Abstract
The epidemiology of lesions identified by magnetic resonance imaging (MRI), along with the use of pre-surgical evaluations and surgery in childhood-onset epilepsy patients has not previously been described. In a prospectively identified community-based cohort of children enrolled from 1993 to 1997, we examined (i) the frequency of lesions identified by MRI; (ii) clinical factors associated with ‘positive’ MRI scans; and (iii) the utilization of comprehensive epilepsy evaluations and neurosurgery. Of the original cohort of 613 children, 518 (85%) had usable MRI scans. Eighty-two (16%) had MRI abnormalities potentially relevant to epilepsy (‘positive’ scans). Idiopathic epilepsy syndromes were identified in 162 (31%) of whom 3% had positive scans. The remainder had non-idiopathic epilepsy syndromes of which 22% had positive MRI findings. Multiple logistic regression analysis identified non-idiopathic epilepsy and abnormal motor-sensory (neurological) examinations as predictors of a positive MRI scan. Of the non-idiopathic patients with normal neurological exams and who were not pharmacoresistant, 10% had positive MRI scans, including four patients with gliomas. Evaluations at comprehensive epilepsy centres occurred in 54 pharmacoresistant cases. To date 5% of the imaged cohort or 8% of non-idiopathic epilepsy patients have undergone surgical procedures (including vagal nerve stimulator implantation) to treat their epilepsy (n = 22) or for tumours (n = 6) without being drug resistant. Applying our findings to the general population of children in the USA, we estimate that there will be 127/1 000 000 new cases per year of pharmacoresistant epilepsy, and 52/1 000 000 childhood-onset epilepsy patients undergoing epilepsy evaluations. In addition, approximately 27/1 000 000 will have an epilepsy-related surgical procedure. These findings support recommendations for the use of MRI in evaluating newly diagnosed paediatric epilepsy patients, especially with non-idiopathic syndromes, and provide estimates on the utilization of comprehensive evaluations and surgery.
Keywords: epidemiology, mesial temporal sclerosis, cortical malformation, epilepsy surgery, pharmacoresistance
Introduction
Structural brain abnormalities are an important cause of epilepsy and are frequently associated with pharmacoresistance. With a few exceptions (Dlugos et al., 2001; Spooner et al., 2006), most of our understanding of these lesions comes from tertiary surgical centres where highly selected patients are thoroughly evaluated. Relatively little is known about the frequency of MRI positive lesions, their association with pharmacoresistance and the use of surgical evaluations and surgery from the community perspective. In its assessment of epilepsy care worldwide, the International League Against Epilepsy (ILAE), Subcommission for Paediatric Epilepsy Surgery noted that insufficient data were available to estimate the number of potential surgical candidates among children with refractory epilepsy and the type and nature of underlying structural lesions associated with new onset epilepsy (Cross et al., 2006). Further, there was little information regarding the proportion of children with refractory epilepsy who were referred for evaluation at comprehensive epilepsy centres, and how many of these received surgery.
The Connecticut Study of Epilepsy is a community-based cohort followed for a median of over a decade in which considerable clinical and research neuroimaging has been performed. This cohort can provide some initial answers to the questions raised in the ILAE's report. In particular, (i) the overall frequency and type of structural abnormalities, identified by MRI, associated with newly diagnosed epilepsy in children; (ii) the clinical features associated with a higher yield of positive MRI findings; and (iii) patterns in the use of comprehensive epilepsy evaluations and surgery.
Methods
Recruitment
The Connecticut Study of Epilepsy is a community-based study that recruited children (1 month to 16 years of age) with newly diagnosed epilepsy from 16 of the 17 offices of practicing paediatric neurologists in the state between 1993 and 1997. Connecticut is a relatively small state with approximately 500 000 children (<16 years old) during the time of recruitment. The US healthcare system relies heavily on specialist care where available. Before recruitment began, paediatricians in the state (paediatricians are considered primary care physicians in the USA) were polled about their practices regarding referral of children with newly diagnosed epilepsy to a paediatric neurologist (a first-level specialist). All paediatricians surveyed responded that their usual practice was to refer to a paediatric neurologist for at least an initial evaluation.
Parents were interviewed at the time of entry to the study. Close contact was maintained with the families, by telephone, every 3–4 months. Permission was obtained to access relevant medical records at initial study entry and on an on-going basis, including records from evaluations at comprehensive epilepsy centres and neurosurgical and histopathology reports. Patients were considered to have had a comprehensive evaluation if they were admitted to an epilepsy centre for prolonged (at least overnight) video electroencephalography (EEG)-telemetry, often with other evaluations e.g. ictal single photon emission computed tomography (SPECT) and Fluorodeoxyglucose-Positron emission tomography (FDG-PET). Other details of the study's recruitment and follow-up methods have been published previously (Berg et al., 1999, 2006). Information regarding sensory and motor neurological deficits (the ‘neurological exam’) was abstracted from the medical records of this examination performed by the neurologist. Cognitive and developmental status was assessed based on information in medical records, school records, special service providers, periodic interviews with parents and, for over half of the cohort, a neuropsychological exam performed for research purposes (Berg et al., 2008).
Clinical characterization of cohort
Each child's seizure type and electro-clinical syndrome were classified according to ILAE criteria (Commission on Classification and Terminology of the International League Against Epilepsy, 1989) and relevant updates (Roger et al., 2002; Panayiotopoulos, 2005). Underlying causes of the epilepsy were classified according to ILAE recommendations (Commission on Epidemiology and Prognosis and International League Against Epilepsy, 1993).
For this presentation, type of epilepsy was classified as ‘idiopathic’ if the epilepsy conformed to one of the well-described traditional idiopathic electro-clinical syndromes and ‘non-idiopathic’ for all other cases. Characterizations of epilepsy syndromes and aetiology were updated as new evidence became available from further EEGs, MRI scans, genetic testing, neurocognitive testing, as well as changes in seizure types. The characterizations of each patient's epilepsy were based on the most recent systematic reassessments done 9 years after initial diagnosis. In some instances, the relevance of what appeared to be a potentially epileptogenic lesion on MRI, to a particular individual's epilepsy, was unclear. Such cases were included in our analyses although it was probable that the MRI finding was coincidental in the context of the patient's specific forms of epilepsy. Pharmacoresistance was defined as the failure of two different appropriate anti-epileptic drugs (AEDs) to bring seizures under complete control when used as prescribed and pushed to the maximum tolerated levels (Berg et al., 2006). This is essentially the definition proposed by the ILAE Task Force on Defining Refractory Epilepsy (French, 2009).
Imaging
More than half of the cohort had MRI scans as part of their initial diagnostic evaluation, from 1993 to 1997 (Berg et al., 2000). The entire cohort was followed, on average, for over a decade and many have had additional clinical neuroimaging. Furthermore, many participated in a phase of the study in which a research MRI scan was performed under a uniform seizure protocol. All research scans and almost all clinical scans were performed on a 1.5 T magnet. Clinical scans used sequences and protocols for the scanners on which they were performed and these represent the standard of care for the local regions. The research scans were performed on either a 1.5 T Siemens Sonata or a 1.5 T General Electric Signa and were originally designed to evaluate mesial temporal anatomy. The sequences used were as follows: sagittal localizer, T1-weighted; coronal gradient echo 1.5 mm thick contiguous sections acquired in a 3D volume acquisition of the entire brain—to evaluate: (i) hippocampal atrophy (HA) and amygdala atrophy and (ii) malformations of cortical development (MCD); coronal 3-mm thick high resolution fast spin echo (FSE) T2W sections through the temporal lobe—to evalaute atrophy in the hippocampus and amygdala and signal change; coronal fluid attenuated inversion recovery (FLAIR) 5-mm thick sections through the brain—to evaluate: (i) anatomic or signal abnormality in the brain and (ii) hippocampal signal changes.
