Abstract
What neural changes underlie individual differences in goal-directed learning? The lateral amygdala (LA) is important for assigning emotional and motivational significance to discrete environmental cues1–4, including those that signal rewarding events5–8. Recognizing that a cue predicts a reward enhances an animal’s ability to acquire that reward; however, the cellular and synaptic mechanisms that underlie cue–reward learning are unclear. Here we show that marked changes in both cue-induced neuronal firing and input-specific synaptic strength occur with the successful acquisition of a cue–reward association within a single training session. We performed both in vivo and ex vivo electrophysiological recordings in the LA of rats trained to self-administer sucrose. We observed that reward-learning success increased in proportion to the number of amygdala neurons that responded phasically to a reward-predictive cue. Furthermore, cue–reward learning induced an AMPA (α-amino-3-hydroxy-5-methylisoxazole propionic acid)-receptor-mediated increase in the strength of thalamic, but not cortical, synapses in the LA that was apparent immediately after the first training session. The level of learning attained by individual subjects was highly correlated with the degree of synaptic strength enhancement. Importantly, intra-LA NMDA (N-methyl-d-aspartate)-receptor blockade impaired reward-learning performance and attenuated the associated increase in synaptic strength. These findings provide evidence of a connection between LA synaptic plasticity and cue–reward learning, potentially representing a key mechanism underlying goal-directed behaviour.
Basolateral amygdala (BLA) neurons are phasically responsive to reward-predictive cues8–11, which is consistent with the idea that cue-evoked neuronal firing emerges as a consequence of cue–reward associations. The BLA is composed of multiple nuclei, including the LA, the first site of convergence for sensory inputs carrying information about conditioned and unconditioned stimuli to the amygdala1,4,12–15. Thus, the LA is a likely initial site for the formation of cue–reward associations that endow the cue with motivational significance that impacts on reward-seeking behaviour.
To test the hypothesis that successful acquisition of cue-directed reward-seeking behaviour is dependent on neuronal plasticity in the LA, we examined LA neuronal firing in response to a reward-predictive cue during training on a sucrose self-administration task (Supplementary Fig. 1). To control for neural activity associated with the motor output of operant responding, and to ensure that the sensory cue predicted reward delivery and not the operant response alone, beam breaks at a nose-poke response port (‘nose-poke operandum’) were reinforced with a cue and sucrose reward after about 50% of nose-pokes (Fig. 1a, b). In rats that successfully acquired this task (see Methods), about half of recorded neurons (49%; 60 of 122 neurons from seven rats during the first session in which each rat met the acquisition criterion) that did not respond to the cue before acquisition developed a robust phasic response to cue onset with acquisition (Fig. 1c, d, and Supplementary Fig. 2). Cue encoding increased across sessions: the cue-evoked population response of all neurons recorded in the third session was enhanced relative to the first session (session × time interaction, F9,1944 = 4.15, P < 0.0001), specifically within the 50 ms after cue onset (P < 0.003; Fig. 1e, Supplementary Fig. 3 and Supplementary Table 1). These changes over sessions were predictive of behaviour: increasing proportions of neurons were recruited to encode the reward-predictive cue as individual rats improved reward-learning performance (Fig. 1f, g). Task efficiency, a behavioural index defined as the number of rewards earned divided by the number of cues presented, and task accuracy, a behavioural index defined as the difference in the number of correct and incorrect port entries divided by the total number of port entries, were significantly correlated (P < 0.0001 and P = 0.0066, respectively; Supplementary Table 2) with the percentage of neurons per rat that showed phasic responses to the reward-predictive cue (Fig. 1f, g). Control studies confirmed that the increase in cue encoding is specific to acquisition of the cue–reward association and is not due to non-associative factors, such as sensitization (Supplementary Figs 4 and 5). These data demonstrate that development of cue-evoked responses in the LA depends on the acquired reward-predictive nature of the cue. Further, the greater the proportion of neurons recruited to encode the reward-predictive cue, the better the rat learned the cue–reward association, and the more successful the rat was at earning rewards.
Figure 1. Reward-related learning success is correlated with rapid increases in cue-related firing.
a, Diagram of operant chamber from above. b, Schematic of behavioural paradigm. c, Temporal dynamics of neuronal population response to the reward-predictive cue. Spike activity of all simultaneously recorded LA units (n = 13) from a rat that successfully acquired the task during the first session; 100-ms bins. This population of neurons develops a response to the onset of the reward-predictive cue with task acquisition (acq.). d, Peri-event histogram of a single LA neuron from a different rat that successfully acquired the task in the first session; 29 trials in each epoch. e, Population histograms (50-ms bins, error bars indicate s.e.m.) of the mean Z-score for all neurons recorded during session 1 (grey; n = 95 neurons) and session 3 (red; n = 123 neurons) for all rats (n = 7). Two asterisks, P < 0.004. f, g, Correlation between proportion of cue-responsive neurons and (f) task efficiency and (g) task accuracy across three sessions. Colours indicate the same rat on different sessions. Only rats with at least six neurons per session were included in scatter plots (n = 6). For all peri-event histograms, time zero indicates cue onset.
