Abstract
Acids 9 a–f as possible bivalent ligands designed as a structural combination of opioid μ-agonist (Fentanyl) and NSAID (Indomethacin) activities and produced compounds which were tested as analgesics. The obtained series of compounds exhibits low affinity and activity both at opioid receptors and as cyclooxygenase (COX) inhibitors. One explanation of the weak opioid activity could be stereochemical peculiarities of these bivalent compounds which differ significantly from the fentanyl skeleton. The absence of significant COX inhibitory properties could be explained by the required substitution of an acyl fragment in the indomethacin structure for 4-piperidyl.
1. Introduction
Since 1990, at least 50 new chemical entities for the treatment of pain have reached clinical development [1], but no one was marketed. Management of chronic pain is often highly individualized and combination therapy on a trial and error basis is not uncommon. During the last few years combined use of non-steroidal anti-inflammatory drugs (NSAIDs) and opioids has been indicated for achieving better analgesia with reduced side effects [2–15].
The synergistic effect in peripheral tissues is thought to result from actions on a common transduction pathway between NSAIDs and opioids [5,16–18], although other mechanisms have been proposed [19]. Multicomponent drugs where two or more agents are co-formulated in a single dosage form are increasingly popular in drug treatment. An alternative strategy is to develop a single chemical entity that is able to modulate multiple targets simultaneously [20–22].
There is an increasing readiness to challenge the current paradigm and to consider developing agents that modulate multiple targets simultaneously with the aim of enhancing efficacy or improving safety relative to drugs that address only a single target [22–29].
For these reasons, we hypothesize that bivalent ligands designed as a combination of two different pharmacophores with μ opioid agonist and NSAID activity could show antinociceptive efficacy in chronic neuropathic pain states. For this paper, Fentanyl molecule was chosen as a structural framework for the creation of possible bivalent ligands for pain relief.
Fentanyl, an opioid with an analgesic potency of about 80 times greater than that of morphine, was introduced into medical practice in the 1960s [30–33].
NSAIDs (COX inhibitors) which are used widely for the treatment of pain and inflammation states have been reviewed [34–37].
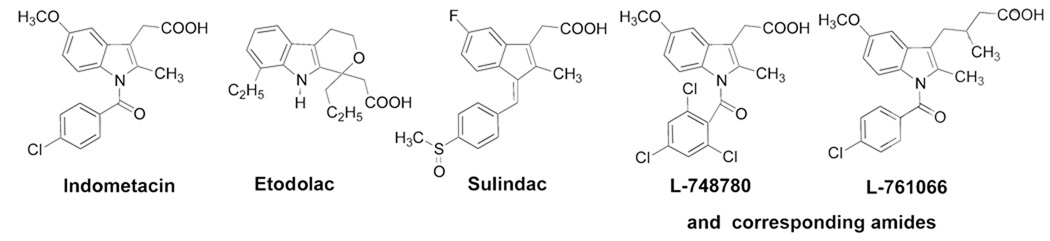
In this paper, we will focus on indolyl-acetic acids in our construction of possible bifunctional opioid agonist/COX inhibitor ligands. The development of indolyl / indene-acetic acids derivatives have involved structural modifications of structures of the most popular drugs of this class of compounds, mainly Indomethacine [38,39]., Many structural changes have been made on indomethacin molecule. Compounds such as Sulindac [40] and Etodolac [41] are already marketed as pharmaceuticals, and others like L-748780 and, L-761066 [42,43] are still under investigation (Fig. 1).
Fig. 1.
Indolyl / indene-acetic acids derivatives.
2. Results and discussion
2. 1. Chemistry
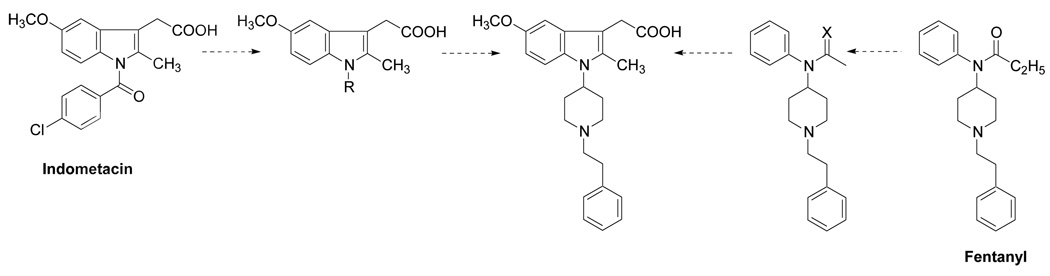
The main goal of this investigation was to develop a single chemical entity combining pharmacophore elements of two powerful analgesics acting by different mechanisms into one molecule. By analogy with approaches to the creation of peptide drugs, where the combination of two active peptides gives excellent results, we hypothesized that combination of the essential parts of two small molecules - the known opioid Fentanyl with the known COX inhibitor Indomethacin might lead to new pharmacological properties (Fig. 2).
Fig. 2.
Combination of the essential parts of the known opioid fentanyl with the known COX inhibitor indomethacin.
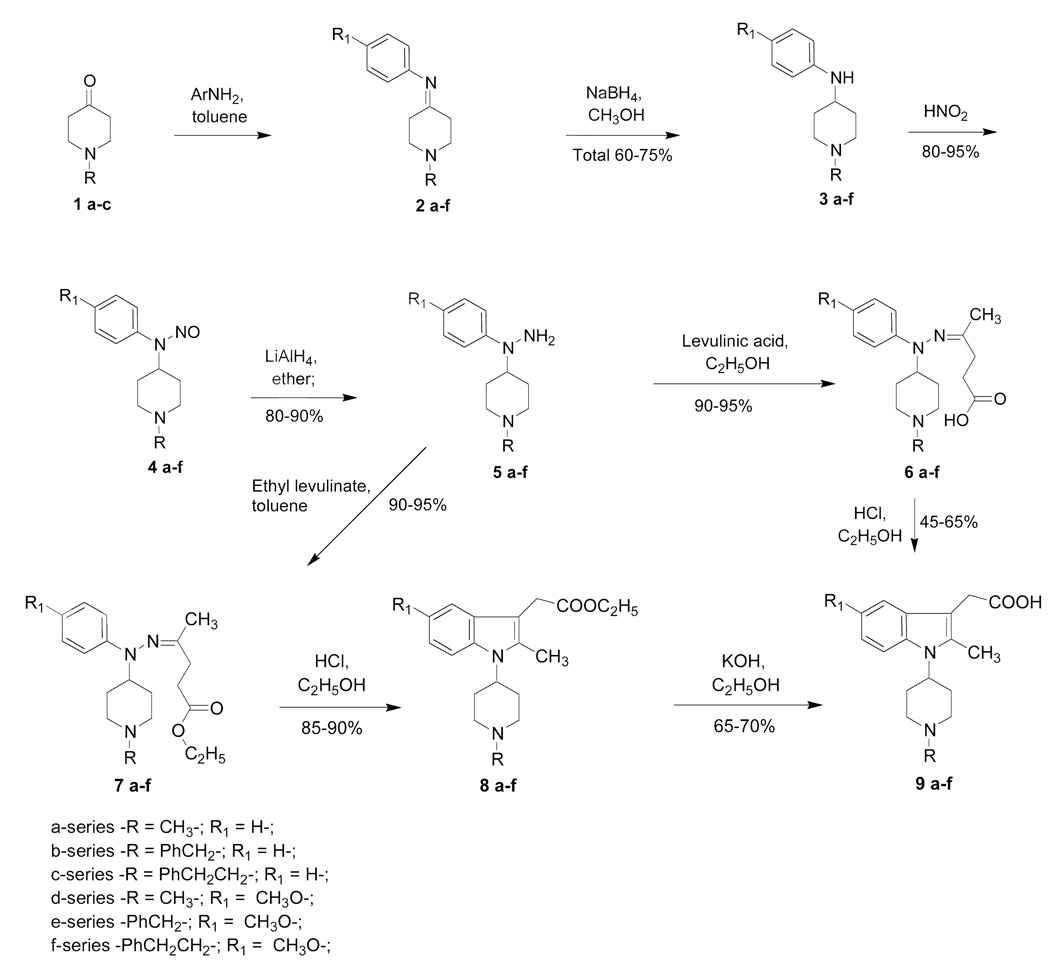
N-(Piperidin-4-yl)-N-phenylhydrazines 5 have been designed as starting materials for creation of indolylpiperidine derivatives (Scheme 1). They were synthesized starting from piperidine-4-ones 1a–c which were condensed with aniline or substituted anilines to give the desired imines (Schiff bases) 2a–f, then hydrogenated with sodium borohydride to obtain 4-anilinopiperidines 3 a–f. These 4-anilinopiperidines were nitrosylated to produce N-nitroso-compounds 4 a–f, then hydrogenated to the desired 4-piperidylphenylhydrazines 5 a–f. 4-Piperidylphenylhydrazines 5 a–f were condensed with levulinic acid or its esters and obtained hydrazones 6 a–f and 7 a–f underwent Fisher type reactions which led to indole derivatives – a series of indomethacine analogs 8 a–f and 9 a–f. Different catalysts have been evaluated for this reaction [44,45] and the optimal one was found to be the simplest – HCl in ethanol for both cases 6→9 and 7→8. Basic hydrolysis of 8 a–f led to the desired 9 a–f. The sequence 5→7→8→9 was found optimal for its ease of separation and purification of products. These heterocyclization reactions present unlimited possibilities for creation of a wide diversity of noncondensed indolopiperidines.
Scheme 1.
Synthesis of [2-methyl-1-(1-methyl-piperidin-4-yl)-1H-indol-3-yl]-acetic acids
2.2 Molecular Modeling
Docking of small molecules was performed using FlexiDock within the Sybyl 7.2 suite of programs [46]. Ligand molecules were constructed using Sybyl 7.2/Builder module. The piperidine nitrogen was protonated while the acid group was used as a carboxylate. The X-ray crystal structure of cyclooxygenase-2 with indomethacin (PDB code: 4cox) [47] was used for docking studies. Initially, the ligand molecule is placed within the active site of the protein and this complex was minimized using 1000 steps of minimization. This minimized complex was used as an input for FlexiDock calculations. FlexiDock calculations involved selecting rotatable bonds for the ligand and then exploring orientations of the ligand within the protein active site. FlexiDock results were obtained as various orientations of the ligand within the active site. These different orientations were analyzed on the basis of FlexiDock score as well as interactions between ligand and the enzyme active site.
A homology model for the μ opiate receptor was built using Sybyl / Biopolymer module. The X-ray crystal structure of bovine rhodopsin (PDB code: 1F88) was used as a template protein [48]. Amino acid residues were replaced by the corresponding μ opioid receptor residues and the model structure was refined and used for Flexidock calculations. Flexidock scores for series 9a – f were obtained as described above and the data is presented in the Table 1.
Table 1.
Flexidock Score for molecules 9a–f with COX-2 and μ opiate receptor
| Compound | FlexiDock scores with COX-2 | Compound | FlexiDock scores with opiate μ-receptor |
|---|---|---|---|
| Indomethacin | − 101.7 | Fentanyl | − 25.0 |
| Compound 9a | − 95.3 | Compound 9a | − 79.0 |
| Compound 9b | − 116.1 | Compound 9b | − 57.0 |
| Compound 9c | − 110.9 | Compound 9c | − 36.0 |
| Compound 9d | − 100.0 | Compound 9d | − 67.0 |
| Compound 9e | − 117.3 | Compound 9e | − 68.0 |
| Compound 9f | − 113.4 | Compound 9f | − 77.0 |
Since more negative the FlexiDock scores result in better binding, the compounds show better binding at both COX2 and the μ opiate receptor. Compounds 9a–f show COX-2 inhibitor activity practically equal to indomethacin and opioid activity 2–3 times higher than that of Fentanyl. Thus, docking experiments showed marginal results; but unfortunately, the biological activity data is not consistent with the results from molecular modeling.
