Summary
Repression of mitochondrial respiration represents an evolutionarily ancient cellular adaptation to hypoxia and profoundly influences cell survival and function; however, the underlying molecular mechanisms are incompletely understood. Primarily utilizing pulmonary arterial endothelial cells as a representative hypoxic cell type, we identify the iron-sulfur cluster assembly proteins (ISCU1/2) as direct targets for repression by the hypoxia-induced microRNA-210 (miR-210). ISCU1/2 facilitate the assembly of iron-sulfur clusters, prosthetic groups that are critical for electron transport and mitochondrial oxidation-reduction reactions. Under in vivo conditions of up-regulating miR-210 and repressing ISCU1/2, the integrity of iron-sulfur clusters is disrupted. In turn, by repressing ISCU1/2 during hypoxia, miR-210 decreases the activity of prototypical iron-sulfur proteins controlling mitochondrial metabolism, including Complex I and aconitase. Consequently, miR-210 represses mitochondrial respiration and associated downstream functions. These results identify important mechanistic connections among microRNA, iron-sulfur cluster biology, hypoxia, and mitochondrial function, with broad implications for cellular metabolism and adaptation to cellular stress.
Introduction
The processes that govern cellular adaptation to hypoxia are complex and incompletely defined. One fundamental and highly conserved response to hypoxia was originally described by Louis Pasteur in the nineteenth century who identified a metabolic shift in hypoxic cells, later discovered to result from repression of the tricarboxylic acid (TCA) cycle, mitochondrial electron transport, and oxidative phosphorylation in favor of glycolysis (Aisenberg and Potter, 1957). This “Pasteur effect” profoundly influences cell survival and function in a variety of cell and tissue types and is thought to optimize the efficiency by which energy is generated (ATP) while avoiding excessive generation of toxic reactive oxygen species (ROS) (Semenza, 2007). In particular, this response may be especially important to the pulmonary vascular endothelium, which directly responds to hypoxia by controlling the release of vasoactive effectors, thereby critically influencing normal vascular physiology and the development of pulmonary hypertension (Stenmark et al., 2006).
The underlying mechanisms of this highly coordinated metabolic response are only beginning to be understood at the molecular level. Hypoxia inducible factor-1 alpha (HIF-1α), a master regulator of the cellular hypoxic response, has been shown to control mitochondrial function (Chavez et al., 2008) and is essential for this repression of mitochondrial respiration (Seagroves et al., 2001) during hypoxia. Furthermore, a limited number of factors have been identified that regulate shifts in hypoxic mitochondrial respiration, including pyruvate dehydrogenase kinase (PDK1), lactate dehydrogenase A (LDHA), cytochrome c oxidase (COX subunits 4-1 and 4-2), and the mitochondrial protease LON (Semenza, 2007). However, owing to the still incompletely understood nature of the interaction between HIF-1α and mitochondria, we hypothesized that additional factors likely mediate this fundamental metabolic event.
Endogenous microRNA molecules (miRNA) have been identified as essential mediators of numerous cellular processes, potentially including the response to hypoxia (Ivan et al., 2008). In particular, microRNA-210 (miR-210) is specifically induced by HIF-1α during hypoxia (Kulshreshtha et al., 2007). The cell cycle regulator E2F3 (Giannakakis et al., 2008), the receptor tyrosine kinase ligand ephrin A3 (Fasanaro et al., 2008), and the DNA repair protein RAD52 (Crosby et al., 2009) have been studied as targets for repression by miR-210, but otherwise the complete functions of this miRNA have yet to be delineated. Because miRNAs quickly and efficiently regulate post-transcriptional changes in target gene expression, we hypothesized that this HIF-1α-dependent miRNA represents an ideal factor that may dynamically modulate mitochondrial metabolism.
By analysis of multiple bioinformatic algorithms, a highly conserved binding site for miR-210 is predicted in the 3′ UTR of the transcripts of the iron-sulfur cluster assembly proteins, ISCU1 and ISCU2. In mammalian cells, two splice isoforms of ISCU exist. Both transcripts carry an identical 3′ UTR, but they differ in their location: ISCU1 is located in the cytosol, whereas ISCU2 is located in the mitochondria (Tong and Rouault, 2000). In order to highlight their functional and structural similarities, these proteins are described here by a single term, ISCU1/2. ISCU1/2 facilitate the assembly of [4Fe-4S] and [2Fe-2S] iron-sulfur clusters, prosthetic groups that promote electron transport and oxidation-reduction reactions integral to numerous cellular processes ranging from ribosome biogenesis, purine catabolism, heme biosynthesis, DNA repair, and iron metabolism, among others (Rouault and Tong, 2008). In particular, iron-sulfur clusters are incorporated into enzymes that are responsible for mitochondrial respiration and energy production. These include the enzymatic isoforms of aconitase, which are integral to the TCA cycle, and the mitochondrial respiratory complexes (Complexes I, II, and III), which facilitate electron transport (Rouault and Tong, 2008). Depending upon the level of ambient oxygen exposure, alteration of these and other iron-sulfur dependent mitochondrial activities can lead to distinct downstream consequences on ROS production and cellular survival (Semenza, 2007). Despite their established importance in these cellular redox reactions, regulation and function of these critical prosthetic groups in hypoxic mammalian cells are poorly characterized.
Because of its robust up-regulation of miR-210 in response to hypoxia and its pivotal function in mediating hypoxic effects in pulmonary vascular tissue, the human pulmonary arterial endothelial cell (HPAEC) was selected as a model cell type to study the specific functions of miR-210 during hypoxic stress. In doing so, we identify ISCU1/2 as direct targets for repression by miR-210. By down-regulating expression of ISCU1/2 during hypoxia, miR-210 decreases the specific activity of prototypical iron-sulfur enzymes regulating mitochondrial function, mitochondrial Complex I and aconitase, leading to downstream phenotypic consequences integral to the Pasteur effect. As a result, both miR-210 and ISCU1/2 are identified as important and essential factors in the regulation of mitochondrial respiration and metabolism during hypoxic stress. These results augment our fundamental understanding of this critical metabolic event, common to primary mammalian cell types and nearly all eukaryotes.
Results
MiR-210 is Robustly Induced by Hypoxia in Primary and Transformed Cells
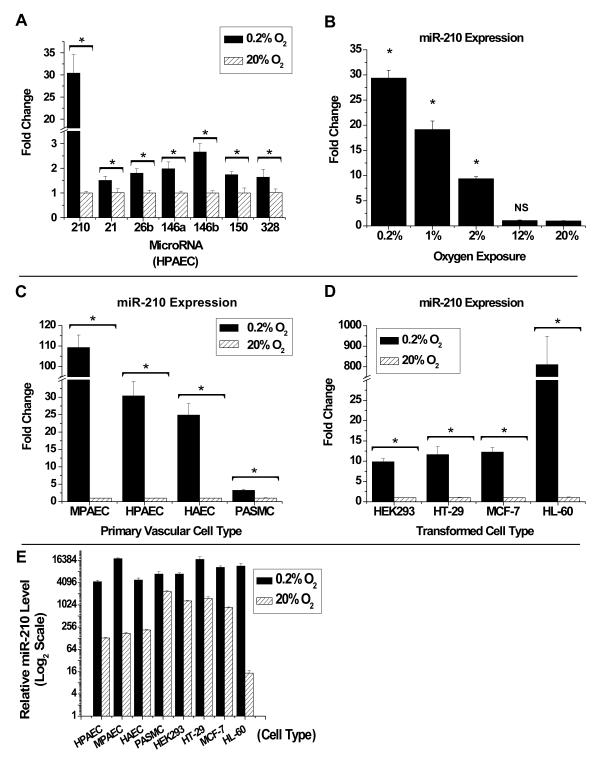
Hypoxic stress in cell culture was utilized to screen for quantitative changes in mature forms of miRNA in HPAECs. After exposure of HPAECs to certain levels of hypoxia (0.2%-12% O2) for 24 hours as compared with standard non-hypoxic cell culture conditions (20% O2), miRNA levels were quantified by reverse transcription and quantitative polymerase chain reaction (RT-QPCR). This approach was confirmed as a standardized system of hypoxic stress by the ability to recapitulate changes in transcript levels of known hypoxia-related genes (data not shown). To identify a complete profile of up-regulated miRNA, a Taqman Low Density Array (TLDA) card (Applied Biosystems) was utilized to screen for the expression levels of 365 mature human miRNA species via RT-QPCR high-throughput amplification followed by validation of these results by standard RT-QPCR. Furthermore, the levels of specific miRNA were assessed, if they were previously reported as up-regulated by hypoxia (Kulshreshtha et al., 2008) or by hypoxia-associated stimuli (such as inflammation).
