Abstract
Dopamine signaling is involved in a number of brain pathways and its disruption has been suggested to be involved in the several disease states, including Parkinson’s disease (PD), schizophrenia, and attention deficit hyperactivity disorder (ADHD). It has been hypothesized that altered storage, release, and reuptake of dopamine contributes to both the hypo- and hyperdopaminergic states that exist in various diseases. Here we use our recently described mathematical model of dopamine metabolism, combined with a comprehensive Monte Carlo simulation analysis, to identify key determinants of dopamine metabolism associated with the dysregulation of dopamine homeostasis that may contribute to the pathogenesis of dopamine-based disorders. Our model reveals that the dopamine transporter (DAT), the vesicular monoamine transporter (VMAT2), and the enzyme monoamine oxidase (MAO) are the most influential components controlling the synaptic level of dopamine and the formation of toxic intracellular metabolites. The results are consistent with experimental observations and point to metabolic processes and combinations of processes that may be biochemical drivers of dopamine neuron degeneration. Since many of the identified components can be targeted therapeutically, the model may aid in the design of combined therapeutic regimens aimed at restoring proper dopamine signaling with toxic intermediates under control.
Keywords: Biochemical Systems Theory, dopamine metabolism, dopamine nerve terminal, Monte Carlo method, metabolic profile, Parkinson’s disease, personalized medicine
Introduction
Dopamine plays a key role as a physiologically, pathologically and pharmacologically crucial neurotransmitter in a variety of disease states, such as Parkinson’s disease, schizophrenia, and attention deficit hyperactivity disorder (ADHD) (Carlsson, 1978; Carlsson, 1988; Graham, 1978; Levy, 1991; Stokes et al., 1999; Swanson et al., 2007). Many of the symptoms of the disease result from altered dopamine signaling. In Parkinson’s disease the hypodopaminergic state is primarily due to the loss of the dopamine producing neurons in the substantia nigra pars compacta, while in schizophrenia and ADHD there appears to be a hyperdopaminergic state. It has been hypothesized that altered handling and storage of dopamine contributes both the hypo- and hyperdopaminergic states in these disorders. Correspondingly, many current treatments attempt to restore dopamine function through either activation or inhibition of various dopamine pathways.
Dopamine metabolism is located primarily in the presynaptic neuron and synaptic cleft, where dopamine synthesis, degradation, compartmentalization, release, reuptake, and numerous regulating mechanisms occur. There are very many biological components and processes involved, and that these components and processes are highly interactive. In the context of the whole system, these components and processes have qualitatively and/or quantitatively different contribution to dopamine signal, under normal status and its dysfunction. Identification of key determinants of dopamine metabolism associated with the dysregulation of dopamine homeostasis may improve our understanding of dopamine-based disorders. However, this type of complex analysis eludes the unaided mental capacities of the human brain and can only be preformed reliably with mathematical and computational models that permit the tracking of hundreds of variables and the quantitative characterization of their responses.
Recently we developed, implemented, tested, and validated a computational model of dopamine metabolism (Qi et al., 2008a; Qi et al., 2008b) which may enhance our understanding of the complexity of the disease. In this article, we utilize our earlier model of dopamine metabolism further with the goal of identifying key determinants within dopaminergic terminal and synaptic cleft, and their combinations, for controlling synaptic dopamine signals. Specifically, we describe a large-scale simulation study that addresses singular, dual, and triple perturbations in the dopamine network and reveals their individual and combined consequences on dopamine itself and on toxic species that emerge in the metabolic system under adverse conditions. The simulations yield population averages, as well as “personalized scenarios,” where rare, unfortunate combinations of factors lead to changes in metabolic profile that may have implications in dopamine-based disorders.
Materials and Methods
Mathematical Model Structure
The model for our simulation studies of dopamine metabolism is based on Biochemical Systems Theory (BST) (Savageau, 1969a; Savageau, 1969b; Savageau, 1970). The main rationale for choosing BST is that it permits model design and implementation, as well as mathematical analyses and simulations, under a minimal set of quantitative assumptions. BST has been described and reviewed numerous times, and detailed reviews are available (Alvarez-Vasquez et al., 2005; Savageau, 1976; Torres and Voit, 2002; Voit, 1991; Voit, 2000a; Voit, 2000b). The present study is based specifically on a recent BST model of dopamine metabolism (Qi et al., 2008a; Qi et al., 2008b). This model was developed in the format of a Generalized Mass Action (GMA) system, in which each biochemical process or transport step is represented as a product of power-law functions. These products include dependent state variables, representing key components like dopamine, as well as independent variables that represent enzymes and environmental factors, which can be manipulated mathematically to mimic disease related deficiencies, risk factors, and lifestyle choices. Simulation studies reveal the consequences of such manipulations, either one at a time, or in arbitrary combinations. Each power-law term also contains a rate constant γ, which characterizes the turnover rate of a process between pools or variables, and kinetic orders f, each of which reflects the strength of effect that the associated variable has on a given process. The rate constants and kinetic orders constitute the baseline parameter sets for the simulations. Our current computational model of dopamine metabolism contains over 200 independent variables and parameters, which render the simulating task a considerable challenge.
Monte Carlo Simulation
It is standard routine to diagnose a BST model with logarithmic gain (log gain) and parameter sensitivity analyses. Log gains measure the percent change in the steady-state value of a metabolite or flux that is due to a 1% change in an independent variable. Strictly speaking, log gains refer to infinitesimally small, single perturbations, and while these often translate into valid predictions of larger changes, there is no guarantee that these predictions are correct in GMA systems. The same is true for sensitivity analyses, which quantify the effects of infinitesimally small changes in parameter values. Because larger, and possibly combined deviations from the normal metabolic state are of relevance in disease development, we decided to complement the gain and sensitivity analyses with simulations of larger deviations.
A fully comprehensive analysis of this type is not feasible. Even if only three increased and three decreased values for each parameter or independent variable were to be assessed, 6200 simulations would have to be executed, and this number would overwhelm the capacity even of a very large computer. Instead, we decided to use the strategy of a Monte Carlo simulation. Different from deterministic simulation methods, the Monte Carlo method is stochastic and utilizes (pseudo-) random numbers. Specifically, values for each parameter are randomly sampled from a statistical distribution that mimics the known or alleged variations of this parameter within human populations. The sampled values for all parameters of interest are entered into the model, while all unaltered parameters are retained at their normal baseline values, and the dynamics of the system toward some final (steady) state is numerically computed. This solution constitutes one iteration within the simulation study, which typically consists of thousands or millions of such iterations. In this fashion, single, double, and triple perturbations can be assessed in a representative, although not exhaustive fashion, allowing one, two, or three parameters to vary (simultaneously) from the norm. The results are collected and interpreted in terms of likely, most likely or extreme scenarios.
