Abstract
Objective
Cerebrospinal fluid (CSF) levels of Aβ peptide 1-42 (Aβ42), tau, and phosphorylated tau (ptau) are potential biomarkers of Alzheimer's disease (AD). We hypothesized that these biomarkers might predict the rate of cognitive change in individuals with very mild dementia of the Alzheimer type (DAT).
Design
Retrospective analysis of CSF biomarkers and clinical data.
Setting
An academic Alzheimer's Disease Research Center.
Participants
Research volunteers in a longitudinal study of aging and cognition. Participants (n=49) had a clinical diagnosis of very mild DAT with a Clinical Dementia Rating (CDR) of 0.5 at the time of lumbar puncture. All participants had at least one follow-up assessment (mean years of follow-up = 3.5 ± 1.8 years).
Main outcome measures
Baseline CSF levels of Aβ42, Aβ40, tau and tau phosphorylated at threonine 181 (ptau181), rate of dementia progression as measured by CDR-sum of boxes (CDR-SB) and by psychometric performance,
Results
The rate of dementia progression was significantly more rapid in individuals with lower baseline CSF Aβ42, with higher tau or ptau181, or high tau/Aβ42 ratio. For example, the annual change in CDR-SB was 1.1 for the lowest two tertiles of Aβ42 values and 0.3 for the highest tertile of Aβ42 values.
Conclusions
In individuals with very mild DAT, lower CSF Aβ42, high tau or ptau181, or a high tau/Aβ42 ratio quantitatively predict more rapid progression of cognitive deficits and dementia. CSF biomarkers may be useful prognostically and to identify individuals who are more likely to progress for participation in therapeutic clinical trials.
Keywords: amyloid beta, Aβ, tau, biomarker, dementia progression
Introduction
The efficacy of treatments for Alzheimer's disease (AD) will likely depend on accurately identifying individuals with underlying AD pathology (e.g plaques and tangles) early in the course of disease. While the clinical diagnosis of dementia of the Alzheimer type (DAT) is accurate in specialized centers, the sensitivity of diagnosis, particularly at milder stages of disease or with a single clinical evaluation, may be much lower1-3. Because there is a growing emphasis on enrolling less cognitively impaired individuals into clinical trials of putative anti-AD agents, methods are needed that will identify those individuals with very mild DAT who are more likely to exhibit measurable cognitive decline over the study period.
Disease-specific biomarkers, such as levels of Aβ42, tau, and p-tau in CSF have reasonable levels of sensitivity and specificity for the diagnosis of DAT (reviewed in 4, 5, 6). Importantly, recent studies combining amyloid imaging using Pittsburgh Compound B with analysis of CSF biomarkers have shown that levels of CSF Aβ42 can accurately separate individuals who have appreciable deposits of neocortical amyloid from those who do not7, 8. In one recent study, individuals diagnosed with “mild cognitive impairment” (MCI9, 10) who had “pathological” concentrations of CSF tau or Aβ42 had a 17.7 fold increased risk of progressing to diagnosed DAT over a 5 year period11. The CSF Aβ42/tau and Aβ42/p-tau ratio also identifies cognitively normal individuals who have a 4-5 fold increased risk for developing very mild DAT (Clinical Dementia Rating (CDR) of 0.5) within 3-4 years8, 12.
If CSF biomarkers reflect underlying pathophysiological mechanisms governing Aβ deposition and injury to neurons, levels of these biomarkers might correlate with the actual rate of cognitive decline in individuals with MCI or very mild dementia. We hypothesized that those individuals with very mild DAT who had low CSF Aβ42 or a high tau/Aβ42 ratio might undergo more rapid cognitive decline than mildly impaired individuals with higher CSF Aβ42 or a lower tau/Aβ42 ratio. The ability of these CSF markers to predict rate of disease progression has implications for diagnosis and treatment of very mild DAT and for clinical trial design.