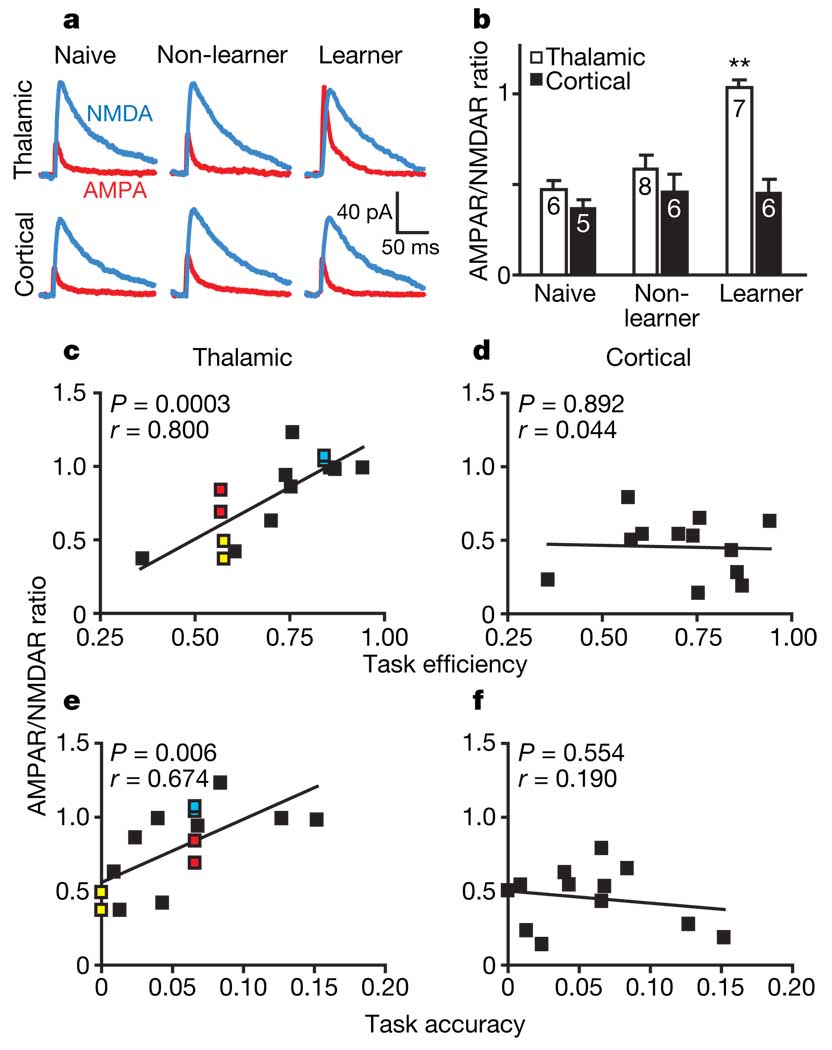
Because our in vivo recordings showed rapidly occurring changes in cue-related firing in the LA during successful cue–reward learning, we proposed that the mechanism underlying these changes was an increase in synaptic strength of thalamic or cortical sensory afferents onto LA neurons; we tested this hypothesis with ex vivo experimentation (Supplementary Fig. 6). Rats were trained on a single session of the same behavioural model and classified as learners (top 50%) or non-learners (bottom 50%) as defined by our learning indices of task efficiency and task accuracy (Supplementary Fig. 7). Any unearned sucrose was delivered in the home cage immediately after the session, ensuring that all rats received the same amount of sucrose. Brains were collected about 30 min after the end of the session for the preparation of acute slices of the LA. We stimulated the internal or external capsule to evoke excitatory postsynaptic currents (EPSCs) from thalamic or cortical afferents, respectively, and used whole-cell patch-clamp techniques within visually identified pyramidal neurons to measure EPSCs containing AMPA receptor (AMPAR)-mediated and NMDA receptor (NMDAR)-mediated currents. We found that the AMPAR/NMDAR ratio, an index of glutamatergic synaptic strength16,17, varied with task performance and afferent (main effects of group, F2,29 = 11.01, P < 0.001; afferent, F1,29 = 22.13, P < 0.001; group × afferent interaction, F2,29 = 7.38, P < 0.004) such that learners had a larger AMPAR/NMDAR ratio at thalamic synapses (P < 0.001; learners, 1.03 ± 0.04; non-learners, 0.58 ± 0.08; naives, 0.47 ± 0.05 (means ± s.e.m.)) but not cortical synapses (learners, 0.45 ± 0.08; non-learners, 0.46 ± 0.10; naives, 0.47 ± 0.04) in the LA relative to non-learners and naives, which did not differ from each other (P = 0.84; Fig. 2a, b). We determined the correlation between each rat’s behavioural performance, as measured by either task efficiency or task accuracy, and the AMPAR/NMDAR ratio, and found a significant positive relationship at thalamic inputs (P = 0.0003 and P = 0.006, respectively) but not cortical inputs (P = 0.89 and P = 0.55, respectively; Fig. 2c–f and Supplementary Table 3). Hence, thalamo-amygdalar synaptic strength predicted the success of individual rats’ reward-learning performance.
