Abstract
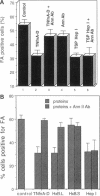
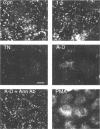
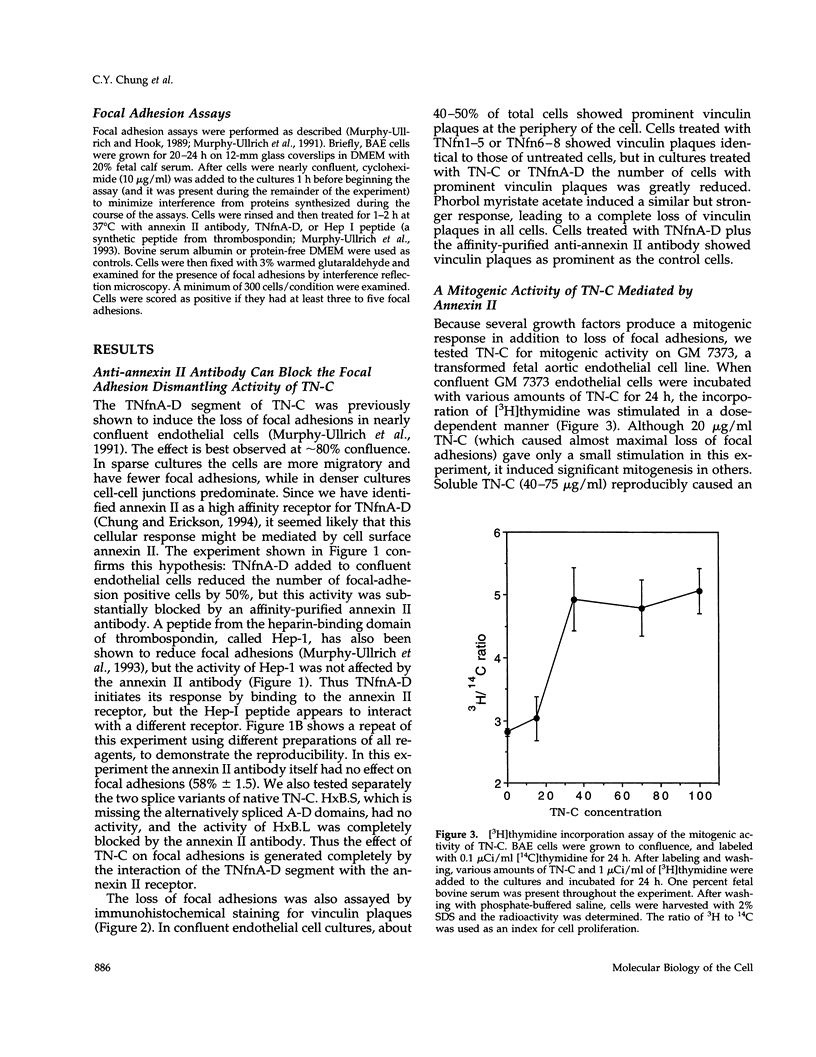
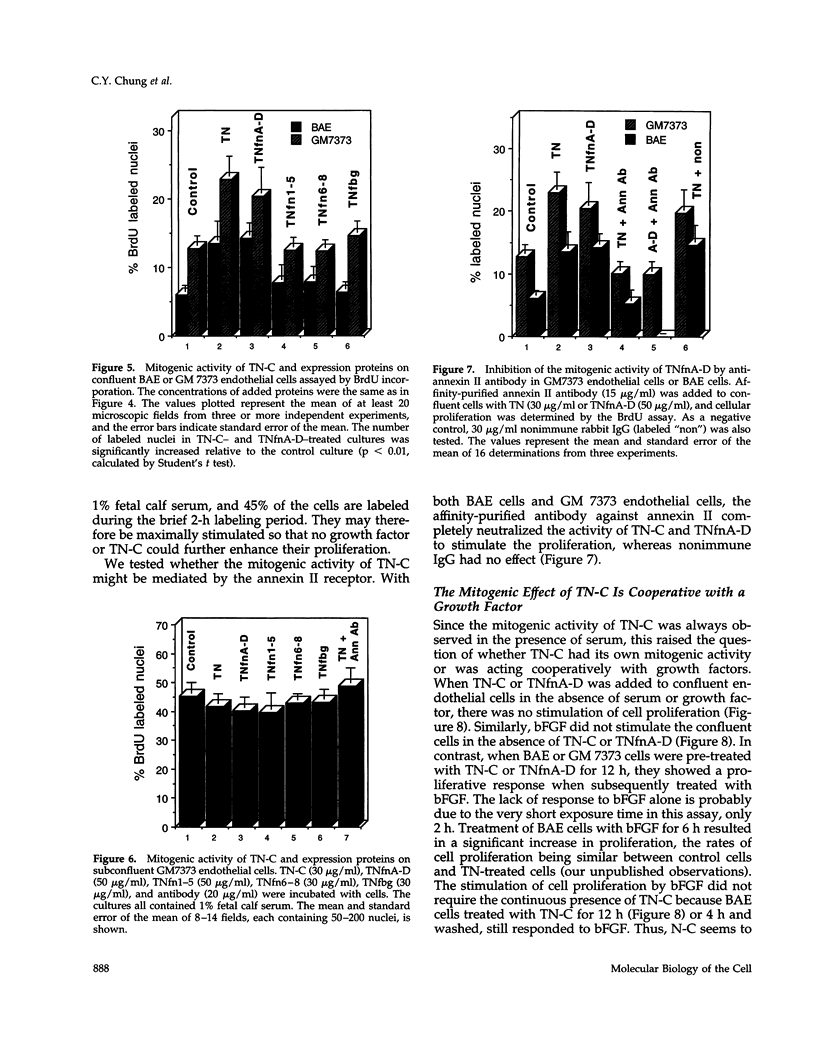
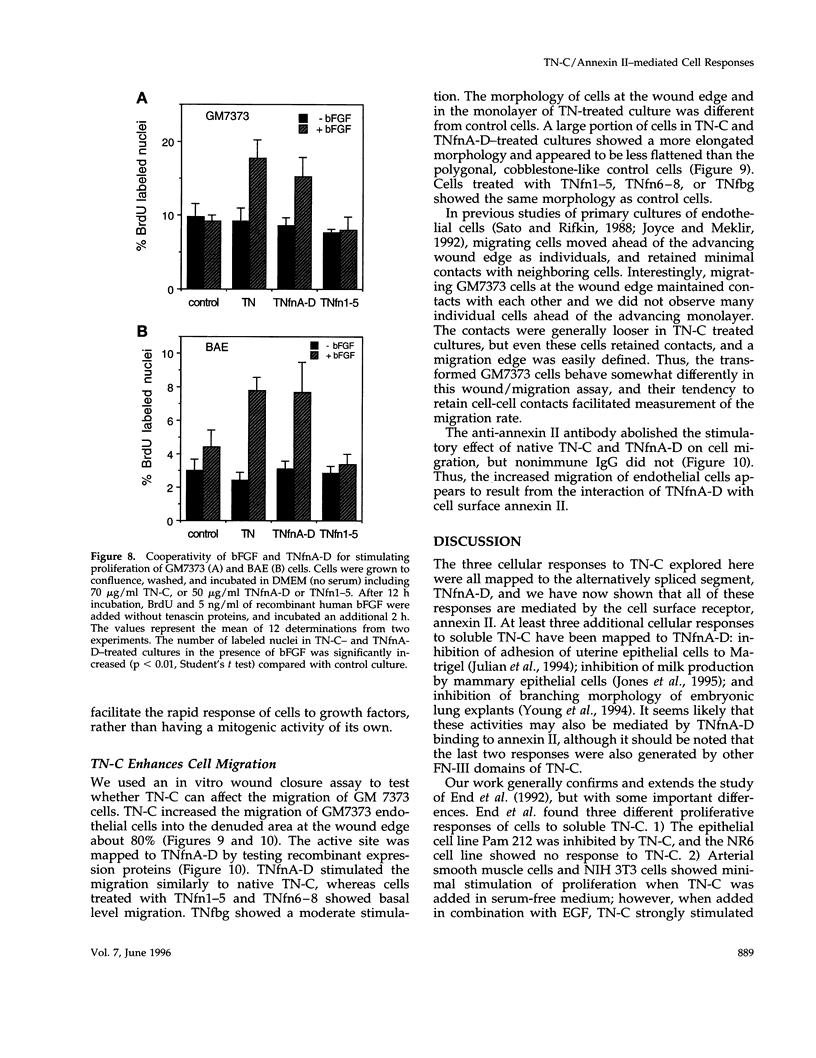
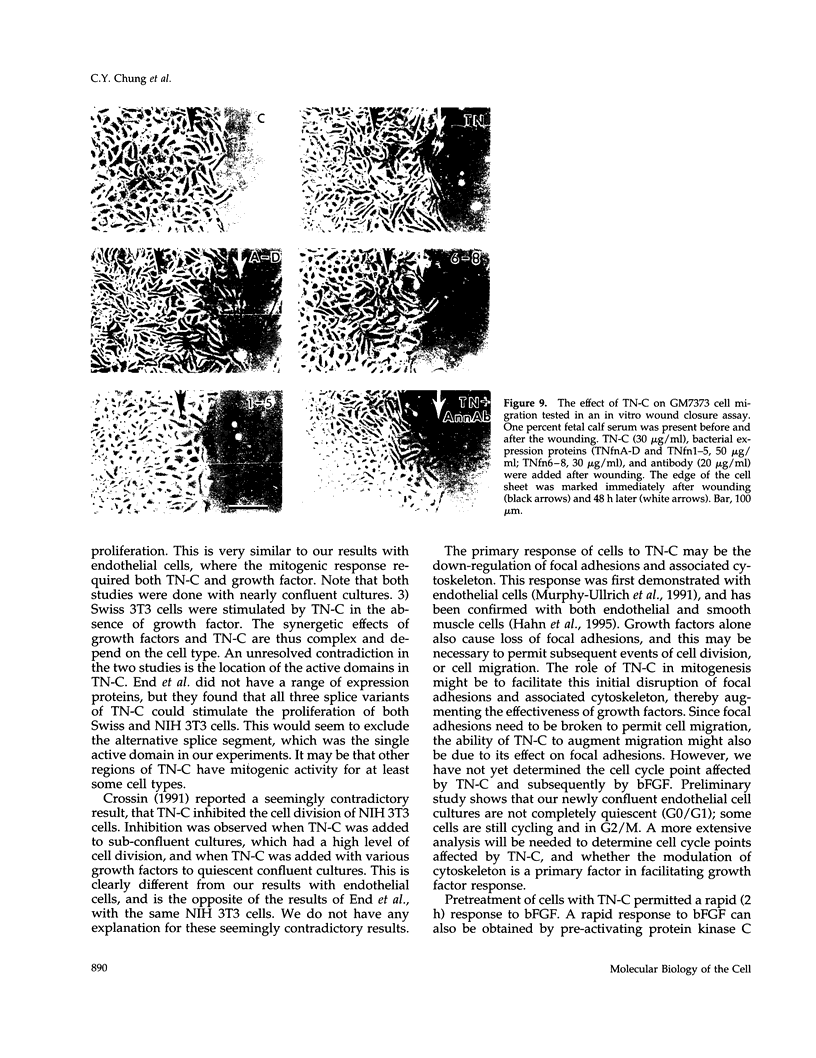
In a previous study we demonstrated that the alternatively spliced region of tenascin-C, TNfnA-D, bound with high affinity to a cell surface receptor, annexin II. In the present study we demonstrate three changes in cellular activity that are produced by adding intact tenascin-C or TNfnA-D to cells, and we show that all three activities are blocked by antibodies against annexin II. 1) TNfnA-D added to confluent endothelial cells induced loss of focal adhesions. 2) TNfnA-D produced a mitogenic response of confluent, growth-arrested endothelial cells in 1% serum. TNfnA-D stimulated mitogenesis only when it was added to cells before or during exposure to other mitogens, such as basic fibroblast growth factor or serum. Thus the effect of TNfnA-D seems to be to facilitate the subsequent response to growth factors. 3) TNfnA-D enhanced cell migration in a cell culture wound assay. Antibodies to annexin II blocked all three cellular responses to TNfnA-D. These data show that annexin II receptors on endothelial cells mediate several cell regulatory functions attributed to tenascin-C, potentially through modulation of intracellular signalling pathways.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aukhil I., Joshi P., Yan Y., Erickson H. P. Cell- and heparin-binding domains of the hexabrachion arm identified by tenascin expression proteins. J Biol Chem. 1993 Feb 5;268(4):2542–2553. [PubMed] [Google Scholar]
- Aukhil I., Slemp C. C., Lightner V. A., Nishimura K., Briscoe G., Erickson H. P. Purification of hexabrachion (tenascin) from cell culture conditioned medium, and separation from a cell adhesion factor. Matrix. 1990 May;10(2):98–111. doi: 10.1016/s0934-8832(11)80176-9. [DOI] [PubMed] [Google Scholar]
