Abstract
The present study investigated the efficacy of nefazodone and bupropion-sustained release for treating cannabis dependence. A double blind, placebo controlled, piggy back design was employed to assess if nefazodone and bupropion-sustained release increased the probability of abstinence from cannabis and reduced the severity of cannabis dependence and cannabis withdrawal symptoms during a 13-week outpatient treatment program. One-hundred and six participants (M=32 years; Females n=25) were randomized to one of three medication conditions (nefazodone, bupropion-sustained release, or placebo) and participated in a weekly individually based coping skills therapy program. Results indicated a an increased probability of achieving abstinence over the course of treatment and a decrease in the severity of cannabis dependence and the withdrawal symptom of irritability. There were no significant effects demonstrated for nefazodone and bupropion-sustained release on cannabis use or cannabis withdrawal symptoms. The results indicate nefazodone and bupropion-sustained release may have limited efficacy in treating cannabis dependence.
Keywords: Nefazodone, Bupropion, cannabis dependence, marijuana dependence
Introduction
Cannabis preparations are the most commonly used illicit substances in the United States.1 Approximately 9% of the American adult population has met criteria for a lifetime cannabis use disorder and evidence suggests there has been an increase in these disorders over the past 10 years.2 Heavy chronic use of marijuana carries with it significant negative personal, social, and medical consequences.3–7 Further, documented increases in the potency of cannabis suggest a greater probability of developing dependence among those who regularly use cannabis.8–9 Separate lines of evidence also point to a growing need for cannabis specific treatment programs. Only a minority of individuals meeting criteria for a cannabis use disorder in the general population has ever received formal drug treatment and there has been a notable interest in cannabis specific treatment programs among cannabis users in the community.2 Further, approximately 15% of adults seeking substance abuse treatment report that cannabis is their primary drug of abuse.10 Together this evidence suggests that developing effective cannabis specific interventions may help address a growing public health concern and meet the needs of an increasing population seeking help for cannabis specific problems.
Motivational Enhancement Therapy (MET) and Coping Skills based Relapse Prevention programs significantly reduce cannabis use and its associated negative social and personal consequences.11–14 These findings demonstrate therapeutic procedures originally developed for treating other substance dependent populations can be modified to influence cannabis use.15–16 However, they also highlight that a notable proportion of individuals continue to smoke cannabis following exposure to these treatment strategies. Thus, there is room for improving our psychosocial intervention efforts. Budney et al. (2000) significantly improved the outcome of a combined MET and Coping-Skills treatment program by including a voucher based incentive system in the context of this treatment approach.17 The incremental improvement of psychosocial interventions by adding other therapeutic techniques offers a useful blueprint for guiding the continued development of more effective cannabis treatment strategies. The continued need for alternative treatment strategies is also highlighted by the finding that 65% of participants in the voucher-enhanced psychosocial treatment continued to smoke cannabis at the last week of treatment.17
Several lines of evidence suggest pharmacological interventions may provide an alternative strategy for treating cannabis dependence. First, pharmacological interventions have demonstrated efficacy in treating other substance use disorders suggesting dependence can be amenable to pharmacological interventions.18 Second, decreases in cannabis use have been demonstrated among subgroups of substance dependent populations enrolled in pharmacotherapy trials for other psychiatric disorders.19–20 These findings highlight the possibility that cannabis use maybe reactive to specific types of pharmacological agents. Third, recent investigations have identified a clear constellation of psychological and physical symptoms associated with cannabis withdrawal that are similar in presentation to depressive disorders and the nicotine withdrawal (i.e. anxiety, dysphoria, irritability, changes in appetite and intake, and decreased quantity and quality of sleep).21–26 The similarities in symptom presentation offers the possibility that successful pharmacological interventions for both depressive and nicotine dependence disorders, such as noradrenergic reuptake inhibitors, may be particularly useful for ameliorating cannabis withdrawal symptoms among patients seeking treatment for their cannabis dependence.27 Reducing the uncomfortable physiological response to abstinence my increase the overall effectiveness of coping skills based relapse prevention strategies particularly during the periods of treatment in which skills are first being introduced.
Bupropion hydrochloride (BPR) and nefazodone (NEF), noradrenergic reuptake inhibitors with different mechanisms of action, have demonstrated promise as pharmacological treatments for cannabis dependence. BPR, a weak blocker of neuronal uptake of serotonin and norepinephrine as well as a re-uptake blocker of dopamine, is an effective, well tolerated antidepressant, with a favorable side effect profile.28 Initial laboratory investigations among non-treatment seeking cannabis dependent participants indicated sustained-release BPR increased irritability and depressed mood during a period of abstinence compared to placebo.29 However, the use of a restricted dosage range in a non-clinical context may limit the generalizability of these findings to clinical populations.20 Among treatment seeking populations, BPR has reduced cocaine use30–31, metamphetamine abuse32, and cigarette smoking and craving.29,33 While the efficacy of BPR as an aid for smoking cessation appears to be independent of a history of major depression, it is hypothesized to exert its effect by reducing the negative mood symptoms associated with nicotine withdrawal which suggests it may also be a useful pharmacological intervention for cannabis withdrawal among treatment seekers.
NEF, an antidepressant with dual action on serotonin and norepinephrine reuptake as well as 5-HT (2A) receptor antagonist effects, has been found to be useful in reducing anxiety and insomnia, has no reported abuse potential, and was a commonly used antidepressant at the time this outpatient trial was conducted.34–35 While laboratory investigations among non-treatment seeking cannabis dependent individuals indicated minimal effects on sleep disturbances and irritability, significant decreases in anxiety and muscle pain during periods of cannabis withdrawal have been demonstrated.36 Furthermore, among cocaine users, evidence suggests NEF may have more impact reducing drug craving then directly preventing relapse.37–38 This evidence suggests NEF therapeutic effects may be mediated by changes in the subjective and physiological reactivity associated with a history of chronic drug use.
To date there have been few published outpatient randomized pharmacologic trials that directly targeted cannabis-dependence among individuals seeking treatment.39–40 This study evaluated the efficacy of sustained release BPR hydrochloride (BPR-SR) and NEF, two antidepressant medications that have demonstrated some efficacy in the treatment of substance use disorders and symptom constellations that characterize the cannabis withdrawal syndrome. Similar to successful trials with nicotine dependent individuals, the use of antidepressant medications in the context of a rigorous psychosocial treatment was chosen to promote initiation and maintenance of abstinence from cannabis, enhance retention, and promote treatment compliance.41 We hypothesized that BPR-SR and/or NEF would be superior to placebo for promoting abstinence among cannabis dependent patients receiving a skill based psychosocial treatment. Secondary analyses investigated if BPR-SR and/or NEF were superior to placebo in reducing the severity of cannabis dependence and the withdrawal symptoms of anxiety, irritability, and sleep disturbances.
Methods
Participants
All participants were seeking outpatient treatment for problems related to cannabis use and were recruited by local advertising or by referrals in the New York City metropolitan area. The medical screening included a complete history and physical exam, an electrocardiogram, and laboratory tests (including hematology, blood chemistry [including liver function tests], and blood pregnancy test for females). The psychiatric evaluation included the Structured Clinical Interview (SCID) for Diagnostic and Statistical Manual of Mental Disorders- Axis I disorders (DSM-IV).42–43 Participants were treated at the Substance Treatment and Research Service (STARS) of Columbia University/ New York State Psychiatric Institute.
