Abstract
Integrins regulate adhesion-dependent growth, survival and invasion of tumor cells. In particular, expression of integrin αvβ3 is associated with progression of a variety of human tumors. Here, we reveal a novel adhesion-independent role for integrin αvβ3 in pancreatic cancer and other carcinomas. Specifically, αvβ3 expressed in carcinoma cells enhanced anchorage-independent tumor growth in vitro and increased lymph node metastases in vivo. This required recruitment of c-src to the β3 integrin cytoplasmic tail, leading to c-src activation, crk-associated substrate (CAS) phosphorylation and tumor cell survival that, surprisingly, was independent of cell adhesion or focal adhesion kinase (FAK) activation. Reduced expression of endogenous αvβ3 or c-src not only suppressed anchorage-independent growth, but also decreased metastasis in vivo, yet did not affect migration/invasion. These data define an unexpected role for an integrin as a mediator of anchorage-independence suggesting that an αvβ3/c-src signaling module may account for the aggressive behavior of αvβ3-expressing tumors in man.
While anchorage-independent growth is a hallmark of transformed cells, tumor growth and metastasis depend on tumor cell interactions with the extracellular matrix, mediated by the integrin family of adhesion receptors. Integrins promote a wide range of adhesion-dependent effects in tumor cells including proliferation, survival, migration/invasion and chemotherapeutic resistance1 attributed to activation of FAK2,3-5 which recruits other signaling molecules including c-src6, a kinase whose activity is associated with enhanced malignancy7. Following adhesion, c-src phosphorylates CAS, a large adaptor protein implicated in cell invasion and survival8-10.
Integrin αvβ3 is expressed in some of the most aggressive tumor cells in a variety of cancers, including: melanoma and carcinomas of the prostate, breast, cervix and pancreas. In melanoma, αvβ3 expression initiates the transition from the benign radial growth phase to the malignant vertical growth phase11,12. In both breast and prostate carcinomas αvβ3 mediates bone metastasis through enhanced tumor cell adhesion13-16. Expression of αvβ3 correlates with disease progression and shorter survival in patients with cervical carcinoma17. In pancreatic ductal adenocarcinoma αvβ3 expression occurs in approximately 58% of human tumors and is associated with increased lymph node metastasis18.
Integrins provide context-dependent cues to both normal and transformed cells that paradoxically promote both cell survival and initiate apoptosis. While expression of some integrins enhances malignancy, others inhibit malignant progression19,20. We recently demonstrated that in some tumors the expression of an unligated integrin induces apoptosis through recruitment and activation of caspase-8, a process termed integrin-mediated death (IMD)21. Tumors lacking caspase-8 were resistant to IMD and exhibited increased metastatic potential22. Here, we describe a novel role for an integrin as a mediator of anchorage-independence and suggest this may account for the enhanced malignancy associated with αvβ3 expression in pancreatic carcinoma and a wide array of other tumors.
Results
Expression of αvβ3 correlates with metastatic potential
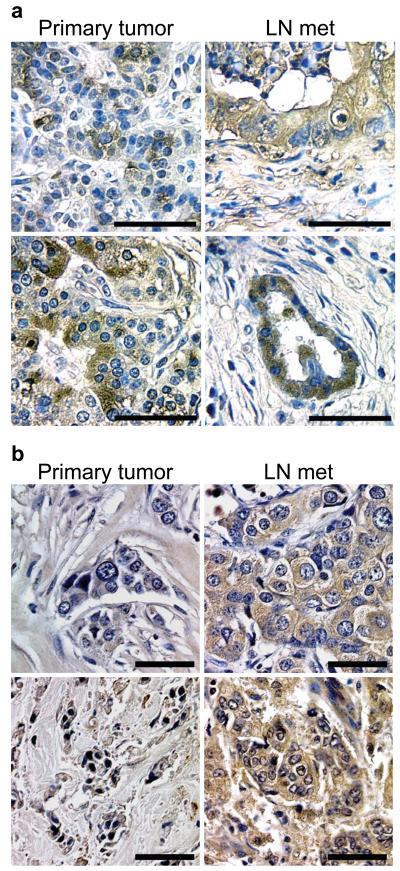
We compared αvβ3 expression in multiple matched pairs of primary tumor and lymph node metastases from pancreatic and breast cancer patients. Interestingly, in pancreatic cancer specimens, cells in the primary tumor showed heterogeneous staining for αvβ3, however most of the tumor cells in the lymph nodes were αvβ3 positive (Fig. 1a) (Supplementary Fig. 1a,b). Similarly, in breast cancer, several examples were observed in which αvβ3 expression was enriched in the lymph node metastases relative to the primary tumor (Fig. 1b). These data suggest that αvβ3 may be a marker of the metastatic cells within these tumors.
Figure 1.
Integrin αvβ3 is expressed in a sub-population of human carcinoma cells and correlates with lymph node invasion. (a,b) Representative images of immunohistochemical staining for the integrin β3 subunit in matched pairs of primary tumors (left panels) and lymph node metastases (right panels) from pancreatic (n=7) (a) and breast (n=50) (b) cancer patients. (a) In pancreatic cancer, β3 was expressed heterogeneously throughout all of the primary tumors, however, tumor cells invading lymph nodes from the same patient were primarily β3-positive in 6 of 7 patients. (b) In breast cancer specimens, β3 was expressed in 28 of 50 primary tumors (56%) however, we observed expression of this integrin in 36 of 50 lymph node metastases (72%) in these same patients. Scale bars, 50 μm.
