Abstract
Background
Only a minority of islet transplant recipients maintain insulin independence at 5 years under the Edmonton protocol of immunosuppression. New immunosuppressive strategies are required in order to improve long term outcomes.
Materials and Methods
Three subjects with unstable type 1 DM underwent islet transplantation with alemtuzumab induction and sirolimus-tacrolimus maintenance for three months then sirolimus-mycophenolic acid maintenance thereafter. Follow-up was >2 years. Comparison was with sixteen historical subjects transplanted under the Miami version of the Edmonton protocol.
Results
Insulin independence was achieved in 2/3 alemtuzumab and 14/16 historical subjects. Those who did not achieve insulin independence only received a single islet infusion. Insulin independence rates remained unchanged in the Alemtuzumab group, but decreased from 14/16 (88%) to 6/16 (38%) in the Historical group over two years. Insulin requirements increased in the Historical group while remaining stable in the Alemtuzumab group. Comparison of functional measures at 3 months suggested better engraftment with alemtuzumab (NS). Further comparison of Alemtuzumab versus Historical groups, up to 24 months, demonstrated significantly better: Mixed Meal Stimulation Index (24 months 1.0±0.08 n=3 vs 0.5±0.06 pmol/mL n=6, p<0.01), Mixed Meal peak C-peptide (24 months 5.0±0.5 n=3 vs 3.1±0.3 nmol/mL n=6, p<0.05), HbA1c (24 months 5.4±0.15 n=3 vs 6.3±0.12pmol/mL n=10, p<0.01).
Administration of alemtuzumab was well tolerated. There was no increased incidence of infections in alemtuzumab subjects despite profound, prolonged lymphocyte depletion.
Conclusions
Islet transplantation with alemtuzumab induction was well tolerated and resulted in improved short and long term outcomes. Further investigation is underway for validation.
INTRODUCTION
The beginning of the last decade witnessed unprecedented advances in the field of islet transplantation (IT) with the results of the Edmonton protocol (14; 15; 19). The short term success has been duplicated in multiple centers, however longer term outcomes have been less successful with insulin independence only maintained in the minority of recipients at 5 years (16; 20).
Although several factors may be responsible for the low long-term insulin independence rates, it is clear that newer immunosuppressive strategies with lower toxicity profiles and aimed towards achieving better long-term outcomes are required.
Currently, maintenance immunosuppression with sirolimus and tacrolimus is the standard in IT. Paradoxically, calcineurin inhibitors (CNI) are associated with ß-cell toxicity (13; 21), insulin resistance (3) and are largely responsible for the development of post-transplant diabetes mellitus in the whole organ transplant setting (7; 9; 17). Another concern associated with this regimen is the development of renal dysfunction (18).
Lympho-depleting strategies are commonly used in whole organ transplantation for the prevention of acute allograft rejection. Alemtuzumab (Campath®, Genzyme Corporation, Cambridge, MA 02142) is a humanized monoclonal antibody against the CD-52 human antigen present on the surface of mature B/T lymphocytes, natural killer cells, monocytes, and macrophages but absent on lymphoid progenitors (26). It has been used in several whole organ transplant settings with promising results (5; 10; 22; 23). Recent data in the literature suggest that alemtuzumab may contribute to expansion of regulatory T cells(11; 25; 26) and this property may favorably modulate the allo-immune response thereby improving long term survival.
The effect of alemtuzumab on multiple inflammatory cell types, for example, macrophages, may prevent the production of pro-inflammatory mediators by intrahepatic macrophages and endothelial cells, thus reducing early islet losses secondary to the deleterious effects of cytokines at the time of islet infusion.
Although its administration results in profound and long-term lympho-depletion, incidence of opportunistic infections seems comparable to that associated with other induction agents (24).
In order to improve IT outcomes by addressing two major areas, engraftment and immunosuppression related toxicity, we developed an immunosuppressive protocol consisting on alemtuzumab induction and long-term CNI-free maintenance immunosuppression. Herein, we report preliminary data on 3 subjects who underwent IT at our center under this novel regimen.
MATERIALS AND METHODS
Three subjects with T1DM have been transplanted to date and have completed at least 2 years follow-up after their final islet infusion. All subjects had hypoglycemia unawareness, glycemic lability and progressive complications despite intensive insulin therapy. All had stable renal function without evidence of diabetic nephropathy. The protocol was approved by the University of Miami health research ethics board (IRB) and each subject gave written informed consent.