Interpretation of MRIs and classification of patients
All available research MRI and original clinical MRI scans were interpreted independently by two neuroradiologists with extensive epilepsy imaging experience (RB&RF). The MRI findings were considered as positive if a lesion was identified that could potentially be related to the underlying epilepsy. Minor MRI findings that were not potentially epileptogenic, such as a pineal cyst, were excluded. MRI findings for all subjects were further reviewed by a paediatric epilepsy neurosurgeon (G.W.M.) to characterize the type of MRI abnormality and determine if the lesions were potentially treatable with resective surgery. We defined those lesions involving portions of one cerebral hemisphere as ‘potentially surgical’. In addition, cases of hypothalamic hamartomas, and tubers associated with tuberous sclerosis complex (TSC) were counted as potential surgical candidates (Weiner et al., 2006).
Preferred source of imaging information
When multiple scan types were available, the following sources of imaging information were relied upon, in descending order of preference: (i) research MRI (n = 299); (ii) clinical MRI re-reviewed and interpreted by study neuroradiologists (n = 107); and (iii) clinical MRI for which only the written report was available (n = 113). When multiple scans from the same source were available (e.g. several re-interpreted clinical scans), one was selected based on the following preferred criteria: (i) a preoperative study; (ii) the best quality study; and (iii) a study done at an older age, particularly when the participant was an infant at the onset of epilepsy. In some instances, the research MRI was performed after a subject had already had surgery. In these cases, we included information from the pre-surgical scan regarding the imaging characterization of the lesion with that from the research scan, which also considered residual abnormalities. When possible, we compared the interpretation of definite abnormalities from clinical reports to those identified based on our own central review in order to identify any potential inadequacies in using clinical reports when original scans were not available.
This report focuses on the MRI abnormalities considered to be definitely or potentially relevant to epilepsy (‘positive’ scans). Incidental (e.g. a pineal cyst) and equivocal MRI abnormalities were considered as negative for this study. Because MRI is the preferred mode for neuroimaging in epilepsy evaluations (Hirtz et al., 2000), subjects for whom only computed tomography (CT) results were available (n = 42) were not included in our analysis although we provide the information from this group in Table 1.
Table 1.
Comparisons of study subjects with research MRIs, clinical MRI scans that were re-read, and clinical MRI scans that were not-re-read (radiology report only)
| Feature | Research MRI (n = 298a) n (%) | Re-read clinical MRI (n = 107) n (%) | Not re-read clinical MRI (n = 113a) n (%) | CT scan only (n = 42) n (%) | No imaging (n = 51) n (%) |
|---|---|---|---|---|---|
| Overall interpretation of scan | |||||
| MRI negative | 267 (89.6) | 84 (78.5) | 85 (75.2) | 32 (76.2) | |
| MRI positive | 31 (10.4) | 23 (21.5) | 28 (24.8) | 10 (23.8)b | |
| Extent of lesion for abnormal scans | |||||
| Uni-hemispheric | 22 (71.0) | 9 (39.1) | 13 (46.4) | 2 (33)c | |
| Bi-hemispheric | 9 (29.0) | 14 (60.9) | 15 (53.6) | 4 (67) | |
| Pharmacoresistance | |||||
| Absent | 237 (79.5) | 73 (68.2) | 76 (67.3) | 37 (88.1) | 48 (94.1) |
| Present | 61 (20.5) | 34 (31.8) | 37 (32.7) | 5 (11.9) | 3 (5.9) |
| Cognitive status | |||||
| IQ ≥ 80 | 254 (85.2) | 62 (57.9) | 66 (58.4) | 27 (64.3) | 41 (80.4) |
| IQ < 80 | 44 (14.8) | 45 (42.1) | 47 (41.6) | 15 (35.7) | 10 (19.6) |
| Neurological exam | |||||
| Normal | 285 (95.6) | 78 (72.9) | 89 (78.8) | 31 (73.8) | 47 (92.2) |
| Abnormal | 13 (4.4) | 29 (27.1) | 24 (21.2) | 11 (26.2) | 4 (7.8) |
| Deceased | |||||
| No | 296 (99.3) | 102 (95.3) | 107 (94.7) | 39 (92.9) | 51 (100) |
| Yes | 2 (0.7) | 5 (4.7) | 6 (5.3) | 3 (7.1) | 0 |
a Two subjects who did have MRIs were excluded, one because the original scan and report were unavailable, and the information provided in the records was inadequate to characterize the findings beyond being abnormal; the other because of a severe head injury that occurred just prior to the only scan (research MRI) that was done, but several years after the onset of epilepsy.
b Three treated hydrocephalus, five pre-perinatal strokes, one lissencephaly and one Pfeiffer syndrome.
c Extent of lesion could not be determined from the CT report in four cases.
Analyses
Comparisons were tested with chi-squared and t-tests as appropriate for the data. Logistic regression was used for multivariable analysis to identify independent indicators of positive MRI findings.
Ethics
The procedures used throughout this study were approved by the Institutional Review Boards of all participating institutions. The initial procedures for obtaining the parents’ informed permission and, when possible, the children's informed assent conformed to the provisions of the Declaration of Helsinki as did procedures for obtaining informed consent once study subjects reached 18 years of age.
Results
Imaging in the cohort
Of the 613 children with newly diagnosed epilepsy, 520 (84.8%) had at least one MRI scan. An additional 42 (6.9%) children had a CT scan without MRI and 51 (8.3%) had no imaging study performed. Two subjects with MRIs were excluded from our analysis. The first case was excluded because the original MRI and radiology report were not available and the description of the abnormalities obtained from the physician's notes was inadequate for classifying the lesion. The second case was excluded because of head trauma (with associated imaging abnormalities) that occured several years after the onset of epilepsy and just prior to the only available MRI scan. Hence, 84.5% (518/613) had usable MRI scans. There were some differences between those who had MRI scans, CT only, and no scans in terms of neurological and cognitive examination, pharmacoresistance and mortality (Table 1). Those without any neuroimaging were more likely to have idiopathic generalized electro-clinical syndromes than those with CT or MRI (41% versus 20%, P = 0.0004).
Source of positive MRI scans
There were differences in the frequency of positive scans and clinical characteristics between subjects who had research MRIs compared with those with only clinical scans (Table 1). Those who participated in the non-sedated research MRI were more likely to have a normal scan (P < 0.001), a normal neurological exam (P < 0.0001), an IQ score above 80 (P < 0.0001) and to still be alive (P = 0.002) compared with those with re-read and not re-read (combined) clinical scans. If research scans were positive, the MRI findings were more likely to be uni-hemispheric and focal than findings from clinical scans (P = 0.01). In addition, because the research scans were performed 8–9 years after study entry, the research scan group was older at the time of their imaging than the participants for whom we used clinical scans (15.5 years ± 4.1 versus 6.1 years ± 4.6). Subjects with re-read versus non-re-read scans were similar in terms of age when scanned (5.6 years for re-read and 6.6 years for non-re-read scans), other patient characteristics and MRI findings.
The identification of definite abnormalities obtained from the original radiological report was comparable with the results obtained when the original MRI images were re-interpreted for the study purposes. Kappa for the agreement over whether the findings were normal (including equivocal and incidental abnormalities) versus definitely abnormal was 0.85 [95% confidence interval (CI) 0.73–0.98]. The specific interpretations of the MRI abnormalities (location and nature of lesion) were also very similar when we compared the clinical with the research interpretations for each positive scan. The excellent agreement between research and original clinical interpretations for definite MRI abnormalities indicates that our reliance on clinical reports for a proportion of study subjects did not introduce major error in the identification of obvious structural abnormalities.
Neuroimaging findings
Of the 518 subjects included in the analyses, 82 (15.8%, 95% CI = 12.7–19.0) had evidence of structural lesions that were considered potentially relevant to epilepsy (Table 2). Thirty (37%) were considered acquired (mostly pre- and perinatal cerebral injuries), 22 (27%) MCD, 18 (22%) discrete lesions (e.g. tumours) and 12 (15%) lesions associated with a variety of genetic conditions.
Table 2.
