animals (Gunasena et al., 1997; Aubard et al., 1999; Almodin et al., 2004a; Liu et al., 2008; Sauvat et al., 2008). Caution should be taken concerning studies on mice, as ovariectomy procedures can result in incomplete removal of the host ovary. Some authors evaluate that 3–36% of the litter obtained from grafted animals could be derived from the remaining ovarian host fragments (Sztein et al., 1998; Candy et al., 2000) and a suitable (non-graft) control should be always employed to validate studies on mice.
Decreases in the fertility rate after transplantation is actually directly correlated with follicular depletion induced by the ischemic processes, however, others factors may be involved. The reduction in litter size may be linked to abnormal epigenetic status. In mice, methylation status of H19 and LIT1 genes, both sensitive to external conditions, were not modified after ovarian transplantation (Sauvat et al., 2008). Despite the correct imprinting of at least two genes, the reduction in the litter size observed in most studies could also reflect spontaneous miscarriages due to malformations linked with imprinting genes.
Factors affecting graft function after ovarian tissue transplantation
The cryopreservation procedure
The tolerance of human ovarian tissue to the freezing-thawing procedure has been now well studied, with a follicular survival rate reaching 70–80% after slow freezing with appropriate cryoprotectants (Hovatta et al., 1996; Gook et al., 2000; Fabbri et al., 2003; Hreinsson et al., 2003; Maltaris et al., 2006a). The slow-freezing cryopreservation procedure may, however, influence the reproductive outcome after graft.
Although some authors did not observe differences in the litter size between cryopreserved and fresh mouse ovarian grafts (Gunasena et al., 1997; Candy et al., 2000; Shaw et al., 2000), others have suggested that cryopreservation procedure before grafting reduced litter size (Sztein et al., 1998). Immature follicles can be cryopreserved without subsequent DNA fragmentation (Demirci et al., 2002), but the integrity of the granulosa cell structure and function after this process has been questioned (Siebzehnrubl et al., 2000; Navarro-Costa et al., 2005). Using microarray technologies, abnormal gene expression in the granulosa cells has been reported after cryopreserved tissue transplantation compared with normal unmanipulated tissue (Lee et al., 2008). Whether the higher rate of apoptosis and the abnormal gene expression observed in this study can be attributed directly to the cryopreservation procedure or to the transplantation remains to be seen.
Others also describe a decrease in the number of growing follicles after 5 days of culture of frozen-thawed 1-day-old mouse ovaries compared with fresh cultured tissue (Choi et al., 2007). This may be caused by the apoptosis and necrosis phenomenon observed after cryopreservation. Despite the lower development rate of primordial follicles, no significant difference was observed between the level of mRNA expression of markers such as growth differentiation factor GDF-9, inhibin-α or ZP3 for the developing follicle in fresh and frozen-thawed ovaries cultured for 5 days (Choi et al., 2007).
The xenograft model, frequently used as an experimental model to evaluate follicular viability and oocyte competence after transplantation (Newton et al., 1996; Oktay et al., 1998, 2000; Nisolle et al., 2000; Gook et al., 2001, 2005; Van den Broecke et al., 2001; Kim et al., 2002, 2005; Maltaris et al., 2006b), has also been described in the study of factors affecting the neovascularization process. Active angiogenesis was demonstrated 24 days after a human tissue xenograft into nude mice (Nisolle et al., 2000), but fibrosis relative to the surface area was significantly higher after xenotransplantation of cryopreserved tissue compared with fresh tissue xenotransplantation. This difference did not, however, affect follicular depletion rate or the vascularization process. In conclusion, the effect of the cryopreservation of ovarian tissue on the follicular developmental ability and oocyte competence requires further elucidation.
Vitrification procedure has been newly applied to ovarian tissue cryopreservation as an alternative approach to the slow-freezing method in various species such as in mice (Chen et al., 2006b; Aerts et al., 2008), sheep (Bordes et al., 2005; Wang et al., 2008), dogs (Ishijima et al., 2006), bovines, pigs (Gandolfi et al., 2006) and humans (Huang et al., 2008b; Wang et al., 2008). This promising technique may have the advantage of preserving the stromal cells, the collagen bundles, the intercellular space as well as the primordial follicles (Chen et al., 2006b; Wang et al., 2008), but the efficiency and the safety of this procedure should be proved before clinical use.
The clinical factors
The life span of the heterotopic or orthotopic graft is likely to be influenced by several clinical factors such as the age of the patient at the time of cryopreservation, the previous gonadotoxic treatment and the volume of ovarian tissue transplanted. A correlation between theses factors and the life span of the graft is not always easy to establish. Previous chemotherapy before the cryopreservation procedure and the localization of the ovarian graft could interact with the revascularization process of the transplanted tissue. Blood vessel injuries and cortical fibrosis have both been implicated in the follicular loss phenomenon induced by chemotherapy (Meirow et al., 2007b). These cortical injuries could also influence the neovascularization processes after ovarian tissue transplantation for patients receiving chemotherapy prior to the cryopreservation procedure.
Through cortical injury, both the ovarian tissue cryopreservation procedure itself and previous chemotherapy may interfere with neovascularization process after transplantation, inducing higher fibrosis rates in the graft.
Role of exogenous factors
Multiple attempts have been reported to shorten the ischemic period and increase the viability and fertility potential after ovarian graft (Table I).
Table I.
Different options investigated in order to reduce ischemic injuries during ovarian tissue transplantation without vascular anastomosis
| Donor/recipient | Graft site | Effect | References | |
|---|---|---|---|---|
| Vitamin E | Human/mice, Mice/mice | Kidney caps. | Improve survival rate, reduction of lipid peroxide and malondialdehyde | Nugent et al. (1998) |
| Mice/mice | Not precise | No beneficial effect | Weissman et al. (1999) | |
| Melatonin Oxytetracyclin | Rat/rat | ip | Reduce ovarian necrosis | Sapmaz et al. (2003) |
| VEGF | Monkey/monkey | sc | Decrease graft viability | Schnorr et al. (2002) |
| Human/mice | ip | No vascularization improvement | Donnez et al. (2006a) | |
| Androgen (male or testosterone treated hosts) | Human/mice | sc | Increase follicular development after stimulation | Weissman et al. (1999) |
| Hamster/hamster | Kidney caps. | Increase follicular population | Arrau et al. (1983) | |
| Mice/rat | Kidney caps. | Increase oocyte yield after stimulation | Snow et al. (2002) | |
| Mice/mice | Kidney caps. | Implantation rate, fetal development unaffected | Waterhouse et al. (2004) | |
| Gonadotrophins after graft (recipient) | Mice/mice | Kidney caps. | No difference in follicular survival | Nugent et al. (1998) |
| Mice/mice | abd. wall | No difference compared with untreated recipients | Imthurn et al. (2000) | |
| Human/mice | sc/kidney caps. | Earlier initiation of follicular development | Van den Broecke et al. (2001) | |
| Human/mice | Neck muscle | Depletion of primordial follicles | Maltaris et al. (2007a) | |
| Human/mice | Kidney caps. | Promote follicular growth | Oktay et al. (1998) | |
| Gonadotrophins before graft (recipient) | Mice/mice | sc | Increase of the growing follicles survival | Wang et al. (2002a) |
| Mice/mice | abd. wall | Increase of the growing follicles survival | Imthurn et al. (2000) | |
| GnRHa (±GnRH) | Human/mice | im | No or detrimental effect on follicular loss prevention | Maltaris et al. (2007b) |
| GnRHa + estradiol | Sheep/sheep | Ovarian pedicle | No difference in the number of primordial follicle, reduce follicular growth | Campbell et al. (2000) |
| Graft into granulation tissue | Rat/mice | im | Improve graft perfusion and follicular survival | Israely et al. (2006) |
| EPO | Dog/mice | Ov. bursa | Enhanced follicular survival | Suzuki et al. (2008) |
sc: subcutaneous; ip: intraperitoneal; im: intramuscular; abd, abdominal; ov: ovarian; caps.: capsule; VEGF: vascular endothelial growth factor; EPO: erythropoietin; GnRH: gonadotrophin releasing hormone.
Antioxidants factors
During ischemia-reperfusion processes, oxygen free radicals constitute the most important component that induces damage of the cell membrane proteins and decreases mitochondrial function and lipid peroxidation (Kupiec-Weglinski and Busuttil, 2005). Endogen antioxidant molecules are able to neutralise these oxygen free radicals produced in excess during the ischemic process. This system, however, can be rapidly overwhelmed. During solid organ transplantation, exogen antioxidants are used to quench free radicals and preserve organs. Both ascorbic acid and mannitol have been shown to be effective in reducing surgically-induced ovarian ischemic injury in a rat model (Sagsoz et al., 2002). A potential benefit of antioxidants administration was also tested during ovarian tissue transplantation. Local antioxidant injection of vitamin E before graft could improve follicular survival rate (Nugent et al., 1998), but these results were not confirmed by others (Weissman et al., 1999). Other antioxidants such as melatonin and oxytetracycline locally administered during intraperitoneal rat ovarian graft were effective to reduce ovarian necrosis (Sapmaz et al., 2003). Kim et al. (2004a) evaluated the efficiency of ascorbic acid to reduce apoptosis of primordial follicles and stromal cells after deprivation of bovine ovarian cortex blood supply for up to 48 h. They showed that stromal cells were more sensitive to ischemic injury than primordial follicles, and that apoptosis was reduced when the tissue was incubated with ascorbic acid up for to 24 h, but not later.
No beneficial effect of antioxidant agents on the follicular survival rate after ovarian transplantation has been yet demonstrated. Moreover, the use of these agents should be further investigated in vitro and in vivo to guarantee their safety.
Growth factors
Multiple growth factors such as fibroblast growth factor, transforming-growth factor (TGFβ-α) or vascular endothelial growth factor (VEGF) are involved in the invasion of the tissue by new vessels. The invasion of the rat cortex by vessels 48 h after a graft is associated with a 5- and 10-fold increase in the expression of mRNA in the outer cortex for TGFβ1 and VEGF, respectively (Dissen et al., 1994). Surprisingly, angiogenic factors such as VEGF failed to have beneficial effects on primate graft function (Schnorr et al., 2002). In contrast, erythropoietin (EPO) may enhance the survival of transplanted tissue, as it promotes the differentiation and proliferation of erythroid progenitor cells as well as preventing apoptosis (Suzuki et al., 2008). The effect of growth factors is still controversial but recent results are encouraging. Their beneficial effect on further follicular development or fertility restoration should be confirmed.
Hormonal factors
Gonadotrophin administration, starting immediately after ovarian tissue transplantation for 3–4 days with the aim of up-regulating VEGF mRNA levels, did not improve the primordial or growing follicles survival rate in the grafts compared with untreated recipients (Nugent et al., 1998; Imthurn et al., 2000). Imthurn et al. (2000) evaluated the effect of 4 days of intraperitoneal administration of gonadotrophins (recombinant human FSH and LH, 3 IU) beginning 2 or 4 days prior to, or on the day of the ovarian tissue graft at a poorly vascularised site (the abdominal wall). They showed that gonadotrophins stimulation 2 days before and 2 days after grafting increased the total number of growing follicles in the graft. Wang et al. (2002a) showed an increase in the growing follicular population when gonadotrophins (human menopausal gonadotrophin, hMG, 5 IU/d) were administered to recipients 4 days before the graft compared with untreated grafted recipients. Angiogenic factors as VEGF, up-regulated by gonadotrophins, may be required to be present in effective amounts before transplantation to be efficient.