Methods
Participants and clinical assessments
Participants were a subset of individuals enrolled in longitudinal studies of healthy aging and dementia at the Washington University Alzheimer's Disease Research Center. All participants enrolled in the longitudinal studies are ≥ 60 years of age at enrollment and in good general health. All agree to lumbar puncture (LP); historically 72% of participants enrolled complete LP and 78% return for annual follow-up. Individuals were selected for analysis here if they had a diagnosis of DAT with a Clinical Dementia Rating (CDR)13 of 0.5 (very mild impairment) at the time of LP and had at least one follow-up clinical assessment after the LP. Participants underwent annual assessments that include assignment of CDR and a 1.5 hr psychometric test battery 14. The CDR, an assessment of the presence or absence of dementia and of dementia severity, is based on semistructured interviews with the individual and a collateral source. CDR and diagnosis were determined independently of psychometric test results. The sum of the CDR boxes (CDR-SB) is a more quantitative representation of the CDR15. Demographic features, health history, language function, medications and depressive features were also assessed. Participants underwent a neurological examination and blood draw for ApoE genotyping.
The psychometric test battery included measures of episodic memory (Forward and Backward Digit Span, Associate Memory subtests from the Wechsler Memory Scale (WMS), and the Benton Visual Retention test), semantic memory and language (information subtest of the Wechsler Adult Intelligence Scale (WAIS) and the Boston Naming Test), executive function (digit span measures from the WMS, a word fluency test, and the WMS mental control subtest) and speeded visuospatial measures (WAIS block design and digit symbol and Trail-Making Test A). The general psychometric composite score16 used was prorated based on the other tests used to generate the original composite because of changes in the psychometric test battery across the study period. Better cognitive functioning is indicated by lower scores on the CDR-SB and by higher scores on the psychometric composite.
All clinical diagnoses, including DAT and depression, were made in accordance with standard criteria17. At our center, a CDR 0.5 originally denoted individuals who were “neither clearly demented nor healthy”18; criteria for MCI also include a CDR 0.5 to indicate the absence of clear dementia. With experience, our center has been able to successfully identify the subset of individuals for whom the mild cognitive impairment is caused by underlying AD as subsequently determined by progression to greater stages of dementia severity and by histopathological confirmation of AD14, 19. The CDR 0.5 designation in our ADRC now denotes very mild dementia; in all the CDR 0.5 individuals included here, the clinical diagnosis was DAT. Some of these individuals would be considered as MCI at other centers, but many were insufficiently impaired to meet criteria for MCI and might be designated as “preMCI”14. For comparison, we applied revised criteria for MCI20 to identify CDR 0.5/DAT individuals who scored 1.5 or more standard deviations below the mean of a comparison group of nondemented individuals on a measure of episodic memory (the Associate Memory subscale of the WMS).
Studies were approved by the Institutional Review Board at Washington University, and informed consent was obtained from all participants.
CSF Collection and Analysis
All individuals underwent LP for the collection of CSF using a standard procedure7. CSF samples were analyzed for total tau, phosphorylated tau181 (ptau181) and Aβ42 by commercial enzyme-linked immunosorbant assay (ELISA, Innotest; Innogenetics, Ghent, Belgium) as previously described7, 8. Aβ1-40 (Aβ40 ) was analyzed by ELISA as previously reported21.
Statistical Analyses
Associations between each of the CSF biomarkers at the time of LP and years of education and age were tested using Pearson product-moment correlations. T-tests for independent samples were used to determine whether mean biomarker values differed by gender or by the presence of at least one APOE4 allele. General linear models (PROC GLM, SAS Institute Inc., Cary, North Carolina) were used to test whether there was a significant association between each of the CSF biomarkers and having a depression diagnosis while adjusting for the effects of age, gender, and education. We used mixed linear models (PROC MIXED, SAS Institute Inc.) to determine if there was a relationship between the slope of the CDR-SB with time after the LP as a function of biomarker values, after controlling for age, sex and education. Similar analyses were conducted to examine biomarker-related differences in the slope of the psychometric composite scores following the LP.
Results
Demographic and biomarker values at baseline assessment in individuals with very mild DAT
Forty-nine CDR 0.5/DAT participants underwent LP and had at least one follow-up clinical assessment. Follow-up interval varied as enrollment was ongoing. Demographic variables at baseline assessment (before LP) are shown in Table 1 and CSF biomarker values are shown in Table 2. More than half of these participants performed better than the cut-off score for MCI on episodic memory performance and can be considered as “PreMCI”14. 29 of these participants were included in the dataset in Fagan et al., 20078.
Table 1.
