Abstract
Limited research has been conducted on the structure of the pars triangularis (PT) in dyslexia despite functional neuroimaging research finding it may play a role in phonological processing. Furthermore, research to date has not examined PT size in ADHD even though the right inferior frontal region has been implicated in the disorder. Hence, one of the purposes of this study was to examine the structure of the PT in dyslexia and ADHD. The other purposes included examining the PT in relation to overall expressive language ability and in relation to several specific linguistic functions given language functioning often is affected in both dyslexia and ADHD. Participants included 50 children: 10 with dyslexia, 15 with comorbid dyslexia/ADHD, 15 with ADHD, and 10 controls. Using a 2 (dyslexia or not) X 2 (ADHD or not) MANCOVA, findings revealed PT length and shape were comparable between those with and without dyslexia. However, children with ADHD had smaller right PT lengths than those without ADHD, and right anterior ascending ramus length was related to attention problems in the total sample. In terms of linguistic functioning, presence of an extra sulcus in the left PT was related to poor expressive language ability. In those with adequate expressive language functioning, left PT length was related to phonological awareness, phonological short-term memory and rapid automatic naming (RAN). Right PT length was related to RAN and semantic processing. Further work on PT morphology in relation to ADHD and linguistic functioning is warranted.
Keywords: Dyslexia, reading disabilities, Attention Deficit Disorder with Hyperactivity, Attention Deficit Disorder, phonological awareness, naming, semantics, syntax, short-term memory, Magnetic Resonance Imaging
Developmental dyslexia is frequently defined as a deficit in word recognition despite adequate intelligence, motivation, and educational background (World Federation of Neurology, 1968). It is believed by many to be caused by poor phonological processing (for a review see Bishop & Snowling, 2004; Rack, Snowling, & Olson, 1992; Swank, 1994). Moreover, a deficit in phonological processing occurs so frequently in dyslexia that it is often considered to be the ‘core’ deficit (Liberman & Shankweiler, 1991; Stanovich, 1988; Swank, 1994). Other common linguistic deficits include poor semantic and/or syntactic functioning, verbal short-term memory, and articulation (Bishop & Snowling, 2004; Catts, 1996; Kibby & Cohen, 2008; Kibby, Marks, Morgan, & Long, 2004; Lombardino, Riccio, Hynd, & Pinheiro, 1997). Coinciding with the prevalence of these secondary linguistic deficits, there is a high comorbidity between dyslexia and specific language impairment (SLI), typically around 30% (Riccio & Hynd, 1993). By definition, children with SLI present with poor non-phonological linguistic functioning (comprehension and/or expression of semantics, syntax, and/or grammatical morphemes), but they commonly have reduced phonological processing and reading ability as well (for a review see Bishop & Snowling, 2004, Filipek, 1999; Joanisse & Seidenberg, 1998; Lane, Foundas, & Leonard, 2001). Hence, there is an overlap between the two disorders, although a number of children present with one but not the other. Regardless of whether a child has been diagnosed with dyslexia or SLI, deficits in phonological processing are associated with poor word recognition and decoding, whereas deficits in non-phonological linguistic skills are associated with poor reading comprehension (Bishop & Snowling, 2004; Catts, 1996, Lane et al., 2001; Lombardino et al., 1997).
The pars triangularis (PT), located within Broca’s area, has been implicated in various aspects of linguistic functioning. More specifically, it has been implicated in rhyming ability (Bookheimer & Dapretto, 1996; Cone, Burman, Bitan, Bolger, & Booth, 2008; Paulesu et al., 1996), phonological awareness (Demonet et al., 1992; Georgiewa et al., 1999), verbal working memory (Baddeley, 1998; Badre & Wagner, 2007), lexical decision making and semantic processing (Bookheimer & Dapretto, 1996; Newman, Just, Keller, Roth, & Carpenter, 2003; Papathanassiou et al., 2000), syntactic processing (Caplan, Alpert, & Waters, 1999; Rumsey et al., 1994), phonological decoding (Georgiewa et al.; 1999; Rumsey, Horwitz et al., 1997; Rumsey, Nace et al., 1997) and word identification (Mechelli et al., 2005; Rumsey, Horwitz et al., 1997) in functional neuroimaging studies.
Despite potential PT involvement in phonological processing, other linguistic functions, and reading, few quantitative studies have been published on it in dyslexia. Of that conducted, Eckert and colleagues (2003) found smaller PT size bilaterally in children with dyslexia as compared to controls when utilizing manual tracing. They also found PT size was related to reading, spelling, phonological awareness, and rapid automatic naming abilities, with the latter two skills being aspects of phonological processing that are often affected in dyslexia (Wolf & Bowers, 1999). While right PT size was predictive of phonological awareness, both left and right PT size were predictive of rapid naming performance. However, in a follow-up study using a subset of the 2003 sample, Eckert and colleagues (2005) found only right PT size was smaller in dyslexia than controls when using manual tracing and controlling for cerebral volume. Groups did not differ in PT size when using voxel-based analysis.
Given the dearth of quantitative research on the PT in dyslexia and SLI’s comorbidity with dyslexia, studies examining the PT in SLI are considered next. Gauger, Lombardino and Leonard (1997) found smaller left PT size and atypical PT asymmetry in children with SLI, but they did not find any relationship between PT size and phonological or other linguistic processing. Jernigan, Hesselink, Sowell, and Tallal, (1991) also found atypical asymmetry in the inferior frontal region in children with comorbid SLI and dyslexia; however, they found this region to be comparable in size to controls. Clark and Plante (1998) found adults with a developmental language disorder were more likely to have an extra sulcus in the PT region, and presence of an additional sulcus was associated with poor linguistic skills.
ADHD often co-occurs with dyslexia and SLI, with about a 15-40% comorbidity with dyslexia (Holborow & Berry, 1986; Shaywitz, Fletcher, & Shaywitz, 1994) and a 31-60% comorbidity with SLI (D’Incau, 2000; Riccio & Hynd, 1993), suggesting they may share overlapping etiologies. Concordantly, children with ADHD frequently have subclinical oral language deficits, particularly in comprehension, syntax formation, and/or pragmatics, despite phonological processing often being intact (Bruce, Thernlund, & Nettelbladt, 2006; Camarata & Gibson, 1999; McInnes, Humphries, Hogg-Johnson, & Tannock, 2003; Purvis & Tannock, 2000). Given this, potential PT involvement in ADHD is of interest. To date, research has not examined PT size in ADHD despite a number of studies implicating the frontal lobes in this population. For example, several studies have found reduced prefrontal size in ADHD, particularly in the right hemisphere (Castellanos, Giedd, Marsh, & Hamburger, 1996; Filipek et al., 1997; Mostofsky, Cooper, Kates, Denckla, & Kaufmann, 2002; Swanson, Castellanos, Murias, LaHoste, & Kennedy, 1998), with some studies specifically identifying the inferior frontal gyrus as one of the regions affected (Clark et al., 2007; Filipek et al., 1997; Sowell et al., 2003; Swanson et al., 1998). Furthermore, various aspects of the right inferior frontal gyrus are active during response inhibition and working memory tasks in functional neuroimaging studies (Aron & Poldrack, 2005; Chamberlain & Sahakian, 2007; Westerberg & Klingberg, 2007).