- Borsi L., Carnemolla B., Nicolò G., Spina B., Tanara G., Zardi L. Expression of different tenascin isoforms in normal, hyperplastic and neoplastic human breast tissues. Int J Cancer. 1992 Nov 11;52(5):688–692. doi: 10.1002/ijc.2910520504. [DOI] [PubMed] [Google Scholar]
- Chen J. M., Sheldon A., Pincus M. R. Structure-function correlations of calcium binding and calcium channel activities based on 3-dimensional models of human annexins I, II, III, V and VII. J Biomol Struct Dyn. 1993 Jun;10(6):1067–1089. doi: 10.1080/07391102.1993.10508696. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R., Mackie E. J., Pearson C. A., Sakakura T. Tenascin: an extracellular matrix protein involved in tissue interactions during fetal development and oncogenesis. Cell. 1986 Oct 10;47(1):131–139. doi: 10.1016/0092-8674(86)90374-0. [DOI] [PubMed] [Google Scholar]
- Chung C. Y., Erickson H. P. Cell surface annexin II is a high affinity receptor for the alternatively spliced segment of tenascin-C. J Cell Biol. 1994 Jul;126(2):539–548. doi: 10.1083/jcb.126.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossin K. L. Cytotactin binding: inhibition of stimulated proliferation and intracellular alkalinization in fibroblasts. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11403–11407. doi: 10.1073/pnas.88.24.11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniloff J. K., Crossin K. L., Pinçon-Raymond M., Murawsky M., Rieger F., Edelman G. M. Expression of cytotactin in the normal and regenerating neuromuscular system. J Cell Biol. 1989 Feb;108(2):625–635. doi: 10.1083/jcb.108.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demange P., Voges D., Benz J., Liemann S., Göttig P., Berendes R., Burger A., Huber R. Annexin V: the key to understanding ion selectivity and voltage regulation? Trends Biochem Sci. 1994 Jul;19(7):272–276. doi: 10.1016/0968-0004(94)90002-7. [DOI] [PubMed] [Google Scholar]