Figure 2. Degree of AMPAR/NMDAR enhancement predicts cue–reward learning.
a, EPSCs evoked by stimulation of thalamic or cortical afferents in rats that were naive, non-learners or learners. b, AMPAR/NMDAR ratios evoked from thalamic afferents were significantly increased in learners (n = 6 rats) in comparison with non-learners (n = 6 rats) or naives (n = 5 rats). Numbers in bars indicate numbers of cells; error bars indicate s.e.m. Two asterisks, P < 0.001, significant difference from other groups as well as from cortical afferent. c–f, Correlation between AMPAR/NMDAR ratio and either task efficiency (c, d) or task accuracy (e, f) for EPSCs evoked from thalamic (c, e), but not cortical (d, f), pathways; the subjects were the same as in b. Colours indicate multiple cells recorded from the same rat; black indicates single cells recorded from each rat.
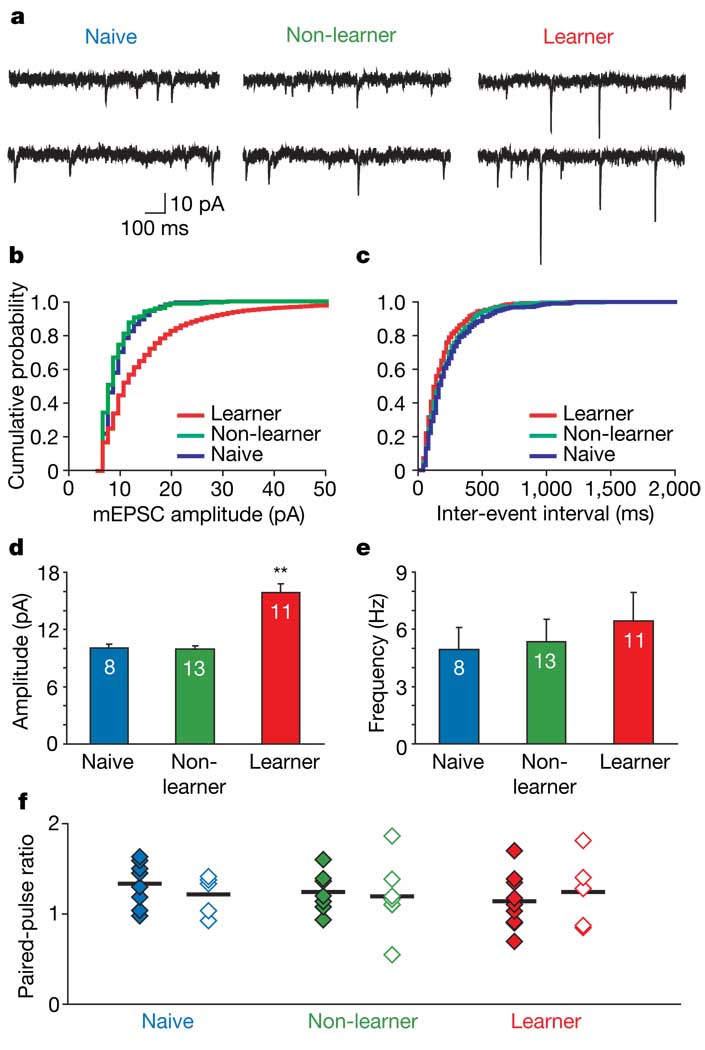
A change in the relative contribution of AMPARs and NMDARs to compound EPSCs may reflect an increase inAMPAR currents and/or a decrease in NMDAR currents at thalamo-amygdalar synapses. To determine whether AMPAR currents were modified during reward learning, we examined AMPAR-mediated miniature EPSCs (mEPSCs), which reflect spontaneously released vesicles of glutamate 18. Typically, an increase in mEPSC amplitude indicates an increase in postsynaptic AMPAR number or function, whereas an increase in mEPSC frequency indicates an increase in the probability of transmitter release (Pr) or in the number of synapses18. mEPSC amplitude was related to task performance (F2,29 = 30.75, P < 0.001), with a greater mean amplitude from LA neurons of learners (P < 0.001; 15.88 ± 0.89 pA) than from those of non-learners (9.98 ± 0.29 pA) or naives (10.05 ± 0.39 pA), which did not differ from each other (P = 0.87; Fig. 3a, b, d). In contrast, the mean mEPSC frequency was not different (F2,29 = 0.5, P = 0.61) in learners (6.45 ± 1.48 Hz), non-learners (5.36 ± 1.16 Hz) and naives (4.96 ± 1.14 Hz) (Fig. 3a, c, e). To examine further whether learning altered Pr, we examined the paired-pulse ratio19 (inter-stimulus interval 50 ms; Fig. 3f). There was no change in the paired-pulse ratio for either afferent (F1,33 = 0.02, P = 0.89) among naives, non-learners or learners (main effect of group, F2,33 = 0.35, P = 0.71; group × afferent interaction, F2,33 = 0.40, P = 0.67), indicating that learning does not cause an immediate change in Pr and that the rapid increase in AMPAR/NMDAR ratio is mediated postsynaptically.