Specifically, using our dopamine model with parameters reflecting the normal state of the pathway system, we successively altered independent variables and parameter values that characterize constituents or magnitudes of metabolic fluxes, and recorded system responses resulting from these variations that could possibly lead to some state of disease. Subsequently screening for disease states, among the many “harmless” combinations of independent variables and parameter values, we were able to associate disease with the most detrimental combinations of perturbations. The key determinants of disease or dopamine dysfunction emerging from these perturbation studies could be considered possible biomarkers for diagnosis and candidate targets for drug treatment. Furthermore, the studies allow the classification of patients into subgroups and to assess the prevalence of these subgroups.
True distributions for parameter values of the dopamine system are not known. As a simple, yet probably relatively representative default, we therefore used triangular distributions with optimal values corresponding to the normal physiology and extremes at about 60% below and 170% above these values (see Supplements for details of the minimum and maximum setting and technical issues). The triangular distribution accounts for a high likelihood of values close to the normal steady state and for decreasing probabilities of stronger variations, which is probably representative of reality.
Our current model of dopamine metabolism contains over 200 independent variables and parameters, which include 35 enzyme activities and environmental factors, 114 kinetic orders, and 66 rate constants. Targeting these independent variables and parameters, we designed three simulation scenarios to account for single-site variations as well as possibly synergistic multi-site changes. First, only one site was selected and randomly sampled from its triangular distribution. This scenario simulates the situation of a single, relevant genetic deficit or the exposure to a toxin. For the second scenario we simultaneously altered two different sites, representing, for instance, exposure of a genetically predisposed population to a toxin, or a general population exposed to one or more toxins that affect two sites simultaneously; a specific example is methamphetamine, which can cause dopamine in vesicles leak into cytosol via VMAT2 and into synaptic cleft through DAT (Hanson et al., 2004; McCann et al., 1998). Finally, in the same vein, we performed simulations addressing a scenario where three different sites are affected simultaneously. This situation might correspond to a rather rare pathogenic mechanism in humans, but was included as a means of searching for potential drug target combinations that may be useful in disease, such as PD.
Selection of Response Variables
To represent different abnormal metabolic states of dopamine neurons associated with altered dopamine function, we selected as indicators the following primary metabolites, toxic species, reactive oxygen species, and reactive nitrogen species, which are highly of disease relevance within dopamine metabolism: extracellular dopamine (DA-e), total dopamine (DA), HO•, •NO2, dopamine quinone (DA-Q), and DOPAC quinone (DOPAC-Q). Extracellular dopamine is the actual signal carrier for movement control. The specific role of total dopamine is unclear, but it greatly decreases during the degeneration of dopaminergic neurons in the brains of PD patients, and thus is a major pathological feature of PD. Historically, the treatment of PD has been based upon attempts to restore dopamine function. However, more recently the underlying degeneration of dopaminergic neurons has been suggested to be due to the generation of toxic species and oxidative stress caused by abnormal dopamine metabolism (Graham, 1978; Stokes et al., 1999). Hence, toxic species and oxidative stress markers were also included as potential disease indicators in this simulation study.
Commonly, relative changes in one dependent variable (metabolite) are measured as consequences of predefined relative changes in independent variables (enzyme activities, risk factors), and the types of correlations between causes and effects, which may be linear or nonlinear, are often ignored. In order to compare effects of changes in different independent variables or parameters with different magnitudes of manipulations, which are due to the large ranges of variations within the chosen triangular distributions, we used ratios of relative changes in a dependent variable to relative changes in an independent variable as indicators of the significance of each variation. For multiple perturbations involving several relative changes in independent variables, possibly with different signs, we used the Euclidean distance as the denominator for the computation of modified ratios.
Results
Because the complete set of results, representing all independent variables and parameters, is very large, we only present findings related to enzyme activities and environmental factors (for a list of these targets under perturbations, see Supplement Table S1).
Single Perturbations
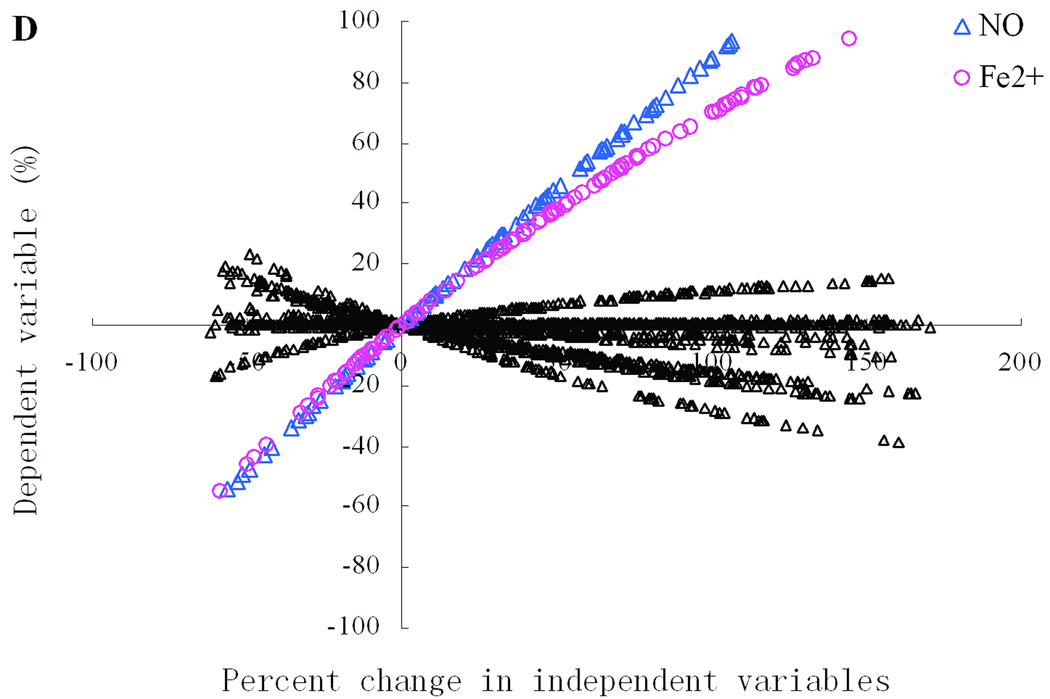
Figure 1 shows the systemic responsiveness of dopamine metabolism to variations in a single enzyme or environmental variable. The x-axis represents relative changes in independent variables, while the y-axis shows corresponding relative changes in dependent variables. At the intersection of each slightly curved line of data points with the y-axis, its slope corresponds to the logarithmic gain (“log gain”), which is an finite quantity often used to characterize the effect of a change in an independent variable on a dependent variable (Savageau, 1976; Voit, 2000b). According to our setting of minimum and maximum of the triangular distribution, predefined relative changes in independent variables are between−60% and 170%. Based on prior experience with gain analyses in general and in the context of our dopamine model (Qi et al., 2008a), we choose ±0.5 as thresholds for significance and assigned distinct symbols and colors to those series with log gains exceeding the thresholds in magnitude.