- End P., Panayotou G., Entwistle A., Waterfield M. D., Chiquet M. Tenascin: a modulator of cell growth. Eur J Biochem. 1992 Nov 1;209(3):1041–1051. doi: 10.1111/j.1432-1033.1992.tb17380.x. [DOI] [PubMed] [Google Scholar]
- Erickson H. P., Bourdon M. A. Tenascin: an extracellular matrix protein prominent in specialized embryonic tissues and tumors. Annu Rev Cell Biol. 1989;5:71–92. doi: 10.1146/annurev.cb.05.110189.000443. [DOI] [PubMed] [Google Scholar]
- Folkman J., Watson K., Ingber D., Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989 May 4;339(6219):58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- Gatchalian C. L., Schachner M., Sanes J. R. Fibroblasts that proliferate near denervated synaptic sites in skeletal muscle synthesize the adhesive molecules tenascin(J1), N-CAM, fibronectin, and a heparan sulfate proteoglycan. J Cell Biol. 1989 May;108(5):1873–1890. doi: 10.1083/jcb.108.5.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinspan J. B., Mueller S. N., Levine E. M. Bovine endothelial cells transformed in vitro by benzo(a)pyrene. J Cell Physiol. 1983 Mar;114(3):328–338. doi: 10.1002/jcp.1041140312. [DOI] [PubMed] [Google Scholar]
- Hahn A. W., Kern F., Jonas U., John M., Bühler F. R., Resink T. J. Functional aspects of vascular tenascin-C expression. J Vasc Res. 1995 May-Jun;32(3):162–174. doi: 10.1159/000159090. [DOI] [PubMed] [Google Scholar]
- Hajjar K. A., Jacovina A. T., Chacko J. An endothelial cell receptor for plasminogen/tissue plasminogen activator. I. Identity with annexin II. J Biol Chem. 1994 Aug 19;269(33):21191–21197. [PubMed] [Google Scholar]