Variation in PT structure is of interest, along with its size/asymmetry, as posterior Sylvian fissure sulcal variability has been shown to correlate with presence of dyslexia (Leonard et al., 1993) and various aspects of linguistic functioning (Hiemenz & Hynd, 2000), along with the size of this region. Moreover, it has been suggested that gyral patterns may reflect the pattern of underlying neuronal connections (Van Essen, 1997). Nonetheless, PT sulcal variability has not been assessed in dyslexia, ADHD, or SLI to date. Foundas, Weisberg, Browning and Weinberger (2001) utilized a classification system which captured PT variability and included four typologies based on the anterior ascending and horizontal rami’s relationship with the Sylvian fissure. Their study, which included only healthy, right-handed men, found type V occurred in 58% of the men, type U occurred in 25%, and type Y occurred in 17%; type J was not found (here the horizontal ramus is poorly formed or absent). It is currently unknown whether children with dyslexia and/or ADHD present with the same frequencies of these typologies. It also is unknown whether these typologies are related to linguistic ability as this was not examined in the Foundas et al. study.
Because of the dearth of research on the pars triangularis in dyslexia and ADHD, the primary goal of this study was to determine if PT morphology (size, asymmetry, shape, presence of an extra sulcus) is affected in these groups. It was hypothesized that presence of dyslexia would be associated with smaller PT length bilaterally based on the findings of Eckert and colleagues (2003) and that presence of ADHD would be associated with smaller right PT length. As non-phonological linguistic deficits are commonly found in both dyslexia and ADHD, the second purpose of this study was to determine if children with poor expressive language functioning, regardless of diagnosis, present with atypical morphology of the PT. The third purpose of this study was to examine the relationship between PT length/shape and phonological processing, non-phonological linguistic functioning, inattention, and hyperactivity/impulsivity given the literature reviewed above. This aspect of the study was exploratory due to the limited and inconsistent findings on whether PT size is related to linguistic functioning (Eckert et al., 2003; Gauger et al., 1997), the lack of research on PT shape and linguistic functioning, and the lack of research on the PT in relation to inattention and hyperactivity/impulsivity.
Method
Participants
Participants included 50 children, ages 8-12 years: 10 with dyslexia, 15 with dyslexia/ADHD, 15 with ADHD, and 10 typically developing controls. The four groups did not differ in gender [X2=2.70, p=.440] or race [X2=2.26, p=.521], with 70% of the sample being male and 96% Caucasian. For all participants, exclusionary criteria included presence of a psychiatric disorder (except ADHD), neurological disorder, medical conditions (except allergies and asthma), and measured intelligence below 80. If taking stimulant medication, children were tested off medication on the day of testing per parent report.
Dyslexia
Dyslexia was defined according to State of Georgia criteria which required at least a 20 point standard score discrepancy between measured intelligence and reading ability, with reading being lower, at the time of data collection. For this study, the Wechsler Intelligence Scale for Children-Third Edition (WISC-III; Wechsler, 1991) was used as the measure of intelligence, and the Reading subtest from the Wide Range Achievement Test-Third Edition (WRAT-3; Wilkinson, 1993) was used as the measure of reading ability. As supplementary measures of reading skill, the Passage Comprehension and Word Attack subtests from the Woodcock Reading Mastery Test-Revised (WRMT; Woodcock, 1987) were administered. In addition, the WRAT-3 Spelling subtest was used as a measure of spelling ability. Spelling was assessed for descriptive purposes as children with dyslexia often have difficulty with spelling.
A discrepancy definition was chosen over a poor reader definition for three main reasons. First, a free neuropsychological report was used to recruit participants, and the State of Georgia used a discrepancy definition at the time of data collection. Second, dyslexia defined with a discrepancy definition may be more likely to have a biological basis than dyslexia defined with a poor reader definition, as the latter group tends to be more heterogeneous in nature and is more likely to include individuals with strong environmental contributions (for reviews see Bishop & Snowling, 2004; Eckert & Leonard, 2000). Third, many studies on the biological basis of dyslexia utilize a discrepancy definition, facilitating comparison across studies.
ADHD
Participants were diagnosed with ADHD according to DSM-IV criteria. The diagnostic process was multi-modal, including use of a semi-structured interview (Schedule for Affective Disorders and Schizophrenia for School-Age Children, updated with DSM-IV criteria; Puig-Antich & Chambers, 1978), parent questionnaires [Behavior Assessment System for Children (BASC; Reynolds & Kampaus, 1993) Parent form and the Child Behavior Checklist (CBCL; Achenbach & Edelbrock, 1983)] and teacher questionnaires [BASC Teacher Form and the Child Behavior Checklist-Teacher Rating Form (TRF; Achenbach & Edelbrock, 1986)] to ensure symptoms were of sufficient number and severity across settings to warrant diagnosis. In the dyslexia/ADHD group, 5 children had ADHD-Predominately Inattentive type (ADHD-PI) and 10 had ADHD-Combined type (ADHD-C). In the ADHD group, 3 children had ADHD-PI and 12 had ADHD-C. The difference in subtype prevalence was not significant, X2=.68, p=.409. All participants in the ADHD group were required to have Average or better WRAT-3 Reading standard scores (≥ 85). Four participants were excluded from the ADHD group because their WRAT-3 Reading scores were below 85 but they did not meet the 20 point discrepancy required for dyslexia/ADHD. This exclusion resulted in the 15 children with ADHD described above.
Controls
Controls had a standard score of 85 or greater on the WRAT-3 Reading subtest, and they did not have a history of grade retention or special education assistance. One participant was excluded from the control group because his WRAT-3 Reading score was below 85 but he did not meet the 20 point discrepancy required for dyslexia diagnosis. This exclusion resulted in the 10 typically developing controls described above.
Materials
All participants underwent a battery of neuropsychological tests including various measures of linguistic functioning. The Clinical Evaluation of Language Fundamentals-Revised (CELF-R; Semel, Wiig, & Secord, 1987) Expressive Language composite score was used to assess overall expressive language functioning; the CELF-R Semantic Relationships subtest was used as a measure of semantic processing, and the Formulated Sentences subtest was used as a measure of syntax formation. The Elision subtest from the Comprehensive Test of Phonological Processing-Experimental Version (CTOPP; Torgesen & Wagner, unpublished test) was used as a measure of phonological awareness. Color and letter naming times from the Rapid Automatized Naming test (RAN; Denckla & Rudel, 1976a, 1976b) were used to assess rapid automatic naming ability. The Boston Naming Test (BNT; Kaplan, Goodglass, & Weintraub, 1983) was used as a measure of confrontational naming ability. The WISC-III Digit Span subtest was used as a measure of phonological short-term memory.
MRI Data Acquisition
Sagittal images (3.1mm) were acquired on a .6T Health Images MRI scanner. The protocol utilized 3-D, gapless slices [TR=51; TE=10 (prior to 9/23/95) or TE=13 (after 9/23/95)]. All scans were assessed by a neurologist and found to be within normal limits. Images were hand-traced using a digitizing tablet with the publicly available Scion Image for Windows software package developed for the National Institutes of Health (NIH).
Brain Measurements
Pars triangularis length
Measurements of the pars triangularis (PT) were based on previously published guidelines (Eckert et al., 2003; Gauger et al., 1997). Lengths of the anterior ascending (AA) and anterior horizontal (AH) rami were initially traced on the sagittal slice providing the best view of the PT for that hemisphere and then on the two slices medial and lateral to the ‘best view’ slice. An average of these five lengths was used for each structure. In addition, the AA and AH average lengths were summed to form an overall pars triangularis length (PTr) for each hemisphere to facilitate comparisons with Eckert et al.’s study.
