Summary
These experiments utilized an enzyme-based microelectrode selective for the second-by-second detection of extracellular glutamate to reveal the α7-based nicotinic modulation of glutamate release in the prefrontal cortex (PFC) of freely moving rats. Rats received intra-cortical infusions of the non-selective nicotinic agonist nicotine (1.0 μg/0.4 μL) or the selective α7 agonist choline (2.0 mM/0.4 μL). The selectivity of drug-induced glutamate release was assessed in subgroups of animals pre-treated with the α7 antagonist, α-bungarotoxin (α-BGT, 10 μM) or kynurenine (10 μM) the precursor of the astrocyte-derived, negative allosteric α7 modulator kynurenic acid. Local administration of nicotine increased glutamate signals (maximum amplitude = 4.3 ± 0.6 μM) that were cleared to baseline levels in 493 ± 80 sec. Pre-treatment with α-BGT or kynurenine attenuated nicotine-induced glutamate by 61% and 60%, respectively. Local administration of choline also increased glutamate signals (maximum amplitude = 6.3 ± 0.9 μM). In contrast to nicotine-evoked glutamate release, choline-evoked signals were cleared more quickly (28 ± 6 sec) and pre-treatment with α-BGT or kynurenine completely blocked the stimulated glutamate release. Using a method that reveals the temporal dynamics of in vivo glutamate release and clearance, these data indicate a nicotinic modulation of cortical glutamate release that is both α7 – and non-α7-mediated. Furthermore, these data may also provide a mechanism underlying the recent focus on α7 full and partial agonists as therapeutic agents in the treatment of cortically-mediated cognitive deficits in schizophrenia.
Keywords: nicotine, alpha 7, glutamate, prefrontal cortex, biosensor, amperometry
Introduction
The literature from both laboratory animals and humans indicates that administration of nicotine (or other nicotinic agonists) modulates neurotransmission within the prefrontal cortex (PFC) and that this modulation is associated with positive effects on attentional processing (Hahn et al., 2003; Kumari et al., 2003; Levin & Simon, 1998; Meinke et al., 2006) and working memory (Chan et al., 2007; Grannon et al., 1995; Kendziorra et al., 2006). These cognitive effects have been demonstrated in healthy individuals as well as in conditions of psychophathology and disease (Levin and Simon, 1998; Levin and Rezvani, 2007; Mansvelder et al., 2006). Dysregulations in nicotinic acetylcholine (nACh) recptors have been linked to the positive and cognitive deficits seen in schizophrenia (for review see Martin et al., 2004). Conversely, the unusually high incidence rate of smoking among schizophrenics has been discussed in terms of a self-medicating practice to offset these deficits (Martin et al., 2004; Masterson & O'Shea, 1984; Nomikos et al., 2000). Collectively, these studies have provided the foundation for extensive investigations on nicotinic agonists as beneficial adjuncts in the pharmacotherapeutic strategy for treating schizophrenia (see Martin et al., 2004 for review).
Nicotine has diverse and complex effects on neurotransmission in the PFC. nACh receptors are found on cortical interneurons (Krenz et al., 2001), cortical projection neurons (Schröder et al., 1989) and a variety of cortical afferents (Cao et al., 2005; Dickinson et al., 2008; Wang et al., 2006) and, as such, can markedly affect cortical information, in complex ways, via the facilitated release of acetylcholine (ACh; Bruno et al., 2006; Tani et al., 1998), dopamine (DA; Cao et al., 2005), norepinephrine (Shearman et al., 2005), GABA (Fallon et al., 2007; López et al., 2001), and glutamate (Fallon et al., 2007; Gioanni et al., 1993; López et al., 2001; Rousseau et al., 2005). Given the close apposition of cholinergic and glutamatergic terminals in frontal cortex (Marchi et al., 2002; Rousseau et al., 2005), the loss of cortical nACh receptors in schizophrenics (Guan et al., 1999), and our interest in the contributions of ACh-glutamate interactions in the cognitive impairments in schizophrenia (Sarter et al., 2005; Kozak et al., 2007) we focused these studies on the nicotinic regulation of glutamate release in PFC.