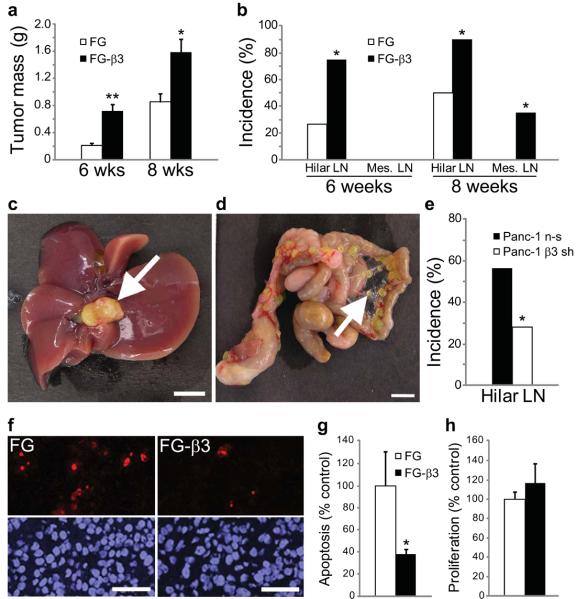
To directly address the role of αvβ3 in tumor malignancy we injected αvβ3 positive or negative GFP-labeled human pancreatic carcinoma cells into the pancreas of nude mice and evaluated primary tumor growth and spontaneous metastasis. Compared to FG cells, which lack αvβ3, FG-β3 cells ectopically expressing αvβ3 (Supplementary Fig. 2) exhibited increased primary tumor mass at both six and eight weeks following injection (Fig. 2a) and significantly enhanced spontaneous metastasis to the hepatic hilar and mesenteric lymph nodes (Fig. 2b). Lymph node metastases were confirmed by anatomical location (Fig. 2c,d), GFP fluorescence (Supplementary Fig. 3a) and histological evaluation (Supplementary Fig. 3b). Interestingly, 25% of the mice with FG-β3 tumors developed severe ascites and wasting emulating the morbidity associated with late stage human pancreatic carcinoma, which was not observed in mice with FG tumors. In support of these findings, knock-down of endogenous β3 in Panc-1 pancreatic cancer cells (Supplementary Fig. 4a) significantly inhibited metastasis to the liver hilar lymph nodes (Fig. 2e), and caused a modest decrease in primary tumor mass (Supplementary Fig. 4b). In summary, these results demonstrate that αvβ3 expression enhances the primary tumor growth and metastasis of these carcinoma cell lines.
Figure 2.
Integrin αvβ3 enhances pancreatic tumor progression and metastasis. (a) Primary tumor mass is enhanced in FG-β3 tumors compared to FG control tumors at both 6 and 8 weeks. (b) At both time-points we also observed enhanced spontaneous metastasis to the hepatic hilar lymph nodes in mice with FG-β3 tumors relative to FG controls. At 8 weeks we observed additional metastasis of FG-β3 cells to the mesenteric lymph nodes. 6 weeks; FG, n=15, FG-β3, n=16. 8 weeks; FG, n=20, FG-β3, n=20. *P<0.05, **P<0.001. (c,d) Representative examples of spontaneous metastases to the hepatic hilar lymph nodes (c) and the mesenteric lymph nodes (d) after orthotopic injection of FG-β3 cells. Scale bar, 5 mm. (e) Knock-down of the β3 integrin subunit in Panc-1 cells (β3 sh) reduced spontaneous metastasis to the liver hilar lymph nodes compared to Panc-1 cells expressing a non-silencing control (n-s). n-s, n=13, β3 sh, n=13. *P<0.05. (f–h) Apoptosis (TUNEL) and proliferation (Ki-67) were assayed in sections from 8 week primary tumors. (f) Representative TUNEL staining results show fewer stained cells in the FG-β3 tumor relative to the FG control. Scale Bar, 50 μm. (g,h) FG-β3 tumors exhibit decreased levels of apoptosis (g) compared to FG controls, with no difference in proliferation (h). FG, n=11; FG-β3, n=10. *P<0.05.
To discern a potential mechanism to account for these findings we analyzed the relative level of cell proliferation, apoptosis and vascular density in FG versus FG-β3 primary tumors. FG-β3 tumors exhibited an approximately 3-fold reduction in apoptosis compared to FG tumors lacking this integrin (Fig. 2f,g), yet we could not detect any difference in proliferation (Fig. 2h) or vascular density (Supplementary Fig. 5a–c). These data demonstrate that αvβ3 expression is associated with increased tumor cell survival.
αvβ3 enhances adhesion-independent activation of c-src
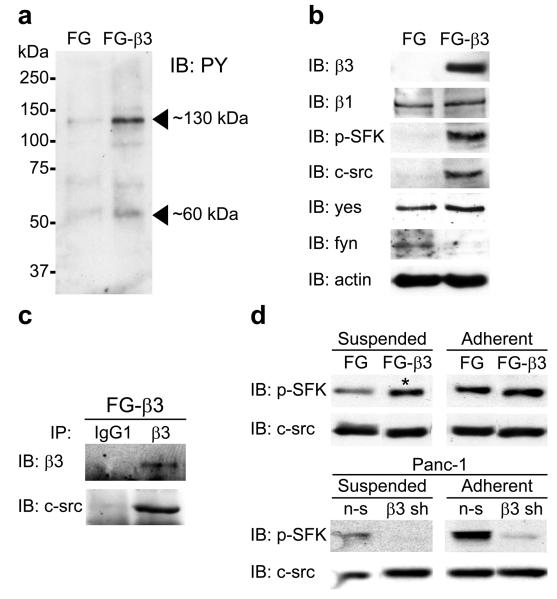
Typically, integrins initiate signaling via cell adhesion to the extracellular matrix where they interact with immobilized matrix proteins and cluster in the plane of the membrane. This facilitates the assembly of a focal contact containing the integrin together with tyrosine kinases such as FAK or c-src and adaptor proteins such as CAS23 that mediate downstream signaling leading to a wide array of cellular activities. FG cell adhesion to fibronectin depends on α5β1 whereas FG-β3 adhesion is mediated by either α5β1 or αvβ3 (Supplementary Fig. 6a). Following adhesion to fibronectin, we identified two prominent phosphoproteins of approximately 60 and 130 kilodaltons in the FG-β3 triton-insoluble lysate relative to the FG control (Fig. 3a). The 60 kDa phosphoprotein was analyzed by immunoblotting with an antibody directed against activated src family kinases (SFK) (pY416). We detected a significant increase in SFK pY416 immunoreactivity in FG-β3 lysates (Fig. 3b) that was verified in focal contacts by immunostaining of adherent, permeabilized cells (Supplementary Fig. 6b). The 130 kDa phosphoprotein(s) most likely represents the SFK substrates FAK (125 kDa) and CAS (130 kDa), as both exhibited enhanced phosphorylation in adherent FG-β3 cells (Supplementary Fig. 7a,b). Thus, while both α5β1 and αvβ3 mediate fibronectin adhesion of FG-β3 cells, only αvβ3 co-localizes with an activated SFK in these cells.
Figure 3.