Furthermore, hormonal pretreatment of the donor before ovary removal appears to also have a beneficial effect on the growing viable population after grafting into recipients (Imthurn et al., 2000). It was suggested that gonadotrophins stimulation (hMG or urofollitrophin) of the recipients 1 or 2 weeks after human tissue xenograft promotes follicular development, however, it seems to contemporaneously deplete primordial follicles pool (Van den Broecke et al., 2001; Maltaris et al., 2007a). In a porcine tissue xenograft model, others showed that gonadotrophin administration (FSH) improves the meiotic competence of the oocytes collected by supporting oocyte growth (Kaneko et al., 2006).
Stimulation with FSH was favourable and required after human tissue xenograft to sustain long-term follicular development beyond the two layers stage in a model using hypogonadic SCID mice (Oktay et al., 1998, 2000). Using another mice strain, however, Gook et al. (2001) reported follicular development up to the antral stage after xenograft into non-hypogonadic SCID mice without exogenous gonadotrophin stimulation.
Elevated endogen gonadotrophin secretion, due to the ovarian failure status before the graft, could also increase the growing follicular proportion but may have a direct toxic effect (Flaws et al., 1997), depleting the primordial follicular pool of the grafted tissue. GnRH agonist, administered to reduce endogenous gonadotrophin levels, surprisingly failed to prevent follicular depletion (Maltaris et al., 2007b) and even severely retarded the re-establishment of normal follicle development (Campbell et al., 2000). The effect of endogenous and exogenous gonadotrophins thus appears to differ.
The results from animal studies lead to options for different approaches in humans. The injection of FSH directly into the subcutaneous site along with an aspirin regimen for 7 days after a heterotopic transplantation procedure was attempted in humans to improve the revascularization process (Oktay et al., 2003). In contrast, Donnez et al. (2006, 2007) suggested the administration of oestro-progesterone tablets before the transplantation along with a GnRH antagonist at the time of the procedure in order to reduce endogen gonadotrophins levels. Meirow et al. (2005) proposed to administer oestro-progesterone tablets during the first post-transplantation month. Another option was to avoid any hormonal treatment after the transplantation procedure (Tryde Schmidt et al., 2004; Schmidt et al., 2005; Demeestere et al., 2006, 2007).
In conclusion, animal experiments show that gonadotrophin stimulation of a recipient or donor initiated at a reasonable time before and continued to suboptimal sites after grafting could have a positive effect on the viable growth follicle rate, but the impact on the long-term ovarian function and fertility of such treatment must be further investigated. The position regarding hormonal treatment before and after ovarian tissue transplantation in human is variable and not yet standardize.
Mechanical factors
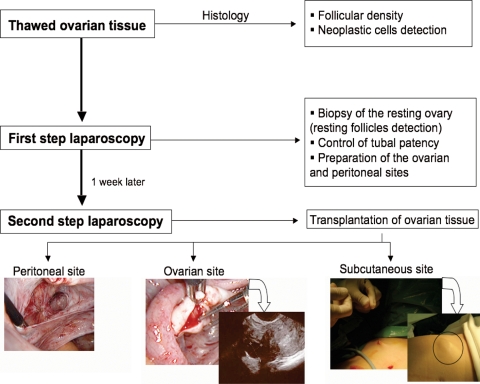
Angiogenesis can also be mechanically stimulated by triggering endogenous processes of new vessel formation. After injury, the inflammatory phase allows collagen deposits to occur although angiogenesis helps to sustain new tissue formation. This physiological phenomenon was used by Donnez et al. (2004) and later by ourselves (Demeestere et al., 2006), inducing neovascularization by creating a peritoneal pocket or longitudinally opening the ovary at the ovarian tissue transplantation sites 1 week before the transplantation procedure (two steps laparoscopy) (Fig. 2). Animal experiments confirmed that the ovarian grafts transplanted into granulation tissue were already perfused at least 24 h prior the intact control grafts (Israely et al., 2006).
Figure 2.
Diagram illustrating the different steps of the cryopreserved ovarian tissue transplantation procedure in human by two-step laparoscopy.
To date, most experiments evaluating different treatments using animal models failed to clearly prevent follicular loss during the ischemic period and increase fertility potential. In humans, the limited number of patients as well as the heterogeneity of the procedure led to the difficult evaluation of the efficiency of the different treatments. The optimal environment before and after transplantation in humans needed to achieve a high follicular survival in the transplanted ovarian tissue remains unclear and needs to be further investigated.
Ovarian transplantation sites: heterotopic or orthotopic
The choice of the transplantation sites constitutes an essential factor involved in future graft viability and in the subsequent oocyte competence. Ovarian tissue can be transplanted back to the original site (orthotopic) or to alternative sites (heterotopic). For each site, clinical considerations such as the possibility of natural conception, ease of the procedure, convenient access for oocyte collection and the volume of tissue transplanted must be taken into consideration (Table II).
Table II.
Advantages and disadvantages of heterotopic and orthotopic sites for ovarian tissue transplantation
| Heterotopic site (subcutaneous) | Orthotopic site | |
|---|---|---|
| Advantages | No limitation of the number of fragments transplanted | Possibility of natural conception |
| Easy transplantation procedure | Restoration of fertility demonstrated | |
| Easy access for follicular monitoring and oocytes collection | Favourable environment for follicular development | |
| Disadvantages | Restoration of fertility not yet demonstrated | Number of fragments transplanted limited by the ovarian size |
| IVF procedure required | Invasive transplantation procedure | |
| Effect of the local environment on the oocyte quality is unknown |
Animal experiments
Animal experiments allow comparison of the follicular development potential at the different orthotopic and heterotopic sites after ovarian tissue autograft or xenograft. Using the xenograft model, Israely et al. (2003) showed that subcutaneous transplantation of rat ovaries into mice is followed by pericyte loss associated with tissue damage, whereas i.m. transplantation allows vascular maintenance and better follicular preservation. In rabbit, histology and ultrastructure of grafted fresh and cryopreserved ovarian tissue into the mesometrium, the ovarian bursa, or the ovary are comparable (Deng et al., 2007).
Other studies concluded that ovarian bursa or kidney capsule sites were more favourable than subcutaneous or intraperitoneal sites (Imthurn et al., 2000; Callejo et al., 2002; Risvanli et al., 2006; Yang et al., 2006). In rat, the subcutaneous site displays fewer primary follicles and corpus luteum than the subperitoneal site (Risvanli et al., 2006). In mice, the grafts placed in subperitoneal pockets contained significantly fewer growing follicles (12%) than non-grafted ovaries and ovaries grafted under the kidney capsule (70%), showing that the transplantation of an ovary to the untreated inner side of the lateral abdominal wall was suboptimal (Imthurn et al., 2000). Subcutaneous grafted ovaries also have a lower oocyte yield compared with those placed under the kidney capsule or in the bursal cavity (orthotopic site) in this species (Yang et al., 2006). Compared with the kidney capsule site, ovarian tissue graft in the back muscle in mice has recently been shown to have a better follicular survival rate (Soleimani et al., 2008).
Considering the endocrine function, no differences in estradiol or FSH levels were observed after 6 months follow-up of rat transplantation at the subcutaneous or intraperitoneal site (Callejo et al., 1999).
As the primary indication for the ovarian tissue transplantation is to restore fertility of women and children facing premature ovarian failure as a result of cancer treatments, the evaluation of the oocyte competence and the normal embryo development after ovarian tissue grafts in various sites constitutes an essential prerequisite for human application.
To date, animal experiments have clearly shown that, depending on the graft site, oocytes collected from graft ovarian tissue have a lower embryo developmental potential than controls (Gunasena et al., 1997; Aubard et al., 1999; Snow et al., 2002; Waterhouse et al., 2004; Yang et al., 2006). The 2-cell cleavage rate from the in vitro matured oocytes was higher when oocytes were derived from graft in the bursal cavity compared with other heterotopic sites. The implantation rate did not differ regarding the graft sites (Yang et al., 2006). Orthotopic as well as heterotopic sites (kidney capsule) led to the birth of normal live young (Table III). One malformed mouse fetus born after fresh ovarian tissue transplantation was reported (Shaw et al., 2000). Vitrification has been also used as an ovarian tissue cryopreservation method and young have been obtained after grafting in different species (Bordes et al., 2005; Chen et al., 2006a; Hasegawa et al., 2006; Bagis et al., 2008). Bordes et al. (2005) reported four lambs born following ovarian tissue vitrification and graft, from which one had a malformation of the leg and oesophagus.
Table III.
Pregnancies and young obtained since 1990 after transplantation of fresh and cryopreserved ovarian tissue in animal models
| Species | Graft site | Tissue transplanted | Pregnancy rate | Total number of pregnancies | Live birth | References |
|---|---|---|---|---|---|---|
| Mice | Ov. bursa | SF Suspend tissue in fibrin clot | 80% (4/5) | 5 pups 6 implants | Normal | Carroll and Gosden (1993) |
| Ov. bursa | SF | 86% | – | Normal | Cox et al. (1996) | |
| Ov. bursa | Fresh/SF | 100%/72% | >50 litters | Normal | Gunasena et al. (1997) | |
| Ov. bursa | Fresh/SF | 70%/57% | 41 pups | Normal | Sztein et al. (1998) | |
| Ov. bursa | Fresh/SF | 92%/83% | – | Normal | Candy et al. (2000) | |
| Ov. bursa | Fresh/SF | 57%/57% | 4 litters/4 litters | 1 malformation | Shaw et al. (2000) | |
| Kidney caps. | Fresh | 33–66% (IR) | 19 pups | Normal | Waterhouse et al. (2004) | |
| sc | Fresh | 70% (IR) | 2 F (day 15) + 4 pups | Low FW | Yang et al. (2006) | |
| Ov. bursa | 65–100% (IR) | 8 F (day 15) + 14 pups | Normal | |||
| Kidney caps. | 53–100% (IR) | 9 F (day 15) + 3 pups | Normal | |||
| Ov. bursa | DCV/CV/SF/fresh | 83/33/60/93% | >100 pups | – | Chen et al. (2006b) | |
| Ov. bursa | Fresh/SF | 70%/87% | <100 litters | – | Liu et al. (2008) | |
| Rat | Ov. bursa | SF | 72% (13/18) | – | Normal | Aubard et al. (1998) |
| Rabbit | Ov. bursa | Fresh (allo- or autograft) | 53% (9/17) | 16 litters | – | Petroianu et al. (2002) |
| Intracortical sowing (ovary) | SF | 100% (5/5) | 7 gestations (22 young) | Normal | Almodin et al. (2004a) | |
| Ov. bursa | Fresh (allo- or autograft) | 37.5–62.5% | 44 litters | – | Petroianu et al. (2006, 2007) | |
| Mice/rat | Kidney caps. | Fresh (xenograft) | 24.2–37.5% (IR) | 5 pups | Normal | Snow et al. (2002) |
| Sheep | Ov. pedicle | Fresh/SF | – | 1 lamb/1 lamb | Normal | Gosden et al. (1994) |
| Ov. pedicle | SF | – | Triplet | Normal | Baird et al. (1999) | |
| Intracortical sowing (ovary) | SF | 100% (2/2) | 4 lambs (1twin) | Normal | Almodin et al. (2004b) | |
| Ov. pedicle | SF | 66% (4/6) | 11 lambs | 5 neonatal death, no congenital abnormalities | Salle et al. (2002, 2003) | |
| Ov. pedicle | Vitrified | 50% (3/6) | 4 lambs | 1 malformed | Bordes et al. (2005) | |
| Monkey | sc | Fresh | 100% (1/1) | 1 young | Normal | Lee et al. (2004) |
Ov.: Ovarian; caps.: capsule; sc: subcutaneous; IR: implantation rate; FW: fetus weight; SF: slow freezing; CV: conventional vitrification; DCV: direct cover vitrification; F: fetuses.
