Abstract
The mechanisms underlying trigeminal pain conditions are incompletely understood. In vitro animal studies have elucidated various targets for pharmacological intervention; however, a lack of clinical models that allow evaluation of viable innervated human tissue has impeded successful translation of many preclinical findings into clinical therapeutics. Therefore, we developed and characterized an in vitro method that evaluates the responsiveness of isolated human nociceptors by measuring basal and stimulated release of neuropeptides from collected dental pulp biopsies.
Informed consent was obtained from patients presenting for extraction of normal wisdom teeth. Patients were anesthetized using nerve block injection, teeth were extracted and bisected, and pulp was removed and superfused in vitro. Basal and capsaicin-evoked peripheral release of immunoreactive calcitonin gene-related peptide (iCGRP) was analyzed by enzyme immunoassay. The presence of nociceptive markers within neurons of the dental pulp was characterized using confocal microscopy.
Capsaicin increased the release of iCGRP from dental pulp biopsies in a concentration-dependent manner. Stimulated release was dependent on extracellular calcium, reversed by a TRPV1 receptor antagonist, and desensitized acutely (tachyphylaxis) and pharmacologically by pretreatment with capsaicin. Superfusion with phorbol 12-myristate 13-acetate (PMA) increased basal and stimulated release, whereas PGE2 augmented only basal release. Compared with vehicle treatment, pretreatment with PGE2 induced competence for DAMGO to inhibit capsaicin-stimulated iCGRP release, similar to observations in animal models where inflammatory mediators induce competence for opioid inhibition.
These results indicate the release of iCGRP from human dental pulp provides a novel tool to determine the effects of pharmacological compounds on human nociceptor sensitivity.
Introduction
Although acute and chronic pain disorders involving the trigeminal system afflict literally millions of patients [53], the precise mechanisms for many of these pain conditions and targets for effective therapeutics remain incompletely understood. Various pain signal transduction cascades have been elucidated in trigeminal neurons using very elegant in vitro animal studies; however, the translation of these findings into diagnostic and pharmacological tools in the clinic has been limited by the paucity of clinical models which allow extraction of viable innervated tissue from humans.
Human dental pulp is composed of multiple cell types, including but not limited to odontoblasts, fibroblasts, inflammatory cells, vascular cells, and sensory and sympathetic neurons. The pulp is innervated densely by unmyelinated C fibers and lightly myelinated Aδ-fibers, both of which contain the putative nociceptive neuropeptides, substance P (SP) and calcitonin gene-related peptide (CGRP) [11,13,14,51,77]. Noxious mechanical and thermal stimulation, as well as chemical activation of sensory neurons, evokes peripheral and central release of SP and CGRP [34,40,48,50,59], while treatment with antagonists to the receptors for SP and CGRP attenuates capsaicin-evoked allodynia [25,74]. These findings suggest that the release of SP and/or CGRP from sensory nerve terminals is a physiological indicator of nociceptive signaling and can be used to determine the effects of inflammatory mediators and pharmacological agents on the basal and stimulated activity of sensory neurons in vitro. A subclass of peptide-containing sensory neurons innervating the dental pulp also express the transient receptor potential channel type 1 (TRPV1), a ligand-gated ion channel activated by capsaicin, the pungent ingredient in chili peppers, which also mediates neuronal activation by other chemical and thermal stimuli [1,83].
In the present study, we hypothesized that the release of neuropeptides from human dental pulp can serve as an in vitro model system to determine the effects of putative algesic and analgesic compounds on the responsiveness of a subset of trigeminal nociceptive neurons. To address this hypothesis, dental pulp collected from extracted third molar teeth of normal healthy patients was superfused to characterize the exocytosis of CGRP in the absence and presence of experimental pharmacological compounds. Confocal microscopy experiments were performed to identify localization of nociceptive modulators on sensory neurons innervating the dental pulp.
Methods
Materials
Capsaicin (Sigma, St. Louis, MO), capsazepine (Tocris, Ellisville, MO), prostaglandin E2 (PGE2; Cayman Chemical, Ann Arbor, MI), and phorbol 12-myristate 13-acetate (PMA; Sigma, St. Louis, MO) were dissolved in 1-methyl-2-pyrrolidinone (MPL) and then diluted in buffer. The final concentration of MPL (<0.1%) was shown not to affect basal or stimulated CGRP release [31]. The mu-opioid agonist, (D-Ala2,N-Me-Phe4,glycinol5)-enkephalin (DAMGO; Bachem, King of Prussia, PA), was dissolved in water and diluted in buffer. All clinical studies were approved by the UTHSCSA Institutional Review Board and patients provided written informed consent to participate in the study.
Recruitment of patients and in vitro superfusion of human dental pulp for this study
Patients presenting to the Oral Surgery Clinics at the University of Texas Health Science Center at San Antonio School of Dentistry were consented to participate in this study. Included in the study were males or non-pregnant premenopausal females between the ages of 18 and 45 who had already elected to have two to four third molars (“wisdom teeth”) extracted. Teeth used in this study had a clinical diagnosis of a normal pulp since they lacked caries or restorations, displayed normal responsiveness to testing and had no periapical radiolucencies upon radiographic evaluation. All teeth used in the study showed fully developed roots to ensure that the innervation of the pulp was complete. All patients were anesthetized using nerve block injection consisting of 2% lidocaine with epinephrine, with 57.9% of patients also receiving intravenous fentanyl and midazolam (50-100μg and 5mg respectively) during extractions. A total of 214 teeth from 62 patients were used to complete the experiments in this study. Following extraction, the teeth were bisected and coronal pulp tissue was removed and placed into wells containing Hanks buffer (138 mM NaCl, 5.3 mM KCl, 0.5 mM MgCl2, 0.4 mM MgSO4, 0.3 mM NaH2PO4, 0.4 mM KH2PO4, 4.0 mM NaHCO3, 1.26 mM CaCl2, 15.5 mM dextrose, 10 mM HEPES, and 0.1% BSA, pH 7.4). To determine capsaicin responsiveness, the pulpal tissue (1 sample per well; 16 ± 2 mg of tissue) was initially incubated with buffer alone for 20 min (basal sample) and then subsequently exposed to selected concentrations of capsaicin with measurement of calcitonin gene related peptide (CGRP) released into the media as the outcome measure. To characterize the capsaicin-stimulated release, pulpal tissue was exposed to buffer alone for 20 min (basal), vehicle, capsazepine (100μM), or calcium-free buffer containing EGTA (10mM) for 20 min and then stimulated with capsaicin (60μM) in the presence or absence of these modulators for 20 min. To determine effects of pretreatment with the phorbol ester, phorbol 12-myristate 13-acetate (PMA), PGE2 or the mu opioid agonist, DAMGO, the pulp tissue was exposed to buffer alone for 20 min (basal), vehicle, PMA (100nM), PGE2 (10μM), DAMGO (1μM) or PGE2 and DAMGO for 20 min, and then stimulated with capsaicin (30μM) in the presence or absence of these modulators for 20 min. Upon completion of the superfusion experiments, tissue samples were lysed to release total cellular stores of iCGRP by freeze/thawing the sample. The release of iCGRP, measured by an enzyme immunoassay (SPI-BIO, Montigny le Bretonneux, France), was expressed as both % of basal release and as % of total content (data not shown).
Immunohistochemistry
Tissue Processing
Extracted third molars (n = 6) were placed in phosphate buffer (PB; 0.1M), split longitudinally, and the pulpal tissues were carefully removed and fixed in 4% paraformaldehyde in PB (pH 7.4) for 30 min. The tissue was rinsed three times in PB for 10 min each and placed in PB with 30% sucrose overnight at 4°C. Samples were placed in cryo-medium, Neg-50 (Richard-Allan Scientific, Kalamazoo, MI), and stored at −80°C until ready for sectioning. Samples were thawed, embedded in Neg-50, serially sectioned in a longitudinal plane at 30 μm with a cryostat, sections placed onto Superfrost glass slides (Fisher Scientific, Pittsburgh, PA), allowed to dry, and stored at −20°C.