Integrin αvβ3 promotes anchorage-independent activation of c-src. (a) Adhesion of FG-β3 cells to fibronectin enriches for phosphotyrosine bands of 60 and 130 kDa in the triton insoluble lysate relative to FG cells. (b) Immunoblotting for pY416 SFK shows enrichment in the FG-β3 triton insoluble lysate. However, only c-src, and not yes or fyn, exhibited a similar pattern of recruitment as pY416 SFK in FG-β3 cells. (c) Co-immunoprecipitation of c-src with αvβ3 from the triton insoluble fraction of FG-β3 cells plated on the αvβ3 substrate vitronectin. (d). While expression of αvβ3 enhanced adhesion-dependent SFK activation, as expected, αvβ3 unexpectedly increased suspension levels of pY416 SFK in FG-β3 cells (asterisk) while β3 knock-down inhibited suspension SFK activation in Panc-1 cells.
To determine which SFK isoform(s) was associated with αvβ3 we examined triton-insoluble lysates for c-src, yes and fyn. This analysis identified c-src as the only isoform associated with αvβ3 (Fig. 3b) suggesting that αvβ3 specifically recruits and activates c-src. To evaluate this further, αvβ3 was immunoprecipitated from FG-β3 cells followed by immunoblotting for c-src. Integrin αvβ3 and c-src formed a complex (Fig. 3c) that was abolished when the C-terminal four amino acids were deleted from the β3 cytoplasmic tail (759x) (Supplementary Fig. 8a) as previously reported for the platelet integrin αIIbβ324. This suggests that αvβ3 recruits c-src in a manner that depends on the terminal four amino acids of the β3 subunit.
We further investigated the αvβ3-mediated activation of c-src by analyzing the kinetics of SFK activation in response to cell adhesion. Consistent with our previous findings (Fig. 3b) expression of αvβ3 in either FG or Panc-1 cells increased SFK activity following adhesion (Fig. 3d). However, to our surprise, αvβ3 also increased SFK activation in cells maintained in suspension, (Fig. 3d and Supplementary Fig. 8b). Interestingly, unlike adherent cells, SFK activation occurred independently of FAK activity when these cells were maintained in suspension (Supplementary Fig. 8c). These findings indicate that integrin αvβ3 recruitment of c-src may promote anchorage-independent signaling distinct from the response induced by this integrin in adherent cells as measured by FAK activation.
αvβ3 promotes anchorage-independence through c-src
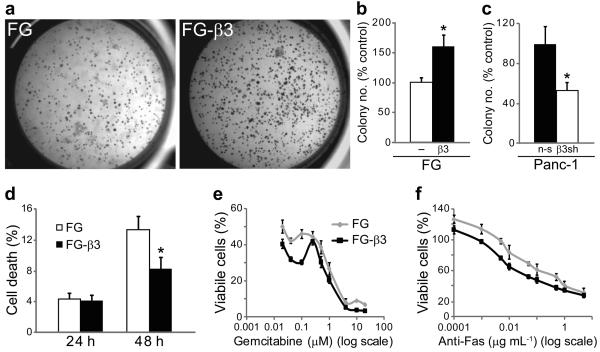
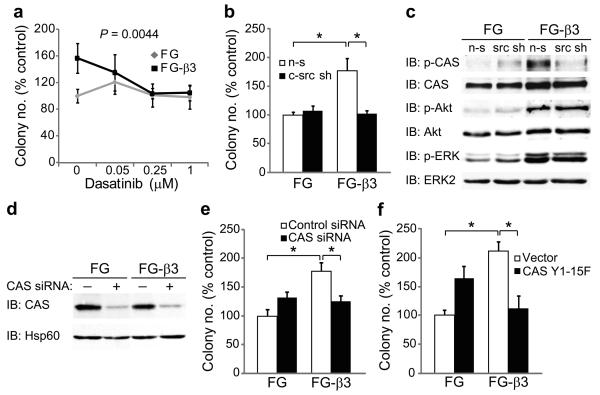
Growth in anchorage-independent conditions is a hallmark of tumor cell transformation and is suggested to play a role in metastasis25,26. Based on our findings that αvβ3 activates c-src in non-adherent FG-β3 cells, we considered whether this might provide an anchorage-independent growth advantage in soft agar. Strikingly, we found that FG-β3 cells formed approximately twice as many colonies as FG cells (Fig. 4a,b) yet these cells showed no change in their growth rate when maintained in adherent culture conditions (Supplementary Fig. 9a). In fact, ligation of αvβ3 did not contribute to the anchorage-independent growth advantage of FG-β3 cells as neither blockade of αvβ3 with the function blocking monoclonal antibody LM60927,28 nor expression of a β3 D119A mutant incapable of binding ligand29 inhibited colony formation (Supplementary Fig. 9b and 10a–c). Similar results were obtained following αvβ3 expression in the αvβ3-negative MiaPaca-2 human pancreatic cell line (Supplementary Fig. 11a), whereas knock-down of endogenous β3 in Panc-1 cells significantly reduced their anchorage-independent growth (Fig. 4c). These effects were also extended to tumor cells of distinct histological origin as αvβ3 expression mediated similar effects on soft agar colony formation in both breast and cervical cancer cell lines (Supplementary Fig. 12a–f). Enhanced colony formation appeared to result from increased survival of αvβ3-expressing cells (Fig. 4d), as observed in vivo, (Fig 2f–h) and not increased proliferation (Supplementary Fig. 11c). In contrast, FG and FG-β3 cells attached to fibronectin showed identical levels of apoptosis in response to either gemcitabine (Fig. 4e) or an anti-Fas antibody (Fig. 4f), suggesting that αvβ3 provides a specific survival benefit under anchorage-independent growth conditions.
Figure 4.
Integrin αvβ3-induces anchorage-independent survival with no affect on survival of adherent cells. (a–c) Expression of αvβ3 in FG cells enhanced colony number in soft agar (a,b) while knock-down of β3 in Panc-1 cells (β3 sh) reduced colony formation compared to cells expressing a non-silencing control (n-s) (c). n=3 independent experiments. *P<0.05. (d) FG and FG-β3 cells were maintained in suspension culture for 24 and 48 h prior to trypan blue staining and counting viable and non-viable cells. Compared to FG control cells, suspension cultures of FG-β3 cells exhibit significantly less cell death. n=4 independent experiments. *P<0.05. (e,f) Ligation of αvβ3 to fibronectin failed to protect FG-β3 cells from apoptosis initiated by either gemcitabine (e) or anti-Fas antibody (CH11) (f). Representative experiments are shown.