- Hedberg K. K., Birrell G. B., Griffith O. H. Phorbol ester-induced actin cytoskeletal reorganization requires a heavy metal ion. Cell Regul. 1991 Dec;2(12):1067–1079. doi: 10.1091/mbc.2.12.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. L., Boudreau N., Myers C. A., Erickson H. P., Bissell M. J. Tenascin-C inhibits extracellular matrix-dependent gene expression in mammary epithelial cells. Localization of active regions using recombinant tenascin fragments. J Cell Sci. 1995 Feb;108(Pt 2):519–527. doi: 10.1242/jcs.108.2.519. [DOI] [PubMed] [Google Scholar]
- Joyce N. C., Meklir B. Protein kinase C activation during corneal endothelial wound repair. Invest Ophthalmol Vis Sci. 1992 May;33(6):1958–1973. [PubMed] [Google Scholar]
- Julian J., Chiquet-Ehrismann R., Erickson H. P., Carson D. D. Tenascin is induced at implantation sites in the mouse uterus and interferes with epithelial cell adhesion. Development. 1994 Mar;120(3):661–671. doi: 10.1242/dev.120.3.661. [DOI] [PubMed] [Google Scholar]
- Koukoulis G. K., Gould V. E., Bhattacharyya A., Gould J. E., Howeedy A. A., Virtanen I. Tenascin in normal, reactive, hyperplastic, and neoplastic tissues: biologic and pathologic implications. Hum Pathol. 1991 Jul;22(7):636–643. doi: 10.1016/0046-8177(91)90285-w. [DOI] [PubMed] [Google Scholar]
- Mackie E. J., Halfter W., Liverani D. Induction of tenascin in healing wounds. J Cell Biol. 1988 Dec;107(6 Pt 2):2757–2767. doi: 10.1083/jcb.107.6.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Gurusiddappa S., Frazier W. A., Hök M. Heparin-binding peptides from thrombospondins 1 and 2 contain focal adhesion-labilizing activity. J Biol Chem. 1993 Dec 15;268(35):26784–26789. [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Hök M. Thrombospondin modulates focal adhesions in endothelial cells. J Cell Biol. 1989 Sep;109(3):1309–1319. doi: 10.1083/jcb.109.