A few reports of live young, obtained after in vitro fertilization of oocytes derived from ovarian tissue grafted subcutaneously, were described in monkey (Lee et al., 2004) and mice (Yang et al., 2006). Embryos were obtained after in vitro fertilization of oocytes collected from ovarian tissue were transplanted subcutaneously in sheep, however, they failed to reach the blastocyst stage (Aubard et al., 1999).
Human experiments
Ovarian xenografts into mice provide a valuable experimental model to study the follicular developmental potential of tissue samples taken from various large mammals including humans (Aubard, 2003). This technique could also be useful to evaluate the gonadotoxicity of various drugs (Oktem and Oktay, 2007) or for the conservation of rare and endangered species (Paris et al., 2004). Most experiments using xenotransplantation of human ovarian tissue into mice also show a difference in the number of resting follicles when grafts are located subcutaneously or under the kidney capsule (Abir et al., 2003; Hernandez-Fonseca et al., 2004), with some exceptions (Van den Broecke et al., 2001). After an average of 24 days, the degree of fibrosis and the relative surface of the capillaries do not differ when intraperitoneal and subcutaneous human ovarian xenografts into mice were compared (Nisolle et al., 2000).
Concerning autotransplantation, the first orthotopic transplantation of cryopreserved ovarian tissue was reported by Oktay (Oktay and Karlikaya, 2000). Since that time, different sites have been investigated in humans to restore ovarian function and fertility. Orthotopic sites included ovarian tissue transplantation in the peritoneum of the ovarian fossa and/or to the remaining ovary (Fig. 2, personal data). Because of the low invasive surgical aspect and its easy access, the subcutaneous site (the abdominal wall or forearm) is regularly chosen as the heterotopic site and is sometimes associated with transplantation at the orthotopic site (Callejo et al., 2001; Oktay et al., 2001, 2003; Wolner-Hanssen et al., 2005; Demeestere et al., 2006; Oktay, 2006) (Fig. 2, personal data). Other heterotopic sites were also tested in humans, such as the uterus, rectus abdominal muscle (Callejo et al., 2001; Kim et al., 2004b), the space between the breast tissue and superficial fascia of the pectoralis muscle (Kim et al., 2004b) as well as the subperitoneal tissue beneath the abdominal fascia between the umbilicus and the pubic bone (Rosendahl et al., 2006). Heterotopic sites were shown to be effective to restore ovarian function but no clinical pregnancy has been reported from oocyte collected, despite the fact that embryos were obtained and transferred (Oktay et al., 2001, 2004; Demeestere et al., 2006). Nevertheless, Rosendahl et al. (2006) recently showed that an ovarian graft at a heterotopic site could result in the production of mature fertilizable oocytes capable of initiating pregnancy (biochemical pregnancy) (Rosendahl et al., 2006).
In all the cases of birth reported after transplantation of ovarian tissue, the fertilized oocytes originated from tissue transplanted at the orthotopic site: to the peritoneum in the ovarian fossa (Donnez et al., 2004) or to the remaining ovary (Meirow et al., 2005; Demeestere et al., 2007; Andersen et al., 2008; Silber et al., 2008a).
Regarding the influence of the ovarian site in humans, additional interesting observations can be drawn from previously published reports as a result of the ability to compare long-term follicular activities at different sites (subcutaneous, peritoneal and ovary) in the same patient (Demeestere et al., 2006, 2007). Over the 14 documented post-transplantation cycles, follicles ≥15 mm diameter at the time of ovulation were observed in 7, 29 and 64% of the cycles at the peritoneal, subcutaneous and ovarian sites, respectively, although the volume of the tissue transplanted at the ovarian site was 2- to 3-fold smaller than at the other sites (personal data). The follicular development is also delayed at the subcutaneous site compared with the ovarian site in the case of concomitant transplantation (Table IV). In contrast, when subcutaneous ovarian tissue transplantation was performed alone, the time necessary to obtain ovarian function recovery was reported to vary from 10 to 15 weeks, which is even shorter than expected (Kim et al., 2004b; Oktay et al., 2004b). Follicular development could therefore occur preferentially at the ovarian site when heterotopic and orthotopic ovarian tissue transplantations are simultaneously performed. Considering the oocyte competence, a total of three oocytes out of seven punctured follicles (four natural cycles) have been collected from the subcutaneous site, however, two of them were degenerated. One 3-cell embryo was transferred after IVF but no pregnancy was observed (Table IV). After subcutaneous ovarian tissue transplantation, Oktay et al. (2004) obtained 20 oocytes from eight consecutive percutaneous oocyte retrievals and six after ovarian stimulation (Oktay et al., 2004). Eight of them were suitable for IVF, five after in vitro maturation, but only two fertilized. One 4-cell embryo was transferred but failed to implant. Finally, it is interesting to note that follicular development seems to be limited at the heterotopic site as most of the follicles failed to grow more than 15 mm in size. The poor oocyte recovery rate and the low fertilization rate obtained suggest that other factors such as temperature, local pressure and environment at the subcutaneous site might contribute to the poor quality of the oocytes.
Table IV.
Follow-up of the follicular development after cryopreserved ovarian tissue transplantation in order to restore fertility of a patient with premature ovarian failure after bone marrow transplantation
| Days post-transplantation | Cycle | Foll. phase length | bFSH | Follicles size at ovulation (mm) |
Post-ovulation decision |
Results | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ovary | Peritoneal | SC right | SC left | Oocytes collected | Fertilization (IVF) | Embryo transfer | |||||
| Nov 2004 (0) | First transplantation (ovary-SC right- peritoneal sites) | ||||||||||
| 148 | Spontaneous | 22 | 12–12.5 | 21 | 13.5 | Timing intercourse | No pregnancy | ||||
| 165 | Spontaneous | 5 | 7 | 16.5–10–8 | 8 | – | Timing intercourse | No pregnancy | |||
| 190 | Spontaneous | 11 | 5 | ND | ND | ND | Timing intercourse | No pregnancy | |||
| 213 | Spontaneous | 10 | 6 | 16–14–13 | – | – | Timing intercourse | No pregnancy | |||
| 237 | Spontaneous | 12 | 6 | 15–11.5 | – | 11 | Timing intercourse | No pregnancy | |||
| 261 | Spontaneous | 11 | 9 | 19.5–10 | – | 10 | Timing intercourse | Miscarriage | |||
| 372 | Spontaneous | 21 | 19 | 18–14 | – | 12 | Timing intercourse | No pregnancy | |||
| 389–409 | Pill | – | 41 | OC 34 | – | – | – | No pregnancy | |||
| 434 | Stimulation | – | – | 18 | – | – | Timing intercourse | No pregnancy | |||
| 518 | Spontaneous | 28 | 19 | 17.5–11.5 | – | 16–11 | 2 | 1 (SC site) | 1 (3 cells) | No pregnancy | |
| May 2006 | Second transplantation (ovary-SC left sites) | ||||||||||
| 583 | Spontaneous | 17 | 25 | – | – | 16.5–10.5 | – | 1 | 0 (deg) | 0 | No pregnancy |
| 608 | Spontaneous | 11 | 24 | – | – | 15 | – | Timing intercourse | No pregnancy | ||
| 635 | Spontaneous | 11 | 6 | 11 | – | 17.5–13 | – | 1 | 0 (deg) | 0 | No pregnancy |
| 650 | Spontaneous | 7 | 6 | 22.5 | – | – | – | Timing intercourse | No pregnancy | ||
| 669 | Spontaneous | 9 | 9 | 15–15 | – | – | – | Timing intercourse | Pregnancy | ||
| June 2007 | Delivery of healthy girl | ||||||||||
| PP-3 months | Spontaneous | 2 follicles | – | – | – | – | – | ||||
| PP-4 months | Spontaneous | ND | ND | – | – | – | – | ||||
| PP-5 months | Spontaneous | 6 | 17.5 | – | 19–14.5 | 12.5 | 0 | 0 | 0 | – | |
| PP-8 months | Spontaneous | 26 | ND | ND | – | – | – | ||||
| PP-15 months | Spontaneous | 17.3 | ND | ND | |||||||
| PP-17 months | Spontaneous | 45 | ND | ND | |||||||
Transplantation procedure has been performed twice in November 2004 and in May 2006 at different sites: ovarian, sub-cutaneous and/or peritoneal. Follicular phase length, basal FSH levels (bFSH), the follicular site at the time of ovulation and the outcomes of each cycle are reported.
deg = degenerated; ND = not done; OC = ovarian cyst; SC = sub-cutaneous, PP = post-partum.
These results are consistent with those obtained in animal studies, showing that follicular development is influenced by the site of transplantation and that the heterotopic site is probably suboptimal compared with the ovarian site.
Despite these considerations, the small size of the atrophic organ (range 0.3–1.3 cm3) limits the volume of ovarian tissue transplantable in the remaining native ovary (Schmidt et al., 2005; Demeestere et al., 2006). Considering the massive loss of the primordial follicle population by an ischemic process, the pool of functional resting follicles of the small amount of ovarian tissue transplantable at the orthotopic site is likely to be limited. Although peritoneal and subcutaneous sites do not appear to be optimal, the graft of a larger amount of ovarian tissue using a combination of heterotopic and orthotopic ovarian tissue transplantation may have a beneficial effect on endocrine function and fertility restoration potential.
Ovarian tissue transplantation versus whole ovarian transplantation with vascular anastomosis
Ovarian transplantation with vascular anastomosis permits an immediate revascularization of the ovarian cortex, significantly reducing the ischemic injury previously described (Bedaiwy and Falcone, 2004). Conversely, the procedure cannot be repeated, and because it is more complex, it requires particular surgical skill. Whole ovary specimens have been transplanted in animal models as well as in humans. Vascular anastomosis of fresh ovary was successfully performed using the ovarian artery, inferior epigastric vessels, carotids vessels or iliac artery in various species (Goding, 1966; Paldi et al., 1975; Scott et al., 1981; Denjean et al., 1982; Wang et al., 2002b). In sheep, the revascularization process was compromised in around 50% of the cases (Jeremias et al., 2002). In humans, ovarian transplantation in the upper arm was performed with success before pelvic irradiation (Leporrier et al., 1987; Hilders et al., 2004). In the first case, a testicular prosthesis was inserted in the forearm of the patients 3 months before the transplantation in order to create a cavity for the transplanted ovary. Over a follow-up period of 16 years, the ovary remained functional (Leporrier et al., 2002). In the second case, the transplantation was performed during the radical hysterectomy for cervical carcinoma and the ovarian cycles remained regular for more than 1 year without local sequelae due to cyclic enlargement of the ovary. Recently, Silber et al. (Silber et al., 2008c) reported a first full-term pregnancy obtained using orthotopic whole fresh ovary transplantation between monozygotic twins who are discordant for premature ovarian failure in order to restore fertility in the affected twin.
The important challenge of the whole ovary procedure concerns the cryopreservation to ensure the diffusion of the cryoprotectant and maintain the healthy structure of the organ. The anti-apoptotic agent (sphingosine-1-phosphate) has been tested without success to increase the cell's survival during the procedure, particularly the endothelial arterial disruption (Onions et al., 2008). Wang et al. (2002b) reported the first pregnancy after transplantation of frozen-thawed rat ovaries, fallopian tubes and upper segment of the uterus in bloc. Ovarian function, however, was restored in only 57% of the rats transplanted with cryopreserved ovaries compared with 100% when fresh organs were transplanted (Yin et al., 2003). Using epigastric vessels or ovarian vascular pedicle, transplantation of a frozen-thawed ovary was also performed with success in sheep (Bedaiwy et al., 2003; Revel et al., 2004; Arav et al., 2005; Bedaiwy and Falcone, 2007) and rabbit (Chen et al., 2006a). In sheep, reanastomosis was successful in only around 60% of the animals due to venous thrombosis or a torn artery (Jeremias et al., 2002; Revel et al., 2004; Imhof et al., 2006). This most likely reflects endothelial damage by the freezing-thawing procedure or by the ischemic time until successful reanastomosis. In the successfully transplanted sheep, cycles were maintained during the 24–36 months period (Arav et al., 2005). The procedure resulted in the birth of a healthy lamb (Imhof et al., 2006). Eighteen months after grafting, the authors reported a massive follicular depletion with less than an 8% follicular survival rate. Other authors reported only 6% of viable follicles and the depletion of the entire follicular population after fresh ovarian and vitrified ovarian grafts, respectively (Courbiere et al., 2008). Ovarian vessel thrombosis was observed in both groups with a higher incidence after whole vitrified ovarian transplantation.