Average rami length was used as the measure of size for three reasons. First, our scanner resolution was weak, but PT sulci could be easily identified and reliably traced (see Figure 1). Second, in her review, Leonard (2001) advocated for cortical sulcal measurements because sulci can be more readily compared across studies than gyral area/volume which is affected by inconsistent tissue segmentation across software packages. Moreover, sulcal length measurements are sensitive to a variety of behavioral and biological inter-individual and group differences (Leonard, 2001). Third, previously published studies on PT size (Eckert et al., 2003, 2005; Gauger et al., 1997) traced its sulci, so our measuring sulcal length facilitated comparisons across studies. Inter-rater reliability between CEH and JMK on 10 brains was 0.91.
Figure 1.
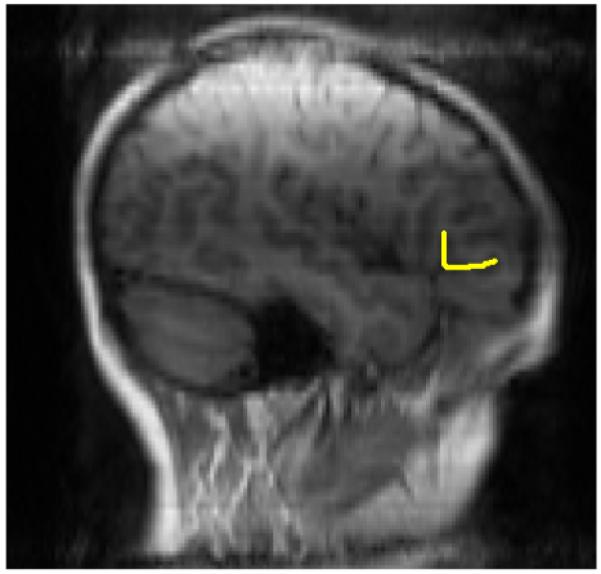
Image depicting measurement of the pars triangularis. Note, this hemisphere has a “Y” typology.
Pars triangularis asymmetry
Asymmetry of the PT was assessed following the work of Gauger et al. (1997) and Jernigan et al. (1991) on this structure. The formula from Gauger and colleagues was used to assess the asymmetry of AA and AH: (Left-Right)/[(Left + Right)*.05]. Hence, a positive value would indicate that the left AA is longer than the right.
Pars triangularis typology
PT morphology was classified in the sagittal plane following the work of Foundas and colleagues (2001). In general, this classification is based upon the relationship of AH and AA to the Sylvian fissure, but type J is assigned when AH is absent or poorly formed. Because V and U typology often co-occur in the same hemisphere (morphology frequently changes from a V to a U when moving laterally in the hemisphere; Foundas et al.) these two patterns were combined for this study. This resulted in three possible morphology types: J, Y, and V/U. Typology was classified for each hemisphere separately. Inter-rater reliability between MYK and HK on 10 brains was 0.98 using Spearman’s Rho. Presence of an extra sulcus within the PT also was coded based on the work of Clark and Plante (1998). This extra sulcus was an additional sulcus attached to the PT proper and not part of the PT coding system (i.e., the stem of the Y typology was not considered an extra sulcus).
Cerebral hemisphere volume
Cerebral hemisphere volume was measured using previously published guidelines (Raz, Gunning-Dixon, Head, Williamson, & Acker, 2001). Specifically, each hemisphere was measured separately and traced on every 4th slice in the coronal plane, starting at the most anterior slice in which a hemisphere was present and continuing until it was absent caudally. The measurements included gray/white matter encompassed by the dura but excluded the optic nerve, tract and chiasm; ventricles; corpus callosum; and septum pallusidum. Cavalieri’s rule for overcorrection was used when calculating volume (as published in Rosen & Harry, 1990).
Results
Participant Characteristics
When using ANOVA, the four groups (controls, dyslexia, dyslexia/ADHD, and ADHD) were comparable in age, maternal education, handedness, and WISC-III Verbal IQ (VIQ), Performance IQ (PIQ), and Full-Scale IQ (FSIQ). Groups differed in reading ability and attention problems, as expected. See Table 1. In terms of reading ability, groups differed in WRAT-3 Reading [F(3,45)=22.46, p=.000], WRAT-3 Spelling [F(3,45)=22.46, p=.000], WRMT-R Passage Comprehension [F(3,44)=18.38, p=.000] and WRMT-R Word Attack [F(3,44)=17.00, p=.000], with the two dyslexia groups performing worse than the ADHD and control groups on all measures when using Games-Howell for unequal ns (ps < .01). The two dyslexia groups were comparable to each other on these measures, as were the ADHD and control groups. In terms of attention problems, hyperactivity, and impulsivity (CBCL Attention Problems scale), group differences were significant, F(3,43)=34.21, p=.000, with the two ADHD groups having worse symptoms than the RD and control groups (ps < .001). The two ADHD groups were comparable to each other, as were the RD and control groups.
Table 1. Participant Descriptive Information.
| Variable | Dyslexia | Dyslexia/ADHD | ADHD | Controls | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 10.34 | 1.12 | 9.42 | 0.99 | 9.56 | 1.03 | 9.73 | 0.87 |
| Edinburgh | 94.00 | 5.16 | 91.33 | 15.17 | 78.13 | 31.03 | 85.63 | 30.87 |
| Maternal Education (years) | 14.29 | 2.06 | 13.47 | 1.46 | 16.60 | 2.61 | 15.89 | 2.42 |
| WISC-III | ||||||||
| Full-Scale IQ | 99.20 | 13.26 | 105.47 | 15.32 | 107.87 | 16.48 | 108.33 | 12.12 |
| Verbal IQ | 95.80 | 13.31 | 104.27 | 17.09 | 108.53 | 13.42 | 111.89 | 14.37 |
| Performance IQ | 103.80 | 12.83 | 106.00 | 13.78 | 106.07 | 18.77 | 103.00 | 9.90 |
| WRAT-3 | ||||||||
| Readinga | 79.00 | 12.32 | 79.40 | 10.82 | 105.20 | 9.10 | 111.00 | 15.62 |
| Spellinga | 81.78 | 11.51 | 83.73 | 7.74 | 99.27 | 10.32 | 105.20 | 15.66 |
| WRMT-R | ||||||||
| Word Attacka | 75.40 | 15.25 | 81.00 | 8.74 | 101.33 | 10.17 | 107.00 | 16.36 |
| Passage Comprehensiona | 76.40 | 14.05 | 80.86 | 11.17 | 98.73 | 9.11 | 108.44 | 12.19 |
| CBCL | ||||||||
| Attention Problemsb | 53.70 | 4.30 | 71.40 | 6.82 | 69.47 | 5.19 | 52.14 | 5.67 |
Note. Edinburgh is measured in percent of tasks performed with the right hand. CBCL is in T-scores, and the rest of the measures are in standard scores.
Those with and without dyslexia differed at ps < .01.
Those with and without ADHD differed at p < .001.