Tissue Staining
Tissue sections were removed from the freezer and stained as previously described [2,55]. This includes PB rinses, incubation in blocking solution (PB with 0.3% Triton X-100, 2% bovine γ-globulin, and 4% normal goat serum (Sigma-Aldrich, St. Louis, MO)) for 90 min, and then incubation overnight with primary antibodies diluted in blocking solution. The primary antibodies used included guinea pig anti-human CGRP (Bachem, King of Prussia, PA; 1:100 dilution), rabbit anti-human TRPV1 (Affinity Bioreagents, Golden, CO; 1:100 dilution), rabbit anti-rat MOR (Immunostar, Hudson, WI; 1:100 dilution), and mouse anti-human N52 (Sigma, St. Louis, MO; 1:2000 dilution). The next day the tissue was rinsed in PB followed by incubation in species-specific Alexa Fluor secondary antibodies (Invitrogen; 488, 568, and 633 nm, diluted 1:100 in blocking solution) for 90 min in a humidifier protected from light. Tissues were rinsed in PB, then water, allowed to dry, coverslipped with Vectashield, (Vector Labs, Burlingame, CA) and stored at 4°C. Tissue specimens were evaluated with a Nikon Eclipse 90i microscope with Nikon C1si confocal laser scan head (Nikon Instruments, Melville, NY) and representative images obtained. EZ-C1 v3.20 (Nikon) was used for acquisition of all images. Final image processing for illustration purposes was done with Adobe Photoshop 7.0.
Data analysis
All experiments were conducted with n = 6 or more. Data are presented as release (fmol/pulp; mean ± SEM) and as percentage of basal release (mean ± SEM). Data were analyzed using GraphPad Prism, version 4 (San Diego, CA). Analysis to determine the concentration of capsaicin necessary to elicit a 50% increase in stimulated release was performed by fitting the concentration responses (0-60μM) to a nonlinear curve (sigmoidal) using GraphPad Prism version 4 to identify the EC50. A one-way ANOVA with Tukey's posttest (p < 0.05) was used to determine significant differences in evoked iCGRP release at increasing concentrations of capsaicin. The effects of treatment with capsazepine, PGE2, PMA, and DAMGO and performing experiments in calcium-free buffer on release of iCGRP were analyzed using one-way ANOVA with Tukey's posttest to identify differences between groups. The statistical significance was tested with p < 0.05.
Results
CGRP and TRPV1 are coexpressed on sensory nerve fibers innervating human coronal pulp
Previous studies have established the presence of TRPV1 receptor immunoreactivity in human dental pulp [57,65], however coexpression of the receptor with CGRP has not been examined. Human coronal pulp (area highlighted in red in the inset of Figure 1A) was examined for immunoreactivity to TRPV1, CGRP, and N52 (as a marker for nerve fibers) in the dental pulp [2,51,55]. As shown in Figure 1, a subpopulation of the many N52-identified nerve fibers located within the coronal pulp also expressed TRPV1 and CGRP. These findings provide an anatomical substrate for interpreting the modulation of CGRP release from capsaicin-sensitive fibers as evaluated below.
Figure 1.
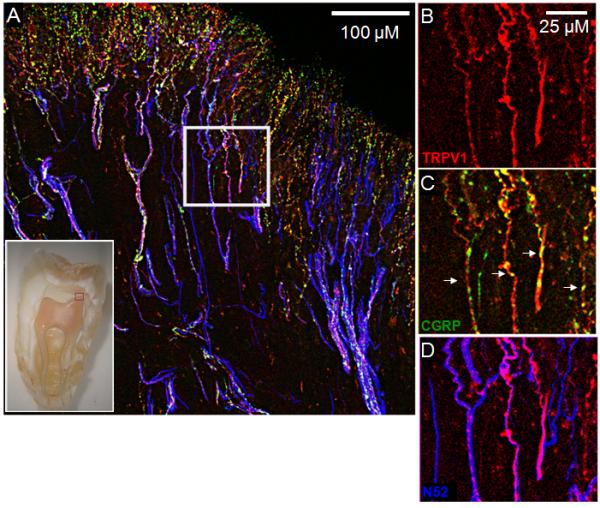
A representative confocal image evaluating the expression patterns of CGRP and TRPV1 in nerve fibers located in the human coronal dental pulp. The inset indicates the area of the pulp represented by the image in panel A. Panels B-D are magnified images from the area enclosed by the white square in panel A. TRPV1 (red) and CGRP (green) immunoreactivities were often coexpressed in the same nerve fibers (Figure 1C). Nerve fibers containing N52 are represented in blue.
Capsaicin elicits release of iCGRP in a concentration-dependent manner
Stimulation of bovine and rat dental pulp with capsaicin elicits the release of iCGRP from this peripheral tissue [37,38]. However, this method has not been applied to peripheral human tissue. Consequently, we examined the release of iCGRP from human pulp biopsies collected from patients. Capsaicin (1–300μM) elicited a concentration-dependent increase in iCGRP release, expressed as fmol of iCGRP from each sample (Figure 2A). Clinical studies are, by their very nature, subject to variance due to differences in gender, age, racial composition, pathology, etc. Therefore, subset analyses were subsequently performed to identify the minimal variance (defined by smallest coefficient of variation) when released levels of iCGRP were either not normalized (eg., fmol/ml; CVEC50: 49.5% and CVpeak: 78.5%), normalized to basal levels of release (% of basal release; CVEC50: 31.6% and CVpeak: 43.2%), or normalized to total tissue content (% of total CGRP content; CVEC50: 75.4% and CVpeak: 50.0%). Although all of the calculations displayed similar results for peak release and EC50 values, the normalization to “% of basal release” yielded the most consistent reduction in CV and accordingly will be used as the outcome measure. As can be seen in Figure 2B, capsaicin produced a concentration-dependent increase in release of iCGRP, with maximal release stimulated by 60μM capsaicin eliciting an almost 4-fold increase in iCGRP release compared to basal release. Because capsaicin induces desensitization of neuronal responses at higher concentrations [21], the concentration-response curve resembles a bell-shaped curve. Based upon these findings, the calculated EC50 for capsaicin-evoked iCGRP release (% of basal) is 19.8 ± 1.9μM.
Figure 2.
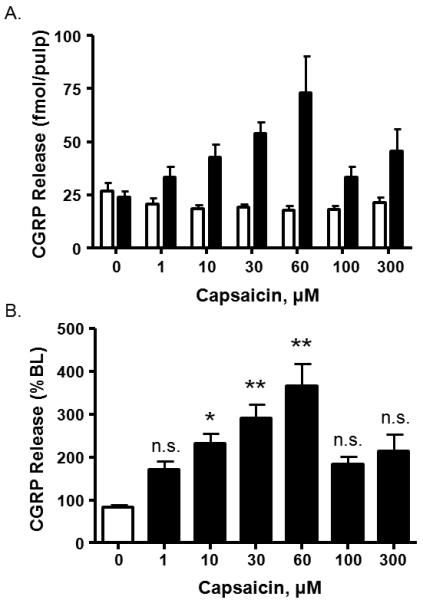
Concentration-response curve of capsaicin-induced iCGRP release from human dental pulp. A. Basal (first column of each pair) and stimulated release of iCGRP in response to capsaicin application (1–300μM, n = 10). Release of iCGRP is expressed as fmol/pulp biopsy. B. Release of iCGRP normalized to the basal release for each biopsy (One-way ANOVA with Tukey's posttest: *p<0.05, **p<0.001). Each datapoint represents the mean ± SEM.