Cryopreservation of a whole ovary using the slow protocol has been performed in humans (Martinez-Madrid et al., 2004, 2007a; Bedaiwy et al., 2006; Martinez-Madrid and Donnez, 2007b), showing vessels and follicular integrity of the ovary after freezing and thawing. Recent advances in whole human ovary cryopreservation procedure using multi-gradient freezing device are also promising (Bromer and Patrizio, 2008). The authors described high follicular viability, normal histological architecture and no evidence of damage to the vessel after this procedure, suggesting a vascular reanastomosis may be feasible.
The transplantation procedure, however, has yet to be attempted in human. Recent data suggest that whole frozen-thawed ovary transplantation is likely to be successful in humans in the future. Despite these encouraging results however, caution is indicated due to the dramatic depletion of follicular density observed after transplantation in animals. The efficiency of transplantation of the whole cryopreserved ovary should be further investigated in animal models.
Conclusion
Considerable advances in the field of fertility preservation have been obtained in the last decade, leading to the introduction of a new dimension of quality of life in many oncological centres. Consequences include an important increase in the request for fertility preservation procedures such as cryopreservation of ovarian tissue. Recent pregnancies published and the birth of healthy babies after cryopreserved ovarian tissue transplantation represent a great hope for these patients. Despite the evidence available for the efficacy of cryopreserved ovarian tissue transplantation to restore fertility, the success rate of the procedure is still limited. Follicular depletion after tissue transplantation without vascular anastomosis is a major concern, limiting the life-span of the transplanted tissue and influencing the hormonal environment after the procedure. Many attempts have already been made to increase the viability of the graft. Most of the exogenous factors used however, have not been efficient or are not applicable in humans.
The transplantation site plays a key role in the neovascularization process and could also influence the subsequent follicular development and oocyte competence through other mechanisms. Based on animal and human experiences, we show that heterotopic sites are suboptimal compared with the orthotopic site. This, however, presents some interesting advantages, justifying further investigation to improve these results.
To avoid ischemic injury, transplantation of a whole cryopreserved ovary may be the better option. Recent data on the viability of the human whole ovary after cryopreservation are encouraging and further research should allow the utilization of this option in the future.
Finally, transplantation of ovarian tissue cannot be proposed for all patients, due to the risk of tumour cell retransmission during the procedure (Kim et al., 2001; Oktay, 2001; Radford, 2004; Sonmezer et al., 2005). Research programmes are needed to develop alternatives for these patients such as isolated follicles transplantation (Dolmans et al., 2007), in vitro follicular culture (Smitz and Cortvrindt, 1999), or pharmacological protection (Paris et al., 2002; Blumenfeld, 2007; Oktay et al., 2007). Recently, the success of 3D culture systems simulating physiological conditions provides a new possibility for the development of in vitro maturation of ovarian follicles in human (Xu et al., 2006).
Author's Role
I.D. is responsible for the fertility preservation project. P.S. is responsible for the Gynaecologic Department and performed surgical procedures (ovarian tissue removal and transplantation). S.E. is the Director of the IVF Laboratory, where the embryo from the oocyte collected at the heterotopic site was obtained. A.D. is responsible for the Fertility Clinic, supported the project and revised the manuscript. Y.E., Director of the Research Laboratory of Human Reproduction and of the Department of Gynaecology and Obstetrics of Erasme Hospital, supported the project and revised the manuscript.
Funding
This study was supported by the Belgian National Fund for Scientific Research (FRSM) and the ‘Fondation Belge contre le Cancer’.
References
- Abir R, Orvieto R, Raanani H, Feldberg D, Nitke S, Fisch B. Parameters affecting successful transplantation of frozen-thawed human fetal ovaries into immunodeficient mice. Fertil Steril. 2003;80:421–428. doi: 10.1016/s0015-0282(03)00658-7. [DOI] [PubMed] [Google Scholar]
- Aerts JM, De Clercq JB, Andries S, Leroy JL, Van Aelst S, Bols PE. Follicle survival and growth to antral stages in short-term murine ovarian cortical transplants after Cryologic solid surface vitrification or slow-rate freezing. Cryobiology. 2008;57:163–169. doi: 10.1016/j.cryobiol.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Almodin CG, Minguetti-Camara VC, Meister H, Ferreira JO, Franco RL, Cavalcante AA, Radaelli MR, Bahls AS, Moron AF, Murta CG. Recovery of fertility after grafting of cryopreserved germinative tissue in female rabbits following radiotherapy. Hum Reprod. 2004;a 19:1287–1293. doi: 10.1093/humrep/deh246. [DOI] [PubMed] [Google Scholar]
- Almodin CG, Minguetti-Camara VC, Meister H, Ceschin AP, Kriger E, Ferreira JO. Recovery of natural fertility after grafting of cryopreserved germinative tissue in ewes subjected to radiotherapy. Fertil Steril. 2004;b 81:160–164. doi: 10.1016/j.fertnstert.2003.05.023. [DOI] [PubMed] [Google Scholar]
- Andersen CY, Rosendahl M, Byskov AG, Loft A, Ottosen C, Dueholm M, Schmidt KL, Andersen AN, Ernst E. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod. 2008;23:2266–2272. doi: 10.1093/humrep/den244. [DOI] [PubMed] [Google Scholar]
- Arav A, Revel A, Nathan Y, Bor A, Gacitua H, Yavin S, Gavish Z, Uri M, Elami A. Oocyte recovery, embryo development and ovarian function after cryopreservation and transplantation of whole sheep ovary. Hum Reprod. 2005;20:3554–3559. doi: 10.1093/humrep/dei278. [DOI] [PubMed] [Google Scholar]
- Arrau J, Roblero L, Cury M, Gonzalez R. Effect of exogenous sex steroids upon the number of germ cells and the growth of foetal ovaries grafted under the kidney capsule of adult ovariectomized hamsters. J Embryol Exp Morphol. 1983;78:33–42. [PubMed] [Google Scholar]
- Aubard Y. Ovarian tissue xenografting. Eur J Obstet Gynecol Reprod Biol. 2003;108:14–18. doi: 10.1016/s0301-2115(02)00424-4. [DOI] [PubMed] [Google Scholar]
- Aubard Y, Newton H, Scheffer G, Gosden R. Conservation of the follicular population in irradiated rats by the cryopreservation and orthotopic autografting of ovarian tissue. Eur J Obstet Gynecol Reprod Biol. 1998;79:83–87. doi: 10.1016/s0301-2115(98)00044-x. [DOI] [PubMed] [Google Scholar]
- Aubard Y, Piver P, Cogni Y, Fermeaux V, Poulin N, Driancourt MA. Orthotopic and heterotopic autografts of frozen-thawed ovarian cortex in sheep. Hum Reprod. 1999;14:2149–2154. doi: 10.1093/humrep/14.8.2149. [DOI] [PubMed] [Google Scholar]
- Bagis H, Akkoc T, Tass A, Aktoprakligil D. Cryogenic effect of antifreeze protein on transgenic mouse ovaries and the production of live offspring by orthotopic transplantation of cryopreserved mouse ovaries. Mol Reprod Dev. 2008;75:608–613. doi: 10.1002/mrd.20799. [DOI] [PubMed] [Google Scholar]
- Baird DT, Webb R, Campbell BK, Harkness LM, Gosden RG. Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at -196 C. Endocrinology. 1999;140:462–471. doi: 10.1210/endo.140.1.6453. [DOI] [PubMed] [Google Scholar]
- Bedaiwy MA, Falcone T. Ovarian tissue banking for cancer patients: reduction of post-transplantation ischaemic injury: intact ovary freezing and transplantation. Hum Reprod. 2004;19:1242–1244. doi: 10.1093/humrep/deh262. [DOI] [PubMed] [Google Scholar]
- Bedaiwy MA, Falcone T. Harvesting and autotransplantation of vascularized ovarian grafts: approaches and techniques. Reprod Biomed Online. 2007;14:360–371. doi: 10.1016/s1472-6483(10)60880-2. [DOI] [PubMed] [Google Scholar]
- Bedaiwy MA, Hussein MR, Biscotti C, Falcone T. Cryopreservation of intact human ovary with its vascular pedicle. Hum Reprod. 2006;21:3258–3269. doi: 10.1093/humrep/del227. [DOI] [PubMed] [Google Scholar]
- Bedaiwy MA, Jeremias E, Gurunluoglu R, Hussein MR, Siemianow M, Biscotti C, Falcone T. Restoration of ovarian function after autotransplantation of intact frozen-thawed sheep ovaries with microvascular anastomosis. Fertil Steril. 2003;79:594–602. doi: 10.1016/s0015-0282(02)04842-2. [DOI] [PubMed] [Google Scholar]
- Bedaiwy MA, El-Nashar SA, El Saman AM, Evers JL, Sandadi S, Desai N, Falcone T. Reproductive outcome after transplantation of ovarian tissue: a systematic review. Hum Reprod. 2008;23:2709–2717. doi: 10.1093/humrep/den301. [DOI] [PubMed] [Google Scholar]
- Blumenfeld Z. How to preserve fertility in young women exposed to chemotherapy? The role of GnRH agonist cotreatment in addition to cryopreservation of embrya, oocytes, or ovaries. Oncologist. 2007;12:1044–1054. doi: 10.1634/theoncologist.12-9-1044. [DOI] [PubMed] [Google Scholar]
- Bordes A, Lornage J, Demirci B, Franck M, Courbiere B, Guerin JF, Salle B. Normal gestations and live births after orthotopic autograft of vitrified-warmed hemi-ovaries into ewes. Hum Reprod. 2005;20:2745–2748. doi: 10.1093/humrep/dei155. [DOI] [PubMed] [Google Scholar]
- Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12:685–718. doi: 10.1093/humupd/dml034. [DOI] [PubMed] [Google Scholar]
- Bromer JG, Patrizio P. Preservation and postponement of female fertility. Placenta. 2008;29:200–205. doi: 10.1016/j.placenta.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Callejo J, Jauregui MT, Valls C, Fernandez ME, Cabre S, Lailla JM. Heterotopic ovarian transplantation without vascular pedicle in syngeneic Lewis rats: six-month control of estradiol and follicle-stimulating hormone concentrations after intraperitoneal and subcutaneous implants. Fertil Steril. 1999;72:513–517. doi: 10.1016/s0015-0282(99)00287-3. [DOI] [PubMed] [Google Scholar]