Demographic characteristics of CDR 0.5/DAT participants.
| TABLE 1. Group Demographics | |
|---|---|
| Age at LP, mean years (SD) | 73.8 (10.0) |
| Gender M:F | 30:19 |
| CDR-SB, mean (SD) | 2.4 (1.2) |
| Education, years, mean (SD) | 15.2 (3.1) |
| Met MCI criteria, number (%) | 20 (40.8%) |
| MMSE, mean (SD) | 26.4 (2.9) |
| Number of assessments after LP, mean (SD) | 3.5 (1.8) |
Abbreviations: LP, lumbar puncture; CDR-SB, Clinical Dementia Rating, Sum of Boxes; MCI, mild cognitive impairment; MMSE, Mini-Mental Status Exam 39. Values shown are mean (SD) at the assessment done prior to the LP.
Table 2.
Baseline CSF biomarker values for mildly impaired individuals included in the analysis and those of a cohort of nondemented individuals.
| TABLE 2 CSF Biomarker Values | CDR 0.5, DAT (n=49) | CDR 0, nondemented (From Fagan et al., 2007, n=90) |
|---|---|---|
| Aβ42 (pg/ml) | 434.1 (211.5) | 567 (207) |
| Aβ40 (pg/ml) | 9672.1 (3397.72) | 9758 (3827) |
| tau (pg/ml) | 564.9 (302.5) | 342 (175) |
| ptau181 (pg/ml) | 85.9 (45.4) | 62 (26) |
Abbreviations: CDR, Clinical Dementia Rating; CSF, cerebrospinal fluid. Values in right column are those for unimpaired individuals (rated CDR 0, no dementia) from an earlier study8 and are included for comparison. Values shown are CSF mean (SD) biomarker values.
There were no significant correlations between the biomarkers and age, years of education, CDR-SB or the psychometric composite score at the time of LP (see Supplemental Table). Individuals with one or more APOε4 alleles had lower average CSF Aβ42 levels than those without an APOε4 allele (average 304.86 pg/ml vs 418.42 pg/ml, P=.0055). Individuals who had been diagnosed with depression or mild mood disorder had significantly higher CSF Aβ42 than those with no depression diagnosis (least-square means were 600.4 pg/ml for those with a depression diagnosis vs. 364.0 pg/ml without, P=.0013 after adjustment for sex, age, and education).
Correlation of biomarker values with subsequent change in CDR-SB
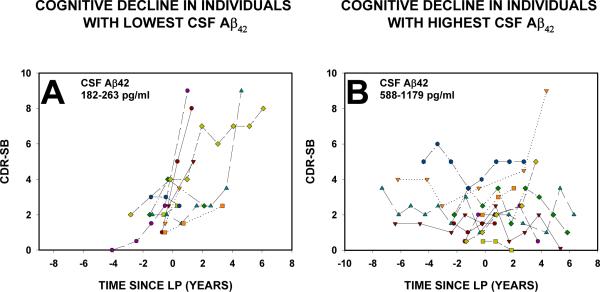
We first compared the unadjusted change in CDR-SB over time in the 10 individuals with the lowest CSF Aβ42 (182-263 pg/ml) to the 10 individuals with the highest Aβ42 (588-1179 pg/ml). As shown in Figure 1, individuals with the lowest CSF Aβ42 had a consistent and more rapid increase in CDR-SB (indicative of more impairment, see Figure 1A) than those with the highest levels of Aβ42 (Figure 1B).
FIGURE 1.
Change in CDR-SB over time. In Panel A, data are shown for the 10 individuals with the lowest CSF Aβ42 levels (actual values were from 182-263 pg/ml). Each data point is the CDR-SB from the clinical assessment at the indicated time relative to the LP (time 0). CDR-SB for each assessment for the 10 individuals with the highest CSF Aβ42 is shown in Panel B. CSF Aβ42 values were 588-1179 pg/ml for this group. The timing of the baseline LP was set at time “0” (indicated by the dotted line). All participants had a global CDR of 0.5 (with CDR-SB between 0.5 and 4.5) at the assessment before the LP.
