Abstract
IL-10 is a potent anti-inflammatory cytokine interfering with antigen presentation by inducing the intracellular sequestration of MHC class II (MHC-II) molecules. Here we studied the contribution of membrane-associated RING-CH (MARCH) ubiquitin ligase family members to the IL-10-induced down-regulation of MHC-II molecules.We found that MARCH1 and MARCH8 proteins are the most potent family members for the down-regulation of MHC-II surface expression in transfected cells, but only MARCH1 mRNA expression is strongly induced by IL-10 in human primary monocytes. We detected mono-and poly-ubiquitinated forms of MHC-II molecules both in IL-10-treated monocytes and in cells transfected with MARCH1. We also show direct interaction between MHC-II and MARCH1 molecules in co-immunoprecipitation assays. Finally, we found that siRNA-mediated knockdown of MARCH1 reverses IL-10-induced MHC-II down-regulation in primary monocytes. Thus, the immunosuppressive effect of IL-10 on antigen presentation is mediated through induced expression of MARCH1.
Keywords: Antigen-presenting cells, Cytokines, Immune regulation, Inflammation, MHC
Introduction
MHC class II (MHC-II) molecules play an essential role during the cellular immune response by presenting pathogen-derived peptides to CD4+ T helper cells. MHC-II genes and molecules show complex and regulated expression patterns controlled both at the transcriptional and post-translational levels. Constitutive and IFN-γ-inducible MHC-II gene expression are largely under the control of the MHC-II transactivator CIITA [1, 2].
Several examples of post-translational control of MHC-II transport have been described, especially in the myeloid lineages of cells. In monocyte-derived immature DC, MHC-II molecules are mainly sequestered in intracellular compartments and DC maturation leads to re-localization of MHC-II molecules at the cell surface, while at the same time MHC-II gene transcription is shut off [3–5]. Interestingly, it has been shown recently that intracellular localization of MHC-II molecules in immature DC coincides with ubiquitination of the cytoplasmic tail of MHC-II β-chains by an as yet unidentified ubiquitin ligase, and that MHC-II ubiquitination is lost during DC maturation [6, 7].
In monocytes, the capacity to stimulate CD4 Tcell responses is strongly inhibited by the immunosuppressive activity of IL-10. This pleiotropic anti-inflammatory cytokine interferes with antigen presentation by down-regulating both MHC-II and the costimulatory molecules CD80 and CD86 [8–10]. In human primary monocytes, IL-10 did not affect MHC-II gene transcription, protein synthesis or loading of the MHC-II αβ complexes with antigenic peptides. Rather, IL-10-treatment caused the intracellular accumulation of mature MHC-II complexes and prevented their display at the plasma membrane by an unidentified mechanism [9].
Membrane-associated RING-CH (MARCH) proteins are cellular homologs of viral transmembrane E3-ubiquitin ligases, such as the KSHV8 K3 (MIR 1) and K5 (MIR 2) molecules, which have been shown to ubiquitinate the cytoplasmic tails of MHC-I molecules [11, 12]. Nine human MARCH protein family members have been described [13]. MARCH8 (c-MIR) was shown to down-regulate CD86 [14], an activity that was shared by MARCH1 and MARCH2 [13]. More recently, transgenic overexpression of MARCH8 led to reduced MHC-II cell surface expression in mice [15], while B cells from MARCH1-deficient mice showed increased expression of MHC-II at the plasma membrane [16].
Thus, IL-10-induced down-regulation of cell surface MHC-II and CD86 in monocytes was reminiscent of the activities of MARCH8 and MARCH1. Here, we identify MARCH1 as the mediator of the IL-10-induced immunosuppressive effect on the MHC-II antigen-presentation pathway.