- Callejo J, Salvador C, Miralles A, Vilaseca S, Lailla JM, Balasch J. Long-term ovarian function evaluation after autografting by implantation with fresh and frozen-thawed human ovarian tissue. J Clin Endocrinol Metab. 2001;86:4489–4494. doi: 10.1210/jcem.86.9.7871. [DOI] [PubMed] [Google Scholar]
- Callejo J, Vilaseca S, Ordi J, Cabre S, Lailla JM, Balasch J. Heterotopic ovarian transplantation without vascular pedicle in syngeneic Lewis rats: long-term evaluation of effects on ovarian structure and function. Fertil Steril. 2002;77:396–402. doi: 10.1016/s0015-0282(01)02970-3. [DOI] [PubMed] [Google Scholar]
- Callejo J, Vilaseca S, Medina M, Salvador C, Valls C, Lailla JM. Inhibin and follicular development in heterotopical ovary transplants without vascular pedicle in syngeneic Lewis rats. Fertil Steril. 2003;79:743–748. doi: 10.1016/s0015-0282(02)04812-4. [DOI] [PubMed] [Google Scholar]
- Campbell BK, Telfer EE, Webb R, Baird DT. Ovarian autografts in sheep as a model for studying folliculogenesis. Mol Cell Endocrinol. 2000;163:131–139. doi: 10.1016/s0303-7207(00)00217-3. [DOI] [PubMed] [Google Scholar]
- Candy CJ, Wood MJ, Whittingham DG. Effect of cryoprotectants on the survival of follicles in frozen mouse ovaries. J Reprod Fertil. 1997;110:11–19. doi: 10.1530/jrf.0.1100011. [DOI] [PubMed] [Google Scholar]
- Candy CJ, Wood MJ, Whittingham DG. Restoration of a normal reproductive lifespan after grafting of cryopreserved mouse ovaries. Hum Reprod. 2000;15:1300–1304. doi: 10.1093/humrep/15.6.1300. [DOI] [PubMed] [Google Scholar]
- Carroll J, Gosden RG. Transplantation of frozen-thawed mouse primordial follicles. Hum Reprod. 1993;8:1163–1167. doi: 10.1093/oxfordjournals.humrep.a138221. [DOI] [PubMed] [Google Scholar]
- Chemoradiotherapy for cervical cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008;26:5802–5812. doi: 10.1200/JCO.2008.16.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Chen SG, Wu GJ, Wang J, Yu CP, Liu JY. Autologous heterotopic transplantation of intact rabbit ovary after frozen banking at -196 degrees C. Fertil Steril. 2006;a 86:1059–1066. doi: 10.1016/j.fertnstert.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Chen SU, Chien CL, Wu MY, Chen TH, Lai SM, Lin CW, Yang YS. Novel direct cover vitrification for cryopreservation of ovarian tissues increases follicle viability and pregnancy capability in mice. Hum Reprod. 2006;b 21:2794–2800. doi: 10.1093/humrep/del210. [DOI] [PubMed] [Google Scholar]
- Choi J, Lee JY, Lee E, Yoon BK, Bae D, Choi D. Cryopreservation of the mouse ovary inhibits the onset of primordial follicle development. Cryobiology. 2007;54:55–62. doi: 10.1016/j.cryobiol.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Courbiere B, Caquant L, Mazoyer C, Franck M, Lornage J, Salle B. Difficulties improving ovarian functional recovery by microvascular transplantation and whole ovary vitrification. Fertil Steril. 2008 doi: 10.1016/j.fertnstert.2008.03.012. In press. [DOI] [PubMed] [Google Scholar]
- Cox SL, Shaw J, Jenkin G. Transplantation of cryopreserved fetal ovarian tissue to adult recipients in mice. J Reprod Fertil. 1996;107:315–322. doi: 10.1530/jrf.0.1070315. [DOI] [PubMed] [Google Scholar]
- Demeestere I, Simon P, Englert Y, Delbaere A. Preliminary experience of ovarian tissue cryopreservation procedure: alternatives, perspectives and feasibility. Reprod Biomed Online. 2003;7:572–579. doi: 10.1016/s1472-6483(10)62074-3. [DOI] [PubMed] [Google Scholar]
- Demeestere I, Simon P, Buxant F, Robin V, Fernandez SA, Centner J, Delbaere A, Englert Y. Ovarian function and spontaneous pregnancy after combined heterotopic and orthotopic cryopreserved ovarian tissue transplantation in a patient previously treated with bone marrow transplantation: case report. Hum Reprod. 2006;21:2010–2014. doi: 10.1093/humrep/del092. [DOI] [PubMed] [Google Scholar]
- Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Fertility preservation: successful transplantation of cryopreserved ovarian tissue in a young patient previously treated for Hodgkin's disease. Oncologist. 2007;12:1437–1442. doi: 10.1634/theoncologist.12-12-1437. [DOI] [PubMed] [Google Scholar]
- Demirci B, Salle B, Frappart L, Franck M, Guerin JF, Lornage J. Morphological alterations and DNA fragmentation in oocytes from primordial and primary follicles after freezing-thawing of ovarian cortex in sheep. Fertil Steril. 2002;77:595–600. doi: 10.1016/s0015-0282(01)03205-8. [DOI] [PubMed] [Google Scholar]
- Deng XH, Xu AR, Chao L, Yu HL, Zhen JH, Hashimoto S, Morimoto Y. Effect of different sites for cryopreserved ovarian tissue implantation in rabbit. Hum Reprod. 2007;22:662–668. doi: 10.1093/humrep/del430. [DOI] [PubMed] [Google Scholar]
- Denjean R, Boeckx W, Gordts S, Brosens I. Ovarian transplantation by selective microvascular anastomoses in the rabbit. Br J Obstet Gynaecol. 1982;89:652–656. doi: 10.1111/j.1471-0528.1982.tb04721.x. [DOI] [PubMed] [Google Scholar]
- Dissen GA, Lara HE, Fahrenbach WH, Costa ME, Ojeda SR. Immature rat ovaries become revascularized rapidly after autotransplantation and show a gonadotropin-dependent increase in angiogenic factor gene expression. Endocrinology. 1994;134:1146–1154. doi: 10.1210/endo.134.3.8119153. [DOI] [PubMed] [Google Scholar]
- Dolmans MM, Demylle D, Martinez-Madrid B, Donnez J. Efficacy of in vitro fertilization after chemotherapy. Fertil Steril. 2005;83:897–901. doi: 10.1016/j.fertnstert.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Dolmans MM, Martinez-Madrid B, Gadisseux E, Guiot Y, Yuan WY, Torre A, Camboni A, Van Langendonckt A, Donnez J. Short-term transplantation of isolated human ovarian follicles and cortical tissue into nude mice. Reproduction. 2007;134:253–262. doi: 10.1530/REP-07-0131. [DOI] [PubMed] [Google Scholar]
- Donnez J, Bassil S. Indications for cryopreservation of ovarian tissue. Hum Reprod Update. 1998;4:248–259. doi: 10.1093/humupd/4.3.248. [DOI] [PubMed] [Google Scholar]
- Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, Martinez-Madrid B, van Langendonckt A. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- Donnez J, Squifflet J, Dolmans MM, Martinez-Madrid B, Jadoul P, Van Langendonckt A. Orthotopic transplantation of fresh ovarian cortex: a report of two cases. Fertil Steril. 2005;84:1018. doi: 10.1016/j.fertnstert.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Donnez J, Martinez-Madrid B, Jadoul P, Van Langendonckt A, Demylle D, Dolmans MM. Ovarian tissue cryopreservation and transplantation: a review. Hum Reprod Update. 2006;12:519–535. doi: 10.1093/humupd/dml032. [DOI] [PubMed] [Google Scholar]
- Donnez J, Dolmans MM, Pirard C, Van Langendonckt A, Demylle D, Jadoul P, Squifflet J. Allograft of ovarian cortex between two genetically non-identical sisters: case report. Hum Reprod. 2007;22:2653–2659. doi: 10.1093/humrep/dem211. [DOI] [PubMed] [Google Scholar]
- Dudzinski DM. Ethical issues in fertility preservation for adolescent cancer survivors: oocyte and ovarian tissue cryopreservation J Pediatr. Adolesc Gynecol. 2004;17:97–102. doi: 10.1016/j.jpag.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Fabbri R, Venturoli S, D'Errico A, Iannascoli C, Gabusi E, Valeri B, Seracchioli R, Grigioni WF. Ovarian tissue banking and fertility preservation in cancer patients: histological and immunohistochemical evaluation. Gynecol Oncol. 2003;89:259–266. doi: 10.1016/s0090-8258(02)00098-7. [DOI] [PubMed] [Google Scholar]
- Flaws JA, Abbud R, Mann RJ, Nilson JH, Hirshfield AN. Chronically elevated luteinizing hormone depletes primordial follicles in the mouse ovary. Biol Reprod. 1997;57:1233–1237. doi: 10.1095/biolreprod57.5.1233. [DOI] [PubMed] [Google Scholar]
- Gandolfi F, Paffoni A, Papasso Brambilla E, Bonetti S, Brevini TA, Ragni G. Efficiency of equilibrium cooling and vitrification procedures for the cryopreservation of ovarian tissue: comparative analysis between human and animal models. Fertil Steril. 2006;85:1150–1156. doi: 10.1016/j.fertnstert.2005.08.062. [DOI] [PubMed] [Google Scholar]
- Gidoni Y, Holzer H, Tulandi T, Tan SL. Fertility preservation in patients with non-oncological conditions. Reprod Biomed Online. 2008;16:792–800. doi: 10.1016/s1472-6483(10)60144-7. [DOI] [PubMed] [Google Scholar]
- Goding JR. Ovarian autotransplantation with vascular anastomoses, and its application to the study of reproductive physiology in the ewe. J Physiol. 1966;186:86P–87P. [PubMed] [Google Scholar]
- Gook DA, Edgar DH, Stern C. The effects of cryopreservation regimens on the morphology of human ovarian tissue. Mol Cell Endocrinol. 2000;169:99–103. doi: 10.1016/s0303-7207(00)00360-9. [DOI] [PubMed] [Google Scholar]
- Gook DA, McCully BA, Edgar DH, McBain JC. Development of antral follicles in human cryopreserved ovarian tissue following xenografting. Hum Reprod. 2001;16:417–422. doi: 10.1093/humrep/16.3.417. [DOI] [PubMed] [Google Scholar]
- Gook DA, Edgar DH, Borg J, Archer J, McBain JC. Diagnostic assessment of the developmental potential of human cryopreserved ovarian tissue from multiple patients using xenografting. Hum Reprod. 2005;20:72–78. doi: 10.1093/humrep/deh550. [DOI] [PubMed] [Google Scholar]
- Gosden RG, Baird DT, Wade JC, Webb R. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at -196 degrees C. Hum Reprod. 1994;9:597–603. doi: 10.1093/oxfordjournals.humrep.a138556. [DOI] [PubMed] [Google Scholar]
- Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17:121–155. doi: 10.1210/edrv-17-2-121. [DOI] [PubMed] [Google Scholar]
- Gunasena KT, Villines PM, Critser ES, Critser JK. Live births after autologous transplant of cryopreserved mouse ovaries. Hum Reprod. 1997;12:101–106. doi: 10.1093/humrep/12.1.101. [DOI] [PubMed] [Google Scholar]
- Hasegawa A, Mochida N, Ogasawara T, Koyama K. Pup birth from mouse oocytes in preantral follicles derived from vitrified and warmed ovaries followed by in vitro growth, in vitro maturation, and in vitro fertilization. Fertil Steril. 2006;86:1182–1192. doi: 10.1016/j.fertnstert.2005.12.082. [DOI] [PubMed] [Google Scholar]