Induction therapy consisted of alemtuzumab 20 mg intravenously (IV) on postoperative day-1 & 0 of initial islet infusion. Each dose required pre-medication with diphenhydramine (50 mg IV), acetaminophen (650 mg PO), and methylprednisolone (125 mg IV). Steroids were not administered with the 2ND of alemtuzumab unless clinically indicated.
Maintenance immunosuppression, commenced postoperative day-1, consisted of sirolimus (Rapamune®, Wyeth, Madison, NJ) titrated to achieve trough levels of 7-10 ng/mL and tacrolimus (Prograf®, Astellas Pharma US, Inc. Deerfield, IL) titrated to attain a trough level of 4-6 ng/mL for 3 months at which time it was discontinued, and mycophenolic acid (Myfortic, Novartis Pharmaceuticals Corporation, NJ) introduced and increased up to a total dose of 720 mg two times per day as clinically tolerated. Subject 3 experienced multiple immunosuppression related adverse events, predominantly sirolimus related. Consequently, at the time of the switch to mycophenolic acid she was continued on tacrolimus and mycophenolic acid. Tacrolimus levels have been below target range since that time, instead, dose of mycophenolic acid was increased to 900mg BID by eight months post transplant.
The antitumor necrosis factor-alpha agent etanercept (Enbrel®, Immunex Corporation, Thousand Oaks, CA) was administered as follows: 50mg IV within one hour of islet infusion and 25 mg subcutaneously twice a week for two weeks after islet infusion. The anti-inflammatory Pentoxifylline, 400 mg orally TID, was administered for three months post-transplantation.
After initial islet infusion, if insulin independence was not achieved within two weeks, a second islet infusion was performed. Induction was with a single 20 mg IV dose of alemtuzumab on the day prior to islet infusion, if lymphocyte percent was greater than 5%, otherwise no induction was given. Pre-medication without steroids was administered prior to the alemtuzumab dose.
Islet isolation and transplantation were performed as previously described (4, 20, 28, 29). There was no difference in the isolation technique between the two groups. In both, DNAase (0.625ml/liter, Pulmozyme (dornase alfa) recombinant® Genentech, Inc.) was used throughout the isolation procedure, including digestion, dilution and wash. Digestion was performed with Liberase® enzyme blend (Roche Pharmaceuticals) in both groups. Islet recovery, average purity, islet number and viability were comparable in both groups. Islets in all subjects underwent a period of culture no less than 24 hours, no more than 72 hours. Post culture islet viability and stimulation index were not different in both groups. IV heparin was given in the infusion bag in both groups (4).
Subjects were followed for insulin independence, graft function, metabolic control and immunologically to evaluate the effects of alemtuzumab. Metabolic testing consisted of a 5 hour mixed meal tolerance test (MMTT) and and intravenous glucose tolerance test (IVGTT) as previously described (4). Parameters compared during MMTT included peak C-peptide, C-peptide AUC (AUC C-pep), and Mixed Meal Stimulation Index (MMSI) and during IVGTT included acute C-peptide release to glucose and AUC C-pep.
Comparison was with sixteen historical islet recipients transplanted under the Miami version of the Edmonton protocol (4). Specifically, induction therapy consisted daclizumab (Zenapax®-Roche, US) 1 mg/kg biweekly for a total of five doses, beginning day of transplant and continued at monthly and bimonthly intervals over the first 2 years post-transplant, respectively. Historical islet infusions were performed between August 2000 and July 2003; alemtuzumab subjects were transplanted between May 2005 and January 2006.
Adverse events (AEs) were compared between groups from the time of commencement of immunosuppression up to 2 years following completion islet infusion. Frequency of events overall were compared as well as severity of events using the National Cancer Institute criteria of adverse events version 3.0.
Serum panel reactive antibody (PRA) activity to HLA-I and HLA-II was determined by complement dependent microlymphocytotoxic technique using a commercial kit (Lambda Cell Tray, OneLambda, Canoga Park, CA) prior to 2006, thereafter, PRA activity was determined by either ELISA using OneLambda antigen tray-mixed standardized HLA-ELISA (OneLambda) or by flow cytometry (Luminex Corporation, Austin, TX).