Anatomical Measures in Dyslexia and ADHD
Pars triangularis size
A 2 (dyslexia or not) X 2 (ADHD or not) MANCOVA was used to compare groups on left AA, AH, and PTr lengths, with left cerebral hemisphere volume as the covariate. This method was chosen rather than merely comparing the four groups to each other so that potential interactions between dyslexia and ADHD could be assessed given their comorbidity. The omnibus tests for dyslexia [F(2,36) < 1.0, ηp2 =.01] and ADHD [F(2,36)=2.35, p=.110; ηp2 =.12] were not significant, but the dyslexia*ADHD interaction was significant, F(2,36)=3.55, p=.039, ηp2 =.17. Nonetheless, none of its univariate ANOVAs was significant. A 2 × 2 MANCOVA also was conducted to compare groups on right AA, AH, and PTr lengths, with right cerebral hemisphere volume as the covariate. The omnibus tests for dyslexia [F(2,37) < 1.0, ηp2 =.02] and dyslexia*ADHD [F(2,37) < 1.0, ηp2 =.04] were not significant, and neither were their univariate ANOVAs. The omnibus tests for ADHD did not reach significance due to variable univariate findings and low power, F(2,37)=2.60, p=.088, ηp2 =.12. Even though the univariate ANOVA was not significant for right AH, F(2,37)=2.31, p=.137, ηp2 =.06, it was significant for right AA, F(1,38)=5.19, p=.028, ηp2 =.12 and PTr, F(1,38)=4.60, p=.038, ηp2 =.11 with children with ADHD having smaller volumes. Groups were comparable in right and left cerebral hemisphere volume. See Table 2.
Table 2. Anatomical Size Variables in Dyslexia and ADHD.
| Variable | Dyslexia | Dyslexia/ADHD | ADHD | Controls | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Left Hemisphere | ||||||||
| AAR Length | 11.33 | 4.03 | 13.56 | 5.02 | 14.34 | 3.50 | 12.25 | 3.43 |
| AHR Length | 13.33 | 3.93 | 11.24 | 4.40 | 14.37 | 14.37 | 11.66 | 5.40 |
| PTr Length | 24.66 | 7.56 | 24.80 | 8.93 | 28.71 | 6.67 | 23.91 | 8.45 |
| Right Hemisphere | ||||||||
| AAR Lengtha | 12.52 | 3.49 | 9.87 | 3.03 | 10.58 | 3.08 | 12.44 | 4.20 |
| AHR Length | 12.30 | 3.29 | 9.03 | 3.66 | 9.87 | 3.55 | 9.95 | 5.06 |
| PTr Lengtha | 24.82 | 5.38 | 18.90 | 5.92 | 20.45 | 5.83 | 22.39 | 8.47 |
| Asymmetry Ratios | ||||||||
| AAR | -.13 | 0.47 | .27 | 0.49 | .20 | 0.43 | .01 | 0.60 |
| AHR | .08 | 0.35 | .22 | 0.50 | .27 | 0.52 | .08 | 0.77 |
| PTr | -.03 | 0.38 | .25 | 0.44 | .23 | 0.44 | .05 | 0.67 |
| Cerebral Hemisphere | ||||||||
| Left Volume | 1563531.56 | 174214.41 | 1559193.30 | 102864.08 | 1564348.97 | 122069.82 | 1603316.07 | 93286.67 |
| Right Volume | 1542643.57 | 169871.15 | 1526861.35 | 98928.58 | 1533219.85 | 122846.71 | 1582245.06 | 88924.39 |
Note. Length measurements are in mm. Cerebral hemisphere volume is in mm3. AAR is short for Anterior Ascending Ramus; AHR is short for Anterior Horizontal Ramus; and PTr is short for Pars Triangularis.
Those with and without ADHD differed at p < .05.
To determine whether findings were due to the definition of dyslexia chosen, these analyses were re-run, comparing below average readers (those with WRAT-3 Reading standard scores < 85) to average or better readers (those with standard scores ≥ 85) following the methodology of Siegel (1988), a major proponent of the ‘poor reader’ definition. The 5 children who were excluded from the study due to low reading levels without a significant discrepancy were included in the poor reader group, causing the total N to be 55. In these analyses both hemisphere volume and FSIQ were used as covariates, since poor readers had significantly lower FSIQ scores than good readers [F(1,52)=39.33, p=.000], consistent with prior literature (for a review see Bishop & Snowling, 2004). The 2 (good versus poor readers) X 2 (ADHD versus no ADHD) MANCOVA comparing groups on left AA, AH and PTr lengths was not significant at the omnibus level for reading ability, F(2,40) < 1.0, ηp2 =.01, ADHD, F(2,40)=2.01, p=.147, ηp2 =.09 or the interaction, F(2,40)=1.83, p=.173, ηp2 =.08. In addition, none of the univariate ANOVAs was significant. The 2 × 2 MANCOVA comparing groups on right AA, AH and PTr lengths was not significant at the omnibus level for reading ability, F(2,40)=2.14, p=.131, ηp2 =.10 or the interaction, F(2,40)=1.57, p=.221, ηp2 =.07. However, with the additional participants the omnibus tests for ADHD were now significant, F(2,40)=4.07, p=.025, ηp2 =.17. Furthermore, all of the univariate ANOVAs were significant: right AA [F(1,41)=6.07, p=.018, ηp2 =.13], AH [F(1,41)=7.19, p=.010, ηp2 =.15] and PTr [F(1,41)=8.23, p=.006, ηp2 =.17].
Given the third purpose of this study and the significant relationship between presence of ADHD and right PT size, Pearson correlations were run between right AA and AH lengths and the parent BASC Attention Problems and Hyperactivity scales using the total sample. The BASC was chosen over the CBCL as it separates attention problems from hyperactivity and impulsivity. Right AA length was negatively correlated with Attention Problems, r=-.36, p=.017, d=.77, indicating smaller size is associated with worse attention problems. The rest of the correlations were small and non-significant (rs < .20 in absolute value).
Pars triangularis asymmetry
A 2 (dyslexia or not) by 2 (ADHD or not) MANCOVA was used to compare groups on asymmetry of the AA and AH, with cerebral hemisphere asymmetry as the covariate. The omnibus tests were not significant for dyslexia [F(2,36) < 1.0, ηp2 =.01], ADHD [F(2,36)=1.55, p=.226, ηp2 =.08] or the interaction [F(2,36) < 1.0, ηp2 =.03], and none of the univariate ANOVAs was significant. See Table 2. Results did not change when re-running the 2 × 2 MANCOVAs, substituting the factor ‘good versus poor readers’ for ‘dyslexia or not’.
Pars triangularis typology and presence of an extra gyrus
Chi-square was used to compare the four groups (dyslexia, dyslexia/ADHD, ADHD and controls) on PT typology. The results for both hemispheres were not significant: left hemisphere, X2(6)=6.09, p=.404 and right hemisphere, X2(6)=2.67, p=.850. Controls’ percentages of Y and V/U typologies were comparable to those of Foundas et al. (2001). Nonetheless, the J typology was found in our study in contrast to the study by Foundas et al. See Table 3. Chi-square also was used to compare the four groups on presence of an extra sulcus in the PT region. Groups were comparable for both hemispheres: left, X2(3)=.40, p=.941 and right, X2(3)=1.55, p=.671.