- Hernandez-Fonseca H, Bosch P, Sirisathien S, Wininger JD, Massey JB, Brackett BG. Effect of site of transplantation on follicular development of human ovarian tissue transplanted into intact or castrated immunodeficient mice. Fertil Steril. 2004;81:888–892. doi: 10.1016/j.fertnstert.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Hilders CG, Baranski AG, Peters L, Ramkhelawan A, Trimbos JB. Successful human ovarian autotransplantation to the upper arm. Cancer. 2004;101:2771–2778. doi: 10.1002/cncr.20715. [DOI] [PubMed] [Google Scholar]
- Hovatta O. Cryopreservation and culture of human primordial and primary ovarian follicles. Mol Cell Endocrinol. 2000;169:95–97. doi: 10.1016/s0303-7207(00)00359-2. [DOI] [PubMed] [Google Scholar]
- Hovatta O, Silye R, Krausz T, Abir R, Margara R, Trew G, Lass A, Winston RM. Cryopreservation of human ovarian tissue using dimethylsulphoxide and propanediol-sucrose as cryoprotectants. Hum Reprod. 1996;11:1268–1272. doi: 10.1093/oxfordjournals.humrep.a019370. [DOI] [PubMed] [Google Scholar]
- Hreinsson JG, Otala M, Fridstrom M, Borgstrom B, Rasmussen C, Lundqvist M, Tuuri T, Simberg N, Mikkola M, Dunkel L, et al. Follicles are found in the ovaries of adolescent girls with Turner's syndrome. J Clin Endocrinol Metab. 2002;87:3618–3623. doi: 10.1210/jcem.87.8.8753. [DOI] [PubMed] [Google Scholar]
- Hreinsson J, Zhang P, Swahn ML, Hultenby K, Hovatta O. Cryopreservation of follicles in human ovarian cortical tissue. Comparison of serum and human serum albumin in the cryoprotectant solutions. Hum Reprod. 2003;18:2420–2428. doi: 10.1093/humrep/deg439. [DOI] [PubMed] [Google Scholar]
- Huang JY, Tulandi T, Holzer H, Lau NM, Macdonald S, Tan SL, Chian RC. Cryopreservation of ovarian tissue and in vitro matured oocytes in a female with mosaic Turner syndrome: case report. Hum Reprod. 2008;a 23:336–339. doi: 10.1093/humrep/dem307. [DOI] [PubMed] [Google Scholar]
- Huang L, Mo Y, Wang W, Li Y, Zhang Q, Yang D. Cryopreservation of human ovarian tissue by solid-surface vitrification. Eur J Obstet Gynecol Reprod Biol. 2008;b 139:193–198. doi: 10.1016/j.ejogrb.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Imhof M, Bergmeister H, Lipovac M, Rudas M, Hofstetter G, Huber J. Orthotopic microvascular reanastomosis of whole cryopreserved ovine ovaries resulting in pregnancy and live birth. Fertil Steril. 2006;85:1208–1215. doi: 10.1016/j.fertnstert.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Imthurn B, Cox SL, Jenkin G, Trounson AO, Shaw JM. Gonadotrophin administration can benefit ovarian tissue grafted to the body wall: implications for human ovarian grafting. Mol Cell Endocrinol. 2000;163:141–146. doi: 10.1016/s0303-7207(00)00218-5. [DOI] [PubMed] [Google Scholar]
- Ishijima T, Kobayashi Y, Lee DS, Ueta YY, Matsui M, Lee JY, Suwa Y, Miyahara K, Suzuki H. Cryopreservation of canine ovaries by vitrification. J Reprod Dev. 2006;52:293–299. doi: 10.1262/jrd.17080. [DOI] [PubMed] [Google Scholar]
- Israely T, Dafni H, Granot D, Nevo N, Tsafriri A, Neeman M. Vascular remodeling and angiogenesis in ectopic ovarian transplants: a crucial role of pericytes and vascular smooth muscle cells in maintenance of ovarian grafts. Biol Reprod. 2003;68:2055–2064. doi: 10.1095/biolreprod.102.011734. [DOI] [PubMed] [Google Scholar]
- Israely T, Dafni H, Nevo N, Tsafriri A, Neeman M. Angiogenesis in ectopic ovarian xenotransplantation: multiparameter characterization of the neovasculature by dynamic contrast-enhanced MRI Magn. Reson Med. 2004;52:741–750. doi: 10.1002/mrm.20203. [DOI] [PubMed] [Google Scholar]
- Israely T, Nevo N, Harmelin A, Neeman M, Tsafriri A. Reducing ischaemic damage in rodent ovarian xenografts transplanted into granulation tissue. Hum Reprod. 2006;21:1368–1379. doi: 10.1093/humrep/del010. [DOI] [PubMed] [Google Scholar]
- Jeremias E, Bedaiwy MA, Gurunluoglu R, Biscotti CV, Siemionow M, Falcone T. Heterotopic autotransplantation of the ovary with microvascular anastomosis: a novel surgical technique. Fertil Steril. 2002;77:1278–1282. doi: 10.1016/s0015-0282(02)03110-2. [DOI] [PubMed] [Google Scholar]
- Kaneko H, Kikuchi K, Noguchi J, Ozawa M, Ohnuma K, Maedomari N, Kashiwazaki N. Effects of gonadotrophin treatments on meiotic and developmental competence of oocytes in porcine primordial follicles following xenografting to nude mice. Reproduction. 2006;131:279–288. doi: 10.1530/rep.1.00957. [DOI] [PubMed] [Google Scholar]
- Kim SS, Radford J, Harris M, Varley J, Rutherford AJ, Lieberman B, Shalet S, Gosden R. Ovarian tissue harvested from lymphoma patients to preserve fertility may be safe for autotransplantation. Hum Reprod. 2001;c 16:2056–2060. doi: 10.1093/humrep/16.10.2056. [DOI] [PubMed] [Google Scholar]
- Kim SS, Soules MR, Battaglia DE. Follicular development, ovulation, and corpus luteum formation in cryopreserved human ovarian tissue after xenotransplantation. Fertil Steril. 2002;78:77–82. doi: 10.1016/s0015-0282(02)03144-8. [DOI] [PubMed] [Google Scholar]
- Kim SS, Yang HW, Kang HG, Lee HH, Lee HC, Ko DS, Gosden RG. Quantitative assessment of ischemic tissue damage in ovarian cortical tissue with or without antioxidant (ascorbic acid) treatment. Fertil Steril. 2004;a 82:679–685. doi: 10.1016/j.fertnstert.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Kim SS, Hwang IT, Lee HC. Heterotopic autotransplantation of cryobanked human ovarian tissue as a strategy to restore ovarian function. Fertil Steril. 2004;b 82:930–932. doi: 10.1016/j.fertnstert.2004.02.137. [DOI] [PubMed] [Google Scholar]
- Kim SS, Kang HG, Kim NH, Lee HC, Lee HH. Assessment of the integrity of human oocytes retrieved from cryopreserved ovarian tissue after xenotransplantation. Hum Reprod. 2005;20:2502–2508. doi: 10.1093/humrep/dei099. [DOI] [PubMed] [Google Scholar]
- Kupiec-Weglinski JW, Busuttil RW. Ischemia and reperfusion injury in liver transplantation. Transplant Proc. 2005;37:1653–1656. doi: 10.1016/j.transproceed.2005.03.134. [DOI] [PubMed] [Google Scholar]
- Langeveld NE, Grootenhuis MA, Voute PA, de Haan RJ, van den Bos C. Quality of life, self-esteem and worries in young adult survivors of childhood cancer. Psychooncology. 2004;13:867–881. doi: 10.1002/pon.800. [DOI] [PubMed] [Google Scholar]
- Lee DM, Yeoman RR, Battaglia DE, Stouffer RL, Zelinski-Wooten MB, Fanton JW, Wolf DP. Live birth after ovarian tissue transplant. Nature. 2004;428:137–138. doi: 10.1038/428137a. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, Beck LN, Brennan LV, Oktay K. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- Lee RK, Li SH, Lu CH, Ho HY, Chen YJ, Yeh HI. Abnormally low expression of connexin 37 and connexin 43 in subcutaneously transplanted cryopreserved mouse ovarian tissue. J Assist Reprod Genet. 2008;25:489–497. doi: 10.1007/s10815-008-9264-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leporrier M, von Theobald P, Roffe JL, Muller G. A new technique to protect ovarian function before pelvic irradiation. Heterotopic ovarian autotransplantation. Cancer. 1987;60:2201–2204. doi: 10.1002/1097-0142(19871101)60:9<2201::aid-cncr2820600915>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Leporrier M, Roffe JL, Von Theobald P, Muller G. Autologous transplantation of whole ovaries vs ovarian cortical strips. JAMA. 2002;287:44–45. doi: 10.1001/jama.287.1.44. [DOI] [PubMed] [Google Scholar]
- Liu L, Wood GA, Morikawa L, Ayearst R, Fleming C, McKerlie C. Restoration of fertility by orthotopic transplantation of frozen adult mouse ovaries. Hum Reprod. 2008;23:122–128. doi: 10.1093/humrep/dem348. [DOI] [PubMed] [Google Scholar]
- Lobo RA. Potential options for preservation of fertility in women. N Engl J Med. 2005;353:64–73. doi: 10.1056/NEJMra043475. [DOI] [PubMed] [Google Scholar]
- Maltaris T, Dragonas C, Hoffmann I, Mueller A, Beckmann MW, Dittrich R. Simple prediction of the survival of follicles in cryopreserved human ovarian tissue. J Reprod Dev. 2006;a 52:577–582. doi: 10.1262/jrd.18012. [DOI] [PubMed] [Google Scholar]
- Maltaris T, Kaya H, Hoffmann I, Mueller A, Beckmann MW, Dittrich R. Comparison of xenografting in SCID mice and LIVE/DEAD assay as a predictor of the developmental potential of cryopreserved ovarian tissue. In Vivo. 2006;b 20:11–16. [PubMed] [Google Scholar]
- Maltaris T, Beckmann MW, Mueller A, Hoffmann I, Kohl J, Dittrich R. Significant loss of primordial follicles after prolonged gonadotropin stimulation in xenografts of cryopreserved human ovarian tissue in severe combined immunodeficient mice. Fertil Steril. 2007;a 87:195–197. doi: 10.1016/j.fertnstert.2006.05.058. [DOI] [PubMed] [Google Scholar]
- Maltaris T, Beckmann MW, Binder H, Mueller A, Hoffmann I, Koelbl H, Dittrich R. The effect of a GnRH agonist on cryopreserved human ovarian grafts in severe combined immunodeficient mice. Reproduction. 2007;b 133:503–509. doi: 10.1530/REP-06-0061. [DOI] [PubMed] [Google Scholar]
- Martinez-Madrid B, Donnez J. Cryopreservation of intact human ovary with its vascular pedicle–or cryopreservation of hemiovaries? Hum Reprod. 2007;b 22:1795–1796. doi: 10.1093/humrep/dem047. author reply 1796–1797. [DOI] [PubMed] [Google Scholar]
- Martinez-Madrid B, Dolmans MM, Van Langendonckt A, Defrere S, Donnez J. Freeze-thawing intact human ovary with its vascular pedicle with a passive cooling device. Fertil Steril. 2004;82:1390–1394. doi: 10.1016/j.fertnstert.2004.06.036. [DOI] [PubMed] [Google Scholar]
- Martinez-Madrid B, Camboni A, Dolmans MM, Nottola S, Van Langendonckt A, Donnez J. Apoptosis and ultrastructural assessment after cryopreservation of whole human ovaries with their vascular pedicle. Fertil Steril. 2007;a 87:1153–1165. doi: 10.1016/j.fertnstert.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Martinez-Madrid B, Donnez J, Van Eyck AS, Veiga-Lopez A, Dolmans MM, Van Langendonckt A. Chick embryo chorioallantoic membrane (CAM) model: a useful tool to study short-term transplantation of cryopreserved human ovarian tissue. Fertil Steril. 2009;91:285–292. doi: 10.1016/j.fertnstert.2007.11.026. [DOI] [PubMed] [Google Scholar]