2.3 Pharmacology
Table 2 shows the functional characterization of compounds 9a–f at δ- and μ-opioid receptors, using guinea pig isolated ileum/longitudinal muscle myenteric plexus (GPI/LMMP) as a source of μ opioid receptors and mouse vas deferens (MVD) as a source of δ opioid receptors.
Table 2.
Results in the MVD and GPI/LMMP
| Assays | MVD | GPI/LMMP | DPDPE Antagonism at 1 µM in the MVD |
PL-017 Antagonism at 1 µM in the GPI/LMMP |
|---|---|---|---|---|
| Compounds | Agonist activity (% inhibition of contraction at 1 µM) or IC50 (nM) ± S.E.M. | |||
| 9a | 17.9 % at 1 µM | 0.7 % at 1 µM | none at 1 µM | none at 1 µM |
| 9b | 1266 +/− 355 | 5164 +/− 2043 | -- | -- |
| 9c | 19.5 % at 1 µM | 3.1 % at 1 µM | none at 1 µM | none at 1 µM |
| 9d | 2.8 % at 1 µM | 0 % at 1 µM | none at 1 µM | none at 1 µM |
| 9e | 8.3 % at 1 µM | 3 % at 1 µM | none at 1 µM | none at 1 µM |
| 9f | 0 % at 1 µM | 6 % at 1 µM | none at 1 µM | none at 1 µM |
Footnotes: DPDPE ([D-Pen2, D-Pen 5]enkephalin) was used to inhibit MVD contraction by selective activation of the delta opioid receptors in this tissue. Compounds 9a–f (at 1 µM) did not reverse the effect of DPDPE. PL-017 was used to inhibit GPI contraction by selective activation of the μ opioid receptors in this tissue. Compounds 9a–f (at 1 µM) did not reverse the effect of PL-017.
The data indicate that the ligands have a very weak range of bioactivities at both δ and μ opioid receptors, practically independent on their respective structures. The weak or lack of opioid activity (agonist or antagonist) is likely due to low affinity of these compounds at opioid receptors, which was confirmed by radioligand competition assays. Compounds 9c and 9f (direct Fentanyl analogues) competed against the binding of the opioid receptor antagonist [3H]diprenorphine in rat brain membranes with inhibition constants in the micromolar range (data not shown).
We tested the effect of 9a–f, at a concentration of 50 nM, on COX-1 or COX-2 mediated prostaglandin production by an enzymatic immunoassay (EIA). We chose 50 nM as the test concentration based on the rationale that a lead compound should have an IC50 value at COX of equal or less than 100 nM, or within an order of magnitude of its affinity (commonly in the nanomolar range) for opioid receptors. The parent compound of 9a–f, indomethacin, inhibits both COX-1 and COX-2, and is more potent at COX-1. The IC50 values for indomethacin in inhibiting prostaglandin production ranged between 40–80 nM for COX-1 and 600–1000 nM for COX-2 [49], whereas COX-2 selective compounds such as celecoxib showed IC50 values in the low nanomolar range for COX-2 in the same assay [49]. However, compounds 9a–f at 50 nM did not inhibit prostaglandin production by COX-1 or COX-2.
Compounds 9a–f were also evaluated in rodent models of acute and chronic pain. The compounds, each given as a single dose of 10 µg into the lumbar spinal cord of naïve Sprague-Dawley rats by intrathecal injection, did not prolong the latency to a noxious thermal stimulus to the hind paw, indicating a lack of antinociceptive activity at least at the dose given (data not shown). In a rat model of neuropathic pain, the same dose of these compounds given intrathecally also had no effect on the abnormal pain state associated with the nerve injury in the injured rats, or on the sensory thresholds of control rats that were given a sham surgery (data not shown). The lack of antinociceptive activity of these compounds is in line with the apparent low affinity and lack of biological activity at opioid receptors indicated by the in vitro assays.
3. Discussions
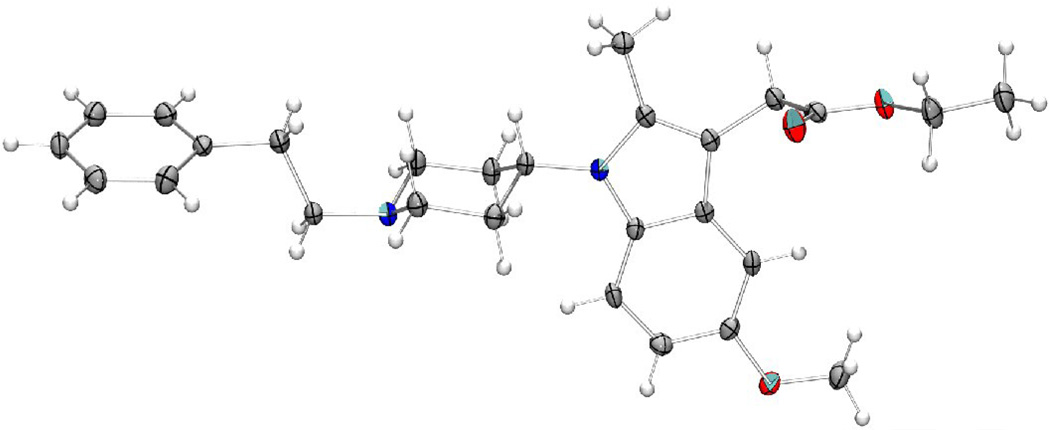
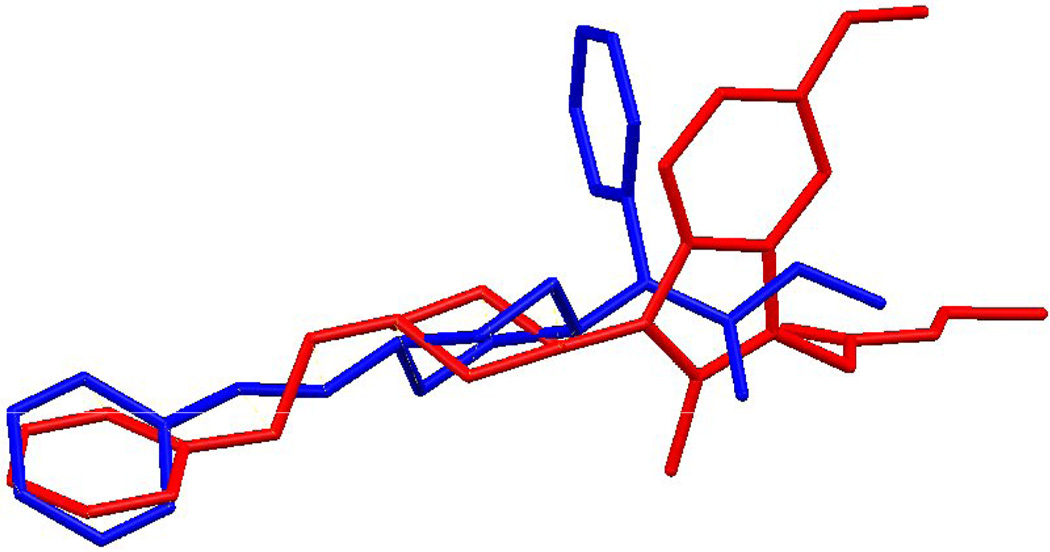
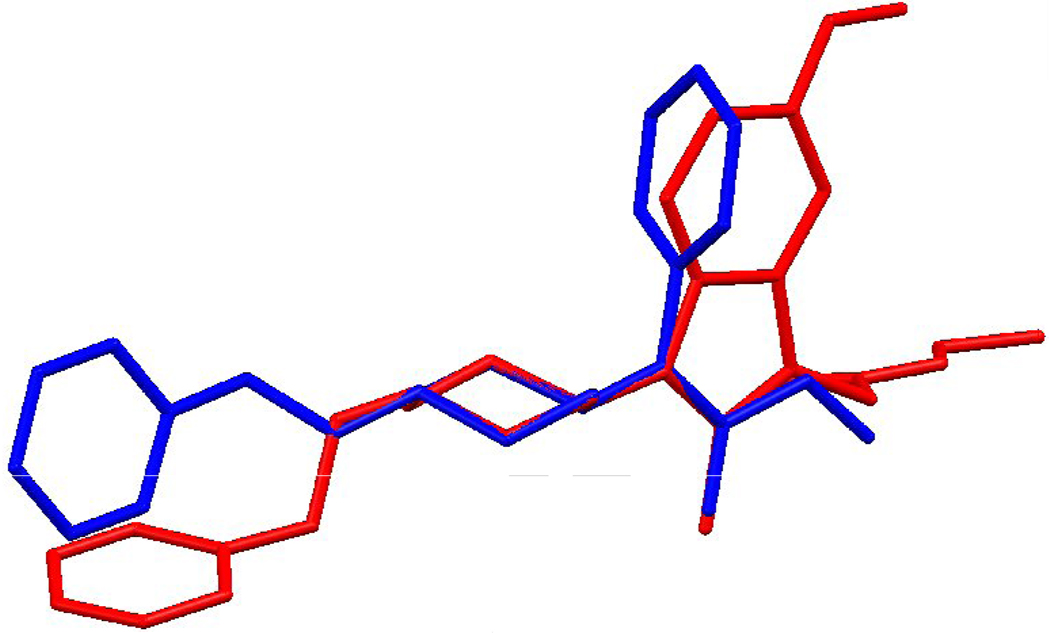
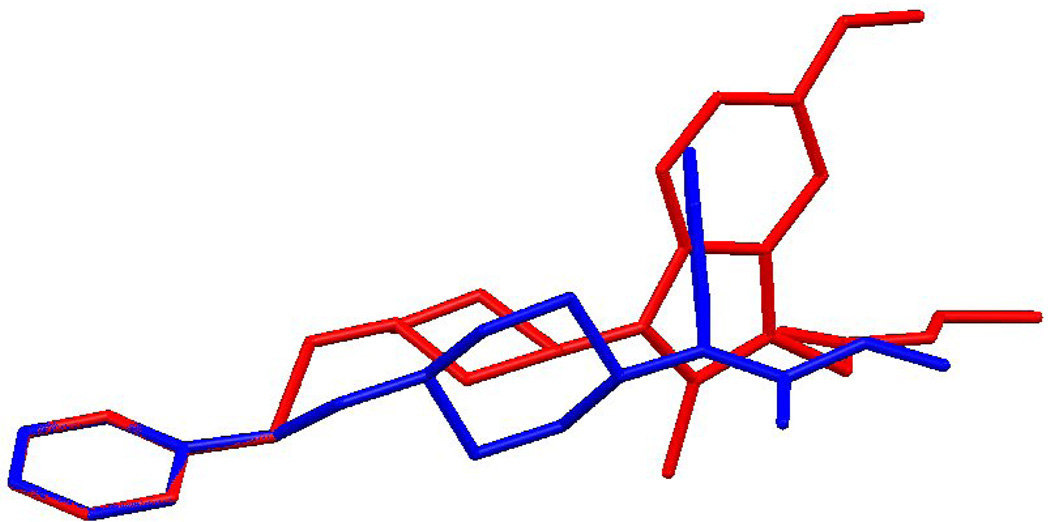
A possible explanation of these results could be related to the stereochemical peculiarities of these compounds which could differ from the fentanyl structure. For a comparison of structures of Fentanyl and the 2-methyl-1-(1-substituted-piperidin-4-yl)-1H-indol-3-yl]-acetic acids, an X-Ray analysis of the 8c (Fig. 3) and superposition of its structure with the X-Ray structure of fentanyl has been examined (Fig. 4–Fig. 6). The Fentanyl data is taken from the Cambridge Structural Database [50, 51].