Figure 3. Successful cue–reward learning induces an increase in mEPSC amplitude but not in frequency or paired-pulse ratio.
a, Sample mEPSCs from rats that were naive, non-learners or learners. b, c, Cumulative probability plots of amplitude (b) and frequency (c) for representative neurons from each group; bins were 1 pA (b) and 20 ms (c). d, e, Learners (n = 6 rats) had increased mEPSC amplitude (d), but not frequency (e), relative to non-learners (n = 6 rats) and naive rats (n = 5 rats). Numbers in bars indicate numbers of cells; errors bars indicate s.e.m. Two asterisks, P < 0.001. f, Lack of change in paired-pulse ratio between the different groups of rats. Filled diamonds, ratios evoked from the thalamic pathway; open diamonds, ratios evoked from the cortical pathway; horizontal lines indicate the means.
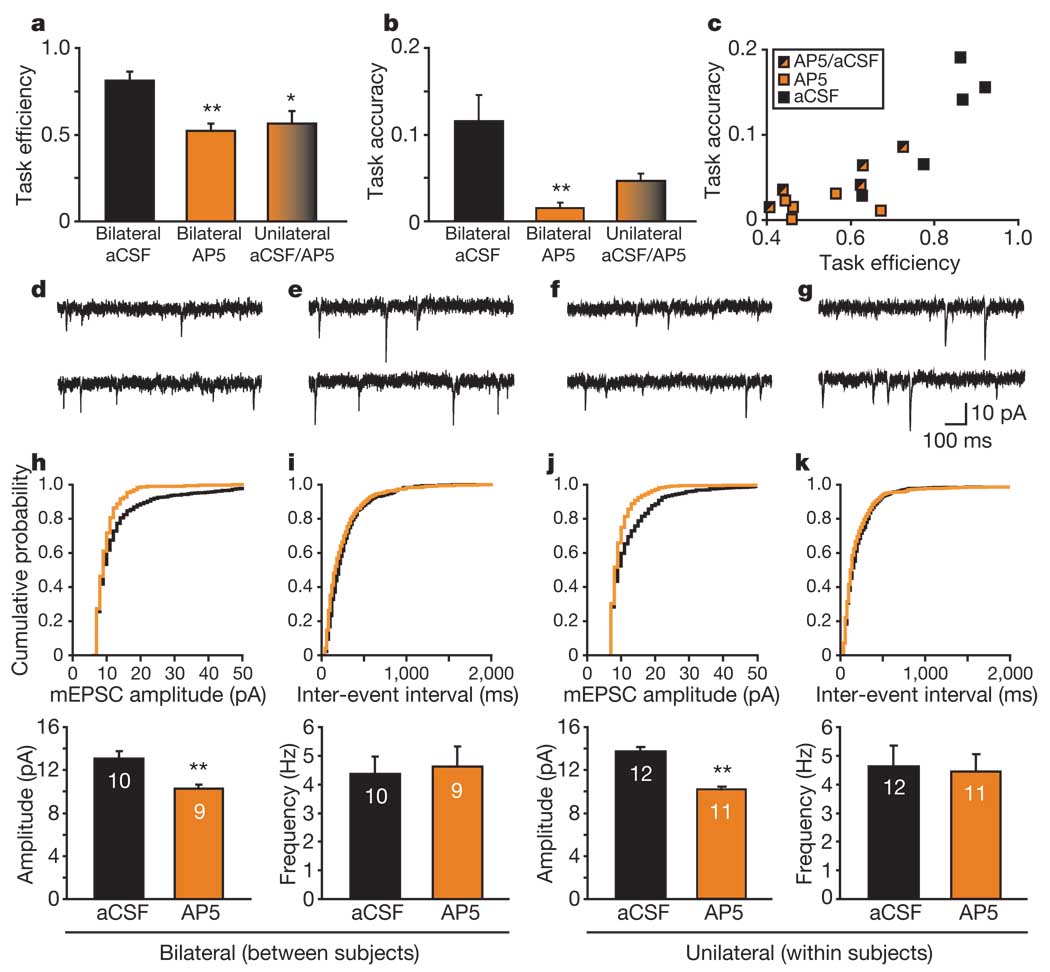
The induction of associative long-term potentiation in the LA depends on the activation of NMDARs20,21, which can lead to increases in AMPAR currents18. In addition, NMDAR blockade within the BLA impairs acquisition, but not performance, in two similar appetitive tasks22,23. To test whether the learning-induced synaptic changes we observed are dependent on NMDAR activation, we locally infused the NMDAR antagonist AP5 (3 µg per side) or vehicle (artificial cerebrospinal fluid; aCSF) into the LA bilaterally before training (Supplementary Fig. 8). To control for the possibility that synaptic changes might be secondary to, rather than causal for, reduced behavioural performance, we included a third group in which rats received unilateral intra-LA infusions of AP5 and contra-lateral infusions of aCSF to provide a within-animal control. Task efficiency was impaired by AP5 (F2,12 = 9.03, P < 0.005) after both bilateral (P < 0.007) and unilateral (P < 0.018) intra-LA pre-training infusions (Fig. 4a, c); bilateral, but not unilateral, intra-LA infusions of AP5 also impaired task accuracy (F2,12 = 7.38, P < 0.009; aCSF versus bilateral AP5, P < 0.009; Fig. 4b, c). The effect of AP5 was not attributable to a spread of drug into the neighbouring central nucleus of the amygdala (Supplementary Fig. 9).