- Meirow D. Reproduction post-chemotherapy in young cancer patients. Mol Cell Endocrinol. 2000;169:123–131. doi: 10.1016/s0303-7207(00)00365-8. [DOI] [PubMed] [Google Scholar]
- Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update. 2001;7:535–543. doi: 10.1093/humupd/7.6.535. [DOI] [PubMed] [Google Scholar]
- Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, Schiff E, Dor J. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353:318–321. doi: 10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- Meirow D, Baum M, Yaron R, Levron J, Hardan I, Schiff E, Nagler A, Yehuda DB, Raanani H, Hourvitz A, et al. Ovarian tissue cryopreservation in hematologic malignancy: ten years’ experience. Leuk Lymphoma. 2007;a 48:1569–1576. doi: 10.1080/10428190701471957. [DOI] [PubMed] [Google Scholar]
- Meirow D, Dor J, Kaufman B, Shrim A, Rabinovici J, Schiff E, Raanani H, Levron J, Fridman E. Cortical fibrosis and blood-vessels damage in human ovaries exposed to chemotherapy. Potential mechanisms of ovarian injury. Hum Reprod. 2007;b 22:1626–1633. doi: 10.1093/humrep/dem027. [DOI] [PubMed] [Google Scholar]
- Moffa F, Biacchiardi CP, Fagioli F, Biasin E, Revelli A, Massobrio M, Madon E. Ovarian tissue cryostorage and grafting: an option to preserve fertility in pediatric patients with malignancies. Pediatr Hematol Oncol. 2007;24:29–44. doi: 10.1080/08880010600970468. [DOI] [PubMed] [Google Scholar]
- Navarro-Costa P, Correia SC, Gouveia-Oliveira A, Negreiro F, Jorge S, Cidadao AJ, Carvalho MJ, Plancha CE. Effects of mouse ovarian tissue cryopreservation on granulosa cell-oocyte interaction. Hum Reprod. 2005;20:1607–1614. doi: 10.1093/humrep/deh787. [DOI] [PubMed] [Google Scholar]
- Newton H, Aubard Y, Rutherford A, Sharma V, Gosden R. Low temperature storage and grafting of human ovarian tissue. Hum Reprod. 1996;11:1487–1491. doi: 10.1093/oxfordjournals.humrep.a019423. [DOI] [PubMed] [Google Scholar]
- Nisolle M, Casanas-Roux F, Qu J, Motta P, Donnez J. Histologic and ultrastructural evaluation of fresh and frozen-thawed human ovarian xenografts in nude mice. Fertil Steril. 2000;74:122–129. doi: 10.1016/s0015-0282(00)00548-3. [DOI] [PubMed] [Google Scholar]
- Nugent D, Meirow D, Brook PF, Aubard Y, Gosden RG. Transplantation in reproductive medicine: previous experience, present knowledge and future prospects. Hum Reprod Update. 1997;3:267–280. doi: 10.1093/humupd/3.3.267. [DOI] [PubMed] [Google Scholar]
- Nugent D, Newton H, Gallivan L, Gosden RG. Protective effect of vitamin E on ischaemia-reperfusion injury in ovarian grafts. J Reprod Fertil. 1998;114:341–346. doi: 10.1530/jrf.0.1140341. [DOI] [PubMed] [Google Scholar]
- Oktay K. Ovarian tissue cryopreservation and transplantation: preliminary findings and implications for cancer patients. Hum Reprod Update. 2001;7:526–534. doi: 10.1093/humupd/7.6.526. [DOI] [PubMed] [Google Scholar]
- Oktay K. Spontaneous conceptions and live birth after heterotopic ovarian transplantation: is there a germline stem cell connection? Hum Reprod. 2006;21:1345–1348. doi: 10.1093/humrep/del007. [DOI] [PubMed] [Google Scholar]
- Oktay K, Karlikaya G. Ovarian function after transplantation of frozen, banked autologous ovarian tissue. N Engl J Med. 2000;342:1919. doi: 10.1056/NEJM200006223422516. [DOI] [PubMed] [Google Scholar]
- Oktay K, Sonmezer M. Ovarian tissue banking for cancer patients: fertility preservation, not just ovarian cryopreservation. Hum Reprod. 2004;19:477–480. doi: 10.1093/humrep/deh152. [DOI] [PubMed] [Google Scholar]
- Oktay K, Oktem O. Ovarian cryopreservation and transplantation for fertility preservation for medical indications: report of an ongoing experience. Fertil Steril. 2008 doi: 10.1016/j.fertnstert.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Oktay K, Newton H, Mullan J, Gosden RG. Development of human primordial follicles to antral stages in SCID/hpg mice stimulated with follicle stimulating hormone. Hum Reprod. 1998;13:1133–1138. doi: 10.1093/humrep/13.5.1133. [DOI] [PubMed] [Google Scholar]
- Oktay K, Newton H, Gosden RG. Transplantation of cryopreserved human ovarian tissue results in follicle growth initiation in SCID mice. Fertil Steril. 2000;73:599–603. doi: 10.1016/s0015-0282(99)00548-8. [DOI] [PubMed] [Google Scholar]
- Oktay K, Buyuk E, Rosenwaks Z, Rucinski J. A technique for transplantation of ovarian cortical strips to the forearm. Fertil Steril. 2003;80:193–198. doi: 10.1016/s0015-0282(03)00568-5. [DOI] [PubMed] [Google Scholar]
- Oktay K, Economos K, Kan M, Rucinski J, Veeck L, Rosenwaks Z. Endocrine function and oocyte retrieval after autologous transplantation of ovarian cortical strips to the forearm. JAMA. 2001;286:1490–1493. doi: 10.1001/jama.286.12.1490. [DOI] [PubMed] [Google Scholar]
- Oktay K, Buyuk E, Veeck L, Zaninovic N, Xu K, Takeuchi T, Opsahl M, Rosenwaks Z. Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;363:837–840. doi: 10.1016/S0140-6736(04)15728-0. [DOI] [PubMed] [Google Scholar]
- Oktay K, Sonmezer M, Oktem O, Fox K, Emons G, Bang H. Absence of conclusive evidence for the safety and efficacy of gonadotropin-releasing hormone analogue treatment in protecting against chemotherapy-induced gonadal injury. Oncologist. 2007;12:1055–1066. doi: 10.1634/theoncologist.12-9-1055. [DOI] [PubMed] [Google Scholar]
- Oktem O, Oktay K. A novel ovarian xenografting model to characterize the impact of chemotherapy agents on human primordial follicle reserve. Cancer Res. 2007;67:10159–10162. doi: 10.1158/0008-5472.CAN-07-2042. [DOI] [PubMed] [Google Scholar]
- Onions VJ, Mitchell MR, Campbell BK, Webb R. Ovarian tissue viability following whole ovine ovary cryopreservation: assessing the effects of sphingosine-1-phosphate inclusion. Hum Reprod. 2008;23:606–618. doi: 10.1093/humrep/dem414. [DOI] [PubMed] [Google Scholar]
- Paldi E, Gal D, Barzilai A, Hampel N, Malberger E. Genital organs. Auto and homotransplantation in forty dogs. Int J Fertil. 1975;20:5–12. [PubMed] [Google Scholar]
- Paris F, Perez GI, Fuks Z, Haimovitz-Friedman A, Nguyen H, Bose M, Ilagan A, Hunt PA, Morgan WF, Tilly JL, et al. Sphingosine 1-phosphate preserves fertility in irradiated female mice without propagating genomic damage in offspring. Nat Med. 2002;8:901–902. doi: 10.1038/nm0902-901. [DOI] [PubMed] [Google Scholar]
- Paris MC, Snow M, Cox SL, Shaw JM. Xenotransplantation: a tool for reproductive biology and animal conservation? Theriogenology. 2004;61:277–291. doi: 10.1016/s0093-691x(03)00234-6. [DOI] [PubMed] [Google Scholar]
- Petroianu A, de Souza Vasconcellos L, Alberti LR, Fonseca de Castro LP, Barbosa Leite JM. Natural pregnancy in rabbits that underwent oophorectomy and orthotopic allogeneic or autologous ovarian transplantation. Fertil Steril. 2002;77:1298–1299. doi: 10.1016/s0015-0282(02)03107-2. [DOI] [PubMed] [Google Scholar]
- Petroianu A, Alberti LR, Vasconcellos LS. Allogeneic ovarian orthotopic transplantation in rabbits without a vascular pedicle: morphological, endocrinologic, and natural pregnancy assessment. Transplant Proc. 2006;38:3092–3093. doi: 10.1016/j.transproceed.2006.08.175. [DOI] [PubMed] [Google Scholar]
- Petroianu A, Alberti LR, Vasconcellos LS. Morphologic, endocrinologic and natural pregnancy assessment of allogeneic ovarian orthotopic transplantation without a vascular pedicle in rabbits. Eur J Obstet Gynecol Reprod Biol. 2007;133:70–75. doi: 10.1016/j.ejogrb.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Picton HM, Harris SE, Muruvi W, Chambers EL. The in vitro growth and maturation of follicles. Reproduction. 2008;136:703–715. doi: 10.1530/REP-08-0290. [DOI] [PubMed] [Google Scholar]
- Poirot C, Vacher-Lavenu MC, Helardot P, Guibert J, Brugieres L, Jouannet P. Human ovarian tissue cryopreservation: indications and feasibility. Hum Reprod. 2002;17:1447–1452. doi: 10.1093/humrep/17.6.1447. [DOI] [PubMed] [Google Scholar]
- Poirot CJ, Martelli H, Genestie C, Golmard JL, Valteau-Couanet D, Helardot P, Pacquement H, Sauvat F, Tabone MD, Philippe-Chomette P, et al. Feasibility of ovarian tissue cryopreservation for prepubertal females with cancer. Pediatr Blood Cancer. 2007;49:74–78. doi: 10.1002/pbc.21027. [DOI] [PubMed] [Google Scholar]
- Radford J. Autotransplantation of ovarian tissue and the risk of disease transmission. Eur J Obstet Gynecol Reprod Biol. 2004;113:S48–S49. doi: 10.1016/j.ejogrb.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Radford JA, Lieberman BA, Brison DR, Smith AR, Critchlow JD, Russell SA, Watson AJ, Clayton JA, Harris M, Gosden RG, et al. Orthotopic reimplantation of cryopreserved ovarian cortical strips after high-dose chemotherapy for Hodgkin's lymphoma. Lancet. 2001;357:1172–1175. doi: 10.1016/s0140-6736(00)04335-x. [DOI] [PubMed] [Google Scholar]
- Revel A, Elami A, Bor A, Yavin S, Natan Y, Arav A. Whole sheep ovary cryopreservation and transplantation. Fertil Steril. 2004;82:1714–1715. doi: 10.1016/j.fertnstert.2004.06.046. [DOI] [PubMed] [Google Scholar]
- Risvanli A, Timurkan H, Akpolat N, Gulacti I, Ulakoglu E. A study of ovarian autotransplantation without vascular a pedicle in rats. J Assist Reprod Genet. 2006;23:401–406. doi: 10.1007/s10815-006-9078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendahl M, Loft A, Byskov AG, Ziebe S, Schmidt KT, Andersen AN, Ottosen C, Andersen CY. Biochemical pregnancy after fertilization of an oocyte aspirated from a heterotopic autotransplant of cryopreserved ovarian tissue: case report. Hum Reprod. 2006;21:2006–2009. doi: 10.1093/humrep/del140. [DOI] [PubMed] [Google Scholar]
- Sagsoz N, Kisa U, Apan A. Ischaemia-reperfusion injury of rat ovary and the effects of vitamin C, mannitol and verapamil. Hum Reprod. 2002;17:2972–2976. doi: 10.1093/humrep/17.11.2972. [DOI] [PubMed] [Google Scholar]