Previous studies, using less direct methods, have revealed a nicotinic modulation of glutamate release in cortex (Gioanni et al., 1999; Lambe et al., 2003; Parikh et al., 2007) consistent with regulations seen in other brain regions (Alkondon et al., 1997; Gray et al., 1996). For example, administration of nicotine in layer V of rat or mouse PFC stimulates glutamate-mediated increases in spontaneous excitatory postsynaptic currents (sEPSCs) on pyramidal neurons (Lambe et al., 2003). The source of glutamate and cortical activation arises from thalamocortical inputs to layer V as electrolytic lesions of this pathway abolished the effect. Likewise, intra-cortical perfusion of nicotine via reverse microdialysis produces a concentration-dependent increase in extracellular glutamate that was both TTX-sensitive and Ca+2-dependent (Gioanni et al., 1993).
The relative contribution of nACh receptor subtypes to the nicotine-induced stimulation of glutamatergic transmission remains unclear. There is evidence for an α4β2-based modulation of this effect (Gioanni et al., 1993; Lambe et al., 2003). However, co-administration of subtype selective nicotinic antagonists along with nicotine suggests that multiple nACh receptor subtypes are involved in this cortical activation. The more potent α4β2 antagonist dihydro-β-erythroidine (DHBE) blocked latency responses in PFC but so did administration of methyllcaconitine (MLA) at concentrations that may have blocked the α7 nACh receptor (Gioanni et a., 1993). Several recent papers provide direct evidence for an α7-based modulation of glutamate release in cortex from synaptosomes (Wang et al., 2006), tissue minces (Rousseau et al., 2005), gliosomes (Patti et al., 2007), and, most recently, an in vivo report (Wu et al., 2008), that is consistent with similar demonstrations in several subcortical brain regions (Alkondon et al., 1997; Gray et al., 1996).
While the electrophysiological and microdialysis studies cited above suggest a nicotinic-based activation of cortical glutamatergic transmission they are constrained by methodological issues. The index of sEPSCs is an indirect measure of glutamate release and the necessary use of anaesthesia may dampen the excitability of both cholinergic (Jansson et al., 2004) and glutamatergic (Westphalen and Hemmings, 2006) systems. Moreover, the use of microdialysis to more directly measure glutamate efflux is limited by poor spatial and temporal resolution.
Recently, enzyme-selective glutamate microelectrodes have been developed that measure extracellular glutamate signals with a spatial and temporal resolution currently not available with conventional neurochemical methods (Day et al., 2006; Kulagina et al., 1999; Rutherford et al., 2007; van der Zeyden et al., 2008). Our laboratory has described the development and validation of a glutamate-sensitive microelectrode array (MEA; Day et al., 2006; Rutherford et al., 2007;) that permits rapid (600 msec) detection of in vivo glutamate signals with a high sensitivity (L.O.D. 0.2-0.5 μM).
The experiments described below utilize the glutamate-sensitive MEA to directly determine the ability of nicotine to locally evoke glutamate release in the prelimbic/infralimbic region of PFC in freely-moving rats. Following demonstration of a robust increase in nicotine-induced glutamate, we assessed the contributions of the α7nACh receptor subtype in two subsequent experiments. First, we determined the ability of the selective α7 nACh receptor antagonist α-bungarotoxin (α-BGT; Castro & Albuquerque, 1995) and kynurenine, a precursor to the astrocyte-derived kynurenic acid, a negative allosteric modulator of α7nACh receptors (Turski et al., 1989), to attenuate the nicotine-induced glutamate signal. Second, we determined whether infusions of choline, an agonist selective at α7nACh receptors (Alkondon et al., 1997), would mimic the stimulatory effects of nicotine on glutamate release and then whether infusion of α-BGT or kynurenine would eliminate the choline-induced signal.
Methods
Subjects
Male Wistar rats (Charles River, Wilmington MA, USA) weighing 250-450 g were used as subjects in these experiments. Animals were maintained in a temperature and humidity controlled room on a 12:12 hour light:dark cycle (lights on at 0600 hr) and individually housed in plastic cages lined with corn cob bedding (Harlan Teklad, Madison, WI, USA). Animals had access to food and water ad libitum. All procedures involving animals were approved by The Ohio State University Institutional Animal Care and Use Committee in accordance with the NIH Guide for the Care and Use of Laboratory Animals. As such, all efforts were made to minimize animal suffering, to reduce the number of animals used, and alternatives to in vivo techniques were considered.