Figure 4. Local NMDAR blockade attenuates reward-related learning and the associated increase in mEPSC amplitude.
a, b, Measures of task performance among groups (n = 5 rats per group). Task efficiency (a) is decreased after either unilateral or bilateral AP5 intra-LA infusion, whereas task accuracy (b) is decreased after bilateral AP5 (asterisk, P < 0.05, two asterisks, P < 0.009, compared with aCSF). No other comparisons were significant. Error bars indicate s.e.m. c, Individual rat performances based on task efficiency and task accuracy. d–g, Sample mEPSCs from rats that received pre-training infusions. d, e, Traces recorded from rats that received bilateral infusions of AP5 (d) and aCSF (e). f, g, Traces recorded from a representative rat that received unilateral intra-LA infusions of AP5 (f) and aCSF (g). h–k, Cumulative probability plots of amplitude (h, j) and frequency (i, k) for mEPSCs in representative cells from rats receiving bilateral infusions of AP5 (orange) or aCSF (black) (h, i) or unilateral infusions of AP5 and aCSF (j, k). Below each probability plot is the corresponding bar graph indicating the group mean and s.e.m. There is a difference in amplitude (h, j), but not in frequency (i, k) for both bilaterally infused (h) and unilaterally infused (j) rats; two asterisks, P < 0.001, compared with aCSF. Numbers in bars indicate numbers of cells.
After intra-LA infusions and the training session, brains from these rats were collected for the preparation of acute slices. Rats that received bilateral intra-LA infusions of AP5 showed a lower mean amplitude of mEPSCs (P = 0.003; 10.26 ± 0.41 pA; Fig. 4d, h) than after infusions with aCSF (13.09 ± 0.68 pA; Fig. 4e, h), whereas there was no change in mEPSC frequency between groups (P = 0.66; Fig. 4d, e, i). The decrease in task efficiency and the decrease in mEPSC amplitude after local infusion of an NMDAR antagonist suggest that cue–reward learning and the associated increase in AMPAR number or function are dependent on NMDAR activation. By comparing mEPSCs from rats with unilateral intra-LA AP5 infusions and contralateral aCSF infusions, we were able to determine with confidence that any differences between LA neurons treated with AP5 or aCSF are due to local NMDAR blockade rather than to an AP5-induced difference in task performance. Within subjects, we found that the amplitude of LA mEPSCs recorded after AP5 infusion into the LA on one side was significantly lower (P < 0.001; Fig. 4f, j) relative to aCSF infusion on the contralateral side (Fig. 4g, j), whereas there was no difference in frequency (P = 0.99; Fig. 4f, g, k). Local NMDAR blockade therefore attenuates the learning-dependent increase in postsynaptic AMPAR currents and impairs the acquisition of reward-directed behaviour.
These results show that, with cue–reward learning, cue-responsive neurons are rapidly recruited in vivo, thalamo-amygdalar synapses are selectively strengthened, and LA neurons show NMDAR-dependent increases and associated potentiation of AMPAR number or function. The proportion of cells recorded in vivo that developed a response to the reward-predictive cue is less than the proportion of cells that showed enhanced synaptic strength with learning (Fig. 2c). This suggests that the integration of multiple inhibitory and excitatory synapses on a given cell may constrain cue-related spike firing3,24,25, even if that cell possesses enhanced thalamic inputs. The thalamic pathway is under strong inhibitory suppression20,24 in vivo, whereas our ex vivo recordings were performed under γ-aminobutyric acid (GABA)A-receptor antagonism to isolate EPSCs.
The parallel emergence of increased synaptic strength and cue-related firing in the LA neurons during reward learning suggests that this excitatory synaptic increase contributes to enhanced spike activity of LA neurons in response to the conditioned stimulus, driven by auditory and visual thalamic inputs that terminate in the LA1,12. Consistent with our results, auditory fear conditioning, which requires an intact LA1,2,4, increases neuronal firing in response to a shock-predictive cue and potentiates transmission at thalamo-amygdalar synapses26 by an NMDAR-dependent mechanism, probably a result of postsynaptic AMPAR trafficking27. Previous work on fear conditioning suggests that plasticity also occurs at cortical28 synapses in the LA, although this enhancement was found at later time points than tested here. Single-unit recordings in the LA show that the thalamic pathway conditions more rapidly than the cortical pathway during fear conditioning1,29. Our findings, viewed in the context of fear conditioning, prompt further experimentation to determine whether rapidly occurring reward-learning-induced plasticity at thalamo-amygdalar synapses facilitates subsequent consolidation at other sites30.