In HPAECs, only a few miRNA are induced by greater than 1.5-fold during hypoxia -- namely, miR-210 as well as miR-21, miR-26b, miR-146a, miR-146b, miR-150, and miR-328 (Fig. 1A). Some, but not all, of these miRNA have been identified in other transformed (Kulshreshtha et al., 2007) or primary endothelial cells (Fasanaro et al., 2008) exposed to hypoxia. Furthermore, in contrast to the other identified miRNA, only miR-210 is up-regulated by hypoxia in a robust (>25-fold increase) (Fig. 1A) and oxygen-dependent manner (Fig. 1B) in HPAECs. In fact, exposure of murine PAECs (MPAECs) to hypoxia leads to >100-fold up-regulation of miR-210 (Fig. 1C). Exposure of human aortic endothelial cells (HAECs) to hypoxia also leads to >20-fold up-regulation of miR-210 (Fig. 1C), suggesting this phenomenon is common to endothelial cells derived from either the pulmonary or peripheral vasculature. In addition, hypoxic treatment increases miR-210 in a variety of transformed cell types (i.e., embryonic kidney, HEK293; colonic adenocarcinoma, HT-29; breast carcinoma, MCF-7; and promyelocytic leukemia, HL-60, Fig. 1D). Notably, the absolute levels of miR-210 induced by hypoxia are mostly similar across these tested cell types (Fig. 1E). However, endothelial cell types and HL-60 cells carry particularly low levels of miR-210 during exposure to 20% O2 (Fig. 1E, Supp. Fig. 1), perhaps suggestive of an augmented action of this miRNA in these cellular contexts when induced by hypoxia. Nonetheless, while heterogeneity exists in the baseline level of expression and relative degree of up-regulation, hypoxia increases expression of miR-210 in a variety of both primary and transformed mammalian cell types, likely reflecting a fundamental and highly conserved action of this miRNA.
Figure 1. MiR-210 is Uniquely and Robustly Up-Regulated by Hypoxia.
(A) RT-QPCR demonstrates up-regulation of a unique panel of miRNA in HPAECs during hypoxia (0.2% O2, black bars) as compared with standard non-hypoxic cell culture conditions (20% O2, hatched bars). (B) RT-QPCR demonstrates induction of miR-210 in decreasing concentrations of oxygen. Statistical significance was determined with a one-way ANOVA and Bonferroni post hoc test. (C) RT-QPCR demonstrates induction of miR-210 under hypoxia (0.2% O2, black bars) as compared with 20% O2 (hatched bars) in vascular cell types, including murine (MPAECs) and human PAECs (HPAECs), human aortic endothelial cells (HAECs), and human pulmonary arterial smooth muscle cells (PASMCs). (D) In transformed cells, RT-QPCR demonstrates induction of miR-210 in hypoxia (0.2% O2, black bar) as compared with 20% O2 (hatched bar). (E) Relative levels of mature miR-210 are up-regulated by 0.2% O2 (black bars) in multiple primary and transformed cell types as compared with exposure to 20% O2 (hatched bars). Levels are based on the formula (2−Ct × 1012). In (A-D), expression of each miRNA under 20% O2 is assigned to a fold change of 1, to which hypoxic expression is compared. In all panels, error bars reflect SEM; * signifies p<0.05 (N≥3), NS signifies p≥0.05 (N≥3).
MiR-210 Directly Represses Expression of ISCU1/2
To consolidate the list of putative mRNA targets of miR-210 from three in silico algorithms (TargetScan, Sanger MiRBase, and MiRTarget2), only predicted target mRNA with 100% complementarity to the miR-210 5′-seed sequence (positions 2-8) were considered. We further restricted putative targets to those potentially associated with the hypoxic cellular response. Using these criteria, one target was represented in all three of the above algorithms: the iron-sulfur cluster assembly proteins ISCU1/2.
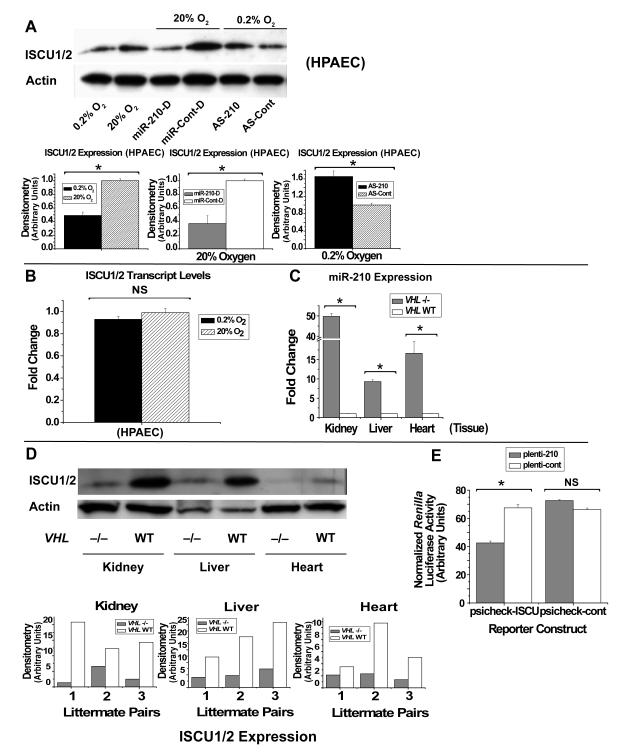
To validate this prediction in HPAECs, we found that ISCU1/2 protein levels (Fig. 2A), but not mRNA transcript levels (Fig. 2B), are significantly down-regulated after exposure to 0.2% O2 as compared with 20% O2. Using gel densitometry, protein levels of ISCU1/2 on average after hypoxic exposure are 0.5-fold of expression at 20% O2 (see Fig. 2A, Supp. Fig. 2A, C). Notably, although typical gel electrophoresis does not routinely resolve the ISCU isoforms due to minimal size discrepancy, suitable assessment of changes in global ISCU protein expression can still be appreciated. Comparable levels of down-regulation occur after exposure to 0.2% O2 or 1% O2 (Supp. Fig. 2A), suggesting a range of physiologically relevant hypoxic conditions in which this pathway is active and perhaps a critical range of miR-210 levels which can induce similar levels of target gene repression. HAECs exhibit similar levels of down-regulation in 0.2% O2 (Supp. Fig. 2A), demonstrating this regulatory pathway in endothelial cells derived from multiple vascular beds. Additionally, ISCU1/2 levels remain repressed for up to 60 hours of hypoxic exposure (Supp. Fig. 2C), reflecting the up-regulation of miR-210 under the same conditions (Supp. Fig. 2B). In contrast to HIF proteins which increase during acute hypoxia but are inactivated with chronic hypoxia (i.e., > 24 hours of hypoxia) (Ginouves et al., 2008), miR-210 and repression of ISCU1/2 display sustained periods of action and, may, therefore reflect a chronic, rather than transient, adaptation to hypoxia.
Figure 2. MiR-210 Recognizes ISCU1/2 as Direct Targets for Repression.