To investigate whether αvβ3–mediated anchorage-independent survival was c-src-dependent, cells were placed in suspension culture in the presence or absence of dasatinib, a clinically approved SFK inhibitor. Treatment of FG-β3 cells with dasatinib, reduced colony formation of FG-β3 cells to the level observed for FG cells (Fig. 5a) suggesting that c-src activity plays a role in the αvβ3-anchorage independent growth advantage of FG-β3 cells. Importantly, dasatinib had no effect on FG cell anchorage-independent growth despite significantly inhibiting SFK activity in these cells (Supplementary Fig. 13). A similar result was also observed in MP-2 cells (Supplementary Fig. 11b). Consistent with the lack of FAK activation in suspended FG and FG-β3 cells, treatment with either of two different FAK inhibitors failed to reduce colony number in either cell type (Supplementary Fig. 14a,b). In support of this pharmacological data, knock-down of c-src in FG-β3 cells (Supplementary Fig. 15) specifically inhibited αvβ3-mediated colony formation to the level observed in FG cells (Fig. 5b). Next, we considered whether the c-src/αvβ3 complex in FG-β3 cells might play a role in αvβ3-mediated anchorage-independent colony formation. To test this, we expressed a truncation mutant of β3 (759x) that fails to interact with c-src24 (Supplementary Fig. 8a). Cells expressing this mutant failed to enhance soft agar colony formation compared to cells expressing the wild-type receptor (Supplementary Fig. 16).
Figure 5.
Integrin αvβ3-induces anchorage-independence through c-src phosphorylation of CAS (a) To discern a role for c-src activation in αvβ3-induced anchorage-independent growth, FG and FG-β3 cells were treated with dasatinib, a clinically approved SFK inhibitor. Treatment with dasatinib specifically reduced colony number in FG-β3 cells compared to vehicle control (DMSO) while no effect was observed in FG cells. n=3 independent experiments. P=0.0044. (b) Knock-down of c-src (c-src sh) selectively inhibited αvβ3-mediated colony formation relative to a non-silencing control (n-s) with no effect on FG cells. n=3 independent experiments. *P<0.05. (c) Expression of αvβ3 potentiates CAS, Akt and ERK signaling in suspension cultured cells, but only CAS phosphorylation required c-src. (d,e) Knock-down of CAS with siRNA specifically decreased αvβ3-mediated growth in soft agar compared to FG control cells. A representative experiment is shown. n=3 independent experiments. *P<0.05. (f) Stable expression of a CAS mutant lacking c-src phosphorylation sites (Y1-15F) specifically inhibited FG-β3 cell colony formation. A representative experiment is shown. n=3 independent experiments. *P<0.05.
In adherent cells, FAK localizes to integrin focal contacts where it recruits and activates c-src resulting in phosphorylation of c-src substrates, including CAS, promoting cell proliferation and migration8,9. While these effects occur in FG-β3 cells attached to fibronectin (Supplementary Fig. 7a,b) or vitronectin (not shown) FG-β3 cells maintained in suspension show increased CAS phosphorylation in the absence of FAK activation. However, CAS phosphorylation under these conditions was c-src-dependent since it was abolished by knockdown of c-src (Fig. 5c). Interestingly, FG-β3 cells in suspension also exhibited increased phosphorylation of Akt and ERK in a manner independent of c-src (Fig. 5c). These findings indicate that αvβ3 expression activates both c-src-dependent and independent signaling pathways yet only the c-src-dependent pathway leads to increased anchorage-independent growth and CAS phosphorylation.
We next considered whether CAS was required for αvβ3-mediated colony formation in soft agar. Knock-down of CAS with siRNA oligonucleotides specifically reduced colony number in FG-β3 cells compared to FG cells (Fig. 5d,e). We further considered whether c-src-dependent phosphorylation of CAS was required for αvβ3-mediated colony formation. To test this, we expressed a dominant negative mutant version of CAS in both FG and FG-β3 cells in which the known c-src tyrosine phosphorylation sites within its substrate domain were mutated to phenylalanines (CAS Y1-15F)30 (Supplementary Fig. 17). Cells expressing this mutant were embedded in soft agar and colony formation was assessed. As expected, control FG-β3 cells showed an approximately 2-fold increase in colony number compared to FG cells. FG-β3 cells expressing the CAS Y1-15F mutant showed no such increase in colony formation (Fig. 5f). These findings indicate that αvβ3-mediated activation of c-src promotes increased anchorage-independent growth based on its capacity to phosphorylate the CAS substrate domain.
c-src mediates αvβ3 tumor cell survival and metastasis
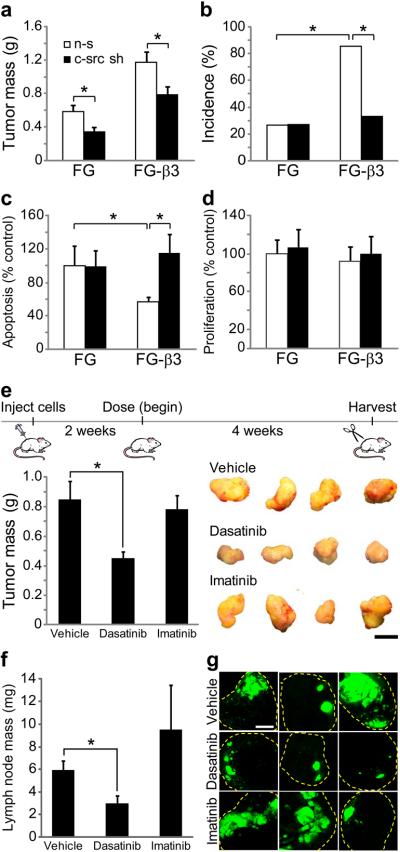
To determine whether αvβ3-mediated c-src activation could lead to increased tumor malignancy, FG and FG-β3 cells expressing control or c-src knockdown shRNA's were injected into the pancreas of nude mice and analyzed after eight weeks. Although c-src knock-down reduced primary tumor mass in both FG and FG-β3 cells (Fig. 6a), it specifically blocked the metastatic advantage of the FG-β3 cells (Fig. 6b). Mechanistically, this appears to be due to effects on c-src-dependent cell survival (Fig. 6c), but not proliferation (Fig. 6d). The c-src binding site on the β3 tail is critical to the in vivo effects of αvβ3 expression as FG-759x cells formed tumors only 3% the mass of FG-β3 cells (Supplementary Fig. 18a,b). These data describe the surprising finding that the in vivo effects of αvβ3 expression critically require c-src and its interaction with the β3 cytoplasmic tail.