All positive MRI findings in MRI-imaged cohort (n = 82)
| Case # | Lesion typea | MRI finding | MTS/HA | Additional clinical notes | Extent of lesion | Lobe | Post-surg. candidate | Pharmaco- resistant | Evaluation | Surgery |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Acquired | Atrophy—bilateral focal | Hypoglycemic encephalopathy; west syndrome | Bi-hemispheric | Parietal–occipital | Yes | EEG Tele and PET/SPECT | VNS | ||
| 2 | Acquired | Atrophy—bilateral-focal | Prenatal ischaemia and cerebral palsy | Bi-hemispheric | Occipital | |||||
| 3 | Acquired | Atrophy—diffuse | Prenatal ischaemia-deceased | Bi-hemispheric | All | |||||
| 4 | Acquired | Atrophy—diffuse | HIE | Bi-hemispheric | All | Yes | ||||
| 5 | Acquired | Atrophy—diffuse | Prenatal ischaemia; west syndrome | Bi-hemispheric | All | Yes | ||||
| 6 | Acquired | Atrophy—diffuse | Post-infection; west syndrome | Bi-hemispheric | All | Yes | ||||
| 7 | Acquired | Atrophy—diffuse | Yes | HIE | Bi-hemispheric | All | ||||
| 8 | Acquired | Atrophy—diffuse and plagiocephaly | Prenatal ischaemia- deceased | Bi-hemispheric | All | Yes | EEG Tele | |||
| 9 | Acquired | Atrophy—diffuse and ventriculomegaly | Yes | HIE | Bi-hemispheric | All | ||||
| 10 | Acquired | Atrophy—focal | Prenatal ischaemia | Uni-hemispheric | Parietal-right | Yes | Yes | EEG Tele and PET/SPECT | VNS | |
| 11 | Acquired | Atrophy—focal | Postnatal CVA | Uni-hemispheric | Occipital-right | Yes | ||||
| 12 | Acquired | Atrophy—focal | Postnatal CVA | Uni-hemispheric | Parietal–occiptial-right | Yes | Yes | |||
| 13 | Acquired | Atrophy—focal | IVH | Uni-hemispheric | Parietal-left | Yes | Yes | |||
| 14 | Acquired | Atrophy—focal | Prenatal ischaemia | Uni-hemispheric | Frontal-right | Yes | Yes | EEG Tele and PET/SPECT | Surgery | |
| 15 | Acquired | Atrophy—focal | Neonatal CVA | Uni-hemispheric | Parietal-left | Yes | ||||
| 16 | Acquired | Atrophy—focal | Uni-hemispheric | Parietal-right | Yes | |||||
| 17 | Acquired | Atrophy—focal | Prenatal ischaemia | Uni-hemispheric | Temporal-left | Yes | ||||
| 18 | Acquired | Atrophy—lacunar infarct | Strep. pneumonia | Uni-hemispheric | Thalamus-right | Yes | ||||
| 19 | Acquired | Atrophy—multilobar | R-MCA stroke | Uni-hemispheric | Frontal–parietal-right | Yes | Yes | EEG Tele and PET/SPECT | Surgery | |
| 20 | Acquired | Atrophy—multilobar and ventriculomegaly | Yes | Prenatal ischaemia | Uni-hemispheric | Temporal–parietal– occipital-right | Yes | Yes | ||
| 21 | Acquired | Congenital Toxoplasmosis Hydrocephalus | Uni-hemispheric | Temporal | Yes | |||||
| 22 | Acquired | Microcephaly and Ventriculomegaly | HIE | Bi-hemispheric | All | |||||
| 23 | Acquired | Porencephaly and Ventriculomegaly | Uni-hemispheric | Hemisphere-left | Yes | |||||
| 24 | Acquired | PVL | H/O Trauma | Bi-hemispheric | Frontal | |||||
| 25 | Acquired | PVL | Spastic deplegia | Bi-hemispheric | Parietal | |||||
| 26 | Acquired | PVL | IVH/absence epilepsy | Uni-hemispheric | Frontal-left | Yes | ||||
| 27 | Acquired | PVL | H/O IVH | Bi-hemispheric | All | Yes | ||||
| 28 | Acquired | PVL | Yes | H/O IVH | Bi-hemispheric | All | Yes | EEG Tele | ||
| 29 | Acquired | PVL | H/O IVH | Bi-hemispheric | All | |||||
| 30 | Acquired | PVL and Porencephaly | H/O IVH; west syndrome | Uni-hemispheric | Frontal–temporal– parietal-right | Yes | Yes | EEG Tele and PET/SPECT | VNS | |
| 31 | MCD | Cortical dysplasia | West syndrome | Bi-hemispheric | Frontal | Yes | VNS | |||
| 32 | MCD | Cortical dysplasia | Yes-Path only | SUDEP | Bi-hemispheric | Parietal | Yes | EEG Tele and PET/SPECT | Surgery and VNS | |
| 33 | MCD | Cortical dysplasia | Uni-hemispheric | Frontal-right | Yes | |||||
| 34 | MCD | Cortical dysplasia | Uni-hemispheric | Temporal-right | Yes | |||||
| 35 | MCD | Cortical dysplasia | Uni-hemispheric | Frontal–parietal-left | Yes | |||||
| 36 | MCD | Cortical dysplasia | Uni-hemispheric | Parietal-left | Yes | |||||
| 37 | MCD | Cortical dysplasia | Yes | Uni-hemispheric | Temporal-left | Yes | Yes | EEG Tele and PET/SPECT | Surgery and VNS | |
| 38 | MCD | Cortical dysplasia | West syndrome | Uni-hemispheric | Temporal-left | Yes | Yes | EEG Tele | ||
| 39 | MCD | Hemimegalencephaly | Uni-hemispheric | Hemisphere-left | Yes | Yes | EEG Tele and PET/SPECT | |||
| 40 | MCD | Heterotopia | JAE/JME | Bi-hemispheric | Frontal | |||||
| 41 | MCD | Heterotopia, Chiari-II | Bi-hemispheric | Temporal–occipital | ||||||
| 42 | MCD | Hetertopia | Yes | Uni-hemispheric | Temporal-right | Yes | Yes | |||
| 43 | MCD | Hetertopia | Uni-hemispheric | Occipital-left | Yes | Yes | EEG Tele and PET/SPECT | |||
| 44 | MCD | Holoprosencephaly | Deceased | Bi-hemispheric | All | |||||
| 45 | MCD | Lissencephaly | West syndrome | Bi-hemispheric | All | Yes | ||||
| 46 | MCD | Pachygyria | Deceased | Bi-hemispheric | All | Yes | ||||
| 47 | MCD | Pachygyria | Bi-hemispheric | All | Yes | EEG Tele | ||||
| 48 | MCD | Pachygyria and colpocephaly | Bi-hemispheric | All | ||||||
| 49 | MCD | Polymicorgyria and colpocephaly | Bi-hemispheric | Frontal–parietal | Yes | |||||
| 50 | MCD | Polymicrogyria | Bi-hemispheric | Frontal–parietal | Yes | EEG Tele and PET/SPECT | VNS | |||
| 51 | MCD | Schizencephaly | Uni-hemispheric | Frontal–parietal-right | Yes | |||||
| 52 | MCD | Schizencephaly | Uni-hemispheric | Frontal-left | Yes | |||||
| 53 | Genetic | Degenerative | Yes | Complex IV deficiency-deceased | Bi-hemispheric | All | Yes | EEG Tele and PET/SPECT | ||
| 54 | Genetic | Degenerative | Batten disease- deceased | Bi-hemispheric | All | Yes | ||||
| 55 | Genetic | Degenerative | Menkes/west syndrome-deceased | Bi-hemispheric | All | Yes | ||||
| 56 | Genetic | Neurofibromatosis | Bi-hemispheric | All | ||||||
| 57 | Genetic | Neurofibromatosis | Yes | Bi-hemispheric | Temporal | |||||
| 58 | Genetic | Tuberous sclerosis complex | West syndrome | Bi-hemispheric | Multiple Tubers | Yes | Yes | |||
| 59 | Genetic | Tuberous sclerosis complex | Bi-hemispheric | Multiple Tubers | Yes | Yes | EEG Tele and PET/SPECT | |||
| 60 | Genetic | Tuberous sclerosis complex | Bi-hemispheric | Multiple Tubers | Yes | |||||
| 61 | Genetic | Tuberous sclerosis complex | West syndrome | Bi-hemispheric | Multiple Tubers | Yes | Yes | |||
| 62 | Genetic | Tuberous sclerosis complex | Bi-hemispheric | Multiple Tubers | Yes | |||||
| 63 | Genetic | Tuberous sclerosis complex | Bi-hemispheric | Multiple Tubers | Yes | Yes | EEG Tele | Surgery and VNS | ||
| 64 | Genetic | Tuberous sclerosis complex | Bi-hemispheric | Multiple Tubers | Yes | Yes | EEG Tele | |||
| 65 | Discrete Lesion | Cavernous angioma | Uni-hemispheric | Tempora–Parietal-left | Yes | Yes | EEG Tele and PET/SPECT | Surgery and VNS | ||