(A) Western blot/gel densitometry analyses in HPAECs reveal down-regulation of ISCU1/2 in 0.2% O2 (black bars) as compared to 20% O2 (hatched bars). ISCU1/2 is repressed by miR-210 duplexes (miR-210-D, grey bars) as compared with control miRNA duplexes (miR-Cont-D, white bars). During hypoxia (0.2% O2), inhibition of miR-210 (AS-210, black bar) up-regulates ISCU1/2 as compared with antisense control (AS-Cont, hatched bar). (B) RT-QPCR demonstrates no change in transcript levels of ISCU1/2 in HPAECs exposed to 0.2% O2 (black bar) as compared with 20% O2 (hatched bar). (C) RT-QPCR demonstrates induction of miR-210 in murine kidney, liver, and heart tissue carrying inactivated VHL (VHL -/-) (grey bars, N=3 mice) as compared with VHL WT tissue (white bars, N=3 mice). The level of miR-210 in each VHL WT tissue is assigned to a fold change of 1, to which expression in VHL -/- tissue is compared. (D) Gel densitometry of Western blots reveals down-regulation of ISCU1/2 protein in tissue derived from 3 littermate pairs of VHL -/- (grey bars) and VHL WT (white bars) mice. A representative Western blot is shown. (E) COS7 cells were co-transfected with either reporter construct, psicheck-ISCU or psicheck-cont, along with expression plasmids encoding for either miR-210 (plenti-210, grey bars) or negative control (plenti-cont, white bars). MiR-210 (plenti-210) reduces Renilla luciferase activity as compared with control (plenti-cont) in the setting of psicheck-ISCU, but induces no significant change in the setting of psicheck-cont. Error bars reflect SEM; * signifies p<0.05 (N≥3), NS signifies p≥0.05 (N≥3). Western blots are representative of experiments performed at least in triplicate; gel densitometry is normalized to actin levels and compared as arbitrary units.
Transfection of miR-210 duplexes (denoted as miR-210-D) in HPAECs was employed to increase intracellular levels of mature miR-210 in the absence of hypoxia. Similar to the hypoxic response, when transfected at relatively low transfection concentrations (2 nM), miR-210-D decreases ISCU1/2 to nearly 0.3-fold of expression with transfected miR-control duplexes (miR-Cont-D) (Fig. 2A). In this setting, ISCU1/2 transcripts are decreased to levels that are 0.4-fold of control (Supp. Fig. 2E). In contrast, at a lower concentration of transfected miR-210-D (0.02 nM), ISCU1/2 are decreased to levels that are 0.6-fold of control, but ISCU1/2 transcripts remain unchanged (Supp. Fig. 2D-E). Therefore, depending on its concentration, this miRNA can suppress ISCU1/2 expression either at the translational level alone or at the pre-translational level. Finally, ISCU1/2 expression during hypoxia was assessed in the setting of antisense inhibition of miR-210 (AS-210). AS-210 increases ISCU1/2 by 1.65-fold during hypoxia as compared to AS-Cont and, thus, substantially rescues expression nearly to non-hypoxic levels (Fig. 2A). Certainly, other factors independent of miR-210 could also contribute to the control of ISCU1/2 during hypoxia. Nonetheless, through both gain-of-function and loss-of-function assays, these data demonstrate that miR-210 is necessary and sufficient for the down-regulation of ISCU1/2 during hypoxia.
To demonstrate down-regulation of ISCU1/2 during HIF-1α expression in vivo, tissue was analyzed from mice carrying tamoxifen-dependent, conditionally inactivated von Hippel Lindau alleles (VHL -/-), an established model of sustained stabilization of HIF proteins during non-hypoxic, or “normoxic,” conditions (Haase et al., 2001). In comparison to tamoxifen-treated VHL flox/flox littermate control mice (referred to as “WT”), VHL -/- null mice demonstrate significantly higher levels of miR-210 in kidney, liver, and heart (Fig. 2C), corresponding with elevated HIF-1α expression (Young et al., 2008). Importantly, VHL -/- null mice also exhibit robust down-regulation of ISCU1/2 expression in kidney, liver, and heart as compared with VHL WT mice (Fig. 2D). Variability in the level of down-regulation among littermate pairs likely reflects variation in the degree of VHL inactivation after tamoxifen exposure (Minamishima et al., 2008). Nonetheless, taken together with the mechanistic data in Fig. 2A and Supp. Fig. 2, these data reflect the in vivo relevance of this HIF-dependent and miR-210-dependent repression of ISCU1/2.
To prove that miR-210 directly recognizes the identical predicted target site in the 3′ UTR of ISCU1 and ISCU2, that target sequence was cloned into the 3′ UTR of a Renilla luciferase reporter vector (psicheck-2, Promega) to generate psicheck-ISCU. COS7 cells were transfected with psicheck-ISCU or a control (psicheck-cont) without a miR-210 target sequence. In each case, these cells were co-transfected with either expression vectors encoding for miR-210 (plenti-210) or control (plenti-cont). Renilla luciferase activity was measured and normalized to the firefly luciferase signal, separately encoded in the psicheck-2 parent vector. MiR-210 (plenti-210) significantly down-regulates Renilla luciferase activity as compared to control (plenti-cont) in the presence of psicheck-ISCU. In contrast, Renilla luciferase activity is not significantly altered in the presence of psicheck-cont (Fig. 2E). Similarly, during hypoxia when endogenous miR-210 is up-regulated, Renilla luciferase activity is more robustly repressed when driven by psicheck-ISCU (AS-Cont) as compared with psicheck-cont (AS-Cont). Demonstrating the essential role of miR-210 in this response, inhibition of miR-210 (AS-210) during hypoxia increases Renilla luciferase activity encoded by psicheck-ISCU, rescuing activity nearly back to the level driven by psicheck-cont (AS-Cont) (Supp. Fig. 2F). Taken together with the bioinformatic predictions and the down-regulation of endogenous ISCU1/2 by miR-210, these results demonstrate that miR-210 represses gene expression by recognizing the predicted target sequence in the 3′ UTR of ISCU1/2.
Disruption of the Integrity of Iron-Sulfur Clusters in VHL -/- Murine Tissue
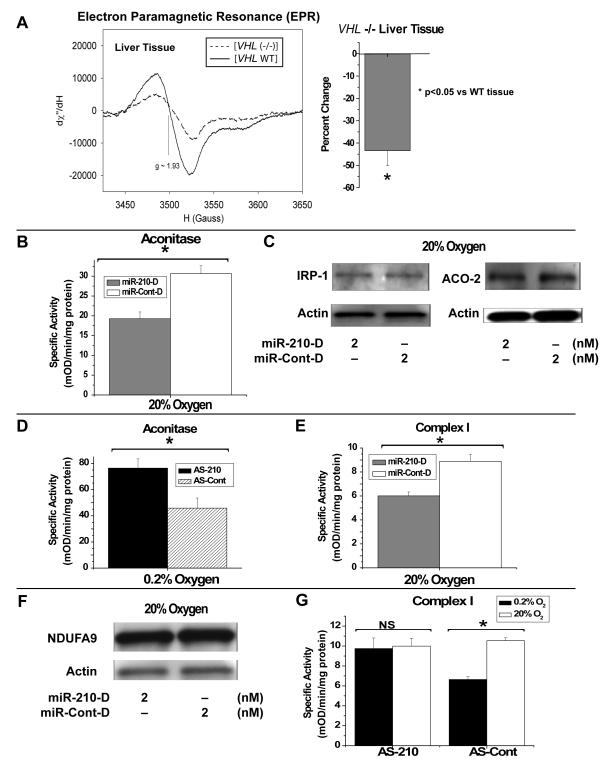
Because ISCU1/2 are essential for the biogenesis of iron-sulfur clusters in vivo, miR-210 and HIF-1α would be expected to decrease levels of these clusters during hypoxia via down-regulation of ISCU1/2. To determine if a HIF-dependent mechanism such as miR-210 up-regulation can modulate the integrity of iron-sulfur clusters, electron paramagnetic resonance spectroscopy (EPR) was performed to quantify iron-sulfur cluster levels in intact cells. Due of high levels of mitochondrial and iron-sulfur cluster content, murine liver, heart, and kidney tissue were selected for analysis by EPR. VHL -/- liver tissue (N=3 mice) displays an EPR spectroscopic signal with a peak-to-peak amplitude (g=1.93, representing intact iron-sulfur clusters) that is nearly 45% lower than that seen in VHL WT tissue (N=3 mice) (Fig. 3A). Comparable decreases are also noted in VHL -/- heart (mean decrease of 43.3%, N=2 mice) and VHL -/- kidney (mean decrease of 45.7%, N=2 mice) tissue, as compared with WT tissue (N=2 mice). As a result, VHL- and HIF-dependent mechanisms increase miR-210, decrease ISCU1/2, and consequently disrupt intact iron-sulfur cluster levels in vivo.