Capsaicin-stimulated release is dependent on extracellular calcium, is reversed by capsazepine, and is desensitized by prior application of capsaicin
To determine whether basal or capsaicin-evoked iCGRP release from dental pulp was calcium-dependent, calcium in the buffer was replaced with EGTA (10mM). The use of calcium-free buffer completely abolished the ability of capsaicin (60μM) to stimulate release of iCGRP (310.1 ± 35.4% vs 82.7 ± 5.7 % of BL in the presence and absence of calcium, respectively, p<0.001; Figure 3A).
Figure 3.
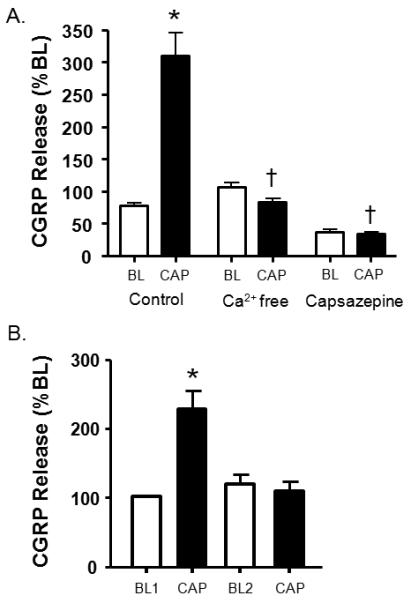
Effect of calcium-free buffer and the capsaicin receptor antagonist capsazepine on basal and capsaicin-evoked iCGRP release and pharmacological desensitization of iCGRP release. A. The first pair of columns represents basal and stimulated release elicited by capsaicin (60μM). The second pair of columns corresponds to basal and capsaicin-stimulated release in calcium-free buffer containing EGTA (10mM). The last pair of columns represents basal and stimulated release in the presence of capsazepine (300μM). An asterisk indicates a statistically significant difference between BL and stimulated release (One-way ANOVA with Tukey's posttest: *p<0.001; n=6) whereas a cross indicates statistically significant differences between control cap-stimulated release and release in the absence of calcium and presence of capsazepine, respectively (One-way ANOVA with Tukey's posttest: †p<0.001; n=6). B. The first two columns represent basal and stimulated release elicited by capsaicin (100μM). The third column represents basal release from the same pulpal biopsies subsequent to capsaicin stimulation and the fourth represents release from a second capsaicin stimulation. An asterisk indicates a statistically significant difference between BL and stimulated release (One-way ANOVA with Tukey's posttest: *p<0.01; n=3). Data are presented as the mean % of basal CGRP release ± SEM.
Capsazepine (300μM), a competitive capsaicin receptor antagonist, did not have an effect on basal rates of spontaneous secretory activity (Figure 3A). However, capsazepine completely inhibited the evoked release of iCGRP compared to capsaicin treatment alone (33.5 ± 3.4% vs. 310.1 ± 35.4% of BL, respectively, p<0.001).
The TRPV1 channel can be desensitized by acute (continued exposure to an agonist) and by pharmacological methods (repeated administration of compounds) [42,49,54,63]. However, comparatively few studies have evaluated this effect using human TRPV1 expressed in native tissue. As seen in Figure 2B, capsaicin-evoked tachyphylaxis was evident at concentrations above 60μM. Similarly, Figure 3B indicates pharmacological desensitization of TRPV1 with repeated administrations of capsaicin (100μM). These studies suggest that human TRPV1 undergoes similar processes of desensitization when expressed at physiologic levels in native tissue.
Capsaicin-stimulated release is not dependent on tooth, clinical status, anesthesia, or patient sex
The effects of several variables on the stimulated release of iCGRP from human dental pulp biopsies were analyzed. As indicated in Table 1, stimulated neuropeptide release from the individual third molars (teeth ID number 1, 16, 17, and 32) was not statistically different. In addition, release from erupted teeth was not different from non-erupted teeth. Release from pulpal biopsies collected from patients undergoing surgery with local nerve block in the absence or presence of intravenous fentanyl/midazolam was similar, suggesting that any opioid effects from fentanyl administration to the patient were washed out by the time the superfusion experiment was performed. Finally, we did not observe a statistically different amount of stimulated release or iCGRP content (361.5 ± 30.9 fmol in males vs. 352.3 ± 23.7 fmol in females) between patient sexes.
Table 1.
Data are presented as the mean % of basal CGRP release ± SEM.
Effects of patient variables on the release of iCGRP from dental pulp (% basal release) stimulated by 30μM capsaicin
| Tooth | |||
| 1 | 16 | 17 | 32 |
| 257.2 ± 21.7 | 256.5 ± 20.2 | 345.4 ± 74.4 | 252.1 ± 26.3 |
| n=21 | n=17 | n=6 | n=10 |
| Clinical status | |||
| Erupted | Non-erupted | ||
| 278.4 ± 27.8 n=37 | 238.5 ± 19.7 n=17 | ||
| Anesthesia | |||
| Nerve block | Nerve block & IV sedation | ||
| 264.3 ± 21.6 n=17 | 266.6 ± 18.5 n=37 | ||
| Sex | |||
| Male | Female | ||
| 288.9 ± 23.2 n=23 | 248.7 ± 17.5 n=31 | ||
The Human Mu Opioid Receptor (MOR) is expressed on peripheral afferent fibers
Although several studies have evaluated MOR expression in human tissues, comparatively little is known about the expression of this opioid receptor on CGRP-containing afferent fibers. As shown in Figure 4, sensory neurons expressing N52 (blue) express both MOR (red) and CGRP (green). These data provide an anatomical substrate for interpreting mu opioid receptor agonist modulation of peptidergic afferent fibers in human tissue.
Figure 4.
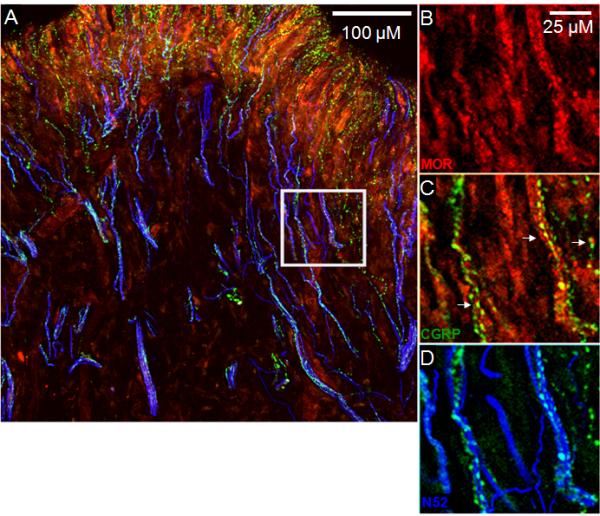
Evaluation of the expression patterns of CGRP and MOR in nerve fibers located in the human coronal dental pulp. Panels B-D are magnified images from the area enclosed by the white square in panel A. MOR (red) and CGRP (green) immunoreactivities were often coexpressed in the same nerve fibers. Nerve fibers containing N52 are represented in blue.
Capsaicin-stimulated release is sensitized by the phorbol ester, phorbol 12-myristate 13-acetate
Numerous reports have demonstrated sensitization of neuropeptide release by the prostanoid, PGE2, and by phorbol esters [3,18,30,35,36,68,72,80,81]. To demonstrate that this model of human nociceptor physiology is sensitive to phorbol esters, we determined the capsaicin-stimulated release of iCGRP in the absence and presence of PMA (100nM) for 20 minutes prior to and throughout capsaicin stimulation. As demonstrated in Figure 5, PMA had a direct excitatory effect on sensory neurons as determined by an increase in the basal iCGRP release. Treatment with PMA also enhanced the stimulated release of iCGRP; capsaicin (30μM) elicited a 2.6-fold increase in iCGRP release over basal levels, whereas PMA augmented this release to 3.7-fold over basal release.