Two tailed t-test analyses were performed between groups at all follow-up time points. Results are expressed as means±SEM. Statistical significance was reached at p<0.05. Subjects in the historical group were no longer considered if they withdrew from immunosuppression or underwent a supplemental infusion, hence the number of subjects in this group decreases over time.
RESULTS
Alemtuzumab and historical groups were compared for demographic variables and no significant differences were found (Table 1).
Table 1.
Demographics. t-tests were performed between groups to denote statistical significance
| Variable | Alemtuzumab Subjects n=3 | Historical Subjects n=16 | p | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (years) | 41.33 | 3.05 | 41.06 | 9.96 | 0.93 |
| T1DM Duration (years) | 29.67 | 12.51 | 27 | 12.40 | 0.76 |
| Wt (Kg) | 65.93 | 2.53 | 71.42 | 13.32 | 0.15 |
| BMI (kg/m2) | 25.34 | 0.95 | 24.62 | 1.84 | 0.35 |
| Ins/day (U/day) | 33.2 | 8.00 | 34.04 | 8.64 | 0.88 |
| Ins/kg /day | 0.51 | 0.14 | 0.48 | 0.08 | 0.75 |
| HbA1c(%) | 6.8 | 0.80 | 7.49 | 1.00 | 0.27 |
| IEQ | 817787 | 455522 | 917708 | 263112 | 0.74 |
| IEQ/kg | 12554.3 | 7173 | 13058.8 | 3540 | 0.92 |
In the alemtuzumab group, two subjects received two islet infusions and both achieved insulin independence after the second. The third subject developed sirolimus toxicity and was switched to tacrolimus-mycophenolic acid at 4 months post-infusion. Despite resolution of side effects, the patient refused a second infusion and did not achieve insulin independence although did have a >50% reduction in insulin requirements. In the historical group, thirteen subjects received two infusions and all achieved insulin independence, three subjects received only one infusion and 1/3 achieved insulin independence, the other two subjects were withdrawn, prior to a second infusion, because of complications of immunosuppression. The number of islet equivalents (IEQ) in single and double infusion subjects was comparable.
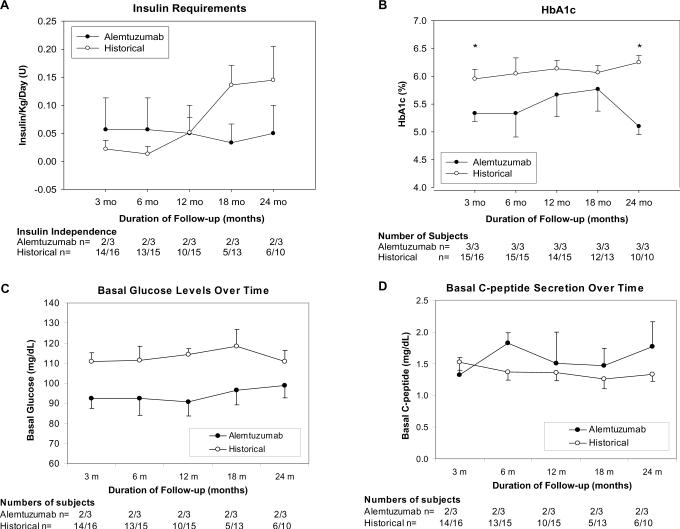
Insulin requirements were considered as the mean of all subjects, including those that were insulin independent, at all time points. Insulin requirements were initially lower in the historical group (p=NS) (Figure 1A). However, by 12 months requirements were identical and thereafter requirements in the historical group continued to increase while in the alemtuzumab group, requirements remained stable over 2 years.
Figure 1.
Insulin Independence rates and Change in Insulin Requirements (A) demonstrate stability in Alemtuzumab group. By 24 months post infusion less than half of the Historical group remain insulin independent. HbA1c (B) is significantly better at 3 and 24 months post completion islet infusion in Alemtuzumab group (24 months 5.1±0.15 vs 6.3±0.12 % p<0.005). Results of routine protocol monitoring of Fasting Plasma Glucose (C) and C-peptide (D) up to 24 months post completion islet infusion demonstrate consistently better levels in the Alemtuzumab group, specifically normoglycemia compared to impaired fasting glucose levels in the Historical group.