Figure 1. Efficiency of variations in an enzyme or an environmental variable on changing level of dopamine, hydroxyl radical, nitrogen dioxide, and toxic species.
| A: DA-e | B: total dopamine (DA) |
| C: HO• | D: •NO2 |
| E: DA-Q | F: DOPAC-Q |
The X-axis shows relative changes in an independent variable, while the Y-axis is relative changes in a dependent variable.
According to our setting of minimum and maximum of the triangular distribution, relative changes in independent variables are between −60 and 170 (%). Distinct symbols and colors were used for those series with ratios of relative change across one of the thresholds. A list of enzymes and environmental substances as well as their abbreviations are presented in Supplement Tables S1. Significant independent variables and their abbreviations are: monoamine oxidase (MAO), vesicular monoamine transporter 2 (VMAT2), dopamine transporter (DAT), catalase (CAT), glutathione peroxidase (GPx), Glutathione (GSH), Iron cation (Fe2+), superoxide dismutase (SOD), S-Adenosyl-L-methionine (SAM), • NO (NO), catechol O-methyltransferase (COMT).
For one-site manipulations, most relationships between changes in dependent and independent variables are close to zero (Fig. 1), which indicates that even large, singular changes do not necessarily move the dopamine pathway out of its normal operational range. In fact, the observation that the vast majority of these relationships are close to zero is a sign of inherent robustness of the model. For DA-e (Fig. 1A), only monoamine oxidase (MAO), vesicular monoamine transporter 2 (VMAT2), and dopamine transporter (DAT) produce effects above the preset thresholds. Among these components, only VMAT2 has positive effects, which implies that changes in VMAT2 level will cause changes in DA-e level in the same direction, while changes in one of the other two variables have inverse effects on extracellular dopamine level. In other words, to increase the amount of DA-e in the synaptic cleft, one could either activate VMAT2 or inhibit MAO or DAT, and these actions would constitute the most efficient means of increasing DA-e among all possible single-site manipulations. Interestingly, DA exhibits responses very similar to those in DA-e, but the effects exerted by DAT manipulations are no longer significant (Fig. 1B).
Oxidative stress and toxic species have been implicated as important causes of the degeneration of dopamine neurons. The effects on relative changes of HO•, •NO2, DA-Q, and DOPAC-Q from single-site mutations are shown in Figures 1C–F, respectively. HO• exhibits the most significant positive effects with respect to iron cation, moderate positive effects with respect to MAO and DAT, and moderate negative effects with respect to catalase (CAT), glutathione peroxidase (GPx), Glutathione (GSH), and VMAT2. As important components of cell defense system, CAT, GPx, and GSH show a similar capability of scavenging highly reactive hydroxyl radicals as VMAT2, which is a member of the toxin-extruding antiporter gene family. Similarly, iron cation cause significant positive ratios in •NO2, and this effect is almost the same as that from •NO, which is one of the main sources of •NO2. Iron cation also has a very significant positive effect on DA-Q, while DAT has a similar but much weaker effect. DA-Q is also characterized by moderate negative effects with respect to MAO, S-adenosyl-L-methionine (SAM), catechol O-methyltransferase (COMT), and VMAT2. In contrast to the negative effects on DA-Q, MAO positively influences the toxic species DOPAC-Q, although with smaller magnitudes.
Double Perturbations
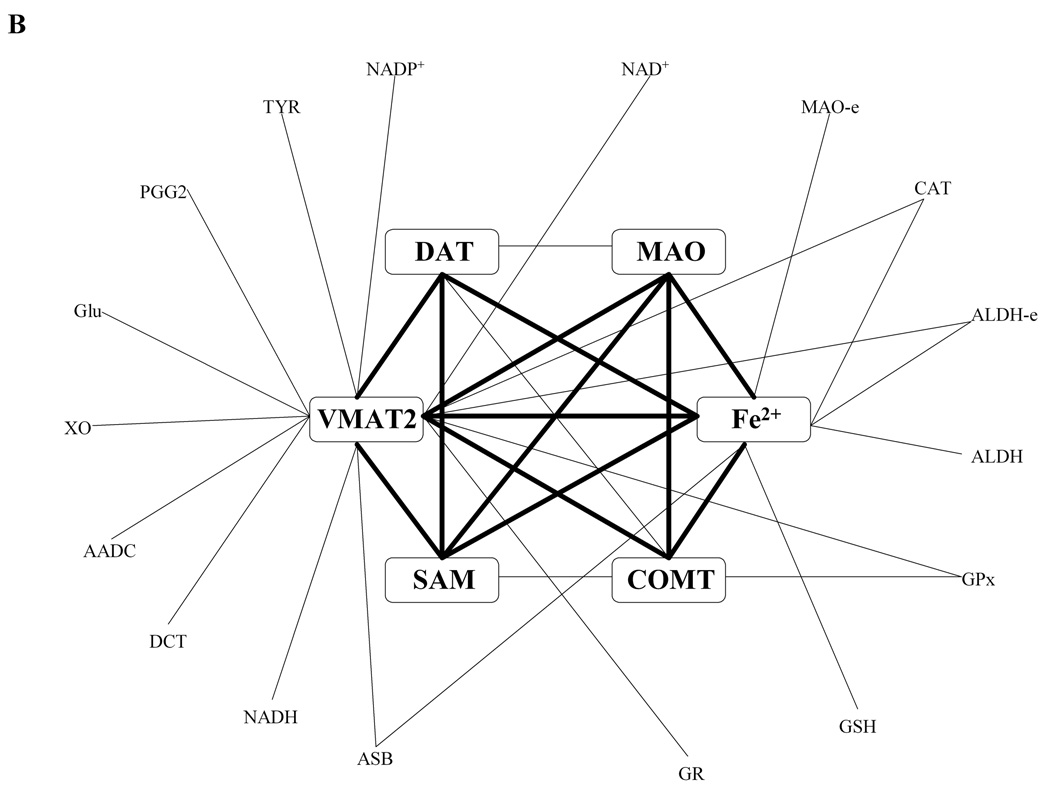
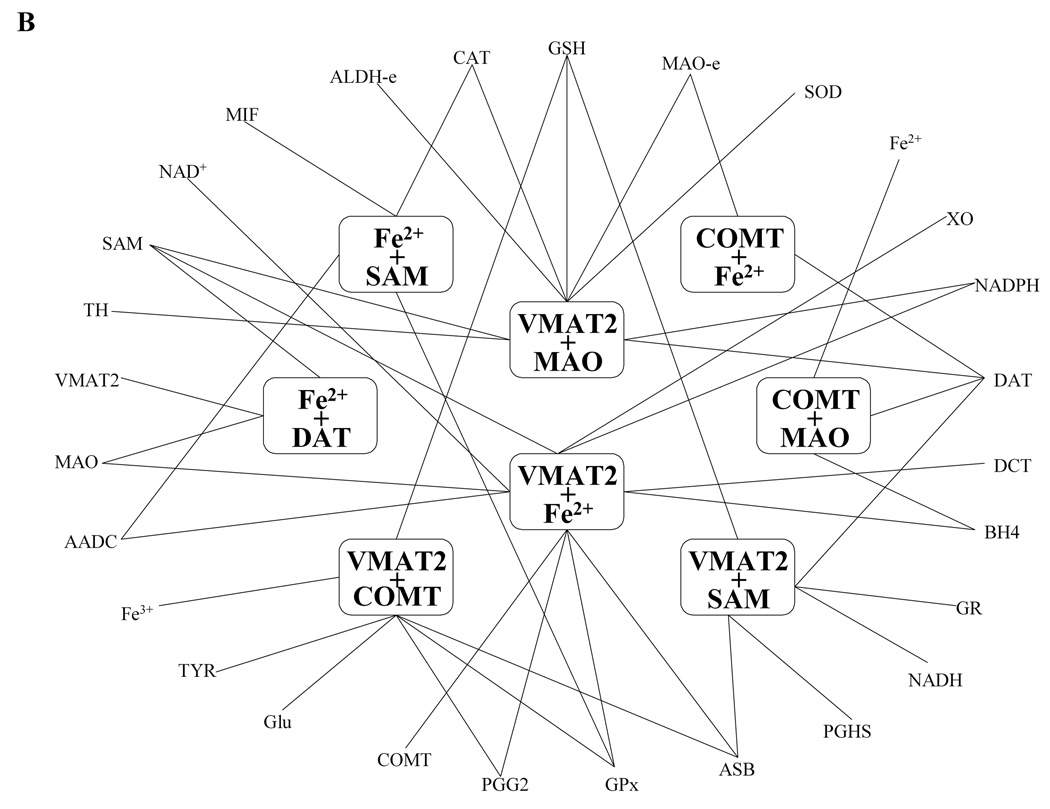
Allowing for the possibility of synergism or antagonism of effects of alterations in enzymes or environmental factors, we investigated two simultaneous perturbations. For fairness of comparisons with single perturbations, we doubled the thresholds for significant combined ratios to ±1.0. Because log gain curves are not feasible for two-site changes, we display a set of significant results, screened out from very many two-site connections, in the format of a network graph. The network for DA-e clearly shows three hot spots (Fig. 2A), namely VMAT2, DAT, and MAO. Maybe not surprisingly, these hot spots were already identified as significant components in the corresponding one-site scenario. Thus, in order to increase the level of DA, the most efficient combinations according to the model analysis are: SAM with MAO; prostaglandin G/H synthase with MAO; xanthine oxidase with MAO; aldehyde dehydrogenase with MAO; MAO with •NO; and MAO with VMAT2. The results clearly reflect the extraordinary importance of MAO for the tissue dopamine level.