Figure 5.
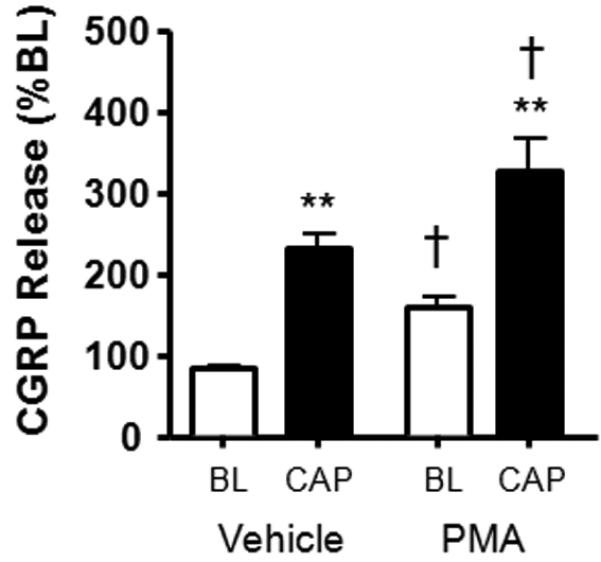
Effects of PMA (100nM) on iCGRP release stimulated by capsaicin (30μM). The white columns represent basal release, whereas the black columns correspond to stimulated release in the absence or presence of PMA. Asterisks indicate a statistically significant difference between BL and stimulated release (One-way ANOVA with Tukey's posttest: **p<0.001; n=12-13) whereas a cross indicates statistically significant differences between basal and cap-stimulated release in the absence and presence of PMA (One-way ANOVA with Tukey's posttest: †p<0.05; n=12-13). Data are presented as the mean % of basal iCGRP release ± SEM.
Capsaicin-stimulated release is not sensitized by PGE2, however, pretreatment with PGE2 enables inhibitory effects of DAMGO on release
As observed with PMA, the application of PGE2 (10μM) also had a small excitatory effect on iCGRP release; the white columns of Figure 6 demonstrate that PGE2 elicited a relative 25% enhancement of basal iCGRP release (96.6 ± 2.9% vs. 125.5 ± 6.1% of BL, respectively, p<0.05). However, in contrast to the sensitization observed with PMA, pretreatment with PGE2 did not augment the capsaicin-stimulated release of iCGRP (Figure 6; black columns).
Figure 6.
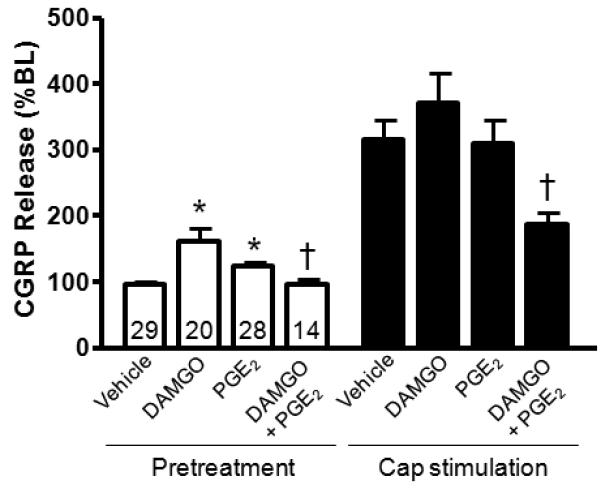
Effects of PGE2 (10μM) and DAMGO (1μM) on basal and capsaicin-stimulated (30μM) iCGRP release. The white columns on the left represent basal release in the absence and presence of PGE2 and DAMGO as indicated, whereas the black columns correspond to stimulated release in the absence or presence of the modulators. An asterisk indicates a statistically significant difference between vehicle and experimental release, whereas a cross indicates a statistically significant difference in the release after DAMGO treatment in the absence and presence of PGE2 (One-way ANOVA with Tukey's posttest: *p<0.05 and †p<0.001; n=14-29 as indicated in the basal release columns). Data are presented as the mean % of basal iCGRP release ± SEM.
Previous reports indicate that agonists at the mu-opioid receptor inhibit the stimulated release of iCGRP from sensory neurons [12,18,71]. To determine the inhibitory effects of a mu-opioid agonist, we measured basal and capsaicin-stimulated release in the absence and presence of the MOR agonist, [D-Ala2,N-Me-Phe4,Gly5-ol]-Enkephalin (DAMGO; 1μM) for 20 minutes prior to and throughout capsaicin stimulation. By itself, DAMGO enhanced the release of iCGRP to 161.7 ± 20.2% of basal release (white columns of Figure 6). The capsaicin-stimulated release was 314.6 ± 30.7% and 371.6 ± 44.3% of basal release in the absence and presence of the mu-opioid agonist, respectively (black columns of Figure 6). Previous studies have demonstrated that the antinociceptive effects of opioids are more pronounced during inflammation, where expression of the mu-opioid receptor and the TRPV1 is enhanced [43,45,64,67]. In addition to increased expression of the opioid receptor, there is acute sensitization of neuropeptide release mediated through an increase in inflammatory mediators. This laboratory and others have demonstrated inhibitory effects of opioid agonists on the capsaicin-stimulated release of iCGRP potentiated by bradykinin or forskolin, but not on unpotentiated release [8,62,63,82], suggesting that acute modulation by inflammatory mediators is necessary to produce inhibitory effects of opioids. Therefore, we next examined whether DAMGO inhibits capsaicin-stimulated release in the presence of PGE2. In these experiments, basal release of iCGRP was collected from pulp biopsies after which the tissue was incubated with buffer containing both PGE2 and DAMGO. Subsequently, the tissues were exposed to capsaicin (30μM) in the presence of PGE2 and DAMGO. Although PGE2 did not sensitize the release of iCGRP, pretreatment with the prostanoid did enable DAMGO to have an inhibitory effect on both baseline and stimulated release of iCGRP. Basal release enhanced by DAMGO alone (161.7 ± 20.2%) was reduced to 96.5 ± 7.1%. In addition, the stimulated release in the presence of DAMGO (371.6 ± 44.3%) was reduced by nearly 50% (to 188.0 ± 17.1%) by PGE2 pretreatment (Figure 6). These findings agree with previous reports which demonstrate inhibitory effects of opioid agonists following activation of PKA or PKC [8,15,62,63,82].
There are mixed reports in the literature regarding sex differences in opioid analgesia. Although some reports claim that females require higher doses of morphine to achieve analgesia comparable to males [4,17,56], other reports claim the reverse is true [20]. We performed a subset analysis of our data to determine whether a sex-difference in peripheral mu-opioid mediated inhibition exists in the dental pulp. We examined the inhibitory effect of DAMGO in the presence of PGE2 by normalizing the stimulated release elicited by DAMGO with PGE2 pretreatment to release in the absence of DAMGO. Interestingly, we observed a greater ability of DAMGO to reduce release in pulpal biopsies contributed by males (54.4%) than those from females (25.1%). The release of CGRP in the presence of PGE2 from female biopsies in the absence and presence of DAMGO was 288.8 ± 44.6 and 216.3 ± 24.4% of baseline, respectively, whereas the release from male biopsies was reduced from 350.0 ± 54.8 to 159.8 ± 20.2 in the absence and presence of DAMGO, respectively (Figure 7).
Figure 7.