In an attempt to evaluate engraftment, outcome measures including fasting plasma glucose (FPG), C-peptide, C-peptide/glucose ratio (CPGR) were compared at one and 3 months post final infusion.
At one month, FPG and C-peptide were significantly lower in the alemtuzumab group (FPG 4.9±0.2 vs 6.2±0.3 mmol/L p<0.01; C-peptide 0.37±0.04 vs 0.57±0.07 nmo/L p<0.05), however, there was no difference in CPGR (1.2±0.14 vs 1.5±0.17 p=0.28). By three months all results were similar (FPG 5.1±0.3 vs 5.9±0.3 mmol/L p=0.13; C-peptide 0.43±0.1 vs 0.47±0.03 nmol/L p=0.65; CPGR 1.3±0.25 vs 1.4±0.09 p=0.98). HbA1c, however, was significantly lower in the alemtuzumab group (5.3±0.15 vs 6.0±0.17 % p<0.05) (figure 1B).
Longitudinal assessment of graft function demonstrated lower HbA1c values at all time points in the Alemtuzumab group reaching statistical significance again at 24 months (5.1±0.15 vs 6.3±0.12 % p<0.005) (figure 1B). FPG was lower in the Alemtuzumab group at all time points, specifically in the normal range compared to historical subjects who demonstrated impaired fasting glucose at all time points (figure 1C). C-peptide levels were slightly higher in the Alemtuzumab group despite lower glucose levels (figure 1D).
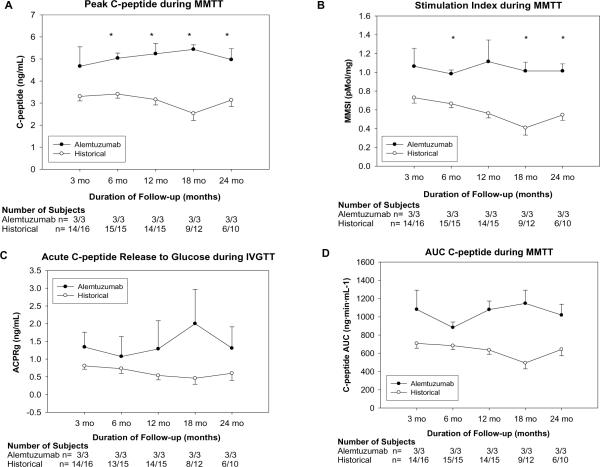
During MMTT, the peak C-peptide stimulation was significantly higher, at all time points except 3 months, in the Alemtuzumab group (3 months 1.57±0.29 nmol/L n=3 vs 1.17±0.06 nmol/L n=14 p=0.3; 24 months 1.67±0.17 nmol/L n=3 vs 1.17±0.10 nmol/L n=6 p<0.05) (figure 2A).
Figure 2.
Results of stimulation testing. Mixed Meal Peak C-peptide (A) and Stimulation Index (B) demonstrate significantly higher levels in the Alemtuzumab group (Peak C-peptide 24 months 1.67±0.17 nmol/L n=3 vs 1.17±0.10 nmol/L n=6 p<0.05, MMSI 24 months 1.0±0.08 n=3 vs 0.5±0.06 pmol/mL n=6, p<0.01). IVGTT Acute C-peptide Release (C) and MMTT C-peptide AUC (D), up to 24 months post completion islet infusion are consistently better in the Alemtuzumab group.
MMSI was higher in the alemtuzumab group at all time points after 3 months, significantly so at 6, 18, and 24 months (3 months, 1.1_0.19 n=3 vs. 0.7+ 0.06 pmol/mL n=14, P=0.22; 24 months, 1.0+0.08 n=3 vs. 0.5+0.06 pmol/mL n=6, P=0.01) (figure 2B). AUC C-peptide was higher in the alemtuzumab group at all time points although this did not reach statistical significance (Fig. 2D)
During IVGTT, ACPRg was higher in the alemtuzumab group at all time points although this never reached statistical significance. Similar findings were noted with the AUC C-peptide during IVGTT (data not shown).