Fig. 3.
The molecular structure of 8c with anisotropic displacement ellipsoids at the 50% probability level.
Fig. 4.
Least-squares average overlay of piperidine rings of Fentanyl (blue) and 8c (red).
Fig. 6.
Overlapping the 4-(N-Phenyl) phenyl rings of fentanyl (blue) and 8c (red).
An exact overlay of the phenyl and piperidine rings with the linker is prevented by differences in their torsion angles. Overlay 1 (Fig. 4) is a least-squares average overlay of these two rings. Overlay 2 (Fig. 5) has only the phenyl rings overlapping and Overlay 3 (Fig. 4) overlaps only the piperidine rings. In all three diagrams compound 8c is in red.
Fig. 5.
Overlapping the 1-(2-Phenethyl) phenyl rings of fentanyl (blue) and 8c(red).
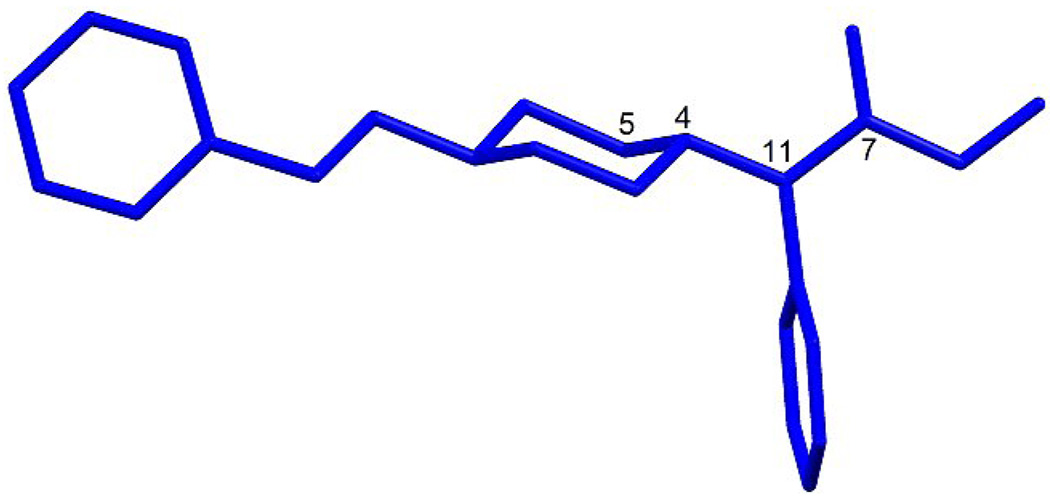
Because it is well documented that the biological activity of Fentanyl and its derivatives is greatly influenced by their stereochemistry[50] the differences in the structure between compounds 9a–f and Fentanyl could account for the loss of affinity and efficacy at μ opioid receptor. For optimal interaction with the μ opioid receptor it is necessary to have both a trans configuration for amide group with the orientation of phenyl ring practically perpendicular to the amide function a 4-N-propionylamide substituent with the ϕ angle (5-4-11-7) in the range of 0 – 30° and an extended conformation of the phenethyl group (Fig 7).
Fig 7.
The orientation of the phenyl rings in fentanyl.
On the other hand, the compounds’ lack of COX inhibitory activity at 50 nM suggests that their IC50 values may be significantly larger than 50 nM, or that the substitution of a necessary acyl fragment in the Indomethacin structure for 4-piperidyl has caused a right shift of the dose effect at COX.
4. Conclusions
The overall conclusion is that this series of novel hybrid fentanyl/indomethacin compounds exhibits low affinity and activity at the opioid receptors and COX. Identification of small molecule bi-ligand drugs with a high binding affinity is a very difficult task. Bi-ligand drug candidates are very dependent on the linker between the two active pharmacophores, its nature, length and flexibility, places of attachments, etc. These factors, which are not very decisive for peptide pairs, become critical for small molecules, which are very sensitive to the minor changes in structure, where arrangement of every atom is crucial. Our attempt to combine opioid and NSAID pharmacophores in one small molecule, which succeeded as a chemical approach, gave unsuccessful pharmacological results. The fentanyl/indomethacin hybrid molecule obtained lost both opioid and COX inhibition properties, which demonstrates that implementation of approaches in peptide science are not necessarily applicable to small molecules.
5. Experimental
5.1. Chemistry
The compounds were characterized by 1H and 13C NMR. Nuclear magnetic resonance spectra were recorded with on Bruker DRX-500 and DRX-600 spectrometers using tetramethylsilane as internal standard. IR spectra were recorded on Nicolet Avatar 360 FT-IR instrument in mineral oil. The compounds were analyzed by electrospray mass spectrometry on a Thermoelectron (Finnigan) LCQ classic ion trap mass spectrometer. Standard ESI conditions were applied and the masses of protonated molecules [MH]+ were measured. Direct infusion (10 uL/min) was applied to ca. 50 uM solutions of the samples in MeOH:H2O 1:1 2% AcOH. High resolution mass spectra were obtained on the 9.4 T Bruker FT-ICR-MS spectrometer in MeOH:ACN 1:1. The purity of compounds was determined by TLC on silica gel plates (Analtech 02521), solvent system MeOH:CHCl3 1:4. Melting points are uncorrected.
5.1.1 General method for the synthesis of the N-(1-Substituted-piperidin-4-yl)anilines 3a–f
A solution of 0.1 mol of a 1-substituted-piperidin-4-one, 0.125 mol of the appropriate phenylamine and 2–3 drops of acetic acid in 200 mL of toluene was heated on stirring under reflux (2–3-hours) with a Dean-Stark trap till the full separation of water. The toluene and excess of phenylamine were removed in vacuo. The residue was dissolved in 200 mL of ether and filtered through a layer of neutral alumina. The remaining pretty pure compound was used for hydrogenation without further purification. In cases with 1-methyl-piperidin-4-ylidene derivatives distillation of the products is recommended 132°C (2 mm Hg) for 2a; 138°C (4 mm Hg) for 2d.
The 1-substituted-piperidin-4-ylidene derivatives 2a–f (0.1 mol) were dissolved in 150 mL of methanol and 3.8 g (0.1 mol) of NaBH4 was added gradually, following stirring during 30 minutes. Obtained solution was stirred for additional 4–5 hours an left over night. The residue was dissolved into ether, dried over MgSO4, filtered and the solvent was removed in vacuum. Remaining solid was crystallized. Recrystallized from hexanes or from diethyl ether. Total yields are varying between 60–76%.
5.1.2. (1-Methyl-piperidin-4-yl)-phenyl-amine (3a)
Crystalline solid (60.7%), mp 81–83 °C, (bp 140–141 °C (4mm Hg). IR: ν (cm−1) = 3390 (N–H); 1 H NMR (600 MHz, CDCl3) δ 7.14 (m, 2H), 6.66 (m, 1H), 6.57 (m, 2H), 3.47 (d, 1 H, J = 7.79 Hz), 3.27 (m, 1 H), 2.81 (d, 2H, J = 11.00 Hz), 2.29 (s, 3 H), 2.13 (t, 2H, J = 11.46 Hz), 2.05 (m, 2H), 1.49 ( 2 H, m). EI-MS: m/z 191; HRMS calcd for C12H19N2: 191.1543; found (ESI, [M+H]+): 191.1539.
5.1.3. (1-Benznyl-piperidin-4-yl)-phenyl-amine (3b)
Crystalline solid (72,3%), mp 84–86 °C. IR: ν (cm−1) = 3392 (N–H). 1H NMR (600 MHz, CDCl3) δ 7.34 (m, 4 H), 7.27 (m, 1H), 7.17 (m, 2H), 6.69 (m, 1H), 6.60 (m, 2H), 3.55 (s, 2H), 3.52 (d, 1H, J = 7.72 Hz), 3.31 (m 1H), 2.87 (d, 2 H, J = 11.71 Hz), 2.17 (m, 2H), 2.05 (m, 2H), 1.50 (m 2H). EI-MS: m/z 267. HRMS calcd for C18H32N2: 267.1856; found (ESI, [M+H]+): 267.1859.
5.1.4. [1-(2-phenyl)-ethyl-piperidin-4-yl]-phenyl-amine (3c)
Crystalline solid (75.1%), mp 96–98 °C. IR: ν (cm−1) = 3390 (N–H). 1H NMR (600 MHz, CDCl3) δ 7.28 (m, 2H), 7.20 (m, 3H), 7.16 (m, 2H), 6.68 (m, 1H), 6.59 (m, 2H), 3.52 (d, 1H, J = 7.85 Hz), 3.32 (m, 1H), 2.98 (d, 2 H, J = 11.40 Hz), 2.83 (m, 2 H), 2.63 (m, 2H), 2.23 (t, 2H, J = 10.96 Hz), 2.09 (d, 2 H, J = 11.86 Hz), 1.53 (m, 2H). EI-MS: m/z 281. HRMS calcd for C19H24N2: 281.2012; found (ESI, [M+H]+): 281.2007.
5.1.5. 4-Methoxyphenyl-(1-Methyl-piperidin-4-yl)-amine (3d)
Crystalline solid (65.0%), mp 46–47 °C, (bp 174–175 °C (4mm Hg). IR: ν (cm−1) = 3391 (N–H). 1H NMR (500 MHz, CDCl3) δ 6.72 (m, 2H), 6.54 (m, 2H), 3.70 (s, 3H), 3.17 (m, 2H), 2.77 (d, 2 H, J = 11.19 Hz), 2.26 (s, 3H), 2.07 (t, 2H, J = 11.20 Hz), 2.00 (d, 2 H, J = 12.60 Hz), 1.43 (m, 2H). EI-MS: m/z 221. HRMS calcd for C13H20N2O: 221.1648; found (ESI, [M+H]+): 221.1655.
5.1.6. 4-Methoxyphenyl-(1-Benznyl-piperidin-4-yl)-amine (3e)
Crystalline solid (76,7%), mp 64–66 °C. IR: ν (cm−1) = 3393 (N–H). 1H NMR (600 MHz, CDCl3) δ 7.32 (m, 4H), 7.25 (m, 1H), 6.75 (m, 2H), 6.56 (m, 2H), 3.72 (s, 3H), 3.53 (s, 2H), 3.20 (m, 1H), 2.85 (d, 2H, J = 11.53 Hz), 2.14 (m, 2H), 2.01 (d, 2H, J = 12.21 Hz), 1.47 (m, 2H). EI-MS: m/z 297. HRMS calcd for C19H25N2O: 297.1961 found (ESI, [M+H]+): 297.1966.