Results and discussion
IL-10 induces the expression of MARCH1
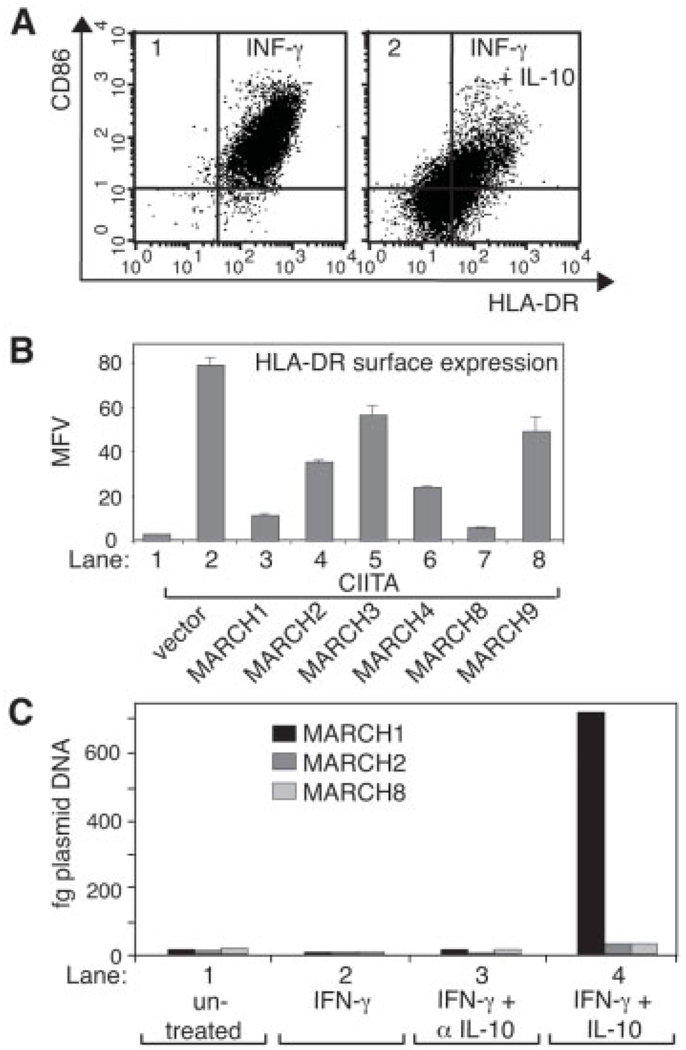
As expected, IL-10 strongly inhibited HLA-DR and CD86 cell surface expression in CD14+ primary monocytes (Fig. 1A) and MHC-II molecules accumulated in internal vesicles in these cells (Supporting Information Fig. S1). We first compared the capacity of MARCH protein family members with a similar membrane topology to MARCH1 and −8 [13] to down-regulate MHC-II cell surface expression. We found that the highly homologous MARCH1 and −8 proteins were the most potent in reducing the CIITA-induced cell surface expression of HLA-DR in transiently transfected 293EBNA cells, but other MARCH proteins also had a certain inhibitory effect under these conditions (Fig. 1B). MARCH4 expression negatively affected cell survival and thus seemed to have a more general effect (data not shown). MARCH1 and −8 efficiently down-regulated all three human MHC-II isotypes; HLA-DR, -DQ, and -DP (Supporting Information Fig. S2).
Figure 1.
IL-10 induces MARCH1 expression. (A) Primary human monocytes were incubated with IFN-γ in the absence (panel 1) or presence (panel 2) of IL-10 for 16 h and analyzed by flow cytometry for HLA-DR and CD86 expression. (B) 293EBNA cells were transiently co-transfected with EGFP (lanes 1–8), CIITA (lanes 2–8), and with MARCH cDNA (lanes 3–8) and analyzed by flow cytometry after 48 h. Shown are mean fluorescence values (MFV) of cell surface HLA-DR expression gated on EGFP-positive cells. Values and SD are derived from duplicates of independent transfections. (C) MARCH1, −2, and −8 mRNA expression in primary human monocytes was analyzed by real-time RT-PCR. Cells were left either untreated (lane 1), or incubated for 16 h in the presence of IFN-γ (lane 2), IFN-γ and an anti-IL-10mAb (20 µg/mL; lane 3) or IFN-γ and IL-10 (lane 4). Samples were normalized for HPRT expression and results are expressed as the amount of equivalent linearized plasmid DNA standard for each MARCH. Shown is a representative example from three independent donors.
Next, we analyzed via quantitative RT-PCR the mRNA expression levels of MARCH family members in cells treated with IL-10 (Fig. 1C). In untreated primary monocytes, the MARCH1, −2 and −8 mRNA were at comparable low levels, which were not affected by treatment with IFN-γ (Fig. 1C, lanes 1 and 2). However, addition of IL-10 for 16 h increased MARCH1 mRNA expression over 40-fold for the representative donor shown in Fig. 1C (lane 4), while MARCH2 and −8 levels increased only 2–3-fold. IL-10-induced expression of MARCH1 was independent of the presence of IFN-γ (data not shown). The lack of sensitivity of MARCH-specific antibodies precluded the analysis of protein expression in these cells. MARCH3, −4 and −9 mRNA expression levels were undetectable or very low and not responsive to IL-10 or IFN-γ in monocytes (data not shown). These results suggested that IL-10-induced MARCH1 expression may be important for the observed immunosuppressive effects in monocytes.