Table 3. Frequencies of Typology and Extra Gyrus by Diagnosis.
| Variable | Dyslexia | Dyslexia/ADHD | ADHD | Controls |
|---|---|---|---|---|
| Left Hemisphere | ||||
| Type J | 1 | 0 | 0 | 1 |
| Type Y | 2 | 7 | 3 | 3 |
| Type V/U | 6 | 7 | 11 | 6 |
| Right Hemisphere | ||||
| Type J | 1 | 3 | 1 | 2 |
| Type Y | 4 | 7 | 7 | 3 |
| Type V/U | 4 | 4 | 6 | 5 |
| Left Hemisphere | ||||
| Extra Gyrus | 2 | 2 | 2 | 2 |
| Right Hemisphere | ||||
| Extra Gyrus | 2 | 3 | 1 | 1 |
Note. Groups did not differ in typology or presence of an extra gyrus for either hemisphere.
Anatomical Analysis by Expressive Language Ability
In accordance with the second purpose of this study, participants were coded as to the presence or absence of poor expressive language ability. Children with CELF-R Expressive Language composite scores ≤ 80 were coded as having “poor” expressive language ability (n=19), whereas children with Expressive Language composites of > 80 were coded as having “adequate” expressive language ability (n=31). The cut-off of 80 was chosen over 85 to focus on children with more substantial expressive language weaknesses.
Pars triangularis size and asymmetry
MANCOVA was used in these analyses, comparing the adequate and poor expressive language groups. FSIQ was used as a covariate because groups differed in VIQ [F(1,47)=22.56, p=.000], PIQ [F(1,47)=15.27, p=.000] and FSIQ [F(1,47)=26.37, p=.000], with the poor expressive language group having lower scores. When left hemisphere volume and FSIQ were used as covariates, the omnibus tests for left AA, AH and PTr were not significant, F(2,37)=1.02, p=.372, ηp2 =.05, nor were any of the univariate ANOVAs. When using right hemisphere volume and FSIQ as covariates, the omnibus tests for right AA, AH and PTr were significant, F(2,37)=3.77, p=.032, ηp2 =.17; however, none of the univariate ANOVAs was significant (ps > .10). In addition, groups were comparable in AA and AH asymmetry, omnibus F(2,37)=1.25, p=.30, ηp2 =.06. See Table 4.
Table 4. Anatomical Size Variables by Expressive Language Group.
| Variable | Poor Expressive Language | Adequate Expressive Language | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Left Hemisphere | ||||
| AAR length | 13.83 | 3.75 | 12.73 | 4.16 |
| AHR length | 13.49 | 4.01 | 12.52 | 4.47 |
| PTr length | 27.33 | 6.94 | 25.25 | 8.05 |
| Right Hemisphere | ||||
| AAR length | 11.90 | 3.47 | 11.20 | 3.72 |
| AHR length | 9.71 | 3.25 | 10.78 | 4.35 |
| PTr length | 21.61 | 5.83 | 21.97 | 7.46 |
| Asymmetry Ratios | ||||
| AAR | .15 | 0.48 | .12 | 0.50 |
| AHR | .30 | 0.52 | .14 | 0.50 |
| PTr | .22 | 0.47 | .13 | 0.45 |
| Cerebral Volume | ||||
| Left | 1539237.20 | 148134.37 | 1583400.21 | 111601.43 |
| Right | 1505127.93 | 139553.15 | 1561763.45 | 113375.32 |
Note. Length measurements are in mm. Cerebral hemisphere volume is in mm3. There were no significant differences in size.
Pars triangularis typology and presence of an extra gyrus
In contrast to size, those with and without expressive language weaknesses differed in the presence of an extra sulcus. Children with poor expressive language had an extra sulcus in the left PT more frequently than those with adequate expressive language [X2(1)=5.53, p=.019, Φ=.33]. Groups were comparable in the presence of a right extra sulcus, X2(1)=0.08, p=.775, Φ=.04. See Table 5. Groups also were comparable in PT typology (left hemisphere, X2(2)=4.10, p=.129; right hemisphere, X2(2)=4.32, p=.116). Nonetheless, for the left hemisphere type J occurred solely in those with poor expressive language functioning.
Table 5. Frequencies of Typology and Extra Gyrus by Expressive Language Group.
| Variable | Poor Expressive Language | Adequate Expressive Language |
|---|---|---|
| Left Hemisphere | ||
| Type J | 2 | 0 |
| Type Y | 5 | 10 |
| Type V/U | 9 | 21 |
| Right Hemisphere | ||
| Type J | 4 | 3 |
| Type Y | 4 | 17 |
| Type V/U | 8 | 11 |
| Left Hemisphere | ||
| Extra Gyrus* | 6 | 2 |
| Right Hemisphere | ||
| Extra Gyrus | 3 | 4 |
Note. Groups did not differ significantly in typology for either hemisphere.
p < .05
Given the third purpose of this study and the finding that groups differed in the presence of a left extra sulcus, t-tests were used to compare those with and without a left extra sulcus (irrespective of expressive language group) on our measures of linguistic ability. Presence of a left extra sulcus was associated with worse performance on the Boston Naming Test, t(48)=2.19, p=.033, d=.63 (extra gyrus M=86.00, SD=12.82; no extra gyrus M=99.60, SD=16.56). Moreover, performance was slightly worse on all linguistic measures for those with a left extra sulcus, including Digit Span, Semantic Relationships, Formulated Sentences, Elision, and Rapid Naming. To determine if these findings were specific to the left hemisphere, analyses were re-run comparing those with and without an extra sulcus in the right PT. Presence of a right extra sulcus was unrelated to linguistic functioning (ps > .10)
Relationship between Pars Triangularis Size and Shape and Linguistic Ability
Because children with poor expressive language performed worse than those with adequate expressive language on all of our phonological and non-phonological linguistic measures (ps < .05), correlations were run within expressive language groups to address the third purpose of our study. Pearson correlations were run between right and left AA and AH lengths and our linguistic measures in an exploratory fashion with alpha level set at .05. For those with good expressive language ability (n=31) moderate or greater correlations were as follows: left AA with Elision [r=.40, p=.032, d=.87], Digit Span [r=.38, p=.042, d=.82], and Color Time [r=-.37, p=.050, d=.80]; left AH with Elision [r=.49, p=.008, d=1.13]; right AA with Semantic Relationships [r=.37, p=.047, d=.80] and Color Time [r=-.41, p=.029, d=.90]; and right AH with Color Time [r=-.43, p=.021, d=.95]. Correlations were non-significant and modest (rs < .25 in absolute value) between PT lengths and Letter Time, Boston Naming Test and Formulated Sentences for both hemispheres. For those with poor expressive language (n=19), left AH was associated with Boston Naming Test performance [r=.65, p=.004, d=1.71], and right AH was moderately correlated with Formulated Sentences [r=.46, p=.053, d=1.04].
To determine if typology is related to linguistic ability, correlations were run between left and right typology and our linguistic measures in an exploratory fashion using Spearman’s rho. For those with good expressive language, right typology was related to Color Time [r=-.49, p=.006, d=1.13] and Letter Time [r=-.44, p=.014, d=.98], with less frequent typologies being associated with longer naming times. For those with poor expressive language, right typology was related to Formulated Sentences [r=.50, p=.046, d=1.54], with less frequent typologies being associated with worse performance.
Discussion
This study had three purposes given the dearth of research on the pars triangularis (PT) in dyslexia and ADHD. The first purpose was to assess the relationship between PT morphology and presence of dyslexia and ADHD. The second purpose was to compare PT morphology between those with and without expressive language weaknesses. The third purpose was to examine the relationship between PT morphology and linguistic functioning, attention problems, and hyperactivity/impulsivity in an exploratory fashion due to the dearth of literature on this topic.