Figure 2. Graph of significant two-site combinations.
Two-site combinations of alterations that most significantly affect the extracellular dopamine level (DA-e; panel A) and the level of dopamine quinone (DA-Q; panel B) form network graphs with each edge represents a combination and each vertex represents a site in these connections. These graphs identify relatively small, highly connected “hubs.” In the case of DA-e, the hub consists of VMAT2, DAT and MAO; pairs of these (red lines) affect DA-e much more strongly than any of the other combinations. In the case of DA-Q, the hubs also contain SAM, COMT, and iron cation. In both cases, every significant combination uses one of these hubs as a necessary vertex.
Corresponding to the effect of iron cation on HO•, as detected in the one-site scenario above, iron cation forms a unique network hub in the network of significant two-site connections for HO• (see Supplement Fig. S1). In other words, all significant two-site combinations require iron cation as a necessary node. The reason is presumably that the highly reactive hydroxyl radical is generated from iron cation and hydrogen peroxide by the Fenton reaction. With respect to •NO2, the significant two-site combinations are: CAT with •NO; Superoxide dismutase (SOD) with •NO; SOD with iron cation; GPx with •NO; •NO with GSH; •NO with ascorbate; and •NO with iron cation.
The network for the toxic species DA-Q identifies iron cation and VMAT2 as hot spots (Fig. 2B). In addition, MAO, COMT, DAT, and SAM form local hubs. Each significant two-site combination has to connect to one of these hubs, which again echoes their significant roles among the one-site alterations. Under the given setting of thresholds, we did not discover any two-site combination with a significant effect on DOPAC-Q, which may imply that this toxic species is difficult to manipulate or control.
The Euclidean distance is useful for screening significant combinations of multiple sites. However, it does not take into account the direction of a manipulation, which may be activating or inhibiting. To alleviate this problem, we performed investigations of systemic responses under different combinations of manipulations, differentiating activation and inhibition for each significant two-site combination, and compared the results qualitatively and quantitatively. As the most pertinent example, we describe directed two-site combinations between MAO and VMAT2 and between COMT and DAT.
Figure 3 illustrates synergisms and antagonisms with scatter plots of DA-e, HO•, and DA-Q in response to directed two-site manipulations; Supplement Figure S2 shows scatter plots associated with DA, •NO2, and DOPAC-Q. The relative changes in each independent variable are limited to ±50% ranges, so that even relatively limited sample sizes are sufficient to cover the space of manipulations with high density. The results show that the most elevated levels of DA-e appear in the 2nd quadrant for the combination MAO with VMAT2 and in the 3rd quadrant for the COMT with DAT combination. Roughly in North-West direction, which is to be interpreted as simultaneous down-regulation of MAO and up-regulation of VMAT2, DA-e levels change from −60% to close to zero at the center and to over +80%. In other words, 40% inhibition of MAO combined with 50% activation of VMAT2 essentially doubles the DA-e level. For the combination of COMT with DAT, the direction is roughly South-West, and the relative effects on DA-e are smaller. These findings quantify the antagonistic effects between MAO and VMAT2 and the synergistic effect between COMT and DAT on the DA-e level.
Figure 3. Scatter plots of extracellular dopamine, hydroxyl radicals, and dopamine quinone in response to manipulations of MAO with VMAT2 (left column) and COMT with DAT (right column).
Each axis is relative changes in one of the manipulated sites within a range of ±50%. The color of each of the 40,000 data points represents the resulting relative change in one of the affected metabolites, as indicated.