MARCH1 ubiquitinates HLA-DR molecules
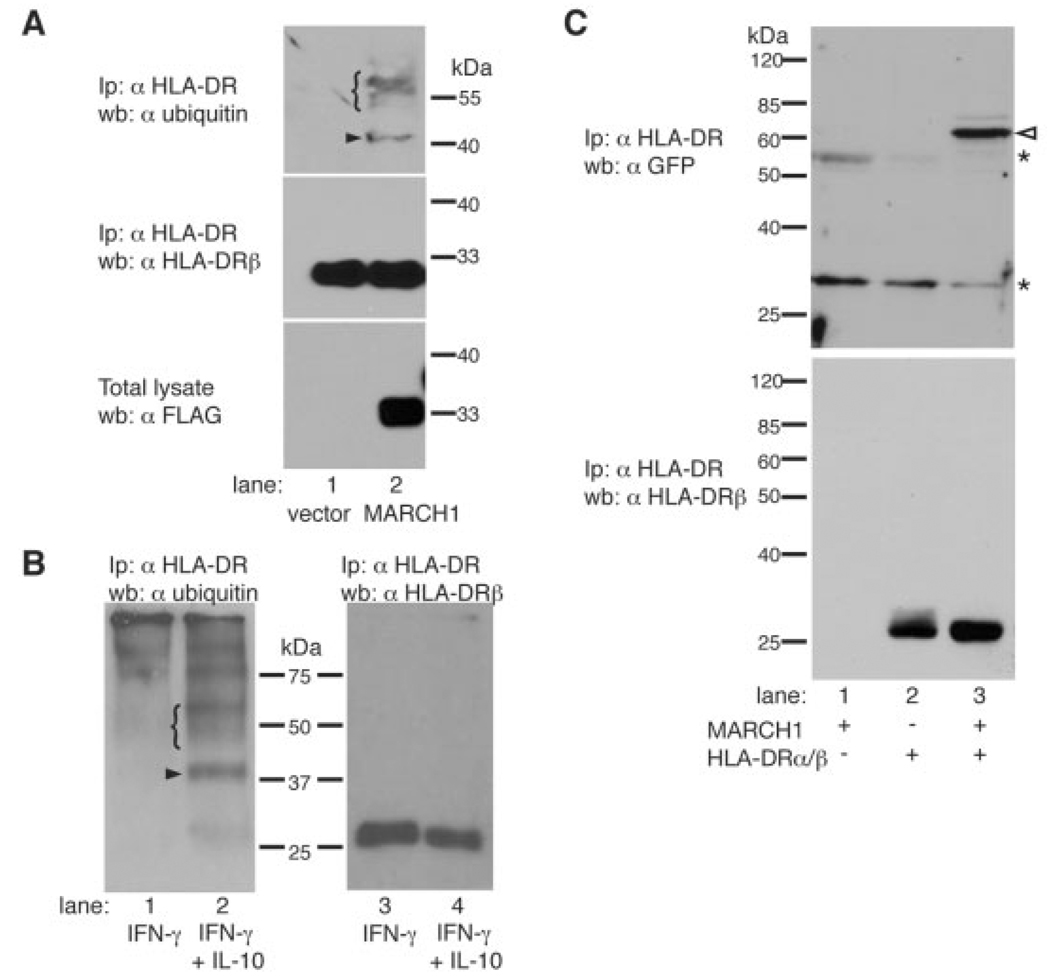
Ubiquitination of mouse MHC-II β-chains by MARCH proteins has been described recently [15, 16].We thus looked for ubiquitinated forms of HLA-DR in HeLa/CIITA cells expressing MARCH1. HLA-DR molecules were immunoprecipitated with mAb L243 and analyzed by western blotting using the ubiquitin-specific mAb P4D1 (Fig. 2A, upper panel). HLA-DR molecules showed the presence of a band of 40–42 kDa, which is compatible with the size of an HLA-DR β-chain (28–30 kDa) bearing one ubiquitin moiety (arrowhead), only in MARCH1-positive and not in vector-transfected cells (Fig. 2A). A band of similar size corresponding to mono-ubiquitinated MHC-II I-Aβ was observed in immature mouse DC [6]. Interestingly, a typical ladder of ubiquitinated material (bracket) was detected at higher molecular weights, suggesting that the β-chain is also poly-ubiquitinated in MARCH1-positive cells. L243 recognizes peptide-loaded MHC-II [17], indicating that MARCH1 can ubiquitinate mature HLA-DR molecules.
