CONSPECTUS
Chemotherapy can destroy tumors and arrest cancer progress. Unfortunately, severe side effects—treatment is usually a series of injections of highly toxic drugs—often restrict the frequency and size of dosages, much to the detriment of tumor inhibition. Most chemotherapeutic drugs have pharmacokinetic profiles with tremendous potential for improvement.
Water-soluble polymers offer the potential to increase drug circulation time, improve drug solubility, prolong drug residence time in a tumor, and reduce toxicity. Cytotoxic drugs that are covalently attached to water-soluble polymers via reversible linkages more effectively target tumor tissue than the drugs alone. Macromolecules passively target solid tumor tissue through a combination of reduced renal clearance and exploitation of the enhanced permeation and retention (EPR) effect, which prevails for fast-growing tumors.
Effective drug delivery involves a balance between (i) elimination of the polymeric drug conjugate from the bloodstream by the kidneys, liver, and other organs and (ii) movement of the drug out of the blood vasculature and into the tumor (that is, extravasation). Polymers are eliminated in the kidney by filtration through pores with a size comparable to the hydrodynamic diameter of the polymer; in contrast, the openings in the blood vessel structures that traverse tumors are an order of magnitude greater than the diameter of the polymer. Thus, features that may broadly be grouped as the “molecular architecture” of the polymer—such as its hydrodynamic volume (or molecular weight), molecular conformation, chain flexibility, branching, and location of the attached drug—can greatly impact elimination of the polymer from the body through the kidney but have a much smaller effect on the extravasation of the polymer into the tumor. Molecular architecture can in theory be adjusted to assert essentially independent control over elimination and extravasation. Understanding how molecular architecture affects passage of a polymer through a pore is therefore essential for designing polymer drug carriers that are effective in passively delivering a drug payload while conforming to the requirement that the polymers must eventually be eliminated from the body.
In this Account, we discuss examples from in vivo studies that demonstrate how polymer architectural features impact the renal filtration of a polymer as well as tumor penetration and tumor accumulation. In brief, features that inhibit passage of a polymer through a pore—such as higher molecular weight, decreased flexibility, and an increased number of polymer chain ends—help prevent elimination of the polymer by the kidneys and can improve blood circulation times and tumor accumulation, thus improving therapeutic effectiveness.
INTRODUCTION
Patients given chemotherapy to treat cancer are typically administered a cocktail of highly toxic drugs by intravenous injection. The treatment dose and frequency is limited due to severe side effects that include nausea, bone-marrow toxicity, increased susceptibility to illness, hair loss, and cardiotoxicity. These side effects restrict the amount of drug that can be used to treat the cancer, and often too little drug is administered to effectively inhibit growth of the cancer. These restrictive, toxic side effects and insufficient delivery of drug to the cancer are due to the inappropriate or sub-optimal pharmacokinetics that affect the vast majority of chemotherapeutic drugs.
Polymer-drug conjugates have been studied for three decades as a potential solution to overcome the limitations of current chemotherapy. In the 1970s, Ringsdorf and Kopeček described a model polymer-based drug carrier1,2 in which a soluble linear macromolecular backbone linked to a targeting moiety serves as a scaffold to carry a therapeutic molecule. The premise behind macromolecular drug delivery is that the pharmacokinetics of the macromolecule-drug conjugate will mimic that of the macromolecule alone, thus enabling better control of drug delivery and improved drug availability to the targeted tissue. Successes include PEGylated proteins (Oncaspar®, Neulasta®) in which poly(ethyleneglycol) (PEG) is attached to a therapeutic polypeptide to improve its circulation time.3 Similarly, the polymer coated liposomal delivery system DOXIL®, carrying the chemotherapeutic agent doxorubicin, is significantly less toxic than doxorubicin alone. In spite of these successes, there are no polymer-drug conjugates approved to treat cancer in the United States.3
While many reviews on “polymer therapeutics” detail advances made in this field3,4, this review will focus on relating the architectural features of soluble, non-interactive, monomolecular polymers and dendrimers5,6 drug carriers to their resultant pharmacokinetic and biodistribution properties and their ability to passively target tumors.7
WHAT HAPPENS TO SOLUBLE POLYMERS IN VIVO?
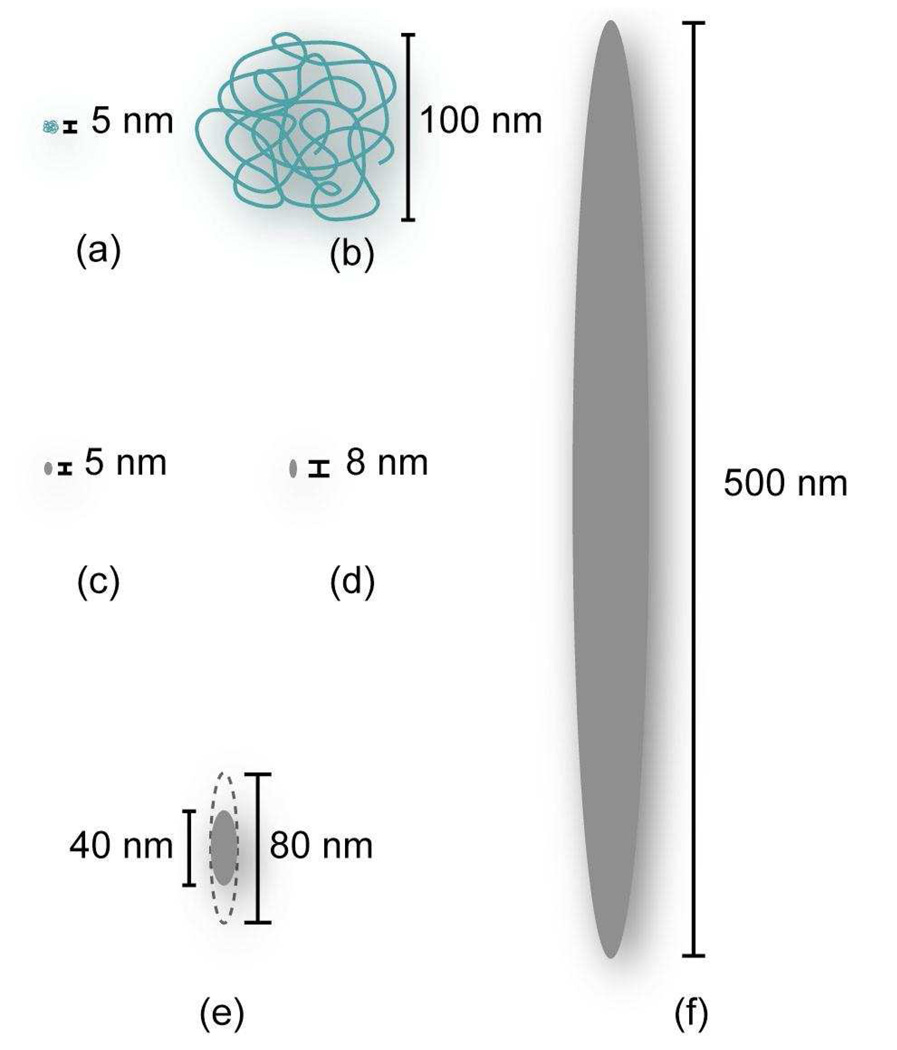
The size discrepancy between macromolecules and classical small drug molecules in solution leads to very different behaviors in vivo. The hydrodynamic diameter (Dh) of the macromolecules is of the same order of magnitude as that of the pores and openings present in the vasculature and elimination systems of the body (Figure 1). In vivo, polymer size has dramatic effects on the transport of polymers throughout the body, their removal by the reticuloendothelial system (RES), kidneys and intestines, and their interaction with extracellular matrix and cells within tissues. Furthermore, polymer features such as branching and conformation in solution can impact how they pass through these pores.