| 66 | Discrete Lesion | Cyst | EEG focus contralateral | Uni-hemispheric | Parietal-right | Yes | ||||
| 67 | Discrete Lesion | Cyst | BRE | Uni-hemispheric | Temporal-right | |||||
| 68 | Discrete Lesion | HA/Sclerosis | Yes | IGE | Bi-hemispheric | Temporal | ||||
| 69 | Discrete Lesion | HA/Sclerosis | Yes | Uni-hemispheric | Temporal-left | Yes | ||||
| 70 | Discrete Lesion | HA/Sclerosis | Yes | BRE | Uni-hemispheric | Temporal-left | ||||
| 71 | Discrete Lesion | HA/Sclerosis | Yes | Uni-hemispheric | Temporal-right | Yes | ||||
| 72 | Discrete Lesion | HA/Sclerosis | Yes | Prenatal ischaemia | Uni-hemispheric | Temporal-right | Yes | |||
| 73 | Discrete Lesion | HA/Sclerosis | Yes | Uni-hemispheric | Temporal-right | Yes | Yes | EEG Tele | ||
| 74 | Discrete Lesion | Hypothalamic Hamartoma | Uni-hemispheric | Hypothalamus-right | Yes | Yes | EEG Tele and PET/SPECT | Surgery and VNS | ||
| 75 | Discrete Lesion | Hypothalamic Hamartoma | Uni-hemispheric | Hypothalamus-right | Yes | Yes | EEG Tele and PET/SPECT | Surgery | ||
| 76 | Discrete Lesion | Tumour—Grade II Astrocytoma | Uni-hemispheric | Temporal-left | Yes | Yes | EEG Tele | Surgery | ||
| 77 | Discrete Lesion | Tumour—low grade Glioma | Uni-hemispheric | Parietal–occipital-left | Yes | Surgery | ||||
| 78 | Discrete Lesion | Tumour—Anaplastic Astrocytoma | Uni-hemispheric | Occipital-right | Yes | Surgery | ||||
| 79 | Discrete Lesion | Tumour—DNET | Uni-hemispheric | Frontal-right | Yes | Surgery | ||||
| 80 | Discrete Lesion | Tumour—DNET | Uni-hemispheric | Frontal–parietal-left | Yes | Surgery | ||||
| 81 | Discrete Lesion | Tumour—Ganglioglioma | Uni-hemispheric | Temporal-right | Yes | Surgery | ||||
| 82 | Discrete Lesion | Tumour—Oligodendroglioma | Yes-Post Op | Uni-hemispheric | Temporal-right | Yes | Surgery | |||
a Based on the MRI finding and clinical information, lesions were classified as ‘Acquired’ if there was evidence of destructive cortical process and atrophy from an insult (e.g. ischaemia, stroke), as malformation of cortical development (MCD; e.g. cortical dysplasia, lissencephaly), a ‘Discrete Lesion’ for uni-hemispheric, focal lesions of the type often treated surgically (e.g. tumours, cavernous malformations) and ‘Genetic’ for MRI findings characteristic of and associated with specific identified genetic disorders (e.g. Batten's Disease, tuberous sclerosis) although we recognize that some lesions could have been classified in more than one category (e.g. lissencephaly; MCD and genetic).
BRE = benign rolandic epilepsy; CVA = cerebrovascular accident; DNET = dysembryoplastic neuroepithelial tumour; HIE = hypoxic-ischemic encephalopathy; IGE = idiopathic generalized epilepsy; IVH = intraventricular hemorrhage; JAE = juvenile absence epilepsy; JME = juvenile myoclonic epilepsy; MCD = malformation of cortical development; PVL = periventricular leucomalacia; R-MCA = right middle cerebral artery; SUDEP = sudden unexpected death in epilepsy; VNS = vagal nerve stimulator
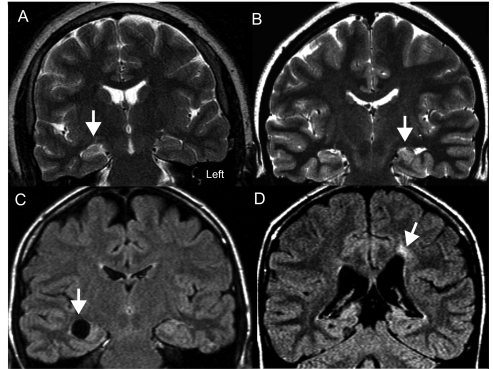
Five subjects (#26, #40, #67, #68 and #70) had structural lesions that were difficult to reconcile with their idiopathic electro-clinical syndromes (Table 2) and which, under other circumstances, might be considered relevant to the cause of a patient's epilepsy. These included two with evidence of HA (Table 2, #68 and #70). In these five cases, however, the findings were ultimately deemed as probably coincidental. Two more patients had clinical histories consistent with acquired insults. The structural abnormalities visible on the MRI, however, did not correspond to their seizures (small thalamic lacunar infarct and a subependymal cyst contralateral to the EEG interictal focus; Table 2, Cases #18 and #66). Examples of some of the MRI findings, including those considered to be coincidental, are provided in Fig. 1.
Figure 1.
(A) Example of a patient with pharmacoresistant epilepsy with EEG suggesting a right temporal focus. MRI shows increased T2 signal in the right hippocampus (arrow). This is the only case so far of a patient with MRI MTS whose seizures are not controlled by drugs and with a concordant EEG. (Table 2; Case #73). (B) Example of another patient with TLE whose seizures are controlled by drugs. Interictal EEG shows greater left than right abnormalities. MRI discloses left HA with some T2 signal changes (arrow; Table 2; Case #69). (C) This patient has electro-clinical findings most consistent with benign rolandic epilepsy including characteristic epileptiform discharges activated during sleep. MRI revealed a cyst in the right mesial temporal lobe (arrow). The MRI finding was considered coincidental in this particular clinical context (Table 2; Case #67). (D) This patient has childhood absence epilepsy, a diagnosis based on the characteristic 3 Hz generalized spike and wave on EEG associated with absence spells induced with hyperventilation. MRI disclosed evidence of an old left intraventricular haemorrhage (arrow) and this patient also has a mild right hemiparesis. The MRI lesion was considered coincidental in the clinical context (Table 2; Case #26).
Positive MRI scans were found in 21.6% (77/356) of patients with non-idiopathic epilepsy compared with 3% (5/162) for those in the idiopathic group. Compared with those with negative MRI scans, patients with positive imaging findings had a younger age at onset, and a higher frequency of abnormalities in both their motor-sensory neurological exams and cognitive status (Table 3). Drug resistance and mortality were also higher in the MRI positive group compared with the negative group. In a multiple logistic regression analysis of all imaged patients, the type of epilepsy (idiopathic versus non-idiopathic) and abnormal neurological exam were the strongest predictors of having a positive MRI scan (Table 4). In a multiple logistic regression analysis limited to study subjects with non-idiopathic forms of epilepsy (n = 356), an abnormal neurological exam (P < 0.0001) and pharmacoresistance (P = 0.04) were independent correlates of a positive MRI. In children with an abnormal neurological exam, 65.7% (23/35) who were pharmacoresistant versus 53.3% (16/30) that were not pharmacoresistant had a positive MRI (P = 0.31). In those with a normal neurological exam, however, 20.7% (17/82) who were pharmacoresistant versus 10.1% (21/209) who were not pharmacoresistant had a positive MRI (P = 0.02). This last group contained four patients with gliomas. A total of 24 patients with normal neurological and cognitive exams and no history suggesting a previous insult or condition (e.g. head trauma, bacterial meningitis) had positive MRI findings. This represents almost a third of all positive MRI scans among patients with non-idiopathic epilepsies.
Table 3.