Materials
All chemicals were used as received unless otherwise stated. Nafion® [5% (w/v) in a mixture of aliphatic alcohols and water], L-ascorbic acid (AA), DA, L-glutamate monosodium salt, glutaraldehyde [25% (w/w) in water], bovine serum albumin (BSA), H2O2, nicotine, choline, and kynurenine were obtained from Sigma Aldrich Corp. (St. Louis, MO, USA). α-BGT was obtained from Tocris Bioscience (Ellisville, MO, USA). L-glutamate oxidase (EC 1.4.3.11) (Glu Ox) was purchased from Seikaghaku America, Inc. (East Falmouth, MA, USA). All solutions were prepared using distilled, deionized water. Solutions used for intracranial injections were prepared in 0.9% saline, adjusted to pH 7.1-7.4. Special care was taken to ensure that stock solutions of BSA, glutaraldehyde, and Nafion® were replaced 1 month after first use.
Coating of glutamate-sensitive microelectrode array
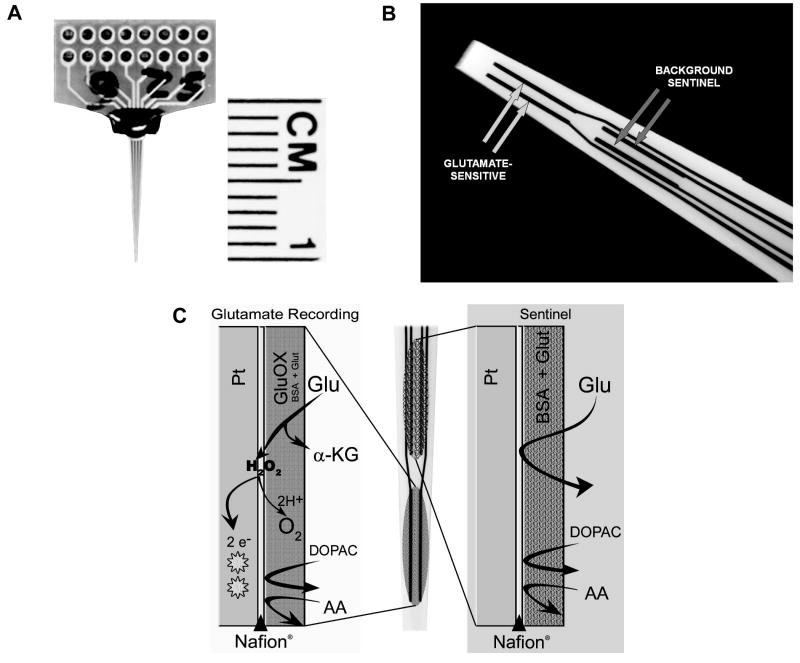
The photograph in Fig. 1A illustrates the MEA for freely-moving animals. The paddle of the MEA interfaces with a pre-amp headstage (‘Rat Hat’, not shown; see Rutherford et al., 2007 for more details on this assemblage). The photograph in Fig. 1B is a magnified view of the tip of the MEA, revealing the four 15 × 333 μm platinum (Pt) recording sites arranged in pairs beginning approximately 100 μm from the electrode tip. Prior to enzyme coating, the tip of the MEA (containing all 4 recording sites) was dipped (5 sec) in a solution of Nafion® (5%) in order to prevent anions (e.g. AA) from reaching the Pt recording sites and generating a significant oxidation current (Burmeister & Gerhardt, 2001). The Nafion®-dipped tips were oven-dried (175°C, 4 min). The MEA was allowed to reach room temperature for at least 30 min prior to coating the recording sites. One pair of recording sites was designated to be sensitive to glutamate plus other endogenous electroactive species and was coated with glutamate oxidase (Glu Ox, 2%, 1 unit/1 μL, 100 nL), (BSA, 1%), and glutaraldehyde (0.125%). The remaining pair served as a sentinel site, sensitive to the oxidation of the same molecules but not glutamate. This coating arrangement and the design of the MEA allows for a self-referencing procedure (see below) in which the current derived exclusively from the oxidation of glutamate can be isolated (Burmeister and Gerhardt, 2001; Day et al., 2006; Rutherford et al., 2007). The relative positioning of the glutamate-sensitive vs sentinel sites, relative to the tip of the MEA (i.e. more ventral vs more dorsal pair) was counterbalanced within a treatment condition. Coated MEAs were allowed to dry for ≥ 2 days at room temperature (25° C) and low humidity prior to in vitro calibrations.