Study inclusion required that participants were between the ages of 18–65, met DSM-IV criteria for a current cannabis dependence diagnosis, and were smoking at least 5 cannabis joints per week. Participants were excluded if they 1) met DSM-IV criteria for current significant and unstable psychiatric condition which required psychiatric intervention (i.e. Schizophrenia or Bipolar disorder), 2) had a chronic organic mental disorder 3) met DSM-IV dependence criteria for another substance (e.g. alcohol), 4) had significant current suicidal risk, 5) had a history of seizures or unexplained loss of consciousness, 6) had taken an MAOI in the previous two weeks, 7) were currently taking terfenedine, cisapride, astemizole, or pimozide, 8) had an unstable physical condition such as hypertension, elevated transaminase levels, or uncontrolled diabetes, 9) had clinically significant coronary vascular disease as indicated by history or suspected by abnormal ECG or history of cardiac symptoms, 10) had a history of allergic reaction to BPR or NEF, or 11) were nursing and/or pregnant and/or unwilling to use an effective method of birth control.
All participants gave written informed consent before the screening procedures were initiated and if accepted to the study, again, prior to beginning the treatment protocol. The study was approved by the Institutional Review Board of the New York State Psychiatric Institute and Columbia University.
Study Procedures
This study was a double-blind, placebo-controlled, randomized trial comparing NEF, BPR-SR, and placebo (PBO) for promoting abstinence from cannabis and reducing symptoms of cannabis withdrawal. The trial was 13 weeks long and included a one-week PBO lead-in phase, a ten-week medication phase (including dose titration), and a two week placebo lead-out phase. Participants were scheduled to attend the treatment clinic twice per week.
A double blind, piggy back design was used, with participants in all three treatment conditions receiving two capsules, three times a day (a total of 6 capsules per day), for the duration of the trial; this included the lead-in and lead-out phases. All capsules were prepared at the research pharmacy and looked identical for all three treatment conditions.. Study medication was provided to the patient on a weekly basis. Participants were asked to return all packaging and unused medication to the nursing station at each visit. The nurse documented any unused or missed medication.
Following the placebo lead-in phase, participants were randomized into either NEF, BPR-SR or a PBO condition. A research pharmacist, who was independent of the research team, conducted the randomization. During the dose titration, NEF began at 150 mg per day at bedtime. Every five days the dose was increased by 150 mg if tolerated, to a maximum dosage of 600 mg per day, (300 mg BID). The NEF group received 300 mg each in the morning and at bedtime; the afternoon dose was two placebo capsules. BPR-SR was begun at 150 mg in the morning, and after three days was increased to a total of 300 mg per day (150 mg BID), if tolerated. The BPR-SR group received 150 mg in the morning and afternoon, the bedtime dose was two placebo capsules. Riboflavin (approximately 100 mg/day) was added to all capsules to track compliance. At each clinic visit urine samples were examined under an ultraviolet (UV) lamp in order to observe fluorescence signifying ingestion of the study capsules.
Participants were asked to provide a urine specimen, complete self-report instruments, have their vital signs and side effects assessed, and received medication until their next visit at each of the two weekly visits. They were compensated $5.00 in cash for transportation costs. All clinical assessments of drug use were conducted on a weekly basis. Monthly blood pregnancy tests for women were performed.
All participants received a coping skills based psychosocial intervention that was administered once per week in an individually based format. Sessions focused on developing cognitive and behavioral skills to decrease the probability of cannabis use. The treatment focused on several core modules: (a) a Functional Analysis of Cannabis Use (b) Coping with Cannabis Cravings and Urges (c) Managing Thoughts about smoking Cannabis (d) Cannabis Refusal Skills (e) Seemingly Irrelevant Decisions related to cannabis use and (f) Planning for Emergencies. Role plays and out-of –session assignments were provided throughout the treatment to bolster the acquisition of the skills discussed during each treatment session. Elective topics included Anger Management and Managing Negative Moods and Depression. The cannabis treatment modules were adapted from manualized skills based treatment programs used to treat other substance dependence disorders44–45 and were similar in scope to more recently developed cannabis specific treatments.11 Therapy sessions were provided by doctoral or master’s level therapists who had received extensive training in the delivery of coping skills based treatments for substance dependence. Sessions were audio-taped and randomly selected to be reviewed during weekly supervision with a senior licensed clinical psychologist.
Assessments
Screening
The Structured Clinical Interview for DSM-IV Disorders (SCID-IV) was administered to establish the presence of cannabis dependence and to rule out the presence of other psychiatric disorders that would exclude participation.42 During the assessment of substance use disorders patients were queried on their current and past use of cannabis and other substances (i.e. alcohol, cocaine, opiates, sedatives, amphetamines, hallucinogens). Substance use assessment included the age of first use, age of regular use, number of days in the past 30 days that the substance was used, number of days in the past week that the substance use used, and the amount of the substance used per using day (i.e. joints per day for cannabis).
Cannabis Use and Dependence Severity
Cannabis use was assessed by urine toxicology and participant self-report. Participants provided a urine sample under the observation of a staff member at every clinic visit and completed a self-report questionnaire assessing the number of days cannabis was smoked, the amount smoked, and the amount of money spent on cannabis since their last appointment. Urine samples were tested for cannabinoids, opiates, methadone, barbiturates, amphetamine and cocaine using fluorescence-polarization immune analysis (FPIA) and scored as positive or negative based on standard NIDA guidelines for cut-off points. An exception to standard cut-off guidelines was made for cannabinoids. The NIDA cut-off is 50 ng/ml for the major cannabinoid metabolite 11-nor-Δ-9-THC-9-carboxylic acid.46 However, cannabinoid metabolites may remain in the urine for several weeks after heavy or chronic use. Thus, using the strict cut-off of 50 ng/ml may increase the probability of false positives when assessing for more recent cannabis use. In the present study a 100 ng/ml was used as the cut-off point to decrease the probability of obtaining a false positive outcome. This cutoff level has been used to minimize false positives by other treatment investigators.17 Sensitivity and specificity analyses using urine toxicology as the gold standard indicated good concordance between UA results and participant self-report (Sensitivity = .73 and a Specificity = 0.86). Furthermore, a positive predicted value of 0.68 indicated that self-reports of cannabis use were consistent with a positive urine toxicology and the negative predictive value of a 0.89 indicated self-reported abstinence was highly consistent with negative toxicology results.
The severity of cannabis dependence symptoms was rated every week by a research psychiatrist using the Clinical Global Impression (CGI).47 The CGI is a clinician rated instrument that assesses the severity and the improvement of the cannabis dependence symptoms. Severity scores ranged from “1” (no pathology) to “7” (extreme pathology). Improvement ratings are based on a comparison between the severity of current symptoms and the severity level rated at baseline. The improvement ratings could range from “1” (very much improved) to “7” (very much worse).
Cannabis Withdrawal
The Cannabis withdrawal symptoms of sleep disturbance, anxiety, and irritability were targeted in this study. Several clinical instruments were used to assess these symptoms. The St Mary’s Hospital Sleep Questionnaire (SMHSQ) is a self-report instrument developed for use with psychiatric and medical in-patients48–49 that has also been used to assess the quality of sleep among substance dependent individuals during periods of withdrawal.50–51 The SMHSQ assesses the overall quality of sleep, the number of awakenings, difficulty in falling asleep, and the number of hours spent falling asleep. It was administered once per week.
The Snaith Irritabiliy Scale is an 18 item self-report instrument that assesses symptoms of depression, anxiety, and irritability.52 The two irritability subscales assess outwardly directed symptoms (e.g. “I lose my temper and shout and snap at others.”) and inwardly directed symptoms (e.g. “I have been getting annoyed with myself”). The four items on each subscale range from “0” to “3” yielding total scores that can range from 0 to 12, with a higher score indicating greater irritability. The Snaith was administered every other week.