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Lightner V. A., Aukhil I., Yan Y. Z., Erickson H. P., Hök M. Focal adhesion integrity is downregulated by the alternatively spliced domain of human tenascin. J Cell Biol. 1991 Nov;115(4):1127–1136. doi: 10.1083/jcb.115.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynal P., Pollard H. B. Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim Biophys Acta. 1994 Apr 5;1197(1):63–93. doi: 10.1016/0304-4157(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Saga Y., Yagi T., Ikawa Y., Sakakura T., Aizawa S. Mice develop normally without tenascin. Genes Dev. 1992 Oct;6(10):1821–1831. doi: 10.1101/gad.6.10.1821. [DOI] [PubMed] [Google Scholar]
- Sato Y., Rifkin D. B. Autocrine activities of basic fibroblast growth factor: regulation of endothelial cell movement, plasminogen activator synthesis, and DNA synthesis. J Cell Biol. 1988 Sep;107(3):1199–1205. doi: 10.1083/jcb.107.3.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliwa M., Nakamura T., Porter K. R., Euteneuer U. A tumor promoter induces rapid and coordinated reorganization of actin and vinculin in cultured cells. J Cell Biol. 1984 Sep;99(3):1045–1059. doi: 10.1083/jcb.99.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitby D. J., Ferguson M. W. The extracellular matrix of lip wounds in fetal, neonatal and adult mice. Development. 1991 Jun;112(2):651–668. doi: 10.1242/dev.112.2.651. [DOI] [PubMed] [Google Scholar]
- Young S. L., Chang L. Y., Erickson H. P. Tenascin-C in rat lung: distribution, ontogeny and role in branching morphogenesis. Dev Biol. 1994 Feb;161(2):615–625. doi: 10.1006/dbio.1994.1057. [DOI] [PubMed] [Google Scholar]
- Zagzag D., Friedlander D. R., Miller D. C., Dosik J., Cangiarella J., Kostianovsky M., Cohen H., Grumet M., Greco M. A. Tenascin expression in astrocytomas correlates with angiogenesis. Cancer Res. 1995 Feb 15;55(4):907–914. [PubMed] [Google Scholar]