Figure 1.
Relative sizes of two different MW polymers and various pores in the body: (a) PVA chain with MW=13.5kDa22 (b) PVA chain with MW=580kDa22 (c) kidney glomerulus pore10 (d) very large interendothelial junction in healthy tissue13 (e) typical range of tumor pore diameters13 (f) very large interendothelial junction in cancerous tissue.
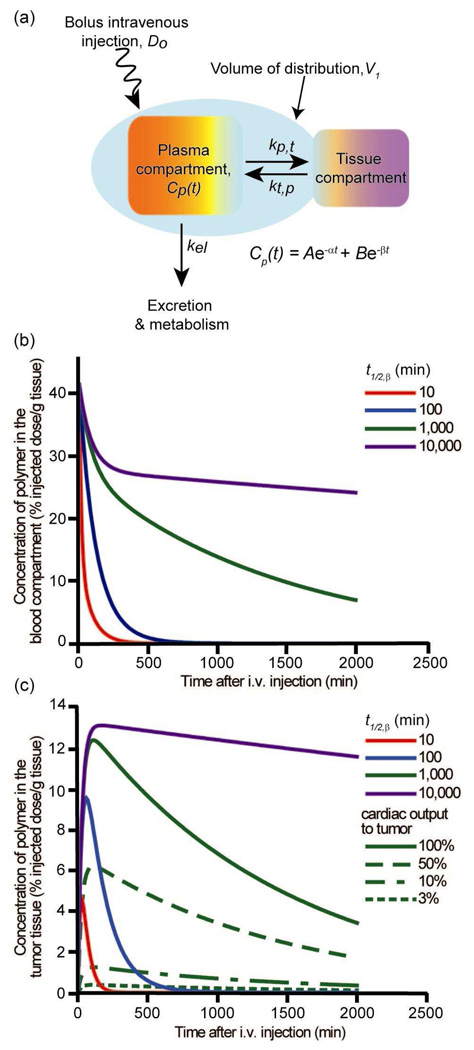
The majority of chemotherapeutic drugs are delivered by intravenous injection, allowing the drug to be administered directly to the blood for rapid presentation to the entire body. When a polymer-bound drug is administered via intravenous injection, the time-dependent concentration of polymer-drug conjugate in the blood, Cp(t), is typically described8 by the two-compartment model (Figure 2a) which assumes transport of the polymer through the blood compartment and the tissue compartment. Once administered by bolus injection (Do), polymer rapidly distributes itself through the blood compartment. The initial volume occupied is called the volume of distribution (V1), and for macromolecules it is often restricted to the blood compartment. From the blood compartment, the polymer can either leave the body by excretion and metabolism, described by the rate constant kel, or pass into the tissues, with a net rate constant (kp,t – kt,p). Cp(t) is described by a bi-exponential equation, with an initial rapid loss of polymer in the blood through distribution to the tissues, followed by a slower decrease in polymer in the blood as it is removed from the body (Figure 2b). The pharmacokinetic properties describing polymer behavior in the blood compartment can be calculated from the bi-exponential equation. One of the most important properties is the beta-phase blood circulation half-life, t1/2,β, which describes the long-term polymer concentration in the blood and removal of polymer from the body.
Figure 2.
Two-compartment model for predicting polymer concentration in the blood and in the tumor of a mouse (% injected dose/g tissue): (a) schematic representation of the two-compartment model. The concentration of polymer in the blood compartment is given by Cp(t), in which the variables A, B, α, and β are functions of Do, V1, and the rate constants; (b) calculated blood concentration curves versus time. The curves represent polymer concentration profiles for polymers with t1/2,β=10min, 100min, 1,000min, and 10,000min, respectively; (c) solid lines - calculated tumor concentration versus time for polymers with blood t1/2,β=10min, 100min, 1,000min, and 10,000 min, respectively, with 100% of cardiac output passing through the tumor; green lines – calculated tumor concentration versus time for a polymer with t1/2,β=1,000min with different fractions of cardiac output. All calculations assumed mouse with ~2.5g blood, and polymer with kb,t=0.012min−1, kt,b=0.025min−1, and kel=0.0006min−1.
Elimination of polymer from the body is typically through kidney clearance.9 As blood passes through the kidneys, the glomerulus filters out solutes, waste products, and excess water. The glomerular basement membrane and epithelial cell coat of the glomerulus consist of a matrix of collagen-like moieties and glycoproteins, which form pores approximately 4nm by 14nm in size. Macromolecules with hydrodynamic radii smaller than glomerular pores readily permeate the pores of the glomerulus, and are removed from the body via urine.10 The threshold for renal filtration of polymers corresponds very roughly with molecular weights ranging from 30kDa to 50kDa, depending on the polymer chemistry, shape, molecular conformation, and flexibility.3 Non-degradable polymers that are too large to be excreted can be retained in the body for extended periods of time.9
The liver and components of the RES can also remove polymer from the blood compartment. In a process called opsonization, opsonin proteins present in the blood bind to foreign particles to increase their recognizability to phagocytic cells. Aggregates or particles above 200nm in diameter are readily bound with opsonin proteins and cleared by the liver and spleen. Opsonin proteins tend to bind and clear hydrophobic or charged polymers more than hydrophilic and neutral polymers, which can accelerate their removal by the liver.11 Additionally, the RES can eliminate polymers indirectly by degradation; this is particularly true for polymers containing peptide or ester linkages.