Comparisons of subjects with negative and positive MRI scans with respect to clinical variables
| MRI |
||||
|---|---|---|---|---|
| Total (n = 518) n (%) | Negative (n = 436, 84.2%) n (%) | Positive (n = 82, 15.8%) n (%) | P-value | |
| Traditional idiopathic syndromes | 162 (31.3) | 157 (36.0) | 5a (6.1) | <0.0001 |
| Age at onset <2 years | 113 (21.8) | 83 (19.0) | 30 (36.6) | 0.0004 |
| Pharmacoresistant | 132 (25.5) | 92 (21.1) | 40 (48.8) | <0.0001 |
| Deceased | 13 (2.5) | 5 (1.2) | 8 (9.8) | <0.0001 |
| FSIQ <80 or equivalent | 136 (26.3) | 92 (21.1) | 44 (53.7) | <0.0001 |
| Abnormal neurological exam | 66 (12.7) | 26 (6.0) | 40 (48.8) | <0.0001 |
| Length of follow-up (years, SD) | 11.7 (2.9) | 10.5 (3.9) | 0.009 | |
| Time to second AED failure (n = 92, years, SD) | 2.7 (3.1) | 1.8 (2.4) | 0.08 | |
a Five cases with idiopathic electro-clinical syndromes and probably incidental structural abnormalities as described in the text. AED = anti-epileptic drug; FSIQ = full scale intelligence quotient.
Table 4.
Multivariable logistic regression model of predictors of positive MRI scan for all patients and for patients with non-idiopathic forms of epilepsy
| Predictor | Relative risk | 95% CI | P-value |
|---|---|---|---|
| Predictors of positive MRIs | |||
| Idiopathic syndrome | 0.26 | 0.10–0.65 | 0.004 |
| Abnormal neurological exam | 4.24 | 2.89–6.22 | <0.0001 |
| Pharmacoresistant | 1.42 | 0.99–2.02 | 0.06 |
| IQ <80a | 1.08 | 0.68–1.70 | 0.74 |
| Age at onset <2 yearsa | 0.88 | 0.63–1.23 | 0.45 |
| Patients with non-idiopathic forms of epilepsy | |||
| Abnormal neurological exam | 4.05 | 2.76–5.93 | <0.0001 |
| Pharmacoresistant | 1.47 | 1.02–2.12 | 0.04 |
| IQ <80a | 1.14 | 0.70–1.85 | 0.60 |
| Age at onset <2 yearsa | 0.88 | 0.63–1.24 | 0.48 |
Death is not considered a predictor of the MRI findings but as an outcome.
a Estimates for IQ<80 and for age at onset were obtained after adjustment for ineurological exam and pharmacoresistance as well as, in the first half of the table, idiopathic syndromes. These three factors were adjusted for each other but not for IQ or age at onset.
In addition to being associated with pharmacoresistance, patients with positive MRI findings were less likely to experience remission periods after second drug failure. Of those with non-idiopathic epilepsies who had tried at least a second drug and been followed at least 3 years after second drug failure (n = 104), 19/68 (27.9%) with negative scans and 1/36 (2.8%) with positive scans were seizure-free for >3 years at last contact (P = 0.002). None of the cases with idiopathic syndromes and positive MRI scans was pharmacoresistant.
Uni- versus bi-hemispheric MRI abnormalities
Forty-four (54%) of the subjects had uni-hemispheric (including subcortical) and 38 (46%) had bi-hemispheric lesions. Compared with patients with uni-hemispheric lesions, those with bi-hemispheric lesions had a younger age at seizure onset, and a higher proportion had an abnormal cognitive or neurological status, as well as higher mortality (Table 5). Pharmacoresistant epilepsy was slightly, but not significantly, more frequent in patients with bi- versus uni-hemispheric lesions (57.9% versus 40.9%, P = 0.12). This was true even after excluding five individuals with idiopathic forms of epilepsy and positive MRI scans.
Table 5.
Comparison of patients with uni-hemispheric versus bi-hemispheric structural abnormalities on MRI
| Uni-hemispheric abnormalitiesa (n = 44) | Bi-hemispheric abnormalities (n = 38) | P-value | |
|---|---|---|---|
| Years (SD) | Years (SD) | ||
| Average age at onset | 6.1 (4.5) | 3.6 (3.8) | 0.008 |
| Average time to second drug failure | 1.9 (2.5) | 1.7 (2.4) | 0.73 |
| Average follow-upa | 11.6 (2.8) | 9.3 (4.5) | 0.008 |
| N (%) | N (%) | ||
| Pharmacoresistant | 18 (40.9) | 22 (57.9) | 0.12 |
| Deceased | 0 (0) | 8 (21.1) | 0.001 |
| FSIQ <80 | 15 (34.1) | 29 (76.3) | 0.0001 |
| Abnormal neurological exam | 15 (34.1) | 25 (65.8) | 0.004 |
| Type of epilepsy | |||
| Focal | 38 (86.4) | 21 (55.3) | 0.0002 |
| Other generalized | 2 (4.6) | 16 (42.1) | |
| Idiopathic | 4 (9.1) | 1 (2.6) |
Of the uni-hemispheric lesions, 39 were focal, 4 multi-lobar and 1 involved the entire hemisphere. In the bi-hemispheric group, 12 involved bilateral homologous regions (e.g. bi-temporal), 2 were multi-lobar, 9 multifocal and 15 involved both hemispheres diffusely.
The P-value is driven by the differences in the focal and other generalized group.
Most (although not quite all) of the difference in follow-up is due to the higher mortality in the bilateral MRI positive group.
Of those with refractory non-idiopathic epilepsy and positive MRI scans, we identified 22 (55.0%) as potential surgical candidates. Thus, 4.2% (95% CI 2.5%, 6.0%) of the MRI-imaged cohort had pharmacoresistant epilepsy associated with potentially resectable structural abnormalities. This represents 6.2% (95% CI 3.7–8.9%) of patients with non-idiopathic epilepsy.
Mesial temporal sclerosis/HA
Sixteen subjects had mesial temporal sclerosis (MTS) or HA. In one patient with bi-hemispheric MRI abnormalities, MTS was found on histopathology only (Table 2, #32). In another patient MTS/HA was interpreted from the MRI as being secondary to postoperative changes (Table 2; #82). In 14 others, MTS was diagnosed based on visual assessment of hippocampal volumetric loss, increased FLAIR or T2 signal change, or both. Information came from five research, eight re-read clinical and one not re-read clinical scans. Of the 14 patients with MRI identified MTS/HA, seven had evidence of other structural abnormalities on their scans. One patient, with a negative MRI, had subtle cortical dysplasia identified at histopathology, and the last patient also had neurofibromatosis. Only five patients had isolated MTS/HA on their MRI scans. As previously mentioned, two of these patients had idiopathic electro-clinical syndromes and well-controlled seizures. Of the other three cases, two were in remission on AEDs and the third was pharmacoresistant with an interictal EEG focus consistent with mesial temporal lobe origin ipsilateral to the imaging findings (Fig. 1A).
Comprehensive evaluations and surgery
Evaluations at comprehensive epilepsy centres were conducted in 54/132 (40.9%) of pharmacoresistant patients and in 53/117 (45.3%) of non-idiopathic, pharmacoresistant patients. In the non-idiopathic group, 30/77 (39%) patients with negative MRI scans and 23/40 (58%) with positive MRI scans were evaluated (Table 6). This last group included 16/22 (72.7%) of those with potential surgical lesions. Of these 16, the median time from failure of second drug to first in-hospital monitoring was 2 years (five were within 1 year, three within 1–2 years, four within 2–3 years and four after >3 years). For the remaining six potential surgery cases with pharmacoresistant epilepsy, two had less than one seizure per month and another was followed for <1 year. Comprehensive evaluations were also performed in 7/18 (38.8%) with abnormal scans, but who were not considered likely surgical candidates and in 30/76 (28.8%) with normal MRI scans. Their median time from failure of the second drug to evaluation was 1.3 years (14 within 1 year, nine within 1–2 years, five within 2–3 years and six after >3 years).
Table 6.