For the highly reactive hydroxyl radical, the combination of MAO with VMAT2 has a roughly South-East direction for increasing this toxic species. Along this direction, HO• decreases under the COMT with DAT combination, indicating that VMAT2 and COMT are both negatively correlated with hydroxyl radical level, while MAO and DAT exhibit positive correlations. For the toxic DA-Q, the same sets of combined manipulations show South-West and North-West directions of elevation, respectively. In contrast to the positive relationship with HO•, MAO has a negative correlation with DA-Q. The other three independent variables have the same type of relationships with DA-Q as they do with HO•.
To summarize the most important results of double perturbations, we find that activating VMAT2 combined with inhibiting MAO seems to form a strategy for significantly increasing DA-e level, while at the same time alleviating highly reactive hydroxyl radical and controlling toxic DA-Q. Moreover, inhibition of both COMT and DAT leads to an increased DA-e level and concomitantly keeps hydroxyl radical and toxic DA-Q under control. It seems that the combination of MAO with VMAT2 is superior in terms of the efficiency of increasing the DA-e level and the advantage of removing highly reactive hydroxyl radical.
Triple Perturbations
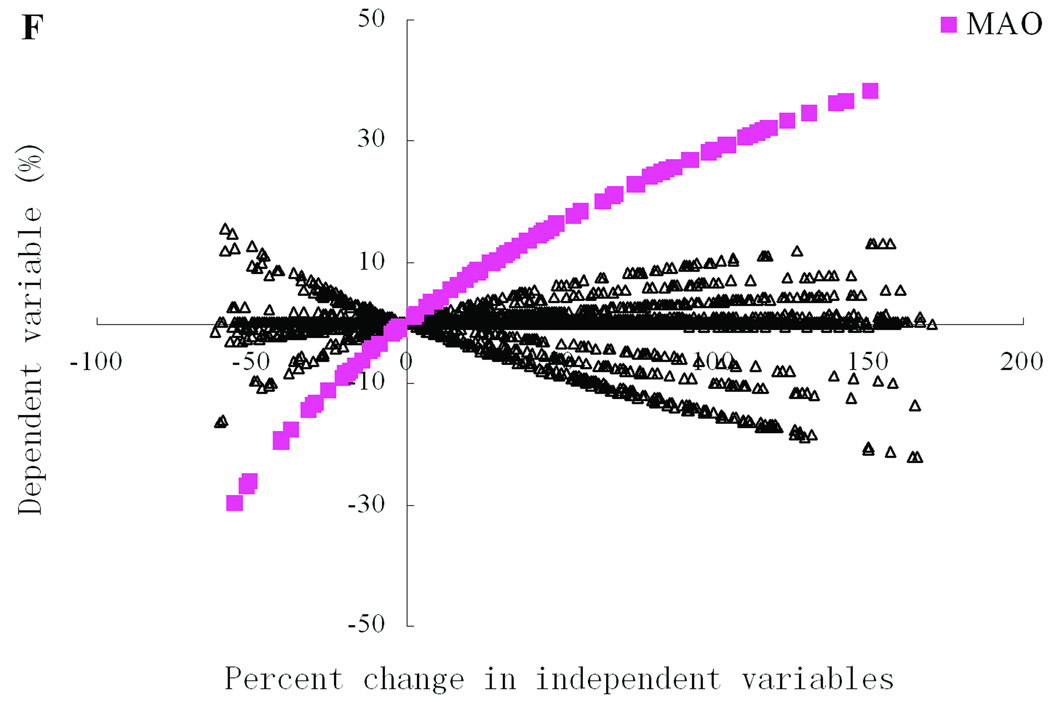
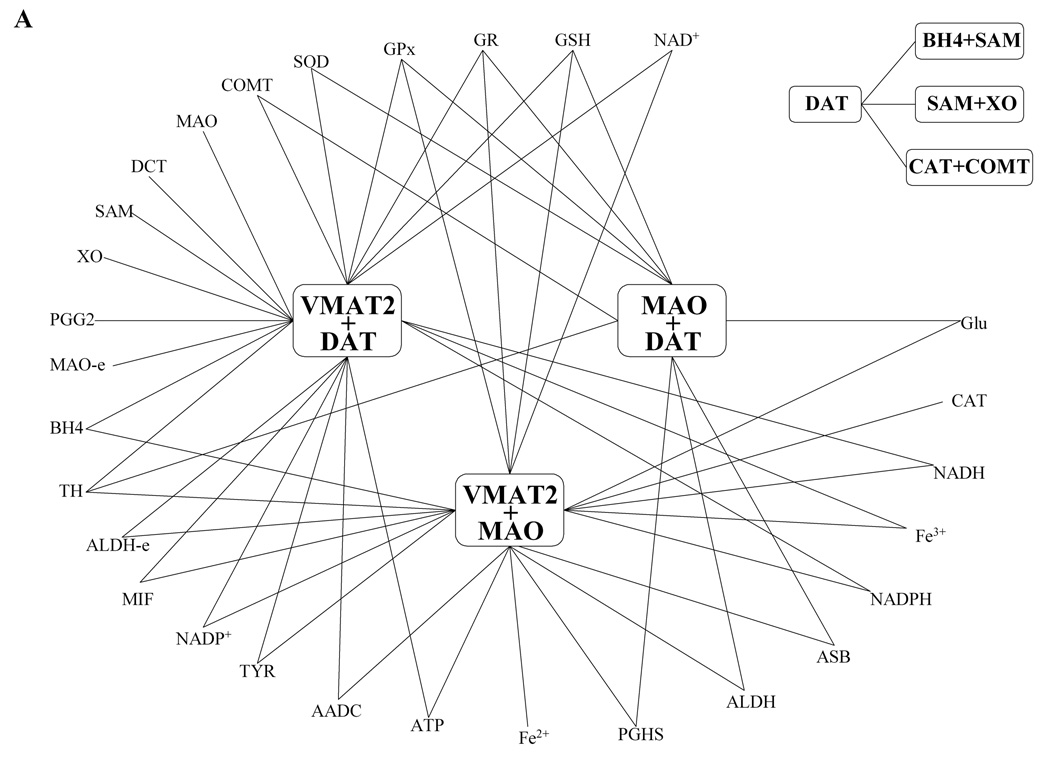
Simulations of three simultaneous variations were performed in a manner similar to double perturbations, and significant three-site combinations were screened out for further analysis and display (Fig. 4A–B). Accounting for the three-fold change, we set the thresholds of significance here to ±1.5. Preliminary analysis indicated that the combinations of VMAT2, DAT, and MAO, which had stood out as hubs for significant two-site combinations in the previous section, were also crucial in three-way perturbations. Therefore, in order to exhibit the networks of significant triple combinations as clearly as possible, we used the combinations VMAT2 + DAT, MAO + DAT, and MAO + VMAT2 as hubs (Figure 4A). Further analysis revealed a clique, which includes connections between DAT and three different two-site combinations including SAM + tetrahydrobiopterin, SAM + xanthine oxidase, and CAT + COMT. These three triple combinations all include one of the significant two-site combinations identified by previous scenario. Because no new significant combinations emerge, it is reasonable to assume that even greater combinations might follow a similar pattern that includes hot spots and cliques from “lower levels.” If so, the resultant hierarchy would implicate a substantial reduction in the space to be searched by multiple-site scenarios.