5.1.7. 4-Methoxyphenyl-[1-(2-phenyl)-ethyl-piperidin-4-yl]-amine (3f)
Crystalline solid (74,8 %), mp 93–95 °C. IR: ν (cm−1) = 3390 (N–H). 1H NMR (600 MHz, CDCl3) δ 7.27 (m, 2H), 7.19 (m, 3H), 6.76 (m, 2H), 6.57 (m, 2H), 3.73 (s, 3H), 3.24 (m, 1H), 3.00 (m, 2H), 2.84 (m, 2H), 2.65 (m, 2H), 2.24 (m, 2H), 2.08 (m, 2H), 1.53 (m, 2H). EI-MS: m/z 311. HRMS calcd for C20H27N2O: 311.2118 found (ESI, [M+H]+): 311.2126.
5.1.8. General method for the synthesis of N-(1-Substituted-piperidin-4-yl)-nitrosoanilines (4 a–f)
To a stirred cooled (ice) mixture of 0,1 mole of N-(1-Substituted-piperidin-4-yl)aniline 3(a–f) dissolved in 100 mL of ethanol and of 200 g of ice, was added (reaction was carried out under an argon atmosphere) dropvise, during 0.5 hour, 0.5 mol (~50mL of HCl conc.) diluted with 150 mL of ice cold water for N-Methyl derivatives 4a,d and 0,75 mol (~75mL of HCl conc.) diluted with 225 mL of ice cold water for derivatives 4b,c,e,f
After 15 min. to an ice cold solution of 0.2 mol of NaNO2 in 40 mL of water was added dropvise, at −8°–5 ° C. Then the obtained mixture was stirred for an additional 4 hours at the same temperature. 200 mL of dichloromethane was added to the mixture and at the temperature below 0° C the mixture was made basic with a cold solution of 0.5 mol (for N-Methyl derivatives 4a,d) and 0,75 mol (for derivatives 4b,c,e,f) of K2CO3 in 125 (187.5) mL of water. Dichloromethane layer was separated, and the water layer was extracted two times with 50 mL portions of dichloromethane and the combained DCM dried over MgSO4. The solvent was removed in vacuo. The residue was dissolved in 200 mL of ether and filtered through a layer of neutral alumina. Remaining after evaporation of ether pure (TLC) nitroso-compounds (sometime crystalline) were immediately used for hydrogenation without further purification. Total yields were vary between 80–95%. (Attempts to distill obtained compounds were unsuccessful due to very quick disintegration processes close to explosion which started during heating). Nevertheless Mass- Spectras registered immediately after preparation showed 100% presence of [MH]+ for all cases.
5.1.9. N-(1-methyl-piperidin-4-yl)-nitrosoaniline (4a)
Crystalline residue (84.0%). IR: ν (cm−1) = 1592 (N=O). EI-MS: m/z 220 [M+H]+. MS/MS fragmentation base peak m/z 180 [M+H−NO]+.
5.1.10. N-(1-benzyl-piperidin-4-yl)-nitrosoaniline (4b)
Crystalline residue (89.5%). IR: ν (cm−1) = 1591 (N=O). EI-MS: m/z 296 [M+H]+. MS/MS fragmentation base peak m/z 266 [M+H−NO]+.
5.1.11. N-[1-(2-phenyl)-ethyl-piperidin-4-yl]-nitrosoaniline (4c)
Crystalline residue (95.0%). EI-MS: m/z 310 [M+H]+. IR: ν (cm−1) = 1590 (N=O). MS/MS fragmentation base peak m/z 280 [M+H−NO]+.
5.1.12. 4-Methoxyphenyl-(1-methyl-piperidin-4-yl)-N-nitrosoamine (4d)
Light yellow liquid (77.5%). IR: ν (cm−1) = 1590 (N=O). EI-MS: m/z 250 [M+H]+. MS/MS fragmentation base peak m/z 220 [M+H−NO]+.
5.1.13. 4-Methoxyphenyl-(1-benzyl-piperidin-4-yl)-N-nitrosoamine(4e)
Light yellow liquid (77.8%). IR: ν (cm−1) = 1593 (N=O). EI-MS: m/z 326 [M+H]+. MS/MS fragmentation base peak m/z 296 [M+H−NO]+.
5.1.14. 4-Methoxyphenyl-[1-(2-phenyl)-ethyl-piperidin-4-yl]- N-nitrosamine (4f)
Crystalline residue (96%). IR: ν (cm−1) = 1591 (N=O). EI-MS: m/z 340 [M+H]+. MS/MS fragmentation base peak m/z 310 [M+H−NO]+.
5.1.15. General method for the synthesis of (1-substituted-piperidin-4-yl)-N-phenyl-hydrazines (5a–f)
A N-(1-Substituted-piperidin-4-yl)-nitrosoaniline 4a–f (0,1 mol) was dissolved in the mixture of 150 mL of dry THF and 50 mL of dry ether was dropped cautionously during one hour to a stirring and gently boiling (reaction was carried out under an argon atmosphere) mixture of 0.125 mol of LiAlH4 in 450 mL of dry ether. Stirring and gentle boiling was continued for 3 hours and the mixture was left under Argon at room temperature overnight. Then the mixture which was cooled to −5°– 0° C and X very slowly, cautionously was added 3.8 mL of water, then after 10–15 min. 3.8 mL of 15% NaOH, and then after additional 10–15 min. 11,4 mL of water. The mixture was stirred at −5°– 0° C for additional 1–2 hours untill full disappearance of characteristic gray color which gave clear pale yellow solution with separated aluminum salts. This was filtered through Buchner funnel and the solution dried over MgSO4. The solution was filtered through a layer of neutral alumina and the solvents were removed in vacuo. The remaining pure compound is possible to distill for cases 5a and 5d or crystallize from ether, hexanes or ethanol for cases 5b,c,e,f. Total yields vary between 58–92%.
5.1.16. N-(1-methyl-piperidin-4-yl)- N-phenyl-hydrazine (5a)
Crystalline solid (71.5%), mp 58–60 °C. IR: ν (cm−1) = 3352 (Hydrazine N-H). 1H NMR (600 MHz, CDCl3) δ 7.25 (m, 2H), 7.01 (m, 2H), 6.78 (m, 1H), 3.58 (tt, 1 H, J = 11.43, 3.90 Hz), 3.31 (bs, 2H), 2.96 (m, 2 H), 2.31 (s, 3H), 2.07 (dt, 2H, J = 2.00, 11.99 Hz), 1.95 (dq, 2H, J = 3.63, 12.20 Hz), 1.69 (m, 2H). EI-MS: m/z 206. HRMS calcd for C12H20N3: 206.1652 found (ESI, [M+H]+): 206.1660.
5.1.17. N-(1-benzyl-piperidin-4-yl)- N-phenyl-hydrazine (5b)
Crystalline solid (77.4%), mp 103–105 °C. IR: ν (cm−1) = 3350 (Hydrazine N-H). 1H NMR (600 MHz, CDCl3) δ 7.36 (m, 4H), 7.27 (m, 3H), 7.01 (d, 2H, J = 8.16 Hz), 6.79 (t, 1H, J = 7.24 Hz), 3.62 (m, 3H), 3.35 (bs, 2H), 3.08 (d, 2H, J = 11.16 Hz), 2.20 (m, 2H), 2.05 (m, 2H), 1.71 (m, 2H). EI-MS: m/z 282. HRMS calcd for C18H24N3: 282.1965 found (ESI, [M+H]+): 282.1972.
5.1.18. N-[1-(2-phenyl)-ethyl-piperidin-4-yl]- N-phenyl-hydrazine (5c)
Crystalline solid (92.8%), mp 72–74 °C. IR: ν (cm−1) = 3354 (Hydrazine N-H). 1H NMR (600 MHz, CDCl3) δ 7.30 (m, 4H), 7.24 (m 3H), 7.05 (m, 2H), 6.81 (m, 1H), 3.65 (tt, 1H, J = 11.48, 3.88 Hz), 3.35 (s, 2H), 3.17 (d, 2H, J = 11.67 Hz), 2.86 (m, 2H), 2.67 (m, 2H), 2.19 (t, 2H, J = 11.51 Hz), 2.02 (dq, 2 H, J = 3.68, 12.25 Hz), 1.77 (m, 2H). EI-MS: m/z 296. HRMS calcd for C19H26N3: 296.2121 found (ESI, [M+H]+): 296.2128.
5.1.19. 4-Methoxy-phenyl-(1-Methyl-piperidin-4-yl)-hydrazine (5d)
Light yellow liquid (64.5%). IR: ν (cm−1) = 3351 (Hydrazine N-H). 1H NMR (500 MHz, CDCl3) δ 6.90 (m, 2H), 6.74 (m, 2H), 3.66 (s, 3H), 3.64 (m, 2H), 3.36 (bs, 2H), 3.26 (tt, 1H, J = 3.92, 11.38 Hz), 2.84 (m, 2 H), 1.93 (dt, 2 H, J = 2.49, 11.86 Hz), 1.78 (m, 2H), 1.59 (m, 2H). EI-MS: m/z 236. HRMS calcd for C13H22N3O: 236.1757 found (ESI, [M+H]+): 236.1748.
5.1.20. 4-Methoxy-phenyl-(1-Benzyl-piperidin-4-yl)- hydrazine (5e)
Crystalline solid (60.0%), mp 68–70 °C. IR: ν (cm−1) = 3353 (Hydrazine N-H). 1H NMR (600 MHz, CDCl3) δ 7.31 (m, 4H), 7.24 (m, 1H), 6.97 (m, 2H), 6.81 (m, 2H), 3.74 (s, 3H), 3.55 (s, 2H), 3.37 (tt, 1 H, J = 11.25, 3.79 Hz), 3.30 (s, 1H), 2.99 (d, 2 H, J = 11.70 Hz), 2.09 (m, 2H), 1.87 (m, 2H), 1.68 (d, 2 H, J = 11.02 Hz). EI-MS: m/z 312. HRMS calcd for C19H26N3O: 312.2070 found (ESI, [M+H]+): 312.2063.
5.1.21. 4-Methoxy-phenyl-[1-(2-phenyl)-ethyl-piperidin-4-yl]- hydrazine (5f)
Crystalline solid (81.5%), mp 95–96 °C. IR: ν (cm−1) = 3350 (Hydrazine N-H). 1H NMR (600 MHz, CDCl3) δ 7.27 (m, 2H), 7.19 (m, 3H), 6.99 (m, 2H), 6.83 (m, 2H), 3.75 (s, 3H), 3.39 (tt, 1H, J = 11.05, 3.79 Hz), 3.30 (s, 2H), 3.13 (m, 2H), 2.85 (m, 2H), 2.64 (s, 2H), 2.17 (s, 2H), 1.91 (m, 2H), 1.76 (m, 2H). EI-MS: m/z 326. HRMS calcd for C20H28N3O: 326.2227 found (ESI, [M+H]+): 326.2222.
5.1.22. General method for the synthesis of N-[(1-substitutedl-piperidin-4-yl)-phenyl-hydrazono]-pentanoic acids (6a–f)
To a solution of 0,005 mol of any of the (1-substituted-piperidin-4-yl)-N-phenyl-hydrazines 5a–f in 10 mL of ethanol was added drop by drop 0.0055 mol of levulinic acid in 5 mL of ethanol. The mixture was left overnight at room temperature. The crystals were filtered and dried in air. Sometimes it was necessary to separate product by flush chromatography on silica (CHCl3/MeOH; 4/1). Yields were quantitative.
