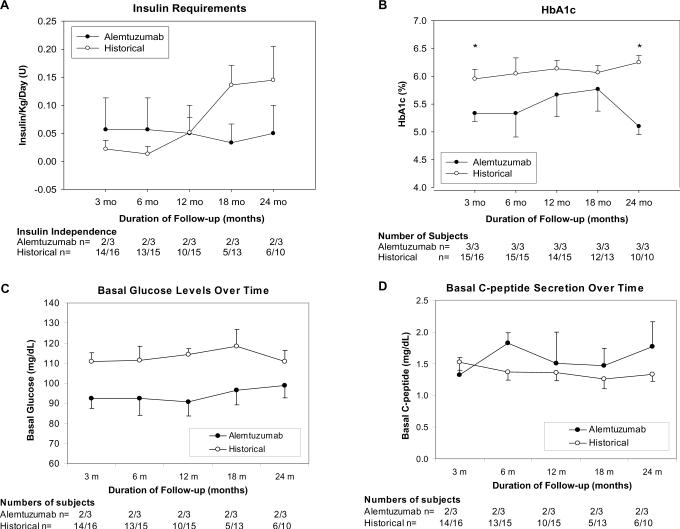
Figure 1.
Insulin Independence rates and Change in Insulin Requirements (A) demonstrate stability in Alemtuzumab group. By 24 months post infusion less than half of the Historical group remain insulin independent. HbA1c (B) is significantly better at 3 and 24 months post completion islet infusion in Alemtuzumab group (24 months 5.1±0.15 vs 6.3±0.12 % p<0.005). Results of routine protocol monitoring of Fasting Plasma Glucose (C) and C-peptide (D) up to 24 months post completion islet infusion demonstrate consistently better levels in the Alemtuzumab group, specifically normoglycemia compared to impaired fasting glucose levels in the Historical group.