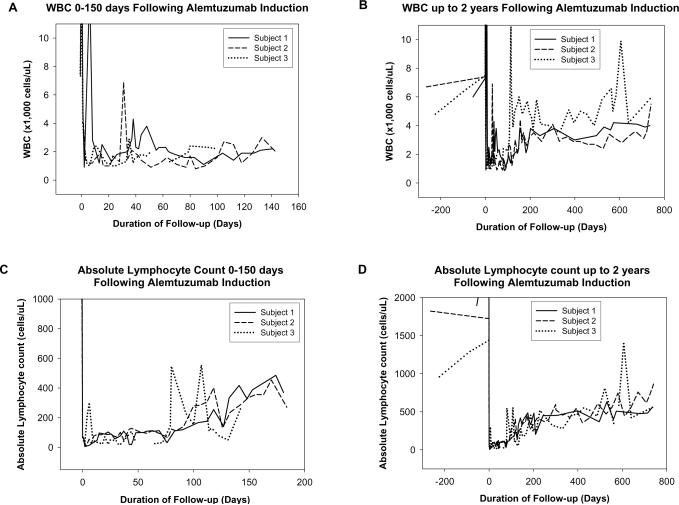
Administration of alemtuzumab resulted in marked leucopenia and lymphopenia. White blood cell count (WBC) in all patients fell to 1,000-3,000 cells/uL, (normal range 4,800-10,800 cells/uL) after a single dose and this lasted 3-4 months, regardless of a single or double induction dose. Initial peak at time of alemtuzumab infusion is secondary to steroid administration during pre-medication (figure 3A and B). WBC in subject 3 increased at 5 months due to previously described changes in immunosuppression, lymphocyte counts remained unchanged (figure 3B and D). Peak in WBC in subject 2 in the second month is due to granulocyte colony stimulating factor (G-CSF) administration at time of second infusion. Lymphocyte counts did not change. Absolute lymphocyte counts remained <200 cells/uL for almost 3 months in all subjects (normal range, 1,200–3,500 cells/uL) (Fig 3C). Even by two years, levels have not yet returned to normal (Fig 3D). The lymphocyte reduction was minimal in the Historical group. G-CSF was used twice in one alemtuzumab subject and thirteen times in four historical subjects over the same time period.
Figure 3.
Hematological Effects of Alemtuzumab Induction. White blood cell count 0-150 days (A) and up to two years (B) and absolute lymphocyte count 0-150 days (C) and up to two years (D), following initial Alemtuzumab administration demonstrate profound long lasting depletion. Changes in subject 3 noted at 4-5 months post transplant coincide with change in immunosuppression (see text).
In the Alemtuzumab group, sirolimus levels were slightly lower during the first 3 months compared with the Historical group (target 10-12 ng/mL compared with 12-15 ng/mL). After 3 months, levels were comparable. Tacrolimus levels were the same in both groups at all time points.
Evaluation of autoantibodies IA2 and GAD65 in alemtuzumab subjects was negative in two before and after islet transplantation, one became positive for GAD65 (2-6x normal ratio) post transplant and positivity for IA2 remained unchanged. This subject continues to demonstrate stable function. PRA levels have remained below 5% in all subjects in both groups.
The administration of alemtuzumab was well tolerated in all subjects, the worst side effect being a skin rash which occurred in all subjects. Despite the profound decrease in WBC, particularly lymphocytes, there has been no increased incidence of infections.
The total number of AEs was similar in both groups (Alemtuzumab 36.3±4.4 (SE) versus Historical 40.3±6 events NS). Similarly, the number of each level of severity and frequency of infections were also similar.
DISCUSSION
The data presented above from three subjects in a novel islet transplant protocol demonstrate a striking improvement in long-term function and stability of transplanted islets.
In these two groups of subjects, differences favoring the Alemtuzumab group were already evident at 1 and 3 months post infusion although they did not reach statistical significance. FPG levels at 1 and 3 months were lower and in the normoglycemic range in the Alemtuzumab group while the Historical group maintained FPG levels in the impaired fasting glucose range suggesting a higher functional mass secondary to improved engraftment in the Alemtuzumab group.
Stimulation testing by MMTT demonstrated a higher β-cell secretory capacity in the Alemtuzumab group. A similar trend was observed in response to IVGTT although differences between groups were not significant.