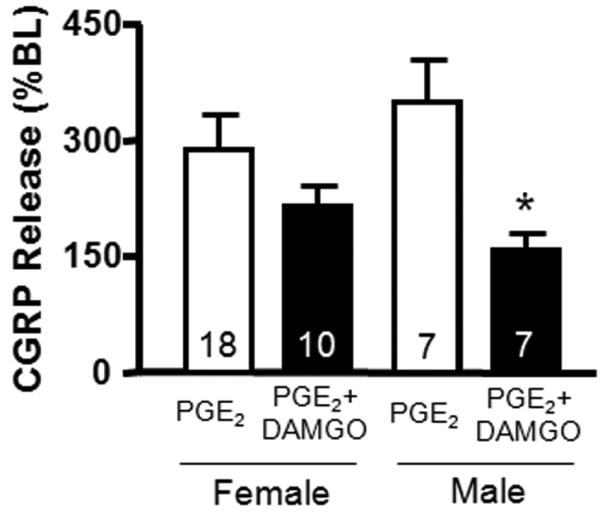
Effects of patient sex on the inhibition of stimulated (30μM capsaicin/10μM PGE2) iCGRP release by DAMGO (1μM). The white columns represent stimulated release in the presence of PGE2, whereas the black columns correspond to stimulated release in the presence of PGE2 and DAMGO. An asterisk indicates a statistically significant difference in the release from male biopsies in the absence and presence of DAMGO (Two-way ANOVA with Bonferroni posttest, *p<0.05; n=7-18 as indicated in the columns). Data are presented as the mean % of basal iCGRP release ± SEM.
Discussion
The present findings provide a model for evaluating mechanisms regulating the release of neuropeptides from isolated peripheral terminals of human sensory neurons innervating dental pulp. Unlike many other surgical procedures in humans, this elective procedure occurs with high frequency suggesting that this method might have wide application in studies evaluating peripheral mechanisms of analgesic drugs or nociceptor activity. In these experiments, we used capsaicin to evoke neuronal activity. Capsaicin has been used extensively in vivo to induce experimental pain in humans by producing neurogenic inflammation and primary and secondary hyperalgesia [5,60,75,76]. The ion channel activated by capsaicin, TRPV1, is a ligand-gated channel activated by noxious heat (≥ 43°C), low pH, and polyunsaturated fatty acids [46] and is essential for the development of inflammatory hyperalgesia [16,26]. The TRPV1 is expressed on both peptidergic and nonpeptidergic sensory neurons innervating human tooth pulp (Figure 1). Capsaicin concentration-dependently elicits the release of iCGRP: in the present study, we observed an EC50 of ∼20μM, which is consistent with other studies requiring micromolar capsaicin concentrations to elicit release of neuropeptides from acutely isolated and perfused tissues [28,34,48,66,78]. Capsaicin-induced activation of the TRPV1 in sensory neurons results in an influx of ions, including in particular, calcium [10]. Increases in intracellular calcium trigger exocytosis of transmitters, including CGRP, into the extracellular space. In this study, we demonstrate that treatment with capsaicin in calcium-free buffer containing EGTA (10mM) prevents the stimulated release of iCGRP from nerve terminals in the dental pulp, suggesting that iCGRP release is mediated through a calcium-dependent exocytotic mechanism. Likewise, pretreatment of dental pulp tissue with capsazepine, a TRPV1 antagonist [9,79], also inhibits the capsaicin-stimulated release of iCGRP, suggesting that the release we observe is mediated through capsaicin activation of TRPV1 channels and subsequent activation of exocytosis. We also demonstrated that TRPV1 desensitization by pretreatment with an equimolar concentration of capsaicin ameliorates the release of iCGRP elicited by capsaicin. This finding is consistent with previous reports by our laboratory and others which demonstrate homologous desensitization of the receptor [27,66]. Calcium-dependent desensitization of capsaicin-induced nociception occurs in vivo [66], thus a strength of this model is that in vivo properties of the TRPV1 can be replicated in this in vitro assay evaluating physiologic expression levels in native tissue.
An important property of the TRPV1 channel is that it can be sensitized, and thus can alter the threshold for activation of sensory neurons in the presence of inflammatory mediators such as bradykinin, prostaglandins, nerve growth factor, serotonin, and ATP [22,39,58,73]. We examined the ability of this assay to detect excitatory and sensitizing effects of proinflammatory mediators using a direct activator of the PKC signaling cascade (PMA) and observed both stimulatory and sensitizing effects of PMA. Receptors for PGE2 are expressed in human dental pulp [19], thus we also examined the effects of PGE2 on iCGRP release. Although we observed a small direct stimulation with PGE2 treatment, we did not detect sensitization of capsaicin-stimulated release by PGE2 as observed from sensory neurons in culture and from spinal cord [39,69,80]. The muopioid receptor (MOR) is also present on nerve fibers in the dental pulp [41], therefore we examined the ability of our assay to detect inhibition of capsaicin-stimulated iCGRP release with the MOR agonist, DAMGO. In contrast to previous reports which demonstrate morphine inhibition of neuropeptide release upon depolarization with high extracellular potassium or electrical stimulation [12,18,71], we did not observe a DAMGO-mediated attenuation of neuropeptide release upon stimulation with capsaicin. Our results parallel in vivo findings that morphine does not cause inhibition of thermal or mechanical hyperalgesia in vivo in the absence of an inflammatory stimulus [45,70]. The lack of observable opioid inhibition may be because the opioid receptors are not competent to signal and attenuate neuropeptide release. Previous studies in this laboratory have demonstrated that mu- and delta-opioid receptor competence, established via pretreatment with bradykinin or an agonist at the PAR-2 receptor, enables inhibitory signaling via an opioid agonist while the agonist in the absence of this priming has no effect on release [8,62,63]. Although we did not demonstrate inhibition of capsaicin-stimulated release with the mu-opioid agonist alone, pretreatment with PGE2 did induce an inhibitory effect of DAMGO, demonstrating that the mu-opioid receptor is present and functional on nerve terminals in the dental pulp. These observations parallel in vivo findings that morphine reverses prostaglandin-induced hyperalgesia via peripheral opioid receptors [32].
Numerous researchers have demonstrated higher potency of mu-opioid agonists in male compared to female rats [7,23,24,44,47,61], however this trend is not as consistent in clinical studies [17,33,56]. To date, most behavioral and psychophysical studies have examined the central effects of opioids on nociception or pain, yet it is known that morphine has an antinociceptive role in the periphery [32,45,52,70]. There are few studies which have examined sex-dependent differences in peripheral mu-opioid inhibition and these studies have generated mixed results. Barrett and colleagues found that a mu-opioid agonist had greater analgesic effects on tonic pain in female rats [6], whereas Ji and colleagues found that a peripherally restricted mu-opioid agonist had greater potency in male rats in a model of visceral pain [44]. We examined only one concentration of DAMGO, however the concentration that was used was near the Emax of the agonist (1μM) [84]. The data generated using this novel in vitro assay demonstrates greater DAMGO inhibition in biopsies from males compared to females and suggests that sex differences in analgesia may originate in the periphery.
In the present study, we have described a novel in vitro method for determining excitatory and inhibitory effects of pharmacological compounds on the sensitivity of isolated peripheral human trigeminal nociceptors. Despite patient heterogeneity, the variability in the capsaicin-stimulated release of iCGRP from the dental pulp was relatively low. The discovery and development of clinical therapeutics and diagnostics has diminished since basic science methods shifted away from whole animal physiology towards molecular and genetic based approaches [29]. The utilization of cell expression systems and genetic manipulation has driven the understanding of microcellular components and enabled the development of a wide array of pharmacological tools, however these tools do not necessarily lead to successful therapeutics [29], in part because the cellular conditions of expression systems may not adequately model native tissue. Integration of the knowledge obtained using molecular biology into more physiologically relevant mechanistic research in intact human tissues has been restricted because of availability of viable innervated human tissue. In this model, we are able to examine sensory nerve function in human tissue from patients who are healthy and have minimal to no concurrent medications, an important consideration for the design of clinical research into pain mechanisms in humans. In addition, our model also allows for collection of multiple samples of clinically healthy tissue from the same patient, allowing for paired studies examining the effects of pharmacological tools on samples from genetically identical tissues. In summary, we believe that this will serve as a valuable tool to study the effects of candidate drugs or drug combinations without risk of in vivo treatment of human patients.