Comprehensive epilepsy evaluations, epilepsy surgery and lesion surgery
| Refractory (n = 132) |
Not refractory MRI positive (n = 42) | |||
|---|---|---|---|---|
| MRI positive (n = 40) |
MRI negative (n = 92 including 15 idiopathic cases) | |||
| Not potential surgical (n = 18) | Potential surgical (n = 22) | |||
| Comprehensive evaluation (including PET/SPECT)a | 7 (4) | 16 (11) | 31b (16) | NA |
| No comprehensive evaluation | 11 | 6 | 61 | NA |
| Resective surgery | 1 | 7 | 3 | 6 |
| Repeat surgeries | 1 | 2 | 0 | 0 |
| Multiple subpial transections | 0 | 0 | 1 | 0 |
| Corpus callosotomy | 0 | 2c | 0 | 0 |
| Vagal nerve stimulator | 4 (1d) | 6 (4d) | 5 (1d) | 0 |
a Numbers within parenthesis represents a subset of those evaluated.
b One case with an idiopathic form of epilepsy underwent inpatient video monitoring.
c One of these two is also counted above with repeated focal resection before corpus callosotomy.
d Indicates the number who also had some other kind of surgery.
Of 61 patients with normal MRI scans who were pharmacoresistant and who did not have comprehensive evaluations, 14 (23.0%) had idiopathic electro-clinical syndromes and 34 (55.6%) were seizure-free for at least 1 year at last contact. Of the 11 pharmacoresistant patients with abnormal MRI scans, but who were not considered likely surgical candidates and who did not have comprehensive evaluations, eight (72.7%) had other generalized electro-clinical syndromes including three with neurodegenerative conditions.
During a median follow-up period of 11.5 years, 28 (5.4%) patients in the MRI cohort have undergone some surgical procedure. Surgery was performed in 16.7% (22/132) of those with pharmacoresistant epilepsy. This represents 4.2% (22/518) of our imaged cohort, 18.8% (22/117) of non-idiopathic pharmacoresistant patients and 41.5% (22/53) of non-idiopathic patients who underwent an evaluation. Resective or disconnection epilepsy surgery (for seizure control) was performed on 13 patients, one of whom first had a lesion resected (i.e. was not pharmacoresistant at the time of the first procedure), but then later required epilepsy surgery (Table 7). Four patients with negative MRI scans and cryptogenic epilepsy had surgery. Abnormal histopathology was documented in two of these cases. Vagal nerve stimulators (VNS) were used in 15 patients (11.4% of those with refractory seizures), and included six patients who underwent other procedures either before or after VNS implantation. Following resective procedures, five pharmacoresistant patients became seizure-free. The other six lesion resection-only patients are also seizure-free. Two patients who were not seizure-free after surgery have died.
Table 7.
Details of 19 surgical cases
| Case # from Table 2 | Primary MRI abnormality | Lesion/histopathology | Type of surgery | ≥1 year seizure-free at last contact |
|---|---|---|---|---|
| Uni-hemispheric Lesion on MRI and pharmacoresistant (n = 7) | ||||
| #74 | Hypothalamic hamartoma | Hypothalamic hamartoma | Focal resection | No |
| #75 | Hypothalamic hamartoma | Hypothalamic hamartoma | Focal resection | Yes |
| #37 | HA | Cortical dysplasia + HA | Two focal resections + callosotomy | No |
| #19 | MCA infarct | MCA infarct | Multilobar resection | Yes |
| #14 | Cystic encephalomalacia | Non-specific gliois | Focal resection | No |
| #76 | Neoplasm | Grade II astrocytoma | Focal resection | Yes |
| #65 | Cavernous angioma | Cavernous angioma | Two focal resectionsa | Yes |
| Uni-hemispheric lesions and not pharmacoresistant (n = 6) | ||||
| #81 | Neoplasm | Ganglioma | Focal resection | Yes |
| #79 | DNET | DNET | Focal resection (prior to seizure onset) | Yes |
| #80 | DNET | DNET | Focal resection | Yes |
| #77 | Neoplasm | Low grade glioma | Focal resection | Yes |
| #78 | Neoplasm | Anaplastic astrocytoma | Focal resection | Yes |
| #82 | Neoplasm | Oligodendroglioma | Focal resection | Yes |
| Bi-hemispheric lesions on MRI and pharmacoresistant (n = 2) | ||||
| #32 | Bi-parietal atrophy + generalized atrophy | MMCD + MTSb | Two focal resections | Noc |
| #63 | TSC | — | Callosotomy | No |
| Negative MRI and pharmacoresistant (n = 4) | ||||
| Neg1 | Equivocal MCD | Normal | Focal resection | Noc |
| Neg2 | Normal | Polymicrogyria | Focal resection | Yes |
| Neg3 | Normal | No Pathology | Mutiple subpial transection | No |
| Neg4 | Normal | Dysplasia | Focal resection | No |
a Surgery was done immediately for the initial lesion. Some years later, seizures recurred and were refractory to pharmacologic treatment and VNS. A second resective procedure was performed and the patient has been seizure-free since surgery.
b MMCD = minimal or microscopic cortical dysplasia (Palmini et al., 2004). MTS was found only on pathology, not on presurgical MRI.
c Deceased, sudden unexpected death.
Estimates for comprehensive evaluation and surgery for the general population
Our findings provide some preliminary estimates of the use of comprehensive evaluations and surgery at a national level, based on 10 years of follow-up. Canadian and Icelandic studies estimate the annual incidence rate of epilepsy in children, under 15 or 16 years of age, at between 410 (Camfield et al., 1996) and 637/1 000 000 (Olafsson et al., 2005) per year. Using an approximate average of 500/1 000 000 newly diagnosed paediatric cases of epilepsy per year and assuming clinical practice comparable to what we observed in this study, there will be approximately 127/1 000 000 new cases of childhood-onset pharmacoresistant epilepsy per year. Further, about 52/1 000 000 children will undergo comprehensive epilepsy evaluations. Approximately 27/1 000 000 from this age group will have surgical procedures, 21/1 000 000 for treatment of seizures and 6/1 000 000 for lesion resection only.
Discussion
We can provide some preliminary answers to questions raised in the ILAE Sub-Commission report (Cross et al., 2006) based upon our representative study of children with newly diagnosed epilepsy in whom 85% had MRI scans. MRI scans were positive with structural abnormalities possibly related to epilepsy in 15.8% of this cohort and in one of five of those who had non-idiopathic syndromes. A proportion of abnormalities found on MRI scans were probably coincidental and likely not to be related to those patients’ specific types of epilepsy.
While the yield of MRI was high (∼25%) in the <2 year onset group, in fact, the strongest correlate of having a positive scan was the type of epilepsy (non-idiopathic) and an abnormal neurological exam. Among those with non-idiopathic epilepsies, the neurological exam was the single strongest predictor of a positive MRI, followed by pharmacoresistance. However, even if the neurological exam was normal and seizures were not pharmacoresistant, one in 10 had a positive MRI. These included four children with gliomas which, at the very least, warrant periodic monitoring although the standard of practice in the USA is to offer surgery. We note that a recent study of surgery for low-grade gliomas indicated that seizure control was much more likely if surgery occurred within 1 year of seizure onset (Chang et al., 2008). Our finding supports the recommendations that MRI be used in evaluating children with new seizures unless a traditional idiopathic electro-clinical syndrome can be identified with confidence (Hirtz et al., 2000, Gaillard et al., 2009). In addition, we previously showed that many individuals who failed trials of two drugs may still experience subsequent remissions (Berg et al., 2009). The current analysis demonstrates that those with positive MRI scans are very unlikely to be in remission at last contact. This finding further supports the recommendations of the ILAE's Commission on Paediatrics that children who are pharmacoresistant be evaluated at a comprehensive centre, which should include a higher quality MRI. In essence, for any child whose seizures are not fully controlled by medication, a reason should be sought, and an MRI scan is an important tool in those with non-idiopathic epilepsy.
Connecticut is a small state in the north east of the USA. There is one well-established comprehensive epilepsy centre in the state and several others in neighbouring states (New York City, NY and Boston, MA). Other areas of the country and regions of the world may not have similar access to comprehensive epilepsy services. Our study reflects the current use of these resources as practiced in the community when there is good geographic access.