Figure 4. Graph of significant three-site combinations.
| A: DA-e | B: DA-Q |
The most frequent two-site hubs (see Figure 2) are contained in three-site hubs. In the network graphs, each edge represents one of these significant combinations and each vertex represents a site or a two-site combination. Every significant connection uses one of these hubs as a necessary vertex.
For toxic DA-Q (Fig. 4B), there are eight hubs including MAO + VMAT2, Iron + VMAT2, Iron + SAM, COMT + Iron, DAT + Iron, COMT + MAO, COMT + VMAT2, and SAM + VMAT2. Each significant three-site combination is connected to one of these hubs. These eight hubs are all composed of hot spots from the single-site scenario as well as hubs from two-site scenario, suggesting an inheritable characteristic that searching significant N site combinations could be based on results of the N-1 site scenario. As a consequence, the N-1 site scenario should be performed and analyzed first, as they seem to provide the most promising candidates for hubs of the network of the N-site combinations. As in the two-site scenario, iron cation forms a unique hub in the network of all significant three-site combinations with respect to HO• (data not shown).
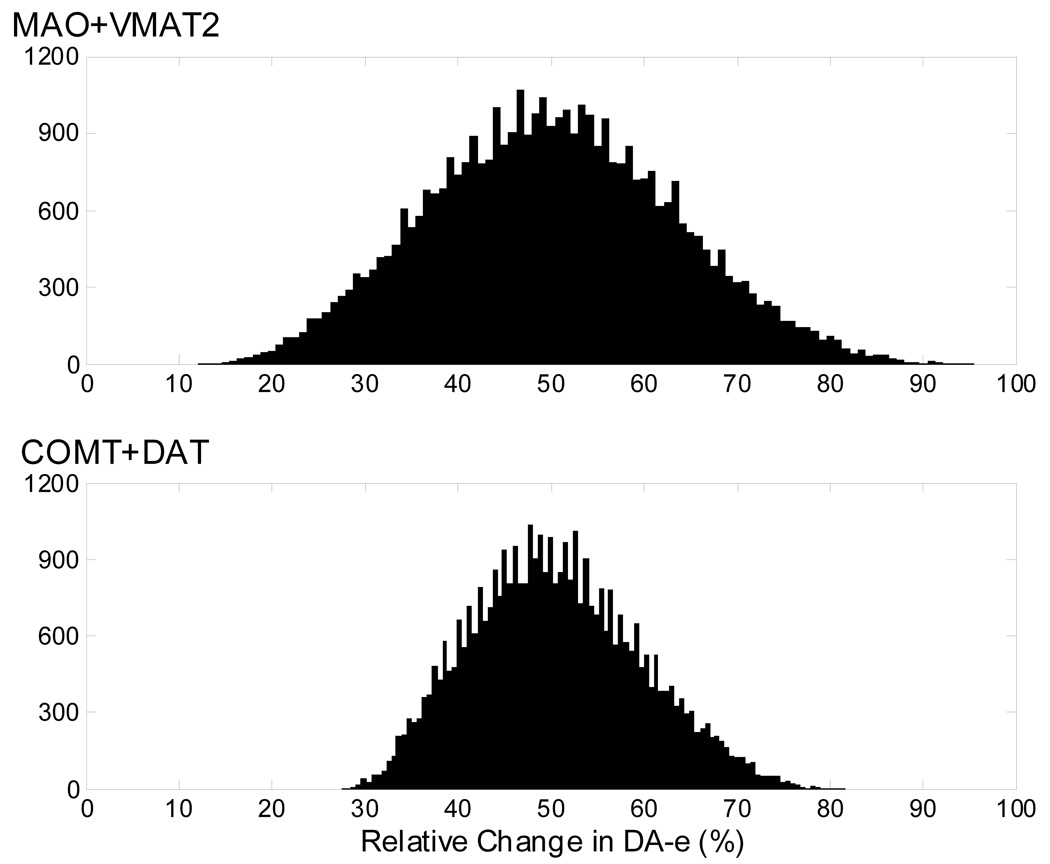
In order to affect the status of a neuron in a predetermined fashion, the mathematical model could be used to compute the optimal combination of introduced alterations, given a desired target state. Of course, it would be difficult to implement these in exact proportions in an actual organism. It is therefore of interest to ask how sensitive the results of combined interventions are with respect to unavoidable imprecision of implementation and to compare profiles of sensitivity between different choices of combined interventions. As an example, suppose the goal is to increase the DA-e level by 50% via a combined alteration in MAO and VMAT2 or in COMT and DAT. Suppose further that manipulations can only be achieved with an accuracy of ±20% around the optimal values. Figure 5 shows the distribution of relative changes in DA-e level in response to imprecise implementations of the two sets of manipulations. Comparing the histograms demonstrates that DA-e has a significantly narrower distribution around its target level under combined manipulations of COMT with DAT, rather than that under the combination of MAO and VMAT2, rendering the combination of COMT with DAT the superior candidate in this respect.
Figure 5. Relative changes in extracellular dopamine in response to imprecise two-site manipulations.
The histograms show the frequencies of a particular level of DA-e obtained from 40,000 simulated imprecise implementations of combined alterations in MAO and VMAT2 (top panel) and in COMT and DAT (bottom panel). Manipulations at each site were allowed to vary uniformly within a range of ±20% around the computed optimal value needed to achieve a 50% increase in DA-e.
Discussion
In disorders with altered dopamine signaling, therapeutic strategies are aimed at restoring signaling by manipulating dopamine precursors, receptors, or enzymes. However, a lot of therapeutic interventions have time-limited utility accompanied by debilitating side effects. The complexity of presynaptic dopamine dynamics and postsynaptic signaling, which is due to the large number of contributing components and an even greater number of nonlinear interactions, makes it difficult to evaluate all of the potential effects of therapy. We propose that an integrated computational model of presynaptic dopamine dynamics could enhance the evaluation of new treatments and approaches to restore dopamine signaling. We have previously developed an initial model of presynaptic dopamine dynamics (Qi et al., 2008a; Qi et al., 2008b). In this study, we subjected the model to a series of Monte Carlo simulations in order to discover key determinants of dopamine metabolism. Specifically, we demonstrated that, among the 35 enzymes and environmental factors we assessed, MAO, VMAT2, and DAT are the most sensitive and influential components for regulating the extracellular DA level. Moreover, MAO and VMAT2 turned also out to be the sites most critical for regulation of the total dopamine level.