Figure 1.
The glutamate-sensitive microelectrode array (MEA) and the enzyme scheme used in the detection of glutamate. (A) photograph of the MEA with ceramic wafer and recording channels. (B) high magnification of the tip of the MEA illustrating the 2 pairs of recording sites, one pair sensitive to glutamate and the remaining pair as a sentinel for background (non-glutamate derived) signals. (C, left) coatings on the glutamate-sensitive recording sites allowing for the measurement of glutamate-derived current at the Pt microelectrode. (C, right) coatings on the sentinel recording sites for the measurement of background current (see Method for details).
Detection of glutamate-generated signals
The enzyme detection scheme responsible for generating the current due to the selective oxidation of glutamate is depicted in Figure 1C. The various coatings of the Pt electrode surface for the two glutamate-sensitive channels are indicated by the shaded columns on the left and a schematic illustrating the 2 pairs of MEA recording sites appears in the middle. The shaded columns on the far right depict the events occurring at the electrode surface of the sentinel channels. In all channels, access to the Pt surface by AA is essentially blocked by Nafion® (see Figure 2 below). On the glutamate-sensitive channels (left), glutamate is oxidized by Glu Ox, generating α-ketoglutarate and H2O2. Because the MEA is maintained at a constant potential of +0.7V the H2O2 reporting molecule is oxidized, yielding two electrons. The resulting current is then amplified and recorded by a FAST-16 mkI recording system (Quanteon, LLC, Nicholasville, KY). On the sentinel channels, extracellular glutamate reaches the Pt surface but in the absence of Glu Ox no oxidation current is generated. Any current detected is due to endogenous electroactive molecules other than glutamate.
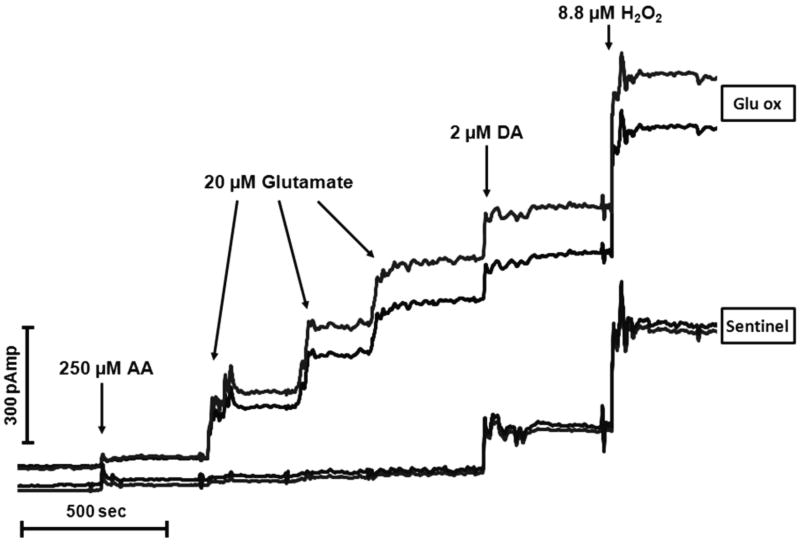
Figure 2.