Figure 6.
Integrin αvβ3 requires c-src for tumor cell survival and metastasis in vivo, but not for migration in vitro. (a–d) FG and FG-β3 non-silencing (n-s) and c-src knock-down cells (c-src sh) were injected into the pancreas of nude mice and assessed for primary tumor growth, metastasis, apoptosis and proliferation after 8 weeks. (a) Knock-down of c-src decreased primary tumor mass in both FG and FG-β3 tumors relative to non-silencing controls. (b) However, in these same mice, knock-down of c-src selectively inhibited αvβ3-mediated metastasis to the hepatic hilar lymph nodes with no affect on FG cells. (a,b) FG non-silencing, n=15, FG-β3 non-silencing, n=14, FG c-src shRNA, n=22, FG-β3 c-src shRNA, n=21. *P<0.05. (c) In primary tumor sections, knock-down of c-src increased apoptosis in FG-β3 tumors to the level of FG controls. (d) The percentage of proliferating cells in primary tumor sections was unaffected by c-src knock-down. (c,d) FG non-silencing, n=8, FG-β3 non-silencing, n=7, FG c-src shRNA, n=7, FG-β3 c-src shRNA, n=6; *P<0.05. (e–g) Treatment with the SFK inhibitor dasatinib decreased FG-β3 cell primary tumor mass (e) and metastasis to the liver hilar lymph nodes (f,g) while imatinib had no effect. Representative tumors are shown in (e). The three largest lymph node metastases from each group are compared in (g). Vehicle, n=12, Dasatinib, n=12, Imatinib, n=12; *P<0.05. Scale bar, 1 mm.
To validate the therapeutic relevance of our findings, we compared the SFK/abl inhibitor dasatinib with the abl inhibitor imatinib for their ability to reduce tumor burden and metastasis of αvβ3-expressing tumors. Orthotopically injected FG-β3 tumor cells were established for two weeks prior to dosing with vehicle (b.i.d.), 30 mg kg−1 dasatinib (b.i.d.) or 50 mg kg−1 imatinib (q.d.) by oral gavage for 4 weeks. Dasatinib treatment inhibited primary tumor mass relative to the vehicle control while imatinib had no effect (Fig. 6e). Importantly, dasatinib appeared to inhibit the enhanced tumor growth associated with αvβ3 expression. While the incidence of metastasis to the hepatic hilar lymph node was relatively unchanged (dasatinib, 7/12; vehicle, 9/12; imatinib, 10/12) the size and extent of the metastatic lesions was significantly reduced (Fig. 6f,g).
Previous studies have linked αvβ3 expression or c-src activation with increased tumor cell migration and invasion27,31. While αvβ3-bearing cells were more migratory on both vitronectin and fibronectin (Supplementary Fig. 19a,c), αvβ3 failed to potentiate invasion of FG cells into Matrigel (data not shown). Interestingly, knock-down or pharmacological inhibition of c-src did not suppress the migration of either FG or FG-β3 cells (Supplementary Fig. 19a–c) despite inhibiting both anchorage-independent growth and metastasis (Fig. 5b and 6b). These findings demonstrate that αvβ3 recruitment and activation of c-src increases the malignant properties of pancreatic tumor cells without influencing their ability to migrate.
Discussion
Anchorage-independence is a hallmark of transformed cells and is suggested to play a role in the growth of solid tumors and survival of circulating tumor cells25,26. However, tumor cell adhesion and migration on extracellular matrix proteins, mediated by members of the integrin family, is linked to tumor cell growth and malignancy. Once ligated, integrins activate FAK and other downstream signaling molecules leading to anchorage-dependent survival and proliferation32,33. However, unligated integrins can negatively influence the malignant properties of tumor cells19-21 by activation of apoptotic pathways inducing a form of death known as IMD. Interestingly, the tumor cells studied here have developed mechanism(s) to escape IMD which contributes to their metastatic behavior22.
Integrin αvβ3 expression is linked to metastasis in several cancers including melanoma, as well as breast, prostate, cervical and pancreatic carcinomas11-18 and enhances tumor cell migration, survival and increased growth factor release27,34-38. Here, we present the unexpected result that integrin αvβ3 contributes to tumor progression and metastatic potential by enhancing anchorage-independent growth. This effect requires integrin αvβ3 recruitment and activation of c-src in a manner that is independent of tumor cell adhesion or the activation of FAK. Importantly, αvβ3 expression increases colony formation and cell survival in soft agar, even in the presence of a function-blocking antibody that prevents ligation to either soluble39 or immobilized27 ligands. In addition, expression of a mutant integrin incapable of binding ligand also showed increased anchorage-independence. A similar increase in cell survival was observed in αvβ3-bearing pancreatic tumors grown in mice, suggesting that αvβ3-mediated survival contributes to both anchorage-independence in vitro and tumor malignancy in vivo. Accordingly, knock-down of endogenous β3 decreased the anchorage-independence and metastasis of pancreatic cancer cells.
Surprisingly, integrin αvβ3 was found to promote c-src-dependent, but FAK independent, phosphorylation of the CAS substrate domain in non-adherent cells. CAS phosphorylation promotes adhesion-mediated cell survival10,40 through FAK and c-src recruitment to integrin containing focal contacts41. In fibroblasts transformed with v-src or v-crk, CAS forms complexes with these molecules in a phosphorylation-dependent manner42 and deletion of CAS prevents v-src mediated transformation43, implicating CAS in oncogenesis. Importantly, we demonstrate that both knock-down of CAS or expression of a non-phosphorylated form of CAS abolished the αvβ3/c-src-mediated colony formation in soft agar.
Anchorage-independence and tumor progression commonly result from oncogene expression. For example, the v-src oncogene potently stimulates anchorage-independent growth in fibroblasts44 and v-src is associated with enhanced cell invasion8. Expression of an activated mutant of c-src together with integrin αvβ3 promoted the transformation of a mouse pseudo-epithelial cell line, suggesting cooperativity between mutationally activate src and αvβ345,46. However, in some circumstances normal cellular derivatives of oncogenes, such as c-src, also contribute to tumor progression31 by stimulating cell migration and invasion. Here, we define a novel integrin-mediated pathway leading to activation of c-src, promoting increased anchorage-independence and tumor cell malignancy that does not impact cell migration.