Earlier epidemiological studies of patients with epilepsy were performed before modern imaging was readily available and provided information about presumed structural brain abnormalities based on clinical history and evidence of functional impairment. Even the more recent epidemiological studies did not have widespread use of MRI (Camfield and Camfield, 2003; Jallon et al., 2001; Arts et al., 2004). There is also a large hospital-based series of new-onset seizure patients, mostly (∼80%) adults who were all evaluated with MRI (King et al., 1998). Thirteen percent of the scans were abnormal, and the MRI findings reflected the older age of the group (45% tumours, 16% trauma). There are, however, two paediatric series of patients with temporal lobe epilepsy (TLE) in which all children were evaluated with MRI and drug resistance (failure of two drugs) was assessed. One study found positive MRI scans in 32% of patients with TLE. Pharmacoresistance was present in 70% of the children with positive and 24% of those with negative scans (Dlugos et al., 2001). The other study reported that 48% of children with TLE had a positive scan. All cases with a positive scan were pharmacoresistant compared with 44% with negative scans (Spooner et al., 2006). Certain methodological differences make direct comparison difficult; however, these figures are within a range that correlates with our findings and collectively they emphasize the importance of MRI in evaluating all patients with newly diagnosed childhood-onset epilepsy.
To provide estimates that reflect comprehensive epilepsy care for a population, it is necessary to have a population-based or representative study group and sufficient clinical detail about imaging and current practice. There are several large epidemiological studies, which are purportedly population based or highly representative; however, MRI utilization was sparse, selective or not reported. Our study is reasonably representative of the population and has the highest MRI coverage of any such study reported to date. Our findings also indicate that the lack of MRI neuroimaging in most prior epidemiological probably results in an underestimate of the proportion of cases who have brain lesions, many of whom are probably classified as ‘cryptogenic’. Future epidemiological studies should be designed to incorporate MRI imaging as a routine part of the epilepsy assessment for all study subjects, particularly those with non-idiopathic forms of epilepsy.
There are two large series of paediatric epilepsy surgery cases, one from the USA (Mathern et al., 1999) and an international survey sponsored by the ILAE (Harvey et al., 2008). For the more common types of surgical substrates (lesions associated with cerebral atrophy, MCD and tumours) the proportion of surgical candidates is comparable in these two series and within a range that correlates with our findings. These two large surveys also contained patients with lesions that were so rare that they were not represented in our community-based series (e.g. Rasmussen and Sturge-Weber). To be reasonably sure of ascertaining even one or two such rare events, future epidemiological studies would have to be on the order of at least two to three times as large as ours.
There was also a group of clinical MRI scans for which we relied on the written reports. While imperfect, our comparison between the clinical reports and our own interpretation of the original scans, when we could obtain them, indicated excellent agreement. Such findings enhance our confidence that this study captured the most obvious structural MRI abnormalities present in the clinical MRI scans. Furthermore, inclusion of cases for which we relied on clinical reports was essential in maintaining the representativeness of our cohort. As shown, those with clinical scans only (half of which were not re-read) were more likely to have IQ < 80, abnormal neurological exams, pharmacoresistant epilepsy and ultimately a higher proportion with structural brain abnormalities. Their exclusion would have systematically biased the composition of the cohort.
Both research and clinical MRI scans may have missed subtle abnormalities (Spooner et al., 2006; Lerner et al., 2009). For example, at least two patients with negative MRI scans in this cohort had positive histopathology for MCD (including one MCD, Type I) after resective neurosurgery. Thus, consistent with previous reports, standard structural MRI scans will not detect all pathologies in patients with refractory epilepsy (Salamon et al., 2006). Such findings are consistent with the ILAE's Sub-commission recommendation that most if not all children with refractory epilepsy should be referred to a specialty centre for comprehensive evaluation, as advanced neuroimaging protocols may detect subtle cortical lesions responsible for the intractable seizures (Cross et al., 2006).
Although the Connecticut study has perhaps the highest coverage for MRI reported in a representative, epidemiological cohort to date, 15% of study participants did not have scans. Of the 93 who did not have an MRI scan, eight met criteria for pharmacoresistance. Thus, we have captured the great majority of pharmacoresistant patients (94.3%) who might qualify for comprehensive evaluations and possibly epilepsy surgery. We report the frequency of pharmacoresistance with mean follow-up of 10 years. While this interval should capture the most aggressive epilepsy syndromes, some patients may not yet have progressed to pharmacoresistance.
Our cohort is not, strictly speaking, population based because we recruited children from paediatric neurologists. However, because all participants were evaluated by paediatric neurologists, we are confident about the accuracy of the diagnosis of epilepsy and of the MRI lesions. This is not necessarily the case in studies that recruit from primary care physicians, as many disorders can be mistaken for epilepsy (Benbadis, 2006; Pellock, 2006). The potential error caused by this lack of diagnostic specificity in epidemiological studies has been reported (Gallitto et al., 2005; Christensen et al., 2007). The results of our study are highly comparable to another study from North America, which is considered population based, in terms of age at onset, gender and proportion with specific well-recognized epilepsy syndromes (Camfield et al., 1996; Berg et al., 1999; Camfield and Camfield, 2003), the proportion with mental retardation (Camfield and Camfield, 2007; Berg et al., 2008) and mortality (Camfield et al., 2002; Berg et al., 2004). Furthermore, the ethnic composition of our cohort was highly comparable to the State of Connecticut, based on the 1990 census. These findings strongly suggest that it is likely that our results are representative of the population in which the study was performed. As such they provide a first estimate of the frequency of new cases of pharmacoresistant epilepsy per year arising from the age group studied, the expected types and frequency of MRI positive lesions in that group, and an assessment of the current utilization of epilepsy evaluations and neurosurgery for pharmacoresistant epilepsy of childhood onset from the perspective of practice in the community.
Funding
National Institutes of Health; National Institute of Neurologic Disorders and Stroke (R37 NS31146 to A.T.B., R.A.B., R.K.F., F.D., S.R.L., F.M.T.) and by (R01 NS38992 to G.W.M.).
Acknowledgements
We are very grateful to all the physicians in Connecticut who have made it possible for us to recruit and follow their patients all these years. We also thank Carol and Peter Camfield who provided helpful comments on this manuscript, as well as Eugene Shapiro who provided essential administrative help throughout and Shlomo Shinnar who participated in earlier phases of this study. This study would not have been possible without the generous help of the many families who have participated since its inception.