Pars Triangularis Morphology in Dyslexia
In contrast to what was hypothesized, those with and without dyslexia were comparable in PT length and asymmetry. They also were comparable in PT typology. Although our findings are inconsistent with the work of Eckert and colleagues (2003), even Eckert et al. (2005) failed to find group differences in PT size when using a voxel-based approach. As the Eckert et al. (2003) study is the only one to find smaller PT size in dyslexia, it is possible that any group differences present in PT size are slight and more power and/or a different operational definition of dyslexia is required to find them. The notion of minimal to slight group differences corresponds with functional neuroimaging research which suggests that the inferior frontal region functions effectively in dyslexia (Pauesu, 1996; Rumsey, Nace et al., 1997) and that children with dyslexia may use it to compensate for a dysfunctional left temporo-parietal region (Shaywitz et al., 1998). Consistent with this notion, Chiron and colleagues (1999) found atypical activation of Broca’s area is associated with developmental oral language disorders but not reading disorders. Further work is indicated to determine whether the findings of Eckert et al. (2003) can be replicated.
Pars Triangularis Morphology in ADHD
Consistent with what was hypothesized, children with ADHD had smaller right PT length. Furthermore, length of the right anterior ascending ramus was related to attention problems in the total sample, such that shorter length was related to worse inattention. Thus, our findings are consistent with prior research implicating the inferior frontal region in ADHD (Filipek et al., 1997; Sowell, 2003; Swanson, 1998). However, our study is the first to implicate length of the right PT specifically in ADHD. Further work on the PT in ADHD is warranted to replicate these findings and to determine if this relationship varies by subtype of ADHD.
Pars Triangularis Morphology and Expressive Language Functioning
Although those with and without expressive language weaknesses were comparable in PT length and asymmetry, they differed in presence of an extra sulcus such that children with expressive language weaknesses were more likely to have an extra sulcus in the left PT, consistent with the work of Clark and Plante (1998) on adults with developmental language disorders. Furthermore, presence of an extra sulcus in the left PT was associated with worse confrontational naming, along with mildly worse language functioning in general, also consistent with the findings of Clark and Plante. In our study this relationship was specific to the left hemisphere, as presence of an extra sulcus in the right PT was not related to poor expressive language functioning in general or to any specific linguistic ability. Moreover, for the left hemisphere, type J was only found in those with expressive language weaknesses. Type J is associated with an absent or poorly formed anterior horizontal ramus. Thus, presence of an extra sulcus or absence of a typical sulcus in the left PT may be associated with expressive language weaknesses. Such brain abnormalities may be due to pruning and/or migration errors in utero (Lane et al., 2001) and/or differences in use/stimulation post-birth (Leonard, 2001).
Examination of Pars Triangularis Morphology and Linguistic Functioning
In our exploratory analysis, for children without expressive language weaknesses left anterior ascending and horizontal rami lengths were related to phonological awareness, and ascending ramus length was related to phonological short-term memory and rapid color naming. In the right hemisphere, anterior ascending and horizontal rami lengths were related to rapid color naming, and ascending ramus length was related to semantic functioning. For all of these correlations, longer length was associated with better performance. Furthermore, right PT typology was related to rapid color and letter naming, such that less frequent typologies were associated with worse rapid naming performance. These findings replicate the work of Eckert and colleagues (2003) who found PT size was predictive of phonological awareness and rapid automatic naming performance. Eckert et al.’s study did not assess phonological short-term memory or semantic language functioning. Given these structural findings and the functional neuroimaging literature reviewed previously, the pars triangularis may play an important role in multiple aspects of phonological processing, including phonological awareness, phonological short-term memory, and rapid automatic naming.
When examining children with weak expressive language functioning overall, limited associations were found between PT size and various specific linguistic functions, but sample size was small. Nonetheless, left anterior horizontal ramus length was moderately correlated with confrontational naming performance. Moreover, right anterior horizontal ramus length was moderately correlated with syntax formation, as was right PT typology. Hence, both an extra sulcus in the left PT and left anterior horizontal ramus length may be associated with confrontational naming performance in those with expressive language weaknesses. Of interest is the relationship between right PT morphology and syntax formation in those with expressive language weaknesses, as syntax tends to be strongly lateralized to the left hemisphere in those with typical language functioning (Kolb & Whishaw, 2003). Perhaps some PT structure/function relationships are atypical in children with expressive language difficulties, consistent with functional neuroimaging research in this area (Chiron et al., 1999; Hugdahl et al., 2004), but further research is indicated given the exploratory nature of our findings.
Study Limitations and Future Directions
Despite small sample size significant results were found, indicating further examination of the structure of the pars triangularis is warranted, particularly in relation to ADHD and linguistic functioning. To address this study’s limitations, future work should include a larger sample with a stronger scanner. It also should assess gyral area/volume and indices of convolutedness for the PT to determine if findings are consistent with those from studies analyzing sulcal length. Lastly, further work is warranted on the presence of an extra sulcus or absence of a typical one in the left PT, especially in relation to linguistic functioning.
Acknowledgements
Data collection was funded by a grant from the National Institutes of Health (NIH)/National Institute of Child Health and Human Development (NICHD) to the last author (GWH; R01 HD26890). Data analysis and write-up were supported in part by a NIH/NICHD grant to the first author (MYK; R03 HD048752). Portions of this data were presented at the International Neuropsychological Society 2005 conference.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach TM, Edelbrock C. Child Behavior Checklist. Thomas Achenbach; Burlington, VT: 1983. [Google Scholar]
- Achenbach TM, Edelbrock C. Teacher’s Report Form. Thomas Achenbach; Burlington, VT: 1986. [Google Scholar]