Figure 3. Direct Disruption of Iron-Sulfur Clusters in VHL -/- Tissue In Vivo Correlates with miR-210-Dependent Repression of Iron-Sulfur Cluster Enzyme Activities in HPAECs.
(A) Electron paramagnetic resonance (EPR) spectroscopy at 15°K of miR-210-positive VHL -/- liver tissue (dotted line) reveals a decrease in the peak-to-peak amplitude of the iron-sulfur cluster signal (g=1.93) as compared with VHL WT murine liver tissue (solid line). The spectroscopic signal in VHL -/- liver tissue (N=3 mice) is nearly 45% lower than that measured in VHL WT liver tissue (N=3 mice) (bar graph). (B) Specific activity of aconitase in HPAECs is decreased by nearly 40% by miR-210-D (2 nM, grey bar) as compared to miR-Cont-D (2 nM, white bar). (C) As measured by Western blot (48 hours post-transfection), miR-210-D (2 nM) does not alter protein expression of IRP-1/cytoplasmic aconitase or ACO-2/mitochondrial aconitase, as compared to miR-Cont-D (2 nM). (D) During hypoxia (0.2% O2), inhibition of miR-210 (AS-210, black bar) increases the specific activity of aconitase by > 60%, as compared with antisense control (AS-Cont, hatched bar). (E) MiR-210-D (2 nM, grey bar) represses Complex I activity by approximately 25%, as compared with miR-Cont-D (2 nM, white bar). (F) As measured by Western blot, miR-210-D (2 nM) does not significantly alter protein expression of mitochondrial Complex I protein NDUFA9, as compared to miR-Cont-D (2 nM). (G) In the presence of control antisense inhibitor (AS-Cont), hypoxia (black bar) induces an approximately 30% decrease in Complex I activity as compared with 20% O2 (white bar). Inhibition of miR-210 (AS-210) abrogates this effect. Error bars reflect SEM; * signifies p<0.05 (N≥3), NS signifies p≥0.05 (N≥3). All blots are representative of experiments performed at least in triplicate.
MiR-210 Disrupts the Activity of Iron-Sulfur Cluster-Dependent Metabolic Proteins
To explore a link between miR-210 and the activity of prototypical iron-sulfur enzymes controlling metabolism, regulation of aconitase activity by miR-210 was assessed. In HPAECs, miR-210-D leads to a nearly 40% reduction in the specific activity of aconitase (Fig. 3B). Conversely, inhibition of miR-210 (AS-210) during hypoxia increases the specific activity of aconitase by greater than 60%, as compared with antisense control (AS-Cont) (Fig. 3D), thus demonstrating that endogenous miR-210 represses aconitase activity during hypoxia. Notably, miR-210-D induces negligible changes in protein levels of either isoform of aconitase (IRP-1/cytoplasmic aconitase or ACO-2/mitochondrial aconitase) over a time frame of 48 hours post-transfection (Fig. 3C). Therefore, correlating with the known function of ISCU1/2 in maintaining the iron-sulfur clusters in aconitase (Tong and Rouault, 2006) and the disruption of iron-sulfur clusters in miR-210-positive tissue (Fig. 3A), this disruption of aconitase activity likely results from miR-210- and ISCU-dependent repression of iron-sulfur cluster biogenesis and integrity.
To ascertain a link between miR-210 and activity of mitochondrial iron-sulfur proteins essential for electron transport, Complex I activity was assessed, given its dependence on multiple iron-sulfur clusters for proper function (Hinchliffe and Sazanov, 2005). In HPAECs, miR-210-D decreases the specific activity of Complex I by 25% (Fig. 3E), and does so in the absence of expression changes of a representative component of Complex I, NDUFA9 (Fig. 3F). In correlation, hypoxia decreases specific activity of Complex I by 30%, as compared with exposure to 20% O2 (Fig. 3G). Repression of Complex I activity is abolished by inhibition of miR-210 (AS-210). Thus, miR-210 is necessary and sufficient to disrupt Complex I activity during hypoxia. These data again suggest that disruption of iron-sulfur cluster assembly, independent of down-regulation of Complex I subunit expression, likely drives the decrease of electron transport activity.
MiR-210 and ISCU1/2 Control Identical Downstream Metabolic Functions
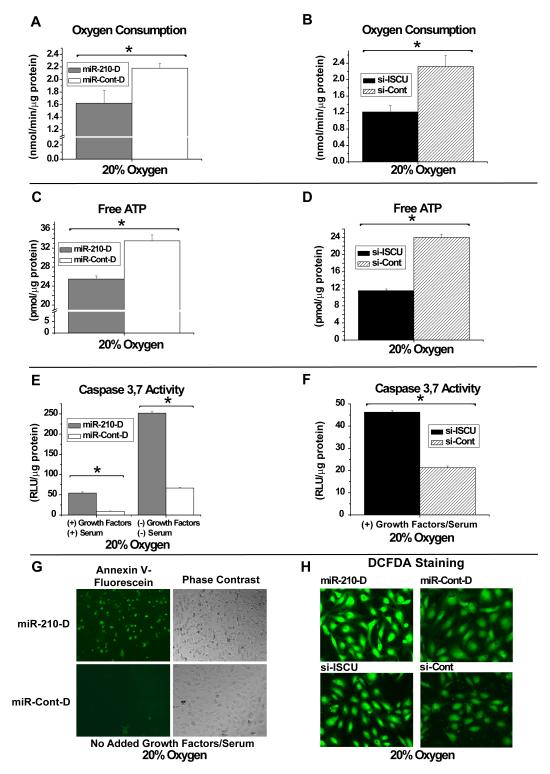
Under normoxic conditions, disruption of Complex I activity and mitochondrial electron transport leads to direct downstream functional consequences on energy production, including decreased oxygen consumption, decreased oxidative phosphorylation (OXPHOS), and reduced ATP generation. Correspondingly, in 20% O2, transfection of miR-210-D leads to an approximate 25% decrease in total oxygen consumption (Fig. 4A) in HPAECs and an approximate 18% decrease in free ATP levels (Fig. 4C). The degree of alteration in these parameters is consistent with the dependence of cultured endothelial cells on OXPHOS for a minority of total ATP production (Mann et al., 2003). In correlation, after transfection of RNAi (si-ISCU), which represses ISCU1/2 expression in HPAECs (data not shown), similar phenotypes are induced, independent of the presence of miR-210 (Fig. 4B, 4D). Thus, miR-210 and ISCU1/2 control similar functions in maintaining mitochondrial respiration and energy production.
Figure 4. MiR-210 and ISCU1/2 Control Identical Mitochondrial Metabolic Functions in HPAECs.