Glossary
Abbreviations
- HA
hippocampal atrophy
- ILAE
International League Against Epilepsy
- MCD
malformations of cortical development
- MTS
mesial temporal sclerosis
- TLE
temporal lobe epilepsy
- VNS
Vagal nerve stimulators
References
- Arts WFM, Brouwer OF, Peters ACB, Stroink H, Peeters EAJ, Schmitz PIM, et al. Course and prognosis of childhood epilepsy: 5-year follow-up of the Dutch study of epilepsy in childhood. Brain. 2004;127:1774–84. doi: 10.1093/brain/awh200. [DOI] [PubMed] [Google Scholar]
- Benbadis S. Psychogenic nonepileptic seizures. In: Wyllie E, Gupta A, Lachhwani DK, editors. The treatment of epilepsy: principles and practice. Philadelphia: Lipincott Williams & Wilkins; 2006. pp. 623–30. [Google Scholar]
- Berg AT, Langfitt JT, Testa FM, Levy SR, DiMario F, Westerveld M, et al. Global cognitive function in children with epilepsy: a community-based study. Epilepsia. 2008;49:608–14. doi: 10.1111/j.1528-1167.2007.01461.x. [DOI] [PubMed] [Google Scholar]
- Berg AT, Levy SR, Testa FM, D'Souza R. Remission of epilepsy after 2 drug failures in children: a prospective study. Ann Neurol. 2009;65:510–19. doi: 10.1002/ana.21642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AT, Shinnar S, Levy SR, Testa FM. Newly diagnosed epilepsy in children: presentation at diagnosis. Epilepsia. 1999;40:445–52. doi: 10.1111/j.1528-1157.1999.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Berg AT, Shinnar S, Testa FM, Levy SR, Smith SN, Beckerman B. Mortality in childhood-onset epilepsy. Arch Pediatr Adolesc Med. 2004;158:1147–52. doi: 10.1001/archpedi.158.12.1147. [DOI] [PubMed] [Google Scholar]
- Berg AT, Testa FM, Levy SR, Shinnar S. Neuroimaging in children with newly diagnosed epilepsy: a community-based study. Pediatrics. 2000;106:527–32. doi: 10.1542/peds.106.3.527. [DOI] [PubMed] [Google Scholar]
- Berg AT, Vickrey BG, Testa FM, Levy SR, Shinnar S, DiMario F, et al. How long does it take epilepsy to become intractable? A prospective investigation. Ann Neurol. 2006;60:73–9. doi: 10.1002/ana.20852. [DOI] [PubMed] [Google Scholar]
- Camfield C, Camfield P. Preventable and unpreventable causes of childhood-onset epilepsy plus mental retardation. Pediatrics. 2007;120:e52–5. doi: 10.1542/peds.2006-3290. [DOI] [PubMed] [Google Scholar]
- Camfield CS, Camfield PR, Veugelers PJ. Death in children with epilepsy: a population-based study. Lancet. 2002;359:1891–5. doi: 10.1016/S0140-6736(02)08779-2. [DOI] [PubMed] [Google Scholar]
- Camfield CS, Camfield PR, Gordon K, Wirrell E, Dooley JM. Incidence of epilepsy in childhood and adolescence: a population-based study in Nova Scotia from 1977 to 1985. Epilepsia. 1996;37:19–23. doi: 10.1111/j.1528-1157.1996.tb00506.x. [DOI] [PubMed] [Google Scholar]
- Camfield P, Camfield C. Nova Scotia pediatric epilepsy study. In: Jallon P, Berg A, Dulac O, Hauser A, editors. Prognosis of epilepsies. Montrouge France: John Libbey, Eurotext; 2003. pp. 113–26. [Google Scholar]
- Chang EF, Potts MB, Keles E, Lamborn KR, Chang SM, Barbaro NM, et al. Seizure characteristics and control following resection in 332 patients with low-grade gliomas. J Neurosurg. 2008;108:227–35. doi: 10.3171/JNS/2008/108/2/0227. [DOI] [PubMed] [Google Scholar]
- Christensen J, Vestergaard M, Olsen J, Sidenius P. Validation of epilepsy diagnoses in the Danish National Hospital Register. Epilepsy Res. 2007;75:162–70. doi: 10.1016/j.eplepsyres.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989;30:389–99. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- Commission on Epidemiology and Prognosis, International League Against Epilepsy. Guidelines for epidemiologic studies on epilepsy. Epilepsia. 1993;34:592–6. doi: 10.1111/j.1528-1157.1993.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Cross JH, Jaykar P, Nordli D, Delalande O, Duchowny M, Wieser HG, et al. Proposed criteria for referral and evaluation of children for epilepsy surgery: Recommendations of the Subcomission for Pediatric Epilepsy Surgery. Epilepsia. 2006;47:953–9. doi: 10.1111/j.1528-1167.2006.00569.x. [DOI] [PubMed] [Google Scholar]
- Dlugos D, Sammel M, Strom B, Farrar J. Response to first drug trial predicts outcome in childhood temporal lobe epilepsy. Neurology. 2001;57:2259–64. doi: 10.1212/wnl.57.12.2259. [DOI] [PubMed] [Google Scholar]
- French JA. Is the epilepsy responsive or resistant? Only time will tell. Ann Neurol. 2009;65:489–90. doi: 10.1002/ana.21679. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Chiron C, Cross JH, Harvey SA, Kuzniecky R, Hertz-Panier L, Vezina GL. Guidelines for imaging infants and children with recent-onset epilepsy. Epilepsia. 2009 doi: 10.1111/j.1528-1167.2009.02075.x. Advance Access published on April 6, 2009. DOI: 10.1111/j.1528-1167.2009.02075.x. [DOI] [PubMed] [Google Scholar]
- Gallitto G, Serra S, La Spina P, Postorino P, Lagana A, Tripodi F, et al. Prevalence and characteristics of epilepsy in the Aeolian Islands. Epilepsia. 2005;46:1828–35. doi: 10.1111/j.1528-1167.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- Harvey S, Cross JH, Shinnar S, Mathern GW. Defining the spectrum of international practice in pediatric epilepsy surgery patients. Epilepsia. 2008;49:146–55. doi: 10.1111/j.1528-1167.2007.01421.x. [DOI] [PubMed] [Google Scholar]
- Hirtz D, Ashwal S, Berg A, Bettis D, Camfield C, Camfield P, et al. Practice parameter: evaluating a first nonfebrile seizure in children. Report of the quality standards subcommittee of the American academy of neurology, the child neurology society, and the American epilepsy society. Neurology. 2000;55:616–23. doi: 10.1212/wnl.55.5.616. [DOI] [PubMed] [Google Scholar]
- Jallon P, Loiseau P, Loiseau J. Newly diagnosed unprovoked epileptic seizures: presentation at diagnosis in CAROLE study. Epilepsia. 2001;42:464–75. doi: 10.1046/j.1528-1157.2001.31400.x. [DOI] [PubMed] [Google Scholar]
- King MA, Newton MR, Graeme GD, Fitt GJ, Mitchell LA, Silvapulle MJ, et al. Epileptology of the first seizure presentation: a clinical, electroencephalographic, and magnetic resonance imaging study of 300 consecutive cases. Lancet. 1998;352:1007–11. doi: 10.1016/S0140-6736(98)03543-0. [DOI] [PubMed] [Google Scholar]
- Lerner JT, Salamon N, Hauptman JS, Velasco TR, Hemb M, Wu JY, et al. Assessment and surgical outcomes for mild type I and severe type II cortical dysplasia: a critical review and the UCLA experience. Epilepsia. 2009;50:1310–35. doi: 10.1111/j.1528-1167.2008.01998.x. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Giza CC, Yudovin S, Vinters HV, Peacock WJ, Shewmon DA, et al. Postoperative seizure control and antiepileptic drug use in pediatric epilepsy surgery patients: the UCLA experience, 1986–1997. Epilepsia. 1999;40:1740–9. doi: 10.1111/j.1528-1157.1999.tb01592.x. [DOI] [PubMed] [Google Scholar]
- Olafsson E, Ludvigsson P, Gudmundsson G, Hesdorffer D, Kjartansson O, Hauser WA. Incidence of unprovoked seizures and epilepsy in Iceland and assessment of the epilepsy syndrome classification: a prospective study. Lancet Neurol. 2005;4:627–34. doi: 10.1016/S1474-4422(05)70172-1. [DOI] [PubMed] [Google Scholar]
- Panayiotopoulos CP. The epilepsies: seizures, syndromes, and management. Chipping Norton: Bladon Medical Publishing; 2005. [PubMed] [Google Scholar]
- Pellock JM. Other nonepileptic paroxysmal disorders. In: Wyllie E, Gupta A, Lachhwani DK, editors. The treatment of epilepsy: principles and practice. Philadelphia: Lipincott Williams & Wilkins; 2006. pp. 631–42. [Google Scholar]
- Roger J, Bureau M, Dravet C, Genton P, Tassinari CA, Wolf P. Epileptic syndromes in infancy, childhood, and adolescence. Eastleigh: John Libbey; 2002. [Google Scholar]
- Salamon N, Kung J, Shaw SJ, Koo J, Koh S, Wu JY, et al. FDG-PET/MRI coregistration improves detection of cortical dysplasia in patients with epilepsy. Neurology. 2008;71:1594–601. doi: 10.1212/01.wnl.0000334752.41807.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner CG, Berkovic SF, Mitchell LA, Wrennall JA, Harvey AS. New onset temporal lobe epilepsy in children: lesion on MRI predicts poor seizure outcome. Neurology. 2006;67:2147–53. doi: 10.1212/01.wnl.0000248189.93630.4f. [DOI] [PubMed] [Google Scholar]
- Weiner HL, Carlson C, Ridgway EB, Zaroff CM, Miles D, LaJoie J, et al. Epilepsy surgery in young children with tuberous sclerosis: results of a novel approach. Pediatrics. 2006;117:1494–502. doi: 10.1542/peds.2005-1206. [DOI] [PubMed] [Google Scholar]