Functional islet mass at any time point is dependent on two factors, the surviving mass of islets at time of engraftment and the survival of functional islet mass over time. Large losses occur at implantation due to a relatively hostile environment and vulnerability prior to establishment of an adequate blood supply, triggering of the innate immune system secondary to events such as IBMIR (1; 12) and/or activation of other components secondary to cytokine release.
The surviving mass is typically marginal which likely contributes to chronic loss over time due to over stimulation and islet exhaustion. In these two groups the differences over time become increasingly pronounced. The number of subjects maintaining insulin independence, the insulin requirements and the functional parameters all consistently demonstrate stability in alemtuzumab subjects while historical subjects demonstrate a progressive deterioration. This finding is a clear indicator that different immunosuppressive therapies such as this can play an important role in changing the long-term outcomes of islet grafts.
In the long term these data may indicate that chronic islet loss is not occurring due to the long term depletion of effector T cells (Teff). New data also suggest that the cell populations that return first may have a regulatory effect rather than a rejection effect. In a trial of renal transplantation using alemtuzumab there was an increase in CD4+CD25+FOXP3+ lymphocytes skewing the regulatory T cell:effector T cell (Tregs/Teff) ratio for years whereas induction with basiliximab, an anti CD-25 agent, resulted in a sustained decrease in Tregs (2). Expansion of Tregs may contribute in regulating rejection responses and allowing long term survival of islet allografts. In historical subjects, CD25+ cells remained undetectable throughout the entire initial two years post completion due to continued daclizumab treatment.
The profound reduction in lymphocytes secondary to alemtuzumab administration occurs within hours and remains striking even at 2 years post administration even taking into consideration that humans take considerably longer to reconstitute compared with data in smaller mammals. While alemtuzumab does not effect stem cell populations, it might cause destruction of a long lived population of cells that is responsible for repopulation. The absolute suppression of lymphocyte appears to allow for graft survival even when immunosuppression levels are subtherapeutic. Clinically it is of concern to have counts this low and our concern led to the use of a colony stimulating factor on two occasions in the same subject when neutrophil counts approached life threatening levels and we noted a preserved neutrophil response to G-CSF without changes in lymphocyte levels. The frequency of G-CSF use was not different to historical controls. We also observed subjects very closely for infectious complications but did not see any increased frequency or severity compared with historical controls. We agree this is a small number to be concluding that devastating complications will not occur, however our findings concur with those in the literature and experiences at other centers using this agent. Long term follow up will further allow us to evaluate leukocyte repopulation and the potential underlying risk to recipient health.
Steroids were also administered in this protocol the day prior to initial islet infusion in order to avoid the cytokine storm associated with alemtuzumab cell lysis, but could have been beneficial for engraftment. Interestingly, it has been recently suggested that brief exposure of human islets in vivo and in vitro to methylprednisolone leads to a reduction in mRNA and protein levels of tissue factor, macrophage chemoattractan protein-1 and IL-8 and enhances ATP content without resulting in permanent effects on insulin secretion and metabolism(8).
Removal of CNI in the long-term has subjectively improved the tolerability of long-term immunosuppression although objective analysis of this is ongoing. While the detrimental effects of CNI on islet function are well known (13; 21), sirolimus is not without its limitations, particularly the prevention of proliferation (27). One subject in the Alemtuzumab group who received a single islet infusion developed severe toxicity to sirolimus and was switched to tacrolimus-MMF. The subject is currently on 7U of Lantus daily with excellent glycemic control with 5.6% HbA1c. Kaplan et al recently reported the case of an insulin independent islet transplant recipient who was switched from sirolimus-tacrolimus maintenance to low dose tacrolimus-MMF and monthly daclizumab infusions due to several sirolimus associated toxicities (6). By 12 months post immunosuppression conversion, the patient remained insulin independent. While both regimens are effective, individual subjects can have unique responses to different immunosuppressive agents. Since one of the goals in islet transplantation is to find a regimen that is more tolerable for recipients, this may be guided more by the individual side effect profile rather than the theoretical long term effect on islet function. An analysis of the side effect profile of each regimen is ongoing.