Previous studies have shown that the platelet integrin αIIbβ3 can recruit and activate c-src in a manner that depends on the C-terminal portion of β3 cytoplasmic tail24. We show that αvβ3 expressing tumor cells also recruit and activate c-src and, similar to the platelet studies, a β3 truncation mutant (759x) prevented c-src recruitment to the integrin. Importantly, cells expressing this truncation mutant failed to increase anchorage-independent growth in vitro or metastasis in vivo. While c-src associates with integrin αvβ3, we could not detect c-src recruitment to other integrins in these cells suggesting that the β3 integrin is unique in this regard. Thus, we conclude that unligated αvβ3 and its ability to recruit c-src contributes to the malignant properties of pancreatic cancer suggesting αvβ3/c-src can function as an oncogenic unit thereby contributing to tumor malignancy.
Expression of αvβ3 is associated with the metastatic potential of several cancers 18,47,48. While antagonists of αvβ3 have proven efficacious as angiogenesis inhibitors in mouse tumor models49, and are now in phase III clinical trials in patients with glioblastoma, our studies suggest that direct inhibition of αvβ3 ligation on tumor cells may provide limited clinical benefit given that αvβ3 activates c-src in a ligand-independent manner. As such, we define a novel oncogenic signaling module comprised of unligated integrin αvβ3 and c-src that occurs in a subset of tumors resistant to IMD. Further, our study shows that dasatinib, a clinically approved SFK inhibitor, or c-src knock-down, not only blocked αvβ3-mediated anchorage-independent growth of pancreatic cancer cells in vitro but suppressed their metastatic properties in vivo. This suggests that c-src kinase inhibition may represent a therapeutic approach for those highly malignant tumors known to express integrin αvβ3.
Methods
Immunohistochemistry
We cut 8 μm sections from formalin-fixed, paraffin-embedded primary tumor specimens from eighteen human patients diagnosed with pancreatic ductal adenocarcinoma (7 with matching lymph node metastases). We also stained a breast cancer tissue microarray containing 50 matched pairs of primary tumor/lymph node metastases (Cat# BR1004; BioMax). We deparaffinized and digested the sections with proteinase K 15 min at room temperature prior to quenching with 0.3% H2O2/0.3% normal serum. After washing, we blocked the sections in normal serum and probed with 1:100 primary antibody overnight at 4 °C. We then incubated the sections for 45 min with a biotinylated secondary antibody (1:2,000) followed by 30 min in Vectastain Elite ABC Reagent (Vector Labs). Staining was performed with DAB substrate for 1–2 min prior to counterstaining with hematoxylin and mounting.
Orthotopic pancreatic tumors
Tumors were generated by injection of GFP-labeled human pancreatic carcinoma cells (1×106 tumor cells in 50 μl of sterile PBS) into the tail of the pancreas of 6–8 week old male nude mice. See Supplementary Methods for details regarding the generation of cell lines. After 6 or 8 weeks, we resected both the primary tumors and the hepatic hilar lymph nodes. Primary tumor mass was determined by measuring the wet weight of the resected tumors. We reported metastasis as the incidence of GFP-expressing cells present in the resected lymph nodes. For the dasatinib treatment experiment, 36 mice were injected with GFP-labeled FG-β3 cells and randomized into three groups of twelve. Tumors established for 2 weeks before beginning dosing. Mice were dosed by oral gavage with the citric acid vehicle (b.i.d.), 30 mg kg−1 dasatinib (b.i.d.) or 50 mg kg−1 imatinib (q.d.) for 4 weeks prior to harvest. All research was conducted under protocols approved by the UCSD animal subjects committee and is in accordance with the guidelines set forth in the NIH Guide for the Care and Use of Laboratory Animals.
In vivo apoptosis and proliferation
Analysis of both apoptosis and proliferation was performed on OCT-embedded frozen primary tumor sections. We assessed apoptosis in vivo by TUNEL staining using the ApopTag Red kit (Millipore). Proliferation was examined by immunofluorescent staining for Ki-67 using the manufacturer's instructions (Abcam). We measured both TUNEL and Ki-67 by capturing images from four 20× fields per tumor section and quantifying the number of stained cells using metamorph software. All data were normalized to total cell number (by co-staining with TOPRO-3 nuclear dye) and expressed as the percent TUNEL or Ki-67 positive cells per field.
Triton-soluble and -insoluble lysates
To isolate focal adhesions, serum-starved FG and FG-β3 cells were allowed to specifically adhere and spread for 2 h on dishes coated with 5 μg mL−1 fibronectin. Non-adherent cells are gently washed away with PBS and the remaining adherent cells were lysed in triton lysis buffer (50 mM Tris, pH 7.4, 150 mM NaCl, and 1% Triton X-100, 50 mM NaF, Protease inhibitor cocktail (Roche), 2 mM PMSF, 2mM sodium orthovanadate) to generate the triton-soluble lysate. The triton-insoluble lysate was prepared by washing the lysed cells twice with ice-cold PBS before adding RIPA lysis buffer (100 mM Tris pH 7.5, 150 mM sodium chloride, 0.1% deoxycholate, 0.1% SDS, 50 mM NaF, Protease inhibitor cocktail (Roche), 2 mM PMSF, 2mM sodium orthovanadate) and concentrating the lysate in a minimal volume.
Soft agar assays
We suspended cells in 0.3% agar/complete media and cultured them on a bottom layer of 1% agar/complete media in 48 or 24-well dishes. We then added additional media and cultured cells for 7–10 days prior to counting colonies consisting of at least 5 cells from 10× fields or whole wells. For dasatinib treatment experiments, colonies were grown in vehicle (DMSO), 50 nM, 250 nM or 1 μM dasatinib diluted in DMSO. We replaced the media with fresh inhibitor every other day. To knock-down CAS we transfected 5×106 FG or FG-β3 cells with 250 nM of control or CAS siRNA oligonucleotides (Qiagen) in 100 μL of Nucleofector V (Amaxa). We embedded the transfected cells in soft agar 48 h post-transfection.
Suspension viability
To directly assay for anchorage-independent survival and proliferation we cultured 1×106 FG or FG-β3 cells in suspension on 1% agar-coated wells in DMEM/10% FBS for 24 and 48 h prior to trypsinizing, staining with trypan blue and counting viable and non-viable cells on a hemocytometer.