The Hamilton Anxiety Scale (HAM-A) is a clinician-administered interview that assesses the affective and vegetative symptoms of anxiety. It consists of 14 items, each rated on a 5 point scale ranging from 0 (none) to 4 (very severe). The total score ranges from 0–56 with greater scores reflecting more significant symptomatology. The HAM-A was administered every other week.53–54
Side Effects
Side effects were recorded and rated on a scale of 0–3 (0=none, 1= mild, 2=moderate, 3= severe) employing the National Institute of Drug Abuse/VA Medication Development Research Unit Adverse Event form (version date 1/15/98).
Outcomes
Weekly cannabis use was the primary outcome measure for this study. It was operationalized by combining the quantitative cannabinoid levels derived from urine toxicology and self-reported cannabis use for a given treatment week. A week of no cannabis use was recorded if all urine samples collected during that week were negative (i.e. < 100ng/ml) and if no use was self-reported in that week. If either urine or self-report data were missing for a given week it was considered to be a non-abstinent week. Participants were categorized as being abstinent if they demonstrated a minimum of three consecutive weeks of abstinence. Separate analyses were also conducted for the abstinence outcome based on either self-reported cannabis use or urine toxicology results.
Secondary outcome measures included (1) improvement in the severity of cannabis dependence symptoms defined as the proportion of participants achieving a CGI dependence improvement score of less than three (indicative of clinical improvement), the change in the CGI severity score over time and (2) changes in the cannabis withdrawal symptoms of sleep disturbances (SMHSQ; “difficulty in falling asleep” and “the number of awakenings”), irritability (Snaith; Inward and Outward symptoms), and anxiety (HAM-A).
Data Analysis
All participants randomized to a treatment condition were included in the outcome analyses. All statistical tests were 2-tailed and employed an alpha significance level set at .05. Demographic variables and baseline clinical characteristics were compared among the groups using a chi-square test for categorical variables and independent one-way ANOVAs for continuous variables. Treatment group differences in retention analyzed using Kaplan-Meier survival curves and the log-rank test. Longitudinal analyses of the cannabis use and withdrawal symptom measures were performed using generalized estimating equations (GEE) as implemented by SAS’s PROC GENMOD. GEE estimations account for the uneven number of observations among treatment participants (i.e. missing data). Dichotomous outcomes (e.g. abstinent vs not abstinent) were modeled using a Log-Link function. Continuous outcomes (e.g. withdrawal symptoms) were modeled using linear regression. All outcome measures were modeled as a function of time, treatment assignment, and a time by treatment assignment interaction. Further, all models controlled for baseline levels of the outcome measure in question (i.e. cannabis use, sleep disturbances, irritability, and anxiety), a baseline by treatment group interaction, and the difference in gender distribution among the treatment groups.
Results
Recruitment Flow
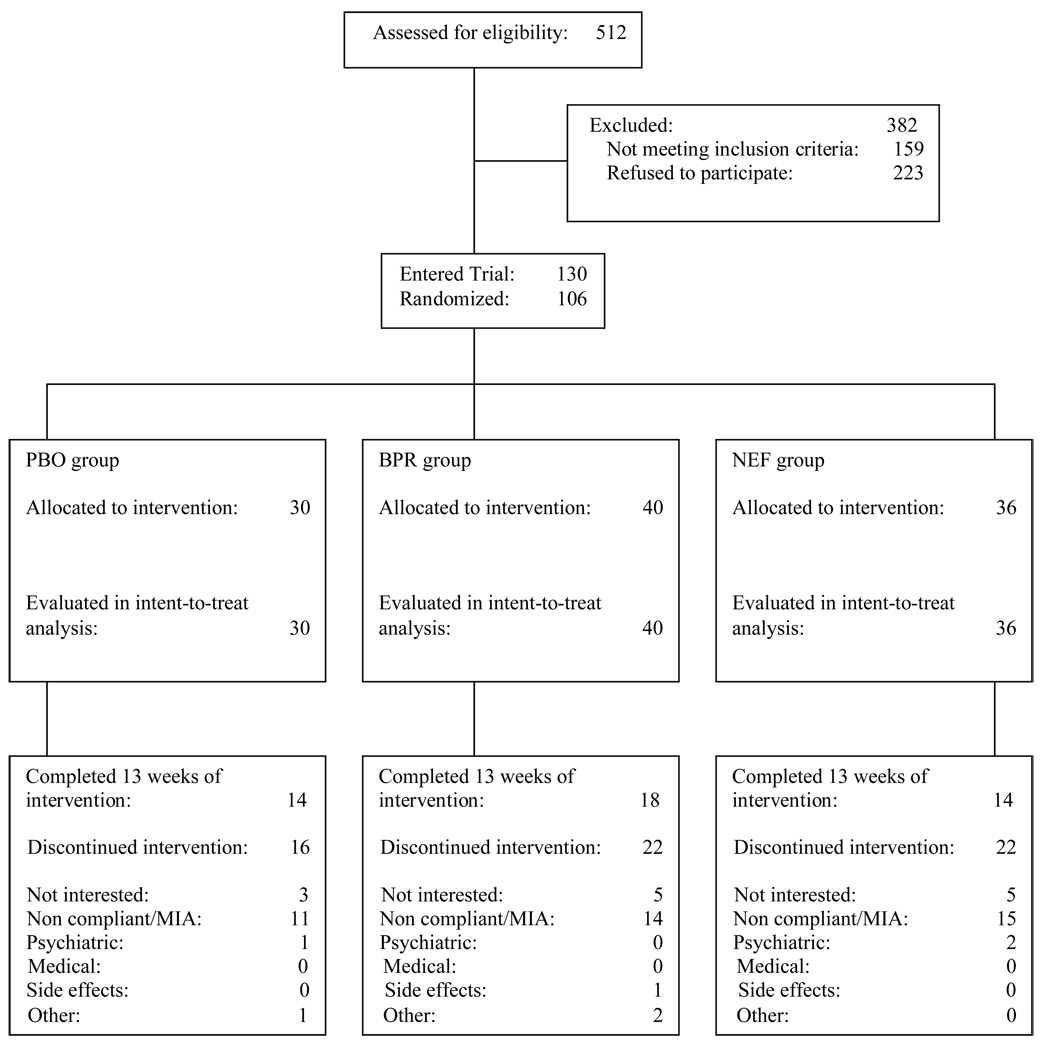
Figure 1 outlines the participant flow during the screening process and throughout the randomized trial. A total of 512 cannabis-dependent treatment seekers began the screening process. One hundred and thirty participants completed screening, met inclusion/exclusion criteria, and consented to begin the study. Of the 130 participants consented to begin the study, 7 participants did not initiate study medication following informed consent. One-hundred-twenty three participants started medications; 17 of which did not complete the placebo lead-in phase. Of the 17 participants not completing the placebo lead-in, 10 were lost to follow-up and stopped attending clinic appointments and 7 left the study for various reasons (5 stated they were no longer interested in treatment, 1 participant was no longer interested in taking medication, and 1 participant started a new job and was no longer able to attend appointments). One hundred and six participants completed the placebo lead-in and were randomized to either the PBO group (n=30), the BPR-SR group (n=40) or the NEF group (n=36).
Figure 1.
Participants’ progress through the screening, entry, randomization and medication phases of the treatment trial.