Figure 2.
HLA-DR is ubiquitinated in MARCH1-transfected cells as well as in IL-10 treated monocytes, and interacts with MARCH1. (A) HeLa/CIITA cells were transfected with empty vector (lane 1) or an expression plasmid for FLAG-tagged MARCH1 (lane 2). At 24 h post transfection, cells were lysed and HLA-DR was immunoprecipitated using L243. Samples were separated by SDS-PAGE and analyzed on immunoblots using the ubiquitin-specific mAb P4D1 (top panel) and HLA-DRβ-specific mAb XD5.117 (middle panel). Total lysates were analyzed by immunoblotting with FLAG-specific mAb M2 (bottom panel). The arrowhead indicates the presence of mono-ubiquitinated HLA-DRβ, the bracket that of polyubiquitinated molecules. (B) Primary human monocytes were cultured for 16 h in the presence of IFN-γ (lanes 1, 3) or IFN-γ plus IL-10 (lanes 2, 4). Cells were lysed and HLA-DR molecules were immunoprecipitated with L243. Non-reduced samples were separated by SDS-PAGE and analyzed for ubiquitin by immunoblotting using P4D1 (left panel). The membrane was stripped and incubated with the HLA-DRβ-specific mAb XD5.117 (right panel). Similar results were obtained using cells from a second donor (data not shown). (C) 293T cells were transiently transfected with EYFP-MARCH1 alone (lane 1),with HLA-DRα/β (lane 2), or co-transfected with HLA-DRα/β and EYFP-MARCH1 (lane 3). At 24 h post transfection, cells were lysed and HLA-DR molecules were immunoprecipitatedwith mAb L243. Samples were separated by SDS-PAGE and analyzed on an immunoblot with a GFP-specific antiserum (top panel). The arrowhead indicates the presence of the EYFP-MARCH1 fusion protein (predicted molecular mass 58.4 kDa) and the asterisks indicate the Ig heavy and light chains. EYFP-MARCH1 expression in samples from lanes 1 and 3 was verified by flow cytometry (data not shown). The membrane was stripped and incubated with the HLA-DRβ specific mAb XD5.117 (bottom panel).
IL-10 induces ubiquitination of MHC-II in monocytes
We next immunoprecipitated HLA-DR molecules from IL-10-treated monocytes. western blotting for ubiquitin revealed the presence of a major band of about 40 kDa and a smear of higher molecular weight in the sample treated with IL-10 (Fig. 2B, left panel). This is very similar to the pattern observed in HeLa/CIITA cells transfected with MARCH1 (Fig. 2A), suggesting that IL-10 induces both mono- and poly-ubiquitination of MHC-II molecules. Bands corresponding to ubiquitinated HLA-DRβ chains could not be revealed when the immunoprecipitated material was re-blotted with XD5.117, probably due to their low abundance (Fig. 2B, right panel). These results clearly indicate that IL-10 induces ubiquitination of HLA-DR molecules in monocytes.
MARCH1 interacts with HLA-DR
The presence of a RING-CH E3-type ubiquitin ligase domain in MARCH1 suggests that the latter comes close to MHC-II molecules in a multimolecular ubiquitination complex. We tested for direct interaction between HLA-DR molecules and MARCH1 by co-immunoprecipitation. 293T cells were transiently transfected with HLA-DR and an enhanced yellow fluorescent protein (EYFP)-MARCH1 fusion protein either alone or in combination. EYFPMARCH1 was fully functional for the down-regulation of MHC-II molecules (Supporting Information Fig. S3). HLA-DR molecules were immunoprecipitated with mAb L243 and analyzed by western blotting (Fig. 2C). A GFP-specific antiserum revealed co-immunoprecipitated EYFP-MARCH1 only in cells co-expressing HLA-DR and EYFP-MARCH1 (Fig. 2C, top panel, lane 3). We also detected in vivo interactions between HLA-DR and MARCH1 in intracellular vesicles by fluorescence energy transfer (FRET) microscopy (data not shown). These results suggest that HLA-DRβ is a direct substrate for the MARCH1 E3 ubiquitin ligase activity.