A polymer cleared from the blood by the tissues passes through the intercellular junctions in the post-capillary fenestrae and discontinuous capillaries in the tissue vasculature. In healthy tissue, the primary mechanism of transport into the interstitium is diffusion through small gaps in the endothelial wall. The size of these gaps is 2–6nm, although additional barriers to transport through the gaps, such as the glycoprotein coat on endothelial cells, exist. In healthy tissue, polymer that is present extracellularly in the interstitium can be removed by the lymphatic system, which returns the polymer to the blood compartment.12
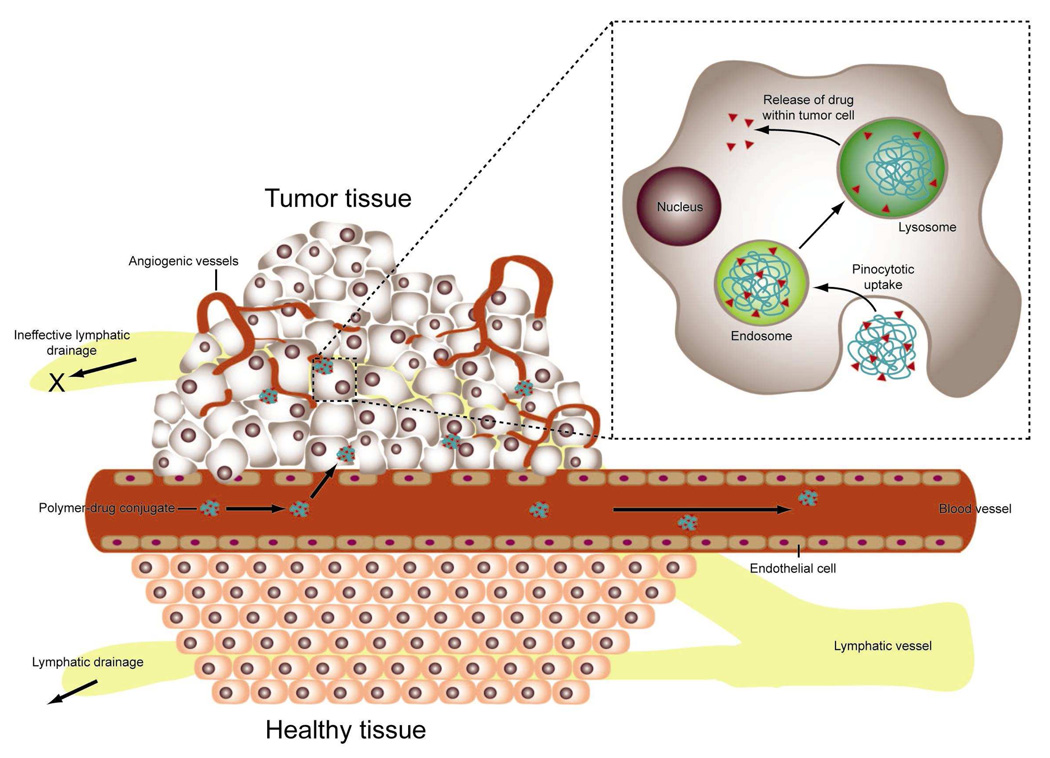
Passage of a polymer into solid tumor tissue and its retention is different than for healthy tissue, due in part to the larger pore size present in tumor vasculature (Figure 1). Pores in the tumor vasculature are believed to typically range in diameter from 40–80nm, however they can be as large as 1µm across.13 This phenomenon called the EPR effect (Figure 3) and the critical feature that allows for passively targeting macromolecules, was first identified by Matsumura and Maeda.14 While studying a polymer conjugate of the anticancer protein neocarzinostatin, they discovered that macromolecules accumulate in tumor tissue at higher levels than in healthy tissue. Due to the rapid and haphazard neovascularization of tumors, tumor vasculature is “leaky” and hence more permeable to macromolecules than the vasculature of healthy tissue. Additionally, in the vicinity of rapidly growing tumors, the lymphatic system is defective or nonexistent, thus eliminating removal of macromolecules through this route.12,14 It has also been observed that the interstitial volume in tumors is larger than the interstitial volume of most healthy tissue. Jain15 suggested that this allows for increased uptake of macromolecules, as there is more “free-fluid” space. On the other hand, Noguchi et al. proposed that the EPR effect results primarily from the prolonged retention of macromolecules.16 Using radiolabeled poly(N-(2-hydroxypropyl)methacrylamide) (HPMA) of different molecular weights (MW), ranging from 4.5 to 800kDa, they observed all polymers were taken-up into tumor tissue at approximately the same rate. However, higher MW polymers were unable to diffuse out of the tissue through the vasculature as rapidly as smaller polymers. Furthermore, the longer blood t1/2,β of polymers resulted in higher accumulation in tumor tissue than in healthy tissue16 (Figure 2c). Another important factor that impacts the total amount of polymeric drug in the tumor is the fraction of circulating blood that passes through the tumor vasculature, the “cardiac output” (Figure 2c). Today, the EPR effect has been observed in many experimental and human tumor models, and is exploited as a mechanism to passively target polymers to solid tumors.12
Figure 3.
Schematic representation of the EPR effect: passive targeting to tumor tissue is achieved by extravasation of polymers through the increased permeability of the tumor vasculature and ineffective lymphatic drainage. Passively-targeted polymer-drug conjugates are taken up by cancer cells through pinocytosis, and processed by endosomes and lysosomes (Inset). Adapted from ref. 48.
Polymer molecules in the interstitial space of healthy tissue and tumor tissue can be taken-up by cells via endocytosis at rates that are dependent on the affinity of the polymer for the cell surface. Most inert, synthetic polymers are thought to be taken-up by fluid-phase pinocytosis.9 Once in the cell, polymers are trafficked to late endosomes and lysosomes for degradation (Figure 3, inset). However, many synthetic polymers may not be degraded by the lysosomal environment and instead accumulate in vesicles within the cell.9 Although not well understood, this accumulation could be significant and result in toxicity over time.
CONTROLLING DELIVERY WITH ARCHITECTURE
For effective delivery of chemotherapeutic agents to solid tumors, properties of a polymer, such as MW, branching, molecular conformation, and flexibility that affect its passage through a pore must be considered. These properties can greatly impact elimination of the polymer from the body by the kidneys, and to a lesser extent, affect extravasation of polymer-drug conjugate by the tumor and penetration into the tumor.
Above all – size matters: molecular weight and hydrodynamic volume
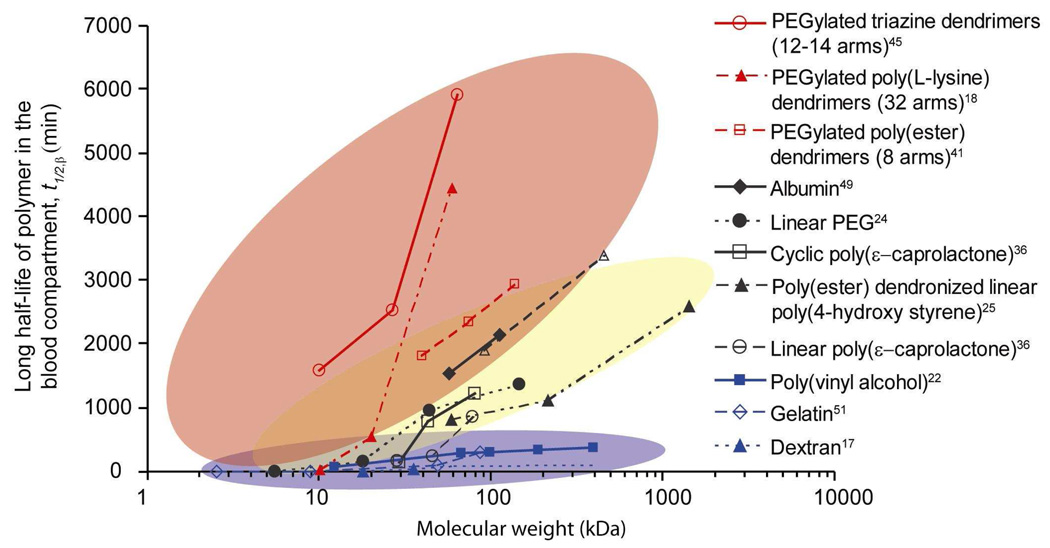
The hydrodynamic volume (Vh), and thus MW, of a polymer plays a critical role in polymer pharmacokinetics and biodistribution. Once the hydrodynamic radius (Rh) of a polymer exceeds the renal threshold of 5nm, the filtration rate of macromolecules decreases with increasing Rh, as long as other factors, such as molecular conformation and chemistry, remain constant.10 Thus the blood circulation t1/2,β increases with increasing MW as a result of decreased filtration by the kidneys. This relationship has been studied by many groups, and a representative sample of results for different biocompatible polymers in mouse models are detailed in Figure 4. As can be seen, the relationship between t1/2,β is typically non-linear, and the change in t1/2,β with MW can vary dramatically depending on both chemistry and architecture.