Sample Description and Retention in Treatment
Table 1 presents demographic and clinical characteristics by treatment group. On average the participants were 32 years of age (SD=10; range 19 to 63). Approximately 34% (n=36) were Caucasian, 28% (n=30) were Hispanic, and 27% (n=29) were African-American. Males comprised 76% of the sample. However, the distribution of gender was not equivalent across the three treatment groups (χ2= 9.49, df = 2, p= .009). BPR had the greatest proportion of females. The treatment groups were not statistically different on other demographic indices, other substance use, or baseline levels of cannabis use.
Table 1.
Baseline Demographic and Clinical Characteristics of Randomized Patients*
| Placebo (N=30) |
Bupropion (N=40) |
Nefazodone (N=36) |
Statistics | p-value | ||
|---|---|---|---|---|---|---|
| Demographics | Mean (SD) | Mean (SD) | Mean (SD) | F2, 102 | ||
| Age | 34 (11) | 33 (9) | 31 (10) | 0.64 | 0.53 | |
| n (%) | n (%) | n (%) | X2 | |||
| Male | 28 (93%) | 25 (63%) | 28 (78%) | 9.10 | 0.01 | |
| Race | Black | 8 (27%) | 8 (20%) | 13 (37%) | 9.70 | 0.14 |
| Hispanic | 9 (30%) | 8 (20%) | 13 (37%) | |||
| White | 11 (37%) | 19 (48%) | 6 (17%) | |||
| Other | 2 (6%) | 5 (12%) | 4 (11%) | |||
| Education (Post high school) | 17 (57%) | 26 (65%) | 18 (50%) | 1.76 | 0.42 | |
| Currently employed** | 27 (90%) | 36 (90%) | 33(92%) | 0.08 | 0.96 | |
| Current Substance Use Disorders | ||||||
| Alcohol abuse | 2 (7%) | 3 (8%) | 1 (3%) | 0.87 | 0.65 | |
| Cocaine abuse | 1 (3%) | 0 (0%) | 2 (6%) | 2.17 | 0.34 | |
| Current Psychiatric Disorders | ||||||
| Affective | 7 (23%) | 9 (23%) | 10 (28%) | 0.32 | 0.85 | |
| Anxiety | 6 (20%) | 8 (20%) | 4 (11%) | 1.33 | 0.51 | |
| Pattern of Marijuana Use | Mean (SD) | Mean (SD) | Mean (SD) | F2, 102 | ||
| CGI severity score | 4.7 (0.8) | 4.6 (0.9) | 4.8 (1.0) | 0.26 | 0.77 | |
| Age at first regular use | 18 (4) | 19 (4) | 18 (4) | 0.13 | 0.88 | |
| Days used in last 30 days | 28 (4) | 29 (4) | 27 (6) | 0.70 | 0.50 | |
| Total amount spent in last 30 days ($) | 106 (97) | 176 (160) | 154 (214) | 1.33 | 0.27 | |
Data obtained during screening for trial prior to initiation of any study procedures. Values in the table are n (%) for categorical variables, or mean (SD) for continuous variables.
Defined as full-time or part-time employment, student or in military service
Of the 106 randomized participants, 49% (n=52) completed the 10-week medication phase of the trial (PBO 53% (n=16), BPR-SR 53% (n=21), NEF 42% (n=15)). Approximately 43% (n=46) completed the entire 13-week trial (PBO 47% (n=14)), BPR-SR 45% (n=18), NEF 39% n=14). Sixty participants left the study after being randomized to a treatment group. In all groups, the majority of participants (n=53) who were dropped from the trial did so because they stopped attending their appointments and did not respond to multiple attempts by the staff to have them return to the clinic to continue participation in the treatment trial (see Figure 1). Of the other seven participants removed from the trial, in the PBO group, one participant was removed from the protocol due to the worsening of pre-existing depressive symptoms and another participant was removed from the trial because a court mandated to a more extensive drug treatment facility. In the BPR-SR group, one participant was removed from the trial because of side effects (see Safety section), and two participants voluntarily left the trial because of employment related scheduling conflicts. In the NEF group, two participants were removed from the trial due to the worsening of depression and anxiety symptoms, respectively. Survival analysis revealed no statistically significant group differences on treatment retention (X2(2) =1.21, p=0.55). There were no differences between those participants who completed the trial and those who did not on demographic indices or baseline substance use measures.
Medication Adherence
The percent of days medication was reported to have been taken and the percentage of riboflavin tests indicating medication capsules were ingested were moderately correlated (r=0.60) suggesting a fair association between the two measures. Detecting the presence of riboflavin in a urine sample is most probable within 24 hrs of ingestion. Thus, the riboflavin procedure is not sensitive to non-adherence that occurs outside of the one day detection window. The restricted time frame for detection limits the utility of riboflavin for documenting adherence over a period of several days. However, riboflavin is a useful tool for detecting non-adherence within 24 hrs of a clinic appointment and it provided the medical staff with an opportunity to discuss adherence issues with a participant, particularly if there is a discrepancy between the UA and self-reported adherence at a clinic visit. Given the narrow window of riboflavin detection, medication adherence was based on participant self-report. Adherence was quantified as the proportion of reported missed doses during the 10-week medication phase of the treatment trial (the number of reported missed doses/ the total number of doses dispensed per week). The treatment groups did differ in the average proportion of self-reported missed doses (F (2,99) = 4.12, p=0.019). Bonferroni corrected paired-wise t-tests indicated that participants on NEF reported a greater proportion of missed doses (M=0.21; SD=0.23) compared to patients on participants on placebo (M=0.10; SD=0.10). All participants reached and were maintained on the maximum tolerated dose. Two participants discontinued their medication at some point in the trial although were re-titrated to the maximum does (see safety below).
Safety
Forty-five percent (n=18) of the BPR-SR group, 42% (n=15) of the NEF group, and 27% (n=8) of the placebo group reported side effects (X2(2)= 2.63, p=0.27). Most of the side effects were of mild severity. Thirteen percent (n=5) of the BPR-SR group, 25% (n=9) of the NEF group, and 3% (n=1) of the placebo group reported side effects as being moderate or severe. The most common side effects in the BPR-SR group were headaches (15%) and nausea (8%). As for the NEF group, the most common side effect was diarrhea (8%), whereas in the placebo group, GI upset was the most common. Furthermore, one patient in the BPR-SR group reported flu-like symptoms following randomization and requested that the medication be discontinued. One patient in the NEF group reported agitation and the medication was discontinued for several days. This patient resumed medication and completed the trial at the maximum dose. Another patient reported diarrhea and the medication was discontinued for several days. Due to the patient’s lactose intolerance the lactose filler in the medication capsules was removed and the patient resumed his medication with resolution of side effects. Table 2 presents the distribution of all the side effects reported during the trial across the three treatment conditions.
Table 2.