The model results are supported by experimental observations and drug developments. MAOB inhibitors, such as Selegiline, have long been used as a therapeutic agent for PD (Fernandez and Chen, 2007; Stocchi et al., 2006; Tyce et al., 1990; Youdim and Bakhle, 2006). This inhibition is thought to prevent the breakdown of extracellular dopamine by MAOB. However, at higher doses it can also inhibit MAOA, which resides within the dopamine neuron. Recent findings show that a reduction of VMAT2 causes a severe reduction of dopamine, nigrostriatal neurodegeneration, increased vulnerability to various toxicants, and motor behavior deficits (Caudle et al., 2007; Colebrooke et al., 2006; Mooslehner et al., 2001; Takahashi et al., 1997). In addition, a human study showed that gene polymorphisms associated with increased VMAT2 expression reduced the incidence of PD in women (Glatt et al., 2006).
Elevated toxic species and oxidative stress are suggested to cause degeneration of dopaminergic neurons, which is the main pathological feature of PD. Our results show that iron cation (Fe2+) is the critical component for regulating the amounts of HO•, •NO2, and DA-Q, while the cellular antioxidant defense system and VMAT2 provide moderate protection by alleviating the level of highly reactive hydroxyl radical. The critical role of iron cation in adverse effects to dopaminergic neurons is supported by several experimental studies (Andersen, 2004; Kaur et al., 2007). Iron levels have been reported to be elevated in the midbrain of Parkinson’s disease patients (Gerlach et al., 1997; Mann et al., 1994). Also, mice administered iron display progressive midbrain neurodegeneration and enhanced vulnerability to toxic injury (Kaur et al., 2007).
Though a drug may exert its efficacy by action on a single site, the beneficial function is often concomitant with undesired side effects. For example, Selegiline’s ability to inhibit MAOB (and to a lesser degree, MAOA) could efficiently increase extracellular DA levels as well as total dopamine content. However, this manipulation increases the amounts of toxic DA-Q as well as that of the toxic species 3-MT and DOPA-Q (data not shown). Commonly, accompanying drugs, which may have different action sites, are used to suppress such deleterious side effects.
Multiple defects at different sites may exhibit interesting synergistic effects with regard to changing the level of dopamine and that of toxic species. Insights into these synergistic combinations might render it possible to develop efficacious means of restoring the dopamine signal in defective state. To discover and explore synergistic and antagonistic combinations we performed two-site and three-site screenings, based on our mathematical model. These combinations could represent simultaneous genetic defects, but also risk factors superimposed on genetic predisposition.
The model analysis led to two observations. First, comparatively small two- or three-site alterations can have the same quantitative effect on DA levels as a much larger alteration in only one site. At the same time, the multiple alterations can have much lower side effects with regard to toxic species. Second, particularly synergistic combinations (“hubs”) of three simultaneous alterations contain hubs of two simultaneous alterations, and these in turn contain highly sensitive components of the system. While this “inheritance” was observed here, there is no guarantee that it constitutes a general design principle of complex systems. Nevertheless, it might be a reasonable default strategy with cost-effectiveness for the development of combined treatment regimens to construct a hierarchy of synergistic hubs, and at the same time to use Monte Carlo method for additional explorations. The feature of inheritance in the hierarchy of synergistic hubs greatly shrinks the search space for multiple alterations. According to our results, 60 out of 7,770 three-site combinations were selected as primary candidates for efficiently regulating extracellular DA level. These could be used as seeds for searching efficient combinations of four or more sites.
The modeling approach offers a list of “chokepoints,” which are the most likely vulnerable sites within a metabolic system and also the most promising targets for medical interventions. Any choices among these points require additional considerations regarding the primary effect, the dose necessary to effect change, and possible side effects. In modeled dopamine system, extracellular and total dopamine levels are both important for normal function, while there are several kinds of toxic species, such as reactive oxygen species and reactive nitrogen species, that are deleterious to dopamine neurons and should be controlled. As shown in our results, manipulation of MAO and VMAT2 seems preferable over simultaneous changes in COMT and DAT, because the MAO/VMAT2 combination has the advantages of a significant elevation in extracellular DA level concomitant with the reduction in highly reactive hydroxyl radical. At the same time, the COMT/DAT intervention is less sensitive to imprecision in the practical implementation of the prescribed manipulations, which might be physiologically advantageous. The final choice would thus be based on extensive considerations and likely be a compromise.
While an experienced biochemist or clinician may not be surprised by the particular two-and three- site combination hubs identified by the model for the dopamine system, the results are generically interesting because they were obtained from the metabolic model without the benefit of additional clinical or physiological insights. They therefore provide proof of concept that computational modeling approaches, while never replacing biochemical and clinical studies, will eventually be able to prescreen likely candidates for efficacious interventions and guide the experimental exploration of potential combinations of defects and treatments. Because of the large numbers of components in complicated physiological systems, such guidance will be a crucial defense against the combinatorial explosion that emerges from the need to test all possible combinations of defects and risk factors.
Supplementary Material
Acknowledgements
This work was supported by a Woodruff Health Science Center Fund grant, NIH grants P01-ES016731 and U54-ES012068, and an endowment from the Georgia Research Alliance.