Knockdown of MARCH1 reverses IL-10-induced down-regulation of MHC-II in monocytes
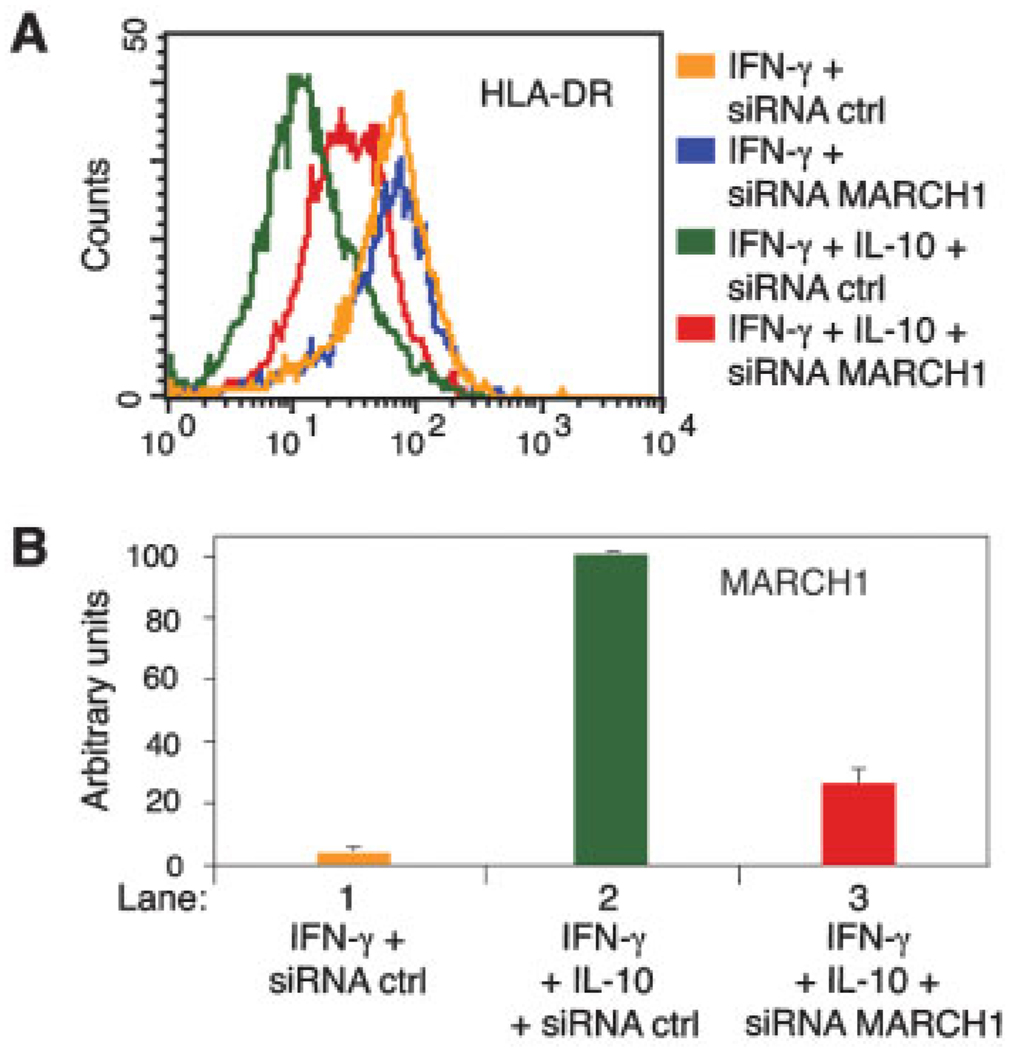
We transfected IFN-γ-stimulated primary monocytes with fluorescent siRNA in the presence or absence of IL-10. Control siRNA had no effect on MHC-II expression under any condition tested, nor did MARCH1-specific siRNA affect the levels of cell surface MHC-II expression in the absence of IL-10 (Fig. 3A). In contrast, knockdown of MARCH1 strongly inhibited IL-10-dependent down-regulation of cell surface HLA-DR (Fig. 3A, compare green and red profiles). MARCH1 protein levels could not be analyzed due to lack of antibody sensitivity, but MARCH1 mRNA levels were reduced by 70% in cells treated with MARCH1-directed siRNA (Fig. 3B). These results demonstrate that MARCH1 is necessary for the IL-10-induced MHC-II down-regulation in monocytes.