Distribution of side effects reported during trial, by treatment condition
| Placebo (N=30) |
Bupropion (N=40) |
Nefazodone (N=36) |
|||
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | X2 | p-value | |
| Side Effect Total | 8(26.7) | 18 (45.0) | 15 (41.7) | 2.63 | 0.27 |
| Reported items | |||||
| Insomnia | 1 (3.3) | 2 (5.0) | 2 (5.6) | NS | NS |
| Irritability | 1 (3.3) | 0 (0) | 0 (0) | NS | NS |
| GI upset | 4(13.3) | 1 (2.5) | 2 (5.6) | NS | NS |
| Urinary discomfort | 0 (0) | 0 (0) | 1 (2.8) | NS | NS |
| Constipation | 0 (0) | 0 (0) | 2 (5.6) | NS | NS |
| Anxiety | 0 (0) | 0 (0) | 1 (2.8) | NS | NS |
| Skin lesion | 0 (0) | 1 (2.5) | 0 (0) | NS | NS |
| Nausea | 0 (0) | 3 (7.5) | 2 (5.6) | NS | NS |
| Vomiting | 1 (3.3) | 0 (0) | 1 (2.8) | NS | NS |
| Dizziness | 2 (6.7) | 0 (0) | 2 (5.6) | NS | NS |
| Sedation | 0 (0) | 0 (0) | 1 (2.8) | NS | NS |
| Motor agitation | 0 (0) | 1 (2.5) | 1 (2.8) | NS | NS |
| Headaches | 1 (3.3) | 6 (15.0) | 2 (5.6) | NS | NS |
| Chest pain | 1 (3.3) | 0 (0) | 0 (0) | NS | NS |
| Diarrhea | 0 (0) | 0 (0) | 3 (8.3) | NS | NS |
| Sweating | 0 (0) | 2 (5.0) | 1 (2.8) | NS | NS |
| Rash | 0 (0) | 1 (2.5) | 1 (2.8) | NS | NS |
| Nightmare | 0 (0) | 0 (0) | 2 (2.8) | NS | NS |
| Muscle twitching | 0 (0) | 1 (2.5) | 0 (0) | NS | NS |
| Chills | 0 (0) | 0 (0) | 1 (2.8) | NS | NS |
| Decreased appetite | 0 (0) | 2 (5.0) | 0 (0) | NS | NS |
| Spacey | 0 (0) | 1 (2.5) | 0 (0) | NS | NS |
| Agitation | 0 (0) | 0 (0) | 1 (2.8) | NS | NS |
| Acne | 0 (0) | 1 (2.5) | 0 (0) | NS | NS |
| Flu | 0 (0) | 2 (5.0) | 0 (0) | NS | NS |
| Tremor | 0 (0) | 2 (5.0) | 0 (0) | NS | NS |
| Frequent urination | 0 (0) | 1 (2.5) | 0 (0) | NS | NS |
| Fatigue | 0 (0) | 1 (2.5) | 0 (0) | NS | NS |
| Dry mouth | 0 (0) | 0 (0) | 1 (2.8) | NS | NS |
| Lucid dreams | 0 (0) | 1 (2.5) | 0 (0) | NS | NS |
| Vaginal discharge | 0 (0) | 1 (2.5) | 0 (0) | NS | NS |
| Ear ache | 1 (3.3) | 0 (0) | 0 (0) | NS | NS |
| Cough | 1 (3.3) | 0 (0) | 0 (0) | NS | NS |
| Erectile dysfunction | 0 (0) | 1 (2.5) | 0 (0) | NS | NS |
| Back pain | 1 (3.3) | 0 (0) | 0 (0) | NS | NS |
Cannabis Use
Table 3 presents the beta estimates of the GEE model for the probability of achieving abstinence over the course of the trial for each treatment condition, adjusting for baseline severity. The results indicated no significant effect of treatment (χ2(1) = 1.08, p= 0.58), suggesting that the probability of abstinence (i.e. three consecutive weeks of no cannabis use) did not differ among the three treatment groups. However, time (χ2(1) = 9.96, p= 0.002) and baseline cannabis use (χ2(2) = 4.05, p=0.04) were found to have significant effects on the probability of achieving abstinence during the trial.a Similar effects were demonstrated when abstinence designation was based on urine toxicology or self-report alone. To assess if medication adherence influenced the effects of treatment outcome, identical models were tested after accounting for medication adherence. The results of these analyses demonstrated no significant effects for treatment condition or a time by treatment interaction. Table 3 presents changes in all of the outcome measures by treatment condition.
Table 3.
Regression (GEE) estimates for abstinence (i.e. three consecutive weeks of no cannabis use) during the medication trial
| Effect | β estimate (SE) | p-value |
|---|---|---|
| Intercept | −3.27 (0.78) | <0.001 |
| Treatment* | ||
| Bupropion | −0.31 (0.53) | 0.567 |
| Nefazodone | −0.60 (0.54) | 0.272 |
| Week** | 0.22 (0.07) | 0.001 |
| Baseline use*** | 0.75 (0.41) | 0.067 |
Treatment is bupropion or nefazodone relative to placebo.
Week corresponds to medication weeks (3–10).
Baseline use is dichotomized into high use vs low use, defined as 30 days or less than 30 days of cannabis use in the month prior to lead-in.
Cannabis Dependence Severity
Results indicated no significant effect of treatment on clinician rated cannabis dependence severity (CGI) scores (F2,90 = 2.04, p=0.14). However, there was a trend baseline severity by treatment interaction (F2,90 = 2.67, p=0.07). This interaction indicated that participants rated as having more severe cannabis dependence symptoms at baseline were more likely to demonstrate a reduction in symptom severity in the BPR-SR and NEF treatment compared to PBO. However, overall severity ratings remained greater in the NEF and BPR-SR groups relative to placebo over the course of the trial.
Results of analyses for clinician rated improvement in cannabis dependence (CGI improvement ratings) indicated no significant effect of treatment (F2,96 = 0.04, p=0.96) or a time by treatment interaction. There was a significant effect of time (F1,491 = 12.42, p=0.0005) indicating the severity of cannabis dependence symptoms was rated as decreasing over the course of treatment for all three treatment groups. At the last observed week, 64% (n=18) of PBO, 54% (n=20) of BPR, and 57% (n=19) of NEF scored less than 3 indicating clinical improvement.
Cannabis Withdrawal
Results of the analysis for sleep difficulty indicated that there was no treatment effect but a significant effect of time (χ2(1) = 5.87, p=0.02) and baseline levels of sleep disturbance (χ2(1) = 5.23, p=0.02). This suggests that overall difficulty falling asleep lessened over time for participants in all treatment conditions. Quantitative results of analysis for difficulty falling asleep (i.e. number of hours it took to fall asleep) indicated no significant effects of treatment (χ2(2) = 5.07, ; p=0.08) or time (χ2(1) = 0.03, ; p=0.87). In terms of sleep disturbances (measured as number of times a patient woke up in his sleep), results indicated no significant effects of treatment (χ2(2) = 1.66,,p=0.44) and time(χ2(1) = 2.66, p=0.10).
Results of the analyses for irritability indicated no significant effects of treatment (F2,82 = 0.55, p=0.58), time(F1,250 = 0.78, p=0.38), or a time by treatment interaction for outward irritability. Similarly, there was no significant effect of treatment (F2,82 = 0.36, p=0.70) or a time by treatment interaction on inward irritability. However, a significant of effect of time (F1,252 = 8.07, p=0.01) was demonstrated indicating inward irritability decreased as the length of treatment increased for all three treatment groups.
Results of the analyses for anxiety symptoms (HAM-A) indicated no significant effects of treatment (F2,81 = 1.16, p=0.32), time (F1.230 = 0.01, p=0.92), or a time by treatment interaction.
Discussion
This study was designed to assess the efficacy of NEF and BPR-SR for the treatment of cannabis dependence. The medications were tolerated fairly well and the side-effect profiles were favorable and consistent with those documented for these medications. The probability of abstinence increased during treatment for all three treatment groups. There was no evidence that either medication was superior to placebo for facilitating abstinence or for reducing the severity of targeted withdrawal symptoms. Participants presenting to treatment with more severe cannabis dependence symptoms demonstrated a greater reduction in dependence severity during treatment independent of the type of medication they received. However, there was a suggestive trend that medications performed relatively worse among participants with a less severe clinical presentation prior to beginning treatment. Overall, the results of this study do not support the use of either NEF or BPR-SR for promoting abstinence or for reducing cannabis withdrawal syndrome symptoms among individuals seeking treatment for cannabis dependence.