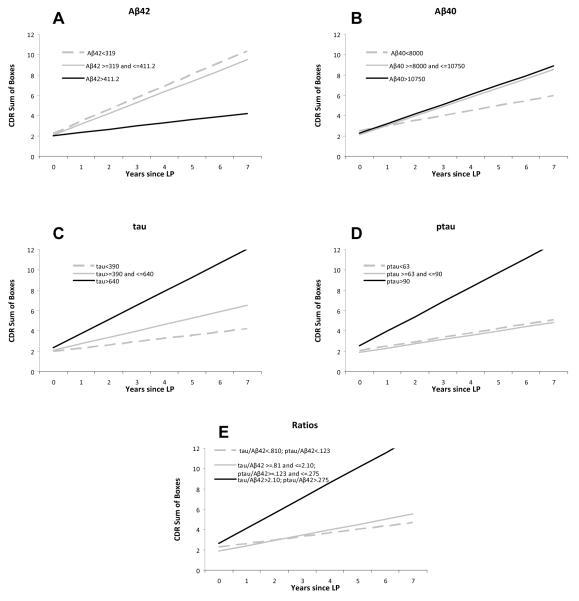
We next analyzed whether CSF biomarker levels at the baseline assessment were associated with the subsequent rate of change of cognitive variables over time for the entire group. Mean follow-up after LP was 3.5 years (range 0.9-7 years). The slope of CDR-SB correlated significantly with baseline levels of CSF Aβ42 (P=0.02), tau (P=0.007), ptau (P=0.0037) and with ratios of tau or ptau/Aβ42 (P=0.003 and 0.0011 respectively), but not with levels of Aβ40 (P=0.4857). For illustrative purposes, we divided the participants into tertiles based on levels of each of the biomarkers and plotted the change in CDR-SB over time for each tertile; mean slopes and intercepts for each tertile for each biomarker and the absolute levels of biomarkers for each tertile are shown in Figure 2. We found that there were differences in the slopes of the CDR-SB between tertiles as a function of CSF Aβ42 (P=0.0265), tau (P=0.0133), and ptau181 (P=0.0092), but not as a function of CSF Aβ40 (P=0.4737). The ratio measures (tau/Aβ42 or ptau181/Aβ42) were closely correlated (r=0.97, P<.0001) and also indicated a difference in the slope of the CDR-SB with time (P=0.0048).
FIGURE 2.
Slope of CDR-SB over time in biomarker tertiles. For each individual biomarker, the sample was divided into tertiles (i.e., low, middle, and high biomarker groups) based on a frequency distribution of the baseline biomarker values. The change in CDR-SB over time was calculated for each tertile. The slope and intercept for each tertile are plotted for each biomarker: (A) CSF Aβ42, (B) CSF Aβ40, (C) CSF tau, (D) CSF ptau181, and (E) tau/ Aβ42 ratio. CSF biomarker values for each tertile are provided in the legend.
Slope values (change in CDR-SB/yr) were 1.1 boxes/yr for the lowest and middle tertiles for CSF Aβ42 and 0.3 boxes/yr for the highest tertile. Findings were similar for those individuals with the highest tertile values of CSF tau, ptau181 and the tau/Aβ42 ratio. Those with values in the highest tertile for tau/Aβ42 had an increase in CDR-SB of 1.5 boxes/yr, the most rapid increase for any of the markers studied here.
Correlation of biomarker values with subsequent change in psychometric composite score
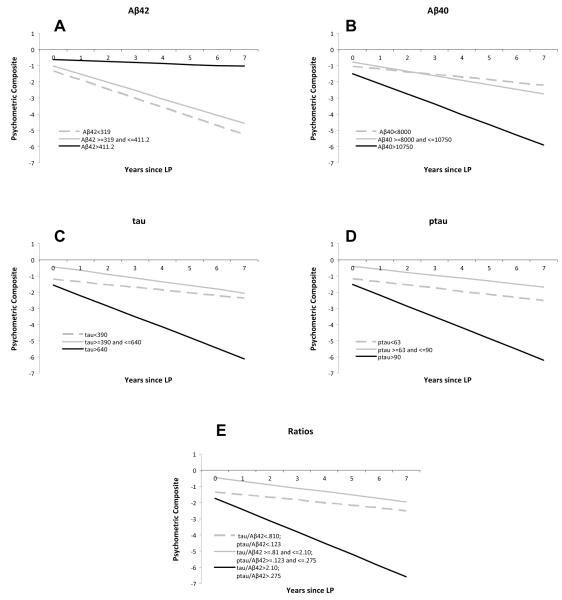
We performed a similar analysis to compare the rate of change in the psychometric composite scores to the CSF biomarker values, again dividing the cohort into tertiles based on the distribution of the biomarker values for illustrative purposes. Those individuals with lower Aβ42 values exhibited a more rapid rate of decline in psychometric composite score after LP than individuals with higher levels (P=0.0263). The slope of change was -0.6 points/yr for the lowest tertile (CSF Aβ42 <319), -0.5 points/yr for the middle tertile (CSF Aβ42 ≥319 pg/ml and ≤ 411.2 pg/ml) and -0.06 points/yr for the highest tertile (CSF Aβ42 > 411.2 pg/ml). There was a faster rate of decline in psychometric composite score for those with higher values of tau (P=0.0511), ptau181 (P=0.0398), and the ratio measures (P=0.0292; Figure 3). Like the CDR-SB, the slope of the psychometric composite was not significantly associated with CSF Aβ40 values (P=0.1639).