Statistical analyses
All data, except the metastasis experiments, are presented as the mean±SEM and statistical differences were evaluated by Student's T-test. For metastasis, bars represent the incidence as a percentage of total mice and statistical evaluation was performed using Chi-square analysis. Colony formation in the presence of dasatinib was evaluated using a two-way repeated measure ANOVA to identify a positive interaction between the drug and the cell line. For all analyses, P < 0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
We wish to thank D. Stupack, L. Acevedo and S. Anand for critical reading of the manuscript. We also want to express our gratitude to M. Bouvet and A. Lowy for their help in obtaining human pancreatic tumor sections. J.S.D. was supported by an NIH Ruth L. Kirschstein National Research Service Award Post-doctoral Fellowship (grant CA123774). This work was supported by funding from the US National Institutes of Health grant numbers CA78045, CA45726, CA95262, CA129660 and HL57900 (to D.A.C.).
References
- 1.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 2.Schlaepfer DD, Mitra SK, Ilic D. Control of motile and invasive cell phenotypes by focal adhesion kinase. Biochim Biophys Acta. 2004;1692:77–102. doi: 10.1016/j.bbamcr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Roberts WG, et al. Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562,271. Cancer research. 2008;68:1935–1944. doi: 10.1158/0008-5472.CAN-07-5155. [DOI] [PubMed] [Google Scholar]
- 4.Pylayeva Y, et al. Ras- and PI3K-dependent breast tumorigenesis in mice and humans requires focal adhesion kinase signaling. The Journal of clinical investigation. 2009;119:252–266. doi: 10.1172/JCI37160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lahlou H, et al. Mammary epithelial-specific disruption of the focal adhesion kinase blocks mammary tumor progression. Proc Natl Acad Sci U S A. 2007;104:20302–20307. doi: 10.1073/pnas.0710091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eide BL, Turck CW, Escobedo JA. Identification of Tyr-397 as the primary site of tyrosine phosphorylation and pp60src association in the focal adhesion kinase, pp125FAK. Molecular and cellular biology. 1995;15:2819–2827. doi: 10.1128/mcb.15.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer cell. 2004;6:209–214. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Brabek J, et al. Crk-associated substrate tyrosine phosphorylation sites are critical for invasion and metastasis of SRC-transformed cells. Mol Cancer Res. 2005;3:307–315. doi: 10.1158/1541-7786.MCR-05-0015. [DOI] [PubMed] [Google Scholar]
- 9.Klemke RL, et al. CAS/Crk coupling serves as a “molecular switch” for induction of cell migration. J Cell Biol. 1998;140:961–972. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho SY, Klemke RL. Extracellular-regulated kinase activation and CAS/Crk coupling regulate cell migration and suppress apoptosis during invasion of the extracellular matrix. J Cell Biol. 2000;149:223–236. doi: 10.1083/jcb.149.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albelda SM, et al. Integrin distribution in malignant melanoma: association of the beta 3 subunit with tumor progression. Cancer research. 1990;50:6757–6764. [PubMed] [Google Scholar]
- 12.Hsu MY, et al. Adenoviral gene transfer of beta3 integrin subunit induces conversion from radial to vertical growth phase in primary human melanoma. The American journal of pathology. 1998;153:1435–1442. doi: 10.1016/s0002-9440(10)65730-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCabe NP, De S, Vasanji A, Brainard J, Byzova TV. Prostate cancer specific integrin alphavbeta3 modulates bone metastatic growth and tissue remodeling. Oncogene. 2007;26:6238–6243. doi: 10.1038/sj.onc.1210429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takayama S, et al. The relationship between bone metastasis from human breast cancer and integrin alpha(v)beta3 expression. Anticancer research. 2005;25:79–83. [PubMed] [Google Scholar]
- 15.Sloan EK, et al. Tumor-specific expression of alphavbeta3 integrin promotes spontaneous metastasis of breast cancer to bone. Breast Cancer Res. 2006;8:R20. doi: 10.1186/bcr1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felding-Habermann B, et al. Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci U S A. 2001;98:1853–1858. doi: 10.1073/pnas.98.4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gruber G, et al. Correlation between the tumoral expression of beta3-integrin and outcome in cervical cancer patients who had undergone radiotherapy. Br J Cancer. 2005;92:41–46. doi: 10.1038/sj.bjc.6602278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hosotani R, et al. Expression of integrin alphaVbeta3 in pancreatic carcinoma: relation to MMP-2 activation and lymph node metastasis. Pancreas. 2002;25:e30–35. doi: 10.1097/00006676-200208000-00021. [DOI] [PubMed] [Google Scholar]
- 19.Giancotti FG, Ruoslahti E. Elevated levels of the alpha 5 beta 1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell. 1990;60:849–859. doi: 10.1016/0092-8674(90)90098-y. [DOI] [PubMed] [Google Scholar]
- 20.Varner JA, Emerson DA, Juliano RL. Integrin alpha 5 beta 1 expression negatively regulates cell growth: reversal by attachment to fibronectin. Molecular biology of the cell. 1995;6:725–740. doi: 10.1091/mbc.6.6.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stupack DG, Puente XS, Boutsaboualoy S, Storgard CM, Cheresh DA. Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J Cell Biol. 2001;155:459–470. doi: 10.1083/jcb.200106070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stupack DG, et al. Potentiation of neuroblastoma metastasis by loss of caspase-8. Nature. 2006;439:95–99. doi: 10.1038/nature04323. [DOI] [PubMed] [Google Scholar]
- 23.Ginsberg MH, Partridge A, Shattil SJ. Integrin regulation. Current opinion in cell biology. 2005;17:509–516. doi: 10.1016/j.ceb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Arias-Salgado EG, et al. Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc Natl Acad Sci U S A. 2003;100:13298–13302. doi: 10.1073/pnas.2336149100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsatsanis C, Spandidos DA. Oncogenic kinase signaling in human neoplasms. Annals of the New York Academy of Sciences. 2004;1028:168–175. doi: 10.1196/annals.1322.019. [DOI] [PubMed] [Google Scholar]