The present results are consistent with laboratory investigations demonstrating that BPR-SR and NEF have minimal effects on the cannabis withdrawal symptoms of sleep disturbances and irritability. This study extended previous results by demonstrating these findings in a cannabis-dependent treatment population exposed to a wider range of medication dosages over a longer period of time. In contrast to previous laboratory investigations, NEF demonstrated limited efficacy in reducing anxiety in this outpatient sample.29,36 The discrepancy in results may be attributable to the different environments in which the medication effects were observed and/or how anxiety was assessed. Participants in the previous laboratory studies stayed in a residential unit and smoked placebo cannabis during the experimental phase in order to induce cannabis withdrawal.36 This procedure guaranteed participants achieved a period of abstinence which increased the probability of experiencing withdrawal symptoms. This clinical trial had less control over abstinence induction and it was a minority of participants who reported maintaining a complete week of abstinence. The continued use of cannabis by a significant proportion of participants may have diminished the severity of withdrawal symptoms experienced thus reducing the therapeutic potential of NEF in this trial. Since NEF has demonstrated minimal impact on the intoxicating effects of cannabis36 it may offer limited therapeutic potential during periods of active cannabis use. In addition, anxiety was assessed across a number of affective and behavior symptoms in this study rather that as a single item measure employed in previous laboratory designs. Variability in measurement may have contributed to differences between this study and previous investigations.
The probability of cannabis abstinence increased over the course of the trial for all three medication groups. The general trend of improvement among participants during treatment parallels the findings of previous treatment studies. Specifically, participants demonstrated a reduction in cannabis use and improvement in dependence symptoms in the context of a cannabis-specific coping-skills based intervention, although a significant proportion of participants did not achieve complete abstinence. While the present findings suggest participating in a formal treatment program for cannabis dependence can promote clinical change, there continues to be room for improvement. A notable challenge, particularly in the context of pharmacotherapy investigations, is how psychosocial interventions can facilitate greater rates of abstinence. Voucher incentives have been effective for improving clinical outcome among cannabis dependent participants receiving coping-skills based interventions, although overall abstinence rates remained low.17,55 Participants in the present study completed a treatment contract and the counseling program emphasized an abstinence goal. However, the program did not formally set a quit date nor did it employ a formal abstinence contract or employ voucher based incentives. These strategies have been employed in other effective treatment programs (smoking and CRA) and may have facilitated greater abstinence rates then demonstrated in the present study. Finding more effective strategies for facilitating abstinence from cannabis would be an important step in constructing a better platform for testing pharmacological interventions that target cannabis withdrawal symptoms in the context of an outpatient treatment study.
The findings of this trial are tempered by several limitations. First, a low retention rate and the small number of participants enrolled in each treatment arm may have impeded our ability to detect treatment differences. However, 62% (n=66) of the patients completed at least 6 weeks of treatment suggesting that there was adequate exposure to medication among those enrolled in the trial and the demonstrated group differences were of questionable clinical significance. Second, the low rates of abstinence demonstrated during the study may have reduced the therapeutic potential of the medications. Third, although participants reported being fairly adherent and there was a modest relationship between riboflavin tests and self-reported adherence, it would have been preferable if we had collected blood levels of the medications administered rather than rely on riboflavin fluorescence and self-report alone. Further, participants in the NEF treatment condition reported missing more medication doses than those in the placebo condition. Although there were no statistically significant differences in the frequency of reported side effects among the treatment conditions, the NEF condition reported almost twice as many total symptoms relative to the placebo condition. This may have been a contributing factor in the difference between self-reported adherence reduced the power to find a significant medication effect. Fourth, it remains unclear as to what is the best way to evaluate a reduction in cannabis use in clinical treatment trials targeting cannabis dependence. In other psychotherapy trials, self-reported use was the primary outcome variable.40 However, this may underestimate the extent of drug use, even when there are no clear negative consequences for reporting drug use. To date, there is no clearly established approach to evaluate urine metabolite data. This study defined a urine as positive if the concentration was >100 ng/ml.17 This is a reasonable cut-off given that urine tests can remain positive for weeks after last use, but will most likely drop below 100 ng/ml in heavy users after 2 weeks of no use.56 One approach is to use quantitative urine cannabis metabolite levels corrected for urine concentration by dividing by urine creatinine levels, however in the present study urine samples were not tested for creatinine, thus this approach could not be utilized.
Despite the limitations of this study, it is clear that there is a substantial group of adult cannabis-dependent individuals who are interested in treatment. Our experience during the screening process was that few prospective patients lost interest in treatment when it was explained that there would be a pharmacologic component. The rationale to study medications to determine if they would help promote abstinence by reducing withdrawal symptoms was accepted by patients as a reasonable approach. Most of the participants were heavy daily cannabis smokers suggesting that facilitating abstinence may need to be the focus of early treatment rather than relapse prevention. In addition, treatment retention needs to be improved in order to better assess medication efficacy. Our research group used a progressive reinforcement strategy that reinforced clinic attendance during an alcohol treatment trial which substantially increased the retention rate.57 This approach might also be useful for pharmacologic trials that target cannabis-dependent individuals. To date, there has been minimal work in the assessment of pharmacologic agents for cannabis-dependent individuals seeking treatment. Given the enormous need for effective treatments for cannabis dependence, more empirical research is warranted.
Table 4.
Baseline and end point means and standard deviations for primary and secondary outcome measures by treatment group.
| Placebo (N=30) | Nefazodone (N=36) | Bupropion (N=40) | Statistic* | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Baseline | Week 10 | Baseline | Week 10 | Baseline | Week 10 | ||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |||
| Abstinent (%) | - | 7 (70%) | - | 8 (50%) | - | 4 (31%) | X2=3.50 | 0.17 |
| Dependence Severity(CGI) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Severity Rating | 4.6 (0.8) | 2.3 (1.6) | 4.6 (0.9) | 2.5 (1.4) | 4.8 (1.0) | 2.7 (1.5) | F2,39=0.26 | 0.77 |
| Improvement Rating | - | 1.4 (0.7) | - | 1.9 (1.0) | - | 1.7 (0.9) | F2,39=1.42 | 0.25 |
| Withdrawal Symptoms | ||||||||
| Snaith Irritability Scale | ||||||||
| Inward Irritability | 3.4 (3.0) | 2.5 (3.4) | 3.4 (2.7) | 1.9 (1.5) | 4.3 (2.8) | 2.6 (3.1) | F2,35=0.28 | 0.75 |
| Outward Irritability | 4.0 (2.5) | 1.9 (1.9) | 4.5 (2.5) | 3.1 (1.9) | 4.6 (2.8) | 4.0 (2.1) | F2,35=3.25 | 0.05 |
| SMHSQ | ||||||||
| Difficulty Falling Asleep | 1.3 (0.6) | 1.1 (0.3) | 1.6 (0.9) | 1.0 (0) | 1.6 (0.7) | 1.5 (0.9) | F2,37=2.97 | 0.06 |
| Sleep Disturbances (# of times awakened) | 1.4 (1.2) | 1.3 (1.2) | 1.6 (1.6) | 1.1 (1.3) | 1.4 (1.7) | 1.0 (1.2) | F2,37=0.16 | 0.86 |
| HAM-A | 7.5 (6.6) | 4.8 (4.3) | 5.6 (4.8) | 2.4 (3.3) | 6.0 (4.9) | 3.9 (5.3) | F2,22=0.63 | 0.54 |
Chi-square statistic was performed for the binary primary outcome. Analysis of variance was performed to compare the mean Week 10 values for the continuous secondary outcomes
Acknowledgements
This research was supported by NIDA grants R01DA13191, K02 00465, K23DA021850. We want to thank the staff of the Substance Treatment and Research Service (STARS) of the New York State Psychiatric Institute. Dr. Levin is a past consultant for Shire Pharmaceuticals, OrthoMcNeil Pharmaceuticals and Eli Lily & Co. and has received grant support from UCB Pharma*,OrthoMcNeil,* Eli Lilly & Company (for medication).