FIGURE 3.
Change in psychometric composite score over time in biomarker tertiles. For each individual biomarker, the sample was divided into tertiles (i.e., low, middle, and high biomarker groups) based on a frequency distribution of the baseline biomarker values as in Figure 2. The change in psychometric composite over time was calculated for each tertile. The slope and intercept for each tertile are plotted for each biomarker: (A) CSF Aβ42, (B) CSF Aβ40, (C) CSF tau, (D) CSF ptau181, and (E) tau/ Aβ42 ratio. CSF biomarker values for each tertile are provided in the legend.
Use of a “cutoff” value to identify individuals with very mild DAT likely to have a more rapid cognitive decline
The utility of CSF biomarkers in clinical practice will require practical guidelines for interpretation of individual results. As an example, we tested the ability of a CSF Aβ42 value of ≤411 pg/ml to predict more rapid disease progression. This values encompasses the lower two tertiles of CSF Aβ42 values and previous studies suggest such individuals will uniformly demonstrate increased cortical binding of Pittsburgh Compound B (PIB22) consistent with deposition of amyloid in the brain7, 8. CSF Aβ42 ≤411 pg/ml predicted a significantly more rapid rate of cognitive decline, as measured by both CDR-SB (P=.0079) and the psychometric factor score (P=.0082). Quantitatively, those with CSF Aβ42 <411 pg/ml had a mean yearly increase in CDR-SB of 1.10 boxes (95% confidence interval 0.74-1.47), while those with CSF Aβ42 > 411 pg/ml had a mean yearly increase in CDR-SB of 0.32 (95% confidence interval -0.11 to 0.74). Alternatively, using the highest tertile of values for the tau/Aβ42 ratio (ratio >0.81), the yearly increase in CDR-SB was 1.49 (95% confidence interval 0.99-1.98), while those with tau/Aβ42ratio ≤0.81 had a mean yearly increase in CDR-SB of 0.43 (95% confidence 0.08-0.76).
Discussion
The main finding of this study is that baseline levels of AD-related CSF biomarkers, Aβ42, tau, ptau181, and the tau/Aβ42 ratio, quantitatively predict rate of cognitive decline over time in individuals with very mild dementia. Our study differs importantly from earlier studies in that we show levels of biomarkers are strongly predictive of the actual rate of decline rather than with a dichotomous assessment of conversion/no conversion from mild impairment to diagnosed DAT. These findings are consistent with previous studies from our group and others showing that CSF levels of Aβ42, tau, and ptau can be used to predict the likelihood that nondemented individuals will develop MCI12 or very mild dementia8 and with studies showing that biomarkers predict progression from MCI to DAT (e.g. see references 11, 23-26). The only published studies correlating AD-related CSF biomarkers with rate of cognitive decline showed that increased levels of three different ptau epitopes (ptau181, ptau231 and ptau199) correlated with decline in MMSE in individuals with MCI followed for 1 year27, 28.