References
- Alvarez-Vasquez F, Sims KJ, Cowart LA, Okamoto Y, Voit EO, Hannun YA. Simulation and validation of modelled sphingolipid metabolism in Saccharomyces cerevisiae. Nature. 2005;433(7024):425–430. doi: 10.1038/nature03232. [DOI] [PubMed] [Google Scholar]
- Andersen JK. Iron dysregulation and Parkinson's disease. J Alzheimers Dis. 2004;6(6 Suppl):S47–S52. doi: 10.3233/jad-2004-6s602. [DOI] [PubMed] [Google Scholar]
- Carlsson A. Antipsychotic drugs, neurotransmitters, and schizophrenia. Am J Psychiatry. 1978;135(2):165–173. doi: 10.1176/ajp.135.2.165. [DOI] [PubMed] [Google Scholar]
- Carlsson A. The current status of the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1988;1(3):179–186. doi: 10.1016/0893-133x(88)90012-7. [DOI] [PubMed] [Google Scholar]
- Caudle WM, Richardson JR, Wang MZ, Taylor TN, Guillot TS, McCormack AL, Colebrooke RE, Di Monte DA, Emson PC, Miller GW. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J Neurosci. 2007;27(30):8138–8148. doi: 10.1523/JNEUROSCI.0319-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebrooke RE, Humby T, Lynch PJ, McGowan DP, Xia J, Emson PC. Age-related decline in striatal dopamine content and motor performance occurs in the absence of nigral cell loss in a genetic mouse model of Parkinson's disease. Eur J Neurosci. 2006;24(9):2622–2630. doi: 10.1111/j.1460-9568.2006.05143.x. [DOI] [PubMed] [Google Scholar]
- Fernandez HH, Chen JJ. Monamine oxidase inhibitors: current and emerging agents for Parkinson disease. Clin Neuropharmacol. 2007;30(3):150–168. doi: 10.1097/01.wnf.0000240956.49315.be. [DOI] [PubMed] [Google Scholar]
- Gerlach M, Double K, Riederer P, Hirsch E, Jellinger K, Jenner P, Trautwein A, Youdim MB. Iron in the Parkinsonian substantia nigra. Mov Disord. 1997;12(2):258–260. [PubMed] [Google Scholar]
- Glatt CE, Wahner AD, White DJ, Ruiz-Linares A, Ritz B. Gain-of-function haplotypes in the vesicular monoamine transporter promoter are protective for Parkinson disease in women. Hum Mol Genet. 2006;15(2):299–305. doi: 10.1093/hmg/ddi445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DG. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol Pharmacol. 1978;14(4):633–643. [PubMed] [Google Scholar]
- Hanson GR, Sandoval V, Riddle E, Fleckenstein AE. Psychostimulants and vesicle trafficking: a novel mechanism and therapeutic implications. Ann N Y Acad Sci. 2004;1025:146–150. doi: 10.1196/annals.1316.019. [DOI] [PubMed] [Google Scholar]
- Kaur D, Peng J, Chinta SJ, Rajagopalan S, Di Monte DA, Cherny RA, Andersen JK. Increased murine neonatal iron intake results in Parkinson-like neurodegeneration with age. Neurobiol Aging. 2007;28(6):907–913. doi: 10.1016/j.neurobiolaging.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Levy F. The dopamine theory of attention deficit hyperactivity disorder (ADHD) Aust N Z J Psychiatry. 1991;25(2):277–283. doi: 10.3109/00048679109077746. [DOI] [PubMed] [Google Scholar]
- Mann VM, Cooper JM, Daniel SE, Srai K, Jenner P, Marsden CD, Schapira AH. Complex I, iron, and ferritin in Parkinson's disease substantia nigra. Ann Neurol. 1994;36(6):876–881. doi: 10.1002/ana.410360612. [DOI] [PubMed] [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci. 1998;18(20):8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooslehner KA, Chan PM, Xu W, Liu L, Smadja C, Humby T, Allen ND, Wilkinson LS, Emson PC. Mice with very low expression of the vesicular monoamine transporter 2 gene survive into adulthood: potential mouse model for parkinsonism. Mol Cell Biol. 2001;21(16):5321–5331. doi: 10.1128/MCB.21.16.5321-5331.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z, Miller GW, Voit EO. Computational systems analysis of dopamine metabolism. PLoS ONE. 2008a;3(6):e2444. doi: 10.1371/journal.pone.0002444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z, Miller GW, Voit EO. A mathematical model of presynaptic dopamine homeostasis: implications for schizophrenia. Pharmacopsychiatry. 2008b;41 Suppl 1:S89–S98. doi: 10.1055/s-2008-1080936. [DOI] [PubMed] [Google Scholar]
- Savageau MA. Biochemical systems analysis. I. Some mathematical properties of the rate law for the component enzymatic reactions. J Theor Biol. 1969a;25(3):365–369. doi: 10.1016/s0022-5193(69)80026-3. [DOI] [PubMed] [Google Scholar]
- Savageau MA. Biochemical systems analysis. II. The steady-state solutions for an n-pool system using a power-law approximation. J Theor Biol. 1969b;25(3):370–379. doi: 10.1016/s0022-5193(69)80027-5. [DOI] [PubMed] [Google Scholar]
- Savageau MA. Biochemical systems analysis. 3. Dynamic solutions using a power-law approximation. J Theor Biol. 1970;26(2):215–226. doi: 10.1016/s0022-5193(70)80013-3. [DOI] [PubMed] [Google Scholar]
- Savageau MA. Biochemical Systems Analysis: A Study of Function and Design in Molecular Biology Reading. MA: Addison-Wesley; 1976. p. 199. [Google Scholar]
- Stocchi F, Vacca L, Grassini P, De Pandis MF, Battaglia G, Cattaneo C, Fariello RG. Symptom relief in Parkinson disease by safinamide: Biochemical and clinical evidence of efficacy beyond MAO-B inhibition. Neurology. 2006;67(7 Suppl 2):S24–S29. doi: 10.1212/wnl.67.7_suppl_2.s24. [DOI] [PubMed] [Google Scholar]
- Stokes AH, Hastings TG, Vrana KE. Cytotoxic and genotoxic potential of dopamine. J Neurosci Res. 1999;55(6):659–665. doi: 10.1002/(SICI)1097-4547(19990315)55:6<659::AID-JNR1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Kinsbourne M, Nigg J, Lanphear B, Stefanatos GA, Volkow N, Taylor E, Casey BJ, Castellanos FX, Wadhwa PD. Etiologic subtypes of attention-deficit/hyperactivity disorder: brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychol Rev. 2007;17(1):39–59. doi: 10.1007/s11065-007-9019-9. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Miner LL, Sora I, Ujike H, Revay RS, Kostic V, Jackson-Lewis V, Przedborski S, Uhl GR. VMAT2 knockout mice: heterozygotes display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion, and enhanced MPTP toxicity. Proc Natl Acad Sci U S A. 1997;94(18):9938–9943. doi: 10.1073/pnas.94.18.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres NV, Voit EO. Pathway Analysis and Optimization in Metabolic Engineering. xiv Cambridge, U.K: Cambridge University Press; 2002. p. + 305. [Google Scholar]
- Tyce GM, Dousa MK, Muenter MD. MAO and L-dopa treatment of Parkinson's disease. J Neural Transm Suppl. 1990;29:233–239. doi: 10.1007/978-3-7091-9050-0_22. [DOI] [PubMed] [Google Scholar]
- Voit EO, editor. S-System Approach to Understanding Complexity. xi. New York, NY: Van Nostrand Reinhold; 1991. Canonical Nonlinear Modeling; p. 365. [Google Scholar]
- Voit EO. Canonical modeling: review of concepts with emphasis on environmental health. Environ Health Perspect. 2000a;108 Suppl 5:895–909. doi: 10.1289/ehp.00108s5895. [DOI] [PubMed] [Google Scholar]
- Voit EO. Computational analysis of biochemical systems: a practical guide for biochemists and molecular biologists. xii. Cambridge, U.K: Cambridge University Press; 2000b. p. 531. [Google Scholar]
- Youdim MB, Bakhle YS. Monoamine oxidase: isoforms and inhibitors in Parkinson's disease and depressive illness. Br J Pharmacol. 2006;147 Suppl 1:S287–S296. doi: 10.1038/sj.bjp.0706464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.