- Aron AR, Poldrack RA. The Cognitive Neuroscience of Response Inhibition: Relevance for Genetic Research in Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry. 2005;57:1285–1292. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Recent developments in working memory. Current Opinion in Neurobiology. 1998;8:234–238. doi: 10.1016/s0959-4388(98)80145-1. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Bishop DVM, Snowling MJ. Developmental Dyslexia and Specific Language Impairment: Same or different? Psychological Bulletin. 2004;130:858–886. doi: 10.1037/0033-2909.130.6.858. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Dapretto M. Functional Neuroimaging of Language in Children: Current Directions and Future Challenges. In: Thatcher RW, Lyon GR, Rumsey J, Krasnegor N, editors. Developmental Neuroimaging: Mapping the development of brain and behavior. Academic Press; San Diego, CA: 1996. pp. 143–155. [Google Scholar]
- Bruce B, Thernlund G, Nettelbladt U. ADHD and language impairment: A study of the parent questionnaire FTF (Five to Fifteen) European Child & Adolescent Psychiatry. 2006;15:52–60. doi: 10.1007/s00787-006-0508-9. [DOI] [PubMed] [Google Scholar]
- Camarata SM, Gibson T. Pragmatic language deficits in attention-deficit hyperactivity disorder (ADHD) Mental Retardation and Developmental Disabilities Research Reviews. 1999;5:207–214. [Google Scholar]
- Caplan D, Alpert N, Waters G. PET studies of syntactic processing with auditory sentence presentation. Neuroimage. 1999;9:343–351. doi: 10.1006/nimg.1998.0412. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Giedd JN, Marsh WL, Hamburger SD. Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Archives of General Psychiatry. 1996;53:607–616. doi: 10.1001/archpsyc.1996.01830070053009. [DOI] [PubMed] [Google Scholar]
- Catts HW. Defining dyslexia as a developmental language disorder: An expanded view. Topics in Language Disorders. 1996;16:14–29. [Google Scholar]
- Chamberlain SR, Sahakian BJ. The neuropsychiatry of impulsivity. Current Opinion in Psychiatry. 2007;20:255–261. doi: 10.1097/YCO.0b013e3280ba4989. [DOI] [PubMed] [Google Scholar]
- Chiron C, Pinton F, Masure MC, Duvelleroy-Hommet C, Leon F, Billard C. Hemispheric specialization using SPECT and stimulation tasks in children with dysphasia and dystrophia. Dev Med Child Neurol. 1999;41:512–520. doi: 10.1017/s0012162299001139. [DOI] [PubMed] [Google Scholar]
- Clark L, Blackwell AD, Aron AR, Turner DC, Dowson J, Robbins TW, et al. Association between response inhibition and working memory in adult ADHD: A link to right frontal cortex pathology? Biological Psychiatry. 2007;61:1395–1401. doi: 10.1016/j.biopsych.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Clark MM, Plante E. Morphology of the inferior frontal gyrus in developmentally language-disordered adults. Brain and Language. 1998;61:288–303. doi: 10.1006/brln.1997.1864. [DOI] [PubMed] [Google Scholar]
- Cone NE, Burman DD, Bitan T, Bolger DJ, Booth JR. Developmental changes in brain regions involved in phonological and orthographic processing during spoken language processing. Neuroimage. 2008;41:623–635. doi: 10.1016/j.neuroimage.2008.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demonet JF, Chollet F, Ramsay S, Cardebat D, Nespoulous JL, Wise R, Rascol A, Frackowiak R. The anatomy of phonological and semantic processing in normal subjects. Brain. 1992;115:1753–1768. doi: 10.1093/brain/115.6.1753. [DOI] [PubMed] [Google Scholar]
- Denckla MB, Rudel R. Rapid automatized naming (R.A.N.): Dyslexia differentiated from other learning disabilities. Neuropsychologia. 1976a;14:471–479. doi: 10.1016/0028-3932(76)90075-0. [DOI] [PubMed] [Google Scholar]
- Denckla MB, Rudel R. Naming of object drawings by dyslexic children and other learning disabled children. Brain and Language. 1976b;3:1–16. doi: 10.1016/0093-934x(76)90001-8. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM. Structural imaging in dyslexia: The planum temporale. Mental Retardation and Developmental Disabilities Research Reviews. 2000;6:198–206. doi: 10.1002/1098-2779(2000)6:3<198::AID-MRDD7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Richards TL, Aylward EH, Thomson J, Berninger VW. Anatomical correlates of dyslexia: Frontal and cerebellar findings. Brain. 2003;126:482–494. doi: 10.1093/brain/awg026. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Wilke M, Eckert M, Richards T, Richards A, Berninger V. Anatomical signatures of dyslexia in children: unique information from manual and voxel based morphometry brain measures. Cortex. 2005;41:304–315. doi: 10.1016/s0010-9452(08)70268-5. [DOI] [PubMed] [Google Scholar]
- Filipek PA. Neuroimaging in the developmental disorders: The state of the science. Journal of Child Psychology and Psychiatry. 1999;40:113–128. [PubMed] [Google Scholar]
- Filipek PA, Semrud-Clikeman M, Steingard RJ, Renshaw PF, Kennedy DN, Biederman J. Volumetric MRI analysis comparing subjects having attention-deficit hyperactivity disorder with normal controls. Neurology. 1997;48:589–601. doi: 10.1212/wnl.48.3.589. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Weisberg A, Browning CA, Weinberger DR. Morphology of the frontal operculum: a volumetric magnetic resonance imaging study of the pars triangularis. J Neuroimaging. 2001;11:153–159. doi: 10.1111/j.1552-6569.2001.tb00026.x. [DOI] [PubMed] [Google Scholar]
- Gauger LM, Lombardino LJ, Leonard CM. Brain morphology in children with specific language impairment. Journal of Speech & Hearing Research. 1997;40:1272–1284. doi: 10.1044/jslhr.4006.1272. [DOI] [PubMed] [Google Scholar]
- Georgiewa P, Rzanny R, Hopf JM, Knab R, Glauche V, Kaiser WA, Blanz B. fMRI during word processing in dyslexic and normal reading children. Neuroreport. 1999;10:3459–3465. doi: 10.1097/00001756-199911080-00036. [DOI] [PubMed] [Google Scholar]
- Hiemenz JR, Hynd GW. Sulcal/gyral pattern morphology of the perisylvian language region in developmental dyslexia. Brain and Language. 2000;74:113–133. doi: 10.1006/brln.2000.2343. [DOI] [PubMed] [Google Scholar]
- Holborow PL, Berry PS. Hyperactivity and learning difficulties. Journal of Learning Disabilities. 1986;19:426–431. doi: 10.1177/002221948601900713. [DOI] [PubMed] [Google Scholar]
- Hugdahl K, Gundersen H, Brekke C, Thomsen T, Rimol LM, Ersland L, Niemi J. fMRI brain activation in a Finnish family with specific language impairment compared with a normal control group. Journal of Speech, Language, and Hearing Research. 2004;47:162–172. doi: 10.1044/1092-4388(2004/014). [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Hesselink JR, Sowell E, Tallal PA. Cerebral structure on magnetic resonance imaging in language- and learning-impaired children. Archives of Neurology. 1991;48:539–545. doi: 10.1001/archneur.1991.00530170103028. [DOI] [PubMed] [Google Scholar]
- Joanisse MF, Seidenberg MS. Specific language impairment: a deficit in grammar or processing? Trends in Cognitive Sciences. 1998;2:240–247. doi: 10.1016/S1364-6613(98)01186-3. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Lea and Febiger; Philadelphia: 1983. [Google Scholar]
- Kibby MY, Cohen MJ. Memory functioning in children with reading disabilities and/or Attention-Deficit/Hyperactivity Disorder: A clinical investigation of their working memory and long-term memory functioning. Child Neuropsychology. 2008;14:525–546. doi: 10.1080/09297040701821752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibby MY, Marks W, Morgan S, Long CJ. Specific impairment in developmental reading disabilities: A working memory approach. Journal of Learning Disabilities. 2004;37:349–363. doi: 10.1177/00222194040370040601. [DOI] [PubMed] [Google Scholar]
- Kolb B, Whishaw IQ. Fundamentals of Human Neuropsychology. 5th ed. Worth Publishers; New York: 2003. [Google Scholar]
- Lane AB, Foundas AL, Leonard CM. The evolution of neuroimaging research and developmental language disorders. Topics in Language Disorders. 2001;21:20–41. [Google Scholar]