(A) MiR-210-D (2 nM, grey bar) represses oxygen consumption as compared with miR-Cont-D (2 nM, white bar) by nearly 25%. (B) Inhibitory RNA recognizing ISCU1/2 (si-ISCU, 40 nM) represses oxygen consumption by nearly 30% as compared with inhibitory RNA control (si-Cont, 40 nM). (C) MiR-210-D (2 nM, grey bar) down-regulates free levels of ATP by approximately 18%. (D) Inhibitory si-ISCU (40 nM) down-regulates free ATP levels by > 40%. (E) MiR-210-D (2 nM, grey bar) induces apoptotic caspase 3,7 activity by >5-fold compared with miR-Cont-D in HPAECs cultured in full growth media; similarly, miR-210-D increases caspase 3,7 activity by >5-fold compared with miR-Cont-D in basal media (without added growth factors or serum) and by >50 fold compared with miR-210-D in full growth media. (F) Inhibitory si-ISCU (40 nM) up-regulates caspase 3,7 activity by >2-fold under full growth media. (G) When cultured in basal media and exposed to annexin V-fluorescein, miR-210 (2 nM) increases fluorescent staining in HPAECs as compared with miR-Cont-D (2 nM). (H) MiR-210-D (2 nM) increases fluorescent staining in HPAECs by the oxidant-sensitive fluorophore DCFDA, as compared with miR-Cont-D (2 nM). Transfection of si-ISCU (40 nM) also increases fluorescent staining by DCFDA, as compared with si-Cont (40 nM). All fluorescent mages were acquired under a green filter (516 nm). Error bars reflect SEM; * signifies p<0.05 (N≥3), NS signifies p≥0.05 (N≥3).
In addition to direct regulation of mitochondrial metabolism, disruption of the balance of electron transport relative to the intracellular levels of oxygen can affect apoptotic potential and ROS production (Semenza, 2007). Under normoxia, alteration of the delivery of electrons in mitochondria, either by pharmacologic inhibitors of electron transport (i.e., rotenone) (Wolvetang et al., 1994) or by disruption of iron-sulfur clusters in the mitochondrial complexes themselves (Napoli et al., 2006), leads to increased apoptosis and increased ROS flux. Accordingly, in 20% O2, miR-210-D increases apoptotic caspase 3,7 activity in full growth media (Fig. 4E), while it further increases caspase activity (Fig. 4E) and annexin V staining (Fig. 4G) to a greater extent when serum and growth factors are withheld. Furthermore, miR-210-D increases ROS flux, as reflected by increased fluorescent staining by the oxidant-sensitive fluorophore, DCFDA (Fig. 4H). Again, similar phenotypes are induced by RNAi repression of ISCU1/2 independent of miR-210 (Fig. 4F, 4H). Therefore, these multiple functions of miR-210 and ISCU1/2 appear convergent, being associated with perturbations in iron-sulfur-dependent aspects of mitochondrial electron transport and respiration.
The miR-210-ISCU1/2 Regulatory Pathway is Active in Transformed Cells
Similar to the results with primary endothelial cells and VHL -/- tissue, hypoxia up-regulates miR-210 (Fig. 1) and down-regulates ISCU1/2 in numerous transformed cell types (Supp. Fig. 3A). Upon further interrogation of HEK293 cells, we found that miR-210-D represses mRNA (Supp. Fig. 3B) and protein expression (Supp. Fig. 3C) of ISCU1/2. Mirroring its effects in HPAECs, miR-210-D represses Complex I activity in HEK293 cells by over 35% (Supp. Fig. 3D), and, in turn, decreases total oxygen consumption by nearly 40% (Supp. Fig. 3E). Therefore, the miR-210-ISCU1/2 axis represents a regulatory pathway shared among differing cell types, likely reflecting its fundamental importance in cellular metabolism during hypoxic stress.
Repression of ISCU1/2 is Necessary for miR-210 to Control Mitochondrial Metabolism
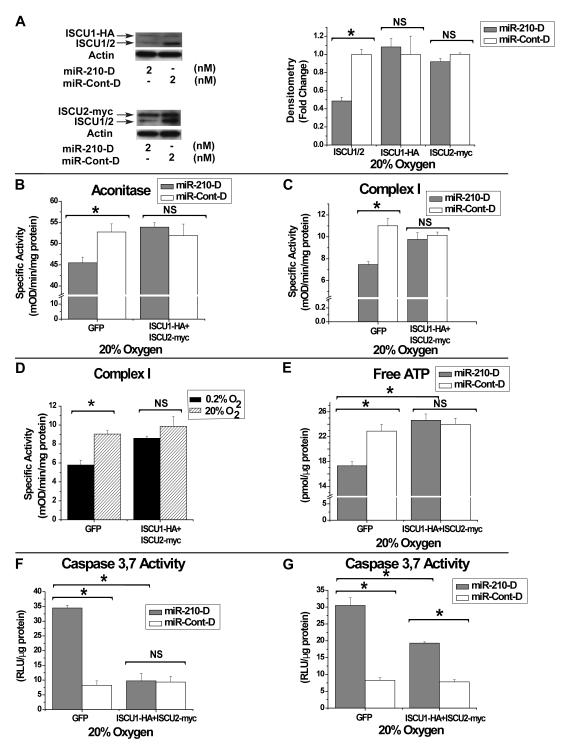
Finally, to demonstrate that the above cellular functions controlled by miR-210 are directly caused by repression of ISCU1/2, alteration of these phenotypes by miR-210 was assessed during constitutive expression of ISCU1/2. To do so, separate lentiviral constructs were made that carry either a hemagglutinin-tagged ISCUI-HA or a myc epitope-tagged ISCU2 (ISCU2-myc), but neither with an intact 3′UTR and, thus, missing a miR-210 target sequence (Tong and Rouault, 2000). While endogenous ISCU1/2 is down-regulated by miR-210-D in HPAECs, protein expression of ISCU1-HA and ISCU2-myc appears unaffected by miR-210-D, consistent with the importance of the 3′UTR target sequence for gene repression (Fig. 5A). In correlation, while miR-210-D decreases both aconitase and Complex I activities in the presence of control GFP expression, miR-210-D induces no significant change in these enzymatic activities in the presence of constitutive expression of ISCU1-HA and ISCU2-myc (Fig. 5B-C). Conversely, while induction of miR-210 in 0.2% O2 decreases Complex I activity as compared to 20% O2 in GFP-expressing HPAECs, hypoxia has negligible effects on Complex I activity during constitutive expression of ISCU1-HA and ISCU2-myc (Fig. 5D). As a result, repression of ISCU1/2 is necessary and essential for down-regulating both aconitase and Complex I activity via miR-210. Furthermore, while miR-210-D decreases ATP, constitutive expression of ISCU1-HA and ISCU2-myc reverses this change in ATP levels (Fig. 5E). Induction of apoptotic caspase 3, 7 activity by miR-210-D is also significantly decreased by constitutive expression of ISCU1-HA and ISCU2-myc (Fig. 5F), yet not fully reversed after longer periods of exposure to miR-210-D (Fig. 5G). Similar to the upstream effects on aconitase and Complex I activity, down-regulation of ISCU function as a whole is necessary for controlling the downstream effects of miR-210 on repressing ATP levels and, at least in part, increasing apoptotic potential. Taken together, these data establish a causative role for ISCU1/2 in miR-210-dependent actions, integral to the repression of mitochondrial respiration that defines the Pasteur effect.
Figure 5. Constitutive Expression of ISCU1 and ISCU2 Inhibits the Action of miR-210 on Iron-Sulfur Cluster-Dependent Metabolic Functions.