These results raise our optimism that both short and long term outcomes of islet transplantation can match those of solid organ transplantation while minimizing risks. Clearly the statistical analysis of these subjects is limited due to low numbers, however, these promising results suggest more attention should be focused in this area and further investigation should help to prove the validity of these results. Stimulation data in the historical group is limited by the fact that subjects are lost during follow-up secondary to withdrawal or supplemental infusion. Nevertheless, even with the `best of the best' remaining in the historical group, the differences remain significant. Induction with alemtuzumab seems to have a beneficial effect on mass preservation at the time of engraftment and during follow-up. Careful selection of subjects should still be performed while the IT community continues to realize the potential of this therapy.
ACKNOWLEDGEMENTS
This work was supported by a grant from JDRF International (4-2004-361), State of Florida and Diabetes Research Institute Foundation.
Footnotes
Conflict of interest statement The authors declare that there is no duality of interest associated with this manuscript.
REFERENCES
- 1.Bennet W, Groth CG, Larsson R, Nilsson B, Korsgren O. Isolated human islets trigger an instant blood mediated inflammatory reaction: implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Ups J Med Sci. 2000;105:125–133. doi: 10.1517/03009734000000059. [DOI] [PubMed] [Google Scholar]
- 2.Bloom DD, Chang Z, Fechner JH, Dar W, Polster SP, Pascual J, et al. CD4+ CD25+ FOXP3+ regulatory T cells increase de novo in kidney transplant patients after immunodepletion with Campath-1H. Am J Transplant. 2008;8:793–802. doi: 10.1111/j.1600-6143.2007.02134.x. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez LA, Lehmann R, Luzi L, Battezzati A, Angelico MC, Ricordi C, et al. The effects of maintenance doses of FK506 versus cyclosporin A on glucose and lipid metabolism after orthotopic liver transplantation. Transplantation. 1999;68:1532–1541. doi: 10.1097/00007890-199911270-00017. [DOI] [PubMed] [Google Scholar]
- 4.Froud T, Ricordi C, Baidal DA, Hafiz MM, Ponte G, Cure P, et al. Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: Miami experience. Am J Transplant. 2005;5:2037–2046. doi: 10.1111/j.1600-6143.2005.00957.x. [DOI] [PubMed] [Google Scholar]
- 5.Huang E, Cho YW, Hayashi R, Bunnapradist S. Alemtuzumab induction in deceased donor kidney transplantation. Transplantation. 2007;84:821–828. doi: 10.1097/01.tp.0000281942.97406.89. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan B, West P, Neeley H, Martellotto J, Iqbal R, Gangemi A, et al. Use of low dose tacrolimus, mycophenolate mofetil and maintenance IL-2 receptor blockade in an islet transplant recipient. Clin Transplant. 2008;22:250–253. doi: 10.1111/j.1399-0012.2007.00757.x. [DOI] [PubMed] [Google Scholar]
- 7.Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003;3:178–185. doi: 10.1034/j.1600-6143.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 8.Lund T, Fosby B, Korsgren O, Scholz H, Foss A. Glucocorticoids reduce pro-inflammatory cytokines and tissue factor in vitro and improve function of transplanted human islets in vivo. Transpl Int. 2008 Jul;21(7):669–78. doi: 10.1111/j.1432-2277.2008.00664.x. [DOI] [PubMed] [Google Scholar]
- 9.Navasa M, Bustamante J, Marroni C, Gonzalez E, Andreu H, Esmatjes E, et al. Diabetes mellitus after liver transplantation: prevalence and predictive factors. J Hepatol. 1996;25:64–71. doi: 10.1016/s0168-8278(96)80329-1. [DOI] [PubMed] [Google Scholar]
- 10.Nishida S, Levi DM, Moon JI, Madariaga JR, Kato T, Selvaggi G, et al. Intestinal transplantation with alemtuzumab (Campath-1H) induction for adult patients. Transplant Proc. 2006;38:1747–1749. doi: 10.1016/j.transproceed.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 11.Noris M, Casiraghi F, Todeschini M, Cravedi P, Cugini D, Monteferrante G, et al. Regulatory T cells and T cell depletion: role of immunosuppressive drugs. J Am Soc Nephrol. 2007;18:1007–1018. doi: 10.1681/ASN.2006101143. [DOI] [PubMed] [Google Scholar]