- Leonard CM. Imaging brain structure in children: Differentiating language disability and reading disability. Learning Disability Quarterly. 2001;24:158–176. [Google Scholar]
- Leonard CM, Voeller KKS, Lombardino LJ, Morris MK, Hynd GW, Alexander AW, Andersen HG, Garofalakis M, Honeyman JC, Mao J, Agree OF, Staab EV. Anomalous cerebral structure in dyslexia revealed with magnetic resonance imaging. Archives of Neurology. 1993;50:461–469. doi: 10.1001/archneur.1993.00540050013008. [DOI] [PubMed] [Google Scholar]
- Liberman IY, Shankweiler D. Phonology and beginning reading: A tutorial. In: Rieben L, Perfetti CA, editors. Learning to read: Basic Research and Its Implications. Lawrence Erlbaum Associates; Hillsdale, NJ: 1991. pp. 3–17. [Google Scholar]
- Lombardino LJ, Riccio C, Hynd GW, Pinheiro S. Linguistic deficits in children with reading disabilities. American Journal of Speech Language Pathology. 1997;6:71–78. [Google Scholar]
- McInnes A, Humphries T, Hogg-Johnson S, Tannock R. Listening comprehension and working memory are impaired in attention-deficit hyperactivity disorder irrespective of language impairment. Journal of Abnormal Child Psychology. 2003;31:427–443. doi: 10.1023/a:1023895602957. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Long S, Friston KJ, Lambon Ralph MA, Patterson K, McClelland JL, Price CJ. Dissociating reading processes on the basis of neuronal interactions. J Cogn Neurosci. 2005;17:1753–1765. doi: 10.1162/089892905774589190. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Cooper KL, Kates WR, Denckla MB, Kaufmann WE. Smaller prefrontal and premotor volumes in boys with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2002;52:785–794. doi: 10.1016/s0006-3223(02)01412-9. [DOI] [PubMed] [Google Scholar]
- Newman SD, Just MA, Keller TA, Roth J, Carpenter PA. Differential effects of syntactic and semantic processing on the subregions of Broca’s area. Brain Res Cogn Brain Res. 2003;16:297–307. doi: 10.1016/s0926-6410(02)00285-9. [DOI] [PubMed] [Google Scholar]
- Papathanassiou D, Etard O, Mellet E, Zago L, Mazoyer B, Tzourio-Mazoyer N. A common language network for comprehension and production: a contribution to the definition of language epicenters with PET. Neuroimage. 2000;11:347–357. doi: 10.1006/nimg.2000.0546. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith U, Snowling M, Gallagher A, Morton J, Frackowiak RS, Frith CD. Is developmental dyslexia a disconnection syndrome? Evidence from PET scanning. Brain. 1996;119:143–157. doi: 10.1093/brain/119.1.143. [DOI] [PubMed] [Google Scholar]
- Puig-Antich J, Chambers W. The Schedule for Affective Disorders and Schizophrenia for School-Age Children. New York State Psychiatric Institute; New York: 1978. [Google Scholar]
- Purvis KL, Tannock R. Phonological processing, not inhibitory control, differentiates ADHD and reading disability. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39:485–494. doi: 10.1097/00004583-200004000-00018. [DOI] [PubMed] [Google Scholar]
- Rack JP, Snowling MJ, Olson RK. The Nonword Reading Deficit in Developmental Dyslexia - a Review. Reading Research Quarterly. 1992;27:28–53. [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Williamson A, Acker JD. Age and sex differences in the cerebellum and the ventral pons: A prospective MR study of healthy adults. American Journal of Neuroradiology. 2001;22:1161–1167. [PMC free article] [PubMed] [Google Scholar]
- Reynolds CR, Kampaus RW. Behavior Assessment System for Children. American Guidance Service; Circle Pines, MN: 1993. [Google Scholar]
- Riccio CA, Hynd GW. Developmental language disorders in children: Relationship with learning disability and Attention Deficit Hyperactivity Disorder. School Psychology Review. 1993;22:696–709. [Google Scholar]
- Rosen GD, Harry JD. Brain volume estimation from serial section measurements: a comparison of methodologies. Journal of Neuroscience Methods. 1990;35:115–124. doi: 10.1016/0165-0270(90)90101-k. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Horwitz B, Donohue B, Nace K, Maisog J, Andreason P. Phonological and orthographic components of word recognition: A PET-rCBF study. Brain. 1997;120:739–759. doi: 10.1093/brain/120.5.739. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Nace K, Donohue B, Wise D, Maisog JM, Andreason P. A positron emission tomographic study of impaired word recognition and phonological processing in dyslexic men. Archives of Neurology. 1997;54:562–573. doi: 10.1001/archneur.1997.00550170042013. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Zametkin AJ, Andreason P, Hanahan AP, Hamburger SD, Aquino T, King AC, Pikus A, Cohen RM. Normal activation of frontotemporal language cortex in dyslexia, as measured with oxygen 15 positron emission tomography. Archives of Neurology. 1994;51:27–38. doi: 10.1001/archneur.1994.00540130037011. [DOI] [PubMed] [Google Scholar]
- Semel E, Wiig E, Secord W. Clinical Evaluation of Language Fundamentals -- Revised. The Psychological Corporation; New York: 1987. [Google Scholar]
- Shaywitz SE, Fletcher JM, Shaywitz BA. Issues in the definition and classification of attention deficit disorder. Topics in Language Disorders. 1994;14:1–25. [Google Scholar]
- Siegel LS. Evidence that IQ scores are irrelevant to the definition and analysis of reading disability. Canadian Journal of Psychology. 1988;42:201–215. doi: 10.1037/h0084184. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Welcome SE, Henkenius AL, Toga AW, Peterson BS. Cortical abnormalities in children and adolescents with attention-deficit hyperactivity disorder. Lancet. 2003;362:1699–1707. doi: 10.1016/S0140-6736(03)14842-8. [DOI] [PubMed] [Google Scholar]
- Stanovich KE. Explaining the differences between the dyslexic and the garden-variety poor reader: The phonological-core variable-difference model. Journal of Learning Disabilities. 1988;21:590–604. doi: 10.1177/002221948802101003. [DOI] [PubMed] [Google Scholar]
- Swank LK. Phonological coding abilities: Identification of impairments related to phonologically based reading problems. Topics in Language Disorders. 1994;14:56–71. [Google Scholar]
- Swanson J, Castellanos F, Murias M, LaHoste G, Kennedy J. Cognitive neuroscience of attention deficit hyperactivity disorder and hyperkinetic disorder. Current Opinion in Neurobiology. 1998;8:263–271. doi: 10.1016/s0959-4388(98)80150-5. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children -- Third Edition. The Psychological Corporation; San Antonio: 1991. [Google Scholar]
- Westerberg H, Klingberg T. Changes in cortical activity after training of working memory--a single-subject analysis. Physiology & Behavior. 2007;92:186–192. doi: 10.1016/j.physbeh.2007.05.041. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS. The Wide Range Achievement Test. 3rd ed. Jastak; Wilmington, DE: 1993. [Google Scholar]
- Wolf M, Bowers PG. The double-deficit hypothesis for the developmental dyslexias. Journal of Educational Psychology. 1999;91:415–438. [Google Scholar]
- Woodcock RW. Woodcock Reading Mastery Test -- Revised. American Guidance Service; Circle Pines, MN: 1987. [Google Scholar]
- World Federation of Neurology . Report of research group on dyslexia and world illiteracy. 1968. [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Constable RT, Mencl WE, Shankweiler DP, Liberman AM, Skudlarski P, Fletcher JM, Katz L, Marchione KE, Lacadie C, Gatenby C, Gore JC. Functional disruption in the organization of the brain for reading in dyslexia. Proc Natl Acad Sci U S A. 1998;95(5):2636–2641. doi: 10.1073/pnas.95.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]