Figure 4.
Effect of MW on blood circulation half-life of intravenously injected polymers for a variety of polymer chemistries and architectures. Red background encompasses polymers with branched and/or globular structures. Yellow background encompasses globular and well-solvated random coil polymers. Blue background encompasses linear polymers that are rapidly cleared from the body. Readers are cautioned to note that the figure is based on reported MW, which does not always scale with actual polymer Vh. Pharmacokinetic experiments for the PEGylated poly(L-lysine) dendrimers and albumin were conducted in normal, healthy rats. The pharmacokinetic data of PVA was collected in tumored mice, however the authors noted that the data was not significantly different from data collected in non-tumored mice. All other data sets were collected from normal, healthy mice.
For example, very large changes in MW of dextran polymers result in very small changes in the blood circulation half-life. Studies of dextran show that above the renal clearance threshold, dextran accumulates and is retained significantly in the liver where enzymes degrade it down to segments less than 40kDa.17 It should be noted that the positive relationship between t1/2,β and MW holds true for polymers that have the same chemistry and structural features such as branching and specific molecular conformations. For example, Kaminskas et al. studied in rats the pharmacokinetics of generation three PEGylated poly(L-lysine) dendrimers with 32 PEG branches by varying the weight of the PEG chains to change the total MW.18 The beta-phase half-lives of the PEGylated dendrimers were reported as 0.7 ± 0.1h, 9.5 ± 0.3h, and 75.4 ± 9.3h for total “molecular weights” of 11.1kDa, 22.4kDa, and 68kDa, respectively, as measured by reverse phase HPLC/MS - a method known to significantly underestimate the MWs of dendritic or branched macromolecules.19 The same authors18 reported that the renal clearance of their PEGylated dendrimers was higher for lower total MW and t1/2,β. However, it should be noted that the trend of increasing blood circulation with increasing MW changes at higher MWs.3 Opsonin proteins and other components of the RES can remove polymers with Dhs of 200nm or larger from blood circulation.11 In short, for polymers with the same chemistry, architecture, and molecular conformation, t1/2,β increases with MW up to the point at which the polymers can be recognized by the RES.
With regards to tumor accumulation, there is also a relationship between polymer MW and tumor uptake. Initially, the concentration of polymer in the tumor increases with MW for polymers above the renal threshold. As polymers circulate longer in the blood compartment, exposure of the tumor vasculature to the polymer increases and tumor uptake increases. However, depending on the tumor type, vasculature, and polymer characteristics, this relationship can level off or even reverse. Murakami et al. studied the relationship between tumor accumulation and MW using different MW PEG in Meth-A fibrosarcoma tumors.20 For six different MW polymers ranging from 31 to ca. 200kDa, they observed an increase in tumor accumulation with an increase in MW. However, for polymers with MW greater than 215kDa, they saw a decrease in tumor accumulation. Seymour et al.21 measured higher levels of accumulation of 297kDa and 556kDa HPMA in Sarcoma 180 tumors than accumulation of 778kDa HPMA. While studying the pharmacokinetics and tumor accumulation of poly(vinyl alcohol) (PVA), Tabata et al. observed an increase in the tumor accumulation after three hours for polymers having a Dh of up to 60nm; above this diameter, accumulation levels decreased.22
One explanation for the reversal of the MW-tumor accumulation relationship at higher MWs is analogous to polymer filtration by the kidneys; the ability of a polymer to pass into the tumor tissue decreases because the polymer Dh is greater than many of the openings in the tumor vasculature walls. Transendothelial openings in fenestrated capillaries of tumor tissue are 40–80nm in diameter, although interendothelial junctions in the discontinuous capillaries can be up to 1µm in size, albeit infrequently.13 Studies of the tumor accumulation of liposomes have shown maximum accumulation to occur with liposomes 100nm in diameter.23 A second explanation for a decrease in tumor accumulation is that hyaluronan, collagen fibers, and other glycoproteins in the interstitial spaces of tumors inhibit the diffusion of higher MW polymers into the tumor.13 Molecules with Dhs larger than the gaps in the tumor endothelial wall and glycoprotein coat will be excluded to a greater extent. It is also known that the interstitial pressure in tumor tissue is higher in the interior than at the periphery, and while convective flow out of the tumor has been suggested but never directly measured, the outward driving force of pressure may decrease the ability of larger polymers to pass into the tumor tissue.13,15 Thus, accumulation in the tumor initially increases with increasing MW, however, this trend can reverse at very high MWs. It should be noted that for polymers above the renal filtration limit, accumulation in healthy tissues can also occur. In particular, multiple researchers have observed increased accumulation in the liver with higher MW polymers.21,22,24,25