Figure 3.
IL-10 induced down-regulation of MHC-II is dependent on MARCH1. Primary human monocytes were transfected with fluorescent-labeled (Alexa 488) control or MARCH1-specific siRNA. Cells were cultured for 16 h in the presence of IFN-γ or IFN-γ plus IL-10. (A) HLA-DR cell surface expression of siRNA-transfected cells was analyzed by flow cytometry. (B) MARCH1 mRNA expression of the cells shown in (A) was analyzed by real-time RT-PCR. Samples were normalized for HPRT expression and MARCH1 expression of the sample in lane 2 was arbitrarily set at 100. Values and SD are from duplicate PCR. Similar results were obtained on another donor.
Concluding remarks
The results presented here provide strong evidence that negative modulation of antigen-presentation function in monocytes by IL-10 is mediated by the induction of MARCH1. Our finding of ubiquitinated HLA-DR molecules in MARCH1-transfected cells and in IL-10-treated monocytes is the first demonstration of this modification in human MHC-II molecules. Down-regulation of murine MHC-II proteins by murine MARCH1 and −8 has been found to be dependent on the single lysine residue in the cytoplasmic tail of the β-chain [15, 16]. As expected, we found that the corresponding lysine residue in the cytoplasmic tail of HLA-DRβ is also important for MARCH1-induced down-regulation of human HLA-DR molecules (Supporting Information Fig. S3). Co-immunoprecipitation indicates that MARCH1 and HLA-DR molecules interact directly. We detected a relatively important fraction of HLA-DR molecules, both in MARCH-transfected cells and in IL-10-treated monocytes, that appeared to be mono-ubiquitinated in addition to poly-ubiquitinated. Mono- and oligo-ubiquitination have been shown to be important sorting signals for membrane proteins, inducing mostly sorting to endosomal and lysosomal vesicles [18]. Accordingly, studies in mouse DC showed that ubiquitination prevents recycling and increases the targeting of MHC-II into luminal vesicles of multivesicular bodies [7, 16].
While MARCH1 and −8 have very similar effects in down-regulating MHC-II, they appear to be expressed quite independently. MARCH8 expression appears to be broadly distributed with highest levels in lung and pancreas [13]. On the other hand, the highest levels of human MARCH1 mRNA expression was found in lymph node and splenic tissues [13], and MARCH1 is important for the regulation of MHC-II in murine B cells [16]. In addition, MARCH1 is expressed in immature human DC and is down-regulated during DC maturation [19]. Our results show that IL-10 modulates mainly the expression of MARCH1 and this is the first demonstration of the regulation of MARCH gene expression by cytokines and its impact under physiological conditions. Analysis of the contribution of MARCH gene regulation to immunopathology will be of great future interest.
Materials and methods
Plasmids
Expression vectors for the different MARCH protein, EBS-CIITA and EBS-enhanced GFP (EGFP) constructs have been described previously [13, 20]. An N-terminal fusion between EYFP (Clontech) and MARCH1 was generated by PCR and cloned into the cDNA expression vector pcDNA3.1 (Invitrogen).
Antibodies
The HLA-DR-specific mAb L243 and XD5.117 have been described previously [21]. L243-allophycocyanin and CD86-PE (IT2.2) were from BD Biosciences. HLA-DR specific HK14-QantumRed and FLAG-specific M2 mAb were from Sigma. P4D1 is specific for ubiquitin (Covance). The GFP-specific rabbit antiserum was from Invitrogen. Rat anti-human IL-10 was from Serotec.
Cell culture
Experiments with human primary monocytes were approved by institutional ethics committees. Human monocytes were isolated by positive selection of CD14+ cells (Miltenyi Biotech) from PBMC obtained by leukapheresis. Cells were incubated for 16 h at 37°C with human recombinant IFN-γ and IL-10 (Peprotech) at 167 and 0.1 ng/mL, respectively. siRNA experiments were performed on fresh monocytes using non-targeting control or MARCH1-specific cocktails of siRNA from Qiagen (1.3 nM; coupled to Alexa 488) and Dharmacon (ON-TARGETplus SMARTpool; 0.6 nM). Cells were electroporated prior to the addition of IFN-γ and IL-10 using an Amaxa Nucleofector II and the Amaxa Monocyte Nucleofection kit. Transfection efficiency was between 20 and 40% and only transfected fluorescent cells were found to be responsive to IL-10. Flow cytometry analysis was performed on live, Alexa 488-positive cells.