5.1.23. N-[(1-methyl-piperidin-4-yl)-phenyl-hydrazono]-pentanoic acid (6a)
Crystalline solid (65.4%), mp 124–126 °C. IR: ν (cm−1) = 1610 (C=N), 1715 (C=O). 1H NMR (600 MHz, CDCl3) δ 10.75 (bs, 1H), 7.21 (t, 2 H, J = 7.86 Hz), 6.89 (t, 1H, J = 7.26 Hz), 6.83 (d, 2H, J = 7.96 Hz), 3.51 (m, 1H,), 3.30 (d, 2H, J = 10.27 Hz), 2.74 (t, 2H, J = 6.93 Hz), 2.68 (m, 2H), 2.55 (s, 3H), 2.37 (m, 4H), 1.75 (d, 2H, J = 11.56 Hz), 1.66 (s, 3H). EI-MS: m/z 304. HRMS calcd for C17H26N3O2: 304.2020 found (ESI, [M+H]+): 304.2022.
5.1.24. N-[(1-benzyl-piperidin-4-yl)-phenyl-hydrazono]-pentanoic acid (6b)
Crystalline solid (72.6%), mp 108–110 °C. IR: ν (cm−1) = 1612 (C=N), 1718 (C=O). 1H NMR (600 MHz, CDCl3) δ 11.63 (bs, 1H), 7.31 (m, 5H), 7.16 (t, 2H, J = 7.76 Hz), 6.84 (t, 1H, J = 7.29 Hz), 6.78 (d, 2H, J = 8.09 Hz), 3.90 (s 2H,), 3.41 (m, 1H), 3.23 (d, 2H, J = 6.88 Hz), 2.74 (t, 2H, J = 7.21 Hz), 2.67 (t, 2H, J = 6.96 Hz), 2.24 (m, 4H), 1.68 (d, 2H, J = 8.96 Hz), 1.64 (s, 3H). EI-MS: m/z 380. HRMS calcd for C23H30N3O: 380.2333 found (ESI, [M+H]+): 380.2340.
5.1.25. 4-[(1-phenethyl-piperidin-4-yl)-hydrazono]-pentanoic acid (6c)
Crystalline solid (91.1%), mp 212–214 °C. IR: ν (cm−1) = 1612 (C=N), 1715 (C=O). 1H NMR (600 MHz, CDCl3) δ 9.85 (bs, 1H), 7.27 (m, 2H), 7.19 (m, 5H), 6.86 (m, 1H), 6.82 (m, 2H), 3.49 (m, 1H), 3.38 (d 2H, J = 5.93 Hz), 2.96 (m, 2H), 2.89 (m, 2H), 2.77 (t, 2H, J = 7.23 Hz), 2.69 (t, 2 H, J = 7.23 Hz), 2.32 (m, 4H), 1.75 (m, 2H), 1.66 (s, 3H). EI-MS: m/z 394. HRMS calcd for C24H32N3O2: 394.2489 found (ESI, [M+H]+): 394.2492.
5.1.26. 4-[(4-Methoxy-phenyl)-(1-methyl-piperidin-4-yl)-hydrazono]-pentanoic acid (6d)
Crystalline solid (67.4%), mp 182–184 °C. IR: ν (cm−1) = 1615 (C=N), 1717 (C=O). 1H NMR (600 MHz, CDCl3) δ 10.75 (bs 1H), 6.90 (m, 2H), 6.74 (m, 2H), 3.76 (s, 3H), 3.51 (m, 1H), 3.30 (d, 2H, J = 10.25 Hz), 2.74 (t, 2H, J = 6.93 Hz), 2.68 (m, 2H), 2.55 (s, 3H), 2.37 (m, 4H), 1.75 (d, 2H, J = 11.58 Hz), 1.66 (s, 3H). EI-MS: m/z 334. HRMS calcd for C18H28N3O3: 334.2125 found (ESI, [M+H]+): 334.2131.
5.1.27. 4-[(1-benzyl-piperidin-4-yl)-(4-methoxy-phenyl) hydrazono]-pentanoic acid (6e)
Light yellow liquid (73.8%). IR: ν (cm−1) = 1614 (C=N), 1714 (C=O). 1H NMR (600 MHz, CDCl3) δ 11.07 (bs, 1H), 7.31 (m, 2H), 7.16 (m, 3H), 6.83 (m, 2H), 6.73 (m, 2H), 3.90 (s, 2H), 3.68 (s, 3H), 3.34 (m, 2H), 3.25 (m, 1H), 2.71 (t, 2H, J = 7.15 Hz), 2.63 (t, 2 H, J = 7.15 Hz), 2.27 (m, 2H), 1.74 (m, 2H), 1.61 (s, 3H). EI-MS: m/z 410. HRMS calcd for C24H32N3O3: 410.2438 found (ESI, [M+H]+): 410.2443.
5.1.28. 4-[(4-Methoxy-phenyl)-1-(2-phenethyl-piperidin-4-yl)-hydrazono]-pentanoic acid (6f)
Crystalline solid (89.3%), mp 177–179 °C. IR: ν (cm−1) = 1610 (C=N), 1715 (C=O). 1H NMR (600 MHz, CDCl3) δ 10.57 (bs, 1H), 7.26 (m, 2H), 7.18 (m, 3H), 6.83 (m, 2H), 6.73 (m, 2H), 3.71 (s, 3H), 3.34 (m, 2H), 3.25 (m 1H), 2.94 (m, 2H), 2.87 (m, 2H), 2.71 (t, 2H, J = 7.15 Hz), 2.63 (t, 2H, J = 7.15 Hz), 2.27 (m, 2H), 1.74 (m, 2H), 1.61 (s, 3H). EI-MS: m/z 424. HRMS calcd for C25H34N3O3: 424.2595 found (ESI, [M+H]+): 424.2602.
5.1.29. General method for the synthesis of N-[(1-substitutedl-piperidin-4-yl)-phenyl-hydrazono]-pentanoic acids ethyl ethers (7a–f)
A solution of 0.01 mol of any of (1-substituted-piperidin-4-yl)-N-phenyl-hydrazines 5a–f, 0.011 mol of levulinic acid ethyl ether and 2–3 drops of acetic acid in 25–30 mL of toluene was heated under reflux for 1.5–2 hours with a Dean-Stark trap till the full separation of water. The toluene and excess of levulinic acid ethyl ether were removed in vacuo. Yields are close to quantitative. For analytical purposes product was purified by flash chromatography on silica (CHCl3/MeOH; 4/1).
5.1.30. N-[(1-methyl-piperidin-4-yl)-phenyl-hydrazono]-pentanoic acid ethyl ether (6a)
Light yellow liquid (81.0%). IR: ν (cm−1) = 1595 (C=N), 1740 (C=O). 1H NMR (600 MHz, CDCl3) δ 7.15 (m, 2H), 6.85 (m, 1H), 6.79 (d, 2 H, J = 8.11 Hz), 4.09 (m, 2H), 3.30 (tt, 1H, J = 11.03, 3.86 Hz), 2.84 (d, 1H, J = 11.59 Hz), 2.69 (t, 1H, J = 6.58 Hz), 2.65 (t, 2H, J = 6.81 Hz), 2.57 (t, 2 H, J = 6.86 Hz), 2.52 (t, 1H, J = 6.58 Hz), 2.23 (m, 3H), 2.14 (s, 1H), 1.93 (t, 2H, J = 11.67 Hz), 1.84 (dq, 2H, J = 3.51, 12.30 Hz), 1.62 (m, 5H), 1.19 (m, 4H). EI-MS: m/z 332. HRMS calcd for C19H30N3O2: 332.2333 found (ESI, [M+H]+): 332.2327.
5.1.31. N-[(1-benzyl-piperidin-4-yl)-phenyl-hydrazono]-pentanoic acid ethyl ether(6b)
Light yellow liquid (87.1%). IR: ν (cm−1) = 1598 (C=N), 1742 (C=O). 1H NMR (600 MHz, CDCl3) δ 11.63 (bs, 1H), 7.31 (m, 5H), 7.16 (t, 2H, J = 7.76 Hz), 6.84 (t, 1H, J = 7.29 Hz), 6.78 (d, 2H, J = 8.09 Hz), 4.15 (q, 2H, J = 7.17 Hz), 3.90 (s, 2H), 3.41 (m, 1H), 3.23 (d, 2H, J = 6.88 Hz), 2.74 (t, 2H, J = 7.21 Hz), 2.67 (t, 2H, J = 6.96 Hz), 2.24 (m, 4H), 1.68 (d, 2H, J = 8.96 Hz), 1.64 (s, 3H), 1.24 (t, 3 H, J = 7.14 Hz). EI-MS: m/z 408. EI-MS: m/z 332. HRMS calcd for C25H34N3O2: 408.2646 found (ESI, [M+H]+): 408.2641
5.1.32. 4-[(1-phenethyl-piperidin-4-yl)-hydrazono]-pentanoic acid ethyl ether (6c)
Light yellow liquid (95.2%). IR: ν (cm−1) = 1598 (C=N), 1740 (C=O). 1H NMR (600 MHz, CDCl3) δ 9.85 (bs, 1H), 7.27 (m, 2H), 7.19 (m, 5H), 6.86 (m, 1H), 6.82 (m, 2H), 4.10 (q, 2H, J = 7.15 Hz), 3.49 (m, 1H), 3.38 (d, 2H, J = 5.93 Hz), 2.96 (m, 2H), 2.89 (m, 2H), 2.77 (t, 2H J = 7.23 Hz), 2.69 (t, 2H, J = 7.23 Hz), 2.32 (m, 4H), 1.75 (m, 2H), 1.66 (s, 3H), 1.22 (t, 3H, J = 7.12 Hz). EI-MS: m/z 422. HRMS calcd for C26H36N3O2: 422.2802 found (ESI, [M+H]+): 422.2797.
5.1.33. 4-[(4-Methoxy-phenyl)-(1-methyl-piperidin-4-yl)-hydrazono]-pentanoic acid ethyl ether (6d)
Light yellow liquid (78.8%). IR: ν (cm−1) = 1596 (C=N), 1741 (C=O). 1H NMR (500 MHz, CDCl3) δ 6.82 (m, 2H), 6.72 (m, 2H), 4.08 (q, 2H, J = 7.15 Hz), 3.69 (s, 3H), 3.05 (tt, 1H, J = 10.74, 3.87 Hz), 2.81 (m, 2H), 2.59 (t, 2H, J = 6.91 Hz), 2.49 (t, 2H, J = 6.80 Hz), 2.20 (s, 3H,), 1.91 (m, 2H), 1.72 (m, 2H), 1.63 (d, 2H, J = 10.78 Hz), 1.56 (s, 3H), 1.20 (t, 3H, J = 7.12 Hz). EI-MS: m/z 362. HRMS calcd for C20H32N3O3: 362.2438 found (ESI, [M+H]+): 362.2430.
5.1.34. 4-[(1-benzyl-piperidin-4-yl)-(4-methoxy-phenyl) hydrazono]-pentanoic acid ethyl ether (6e)
Light yellow liquid (82.5%). IR: ν (cm−1) = 1598 (C=N), 1742 (C=O). 1H NMR (600 MHz, CDCl3) δ 7.29 (m, 4H), 7.22 (m, 1H), 6.86 (m, 2H), 6.76 (m, 2H), 4.16 (q, 2H, J = 7.17 Hz), 3.73 (s, 3H,), 3.47 (s, 2H), 3.12 (tt, 1H, J = 11.06, 3.84 Hz), 2.88 (d, 2H, J = 11.72 Hz), 2.64 (t, 2H, J = 6.70 Hz), 2.56 (t, 2H, J = 6.75 Hz), 1.94 (t, 2H, J = 11.31 Hz), 1.75 (dq, 2H, J = 3.71, 12.09 Hz), 1.65 (d, 2H, J = 11.44 Hz), 1.61 (s, 3H), 1.26 (t, 3H, J = 7.14 Hz). EI-MS: m/z 438. HRMS calcd for C26H36N3O3: 438.2751 found (ESI, [M+H]+): 438.2745.