These findings indicate that rapid synaptic changes in the LA occur during the early stages of cue–reward learning. It is likely that this plasticity permits amygdala neurons to respond selectively to meaningful environmental stimuli and transmit this information to downstream brain regions for the expedited selection of an adaptive behavioural output.
METHODS SUMMARY
Adult male Sprague–Dawley rats (250–350 g) were food-restricted to 90% of free-feeding body weight. Training session length was varied as follows: rats with chronic electrodes were trained daily for 3 h per session, for three sessions; rats with cannulae were trained for one 4-h session to allow the enhanced opportunity to express learning within a single session; and rats with no previous surgery were trained for one 2-h session, the median time required for task acquisition. Nose-poke responses were reinforced on a partial reinforcement schedule, with about 50% of responses reinforced by a 5-s compound light-tone stimulus, and 0.1 ml of 15% sucrose delivered 2 s after cue onset. Task acquisition was defined by more than 80% correct trials in a moving five-trial block. A correct trial was defined as a nose-poke yielding a cue presentation and subsequent port entry (within 10 s or before performing a different behaviour). Incorrect trials were defined as entering the port after a nose-poke without the cue. Phasic neuronal responses were deemed significant if the firing rate of a unit in any of five 100-ms bins in a 0–0.5-s response window after cue onset was different from the firing rate in a 0.5-s baseline epoch by a Wilcoxon signed-rank test. Group values are expressed as means ± s.e.m. The statistical significance of multiple group data was assessed with one- or two-way analyses of variance followed by Bonferroni post-hoc tests when indicated by significant main effects or interactions; two-group data were analysed with two-tailed Student’s t-tests. All correlations were analysed with Pearson’s correlation test. All procedures were approved by the Gallo Center Institutional Animal Care and Use Committee and were in accordance with National Institutes of Health guidelines.
METHODS
Behavioural training
Nose-poke responses were reinforced on about 50% of trials with a subsequent (onset 50 ms after nose-poke) light–tone cue: 3-kHz tone at 80 dB and illumination of two 5-s stimulus lights. At 2 s after the nose-poke, 0.1 ml of 15% sucrose was delivered to a port adjacent to the nose-poke operandum over 3 s. Additional rewards could not be earned until the previous sucrose reward had been consumed (as determined by port entries). Therefore, to maintain contingency between the cue and the reward, whenever sucrose was present in the reward port, all nose-poke responses were paired with the cue. Hence, early learning sessions tended to result in higher percentages of nose-pokes that were presented together with the cue (mean 56%, maximum 70%, minimum 39%), and in higher numbers of cue presentations than sucrose deliveries. Behavioural indices were calculated for each session. The behavioural indices, namely task efficiency and task accuracy, measured distinct aspects of reward-learning success. Task efficiency (rewards earned divided by cues presented) measured the strength of the cue–reward association, because each cue presentation signalled an opportunity for the rats to collect sucrose at the adjacent reward port. Task accuracy (the difference between the number of correct and incorrect trials divided by total port entries) measured each rat’s ability to predict accurately when sucrose would be present in the reward port.
In vivo electrophysiology
Rats were bilaterally implanted with fixed eight-wire electrode arrays (NeuroBiological Laboratories) in the vLA (anteroposterior (AP), −2.8 to −3.3 mm; mediolateral (ML), ±5.0 mm; dorsoventral (DV), 7.2 mm) for chronic neural recording during learning in a custom operant conditioning chamber (MedAssociates) as in ref. 10. Neural activity was recorded, and unit discrimination was performed, with multichannel spike acquisition and sorting software (Plexon Inc.). Responses of single units were deemed statistically significant if the firing rate within one or more 100-ms bins in the response window (0–0.5 s after cue onset) was significantly different (P < 0.01) from a 0.5-s baseline epoch (−2 to −1.5 s) using a Wilcoxon signed-rank test. To determine whether single units developed a within-session cue response, the Mann–Whitney U-test was used to compare trials from the pre-acquisition and post-acquisition epochs for five 100-ms bins in the first 500 ms response window following cue onset. For all comparisons between pre-acquisition and post-acquisition, the number of trials chosen was determined by the epoch (pre-acquisition versus post-acquisition) with the fewest trials, and an equivalent number of trials was randomly selected from the other epoch. For the peri-event surface plot (Fig. 1c), spike counts for each unit were converted to Z-scores by [(FRi – FRmb)/SDb], where FRi is the firing rate in the ith bin of the peri-event period, FRmb is the mean and SDb is the standard deviation of the firing rate of a baseline period across the session (between 1.5 and 2 s before the event). Each unit was then smoothed by averaging each trial with its neighbouring trials (±1) and units were averaged to construct a peri-event surface plot (MATLAB; Mathworks) showing the activity of all recorded units (n = 13) from one rat as the task was acquired. For the population peri-event histogram shown in Fig. 1e, Z-scores were calculated in 50-ms bins for each individual neuron relative to baseline and response periods per trial, and averaged to reveal the population response for sessions 1 and 3.