Immunoprecipitation and western blotting
HeLa/CIITA cells and primary monocytes were lysed in buffer containing 1% Triton X-100, 25 mM N-ethylmaleimide, 5 µM MG132 (all from Sigma) and protease inhibitors (Roche). MHC-II molecules were immunoprecipitated with L243 and heated at 95°C for 5 min in gel-loading buffer. Samples were resolved by SDS-PAGE and analyzed by western blotting. For the co-immunoprecipitation experiment, 293T cells were lysed in buffer containing 1% CHAPS.
Quantitative RT-PCR analysis
Total RNA from 106 frozen monocytes was isolated (Absolutely RNA kit; Stratagene) and first-strand cDNA generated (Expand RT, Roche). Real-time PCR was performed using SYBR Green reagents (Invitrogen) on a Stratagene MX3000P instrument. Samples were normalized for HPRT expression. Quantification was carried out via standard dilution curves of linearized plasmid DNA. All primer pairs except those for MARCH2 are located in different exons or span intron/exon boundaries. All PCR experiments were repeated at least twice with highly comparable results. Primer sequences are shown in the Supporting Information Table 1.
Supplementary Material
Acknowledgements
We thank Rafick Sékaly for cDNA and vectors, and Philippe Pierre for helpful discussions. This work was supported by NIH grants R01 CA/AI094011–04 and R21 CA109674–02, and the Medical Research Foundation to K.F. E.B. is supported by an institutional training grant for molecular pathogenesis (T32 AI007472). J. Thibodeau, A.B. and V.S. are scholars of the Fonds de la recherche en santé du Québec (FRSQ). J. Thibodeau is supported by a Canadian Institutes for Health Research (CIHR)-INSERM International Scientific Exchanges fellowship. D.L. holds the Novartis /Canadian Liver Foundation Hepatology Research Chair. J. Thibodeau and D.L. are supported by the CIHR and INSERM. V.S. is supported by the National Science and Engineering Research Council of Canada (NSERC, grant 262845–03) and the Canadian Foundation for Innovation (CFI).
Abbreviations
- MARCH
membrane-associated RING-CH
- EGFP
enhanced green fluorescent protein
- EYFP
enhanced yellow fluorescent protein
Footnotes
Supporting Information for this article is available at www.wiley-vch.de/contents/jc_2040/2008/37902_s.pdf
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
- 1.Steimle V, Otten LA, Zufferey M, Mach B. Complementation cloning of an MHC class II transactivator mutated in hereditary MHC class II deficiency (or bare lymphocyte syndrome) Cell. 1993;75:135–146. [PubMed] [Google Scholar]
- 2.LeibundGut-Landmann S, Waldburger JM, Krawczyk M, Otten LA, Suter T, Fontana A, Acha-Orbea H, Reith W. Mini-review: Specificity and expression of CIITA, the master regulator of MHC class II genes. Eur. J. Immunol. 2004;34:1513–1525. doi: 10.1002/eji.200424964. [DOI] [PubMed] [Google Scholar]
- 3.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 4.Pierre P, Turley SJ, Gatti E, Hull M, Meltzer J, Mirza A, Inaba K, et al. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388:787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- 5.Landmann S, Muhlethaler-Mottet A, Bernasconi L, Suter T, Waldburger JM, Masternak K, Arrighi JF, et al. Maturation of dendritic cells is accompanied by rapid transcriptional silencing of class II transactivator (CIITA) expression. J. Exp. Med. 2001;194:379–391. doi: 10.1084/jem.194.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin JS, Ebersold M, Pypaert M, Delamarre L, Hartley A, Mellman I. Surface expression of MHC class II in dendritic cells is controlled by regulated ubiquitination. Nature. 2006;444:115–118. doi: 10.1038/nature05261. [DOI] [PubMed] [Google Scholar]