- 12.Ozmen L, Ekdahl KN, Elgue G, Larsson R, Korsgren O, Nilsson B. Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: possible application of the thrombin inhibitor melagatran in clinical islet transplantation. Diabetes. 2002;51:1779–1784. doi: 10.2337/diabetes.51.6.1779. [DOI] [PubMed] [Google Scholar]
- 13.Redmon JB, Olson LK, Armstrong MB, Greene MJ, Robertson RP. Effects of tacrolimus (FK506) on human insulin gene expression, insulin mRNA levels, and insulin secretion in HIT-T15 cells. J Clin Invest. 1996;98:2786–2793. doi: 10.1172/JCI119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan EA, Lakey JR, Paty BW, Imes S, Korbutt GS, Kneteman NM, et al. Successful islet transplantation: continued insulin reserve provides long-term glycemic control. Diabetes. 2002;51:2148–2157. doi: 10.2337/diabetes.51.7.2148. [DOI] [PubMed] [Google Scholar]
- 15.Ryan EA, Lakey JR, Rajotte RV, Korbutt GS, Kin T, Imes S, et al. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes. 2001;50:710–719. doi: 10.2337/diabetes.50.4.710. [DOI] [PubMed] [Google Scholar]
- 16.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 17.Scantlebury V, Shapiro R, Fung J, Tzakis A, McCauley J, Jordan M, et al. New onset of diabetes in FK 506 vs cyclosporine-treated kidney transplant recipients. Transplant Proc. 1991;23:3169–3170. [PMC free article] [PubMed] [Google Scholar]
- 18.Senior PA, Zeman M, Paty BW, Ryan EA, Shapiro AM. Changes in renal function after clinical islet transplantation: four-year observational study. Am J Transplant. 2007;7:91–98. doi: 10.1111/j.1600-6143.2006.01573.x. [DOI] [PubMed] [Google Scholar]
- 19.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 21.Strumph P, Kirsch D, Gooding W, Carroll P. The effect of FK506 on glycemic response as assessed by the hyperglycemic clamp technique. Transplantation. 1995;60:147–151. [PubMed] [Google Scholar]
- 22.Thomas PG, Woodside KJ, Lappin JA, Vaidya S, Rajaraman S, Gugliuzza KK. Alemtuzumab (Campath 1H) induction with tacrolimus monotherapy is safe for high immunological risk renal transplantation. Transplantation. 2007;83:1509–1512. doi: 10.1097/01.tp.0000263344.53000.a1. [DOI] [PubMed] [Google Scholar]
- 23.Tzakis AG, Kato T, Nishida S, Levi DM, Tryphonopoulos P, Madariaga JR, et al. Alemtuzumab (Campath-1H) combined with tacrolimus in intestinal and multivisceral transplantation. Transplantation. 2003;75:1512–1517. doi: 10.1097/01.TP.0000060250.50591.39. [DOI] [PubMed] [Google Scholar]
- 24.Walsh R, Ortiz J, Foster P, Palma-Vargas J, Rosenblatt S, Wright F. Fungal and mycobacterial infections after Campath (alemtuzumab) induction for renal transplantation. Transpl Infect Dis. 2007 Dec 12; doi: 10.1111/j.1399-3062.2007.00292.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Watanabe T, Masuyama J, Sohma Y, Inazawa H, Horie K, Kojima K, et al. CD52 is a novel costimulatory molecule for induction of CD4+ regulatory T cells. Clin Immunol. 2006;120:247–259. doi: 10.1016/j.clim.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Weaver TA, Kirk AD. Alemtuzumab. Transplantation. 2007;84:1545–1547. doi: 10.1097/01.tp.0000296680.75175.67. [DOI] [PubMed] [Google Scholar]
- 27.Zahr E, Molano RD, Pileggi A, Ichii H, Jose SS, Bocca N, et al. Rapamycin impairs in vivo proliferation of islet beta-cells. Transplantation. 2007;84:1576–1583. doi: 10.1097/01.tp.0000296035.48728.28. [DOI] [PubMed] [Google Scholar]
- 28.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988 Apr;37(4):413–20. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 29.Ichii H, Pileggi A, Molano RD, Baidal DA, Khan A, Kuroda Y, et al. Rescue purification maximizes the use of human islet preparations for transplantation. Am J Transplant. 2005 Jan;5(1):21–30. doi: 10.1111/j.1600-6143.2005.00698.x. [DOI] [PubMed] [Google Scholar]