5.1.35. 4-[(4-Methoxy-phenyl)-1-(2-phenethyl-piperidin-4-yl)-hydrazono]-pentanoic acid ethyl ether (6f)
Light yellow liquid (96.1%). IR: ν (cm−1) = 1595 (C=N), 1740 (C=O). 1H NMR (600 MHz, CDCl3) δ 7.22 (m, 2H), 7.13 (m, 3H), 6.84 (m, 2H), 6.74 (m, 2H), 4.11 (q, 2H, J = 7.14 Hz), 3.70 (s, 3H), 3.10 (tt, 1H, J = 10.84, 4.01 Hz), 2.97 (m, 2H), 2.76 (m, 2H), 2.61 (t, 2H, J = 6.81 Hz), 2.52 (m, 4H), 1.98 (m, 2H), 1.75 (m, 2H), 1.68 (m, 2H), 1.59 (s, 3H), 1.23 (t, 3H, J = 7.13 Hz). EI-MS: m/z 452. HRMS calcd for C27H38N3O3: 452.2908 found (ESI, [M+H]+): 452.2989.
5.1.36. General method for the synthesis of [2-methyl-1-(1-methyl-piperidin-4-yl)-1H-indol-3-yl]-acetic acid ethyl esters (8 a–f)
N-[(1-substitutedl-piperidin-4-yl)-phenyl-hydrazono]-pentanoic acids ethyl ethers 7a–f (0,01 mol) was dissolved in 20 mL of 10% ethanol solution of HCl and the solution was heated under reflux for 2.5 hours and then left at room temperature overnight. The solvent was removed in vacuum, and remaining residue was washed 2–3 times with boiling ether. Ether was added to remaining part and on cooling it was neutralized with NH4OH solution. Ether layer was separated and the water layer extracted with ether one more time. Ether layer was dried over MgSO4, filtered through a layer of neutral alumina. The solvent was removed in vacuo. Remaining pure product can be used without further purification. Yields are between 71–87%. For analytical purposes the product was purified by flash chromatography on silica (CHCl3/MeOH; 4/1)for 8a and 8d, (hexane/ethyl acetate; 1/1) for 8 b,c.e.f.
5.1.37. [2-methyl-1-(1-methyl-piperidin-4-yl)-1H-indol-3-yl]-acetic acid ethyl ester (8a)
Light yellow liquid (71.5%). IR: ν (cm−1) = 1746 (C=O). 1H NMR (600 MHz, CDCl3) δ 7.60 (d, 1H, J = 7.66 Hz), 7.55 (d, 1H, J = 7.77 Hz), 7.11 (t, 1H, J = 7.58 Hz), 7.07 (t, 1H, J = 7.36 Hz), 4.21 (m, 1H), 4.12 (q, 2H, J = 7.10 Hz), 3.69 (s, 2H,), 3.12 (d, 2H, J = 11.68 Hz), 2.75 (d, 2 H,J = 11.82 Hz), 2.44 (s, 3H,), 2.42 (s, 3H), 2.25 (t, 2H, J = 11.66 Hz), 1.84 (d, 2H, J = 12.60 Hz), 1.24 (t, 3H, J = 7.12 Hz). EI-MS: m/z 315. EI-MS: m/z 452. HRMS calcd for C19H27N2O2: 315.2067 found (ESI, [M+H]+): 315.2059
5.1.38. [2-methyl-1-(1-benzyl-piperidin-4-yl)-1H-indol-3-yl]-acetic acid ethyl ester (8b)
Light yellow liquid (79.3%). IR: ν (cm−1) = 1747 (C=O). 1H NMR (600 MHz, CDCl3) δ 7.59 (m, 1H), 7.56 (m, 1H), 7.40 (m, 2H), 7.37 (m, 2H), 7.29 (m, 1H), 7.13 (m, 1H), 7.08 (m, 1H), 4.18 (m, 1H), 4.12 (q, 2H, J = 7.26 Hz), 3.90 (s, 2H), 3.69 (s, 2H), 3.62 (s, 2H), 3.10 (d, 2H, J = 11.16 Hz), 2.43 (s, 3H), 1.81 (m, 2H), 1.24 (t, 3H, J = 7.07 Hz). EI-MS: m/z 391. HRMS calcd for C25H31N2O2: 391.2380 found (ESI, [M+H]+): 391.2385.
5.1.39. [2-methyl-1-(1-phenethyl-piperidin-4-yl)-1H-indol-3-yl]-acetic acid ethyl ester (8c)
Crystalline solid (85.6%). mp 93–94 °C. IR: ν (cm−1) = 1744(C=O). 1H NMR (600 MHz, CDCl3) δ 7.47 (d, 1H, J = 8.65 Hz), 7.31 (m, 2H), 7.22 (m, 3H), 7.02 (d, 1H, J = 2.51 Hz), 6.77 (dd, 1H, J = 8.65, 2.51 Hz), 4.11 (q, 2H, J = 7.16 Hz), 3.85 (s, 3H), 3.65 (s, 2H), 3.20 (d, 2H, J = 10.77 Hz), 2.87 (m, 2H), 2.68 (m, 2H), 2.62 (m, 2H), 2.41 (s, 3H), 2.21 (t, 2H, J = 11.23 Hz), 1.83 (m, 2H), 1.24 (t, 3H, J = 7.16 Hz). EI-MS: m/z 405. EI-MS: m/z 391. HRMS calcd for C26H33N2O2: 405.2537 found (ESI, [M+H]+): 405.2543.
5.1.40. [5-methoxy- 2-methyl-1-(1-methyl-piperidin-4-yl)-1H-indol-3-yl]-acetic acid ethyl ester (8d)
Light yellow liquid (75,6%). IR: ν (cm−1) = 1745 (C=O). 1H NMR (500 MHz, CDCl3) δ 7.43 (m, 1H), 6.99 (m, 1H), 6.72 (m, 1H), 4.09 (m+q 3H), 3.81 (s, 3H), 3.61 (s, 2H), 3.01 (d, 2H, J = 11.69 Hz), 2.59 (m, 2H,), 2.37 (s, 3H), 2.33 (s, 3H), 2.11 (m, 2H), 1.77 (m, 2H), 1.21 (m, 3H). EI-MS: m/z 345. HRMS calcd for C20H29N2O3: 345.2173 found (ESI, [M+H]+): 345.2166.
5.1.41. [5-methoxy- 2-methyl-1-(1-benzyl-piperidin-4-yl)-1H-indol-3-yl]-acetic acid ethyl ester (8e)
Light yellow liquid (81.9%). IR: ν (cm−1) = 1747 (C=O). 1H NMR (600 MHz, CDCl3) δ 7.48 (m, 1H), 7.37 (m, 4H), 7.28 (m, 1H), 7.04 (m, 1H), 6.79 (m, 1H), 4.13 (m+q, 3H), 3.86 (s, 3H), 3.66 (s, 2H), 3.60 (s, 2H), 3.07 (m, 2H), 2.60 (m, 2H), 2.41 (s, 3H), 2.15 (m, 2H), 1.79 (m, 2H), 1.25 (t, 3H, J = 6.98 Hz). EI-MS: m/z 421. HRMS calcd for C26H33N2O3: 421.2486 found (ESI, [M+H]+): 421.2493.
5.1.42. [5-methoxy- 2-methyl-1-(1-phenethyl-piperidin-4-yl)-1H-indol-3-yl]-acetic acid ethyl ester (8f)
Light yellow liquid (87.4%). IR: ν (cm−1) = 1745 (C=O). 1H NMR (600 MHz, CDCl3) δ 7.47 (d, 1H, J = 8.65 Hz), 7.31 (m, 2H), 7.22 (m, 3H), 7.02 (d, 1H, J = 2.51 Hz), 6.77 (dd, 1H, J = 8.65, 2.51 Hz), 4.11 (q, 2 H, J = 7.16 Hz), 3.85 (s, 3H), 3.65 (s, 2H), 3.20 (d, 2H, J = 10.77 Hz), 2.87 (m, 2H), 2.68 (m, 2H), 2.62 (m, 2H), 2.41 (s, 3H), 2.21 (t, 2H, J = 11.23 Hz), 1.83 (m, 2H), 1.24 (t, 3H, J = 7.16 Hz). EI-MS: m/z 435. HRMS calcd for C25H31N2O3: 407.2329 found (ESI, [M+H]+): 407.2323.
5.1.43. General method for the synthesis of [2-methyl-1-(1-methyl-piperidin-4-yl)-1H-indol-3-yl]-acetic acids (9a–f)
a. 0,005 mol of one of the N-[(1-substitutedl-piperidin-4-yl)-phenyl-hydrazono]-pentanoic acids ethyl ethers 7a–f dissolved in 8 mL of ethanol was added with cooling (ice) to a stirred solution of 0.00625 mol KOH in 4 mL of ethanol. The solution was left at room temperature for 48 hours. Ethanol was removed in vacuo. Remaining residue was washed several times with boiling ether. A new portion of ether was added and on cooling (ice) 0.00625 mol (0,375 mL) of acetic acid in 1 mL of water was added. Ether layer was separated and remaining part was extracted with CHCl3 and dried (MgSO4). The solvent was removed in vacuo. The product does not need further purification. Yields are between 55–84%.
b. For cases 9a,d the work up during acidification of potassium salts differs. To the remaining residue after ethanol evaporation was added 10 mL acetonitrile, and neutralization with acetic acid (0.00625 mol) is done without water. After stirring the mixture for 30 minutes and filtering the potassium acetate, acetonitrile is evaporated under vacuo giving as a residue of the desired acids 9a,d.
5.1.44. [2-methyl-1-(1-methyl-piperidin-4-yl)-1H-indol-3-yl]-acetic acid (9a)
Crystalline solid (55.2%), mp 83–85 °C. IR: ν (cm−1) = 1718 (C=O). 1H NMR (600 MHz, CDCl3) δ 10.93 (bs, 1H), 7.53 (d, 1H, J = 7.28 Hz), 7.44 (d, 1H, J = 7.70 Hz), 6.97 (m, 2H), 4.08 (m, 1H), 3.60 (s, 2H), 3.11 (d, 2H, J = 11.16 Hz), 2.60 (m, 3H), 2.27 (s, 3H), 2.21 (t, 2H, J = 11.17 Hz), 2.02 (s, 1H), 1.96 (s, 1H), 1.91 (s, 1H), 1.57 (d, 2H, J = 11.53 Hz), 2.38 ( 3 H, s). 13C NMR (125 MHz, MeOD) δ 178.23, 176.56, 133.79, 129.47, 120.78, 118.95, 118.58, 111.05, 107.27, 54.16, 50.68, 42.78, 32.32, 27.65, 21.41, 10.46. EI-MS: m/z 287. HRMS calcd for C17H23N2O2: 287.1754 found (ESI, [M+H +): 287.1761.