Our study differs from those linking CSF biomarkers to “conversion” from MCI to DAT since, although some of these individuals would be diagnosed as MCI at other centers, the majority of individuals diagnosed as very mild DAT here did not have sufficient impairment on objective memory testing to meet MCI criteria. Their mean MMSE scores were similar to those of individuals with MCI in other studies. The mild impairment, slow progression, and higher levels of CSF Aβ42 in some subjects raise the possibility that some of these individuals do not have underlying AD pathology. Clinical diagnosis is subsequently confirmed on neuropathological examination about 90% of the time at our center19, even at such very mild levels of impairment. In one series, in individuals diagnosed with very mild DAT (CDR 0.5) who did not meet MCI criteria, at autopsy 43/47 had AD, one had corticobasal degeneration and 3 had normal brains14. In the present study 5/16 individuals in the highest tertile for CSF Aβ42 had Aβ42 levels >715 pg/ml, the highest level reported to date in autopsy-confirmed AD29, making it unlikely these individuals have underlying AD pathology. CSF biomarker levels may accurately identify the 10% of individuals clinically diagnosed with very mild DAT who do not have underlying AD pathology, but pathological studies will be required to test this hypothesis. The majority of individuals in the highest tertile (11/16) had CSF Aβ42 levels below 715 pg/ml, consistent with possible AD pathology. Although the rate of progression in these individuals (0.3 boxes/year) is slow, such slow progression has been observed in individuals with autopsy confirmed AD. For example, at our center a recent individual with autopsy-confirmed AD had no increase in CDR-SB for the first two years after LP; CSF Aβ42 was 457 pg/ml. Progression may not be linear throughout the course of disease and may be slower at milder stages of dementia30.
The relationship between cerebrospinal fluid Aβ42 and the Aβ42 pools in the brain, both soluble and in plaques, is likely complex and may change during the course of disease21, 31, but there is substantial evidence that once CSF levels of Aβ42 are low, they remain stable over several years in both unimpaired and impaired individuals32-36. The idea that changes in Aβ homeostasis, including the decrease in CSF Aβ42, precede clinically detectable cognitive decline in late-onset AD by at least several years and perhaps by 10-15 years is supported by the correlation between CSF Aβ42 levels and the presence of cortical amyloid deposition even in cognitively normal individuals and the finding that an increased ratio of tau/Aβ42 is predictive of short term decline from normal to very mild dementia7, 8, 12. The recent report by Sluimer et al. showing that change in levels of CSF biomarkers over time in mildly impaired individuals did not correlate with cognitive change as quantified by MMSE supports the idea that biomarker levels remain stable over time even after the onset of impairment 37. These findings support a model in which, in those individuals destined to develop AD, CSF Aβ42 levels decrease from normal levels to a new steady state before any symptoms of cognitive impairment develop. This decrease might be triggered by deposition of Aβ plaques in some brain regions. The present findings suggest that this new “set point” for Aβ42 will correlate with the rate of disease progression once impairment is present.
While the number of subjects in our study was relatively small, the results reported here suggest that CSF biomarkers might be useful as entry criteria for clinical trials of disease modifying therapies for MCI/very mild DAT. Limiting enrollment to individuals with CSF Aβ42 values below a certain cutoff might ameliorate the difficulties caused lack of disease progression in some individuals during the trial. For example, in our study, individuals with CSF Aβ42 values ≤ 411 pg/ml progressed at a rate of 1.11 boxes/per year with a variance of 0.49, while the unselected group of all CDR 0.5 individuals progressed more slowly, at a rate of 0.78 boxes/per year with a variance of 0.70. Using these group characteristics, we calculated how many participants would be needed to power a hypothetical clinical trial, assuming a two-armed study (1:1 treatment vs placebo). If all individuals with a diagnosis of very mild dementia/CDR 0.5 were enrolled, 354 participants would be needed to detect a 50% treatment effect on CDRSB after a 1.5 year period, using a standard normal test at a significance level of 5%, while less than half as many (154) would be needed if CSF Aβ42 <411 pg/ml were included as an inclusion/exclusion criterion to select participants38. These findings are likely to have important implications for both reducing the number of participants needed to show an effect in clinical trials for very mild dementia of the Alzheimer's type/mild cognitive impairment, as well as ultimately to assist in making treatment decisions as more invasive and potentially harmful disease-modifying treatments for AD become available.
Supplementary Material
Acknowledgments
Supported by NIH grants P01AG003991 and P50AG005681 and the Charles and Joanne Knight Alzheimer Research Initiative of Washington University's Alzheimer's Disease Research Center. We are indebted to the Center's Clinical Core for the assessments of participants, to Dr. Martha Storandt for assistance with the psychometric data, and to all our participants. We also thank Eli Lilly for their gift of antibodies to Aβ40. Dr. Snider had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Disclosure: The authors report no conflicts of interest.