Acknowledgments
National Institutes of Health: National Institute of Dental and Craniofacial Research (DE-015576 to MH and DE14318-06 to JF), National Institute of Neurological Disorders and Stroke (NS-58655 to KH), CTSA (U54RR02438 to KH) National Institute for Drug Abuse (DA-16719 to KH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to report.
References
- 1.Alexander SP, Mathie A, Peters JA. Guide to receptors and channels. Br J Pharmacol. (2nd edition) 2006;147(Suppl 3):S1–168. doi: 10.1038/sj.bjp.0706651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarado LT, Perry GM, Hargreaves KM, Henry MA. TRPM8 axonal expression is decreased in painful human teeth with irreversible pulpitis and cold hyperalgesia. J Endod. 2007;33:1167–1171. doi: 10.1016/j.joen.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreeva L, Rang HP. Effect of bradykinin and prostaglandins on the release of calcitonin gene-related peptide-like immunoreactivity from the rat spinal cord in vitro. Br J Pharmacol. 1993;108:185–190. doi: 10.1111/j.1476-5381.1993.tb13460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aubrun F, Salvi N, Coriat P, Riou B. Sex- and age-related differences in morphine requirements for postoperative pain relief. Anesthesiology. 2005;103:156–160. doi: 10.1097/00000542-200507000-00023. [DOI] [PubMed] [Google Scholar]
- 5.Baad-Hansen L, Jensen TS, Svensson P. A human model of intraoral pain and heat hyperalgesia. J Orofac Pain. 2003;17:333–340. [PubMed] [Google Scholar]
- 6.Barrett AC, Smith ES, Picker MJ. Capsaicin-induced hyperalgesia and mu-opioid-induced antihyperalgesia in male and female Fischer 344 rats. J Pharmacol Exp Ther. 2003;307:237–245. doi: 10.1124/jpet.103.054478. [DOI] [PubMed] [Google Scholar]
- 7.Bartok RE, Craft RM. Sex differences in opioid antinociception. J Pharmacol Exp Ther. 1997;282:769–778. [PubMed] [Google Scholar]
- 8.Berg KA, Patwardhan AM, Sanchez TA, Silva YM, Hargreaves KM, Clarke WP. Rapid modulation of micro-opioid receptor signaling in primary sensory neurons. J Pharmacol Exp Ther. 2007;321:839–847. doi: 10.1124/jpet.106.116681. [DOI] [PubMed] [Google Scholar]
- 9.Bevan S, Hothi S, Hughes G, James IF, Rang HP, Shah K, Walpole CS, Yeats JC. Capsazepine: A competitive antagonist of the sensory neurone excitant capsaicin. Br J Pharmacol. 1992;107:544–552. doi: 10.1111/j.1476-5381.1992.tb12781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bevan S, Szolcsanyi J. Sensory neuron-specific actions of capsaicin: Mechanisms and applications. Trends Pharmacol Sci. 1990;11:330–333. doi: 10.1016/0165-6147(90)90237-3. [DOI] [PubMed] [Google Scholar]
- 11.Bhatnagar M, Cintra A, Tinner B, Agnati LF, Kerezoudis N, Edwall L, Fuxe K. Neurotensin-like immunoreactivity in odontoblasts and their processes in rat maxillary molar teeth and the effect of pulpotomy. Regul Pept. 1995;58:141–147. doi: 10.1016/0167-0115(95)00062-g. [DOI] [PubMed] [Google Scholar]
- 12.Brodin E, Gazelius B, Panopoulos P, Olgart L. Morphine inhibits substance P release from peripheral sensory nerve endings. Acta Physiol Scand. 1983;117:567–570. doi: 10.1111/j.1748-1716.1983.tb07228.x. [DOI] [PubMed] [Google Scholar]
- 13.Byers MR. Dynamic plasticity of dental sensory nerve structure and cytochemistry. Arch Oral Biol. 1994;39(Suppl):13S–21S. doi: 10.1016/0003-9969(94)90183-x. [DOI] [PubMed] [Google Scholar]
- 14.Byers MR, Taylor PE. Effect of sensory denervation on the response of rat molar pulp to exposure injury. J Dent Res. 1993;72:613–618. doi: 10.1177/00220345930720031001. [DOI] [PubMed] [Google Scholar]
- 15.Carruthers AM, Sellers LA, Jenkins DW, Jarvie EM, Feniuk W, Humphrey PP. Adenosine A(1) receptor-mediated inhibition of protein kinase a-induced calcitonin gene-related peptide release from rat trigeminal neurons. Mol Pharmacol. 2001;59:1533–1541. doi: 10.1124/mol.59.6.1533. [DOI] [PubMed] [Google Scholar]
- 16.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 17.Cepeda MS, Carr DB. Women experience more pain and require more morphine than men to achieve a similar degree of analgesia. Anesth Analg. 2003;97:1464–1468. doi: 10.1213/01.ANE.0000080153.36643.83. [DOI] [PubMed] [Google Scholar]
- 18.Chang HM, Berde CB, Holz GGt, Steward GF, Kream RM. Sufentanil, morphine, met-enkephalin, and kappa-agonist (U-50,488h) inhibit substance p release from primary sensory neurons: A model for presynaptic spinal opioid actions. Anesthesiology. 1989;70:672–677. doi: 10.1097/00000542-198904000-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang MC, Chen YJ, Tai TF, Tai MR, Li MY, Tsai YL, Lan WH, Wang YL, Jeng JH. Cytokine-induced prostaglandin E2 production and cyclooxygenase-2 expression in dental pulp cells: Downstream calcium signalling via activation of prostaglandin ep receptor. Int Endod J. 2006;39:819–826. doi: 10.1111/j.1365-2591.2006.01156.x. [DOI] [PubMed] [Google Scholar]
- 20.Chia YY, Chow LH, Hung CC, Liu K, Ger LP, Wang PN. Gender and pain upon movement are associated with the requirements for postoperative patient-controlled iv analgesia: A prospective survey of 2,298 chinese patients. Can J Anaesth. 2002;49:249–255. doi: 10.1007/BF03020523. [DOI] [PubMed] [Google Scholar]
- 21.Cholewinski A, Burgess GM, Bevan S. The role of calcium in capsaicin-induced desensitization in rat cultured dorsal root ganglion neurons. Neuroscience. 1993;55:1015–1023. doi: 10.1016/0306-4522(93)90315-7. [DOI] [PubMed] [Google Scholar]
- 22.Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from Ptdins(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 23.Cicero TJ, Nock B, Meyer ER. Gender-related differences in the antinociceptive properties of morphine. J Pharmacol Exp Ther. 1996;279:767–773. [PubMed] [Google Scholar]
- 24.Cook CD, Nickerson MD. Nociceptive sensitivity and opioid antinociception and antihyperalgesia in freund's adjuvant-induced arthritic male and female rats. J Pharmacol Exp Ther. 2005;313:449–459. doi: 10.1124/jpet.104.077792. [DOI] [PubMed] [Google Scholar]
- 25.Davis AJ, Perkins MN. Substance P and capsaicin-induced mechanical hyperalgesia in the rat knee joint; the involvement of bradykinin B1 and B2 receptors. Br J Pharmacol. 1996;118:2206–2212. doi: 10.1111/j.1476-5381.1996.tb15664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 27.Docherty RJ, Yeats JC, Bevan S, Boddeke HW. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflugers Arch. 1996;431:828–837. doi: 10.1007/s004240050074. [DOI] [PubMed] [Google Scholar]