A representative in vitro calibration of the MEA conducted immediately prior to implantation into prefrontal cortex. The top two tracings originate from the glutamate-sensitive (Glu OX) recording channels and the bottom two tracings are from the sentinel background channels. Arrows correspond to the addition of various substances into the calibration beaker. Current (pAmp) is depicted along the vertical and time (sec) along the horizontal axes. The successive additions of glutamate (raising beaker concentration 20 μM/aliquot) produced a linear increase in glutamate signal. Expectedly, there were no changes in current detected related to glutamate on the two sentinel channels. The calibration also reveals equivalent sensitivities on all four channels to the reporting molecule H2O2 and to the potential interferent DA. The effectiveness of the Nafion®-induced blockade of negatively charged compounds is evident by the minor increase in current depicted following the addition of a large concentration of ascorbic acid (AA) to the beaker.
In vitro calibration of microelectrodes
Microelectrodes were calibrated using the FAST-16 mkI electrochemical recording system prior to implantation. Constant potential amperometry was conducted using an applied potential of +0.7V versus a Ag/AgCl reference electrode. Calibrations were performed in a stirred solution of PBS (0.05M, 40 mL, pH 7.4, 37°C). After stabilization, AA (250 μM), glutamate (3 × 20 μM), DA (2 μM), and H2O2 (8.8 μM) were sequentially added to the calibration beaker. Amperometric signals were acquired at a rate of 1.0 Hz. The slope (sensitivity, nA/uM glutamate), limit of detection (LOD, μM glutamate), selectivity (ratio of glutamate over ascorbic acid), and linearity (R2) were calculated. In order to be used for subsequent in vivo recordings, the MEAs had to conform to the following calibration criteria (single electrode mode): i) similar background current (i.e. no greater than a 20 pA difference between the glutamate-sensitive and sentinel channels), ii) linear response to increasing concentrations of glutamate (R2 > 0.998), iii) a minimum slope of -0.003 nA/μM glutamate, iv) a minimum LOD of ≤ 0.5 μM, and v) a high selectivity for glutamate over either AA or DA (i.e. > 50:1).
Implantation of microelectrodes and infusion cannulae
Animals were anaesthetized using isofluorane (2%, 0.6L/min) and implanted with the microelectrode pedestal that contained the MEA and was connected to the miniaturized Rat Hat amplifier (Fig. 1A, left). Full details of the MEA pedestal/Rat Hat assemblage are contained in our previous publication (Rutherford et al., 2007). MEAs were implanted unilaterally in PFC (in mm from bregma: AP +2.7, ML ± 0.6, DV -3.9; hemispheres counterbalanced). Stainless steel guide cannula (26 ga, Plastics One, Roanoke, VA, USA), used for intra-cortical infusions of various drugs, were implanted anterior (20°) to the MEA such that the tip of the infusion cannula (33 ga) was positioned ∼50 μm from the center of the 4 recording sites. A dummy cannula, was positioned in the guide cannula and extended 0.5 mm beyond the tip of the guide. The Ag/AgCl reference electrode was implanted in a contralateral site distant from the recording area. All coordinates were determined from the atlas of Paxinos & Watson (1998).
In vivo recordings and intra-cortical infusions
All recordings of cortical glutamate signals were conducted in freely moving rats in a large wooden box (57.2 cm H × 41.9 cm W × 17.0 cm L) one day after implantation of the MEA and infusion cannula. Animals were placed in the recording box and connected to the head stage. Stable baseline signals were recorded following a 3-4 hr habituation period. Six groups of rats were tested (n = 6 rats/group). The groups differed only with respect to the drugs that were delivered during each of the infusions (all drugs, 0.4 μL over ∼2 s, pH = 7.1-7.4) using a Hamilton PB600-1 manual dispenser (Hamilton Company, Reno, NV, USA). The six experimental groups were; nicotine-saline-nicotine, nicotine-αBGT-nicotine, nicotine-kynurenine-nicotine, choline-saline-choline, choline-αBGT-choline, and choline-kynurenine-choline,
Following the baseline recording, the dummy cannula was removed, and the infusion cannula was inserted. After the stable baseline was re-established, the first infusion [nicotine (12 mM; 1.0 μg/0.4 μL) or choline (2 mM)] was delivered. Forty-five min later, a second infusion [0.9% NaCl, αBGT (10 μM), or kynurenine (10 μM)] was delivered. A third infusion [nicotine (12 mM) or choline (2 mM)] was delivered approximately 5 min after the saline or αBGT and 40 min after the more slowly-acting kynurenine infusions. The effects of the saline, αBGT, or kynurenine infusions on the second choline- and nicotine-induced glutamate signal were compared to the signals evoked following the initial infusions. At the end of each experiment, a control infusion of glutamate (0.25 – 1.0 mM) was delivered in order to confirm that the MEA remained highly sensitive to glutamate even following drug infusions that were predicted to attenuate or block endogenous glutamate signals. In order to ensure that no cross-contamination occurred during the infusion of such small volumes of multiple compounds within a recording session, separate infusion tubings and injection cannula were used for each drug infusion.