REFERENCES
- 1.Hogervorst E, Bandelow S, Combrinck M, Irani SR, Smith AD. The validity and reliability of 6 sets of clinical criteria to classify Alzheimer's disease and vascular dementia in cases confirmed post-mortem: added value of a decision tree approach. Dement Geriatr Cogn Disord. 2003;16(3):170–80. doi: 10.1159/000071006. [DOI] [PubMed] [Google Scholar]
- 2.Ranginwala NA, Hynan LS, Weiner MF, White CL., 3rd. Clinical criteria for the diagnosis of Alzheimer disease: still good after all these years. Am J Geriatr Psychiatry. 2008;16(5):384–8. doi: 10.1097/JGP.0b013e3181629971. [DOI] [PubMed] [Google Scholar]
- 3.Nagy Z, Esiri MM, Hindley NJ, et al. Accuracy of clinical operational diagnostic criteria for Alzheimer's disease in relation to different pathological diagnostic protocols. Dement Geriatr Cogn Disord. 1998;9(4):219–26. doi: 10.1159/000017050. [DOI] [PubMed] [Google Scholar]
- 4.Blennow K, Hampel H. CSF markers for incipient Alzheimer's disease. Lancet Neurol. 2003;2(10):605–13. doi: 10.1016/s1474-4422(03)00530-1. [DOI] [PubMed] [Google Scholar]
- 5.Andreasen N, Blennow K. CSF biomarkers for mild cognitive impairment and early Alzheimer's disease. Clin Neurol Neurosurg. 2005;107(3):165–73. doi: 10.1016/j.clineuro.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Sunderland T, Linker G, Mirza N, et al. Decreased β-amyloid1-42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. Journal of the American Medical Association. 2003;289(16):2094–103. doi: 10.1001/jama.289.16.2094. [DOI] [PubMed] [Google Scholar]
- 7.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid A42 in humans. Ann Neurol. 2006;59(3):512–9. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 8.Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/β-amyloid42 ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64(3):343–9. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 9.Flicker C, Ferris SH, Reisberg B. Mild cognitive impairment in the elderly: predictors of dementia. Neurology. 1991;41(7):1006–9. doi: 10.1212/wnl.41.7.1006. [DOI] [PubMed] [Google Scholar]
- 10.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 11.Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5(3):228–34. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- 12.Li G, Sokal I, Quinn JF, et al. CSF tau/Aβ42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology. 2007;69(7):631–9. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- 13.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 14.Storandt M, Grant EA, Miller JP, Morris JC. Longitudinal course and neuropathologic outcomes in original vs revised MCI and in pre-MCI. Neurology. 2006;67(3):467–73. doi: 10.1212/01.wnl.0000228231.26111.6e. [DOI] [PubMed] [Google Scholar]
- 15.Berg L, Miller JP, Baty J, Rubin EH, Morris JC, Figiel G. Mild senile dementia of the Alzheimer type. 4. Evaluation of intervention. Ann Neurol. 1992;31(3):242–9. doi: 10.1002/ana.410310303. [DOI] [PubMed] [Google Scholar]
- 16.Rubin EH, Storandt M, Miller JP, et al. A prospective study of cognitive function and onset of dementia in cognitively healthy elders. Arch Neurol. 1998;55(3):395–401. doi: 10.1001/archneur.55.3.395. [DOI] [PubMed] [Google Scholar]
- 17.Berg L, McKeel DW, Jr., Miller JP, et al. Clinicopathologic studies in cognitively healthy aging and Alzheimer's disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol. 1998;55(3):326–35. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- 18.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–72. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 19.Morris JC, Storandt M, Miller JP, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58(3):397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 20.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–6. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 21.Cirrito JR, May PC, O'Dell MA, et al. In Vivo Assessment of Brain Interstitial Fluid with Microdialysis Reveals Plaque-Associated Changes in Amyloid-β Metabolism and Half-Life. J. Neurosci. 2003;23(26):8844–8853. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–19. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 23.Hansson O, Zetterberg H, Buchhave P, et al. Prediction of Alzheimer's disease using the CSF Aβ42/Aβ40 ratio in patients with mild cognitive impairment. Dement Geriatr Cogn Disord. 2007;23(5):316–20. doi: 10.1159/000100926. [DOI] [PubMed] [Google Scholar]