5.1.45. [2-methyl-1-(1-benzyl-piperidin-4-yl)-1H-indol-3-yl]-acetic acid (9b)
Crystalline solid (83.0%), mp 78–80 °C. IR: ν (cm−1) = 1720 (C=O). 1 H NMR (600 MHz, CDCl3) δ 11.23 (bs, 1H), 7.39 (m, 7H), 6.99 (m, 1H), 6.91 (m, 1H), 4.02 (m, 1H), 3.89 (s, 2H), 3.61 (s, 2H), 3.17 (d, 2H, J = 10.99 Hz), 2.62 (m, 2H), 2.33 (m, 2H), 2.06 (s, 3H), 1.38 (d, 2H, J = 11.78 Hz). 13C NMR (125 MHz, MeOD) δ 177.04, 175.42, 133.87, 131.21, 129.63, 129.25, 129.13, 120.67, 118.92, 118.39, 111.00, 106.55, 60.60, 52.09, 51.29, 31.59, 27.53, 20.75, 10.43. EI-MS: m/z 363. HRMS calcd for C23H27N2O2: 363.2067 found (ESI, [M+H]+): 363.2061.
5.1.46. [2-methyl-1-(1-phenethyl-piperidin-4-yl)-1H-indol-3-yl]-acetic acid (9c)
Crystalline solid (80.%), mp 83–85 °C. IR: ν (cm−1) = 1717 (C=O). 1H NMR (600 MHz, CDCl3) δ 10.77 (bs, 1H), 8.97 (bs, 1H), 7.52 (m, 1H), 7.28 (m, 2H), 7.20 (m, 4H), 7.00 (m, 1H), 6.95 (m, 1H), 4.08 (m, 1H), 3.62 (s, 2H), 3.30 (d, 2H, J = 10.78 Hz), 2.95 (m, 2H,), 2.91 (m, 2H), 2.62 (d, 2H, J = 9.26 Hz), 2.38 (m, 2H), 2.05 (s, 3H), 1.39 (m, 2H). 13C NMR (125 MHz, MeOD) δ 178.02, 137.55, 133.79, 129.45, 128.88, 128.84, 127.02, 120.80, 118.94, 118.59, 58.18, 52.47, 51.32, 32.38, 31.00, 27.82, 21.30, 10.59. EI-MS: m/z 377. HRMS calcd for C24H29N2O2: 377.2224 found (ESI, [M+H]+): 377.2219.
5.1.47. [5-methoxy- 2-methyl-1-(1-methyl-piperidin-4-yl)-1H-indol-3-yl]-acetic acid (9d)
Crystalline solid (59.2%), mp 58–60 °C. IR : ν(cm−1) = 1721 (C=O). 1H NMR (500 MHz, CDCl3) δ 11.45 (bs, 1H), 7.43 (m, 1H), 6.99 (m, 1H), 6.72 (m, 1H), 4.09 (m, 1H), 3.81 (s, 3H), 3.61 (s, 2H), 3.01 (d, 2H, J = 11.69 Hz), 2.59 (m, 2H), 2.37 (s, 3H), 2.33 (s, 3H), 2.11 (m, 2H), 1.77 (m, 2H). 13 CNMR (125 MHz, MeOD) δ 178.32, 154.03, 134.55, 128.62, 112.25, 110.66, 107.11, 101.30, 55.04, 54.18, 50.30, 43.05, 32.24, 28.31, 10.48. EI-MS: m/z 317. HRMS calcd for C18H25N2O3: 317.1860 found (ESI, [M+H]+): 317.1856.
5.1.48. [5-methoxy- 2-methyl-1-(1-benzyl-piperidin-4-yl)-1H-indol-3-yl]-acetic acid (9e)
Crystalline solid (82%), mp 135–137°C. IR: ν (cm−1) = 1718 (C=O). 1H NMR (600 MHz, CDCl3) δ 10.88 (bs, 1H), 7.48 (m, 1H), 7.37 (m, 4H), 7.28 (m, 1H), 7.04 (m, 1H), 6.79 (m, 1H), 4.13 (m, 1H), 3.86 (s, 3H), 3.66 (s, 2H), 3.60 (s, 2H), 3.07 (m, 2H), 2.60 (m, 2H), 2.41 (s, 3H), 2.15 (m, 2H), 1.79 (m, 2H). 13C NMR (125 MHz, MeOD) δ 179.14, 153.83, 134.37, 133.46, 130.64, 130.16, 129.99, 128.82, 128.76, 111.79, 109.98, 107.11, 101.08, 61.46, 55.32, 52.60, 52.28, 32.97, 28.69, 10.52. EI-MS: m/z 393. HRMS calcd for C24H29N2O3: 393.2173 found (ESI, [M+H]+): 393.2167.
5.1.49. [5-methoxy- 2-methyl-1-(1-phenethyl-piperidin-4-yl)-1H-indol-3-yl]-acetic acid (9f)
Crystalline solid (84.3%), mp 98–100 °C. IR: ν (cm−1) = 1720 (C=O). 1H NMR (600 MHz, CDCl3) δ 11.26 (bs, 1H), 7.42 (s, 1H), 7.29 (m, 2H), 7.21 (m, 4H), 6.99 (d, 1H J = 2.36 Hz), 6.71 (dd, 1H, J = 8.84, 2.36 Hz), 4.08 (m, 1H), 3.71 (s, 3H), 3.59 (s, 2H), 3.33 (d, 2H, J = 10.47 Hz), 3.00 (m 2H), 2.93 (m, 2H), 2.68 (m, 1H), 2.43 (s, 2H), 2.19 (s, 2H,), 2.06 (s, 3H), 1.46 (d, 2H, J = 11.32 Hz). 13C NMR (125 MHz, MeOD) δ 178.48, 153.93, 137.67, 134.45, 130.04, 128.84, 126.97, 111.83, 110.21, 107.24, 101.17, 58.28, 55.29, 52.46, 51.42, 32.80, 31.07, 28.08, 10.61. EI-MS: m/z 407. HRMS calcd for C25H31N2O3: 407.2329 found (ESI, [M+H]+): 407.2333.
5.2. Biological assays
5.2.1. Tissue bioassays
5.2.1.1. Guinea Pig Isolated Ileum/Longitudinal Muscle with Myenteric Plexus
Male Hartley guinea pigs under CO2 anesthesia were sacrificed by decapitation and a nonterminal portion of the ileum removed. The longitudinal muscle with myenteric plexus (LMMP) was carefully separated from the circular muscle and cut into strips as described previously [52]. These tissues were tied to gold chains with suture silk and mounted between platinum wire electrodes in 20 mL organ baths at a tension of 1 g and bathed in oxygenated (95:5 O2:CO2) Kreb's bicarbonate buffer at 37C. They were stimulated electrically (0.1 Hz, 0.4 msec duration) at supramaximal voltage. Following an equilibration period, compounds were added cumulatively to the bath in volumes of 14–60 µl until maximum inhibition was reached. A dose-response curve of PL-017 was constructed to determine tissue integrity before analog testing.
5.2.1.2. Mouse Isolated Vas Deferens Preparation
Male ICR mice under CO2 anesthesia were sacrificed by cervical dislocation and the vasa deferentia removed. Tissues were tied to gold chains with suture silk and mounted between platinum wire electrodes in 20 ml organ baths at a tension of 0.5 g and bathed in oxygenated (95:5 O2:CO2) magnesium free Kreb's buffer at 37C. They were stimulated electrically (0.1 Hz, single pulses, 2.0 msec duration) at supramaximal voltage as previously described [53]. Following an equilibration period, compounds were added to the bath cumulatively in volumes of 14–60 µL until maximum inhibition was reached. A dose-response curve of DPDPE was constructed to determine tissue integrity before analog testing.
5.2.1.3. Agonist and Antagonist testings
Compounds were tested as agonists by adding cumulatively to the bath until a full dose-response curve was constructed or to a concentration of 1 µM. Compounds were tested as antagonists by adding to the bath 2 minutes before beginning the cumulative agonist dose-response curves of the delta (DPDPE) or μ (PL-017) opioid agonists.
5.2.1.4. Analysis
Percentage inhibition was calculated using the average tissue contraction height for 1 min preceding the addition of the agonist divided by the contraction height 3 min after exposure to the dose of agonist. IC50 values represent the mean of not less than 4 tissues. IC50 and Emax estimates were determined by computerized non-linear least-squares analysis (FlashCalc).
5.2.2. Radioligand binding analysis
Crude membranes were prepared from whole rat brains. The protein concentration of the membrane preparations was determined by the Lowry method and the membranes were stored at −80°C until use. Membranes were resuspended in assay buffer (50 mM Tris,pH 7.4, containing 50 µg/mL bacitracin, 30 µM bestatin, 10 µM captopril, 100 µM PMSF, 1 mg/mL BSA). Ten concentrations of a test compound were each incubated with 50 µg of membranes and 500 pM [3H]diprenorphine (55 Ci/mmol). Naloxone at 10 µM was used to define non-specific binding of the radioligand in all assays. All samples were carried out in duplicates. The samples were incubated in a shaking water bath at 25°C for 3 hr, followed by rapid filtration through Whatman GF/B filter paper (Gaithersburg, MD) pre-soaked in 1% polyethyleneimine, washed 4 times each with 2 mL of cold saline, and the radioactivity determined by liquid scintillation counting (Beckman LS5000 TD).
5.2.3. Enzyme immunoassay
A COX inhibitor screening assay kit (Cayman Chemical, catalog#560101) was used to evaluate the effect of compounds 9a–f on COX-1 or COX-2 mediated conversion of arachidonic acid to prostaglandin H2; the latter was quantified by an enzyme immunoassay. The assay was carried out according to the manufacturer’s recommendations. Briefly, compounds (50 nM final concentration) were pre-incubated with COX-1 or COX-2 for 5 min at 37C prior to the addition of arachidonic acid, and the reaction allowed to proceed for 2 min at 37C. Reaction was terminated with 1 M HCl and the amount of prostaglandin produced was determined by ELISA. The IC50 value of the reference compound indomethacin measured by this kit is around 200 nM.
5.2.4. Behavioral assays
Male Sprague-Dawley rats (220–275g) were used for all experiments. All surgical and testing procedures were approved by IACUC and have been previously described [54], including intrathecal catheterization for spinal cord drug delivery, peripheral nerve injury by unilateral ligation of the L5 and L6 spinal nerves of the left sciatic nerve, or sham operation as control, and behavioral testing for the subjects’ response time to an infra read heat source, or their response to mechanical stimuli using a series of von Frey filaments. All drug testing was carried out 7 days after spinal nerve or sham surgery. Each test group consisted of 5–6 rats.
5.3. Crystal Structure for compound 8c
A colorless prism-like specimen of 8c C27H34N2O3, approximate dimensions 0.06 mm × 0.11 mm × 0.15 mm, was used for the X-ray crystallographic analysis. The X-ray intensity data were measured at 100(2) K on a Bruker SMART APEXII system equipped with a graphite monochromator and a Cu Kα fine-focus sealed tube (λ= 1.54178 Å) operated at 1.2 kW power (40kV, 30 mA). The detector was placed at a distance of 3.972 cm. from the crystal.
A total of 3889 frames were collected with a scan width of 0.5° in ω or φ and an exposure time of 10.0 sec/frame. The total data collection time was 15.34 hours. The frames were integrated with the Bruker SAINT software package using a narrow-frame integration algorithm. The integration of the data using a monoclinic unit cell yielded a total of 15841 reflections to a maximum θ angle of 68.17° (0.83 Å resolution), of which 4080 were independent (average redundancy 3.88, completeness = 97.3%, Rint = 5.55%, Rsig = 4.25%) and 3123 (76.54%) were greater than 2σ(F2). The final cell constants of a = 9.9661(3) Å, b = 14.0923(5) Å, c = 16.4655(5) Å, β = 96.182(2)°, volume = 2299.05(13) Å3, are based upon the refinement of the XYZ-centroids of 4640 reflections above 20 σ(I) with 8.279° < 2θ < 135.275°. Analysis of the data showed negligible decay during data collection. Data were corrected for absorption effects using the multi-scan technique (SADABS). The ratio of minimum to maximum apparent transmission was 0.939. The calculated minimum and maximum transmission coefficients (based on crystal size) are 0.9078 and 0.9629.