(A) Western blot/gel densitometry demonstrates that miR-210-D down-regulates ISCU1/2 expression (lower bands) as compared with control; in contrast, no change in protein levels is induced by miR-210-D of 3′UTR-deficient ISCU1-HA (HA-tagged) or ISCU2-myc (myc-tagged). Gel densitometry is normalized to actin levels. Expression levels with miR-Cont-D are assigned to a fold change of 1 (white bars), to which levels with miR-210-D (grey bars) are compared. Blots are representative of experiments performed in triplicate. (B-C) As compared with miR-Cont-D (2 nM, white bars), miR-210-D (2 nM, grey bars) decreases specific activities of aconitase (B) and Complex I (C) in GFP-expressing HPAECs; in contrast, miR-210-D induces no significant alteration of enzyme activities in the presence of constitutively expressed ISCU1/2 (ISCU1-HA+ISCU2-myc). (D) As compared with 20% O2 (hatched bars), Complex I activity is decreased in 0.2% O2 (black bars) in GFP-expressing HPAECs (GFP). This hypoxic repression is abrogated by constitutive expression of ISCU1/2 (ISCU1-HA+ISCU2-myc). (E) MiR-210 (2 nM, grey bars) decreases free ATP levels as compared with miR-Cont-D (2 nM, white bars) in GFP-positive HPAECs; this effect is abrogated by constitutively expressed ISCU1/2 (ISCU1-HA+ISCU2-myc). (F) Thirty-six hours after transfection, miR-210-D (2 nM, grey bars) induces >3-fold up-regulation of apoptotic caspase 3,7 activity in HPAECs expressing GFP as compared with miR-Cont-D (2 nM, white bars); no change in caspase 3,7 activity is induced by miR-210-D in HPAECs constitutively expressing ISCU1/2 (ISCU1-HA+ISCU2-myc). (G) Fifty-four hours after transfection, miR-210-D (2 nM, grey bars) induces a >3-fold increase in caspase 3,7 activity in HPAECs expressing GFP as compared with miR-Cont-D (2 nM, white bars); miR-210-D (2 nM, grey bars) induces significantly lower caspase 3,7 activity in HPAECs constitutively expressing ISCU1/2 (ISCU1-HA+ISCU2-myc). Error bars reflect SEM; * signifies p<0.05 (N≥3), NS signifies p≥0.05 (N≥3).
Discussion
Teleological Significance of the miR-210-ISCU1/2 Pathway on Hypoxic Metabolic Regulation
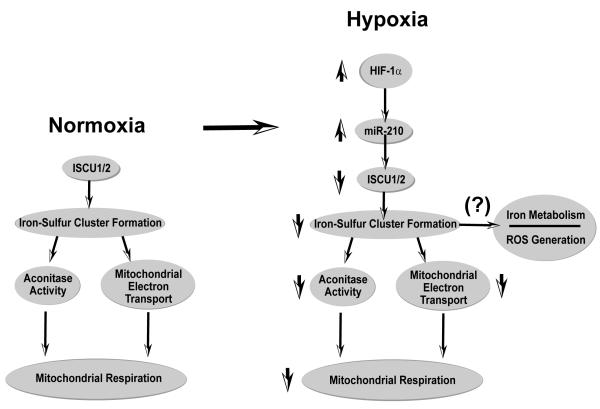
Based on these data, we conclude that miR-210 down-regulates expression of ISCU1/2 during hypoxia in order to repress key metabolic processes that predominate in the Pasteur effect, including mitochondrial electron transport and the TCA cycle (Fig. 6). The elucidation of the miR-210-ISCU1/2 regulatory pathway substantially clarifies the nature of the complex crosstalk between HIF-1α and mitochondria (Seagroves et al., 2001) and complements other HIF-dependent processes proposed to regulate the mismatch of reduced oxygen tension and mitochondrial electron transport. First, by induction of PDK1, HIF-1α decreases the activity of the TCA cycle, increases glycolysis, and reduces mitochondrial electron flux (Kim et al., 2006). Second, HIF-1α induces a COX4-1 to COX4-2 subunit switch during hypoxia to improve the efficiency of electron transfer to oxygen at Complex IV (Fukuda et al., 2007). By targeting the function of iron-sulfur cluster-dependent enzymes in both the TCA cycle (aconitase) and the electron transport chain (Complex I), miR-210 and ISCU1/2 provide overlapping control over this metabolic switch. Thus, these coordinated changes allow HIF-1α to manipulate multiple targets and ensure precise, fail-safe control over energy production and cellular survival during hypoxic stress (Semenza, 2007).
Figure 6. MiR-210 Regulates Iron-Sulfur Cluster-Dependent Metabolic Function during Hypoxia.
A molecular model is presented whereby the hypoxia-induced miR-210 directly represses expression of ISCU1/2 to down-regulate iron-sulfur cluster biogenesis and iron-sulfur-dependent metabolic enzyme activity. In doing so, miR-210 disrupts mitochondrial respiration and potentially other iron-sulfur cluster dependent functions such as iron metabolism and ROS generation. As a result, miR-210 modulates a unique constellation of essential metabolic functions that predominate in the Pasteur effect and influence cellular adaptation to hypoxia in the mammalian cell.
Identification of this pathway also indicates that disruption of iron-sulfur clusters themselves is likely an essential feature of this metabolic transition in hypoxia. Our current in vivo knowledge of deficient iron-sulfur cluster biogenesis in mammalian cells is indirect, based mostly on studies of frataxin, a protein that cooperates with ISCU2 in mitochondrial iron-sulfur cluster synthesis. Studies in frataxin-deficient mice (Thierbach et al., 2005) have revealed its critical role in augmenting OXPHOS and altering iron homeostasis. In correlation, a mutation in ISCU1/2 has recently been linked to a human genetic syndrome leading to myopathy and lactic acidosis (Mochel et al., 2008). When interpreted together with the data presented here, these convergent lines of evidence indicate that not only does ISCU1/2 regulate mitochondrial respiration and metabolism but also that such control during hypoxia likely relies upon maintenance of iron-sulfur cluster integrity. The characterization of this type of prosthetic group as a key determinant of this metabolic shift during hypoxia correlates with the known function in bacteria of iron-sulfur clusters as intracellular sensors of oxygen (Lazazzera et al., 1996). In mammalian cells, it is possible that a combination of acute sensitivity of iron-sulfur cluster integrity to oxidative stress (Beinert et al., 1997) along with repression of iron-sulfur cluster biogenesis via down-regulated ISCU1/2 may provide a real-time redox-sensitive “switch” that is triggered by hypoxia to induce downstream metabolic changes. As a result, a connection linking miR-210 and ISCU1/2 to hypoxia and mitochondrial respiration is logical from a teleological perspective, relying upon the well-conserved iron-sulfur cluster moiety to act as a direct switch that signals for shifts in aerobic respiration.
Although the miR-210-ISCU1/2 regulatory mechanism is active in various cell types, cellular context likely still influences how this pathway is utilized. For example, in contrast to non-transformed cell types, tumor cells display elevated rates of glycolysis and repressed rates of OXPHOS during normoxia (the “Warburg effect”). It is an intriguing possibility that the miR-210-ISCU1/2 pathway may influence both the Pasteur effect as well as the Warburg effect, especially in renal tumors that constitutively express HIF-1α under normoxia. Additionally, miR-210 is specifically induced by HIF-1α but not HIF-2α (Camps et al., 2008), a second transcriptional regulator of the hypoxic response, which carries partial but not full redundancy in function with HIF-1α (Wang et al., 2005). As a result, miR-210 may not be up-regulated to robust levels in all tissue types, especially in those where HIF-1α expression is diminished and HIF-2α predominates (Sowter et al., 2003). In those cases, other putative HIF-dependent miRNA may regulate iron-sulfur cluster biology. Alternatively, miR-210 may modulate metabolic functions via alternative but synergistic mechanisms independent of ISCU1/2. Thus, it may prove worthwhile to validate other in silico predicted targets of miR-210 or HIF-induced miRNA that could work in concert with ISCU1/2 or its protein partners to control iron-sulfur cluster integrity for more efficient metabolism and energy production.
Additional Implications of Disrupted Iron-Sulfur Cluster Biogenesis During Hypoxia
Besides mitochondrial metabolism, the miR-210-ISCU1/2 axis may regulate additional hypoxia-dependent and iron-sulfur cluster-dependent processes. These may include iron metabolism, which is controlled by ISCU1/2 activity (Tong and Rouault, 2006) and hypoxia (Meyron-Holtz et al., 2004), via mechanisms that have only been partially delineated. Furthermore, hypoxic repression of mitochondrial function by miR-210 and ISCU1/2 carries the theoretical potential to trigger mitophagy — a form of autophagy in which defective mitochondria are selectively delivered to lysosomes for degradation. Mitophagy has been characterized as a HIF-dependent mechanism (Zhang et al., 2008). It remains to be seen if miR-210 itself induces such mitophagy and if that would lead to further adaptive or degenerative processes.