- 26.Simpson CD, Anyiwe K, Schimmer AD. Anoikis resistance and tumor metastasis. Cancer letters. 2008 doi: 10.1016/j.canlet.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 27.Leavesley DI, Ferguson GD, Wayner EA, Cheresh DA. Requirement of the integrin beta 3 subunit for carcinoma cell spreading or migration on vitronectin and fibrinogen. J Cell Biol. 1992;117:1101–1107. doi: 10.1083/jcb.117.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyauchi A, et al. Recognition of osteopontin and related peptides by an alpha v beta 3 integrin stimulates immediate cell signals in osteoclasts. J Biol Chem. 1991;266:20369–20374. [PubMed] [Google Scholar]
- 29.Loftus JC, et al. A beta 3 integrin mutation abolishes ligand binding and alters divalent cation-dependent conformation. Science. 1990;249:915–918. doi: 10.1126/science.2392682. [DOI] [PubMed] [Google Scholar]
- 30.Shin NY, et al. Subsets of the major tyrosine phosphorylation sites in Crk-associated substrate (CAS) are sufficient to promote cell migration. J Biol Chem. 2004;279:38331–38337. doi: 10.1074/jbc.M404675200. [DOI] [PubMed] [Google Scholar]
- 31.Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer metastasis reviews. 2003;22:337–358. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- 32.Reddig PJ, Juliano RL. Clinging to life: cell to matrix adhesion and cell survival. Cancer metastasis reviews. 2005;24:425–439. doi: 10.1007/s10555-005-5134-3. [DOI] [PubMed] [Google Scholar]
- 33.Westhoff MA, Serrels B, Fincham VJ, Frame MC, Carragher NO. SRC-mediated phosphorylation of focal adhesion kinase couples actin and adhesion dynamics to survival signaling. Molecular and cellular biology. 2004;24:8113–8133. doi: 10.1128/MCB.24.18.8113-8133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uhm JH, Dooley NP, Kyritsis AP, Rao JS, Gladson CL. Vitronectin, a glioma-derived extracellular matrix protein, protects tumor cells from apoptotic death. Clin Cancer Res. 1999;5:1587–1594. [PubMed] [Google Scholar]
- 35.De S, et al. VEGF-integrin interplay controls tumor growth and vascularization. Proc Natl Acad Sci U S A. 2005;102:7589–7594. doi: 10.1073/pnas.0502935102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matter ML, Ruoslahti E. A signaling pathway from the alpha5beta1 and alpha(v)beta3 integrins that elevates bcl-2 transcription. J Biol Chem. 2001;276:27757–27763. doi: 10.1074/jbc.M102014200. [DOI] [PubMed] [Google Scholar]
- 37.Bao W, Stromblad S. Integrin alphav-mediated inactivation of p53 controls a MEK1-dependent melanoma cell survival pathway in three-dimensional collagen. J Cell Biol. 2004;167:745–756. doi: 10.1083/jcb.200404018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scatena M, et al. NF-kappaB mediates alphavbeta3 integrin-induced endothelial cell survival. J Cell Biol. 1998;141:1083–1093. doi: 10.1083/jcb.141.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Charo IF, Nannizzi L, Smith JW, Cheresh DA. The vitronectin receptor alpha v beta 3 binds fibronectin and acts in concert with alpha 5 beta 1 in promoting cellular attachment and spreading on fibronectin. J Cell Biol. 1990;111:2795–2800. doi: 10.1083/jcb.111.6.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cabodi S, et al. p130Cas as a new regulator of mammary epithelial cell proliferation, survival, and HER2-neu oncogene-dependent breast tumorigenesis. Cancer research. 2006;66:4672–4680. doi: 10.1158/0008-5472.CAN-05-2909. [DOI] [PubMed] [Google Scholar]
- 41.Bouton AH, Riggins RB, Bruce-Staskal PJ. Functions of the adapter protein Cas: signal convergence and the determination of cellular responses. Oncogene. 2001;20:6448–6458. doi: 10.1038/sj.onc.1204785. [DOI] [PubMed] [Google Scholar]
- 42.Sakai R, et al. A novel signaling molecule, p130, forms stable complexes in vivo with v-Crk and v-Src in a tyrosine phosphorylation-dependent manner. Embo J. 1994;13:3748–3756. doi: 10.1002/j.1460-2075.1994.tb06684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Honda H, et al. Cardiovascular anomaly, impaired actin bundling and resistance to Src-induced transformation in mice lacking p130Cas. Nature genetics. 1998;19:361–365. doi: 10.1038/1246. [DOI] [PubMed] [Google Scholar]
- 44.Jove R, Hanafusa H. Cell transformation by the viral src oncogene. Annual review of cell biology. 1987;3:31–56. doi: 10.1146/annurev.cb.03.110187.000335. [DOI] [PubMed] [Google Scholar]
- 45.Huveneers S, et al. Integrin alpha v beta 3 controls activity and oncogenic potential of primed c-Src. Cancer research. 2007;67:2693–2700. doi: 10.1158/0008-5472.CAN-06-3654. [DOI] [PubMed] [Google Scholar]
- 46.Huveneers S, Arslan S, van de Water B, Sonnenberg A, Danen EH. Integrins uncouple Src-induced morphological and oncogenic transformation. J Biol Chem. 2008;283:13243–13251. doi: 10.1074/jbc.M800927200. [DOI] [PubMed] [Google Scholar]
- 47.Nip J, Shibata H, Loskutoff DJ, Cheresh DA, Brodt P. Human melanoma cells derived from lymphatic metastases use integrin alpha v beta 3 to adhere to lymph node vitronectin. The Journal of clinical investigation. 1992;90:1406–1413. doi: 10.1172/JCI116007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Allan AL, et al. Role of the integrin-binding protein osteopontin in lymphatic metastasis of breast cancer. The American journal of pathology. 2006;169:233–246. doi: 10.2353/ajpath.2006.051152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reinmuth N, et al. Alphavbeta3 integrin antagonist S247 decreases colon cancer metastasis and angiogenesis and improves survival in mice. Cancer research. 2003;63:2079–2087. [PubMed] [Google Scholar]
- 50.Vezeridis MP, et al. Heterogeneity of potential for hematogenous metastasis in a human pancreatic carcinoma. The Journal of surgical research. 1990;48:51–55. doi: 10.1016/0022-4804(90)90145-r. [DOI] [PubMed] [Google Scholar]
- 51.Ricono JM, et al. Specific cross-talk between epidermal growth factor receptor and integrin alphavbeta5 promotes carcinoma cell invasion and metastasis. Cancer research. 2009;69:1383–1391. doi: 10.1158/0008-5472.CAN-08-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.