Once again it must be emphasized that the MWs reported in many research articles are frequently not accurate or representative of the polymer’s Vh.26 Therefore, when reading any article, readers should carefully consider how the “MW” was determined before drawing conclusions on issues of Vh relative to other polymers.
Molecular conformation and flexibility
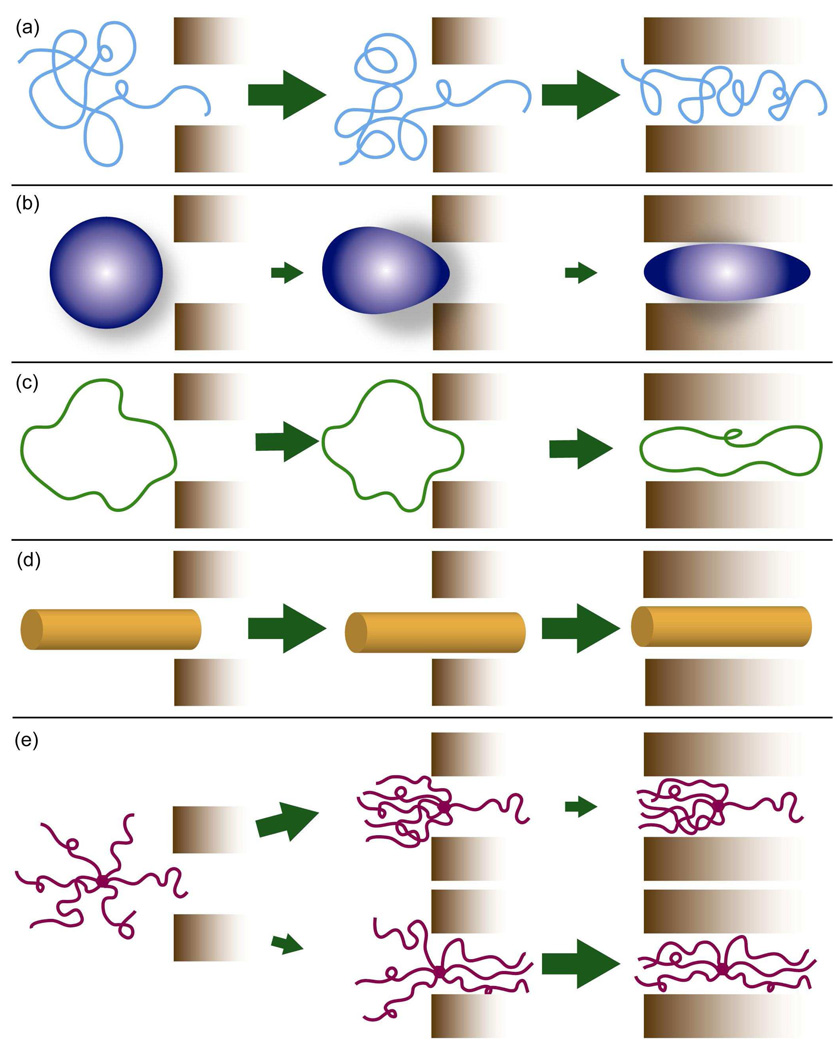
Despite potential minor shortcomings, Figure 4 reveals that the spread of the blood circulation t1/2,β vs. reported MW data can be roughly divided into three areas on the plot, highlighted by red, yellow, and blue backgrounds. Each area can be related to a molecular “shape” of the polymers within it, with red encompassing the PEGylated dendrimers and branched polymers, yellow encompassing globular polymers and well-solvated random coil polymers, and blue encompassing linear polymers rapidly cleared by the liver. The molecular conformation, or “shape” of the macromolecule, can have a significant effect on the pharmacokinetics, and can also impact tumor accumulation and penetration. Examples of a few polymer drug carriers discussed in this review and their representative molecular “shape” in solution are illustrated in Figure 5. In addition to shape, flexibility of the polymer plays a significant role. Figure 6 depicts various possible polymer conformations with approximately the same Vh and the impact of conformation and flexibility on pore penetration. As suggested in Figure 6a, a flexible, loosely coiled polymer could readily deform to pass through a pore. On the other hand, proteins, star and hyperbranched polymers, dendrimers, and poorly solvated polymers, which collapse upon themselves, adopt more rigid conformations. They are denser due to sterics or favorable intramolecular interactions and thus less capable of deformation to pass through a pore (Figure 6b and 6e). Rigid, elongated polymers can also be made depending on the choice of backbone, or through side chains that force the backbone into a rigid conformation (Figure 6d).27,28,29 Cyclic polymers (Figure 6c) constitute another polymer “shape”, and their solution behavior is changed by the lack of a chain end. The theory and methods of characterizing polymer size and shape is detailed elsewhere.26,30
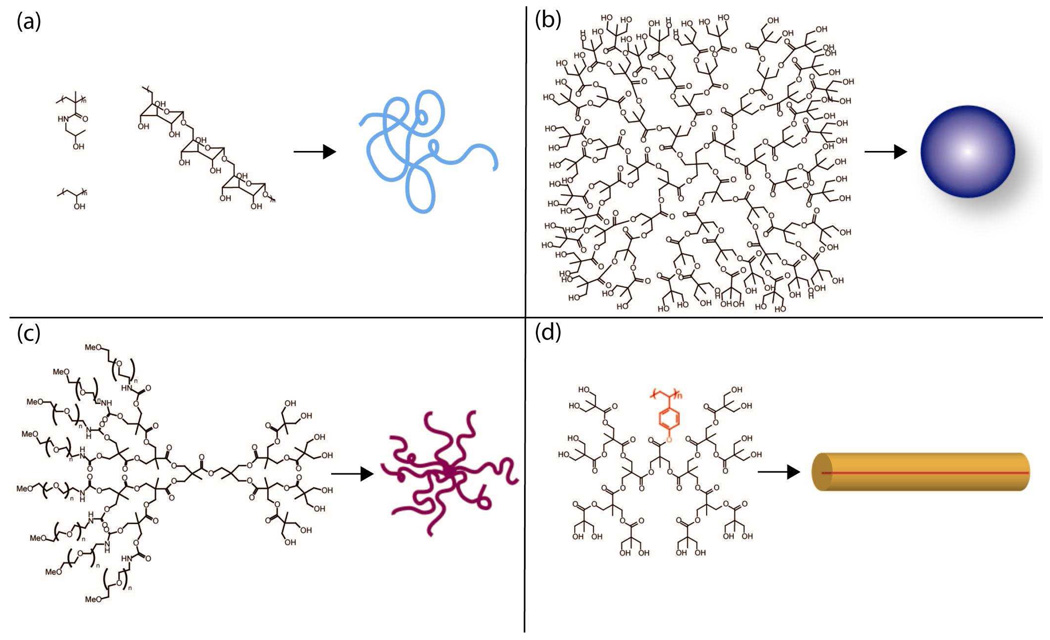
Figure 5.
Structures of polymer drug carriers, and their molecular conformation in solution: (a) linear polymers (HMPA, PVA, dextran) with a loose random coil conformation; (b) Poly(ester) dendrimer39 with pentaerythritol core (c) PEGylated poly(ester) dendrimers form “star-like” structures with many long arms.40 (d) Poly(ester) dendronized linear poly(4-hydroxystyrene) forms a tubular “rigid rod” because the backbone is elongated due to the steric requirements of the dendritic branches emanating from the core.27
Figure 6.
Polymer architecture and the passage of a polymer through a pore for polymers with approximately equivalent Vh: (a) linear random coil polymer readily penetrates and reptates through a pore; (b) polymer with a rigid, globular conformation must deform to pass through; beyond threshold MW both entry and passage through the pore could be difficult; (c) a cyclic polymer lacks a chain end for entering the pore and must deform to enter and pass through it; (d) polymer with a rigid, elongated or tubular conformation easily enters and passes through; (e) arm orientation and distance between chain-ends of branched polymers impact the rate of entry and passage through a pore. While initial entry of only one chain-end (top) may occur rapidly, passage of the entire polymer through the pore is sterically hindered, as the remaining arms must deform for the polymer to pass through;47 a symmetric conformation (bottom) is less likely since multiple chain ends must penetrate the pore at the same time. Once entry has been achieved passage of the polymer would be less hindered than for the asymmetric distribution.
The shape and ability of a macromolecule to deform appear to impact its rate of glomerular filtration and thus blood circulation half-life. Multiple studies have been completed comparing the renal filtration rates of neutral or uncharged plasma proteins and polysaccharides. Therefore, the filtration rate of dextran, with a slightly ellipsoidal random coil conformation, is higher than that of globular proteins with equivalent hydrodynamic radii such as horseradish peroxidase and ficoll.31,32,33 A study of four differently shaped molecules with similar Stokes-Einstein radii – albumin, ficoll, hyaluronan, and bikunin, also concluded that an elongated shape facilitated glomerular filtration.34 Venturoli and Rippe have suggested that the flexibility, and hence molecular deformability, of a macromolecule plays a crucial role in the rate at which it permeates the glomerulus.35 Their measurements showed that glomerular permeability was significantly higher when measured with flexible molecules such as dextran and ficoll than when measured with globular proteins. However, systematic studies comparing the blood circulation half-lives of flexible or elongated polymers with flexible, spherical polymers of similar chemistry have not been completed.
Cyclic polymers also have longer blood circulation in comparison to equivalent MW linear polymers. Nasongkla et al.36 recently studied a family of linear and cyclic PEGylated poly(ε-caprolactone). The cyclic polymers had significantly longer blood circulation times than equivalent MW linear polymers as a result of both the lack of a polymer chain end and an increased resistance to deformation.37 (Figure 6c), which hinders reptation through pores of the kidney.