The structure was solved and refined using the Bruker SHELXTL (Version 2008.2) Software Package, using the space group P2(1)/c, with Z = 4 for the formula unit, C27H34N2O3. The final anisotropic full-matrix least-squares refinement on F2 with 327 variables converged at R1 = 3.88%, for the observed data and wR2 = 9.71% for all data. The goodness-of-fit was 1.009. The largest peak on the final difference electron density synthesis was 0.231 e−/Å3 and the largest hole was −0.184 e−/Å3 with an RMS deviation of 0.040 e−/Å3. On the basis of the final model, the calculated density was 1.255 g/cm3 and F(000), 936 e−.
Acknowledgment
This work was supported from the U.S. Public National Institute of Health DA 13449, DA06784 and DA 06789. The authors thank Ohannes Melemedjian, David Rankin, Nicole Schecter and Janice Oyarzo for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Schmidt WK. In: “Pain” Current Understanding, Emerging Therapies and Novel Approaches to Drug Discovery. Bountra Ch, Munglani R, Schmidt WK., editors. New York, Basel: Marcel Dekker, Inc; 2003. p. 385. [Google Scholar]
- 2.Malmberg AB, Yaksh TL. Anesthesiol. 1993;79:270. doi: 10.1097/00000542-199308000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Miranda HF, Silva E, Pinardi G. Can. J. Physiol. Pharmacol. 2004;82:331. doi: 10.1139/y04-027. [DOI] [PubMed] [Google Scholar]
- 4.Satyanarayana PSV, Jain NK, Singh A, Kulkarni SK. Progr. NeuroPsychopharmacol. Biol. Psych. 2004;28:641. doi: 10.1016/j.pnpbp.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Ingram SL. Progr. Brain Res. 2000;129:483. doi: 10.1016/S0079-6123(00)29035-3. [DOI] [PubMed] [Google Scholar]
- 6.Pinardi G, Prieto JC, Miranda HF. Pharmacol. Biochem. Behav. 2005;82:120. doi: 10.1016/j.pbb.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Miranda HF, Prieto JC, Pinardi G. Brain Res. 2005;1049(2):165. doi: 10.1016/j.brainres.2005.04.068. [DOI] [PubMed] [Google Scholar]
- 8.Maves TJ, Pechman PS, Meller ST, Gebhart GF. Anesthesiol. 1994;80:1094. doi: 10.1097/00000542-199405000-00018. [DOI] [PubMed] [Google Scholar]
- 9.Pyati S, Gan TJ. CNS Drugs. 2007;21:185. doi: 10.2165/00023210-200721030-00002. [DOI] [PubMed] [Google Scholar]
- 10.Kolesnikov Yu, Cristea M, Oksman G, Torosjan A, Wilson R. Brain Res. 2004;1029:217. doi: 10.1016/j.brainres.2004.09.058. [DOI] [PubMed] [Google Scholar]
- 11.Poveda R, Planas E, Pol O, Romero A, Sanchez S, Puig M. Eur. J. Pain. 2003;7:439. doi: 10.1016/S1090-3801(03)00003-X. [DOI] [PubMed] [Google Scholar]
- 12.Montes A, Warner W, Puig MM. Brit. J. Anaesth. 2000;85:217. doi: 10.1093/bja/85.2.217. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher D, Benoist JM, Gautron M, Guilbaud G. Anesthesiol. 1997;87:317. doi: 10.1097/00000542-199708000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Oldfield V, Perry CM. Drugs. 2005;65:2337. doi: 10.2165/00003495-200565160-00011. [DOI] [PubMed] [Google Scholar]
- 15.Loder E. CNS Drugs. 2005;19:769. doi: 10.2165/00023210-200519090-00004. [DOI] [PubMed] [Google Scholar]
- 16.Dahl JB, Kehlet H. Br. J. Anesth. 1991;66:703. doi: 10.1093/bja/66.6.703. [DOI] [PubMed] [Google Scholar]
- 17.Malmberg AB, Yaksh TL. Anesthesiol. 1993;79:270. doi: 10.1097/00000542-199308000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Maves TJ, Pechman PS, Meller ST, Gebhart GF. Anesthesiol. 1994;80:1094. doi: 10.1097/00000542-199405000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Levine JD, Taiwo YO. Neurosci. 1989;32:571. doi: 10.1016/0306-4522(89)90279-0. [DOI] [PubMed] [Google Scholar]
- 20.Morphy R, Kay C, Rankovic Z. Drug Disc. Today. 2004;9:641. doi: 10.1016/S1359-6446(04)03163-0. [DOI] [PubMed] [Google Scholar]
- 21.Roth BL, Sheffler DJ, Kroeze WK. Nat. Rev. Drug Discovery. 2004;3:353. doi: 10.1038/nrd1346. [DOI] [PubMed] [Google Scholar]
- 22.Hruby VJ, Agnes RS, Davis P, Ma S-W, Lee YS, Vanderah TW, Lai J, Porreca F. Life Sciences. 2003;73:699. doi: 10.1016/s0024-3205(03)00390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Law MR, Wald J, Morris JK, Jordan RE. Br. Med. J. 2003;326:1427. doi: 10.1136/bmj.326.7404.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keith CT, Borisy AA, Stockwell BR. Nat. Rev. Drug Discovery. 2005;4:1. doi: 10.1038/nrd1609. [DOI] [PubMed] [Google Scholar]
- 25.Wooden E, Kulkarni V, Agnes RS, Salibay C, Davis P, Ma Sw, Lai J, Porreca F, Hruby VJ. Understanding Biology Using Peptides. In: Blondelle Sylvie E., editor. Proc. American Peptide Symposium 19; Series: American Peptide Symposia, Vol. 9; 2006. p. 361. [Google Scholar]
- 26.Lee YS, Agnes RS, Badghisi H, Davis P, Ma S-W, Lai J, Porreca F, Hruby VJ. J Med. Chem. 2006;49:1773. doi: 10.1021/jm05085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agnes RS, Lee YS, Davis P, Ma S-W, Badghisi H, Porreca F, Lai J, Hruby VJ. J. Med. Chem. 2006;49:2868. doi: 10.1021/jm050921q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hruby VJ, Porreca F, Yamamura HI, Tollin G, Agnes RS, Lee YS, Cai M, Alves I, Cowell S, Varga E, Davis P, Salamon Z, Roeske W, Vanderah T, Lai J. The AAPS Journal. 2006;8:E450–E460. doi: 10.1208/aapsj080353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee YS, Agnes RS, Davis P, Ma S-W, Badghisi H, Lai J, Porreca F, Hruby VJ. J. Med. Chem. 2007;50:165. doi: 10.1021/jm061268p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanley THJ. Pain Sympt. Manag. 2005;29(5 Suppl):67. doi: 10.1016/j.jpainsymman.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Bagley JR, Kudzma LV, Lalinde NL, Colapret JA, Huang B-S, Lin Bor S, Jerussi TP, Benvenga MJ, Doorley BM, Ossipov MH, Spaulding TC, Spencer HK. Med. Res. Rev. 1991;11:403. doi: 10.1002/med.2610110404. [DOI] [PubMed] [Google Scholar]
- 32.Meert TF. Pharm. World Sci. 1996;18:1. doi: 10.1007/BF00449683. [DOI] [PubMed] [Google Scholar]
- 33.Markman JD. Pain. 2008;136:227. doi: 10.1016/j.pain.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Flower RJ. Nat. Rev. Drug Disc. 2003;2:179. doi: 10.1038/nrd1034. [DOI] [PubMed] [Google Scholar]
- 35.Dannhardt G, Kiefer W. Eur. J. Med. Chem. 2001;36:109. doi: 10.1016/s0223-5234(01)01197-7. [DOI] [PubMed] [Google Scholar]
- 36.Gilron I, Coderre TJ. Exp. Opin. Emerg. Drugs. 2007;12:113. doi: 10.1517/14728214.12.1.113. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto H. Chem. Pharm. Bull. 1968;16:17. doi: 10.1248/cpb.16.17. [DOI] [PubMed] [Google Scholar]
- 38.Boltze KH, Brendler O, Jacobi H, Opitz W, Raddatz S, Seidel PR. [PubMed] [Google Scholar]
- 39.Vollbrecht D. Arzneim.-Forsch. 1980;30(8A):1314. [PubMed] [Google Scholar]
- 40.Campos KR, Woo JCS, Lee S, Tillyer RD. Org. Lett. 2004;6:79. doi: 10.1021/ol036113f. [DOI] [PubMed] [Google Scholar]
- 41.Meneghin M. 206241. EP. 1986; Chem. Abstr. 1987;106:84196. [Google Scholar]
- 42.Demerson CA, Humber LG. 4585877. US. 1986; Chem. Abstr. 1986;105:115045. [Google Scholar]
- 43.Black C, Mancini JA, Lau CK, Prasit P, Vickers PJ. 2283745. GB. 1995; Chem. Abstr. 1995;123:227989. [Google Scholar]
- 44.Leblanc Y, Black WC, Chan CC, Charleson S, Delorme D, Denis D, Gauthier JY, Grimm EL, Gordon R, Guay D, Kargman S, Lau CK, Leblanc Y, Mancini J, Ouellet M, Percival D, Roy P, Skorey K, Tagari P, Vickers P, Wong E, Xu L, Prasit P. Bioorg. Med. Chem. Lett. 1996;6:731. [Google Scholar]
- 45.Hughes DL. Org. Prep. Proc. Int. 1993;25:607. [Google Scholar]
- 46.Sybyl 7.2 molecular modeling package is available from Tripos Inc. St. Louis MS
- 47.Kurumbail RG, Stevens AM, Gierse JK, McDonald JJ, Stegeman RA, Pak JY, Gildehaus D, Miyashiro JM, Penning TD, Seibert K, Isakson PC, Stallings WC. Nature. 1996;384:644. doi: 10.1038/384644a0. [DOI] [PubMed] [Google Scholar]
- 48.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Science. 2000;289:739. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 49.Gierse JK, Koboldt CM, Walker MC, Seibert K, Isakson PC. Biochem. J. 1999;339:607. [PMC free article] [PubMed] [Google Scholar]
- 50.Peeters OM, Blaton NM, De Ranter CJ, Van Herk AM, Goubitz KJ. Cryst. Mol. Struct. 1980;9:153. [Google Scholar]
- 51.Allen FH. Acta Crystallogr. Sect.B. 2002;58:380. doi: 10.1107/s0108768102003890. [DOI] [PubMed] [Google Scholar]
- 52.Porreca F, Burks TF. J. Pharm. Exp. Ther. 1983;225:688. [PubMed] [Google Scholar]
- 53.Kramer TH, Davis P, Hruby VJ, Burks TF, Porreca F. J. Pharm. Exp. Ther. 1993;266:577. [PubMed] [Google Scholar]
- 54.Lai J, Gold M, Kim CS, Bian D, Ossipov MH, Hunter JC, Porreca F. Pain. 2002;95:143. doi: 10.1016/s0304-3959(01)00391-8. [DOI] [PubMed] [Google Scholar]