Ex vivo electrophysiology
About 30 min after session end, rats were anaesthetized with 40mg kg−1 pentobarbital and perfused transcardially with 30 ml of modified aCSF (at about 1 °C) for perfusion containing (in mM): 225 sucrose, 126 NaCl, 2.5 KCl, 1.0 NaH2PO4, 4.9 MgCl2, 0.1 CaCl2, 26.2 NaHCO3, 1.25 glucose, 3 kynurenic acid. Coronal sections containing the LA (320 µm) were collected in a holding chamber (superfusion solution, saturated with 95% O2 and 5% CO2, containing (in mM): 126 NaCl, 2.5 KCl, 1.0 NaH2PO4, 1.3 MgCl2, 2.4 CaCl2, 26.2 NaHCO3, 11 glucose, 1 ascorbic acid at 32–34 °C)) to recover for about 1 h before recording with the same superfusion solution without ascorbic acid but with 0.1 mM picrotoxin. Recordings were made from visually identified pyramidal neurons in the ventral aspect of the LA. Recording electrodes (2.8–4.0 MΩ) were filled with (in mM): 120 caesium methansulphonate, 20 HEPES, 0.4 EGTA, 2.8 NaCl, 5 tetraethylammonium chloride, 2.5 MgATP, 0.25 NaGTP (pH 7.25–7.4; 280–290 milli-osM). Series resistance (10–20 MΩ) and input resistance were monitored online. EPSCs were filtered at 2 kHz and collected with custom scripts written in IgorPro software (Wavemetrics). The AMPAR/NMDAR ratio was calculated by averaging 20–30 EPSCs at +40mV before and after application of the NMDAR blocker AP5 (50 µM) for 5 min. NMDAR responses were calculated by subtracting the average response in the presence of AP5 from that seen in its absence. Similar to previous studies20,27,28, electrical stimulation was applied to the internal capsule to evoke EPSCs in LA neurons from thalamic afferents12, and the external capsule to evoke EPSCs from cortical afferents15. In each rat from which a thalamic AMPAR/NMDAR ratio was recorded, a cortical AMPAR/NMDAR ratio was also recorded. mEPSC traces were filtered at 1 kHz, collected with Clampex (Molecular Devices) and analysed with Mini Analysis Program (Synaptosoft). AMPAR mEPSCs were recorded in cells voltage-clamped at −70mV and in the continual presence of lidocaine (500 µM) over 5 min; 300 events were analysed per cell (the detection criterion was set at 7 pA). Behavioural performance was not calculated until after whole-cell recordings had been analysed.
Intra-LA infusions
Rats were implanted with cannulae just dorsal to the LA (AP, −2.8 to −3.3 mm; ML, ± 5.0 mm; DV, 7.0 mm). One week later, rats received sham infusions of aCSF 24 h before the training session; 10–15 min before the training session, aCSF or AP5 (0.4 µl aCSF per side or 3 µg of AP5 per 0.4 µl per side; 0.1 µl min−1) was infused bilaterally. After training, brains were prepared for whole-cell recordings as above, after careful removal of the cannula headstage. Cannula placements were revealed during the slice recording session with an upright microscope under infrared illumination (Supplementary Fig. 6). An additional group received cannulae in the central nucleus of the amygdala (AP, −1.8 to 2.3 mm; ML, ± 4.6 mm; DV, 7.0 mm) for infusion of AP5.
Supplementary Material
Acknowledgements
We thank H. L. Fields, R. A. Nicoll, A. J. Doupe, B. T. Chen, M. J. Wanat and F. W. Hopf for critical comments; W. W. Schairer, J. J. Cone and L. D. Tye for technical assistance; and T. M. Gill and A. D. Milstein for discussion and technical advice. This study was supported by the State of California for Medical Research on Alcohol and Substance Abuse through the University of California at San Francisco (P.H.J. and A.B.), National Institutes of Health grant RO1DA115096 (A.B.) and a National Science Foundation Graduate Research Fellowship (K.M.T.).
Footnotes
Full Methods and any associated references are available in the online version of the paper at www.nature.com/nature.
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions K.M.T. performed the experiments and analyzed the data, with assistance and training in whole-cell recording from G.D.S., who performed pilot mEPSC experiments. B.R. performed cannula surgeries and trained K.M.T. in microinjection techniques. A.B. and P.H.J. provided mentorship and resources. K.M.T., G.D.S., A.B. and P.H.J. contributed to study design, results analysis, interpretation and manuscript writing.
Author Information Reprints and permissions information is available at www.nature.com/reprints.