The importance of both “shape” and flexibility is exemplified when examining tumor uptake of rigid and elongated polymers. Polymers with less flexible, elongated conformations, or “rigid rod” conformations, have shown higher tumor accumulation than more flexible, elongated polymers. A study of polylysine conjugated with the coupling agent DTPA-Gd29,38 showed that at conjugation levels ≥90% the polymer assumed an extended “rigid rod” conformation, leading to significantly higher signal enhancement than observed with randomly coiled, lesser conjugated polymer and albumin-GTPA-Gd controls. In addition, a larger signal was observed in the interior of the tumor than at its periphery, suggesting that the extended polymer was able to penetrate through the tumor interstitium. High tumor accumulation of a rigid rod polymer was also observed in our laboratories25 in the study of the pharmacokinetics and biodistribution of dendronized linear poly(4-hydroxystyrene), with some of the highest published values for tumor accumulation in the murine C26 colon carcinoma tumor. Various in vitro and in vivo studies have shown greater diffusion coefficients for linear macromolecules than for globular macromolecules with equivalent Stokes-Einstein radii.15 It has been suggested that the extended conformation of rigid rod polymers enables them to more easily reptate through openings in the tumor vasculature and through the tumor extracellular matrix, since they are less likely to entangle with the extracellular matrix.38
Branching leads to drastic increases in blood circulation time
Branched polymers provide a definitive example of the role of flexibility in blood circulation time and glomerular permeability. For polymers with similar MW and chemistry, increasing the number of branches or arms increases the blood circulation half-life. Work from our laboratories39 demonstrated that dendrimers under 20kDa were rapidly removed from the body – a finding that correlates with their low molecular weight. A subsequent study with a library of PEGylated polyester “bow-tie” dendrimers40 established the relationship between branching and blood circulation time.39,40,41 For a series of bow-ties with equivalent MW (~40kDa), there was an increase in t1/2,β from 1.4±0.4h for the two-arm dendrimer – essentially a linear polymer − to 26±6h for the four-arm dendrimer, and finally 31±2h for the eight-arm dendrimer.41 Corresponding biodistribution studies in healthy mice show no significant variation in tissue uptake between the three polymers and decreased polymer excreted in the urine with increased branching. Given that the blood circulation half-life increased with the number of arms while polymer excreted in the urine decreased, we believe that the slower rates of renal filtration for bow-ties with more arms resulted from a hindered ability to reptate through pores. This polymer drug carrier studied in C26 colon carcinoma-tumored mice showed long blood circulation times and remarkable efficacy in delivering the chemotherapeutic drug doxorubicin to tumors leading to their complete disappearance in sharp contrast to the free drug which, itself, was ineffective.42
In a related finding, Gerber and Radke43 have separated star and linear polymers by 2D-chromatography on the basis of their number of arms. Although their observations did not involve compounds with strictly-equivalent MW, Bowen et al. observed44 that proteins PEGylated with branched PEG chains also showed prolonged blood circulation half-lives and activities when compared to proteins PEGylated with a single PEG chain. This relationship between the number of branches and t1/2,β also applies when comparing branched polymers to linear polymers. Comparing PEGylated bow-tie dendrimers to similar MW linear PEG, the linear PEG has a shorter blood circulation time.24,41 In a somewhat bold comparison of three families18,41,45 of PEGylated dendrimers that did not possess either the same chemistry or the same branched architecture, Lim et al.45 recently suggested that the most important parameter for determining blood circulation t1/2,β of branched polymers was the number of arms and not MW, chemistry, or branched architecture. Comparing their PEGylated triazine dendrimers with the PEGylated bow-tie dendrimers, they observed an increase in blood circulation half-life with an increase in the number of PEG chains. However, the triazine dendrimers of Lim et al. may self-orient and aggregate in solution, and their Dhs should be measured before these dendrimers can be used to draw such a general conclusion.41,42
At first, the observation of increased t1/2,β with increased branching may appear unexpected since branched polymers have smaller hydrodynamic radii than linear polymers with equivalent MWs.26 A smaller Rh would be expected to result in an increased rate of renal filtration, as the radius of the macromolecule is closer in size to that of the glomerulus pore. Yet, as experimentally observed, this is not the case once a MW threshold is reached. The decreased renal filtration of branched molecules is supported by the work of de Gennes and co-workers46 who used viscosity measurements on polystyrene samples with different branching to show that increasing the number of polymer branches decreases the ability of a polymer to deform and elongate. Given that branching decreases the ability for star polymers to deform, they should be filtered less readily by the glomerulus and circulate in the blood compartment longer. An alternative explanation is that increased branching decreases the ability of a polymer to reptate through a pore.4,41 For branched polymers to pass through a pore, one or more arms must enter the pore and the remainder deform backward (Figure 6e) to enable passage, a requirement that becomes increasingly difficult as the number of arms increases. De Gennes has shown that for a branched polymer in a static solvent, the lowest energy barrier for entry into a pore with a diameter smaller than the polymer Dh occurs with a symmetric distribution of polymer arms,47 as depicted in the lower part of Figure 6e. As the number of arms increases, the energy barrier for entry increases. Thus, both experimental observation and theory suggest that beyond a threshold MW, branching decreases renal filtration and increases t1/2,β.
SUMMARY AND GUIDELINES
Effective chemotherapeutic drug delivery is a balance between clearance by the kidney, uptake by the tumor, and removal of the polymer from the bloodstream by the liver and RES. The key to improved passive targeting with mono-macromolecules lies in the understanding of how polymer architecture affects their passage through a pore. Although additional systematic studies are needed to definitively elucidate the relationship between polymer architectural features and the resultant pharmacokinetics and in vivo distribution, it seems that there are clear trends in the effect of size, molecular conformation in solution, flexibility, and branching on the in vivo behavior of macromolecular carriers.
Based on the limited number of quantitative studies available today, the following general guidelines are presented to future researchers for the control of polymer pharmacokinetics and biodistribution:
Increasing polymer molecular weight decreases renal filtration and increases blood circulation time and tumor accumulation.
Increasing the number of polymer arms decreases renal filtration due to decreased pore penetration, and increases blood circulation time.
Rigid, spherical polymers have decreased renal filtration and longer blood circulation time compared to flexible and elongated polymers.
More rigid, tubular polymers can achieve higher tumor accumulation due to increased pore penetration.
Nonetheless, the clinical usefulness of a polymer drug carrier is limited if the polymer persists in the tissues for long times after administration. Ideally, a polymer drug carrier will be removed from the body once it has delivered its drug payload, thus minimizing side effects from the polymer itself. Renal filtration is the primary mechanism in which polymers are removed from the body, therefore a general “rule of thumb” exists that polymers should be less than ~40kDa and small enough to be filtered by the kidneys.3 However, this limitation could be circumvented by a careful choice of molecular weight, flexibility, molecular conformation, and the incorporation of linkages that are cleavable by enzymes or hydrolysis, allowing for somewhat larger polymers that circulate in the body longer and penetrate tumors to a greater extent and quantity. It is clear that increasing our understanding of the impact of chemistry and architecture on polymer pharmacokinetics is essential for improving the design of polymer drug carriers and furthering their progress in clinical trials.
ACKNOWLEDGEMENTS
We thank the National Institute of Health for financial support (RO1 EB002047).
Biographies
Megan E. Fox received her B.S.E. in Chemical Engineering at Case Western Reserve University (2004). She is currently working towards her Ph.D. in Chemical Engineering at the University of California, Berkeley, under the guidance of Professor Jean Fréchet. Her research interests include dendritic polymer drug carriers for chemotherapy and polymer pharmacokinetics.