*not current
References
- 1.NIDA. NIDA Research Reports. 2005 < www.nida.nih.gov/ResearchReports/Marijuana/>.
- 2.Stinson F, Ruan W, Pickering R, Grant B. Cannabis use disorders in the USA: prevalence, correlates and co-morbidity. Psychological Medicine. 2006;36(10):1447–1460. doi: 10.1017/S0033291706008361. [DOI] [PubMed] [Google Scholar]
- 3.Kalant H. Adverse effects of cannabis on health: an update of the literature since 1996. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2004;28:849–863. doi: 10.1016/j.pnpbp.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 4.Brook JS, Cohen P, Brook DW. Longitudinal study of co-occurring psychiatric disorders and substance use. Journal of American Academic Child and Adolescent Psychiatry. 1998;37(3):322–330. doi: 10.1097/00004583-199803000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Gruber AJ, Pope HG, Hudson JI, Yurgelun-Todd D. Attributes of long-term heavy cannabis users: a case-control study. Psychological Medicine. 2003;33(8):1415–1422. doi: 10.1017/s0033291703008560. [DOI] [PubMed] [Google Scholar]
- 6.Lynskey M, Hall W. The effects of adolescent cannabis use on educational attainment: a review. Addiction. 2000;95(11):1621–1630. doi: 10.1046/j.1360-0443.2000.951116213.x. [DOI] [PubMed] [Google Scholar]
- 7.Tashkin DP, Fligiel S, Wu TC, Gong H, Jr, Barbers RG, Coulson AH, et al. Effects of habitual use of marijuana and/or cocaine on the lung. NIDA Research Monograph. 1990;99:63–87. [PubMed] [Google Scholar]
- 8.ElSohly MA, Ross SA, Mehmedic Z, Arafat R, Yi B, Banahan BF., 3rd Potency trends of delta9-THC and other cannabinoids in confiscated marijuana from 1980–1997. Journal of Forensic Sciences. 2000;45(1):24–30. [PubMed] [Google Scholar]
- 9.Compton WM, Pringle B. Services research on adolescent drug treatment. Commentary on “The Cannabis Youth Treatment (CYT) Study: main findings from two randomized trials”. Journal of Substance Abuse Treatment. 2004;27(3):195–196. doi: 10.1016/j.jsat.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 10.SAMSHA. National Household Survey on Drug Abuse. 2004 < www.samsa.gov/oas/NHSDA/2004>>.
- 11.MTPRG Marijuana Treatment Project Research Group (MTPRG) Brief treatments for cannabis dependence: Findings from a randomized multi-site trial. Journal of Consulting and Clinical Psychology. 2004;72(3):455–466. doi: 10.1037/0022-006X.72.3.455. [DOI] [PubMed] [Google Scholar]
- 12.Litt MD, Kadden RM, Stephens RS. The Marijuana Group Treatment Research Group. Coping and Self-Efficacy in Marijuana Treatment: Results from the Marijuana Treatment Project. Journal of Consulting Clinical Psychology. 2005;73:1015–1025. doi: 10.1037/0022-006X.73.6.1015. [DOI] [PubMed] [Google Scholar]
- 13.Stephens RS, Roffman RA, Simpson EE. Treating adult marijuana dependence: a test of the relapse prevention model. Journal of Consulting and Clinical Psychology. 1994;62(1):92–99. doi: 10.1037//0022-006x.62.1.92. [DOI] [PubMed] [Google Scholar]
- 14.Stephens RS, Roffman RA, Curtin L. Comparison of extended versus brief treatments for marijuana use. Journal of Consulting and Clinical Psychology. 2000;68(5):898–908. [PubMed] [Google Scholar]
- 15.Project MATCH Research group. Matching alcoholism treatments to client heterogeneity: Project MATCH three-year drinking outcomes. Alcoholism: Clinical and Experimental Research. 1998;22:1300–1311. doi: 10.1111/j.1530-0277.1998.tb03912.x. [DOI] [PubMed] [Google Scholar]
- 16.Carroll KM. Relapse prevention as a psychosocial treatment: A review of controlled clinical trials. Experimental and Clinical Psychopharmacology. 1996;4:46–54. [Google Scholar]
- 17.Budney AJ, Higgins ST, Radonovich KJ, Novy PL. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. Journal of Consulting and Clinical Psychology. 2000;68(6):1051–1061. doi: 10.1037//0022-006x.68.6.1051. [DOI] [PubMed] [Google Scholar]
- 18.O'Malley SS, Kosten TR. Pharmacotherapy of Addictive Disorders. In: Miller WR, Carroll KM, editors. Rethinking substance abuse: What the science shows, and what we, should do about it. NY: Guilford Press; 2006. pp. 240–256. [Google Scholar]
- 19.Cornelius J, Salloum I, Haskett R. Fluoxetine versus placebo for the marijuana use of depressed alcohlics. Addictive. Behaviors. 1999;24:111–114. doi: 10.1016/s0306-4603(98)00050-1. [DOI] [PubMed] [Google Scholar]
- 20.McRae A, Budney A, Kathleen K. Treatment of marijuana dependence; a review of the literature. Journal of Substance Abuse Treatment. 2003;24(4):369–376. doi: 10.1016/s0740-5472(03)00041-2. [DOI] [PubMed] [Google Scholar]
- 21.Budney AJ, Hughes JR. The cannabis withdrawal syndrome. Current Opinions in Psychiatry. 2006;19(3):233–238. doi: 10.1097/01.yco.0000218592.00689.e5. [DOI] [PubMed] [Google Scholar]
- 22.Budney AJ, Hughes JR, Moore BA, Vandrey R. Review of the validity and significance of cannabis withdrawal syndrome. American Journal of Psychiatry. 2004;161(11):1967–1977. doi: 10.1176/appi.ajp.161.11.1967. [DOI] [PubMed] [Google Scholar]
- 23.Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following oral THC administration to humans. Psychopharmacology (Berl) 1999;141(4):385–394. doi: 10.1007/s002130050848. [DOI] [PubMed] [Google Scholar]
- 24.Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology (Berl) 1999;141(4):395–404. doi: 10.1007/s002130050849. [DOI] [PubMed] [Google Scholar]
- 25.Georgotas A, Zeidenberg P. Observations on the effects of four weeks of heavy marijuana smoking on group interaction and individual behavior. Comprehensive Psychiatry. 1979;20(5):427–432. doi: 10.1016/0010-440x(79)90027-0. [DOI] [PubMed] [Google Scholar]
- 26.Mendelson J. Marijuana withdrawal syndrome in a woman. American Journal of Psychiatry. 1984;141:1289–1290. doi: 10.1176/ajp.141.10.1289. [DOI] [PubMed] [Google Scholar]
- 27.Glassman A. Psychiatry and cigarettes. Archives of General Psychiatry. 1998;55(8):692–693. doi: 10.1001/archpsyc.55.8.692. [DOI] [PubMed] [Google Scholar]
- 28.Davidson J, Connor K. Bupropion sustained release: a therapeutic overview. J Clin Psychiatry. 1998;59(4):25–31. [PubMed] [Google Scholar]
- 29.Haney M, Ward AS, Comer SD, Hart CL, Foltin RW, Fischman MW. Bupropion SR worsens mood during marijuana withdrawal in humans. Psychopharmacology (Berl) 2001;155(2):171–179. doi: 10.1007/s002130000657. [DOI] [PubMed] [Google Scholar]