The control of apoptosis and ROS flux by the miR-210-ISCU1/2 pathway may offer insight into the complex processes by which ROS levels are regulated by hypoxia. It has been proposed that hypoxia initially up-regulates mitochondrial ROS flux and consequently induces HIF-1α due to a mismatch of electron transport and decreased oxygen partial pressure. Upon induction of HIF-1α and HIF-dependent compensatory mechanisms, however, repression of electron transport corrects the mismatch with reduced oxygen tension, thereby decreasing ROS production, decreasing apoptosis, and optimizing energy production in the hypoxic cell (Semenza, 2007). Based on this model, the repression of ISCU1/2 and iron-sulfur cluster biogenesis by miR-210 during normoxia would create a mismatch in electron transport and oxygen concentration that leads to an increase in toxic ROS production and increased apoptosis, as corroborated by our data (Fig. 4E-H). In contrast, during hypoxia, miR-210 should induce a decrease of ROS flux by acting as a homeostatic mechanism to relieve, rather than aggravate, such a mismatch of electron transport and oxygen concentration. In corroboration, inhibition of miR-210 in HPAECs appears to increase ROS flux after hypoxic exposure (Supp. Fig. 4A) and to decrease ATP levels as compared with antisense control (Supp. Fig. 4B), indicating an adaptive role for this miRNA during hypoxic stress. Notably, apoptotic caspase activity (Supp. Fig. 4C) and cell viability (Supp. Fig. 4D) of HPAECs are not significantly affected by inhibiting miR-210 under these cell-culture conditions of hypoxia. Only under extreme stress of growth factor and serum deprivation in the setting of hypoxia does inhibition of miR-210 induce a modest increase of caspase 3,7 signaling and a correlative decrease in cell viability (Supp. Fig. 4E-F). These subtle effects on cell survival likely reflect the activity of additional compensatory mechanisms to maintain cell viability, which may be especially prominent in the HPAEC, a cell type which is exceptionally resistant to hypoxia-induced cell death (Tretyakov and Farber, 1995). Nonetheless, when interpreted with other studies demonstrating the repression of apoptosis by miR-210 during hypoxia in other cellular contexts (Fasanaro et al., 2008; Kulshreshtha et al., 2007), these data lend credence to a model in which miR-210 carries a maladaptive role during normoxia, causing a mismatch of ambient oxygen and electron transport, but an adaptive role during hypoxia due to the homeostatic correction of the imbalance of electron transport and oxygen exposure.
Complete understanding of this action of miR-210 is nonetheless difficult. While our data suggest a minimal change in ROS levels after hypoxic exposure of HPAECs (Supp. Fig. 4A), controversy exists as to whether hypoxia decreases or increases ROS flux in the pulmonary vasculature, given the technical hurdles of accurately assessing these oxygen species under low oxygen tension (Moudgil et al., 2005). Furthermore, if miR-210 alternatively regulates iron metabolism or preferentially allows for release of free iron from modified iron-sulfur clusters during hypoxia (Beinert et al., 1997), redox-sensitive iron species may serve as additional sources of ROS generation, independent of the activity of mitochondrial electron transport. As a result, future and more definitive studies will be critical for defining the effects of miR-210 on the control of ROS generation. This experimental direction will be particularly important in the endothelium, owing to the importance of potential ROS-dependent phenotypes, such as permeability, thrombosis, vasomotor tone, and angiogenesis.
In conclusion, we identify miR-210 and ISCU1/2 as critical regulatory factors in hypoxia that control mitochondrial respiration and, thus, modulate a fundamental shift in cellular metabolism. Consequently, these data should serve as a foundation for studies designed to explore further the actions of miR-210 and other hypoxia-induced miRNA on mitochondria and metabolism, additional functions of iron-sulfur cluster biology in hypoxia, and the more complex effects of miR-210 and ISCU1/2 in vivo under physiological and hypoxia-dependent disease states.
Experimental Procedures
Cells
Primary HPAECs and human PASMCs were purchased and propagated in EGM-2 and SmGM-2 media, respectively (Lonza). Experiments were performed at passages 5-9. COS7, HEK293, MCF-7, and HT-29 cells were grown in DMEM with 10% serum. HL-60 cells were grown in RPMI1640 with 4 mM L-Glutamine and 10% serum.
Exposure to Hypoxia
HPAECs were exposed for 24 hours (unless otherwise stated) either to standard non-hypoxic cell culture conditions, (20% O2, 5% CO2, with N2 balance at 37°C) or to hypoxia (0.2%-12% O2, 5% CO2, with N2 balance at 37°C), either in a modular hypoxia chamber or tissue culture incubator. Conditions were based on studies of hypoxic exposure of HPAECs (Manalo et al., 2005) to allow for “steady state” adaptation without non-specific cell death.
RNA Extraction and RT-QPCR
RNA extraction (miRNeasy kit, Qiagen) and reverse transcription of 10 ng of total RNA were performed to generate cDNA representing levels of either mRNA (High Fidelity cDNA Amplification Kit, Applied Biosystems) or mature miRNA molecules (MicroRNA Assay Kit, Applied Biosystems). Owing to the stem loop structures of the miRNA primers, only mature miRNA molecules were amplified into cDNA. cDNA was amplified via fluorescently labeled Taqman probe and primer sets using an Applied Biosystems 7900HT Fast Real Time PCR device. Details of high-throughput RT-PCR screening of miRNA are described in Supplemental Methods.
MicroRNA Overexpression and Repression
Chemically synthesized miRNA duplexes (Ambion) were transfected to mimic the mature form of miR-210, as compared with control. Oligonucleotides (Dharmacon) were transfected that carry specific antisense sequences to miR-210, as compared with control. RNA interference of ISCU1/2 expression was performed using custom designed inhibitory RNA (Stealth siRNA from Invitrogen).
Additional Information
See Supplemental Methods for information on in silico miRNA target prediction, EPR spectroscopy, animal manipulation, plasmid construction, lentivirus production, transfection, immunoblotting, and standardized assays of metabolic and mitochondrial functions, ROS flux, and cell survival.
Statistical Analysis
Data are presented either as a representative example of a single experiment, repeated at least in triplicate, or as mean ± standard error of the mean for three or more experiments. Paired samples were compared by Student’s t test; multiple comparisons were made with a one-way ANOVA followed by a Bonferroni post hoc test. Values of p<0.05 are considered significant.