Francis C. Szoka received his M.S. (Microbiology) from the University of Maryland and his Ph.D. (Biochemistry) from the State University of New York (SUNY). His major research interests include applying chemical, biochemical, and biophysical approaches to the study of membrane fusion/destabilization and developing drug/gene delivery systems based upon defined physicochemical mechanisms of membrane destabilization and intracellular trafficking.
Jean M. J. Fréchet, the Henry Rapoport Chair of Organic Chemistry at the University of California, Berkeley, graduated from the Institut de Chimie et Physique Industrielles (now CPE) in Lyon, France, before completing Ph.D. studies in organic and polymer chemistry at SUNY and Syracuse University. His current research focuses on functional polymers from fundamental concepts to applications in areas as varied as therapeutics, catalysis, and energy. Additional details can be found at his research group’s webpage http://frechet.cchem.berkeley.edu.
References
- 1.Bader H, Ringsdorf H, Schmidt B. Water-Soluble Polymers in Medicine. Angew. Makromol. Chem. 1984;123/124:457–485. [Google Scholar]
- 2.Kopecek J. Soluble Biomedical Polymers. Polymers in Medicine. 1977;7:191–221. [PubMed] [Google Scholar]
- 3.Duncan R. The Dawning Era of Polymer Therapeutics. Nature Reviews Drug Discovery. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 4.Lee CC, Mackay JA, Fréchet JMJ, Szoka FC. Designing Dendrimers for Biological Applications. Nat. Biotechnol. 2005;23:1517–1526. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- 5.Liu MJ, Fréchet JMJ. Designing Dendrimers for Drug Delivery. Pharmaceutical Science & Technology Today. 1999;2:393–401. doi: 10.1016/s1461-5347(99)00203-5. [DOI] [PubMed] [Google Scholar]
- 6.Gillies ER, Fréchet JMJ. Dendrimers and Dendritic Polymers in Drug Delivery. Drug Discovery Today. 2005;10:35–43. doi: 10.1016/S1359-6446(04)03276-3. [DOI] [PubMed] [Google Scholar]
- 7.Grayson SM, Godbey WT. The Role of Macromolecular Architecture in Passively Targeted Polymeric Carriers for Drug and Gene Delivery. J. Drug Target. 2008;16:329–356. doi: 10.1080/10611860801969616. [DOI] [PubMed] [Google Scholar]
- 8.Welling PG. Pharmacokinetics: Processes, Mathematics, and Applications. Washington, D.C.: American Chemical Society; 1997. The Two-Compartment Open Model With Intravenous or Oral Administration, Chapter 17. [Google Scholar]
- 9.Duncan R. Soluble Synthetic Polymers As Potential Drug Carriers. Advances in Polymer Science. 1984;57:51–101. [Google Scholar]
- 10.Venkatachalam MA, Rennke HG. The Structual and Molecular Basis of Glomerular Filtration. Circ. Res. 1978;43:337–347. doi: 10.1161/01.res.43.3.337. [DOI] [PubMed] [Google Scholar]
- 11.Owens DE, III, Peppas NA. Opsonization, Biodistribution, and Pharmacokinetics of Polymeric Nanoparticles. Int. J. Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor Vascular Permeability and the EPR Effect in Macromolecular Therapeutics: a Review. J. Controlled Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 13.Jain RK. Transport of Molecules Across Tumor Vasculature. Cancer Metastasis Rev. 1987;6:559–593. doi: 10.1007/BF00047468. [DOI] [PubMed] [Google Scholar]
- 14.Matsumura Y, Maeda H. A New Concept for Macromolecular Therapeutics in Cancer-Chemotherapy - Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 15.Jain RK. Transport of Molecules in the Tumor Interstitium - A Review. Cancer Res. 1987;47:3039–3051. [PubMed] [Google Scholar]
- 16.Noguchi Y, Wu J, Duncan R, Strohalm J, Ulbrich K, Akaike T, Maeda H. Early Phase Tumor Accumulation of Macromolecules: a Great Difference in Clearance Rate Between Tumor and Normal Tissues. Jpn. J. Cancer Res. 1998;89:307–314. doi: 10.1111/j.1349-7006.1998.tb00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaneo Y, Uemura T, Tanaka T, Kanoh S. Polysaccharides As Drug Carriers: Biodisposition of Fluorescein-Labeled Dextrans in Mice. Biological & Pharmaceutical Bulletin. 1997;20:181–187. doi: 10.1248/bpb.20.181. [DOI] [PubMed] [Google Scholar]
- 18.Kaminskas LM, Boyd BJ, Karellas P, Krippner GY, Lessene R, Kelly B, Porter CJH. The Impact of Molecular Weight and PEG Chain Length on the Systemic Pharmacokinetics of PEGylated Poly L-Lysine Dendrimers. Mol. Pharmaceutics. 2008;5:449–463. doi: 10.1021/mp7001208. [DOI] [PubMed] [Google Scholar]
- 19.Hawker CJ, Fréchet JMJ. Preparation of Polymers With Controlled Molecular Architecture - A New Convergent Approach to Dendritic Macromolecules. J. Am. Chem. Soc. 1990;112:7638–7647. [Google Scholar]
- 20.Murakami Y, Tabata Y, Ikada Y. Tumor Accumulation of Poly(Ethylene Glycol) With Different Molecular Weights After Intravenous Injection. Drug Delivery. 1997;4:23–31. [Google Scholar]
- 21.Seymour LW, Miyamoto Y, Maeda H, Brereton M, Strohalm J, Ulbrich K, Duncan R. Influence of Molecular Weight on Passive Tumour Accumulation of a Soluble Marcomolecular Drug Carrier. Eur. J. Cancer. 1995;31A:766–770. doi: 10.1016/0959-8049(94)00514-6. [DOI] [PubMed] [Google Scholar]
- 22.Tabata T, Murakami Y, Ikada Y. Tumor Accumulation of Poly(Vinyl Alcohol) of Different Sizes After Intravenous Injection. J. Controlled Release. 1998;50:123–133. doi: 10.1016/s0168-3659(97)00129-6. [DOI] [PubMed] [Google Scholar]
- 23.Uchiyama K, Nagayasu Y, Yamagiwa T, Nishida H, Harashima H. Kiwada Effect of the Size and Fluidity of Liposomes on Their Accumulation in Tumors: A Presumption of Their Interaciton With Tumors. Int. J. Pharm. 1995;121:195–203. [Google Scholar]
- 24.Yamaoka T, Tabata Y, Ikada Y. Distribution and Tissue Uptake of Poly(Ethylene Glycol) With Different Molecular Weights After Intravenous Administration to Mice. J. Pharm. Sci. 1994;83:601–606. doi: 10.1002/jps.2600830432. [DOI] [PubMed] [Google Scholar]
- 25.Lee CC, Yoshida M, Fréchet JMJ, Dy EE, Szoka FC. In Vitro and in Vivo Evaluation of Hydrophilic Dendronized Linear Polymers. Bioconjug. Chem. 2005;16:535–541. doi: 10.1021/bc0497665. [DOI] [PubMed] [Google Scholar]
- 26.Munk P, Aminabhavi TM. Introduction to Macromolecular Science. New York City: John Wiley & Sons, Inc.; 2002. Macromolecules in Solutions: Hydrodynamics of Macromolecular Solutions, Chapter 3.3. [Google Scholar]