- Salle B, Demirci B, Franck M, Rudigoz RC, Guerin JF, Lornage J. Normal pregnancies and live births after autograft of frozen-thawed hemi-ovaries into ewes. Fertil Steril. 2002;77:403–408. doi: 10.1016/s0015-0282(01)02960-0. [DOI] [PubMed] [Google Scholar]
- Salle B, Demirci B, Franck M, Berthollet C, Lornage J. Long-term follow-up of cryopreserved hemi-ovary autografts in ewes: pregnancies, births, and histologic assessment. Fertil Steril. 2003;80:172–177. doi: 10.1016/s0015-0282(03)00554-5. [DOI] [PubMed] [Google Scholar]
- Sapmaz E, Ayar A, Celik H, Sapmaz T, Kilic N, Yasar MA. Effects of melatonin and oxytetracycline in autologous intraperitoneal ovary transplantation in rats. Neuro Endocrinol Lett. 2003;24:350–354. [PubMed] [Google Scholar]
- Sauvat F, Capito C, Sarnacki S, Poirot C, Bachelot A, Meduri G, Dandolo L, Binart N. Immature cryopreserved ovary restores puberty and fertility in mice without alteration of epigenetic marks. PLoS ONE. 2008;3:e1972. doi: 10.1371/journal.pone.0001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt KL, Ernst E, Byskov AG, Nyboe Andersen A, Yding Andersen C. Survival of primordial follicles following prolonged transportation of ovarian tissue prior to cryopreservation. Hum Reprod. 2003;18:2654–2659. doi: 10.1093/humrep/deg500. [DOI] [PubMed] [Google Scholar]
- Schmidt KL, Andersen CY, Loft A, Byskov AG, Ernst E, Andersen AN. Follow-up of ovarian function post-chemotherapy following ovarian cryopreservation and transplantation. Hum Reprod. 2005;20:3539–3546. doi: 10.1093/humrep/dei250. [DOI] [PubMed] [Google Scholar]
- Schnorr J, Oehninger S, Toner J, Hsiu J, Lanzendorf S, Williams R, Hodgen G. Functional studies of subcutaneous ovarian transplants in non-human primates: steroidogenesis, endometrial development, ovulation, menstrual patterns and gamete morphology. Hum Reprod. 2002;17:612–619. doi: 10.1093/humrep/17.3.612. [DOI] [PubMed] [Google Scholar]
- Scott JR, Keye WR, Poulson AM, Reynolds WA. Microsurgical ovarian transplantation in the primate. Fertil Steril. 1981;36:512–515. doi: 10.1016/s0015-0282(16)45803-6. [DOI] [PubMed] [Google Scholar]
- Shaw JM, Cox SL, Trounson AO, Jenkin G. Evaluation of the long-term function of cryopreserved ovarian grafts in the mouse, implications for human applications. Mol Cell Endocrinol. 2000;161:103–110. doi: 10.1016/s0303-7207(99)00230-0. [DOI] [PubMed] [Google Scholar]
- Siebzehnrubl E, Kohl J, Dittrich R, Wildt L. Freezing of human ovarian tissue – not the oocytes but the granulosa is the problem. Mol Cell Endocrinol. 2000;169:109–111. doi: 10.1016/s0303-7207(00)00362-2. [DOI] [PubMed] [Google Scholar]
- Silber SJ, Gosden RG. Ovarian transplantation in a series of monozygotic twins discordant for ovarian failure. N Engl J Med. 2007;356:1382–1384. doi: 10.1056/NEJMc066574. [DOI] [PubMed] [Google Scholar]
- Silber SJ, Lenahan KM, Levine DJ, Pineda JA, Gorman KS, Friez MJ, Crawford EC, Gosden RG. Ovarian transplantation between monozygotic twins discordant for premature ovarian failure. N Engl J Med. 2005;353:58–63. doi: 10.1056/NEJMoa043157. [DOI] [PubMed] [Google Scholar]
- Silber SJ, DeRosa M, Pineda J, Lenahan K, Grenia D, Gorman K, Gosden RG. A series of monozygotic twins discordant for ovarian failure: ovary transplantation (cortical versus microvascular) and cryopreservation. Hum Reprod. 2008;a 23:1531–1537. doi: 10.1093/humrep/den032. [DOI] [PubMed] [Google Scholar]
- Silber SJ, Derosa M, Pineda J, Lenahan K, Grenia D, Gorman K, Gosden RG. A series of monozygotic twins discordant for ovarian failure: ovary transplantation (cortical versus microvascular) and cryopreservation. Hum Reprod. 2008b doi: 10.1093/humrep/den032. [DOI] [PubMed] [Google Scholar]
- Silber SJ, Grudzinskas G, Gosden RG. Successful pregnancy after microsurgical transplantation of an intact ovary. N Engl J Med. 2008;c 359:2617–2618. doi: 10.1056/NEJMc0804321. [DOI] [PubMed] [Google Scholar]
- Smitz J, Cortvrindt R. Oocyte in-vitro maturation and follicle culture: current clinical achievements and future directions. Hum Reprod. 1999;14:145–161. doi: 10.1093/humrep/14.suppl_1.145. [DOI] [PubMed] [Google Scholar]
- Snow M, Cox SL, Jenkin G, Trounson A, Shaw J. Generation of live young from xenografted mouse ovaries. Science. 2002;297:2227. doi: 10.1126/science.1073693. [DOI] [PubMed] [Google Scholar]
- Soleimani R, Van der Elst J, Heytens E, Van den Broecke R, Gerris J, Dhont M, Cuvelier C, De Sutter P. Back muscle as a promising site for ovarian tissue transplantation, an animal model. Hum Reprod. 2008;23:619–626. doi: 10.1093/humrep/dem405. [DOI] [PubMed] [Google Scholar]
- Sonmezer M, Shamonki MI, Oktay K. Ovarian tissue cryopreservation: benefits and risks. Cell Tissue Res. 2005;322:125–132. doi: 10.1007/s00441-005-1098-4. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Ishijima T, Maruyama S, Yanagimoto Ueta Y, Abe Y, Saitoh H. Beneficial effect of desialylated erythropoietin administration on the frozen-thawed canine ovarian xenotransplantation. J Assist Reprod Genet. 2008;25:571–575. doi: 10.1007/s10815-008-9271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztein J, Sweet H, Farley J, Mobraaten L. Cryopreservation and orthotopic transplantation of mouse ovaries: new approach in gamete banking. Biol Reprod. 1998;58:1071–1074. doi: 10.1095/biolreprod58.4.1071. [DOI] [PubMed] [Google Scholar]
- Tao T, Del Valle A. Human oocyte and ovarian tissue cryopreservation and its application. J Assist Reprod Genet. 2008;25:287–296. doi: 10.1007/s10815-008-9236-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thewes B, Meiser B, Taylor A, Phillips KA, Pendlebury S, Capp A, Dalley D, Goldstein D, Baber R, Friedlander ML. Fertility- and menopause-related information needs of younger women with a diagnosis of early breast cancer. J Clin Oncol. 2005;23:5155–5165. doi: 10.1200/JCO.2005.07.773. [DOI] [PubMed] [Google Scholar]
- Tryde Schmidt KL, Yding Andersen C, Starup J, Loft A, Byskov AG, Nyboe Andersen A. Orthotopic autotransplantation of cryopreserved ovarian tissue to a woman cured of cancer - follicular growth, steroid production and oocyte retrieval. Reprod Biomed Online. 2004;8:448–453. doi: 10.1016/s1472-6483(10)60929-7. [DOI] [PubMed] [Google Scholar]
- Van den Broecke R, Liu J, Handyside A, Van der Elst JC, Krausz T, Dhont M, Winston RM, Hovatta O. Follicular growth in fresh and cryopreserved human ovarian cortical grafts transplanted to immunodeficient mice. Eur J Obstet Gynecol Reprod Biol. 2001;97:193–201. doi: 10.1016/s0301-2115(00)00507-8. [DOI] [PubMed] [Google Scholar]
- Van der Steeg JW, Steures P, Eijkemans MJC, Habbema JDF, Hompes PGA, Broekmans FJ, Bouckaert PXJM, Bossuyt PMM, van der Veen F, Mol BWJ, et al. Predictive value and clinical impact of basal follicle-stimulating hormone in subfertile, ovulatory women. J Clin Endocrinol Metab. 2007;92:2163–2168. doi: 10.1210/jc.2006-2399. 10.1210/jc.2006–2399. [DOI] [PubMed] [Google Scholar]
- Visser JA, Themmen AP. Anti-Mullerian hormone and folliculogenesis. Mol Cell Endocrinol. 2005;234:81–86. doi: 10.1016/j.mce.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Wallace WH, Thomson AB, Kelsey TW. The radiosensitivity of the human oocyte. Hum Reprod. 2003;18:117–121. doi: 10.1093/humrep/deg016. [DOI] [PubMed] [Google Scholar]
- Wang H, Mooney S, Wen Y, Behr B, Polan ML. Follicle development in grafted mouse ovaries after cryopreservation and subcutaneous transplantation. Am J Obstet Gynecol. 2002;a 187:370–374. doi: 10.1067/mob.2002.123606. [DOI] [PubMed] [Google Scholar]
- Wang X, Chen H, Yin H, Kim SS, Lin Tan S, Gosden RG. Fertility after intact ovary transplantation. Nature. 2002;b 415:385. doi: 10.1038/415385a. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xiao Z, Li L, Fan W, Li SW. Novel needle immersed vitrification: a practical and convenient method with potential advantages in mouse and human ovarian tissue cryopreservation. Hum Reprod. 2008;23:2256–2265. doi: 10.1093/humrep/den255. [DOI] [PubMed] [Google Scholar]
- Waterhouse T, Cox SL, Snow M, Jenkin G, Shaw J. Offspring produced from heterotopic ovarian allografts in male and female recipient mice. Reproduction. 2004;127:689–694. doi: 10.1530/rep.1.00081. [DOI] [PubMed] [Google Scholar]
- Weintraub M, Gross E, Kadari A, Ravitsky V, Safran A, Laufer N, Revel A. Should ovarian cryopreservation be offered to girls with cancer. Pediatr Blood Cancer. 2007;48:4–9. doi: 10.1002/pbc.20946. [DOI] [PubMed] [Google Scholar]
- Weissman A, Gotlieb L, Colgan T, Jurisicova A, Greenblatt EM, Casper RF. Preliminary experience with subcutaneous human ovarian cortex transplantation in the NOD-SCID mouse. Biol Reprod. 1999;60:1462–1467. doi: 10.1095/biolreprod60.6.1462. [DOI] [PubMed] [Google Scholar]
- Wo JY, Viswanathan AN. Impact of radiotherapy on fertility, pregnancy, and neonatal outcomes in female cancer patients. Int J Radiat Oncol Biol Phys. 2009;73:1304–1312. doi: 10.1016/j.ijrobp.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolner-Hanssen P, Hagglund L, Ploman F, Ramirez A, Manthorpe R, Thuring A. Autotransplantation of cryopreserved ovarian tissue to the right forearm 4(1/2) years after autologous stem cell transplantation. Acta Obstet Gynecol Scand. 2005;84:695–698. doi: 10.1111/j.0001-6349.2005.00654.x. [DOI] [PubMed] [Google Scholar]
- Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006;12:2739–2746. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HY, Cox SL, Jenkin G, Findlay J, Trounson A, Shaw J. Graft site and gonadotrophin stimulation influences the number and quality of oocytes from murine ovarian tissue grafts. Reproduction. 2006;131:851–859. doi: 10.1530/rep.1.00916. [DOI] [PubMed] [Google Scholar]
- Yin H, Wang X, Kim SS, Chen H, Tan SL, Gosden RG. Transplantation of intact rat gonads using vascular anastomosis: effects of cryopreservation, ischaemia and genotype. Hum Reprod. 2003;18:1165–1172. doi: 10.1093/humrep/deg236. [DOI] [PubMed] [Google Scholar]