- 28.Donnerer J, Schuligoi R, Amann R. Time-course of capsaicin-evoked release of calcitonin gene-related peptide from rat spinal cord in vitro. Effect of concentration and modulation by ruthenium red. Regul Pept. 1992;37:27–37. doi: 10.1016/0167-0115(92)90061-x. [DOI] [PubMed] [Google Scholar]
- 29.Duyk G. Attrition and translation. Science. 2003;302:603–605. doi: 10.1126/science.1090521. [DOI] [PubMed] [Google Scholar]
- 30.Escott KJ, Beattie DT, Connor HE, Brain SD. The modulation of the increase in rat facial skin blood flow observed after trigeminal ganglion stimulation. Eur J Pharmacol. 1995;284:69–76. doi: 10.1016/0014-2999(95)00367-t. [DOI] [PubMed] [Google Scholar]
- 31.Fehrenbacher JC, Taylor CP, Vasko MR. Pregabalin and gabapentin reduce release of substance P and CGRP from rat spinal tissues only after inflammation or activation of protein kinase C. Pain. 2003;105:133–141. doi: 10.1016/s0304-3959(03)00173-8. [DOI] [PubMed] [Google Scholar]
- 32.Ferreira SH, Nakamura M. Ii - prostaglandin hyperalgesia: The peripheral analgesic activity of morphine, enkephalins and opioid antagonists. Prostaglandins. 1979;18:191–200. doi: 10.1016/0090-6980(79)90104-7. [DOI] [PubMed] [Google Scholar]
- 33.Fillingim RB, Gear RW. Sex differences in opioid analgesia: Clinical and experimental findings. Eur J Pain. 2004;8:413–425. doi: 10.1016/j.ejpain.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Flores CM, Leong AS, Dussor GO, Harding-Rose C, Hargreaves KM, Kilo S. Capsaicin-evoked cgrp release from rat buccal mucosa: Development of a model system for studying trigeminal mechanisms of neurogenic inflammation. Eur J Neurosci. 2001;14:1113–1120. doi: 10.1046/j.0953-816x.2001.01736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franco-Cereceda A. Prostaglandins and CGRP release from cardiac sensory nerves. Naunyn Schmiedebergs Arch Pharmacol. 1989;340:180–184. doi: 10.1007/BF00168966. [DOI] [PubMed] [Google Scholar]
- 36.Geppetti P, Del Bianco E, Tramontana M, Vigano T, Folco GC, Maggi CA, Manzini S, Fanciullacci M. Arachidonic acid and bradykinin share a common pathway to release neuropeptide from capsaicin-sensitive sensory nerve fibers of the guinea pig heart. J Pharmacol Exp Ther. 1991;259:759–765. [PubMed] [Google Scholar]
- 37.Goodis HE, Poon A, Hargreaves KM. Tissue pH and temperature regulate pulpal nociceptors. J Dent Res. 2006;85:1046–1049. doi: 10.1177/154405910608501114. [DOI] [PubMed] [Google Scholar]
- 38.Hargreaves KM, Jackson DL, Bowles WR. Adrenergic regulation of capsaicin-sensitive neurons in dental pulp. J Endod. 2003;29:397–399. doi: 10.1097/00004770-200306000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Hingtgen CM, Vasko MR. Prostacyclin enhances the evoked-release of substance P and calcitonin gene-related peptide from rat sensory neurons. Brain Res. 1994;655:51–60. doi: 10.1016/0006-8993(94)91596-2. [DOI] [PubMed] [Google Scholar]
- 40.Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: Involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24:739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- 41.Jaber L, Swaim WD, Dionne RA. Immunohistochemical localization of mu-opioid receptors in human dental pulp. J Endod. 2003;29:108–110. doi: 10.1097/00004770-200302000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Jeske NA, Patwardhan AM, Gamper N, Price TJ, Akopian AN, Hargreaves KM. Cannabinoid WIN 55,212-2 regulates trpv1 phosphorylation in sensory neurons. J Biol Chem. 2006;281:32879–32890. doi: 10.1074/jbc.M603220200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. P38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 44.Ji Y, Murphy AZ, Traub RJ. Sex differences in morphine-induced analgesia of visceral pain are supraspinally and peripherally mediated. Am J Physiol Regul Integr Comp Physiol. 2006;291:R307–314. doi: 10.1152/ajpregu.00824.2005. [DOI] [PubMed] [Google Scholar]
- 45.Joris JL, Dubner R, Hargreaves KM. Opioid analgesia at peripheral sites: A target for opioids released during stress and inflammation? Anesth Analg. 1987;66:1277–1281. [PubMed] [Google Scholar]
- 46.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 47.Kest B, Sarton E, Dahan A. Gender differences in opioid-mediated analgesia: Animal and human studies. Anesthesiology. 2000;93:539–547. doi: 10.1097/00000542-200008000-00034. [DOI] [PubMed] [Google Scholar]
- 48.Kilo S, Harding-Rose C, Hargreaves KM, Flores CM. Peripheral CGRP release as a marker for neurogenic inflammation: A model system for the study of neuropeptide secretion in rat paw skin. Pain. 1997;73:201–207. doi: 10.1016/S0304-3959(97)00108-5. [DOI] [PubMed] [Google Scholar]
- 49.Koplas PA, Rosenberg RL, Oxford GS. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J Neurosci. 1997;17:3525–3537. doi: 10.1523/JNEUROSCI.17-10-03525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuraishi Y, Hirota N, Sato Y, Hino Y, Satoh M, Takagi H. Evidence that substance P and somatostatin transmit separate information related to pain in the spinal dorsal horn. Brain Res. 1985;325:294–298. doi: 10.1016/0006-8993(85)90326-9. [DOI] [PubMed] [Google Scholar]
- 51.Lawson SN, McCarthy PW, Prabhakar E. Electrophysiological properties of neurones with CGRP-like immunoreactivity in rat dorsal root ganglia. J Comp Neurol. 1996;365:355–366. doi: 10.1002/(SICI)1096-9861(19960212)365:3<355::AID-CNE2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 52.Levine JD, Taiwo YO. Involvement of the mu-opiate receptor in peripheral analgesia. Neuroscience. 1989;32:571–575. doi: 10.1016/0306-4522(89)90279-0. [DOI] [PubMed] [Google Scholar]
- 53.Lipton JA, Ship JA, Larach-Robinson D. Estimated prevalence and distribution of reported orofacial pain in the United States. J Am Dent Assoc. 1993;124:115–121. doi: 10.14219/jada.archive.1993.0200. [DOI] [PubMed] [Google Scholar]
- 54.Liu L, Simon SA. Capsaicin-induced currents with distinct desensitization and Ca2+ dependence in rat trigeminal ganglion cells. J Neurophysiol. 1996;75:1503–1514. doi: 10.1152/jn.1996.75.4.1503. [DOI] [PubMed] [Google Scholar]
- 55.Luo S, Perry GM, Levinson SR, Henry MA. NaV1.7 expression is increased in painful human dental pulp. Mol Pain. 2008;4:16. doi: 10.1186/1744-8069-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller PL, Ernst AA. Sex differences in analgesia: A randomized trial of mu versus kappa opioid agonists. South Med J. 2004;97:35–41. doi: 10.1097/01.smj.0000085743.68121.a9. [DOI] [PubMed] [Google Scholar]
- 57.Morgan CR, Rodd HD, Clayton N, Davis JB, Boissonade FM. Vanilloid receptor 1 expression in human tooth pulp in relation to caries and pain. J Orofac Pain. 2005;19:248–260. [PubMed] [Google Scholar]