- 27.Lee CC, Fréchet JMJ. Synthesis and Conformations of Dendronized Poly(L-Lysine) Macromolecules. 2006;39:476–481. doi: 10.1021/ma052078b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Das J, Yoshida M, Fresco ZM, Choi TL, Fréchet JMJ, Chakraborty AKA. Dendronized Polymer Is a Single-Molecule Glass. J. Phys. Chem. B. 2005;109:6535–6543. doi: 10.1021/jp058081e. [DOI] [PubMed] [Google Scholar]
- 29.Uzgiris EE, Cline H, Moasser B, Grimmond B, Amaratunga M, Smith JF, Goddard G. Conformation and Structure of Polymeric Contrast Agents for Medical Imaging. Biomacromolecules. 2004;5:54–61. doi: 10.1021/bm034197+. [DOI] [PubMed] [Google Scholar]
- 30.Harding S. On the Hydrodynamic Analysis of Macromolecular Conformation. Biophys. Chem. 1995;55:69–93. doi: 10.1016/0301-4622(94)00143-8. [DOI] [PubMed] [Google Scholar]
- 31.Rennke HG, Venkatachalam MA. Glomerular Permeability of Macromolecules. J. Clin. Invest. 1979;63:713–717. doi: 10.1172/JCI109354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bohrer MP, Deen WM, Robertson CR, Troy JL, Brenner BM. Influence of Molecular-Configuration on the Passage of Macromolecules Across the Glomerular Capillary Wall. J. Gen. Physiol. 1979;74:583–593. doi: 10.1085/jgp.74.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asgeirsson D, Venturoli D, Fries E, Rippe B, Rippe C. Glomerular Sieving of Three Neutral Polysaccharides, Polyethylene Oxide and Bikunin in Rat. Effects of Molecular Size and Conformation. Acta Physiologica. 2007;191:237–246. doi: 10.1111/j.1748-1716.2007.01733.x. [DOI] [PubMed] [Google Scholar]
- 34.Ohlson M, Sorenson J, Lindstrom K, Blom AM, Fries E, Haraldsson B. Effects of Filtration Rate on the Glomerular Barrier and Clearance of Four Differently Shaped Molecules. American Journal of Physiology-Renal Physiology. 2001;281:F103–F113. doi: 10.1152/ajprenal.2001.281.1.F103. [DOI] [PubMed] [Google Scholar]
- 35.Venturoli D, Rippe B. Ficoll and Dextran Vs. Globular Proteins As Probes for Testing Glomerular Permselectivity: Effects of Molecular Size, Shape, Charge, and Deformability. American Journal of Physiology-Renal Physiology. 2005;288:F605–F613. doi: 10.1152/ajprenal.00171.2004. [DOI] [PubMed] [Google Scholar]
- 36.Nasongkla N, Chen B, Macaraeg N, Fox ME, Fréchet JMJ, Szoka FC. Dependence of Pharmacokinetics and Biodistribution on Polymer Architecture: Effect of Cyclic Versus Linear Polymers. J. Am. Chem. Soc. 2009;131:3842–3843. doi: 10.1021/ja900062u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burchard W. Theory of Cyclic Macromolecules, Chapter 2. In: Semlyen JA, editor. Cyclic Polymers. New York: Elsevier Applied Science Publishers; 1986. [Google Scholar]
- 38.Uzgiris E. The Role of Molecular Conformation on Tumor Uptake of Polymeric Contrast Agents. Invest. Radiol. 2004;39:131–137. doi: 10.1097/01.rli.0000107495.48025.97. [DOI] [PubMed] [Google Scholar]
- 39.De Jesus OLP, Ihre HR, Gagne L, Fréchet JMJ, Szoka FC. Polyester Dendritic Systems for Drug Delivery Applications: In Vitro and in Vivo Evaluation. Bioconjug. Chem. 2002;13:453–461. doi: 10.1021/bc010103m. [DOI] [PubMed] [Google Scholar]
- 40.Gillies ER, Fréchet JMJ. Designing Macromolecules for Therapeutic Applications: Polyester Dendrimer-Poly(Ethylene Oxide) "Bow-Tie" Hybrids With Tunable Molecular Weight and Architecture. J. Am. Chem. Soc. 2002;124:14137–14146. doi: 10.1021/ja028100n. [DOI] [PubMed] [Google Scholar]
- 41.Gillies ER, Dy EE, Fréchet JMJ, Szoka FC. Biological Evaluation of Polyester Dendrimer:Poly(Ethylene Oxide) "Bow-Tie" Hybrids With Tunable Molecular Weight and Architecture. Mol. Pharmaceutics. 2005;2:129–138. doi: 10.1021/mp049886u. [DOI] [PubMed] [Google Scholar]
- 42.Lee CC, Gillies E, Fox ME, Dy E, Fréchet JMJ, Szoka FC. A Single Dose of Bowtie-Conjugated Doxorubicin Cures Mice Tumored With C26 Colon Carcinoma. Proc. Natl. Acad. Sci. U. S. A. 2006;103:16649–16654. doi: 10.1073/pnas.0607705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gerber J, Radke W. Topological Separation of Linear and Star-Shaped Polystyrenes by Off-Line 2D Chromatography. Stars Having High Molar Mass Arms and Quantification of the Star Fraction. Polymer. 2005;46:9224–9229. [Google Scholar]
- 44.Bowen S, Tare N, Inoue T, Yamasaki M, Okabe M, Horii I, Eliason JF. Relationship Between Molecular Mass and Duration of Activity of Polyethylene Glycol Conjugated Granulocyte Colony-Stimulating Factor Mutein. Exp. Hematol. 1999;27:425–432. doi: 10.1016/s0301-472x(98)00051-4. [DOI] [PubMed] [Google Scholar]
- 45.Lim J, Guo Y, Rostollan CL, Stanfield J, Hsieh J, Sun X, Simanek EE. The Role of the Size and Number of Polyethylene Glycol Chains in the Biodistribution and Tumor Localization of Triazine Dendrimers. Mol. Pharmaceutics. 2008;5:540–547. doi: 10.1021/mp8000292. [DOI] [PubMed] [Google Scholar]
- 46.Dondos A, Papanagopoulos D, de Gennes PG, Brochard-Wyart FB. On the Deformation of Star Shaped Polystyrenes in Flowing Solutions. Macromolecular Theory and Simulations. 1999;8:147–150. [Google Scholar]
- 47.de Gennes PG. Flexible Polymers in Nanopores. Advances in Polymer Science. 1999;138:105. [Google Scholar]
- 48.Peer D, Karp JM, Hong S, FaroKHzad OC, Margalit R, Langer R. Nanocarriers As an Emerging Platform for Cancer Therapy. Nature Nanotechnology. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 49.Matsushita S, Chuang VTG, Kanazawa M, Tanase S, Kawai K, Maruyama T, Suenaga A, Otagiri M. Recombinant Human Serum Albumin Dimer Has High Blood Circulation Activity and Low Vascular Permeability in Comparison With Native Human Serum Albumin. Pharm. Res. 2006;23:882–891. doi: 10.1007/s11095-006-9933-1. [DOI] [PubMed] [Google Scholar]
- 50.Kainthan RK, Brooks DE. In Vivo Biological Evaluation of High Molecular Weight Hyperbranched Polyglycerols. Biomaterials. 2007;28:4779–4787. doi: 10.1016/j.biomaterials.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 51.Yamaoka T, Tabata Y, Ikada Y. Body Distribution of Intravenously Administered Gelatin With Different Molecular-Weights. J. Controlled Release. 1994;31:1–8. [Google Scholar]