Supplementary Material
Acknowledgments
This work was supported by MGH Cardiology NIH-T32 grant (S.Y.C.), AHA grant 0825906D (S.Y.C.), and NIH grants HL 61795, NO1 HV 28178, PO1 81587, U54 HL 70819 (J.L., Y.Z.). No conflicts of interest are reported. We thank the Loscalzo laboratory and R. Liao for fruitful discussions; N. Kelly (bioinformatics); W. Kaelin, J. Moslehi, and Y. Minamishima (VHL WT and -/- murine tissue); H. Ardehali (miR-210 expression vector); T. Rouault (ISCU expression vectors); V. Mootha (technical aid in measuring oxygen consumption); S.K. Chan and J.W. Snow (critical reading of the manuscript); and S. Tribuna (administrative assistance).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aisenberg AC, Potter VR. Studies on the Pasteur effect. II. Specific mechanisms. J Biol Chem. 1957;224:1115–1127. [PubMed] [Google Scholar]
- Beinert H, Holm RH, Munck E. Iron-sulfur clusters: nature’s modular, multipurpose structures. Science. 1997;277:653–659. doi: 10.1126/science.277.5326.653. [DOI] [PubMed] [Google Scholar]
- Camps C, Buffa FM, Colella S, Moore J, Sotiriou C, Sheldon H, Harris AL, Gleadle JM, Ragoussis J. hsa-miR-210 Is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin Cancer Res. 2008;14:1340–1348. doi: 10.1158/1078-0432.CCR-07-1755. [DOI] [PubMed] [Google Scholar]
- Chavez A, Miranda LF, Pichiule P, Chavez JC. Mitochondria and hypoxia-induced gene expression mediated by hypoxia-inducible factors. Ann N Y Acad Sci. 2008;1147:312–320. doi: 10.1196/annals.1427.021. [DOI] [PubMed] [Google Scholar]
- Crosby ME, Kulshreshtha R, Ivan M, Glazer PM. MicroRNA Regulation of DNA Repair Gene Expression in Hypoxic Stress. Cancer Res. 2009;69:1221–1229. doi: 10.1158/0008-5472.CAN-08-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasanaro P, D’Alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, Capogrossi M, Martelli F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem. 2008;283:15878–15883. doi: 10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129:111–122. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- Giannakakis A, Sandaltzopoulos R, Greshock J, Liang S, Huang J, Hasegawa K, Li C, O’Brien-Jenkins A, Katsaros D, Weber BL, Simon C, Coukos G, Zhang L. miR-210 links hypoxia with cell cycle regulation and is deleted in human epithelial ovarian cancer. Cancer Biol Ther. 2008;7:255–264. doi: 10.4161/cbt.7.2.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginouves A, Ilc K, Macias N, Pouyssegur J, Berra E. PHDs overactivation during chronic hypoxia “desensitizes” HIFalpha and protects cells from necrosis. Proc Natl Acad Sci U S A. 2008;105:4745–4750. doi: 10.1073/pnas.0705680105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase VH, Glickman JN, Socolovsky M, Jaenisch R. Vascular tumors in livers with targeted inactivation of the von Hippel-Lindau tumor suppressor. Proc Natl Acad Sci U S A. 2001;98:1583–1588. doi: 10.1073/pnas.98.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchliffe P, Sazanov LA. Organization of iron-sulfur clusters in respiratory complex I. Science. 2005;309:771–774. doi: 10.1126/science.1113988. [DOI] [PubMed] [Google Scholar]
- Ivan M, Harris AL, Martelli F, Kulshreshtha R. Hypoxia response and microRNAs: no longer two separate worlds. J Cell Mol Med. 2008;12:1426–1431. doi: 10.1111/j.1582-4934.2008.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Kulshreshtha R, Davuluri RV, Calin GA, Ivan M. A microRNA component of the hypoxic response. Cell Death Differ. 2008;15:667–671. doi: 10.1038/sj.cdd.4402310. [DOI] [PubMed] [Google Scholar]
- Kulshreshtha R, Ferracin M, Wojcik S, Garzon R, Alder H, Agosto-Perez F, Davuluri R, Liu C, Croce C, Negrini M, Calin G, Ivan M. A microRNA signature of hypoxia. Mol Cell Biol. 2007;27:1859–1867. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazazzera BA, Beinert H, Khoroshilova N, Kennedy MC, Kiley PJ. DNA binding and dimerization of the Fe-S-containing FNR protein from Escherichia coli are regulated by oxygen. J Biol Chem. 1996;271:2762–2768. doi: 10.1074/jbc.271.5.2762. [DOI] [PubMed] [Google Scholar]
- Manalo D, Rowan A, Lavoie T, Natarajan L, Kelly B, Ye S, Garcia J, Semenza G. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105:659–669. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- Mann GE, Yudilevich DL, Sobrevia L. Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells. Physiol Rev. 2003;83:183–252. doi: 10.1152/physrev.00022.2002. [DOI] [PubMed] [Google Scholar]
- Meyron-Holtz E, Ghosh M, Rouault T. Mammalian tissue oxygen levels modulate iron-regulatory protein activities in vivo. Science. 2004;306:2087–2090. doi: 10.1126/science.1103786. [DOI] [PubMed] [Google Scholar]
- Minamishima YA, Moslehi J, Bardeesy N, Cullen D, Bronson RT, Kaelin WG., Jr. Somatic inactivation of the PHD2 prolyl hydroxylase causes polycythemia and congestive heart failure. Blood. 2008;111:3236–3244. doi: 10.1182/blood-2007-10-117812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochel F, Knight M, Tong W, Hernandez D, Ayyad K, Taivassalo T, Andersen P, Singleton A, Rouault T, Fischbeck K, Haller R. Splice mutation in the iron-sulfur cluster scaffold protein ISCU causes myopathy with exercise intolerance. Am J Hum Genet. 2008;82:652–660. doi: 10.1016/j.ajhg.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moudgil R, Michelakis E, Archer S. Hypoxic pulmonary vasoconstriction. J Applied Physiol. 2005;98:390–403. doi: 10.1152/japplphysiol.00733.2004. [DOI] [PubMed] [Google Scholar]
- Napoli E, Taroni F, Cortopassi GA. Frataxin, iron-sulfur clusters, heme, ROS, and aging. Antioxid Redox Signal. 2006;8:506–516. doi: 10.1089/ars.2006.8.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault TA, Tong WH. Iron-sulfur cluster biogenesis and human disease. Trends Genet. 2008;24:398–407. doi: 10.1016/j.tig.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagroves TN, Ryan HE, Lu H, Wouters BG, Knapp M, Thibault P, Laderoute K, Johnson RS. Transcription factor HIF-1 is a necessary mediator of the pasteur effect in mammalian cells. Mol Cell Biol. 2001;21:3436–3444. doi: 10.1128/MCB.21.10.3436-3444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G. Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem J. 2007;405:1–9. doi: 10.1042/BJ20070389. [DOI] [PubMed] [Google Scholar]
- Sowter HM, Raval RR, Moore JW, Ratcliffe PJ, Harris AL. Predominant role of hypoxia-inducible transcription factor (Hif)-1alpha versus Hif-2alpha in regulation of the transcriptional response to hypoxia. Cancer Res. 2003;63:6130–6134. [PubMed] [Google Scholar]
- Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006;99:675–691. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- Thierbach R, Schulz T, Isken F, Voigt A, Mietzner B, Drewes G, von Kleist-Retzow J, Wiesner R, Magnuson M, Puccio H, Pfeiffer A, Steinberg P, Ristow M. Targeted disruption of hepatic frataxin expression causes impaired mitochondrial function, decreased life span and tumor growth in mice. Human Mol Genet. 2005;14:3857–3864. doi: 10.1093/hmg/ddi410. [DOI] [PubMed] [Google Scholar]
- Tong WH, Rouault T. Distinct iron-sulfur cluster assembly complexes exist in the cytosol and mitochondria of human cells. EMBO J. 2000;19:5692–5700. doi: 10.1093/emboj/19.21.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong WH, Rouault TA. Functions of mitochondrial ISCU and cytosolic ISCU in mammalian iron-sulfur cluster biogenesis and iron homeostasis. Cell Metab. 2006;3:199–210. doi: 10.1016/j.cmet.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Tretyakov AV, Farber HW. Endothelial cell tolerance to hypoxia. Potential role of purine nucleotide phosphates. J Clin Invest. 1995;95:738–744. doi: 10.1172/JCI117721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang V, Davis DA, Haque M, Huang LE, Yarchoan R. Differential gene up-regulation by hypoxia-inducible factor-1alpha and hypoxia-inducible factor-2alpha in HEK293T cells. Cancer Res. 2005;65:3299–3306. doi: 10.1158/0008-5472.CAN-04-4130. [DOI] [PubMed] [Google Scholar]
- Wolvetang E, Johnson K, Krauer K, Ralph S, Linnane A. Mitochondrial respiratory chain inhibitors induce apoptosis. FEBS Lett. 1994;339:40–44. doi: 10.1016/0014-5793(94)80380-3. [DOI] [PubMed] [Google Scholar]
- Young AP, Schlisio S, Minamishima YA, Zhang Q, Li L, Grisanzio C, Signoretti S, Kaelin WG., Jr. VHL loss actuates a HIF-independent senescence programme mediated by Rb and p400. Nat Cell Biol. 2008;10:361–369. doi: 10.1038/ncb1699. [DOI] [PubMed] [Google Scholar]
- Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, Semenza GL. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283:10892–10903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.