References
- 1.LeDoux J. The emotional brain, fear, and the amygdala. Cell. Mol. Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis M. In: The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Aggleton JP, editor. Chichester, UK: Wiley; 1992. pp. 255–306. [Google Scholar]
- 3.Rosenkranz JA, Grace AA. Dopamine-mediated modulation of odour-evoked amygdala potentials during pavlovian conditioning. Nature. 2002;417:282–287. doi: 10.1038/417282a. [DOI] [PubMed] [Google Scholar]
- 4.Maren S, Quirk GJ. Neuronal signalling of fear memory. Nature Rev. Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 5.Cador M, Robbins TW, Everitt BJ. Involvement of the amygdala in stimulus-reward associations: interaction with the ventral striatum. Neuroscience. 1989;30:77–86. doi: 10.1016/0306-4522(89)90354-0. [DOI] [PubMed] [Google Scholar]
- 6.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 7.Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci. 2006;29:272–279. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Schoenbaum G, Chiba AA, Gallagher M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. J. Neurosci. 1999;19:1876–1884. doi: 10.1523/JNEUROSCI.19-05-01876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uwano T, Nishijo H, Ono T, Tamura R. Neuronal responsiveness to various sensory stimuli, and associative learning in the rat amygdala. Neuroscience. 1995;68:339–361. doi: 10.1016/0306-4522(95)00125-3. [DOI] [PubMed] [Google Scholar]
- 10.Tye KM, Janak PH. Amygdala neurons differentially encode motivation and reinforcement. J. Neurosci. 2007;27:3937–3945. doi: 10.1523/JNEUROSCI.5281-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doron NN, Ledoux JE. Organization of projections to the lateral amygdala from auditory and visual areas of the thalamus in the rat. J. Comp. Neurol. 1999;412:383–409. [PubMed] [Google Scholar]
- 13.Azuma S, Yamamoto T, Kawamura Y. Studies on gustatory responses of amygdaloid neurons in rats. Exp. Brain Res. 1984;56:12–22. doi: 10.1007/BF00237437. [DOI] [PubMed] [Google Scholar]
- 14.Nakashima M, et al. An anterograde and retrograde tract-tracing study on the projections from the thalamic gustatory area in the rat: distribution of neurons projecting to the insular cortex and amygdaloid complex. Neurosci. Res. 2000;36:297–309. doi: 10.1016/s0168-0102(99)00129-7. [DOI] [PubMed] [Google Scholar]
- 15.McDonald AJ. Cortical pathways to the mammalian amygdala. Prog. Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 16.Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- 17.Perkel DJ, Nicoll RA. Evidence for all-or-none regulation of neurotransmitter release: implications for long-term potentiation. J. Physiol. (Lond.) 1993;471:481–500. doi: 10.1113/jphysiol.1993.sp019911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malenka RC, Nicoll RA. Long-term potentiation—a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 19.Hess G, Kuhnt U, Voronin LL. Quantal analysis of paired-pulse facilitation in guinea pig hippocampal slices. Neurosci. Lett. 1987;77:187–192. doi: 10.1016/0304-3940(87)90584-2. [DOI] [PubMed] [Google Scholar]
- 20.Shin RM, Tsvetkov E, Bolshakov VY. Spatiotemporal asymmetry of associative synaptic plasticity in fear conditioning pathways. Neuron. 2006;52:883–896. doi: 10.1016/j.neuron.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Humeau Y, Shaban H, Bissiere S, Luthi A. Presynaptic induction of heterosynaptic associative plasticity in the mammalian brain. Nature. 2003;426:841–845. doi: 10.1038/nature02194. [DOI] [PubMed] [Google Scholar]
- 22.Burns LH, Everitt BJ, Robbins TW. Intra-amygdala infusion of the N-methyl-d-aspartate receptor antagonist AP5 impairs acquisition but not performance of discriminated approach to an appetitive CS. Behav. Neural Biol. 1994;61:242–250. doi: 10.1016/s0163-1047(05)80007-x. [DOI] [PubMed] [Google Scholar]
- 23.Baldwin AE, Holahan MR, Sadeghian K, Kelley AE. N-methyl-d-aspartate receptor-dependent plasticity within a distributed corticostriatal network mediates appetitive instrumental learning. Behav. Neurosci. 2000;114:84–98. doi: 10.1037//0735-7044.114.1.84. [DOI] [PubMed] [Google Scholar]
- 24.Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J. Neurosci. 2003;23:11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samson RD, Pare D. A spatially structured network of inhibitory and excitatory connections directs impulse traffic within the lateral amygdala. Neuroscience. 2006;141:1599–1609. doi: 10.1016/j.neuroscience.2006.04.077. [DOI] [PubMed] [Google Scholar]
- 26.McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390:607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- 27.Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- 28.Tsvetkov E, Carlezon WA, Benes FM, Kandel ER, Bolshakov VY. Fear conditioning occludes LTP-induced presynaptic enhancement of synaptic transmission in the cortical pathway to the lateral amygdala. Neuron. 2002;34:289–300. doi: 10.1016/s0896-6273(02)00645-1. [DOI] [PubMed] [Google Scholar]
- 29.Quirk GJ, Armony JL, LeDoux JE. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997;19:613–624. doi: 10.1016/s0896-6273(00)80375-x. [DOI] [PubMed] [Google Scholar]
- 30.McGaugh JL. Memory consolidation and the amygdala: a systems perspective. Trends Neurosci. 2002;25:456–461. doi: 10.1016/s0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.