- 58.Moriyama T, Iida T, Kobayashi K, Higashi T, Fukuoka T, Tsumura H, Leon C, Suzuki N, Inoue K, Gachet C, Noguchi K, Tominaga M. Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. J Neurosci. 2003;23:6058–6062. doi: 10.1523/JNEUROSCI.23-14-06058.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morton CR, Hutchison WD. Release of sensory neuropeptides in the spinal cord: Studies with calcitonin gene-related peptide and galanin. Neuroscience. 1989;31:807–815. doi: 10.1016/0306-4522(89)90443-0. [DOI] [PubMed] [Google Scholar]
- 60.Ngom PI, Dubray C, Woda A, Dallel R. A human oral capsaicin pain model to assess topical anesthetic-analgesic drugs. Neurosci Lett. 2001;316:149–152. doi: 10.1016/s0304-3940(01)02401-6. [DOI] [PubMed] [Google Scholar]
- 61.Okamoto K, Tashiro A, Hirata H, Bereiter DA. Differential modulation of TMJ neurons in superficial laminae of trigeminal subnucleus caudalis/upper cervical cord junction region of male and cycling female rats by morphine. Pain. 2005;114:203–211. doi: 10.1016/j.pain.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 62.Patwardhan AM, Berg KA, Akopain AN, Jeske NA, Gamper N, Clarke WP, Hargreaves KM. Bradykinin-induced functional competence and trafficking of the delta-opioid receptor in trigeminal nociceptors. J Neurosci. 2005;25:8825–8832. doi: 10.1523/JNEUROSCI.0160-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patwardhan AM, Diogenes A, Berg KA, Fehrenbacher JC, Clarke WP, Akopian AN, Hargreaves KM. PAR-2 agonists activate trigeminal nociceptors and induce functional competence in the delta opioid receptor. Pain. 2006;125:114–124. doi: 10.1016/j.pain.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 64.Pol O, Puig MM. Expression of opioid receptors during peripheral inflammation. Curr Top Med Chem. 2004;4:51–61. doi: 10.2174/1568026043451519. [DOI] [PubMed] [Google Scholar]
- 65.Renton T, Yiangou Y, Baecker PA, Ford AP, Anand P. Capsaicin receptor VR1 and ATP purinoceptor P2X3 in painful and nonpainful human tooth pulp. J Orofac Pain. 2003;17:245–250. [PubMed] [Google Scholar]
- 66.Ruparel NB, Patwardhan AM, Akopian AN, Hargreaves KM. Homologous and heterologous desensitization of capsaicin and mustard oil responses utilize different cellular pathways in nociceptors. Pain. 2008;135:271–279. doi: 10.1016/j.pain.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schafer M, Imai Y, Uhl GR, Stein C. Inflammation enhances peripheral mu-opioid receptor-mediated analgesia, but not mu-opioid receptor transcription in dorsal root ganglia. Eur J Pharmacol. 1995;279:165–169. doi: 10.1016/0014-2999(95)00150-j. [DOI] [PubMed] [Google Scholar]
- 68.Southall MD, Michael RL, Vasko MR. Intrathecal NSAIDs attenuate inflammation-induced neuropeptide release from rat spinal cord slices. Pain. 1998;78:39–48. doi: 10.1016/S0304-3959(98)00113-4. [DOI] [PubMed] [Google Scholar]
- 69.Southall MD, Vasko MR. Prostaglandin E(2)-mediated sensitization of rat sensory neurons is not altered by nerve growth factor. Neurosci Lett. 2000;287:33–36. doi: 10.1016/s0304-3940(00)01158-7. [DOI] [PubMed] [Google Scholar]
- 70.Stein C, Schafer M, Machelska H. Attacking pain at its source: New perspectives on opioids. Nat Med. 2003;9:1003–1008. doi: 10.1038/nm908. [DOI] [PubMed] [Google Scholar]
- 71.Suarez-Roca H, Abdullah L, Zuniga J, Madison S, Maixner W. Multiphasic effect of morphine on the release of substance P from rat trigeminal nucleus slices. Brain Res. 1992;579:187–194. doi: 10.1016/0006-8993(92)90050-j. [DOI] [PubMed] [Google Scholar]
- 72.Suarez-Roca H, Maixner W. Morphine produces a biphasic modulation of substance P release from cultured dorsal root ganglion neurons. Neurosci Lett. 1995;194:41–44. doi: 10.1016/0304-3940(95)11721-8. [DOI] [PubMed] [Google Scholar]
- 73.Sugiuar T, Bielefeldt K, Gebhart GF. TRPV1 function in mouse colon sensory neurons is enhanced by metabotropic 5-hydroxytryptamine receptor activation. J Neurosci. 2004;24:9521–9530. doi: 10.1523/JNEUROSCI.2639-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun RQ, Lawand NB, Willis WD. The role of calcitonin gene-related peptide (CGRP) in the generation and maintenance of mechanical allodynia and hyperalgesia in rats after intradermal injection of capsaicin. Pain. 2003;104:201–208. doi: 10.1016/s0304-3959(03)00008-3. [DOI] [PubMed] [Google Scholar]
- 75.Svensson P, Arendt-Nielsen L, Bjerring P, Bak P, Hjorth T, Troest T. Human mastication modulated by experimental trigeminal and extra-trigeminal painful stimuli. J Oral Rehabil. 1996;23:838–848. doi: 10.1046/j.1365-2842.1996.00441.x. [DOI] [PubMed] [Google Scholar]
- 76.Szolcsanyi J. Forty years in capsaicin research for sensory pharmacology and physiology. Neuropeptides. 2004;38:377–384. doi: 10.1016/j.npep.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 77.Taylor PE, Byers MR, Redd PE. Sprouting of CGRP nerve fibers in response to dentin injury in rat molars. Brain Res. 1988;461:371–376. doi: 10.1016/0006-8993(88)90270-3. [DOI] [PubMed] [Google Scholar]
- 78.Ulrich-Lai YM, Flores CM, Harding-Rose CA, Goodis HE, Hargreaves KM. Capsaicin-evoked release of immunoreactive calcitonin gene-related peptide from rat trigeminal ganglion: Evidence for intraganglionic neurotransmission. Pain. 2001;91:219–226. doi: 10.1016/S0304-3959(00)00439-5. [DOI] [PubMed] [Google Scholar]
- 79.Urban L, Dray A. Capsazepine, a novel capsaicin antagonist, selectively antagonises the effects of capsaicin in the mouse spinal cord in vitro. Neurosci Lett. 1991;134:9–11. doi: 10.1016/0304-3940(91)90496-g. [DOI] [PubMed] [Google Scholar]
- 80.Vasko MR. Prostaglandin-induced neuropeptide release from spinal cord. Prog Brain Res. 1995;104:367–380. doi: 10.1016/s0079-6123(08)61801-4. [DOI] [PubMed] [Google Scholar]
- 81.Vasko MR, Zirkelbach SL, Waite KJ. Prostaglandins stimulate the release of substance P from rat spinal cord slices. Prog Pharmacol Clin Pharmacol. 1993;10:69–89. [Google Scholar]
- 82.Vetter I, Wyse BD, Monteith GR, Roberts-Thomson SJ, Cabot PJ. The mu opioid agonist morphine modulates potentiation of capsaicin-evoked TRPV1 responses through a cyclic AMP-dependent protein kinase A pathway. Mol Pain. 2006;2:22. doi: 10.1186/1744-8069-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430:748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- 84.Zollner C, Shaqura MA, Bopaiah CP, Mousa S, Stein C, Schafer M. Painful inflammation-induced increase in mu-opioid receptor binding and G-protein coupling in primary afferent neurons. Mol Pharmacol. 2003;64:202–210. doi: 10.1124/mol.64.2.202. [DOI] [PubMed] [Google Scholar]