- 7.van Niel G, Wubbolts R, Ten Broeke T, Buschow SI, Ossendorp FA, Melief CJ, Raposo G, et al. Dendritic cells regulate exposure of MHC class II at their plasma membrane by oligoubiquitination. Immunity. 2006;25:885–894. doi: 10.1016/j.immuni.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Willems F, Marchant A, Delville JP, Gerard C, Delvaux A, Velu T, de Boer M, Goldman M. Interleukin-10 inhibits B7 and intercellular adhesion molecule-1 expression on human monocytes. Eur. J. Immunol. 1994;24:1007–1009. doi: 10.1002/eji.1830240435. [DOI] [PubMed] [Google Scholar]
- 9.Koppelman B, Neefjes JJ, de Vries JE, de Waal Malefyt R. Interleukin-10 down-regulates MHC class II alphabeta peptide complexes at the plasma membrane of monocytes by affecting arrival and recycling. Immunity. 1997;7:861–871. doi: 10.1016/s1074-7613(00)80404-5. [DOI] [PubMed] [Google Scholar]
- 10.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 11.Lehner PJ, Hoer S, Dodd R, Duncan LM. Downregulation of cell surface receptors by the K3 family of viral and cellular ubiquitin E3 ligases. Immunol. Rev. 2005;207:112–125. doi: 10.1111/j.0105-2896.2005.00314.x. [DOI] [PubMed] [Google Scholar]
- 12.Ohmura-Hoshino M, Goto E, Matsuki Y, Aoki M, Mito M, Uematsu M, Hotta H, Ishido S. A novel family of membrane-bound e3 ubiquitin ligases. J. Biochem. (Tokyo) 2006;140:147–154. doi: 10.1093/jb/mvj160. [DOI] [PubMed] [Google Scholar]
- 13.Bartee E, Mansouri M, Hovey Nerenberg BT, Gouveia K, Fruh K. Downregulation of major histocompatibility complex class I by human ubiquitin ligases related to viral immune evasion proteins. J. Virol. 2004;78:1109–1120. doi: 10.1128/JVI.78.3.1109-1120.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goto E, Ishido S, Sato Y, Ohgimoto S, Ohgimoto K, Nagano-Fujii M, Hotta H. c-MIR, a human E3 ubiquitin ligase, is a functional homolog of herpesvirus proteins MIR1 and MIR2 and has similar activity. J. Biol. Chem. 2003;278:14657–14668. doi: 10.1074/jbc.M211285200. [DOI] [PubMed] [Google Scholar]
- 15.Ohmura-Hoshino M, Matsuki Y, Aoki M, Goto E, Mito M, Uematsu M, Kakiuchi T, et al. Inhibition of MHC class II expression and immune responses by c-MIR. J. Immunol. 2006;177:341–354. doi: 10.4049/jimmunol.177.1.341. [DOI] [PubMed] [Google Scholar]
- 16.Matsuki Y, Ohmura-Hoshino M, Goto E, Aoki M, Mito-Yoshida M, Uematsu M, Hasegawa T, et al. Novel regulation of MHC class II function in B cells. EMBO J. 2007;26:846–854. doi: 10.1038/sj.emboj.7601556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roche PA, Cresswell P. Invariant chain association with HLA-DR molecules inhibits immunogenic peptide binding. Nature. 1990;345:615–618. doi: 10.1038/345615a0. [DOI] [PubMed] [Google Scholar]
- 18.Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nat. Rev. Mol. Cell. Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- 19.de Gassart A, Camossetto V, Thibodeau J, Ceppi M, Catalan N, Pierre P, Gatti E. MHC class II stabilization at the surface of human dendritic cells is the result of maturation-dependent MARCH I down-regulation. Proc. Natl. Acad. Sci. USA. 2008;105:3491–3496. doi: 10.1073/pnas.0708874105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hake SB, Masternak K, Kammerbauer C, Janzen C, Reith W, Steimle V. CIITA leucine rich repeats control nuclear localisation, in vivo recruitment to the MHC class II enhanceosome, and MHC class II gene transactivation. Mol. Cell. Biol. 2000;20:7716–7725. doi: 10.1128/mcb.20.20.7716-7725.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khalil H, Brunet A, Saba I, Terra R, Sekaly RP, Thibodeau J. The MHC class II beta chain cytoplasmic tail overcomes the invariant chain p35-encoded endoplasmic reticulum retention signal. Int. Immunol. 2003;15:1249–1263. doi: 10.1093/intimm/dxg124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.