- 24.Hansson O, Buchhave P, Zetterberg H, Blennow K, Minthon L, Warkentin S. Combined rCBF and CSF biomarkers predict progression from mild cognitive impairment to Alzheimer's disease. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 25.Hampel H, Teipel SJ, Fuchsberger T, et al. Value of CSF β-amyloid1-42 and tau as predictors of Alzheimer's disease in patients with mild cognitive impairment. Mol Psychiatry. 2004;9(7):705–10. doi: 10.1038/sj.mp.4001473. [DOI] [PubMed] [Google Scholar]
- 26.Herukka SK, Hallikainen M, Soininen H, Pirttila T. CSF Aβ42 and tau or phosphorylated tau and prediction of progressive mild cognitive impairment. Neurology. 2005;64(7):1294–7. doi: 10.1212/01.WNL.0000156914.16988.56. [DOI] [PubMed] [Google Scholar]
- 27.Buerger K, Teipel SJ, Zinkowski R, et al. CSF tau protein phosphorylated at threonine 231 correlates with cognitive decline in MCI subjects. Neurology. 2002;59(4):627–9. doi: 10.1212/wnl.59.4.627. [DOI] [PubMed] [Google Scholar]
- 28.Buerger K, Ewers M, Andreasen N, et al. Phosphorylated tau predicts rate of cognitive decline in MCI subjects: a comparative CSF study. Neurology. 2005;65(9):1502–3. doi: 10.1212/01.wnl.0000183284.92920.f2. [DOI] [PubMed] [Google Scholar]
- 29.Engelborghs S, Sleegers K, Cras P, et al. No association of CSF biomarkers with APOEepsilon4, plaque and tangle burden in definite Alzheimer's disease. Brain. 2007;130(Pt 9):2320–6. doi: 10.1093/brain/awm136. [DOI] [PubMed] [Google Scholar]
- 30.Storandt M, Grant EA, Miller JP, Morris JC. Rates of progression in mild cognitive impairment and early Alzheimer's disease. Neurology. 2002;59(7):1034–41. doi: 10.1212/wnl.59.7.1034. [DOI] [PubMed] [Google Scholar]
- 31.Barten DM, Guss VL, Corsa JA, et al. Dynamics of β-amyloid reductions in brain, cerebrospinal fluid, and plasma of β-amyloid precursor protein transgenic mice treated with a γ-secretase inhibitor. J Pharmacol Exp Ther. 2005;312(2):635–43. doi: 10.1124/jpet.104.075408. [DOI] [PubMed] [Google Scholar]
- 32.Blennow K, Zetterberg H, Minthon L, et al. Longitudinal stability of CSF biomarkers in Alzheimer's disease. Neurosci Lett. 2007;419(1):18–22. doi: 10.1016/j.neulet.2007.03.064. [DOI] [PubMed] [Google Scholar]
- 33.Zetterberg H, Pedersen M, Lind K, et al. Intra-individual stability of CSF biomarkers for Alzheimer's disease over two years. J Alzheimers Dis. 2007;12(3):255–60. doi: 10.3233/jad-2007-12307. [DOI] [PubMed] [Google Scholar]
- 34.Kanai M, Matsubara E, Isoe K, et al. Longitudinal study of cerebrospinal fluid levels of tau, Aβ1-40, and Aβ1-42(43) in Alzheimer's disease: a study in Japan. Ann Neurol. 1998;44(1):17–26. doi: 10.1002/ana.410440108. [DOI] [PubMed] [Google Scholar]
- 35.Andreasen N, Hesse C, Davidsson P, et al. Cerebrospinal fluid β-amyloid1-42 in Alzheimer disease: differences between early- and late-onset Alzheimer disease and stability during the course of disease. Arch Neurol. 1999;56(6):673–80. doi: 10.1001/archneur.56.6.673. [DOI] [PubMed] [Google Scholar]
- 36.Mollenhauer B, Bibl M, Trenkwalder C, et al. Follow-up investigations in cerebrospinal fluid of patients with dementia with Lewy bodies and Alzheimer's disease. J Neural Transm. 2005;112(7):933–48. doi: 10.1007/s00702-004-0235-7. [DOI] [PubMed] [Google Scholar]
- 37.Sluimer JD, Bouwman FH, Vrenken H, et al. Whole-brain atrophy rate and CSF biomarker levels in MCI and AD: A longitudinal study. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 38.Xiong C, Zhu K, Yu K, Miller JP. Statistical Modeling in Biomedical Research: Longitudinal Data Analys. In: Rao DR, Miller JP, Rao DC, editors. Epidemiology and Medical Statistics. 27 vol. Elsevier; Amsterdam, The Netherlands: 2008. [Google Scholar]
- 39.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.