- 30.Levin F, Evans S, McDowell D, Brooks D, Nunes E. Bupropion treatment for cocaine abuse and adult attention-deficit/hyperactivity disorder. Journal of Addictive Disorders. 2002;21(2):1–16. doi: 10.1300/J069v21n02_01. [DOI] [PubMed] [Google Scholar]
- 31.Solhkah R, Wilens T, Daly J, Prince J, VanPatten S, Biederman J. Bupropion SR for the treatment of substance-abusing outpatient adolescents with attention-deficit/hyperactivity disorder and mood disorders. Journal of Child and Adolescent Psychopharmacology. 2005;15(5):777–786. doi: 10.1089/cap.2005.15.777. [DOI] [PubMed] [Google Scholar]
- 32.Elkashef A, Rawson R, Anderson A, Li S, Holmes T, Smith E, Chaing N, Kahn R, Vocci F, Ling W, Pearce V, McCann M, Campbell J, Gorodetzky C, Haning W, Cariton B, Mawhinney J, Weis D. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33(5):1162–1170. doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- 33.Mooney ME, Sofuoglu M. Bupropion for the treatment of nicotine withdrawal and craving. Expert Review of Neurotherapeutics. 2006;6(7):965–981. doi: 10.1586/14737175.6.7.965. [DOI] [PubMed] [Google Scholar]
- 34.Preskorn SH. Comparison of the tolerability of bupropion, fluoxetine, imipramine, nefazodone, paroxetine, sertraline, and venlafaxine. Journal of Clinical Psychiatry. 1995;56 Suppl 6:12–21. [PubMed] [Google Scholar]
- 35.Thase ME. Treatment of severe depression. Journal of Clinical Psychiatry. 2000;61 Suppl 1:17–25. [PubMed] [Google Scholar]
- 36.Haney M, Hart CL, Ward AS, Foltin RW. Nefazodone decreases anxiety during marijuana withdrawal in humans. Psychopharmacology (Berl) 2003;165(2):157–165. doi: 10.1007/s00213-002-1210-3. [DOI] [PubMed] [Google Scholar]
- 37.Passos S, Camacho L, Lopes C, dos Santos M. Nefazodone in out-patient treatment of inhaled cocaine dependence: a randomized double-blind placebo-controlled trial. Addiction. 2005;100(4):489–494. doi: 10.1111/j.1360-0443.2005.01041.x. [DOI] [PubMed] [Google Scholar]
- 38.Ciraulo D, Knapp C, Rotrosen J, Sarid-Segal O, Ciraulo A, LoCastro J, Greenblatt J, Leiderman D. Nefazodone treatment of cocaine-dependence with comorbid depressive symptoms. Addiction. 2005;100 suppl:23–31. doi: 10.1111/j.1360-0443.2005.00984.x. [DOI] [PubMed] [Google Scholar]
- 39.Levin FR, McDowell D, Evans SM, Nunes E, Akerele E, Donovan S, et al. Pharmacotherapy for marijuana dependence: a double-blind, placebo-controlled pilot study of divalproex sodium. American Journal of Addiction. 2004;13(1):21–32. doi: 10.1080/10550490490265280. [DOI] [PubMed] [Google Scholar]
- 40.Nordstrom B, Levin FR. Treatment of cannabis use disorders: A review of the literature. American Journal on Addictions. 2007;16(5):331–342. doi: 10.1080/10550490701525665. [DOI] [PubMed] [Google Scholar]
- 41.Hall S, Reus V, Munez R, Sees K, Humfleet G, Hartz D, Frederick S, Triffleman G. Cognitive behavior therapy in the treatment of cigarette smoking. Archives of General Psychiatry. 1998;55:683–690. doi: 10.1001/archpsyc.55.8.683. [DOI] [PubMed] [Google Scholar]
- 42.First MB, Spitzer RL, Gibbon M, William JBW. Structured Clinical Interview for DSM-IV Axis I Disorders- Patient Edition (SCID-I/P, Patient Verision 2.0) Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 43.APA. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition, Text Revision ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 44.Kadden R, Carroll K, Donovan D, Cooney N, Monti P, Abrams D, Litt M, Hester R. NIAAA Project Match Monograph Series. Volume 3. Rockville, MD: NIH; 1995. Cognitive-Behavioral Coping Skills Therapy Manual: A Clinical Research Guide for Therapists Treating Individuals with Alcohol Abuse and Dependence. [Google Scholar]
- 45.Carroll KM. Bethesda, MD: NIH; 2000. A Cognitive-Behavioral Approach: Treating Cocaine Addiction. [Google Scholar]
- 46.NIDA. NIDA Research Monograph 73. Washington, DC: US Govt Print Office; 1986. [Google Scholar]
- 47.Guy W. Rockville, MD: National Institute for Mental Health; 1976. ECDEU Assessment Manual for Psychopharmacology. [Google Scholar]
- 48.Ellis B, Johns M, Lancaster R, Raptopoulos P, Angelopoulos N, Priest R. The St. Mary’s Hospital sleep questionnaire: a study of reliability. Sleep. 1981;4:93–97. doi: 10.1093/sleep/4.1.93. [DOI] [PubMed] [Google Scholar]
- 49.Leigh T, Bird H, Hindmarch I, Constable P, Wright V. Factor analysis of the St Mary’s Hospital Sleep Questionnaire. Sleep. 1988;11:448–453. doi: 10.1093/sleep/11.5.448. [DOI] [PubMed] [Google Scholar]
- 50.Oyefeso A, Sedgwick P, Ghodse H. Subjective sleep-wake parameters in treatment-seeking opiate addicts. Drug and Alcohol Dependence. 1997;48(1):9–16. doi: 10.1016/s0376-8716(97)00097-5. [DOI] [PubMed] [Google Scholar]
- 51.McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, Wongtan T, White J. The nature, time course and severity of methamphetamine withdrawal. Addiction. 2005;100(9):1320–1329. doi: 10.1111/j.1360-0443.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- 52.Snaith R, Constantopoulos A, Jardine M, McGuffin P. A clinical scale for the self-assessment of irritability. Br J of Psychiatry. 1978;132:164–171. doi: 10.1192/bjp.132.2.164. [DOI] [PubMed] [Google Scholar]
- 53.Hamilton M. The assessment of anxiety states by rating. British Journal of Medical Psychology. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 54.Hamilton M. Diagnosis and rating of anxiety. British Journal of Psychiatry. 1969;3:76–79. [Google Scholar]
- 55.Kadden RM, Litt MD, Kabela-Cormier E, Petry NM. Abstinence rates following behavioral treatments for marijuana dependence. Addictive Behaviors. 2007;32:1220–1236. doi: 10.1016/j.addbeh.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hawks RL, Chiang CN. Examples of specific drug assays. NIDA Research Monograph. 1986;73:84–112. [PubMed] [Google Scholar]
- 57.Evans SM, Levin FR, Brooks DJ, Garawi F. Memantine treatment for alcohol dependence: Implementation of contingency management procedures to enhance clinical trials. Alcoholism: Clinical and Experimental Research. 2007;31(5):775–782. doi: 10.1111/j.1530-0277.2007.00360.x. [DOI] [PubMed] [Google Scholar]