Histology
At the conclusion of each experiment, animals were anaesthetized with isoflourane and then given an overdose of pentobarbital. Brains were removed and stored in formalin (10%) for at least 24 h, and then transferred to a sucrose solution (30%) for at least three days. Brains were sectioned using a cryostat; coronal and sagittal sections (50 μm) were mounted on gelatin-coated slides, stained using Cresyl Violet, and examined under a light microscope for verification of microelectrode and cannula placement.
Data Analyses
Measurements derived from the FAST-16 data file included: (i) maximum amplitude, the peak concentration (μM) of the glutamate signal, (ii) the rise time (sec) from drug infusion to peak amplitude, and (iii) T80, the time in seconds from maximum peak rise to 80% decay of signal (a measure of glutamate clearance). The glutamate signal, initially measured in pA, was transformed to a concentration equivalent (μM) on the basis of the individual calibration curves generated immediately prior to surgery. The signal derived exclusively by the oxidation of glutamate was isolated using a self-referencing procedure between the glutamate-sensitive recording channel and the adjacent background sentinel channel as we have described elsewhere (Burmeister and Gerhardt, 2001; Day et al., 2006; Rutherford et al., 2007). In terms of group comparisons, the effects of saline, α-BGT, or kynurenine on nicotine- or choline-induced glutamate release (maximum amplitude, rise time, and T80) were analyzed by analysis of variance (ANOVA). A limited number of planned comparisons were conducted using t-tests for dependent measures.
Results
Calibration of glutamate-sensitive microelectrodes
A representative in vitro calibration of the glutamate-sensitive MEA is illustrated in Figure 2. This figure illustrates current tracings, from each of the 4 channels, following the administration of AA, glutamate, DA, and H2O2 (indicated by arrows). The top two tracings (Glu Ox) depict current at the glutamate-sensitive channels whereas the bottom two tracings (sentinel) depict current from the sentinel or background channels. The addition of AA to the beaker produced a minor increase in oxidation current that was, importantly, comparable on all 4 channels. This modest increase, relative to that produced by other compounds, illustrates the relative effectiveness of the Nafion® coating. The addition of glutamate (20 μM aliquots) produced large, reproducible, and linear increases in baseline current as the concentration of glutamate in the beaker was progressively elevated. In contrast, the signal on the sentinel channels did not change as a result of the addition of glutamate. The addition of DA produced a response that was roughly equivalent to that of glutamate (the +0.7V potential of the MEA is higher than the oxidation potential of DA) and, as expected, was comparable across the 4 channels. Finally, all 4 recording sites exhibited similar sensitivity to the reporting molecule, H2O2, which is a necessary condition for the self referencing procedure used to isolate the current change due to the oxidation of glutamate.
Subsequent tracings depicting experimental results will illustrate the signal at one of the glutamate-sensitive sites, one of the sentinel sites, and the self-referenced isolated glutamate signal. These tracings will utilize the results of the in vitro calibration of that particular MEA to translate current changes (nAmp) to concentration of glutamate (μM equivalents). An analysis of all of the calibration data from microelectrodes (n = 36) utilized in this experiment revealed the following characteristics (mean ± S.E.M.) of the glutamate-sensitive channels; sensitivity = -0.004 ± 0.0004 nA/μM glutamate, linearity (R2) = 0.999 ± 0.0003; L.O.D. = 0.32 ± 0.07 μM Glu, and selectivity (glutamate/AA) = 84 ± 12. In the self-referenced mode, the L.O.D. improved to 0.14 ± 0.02 μM glutamate.
Intra-cortical placement of microelectrode and infusion cannula
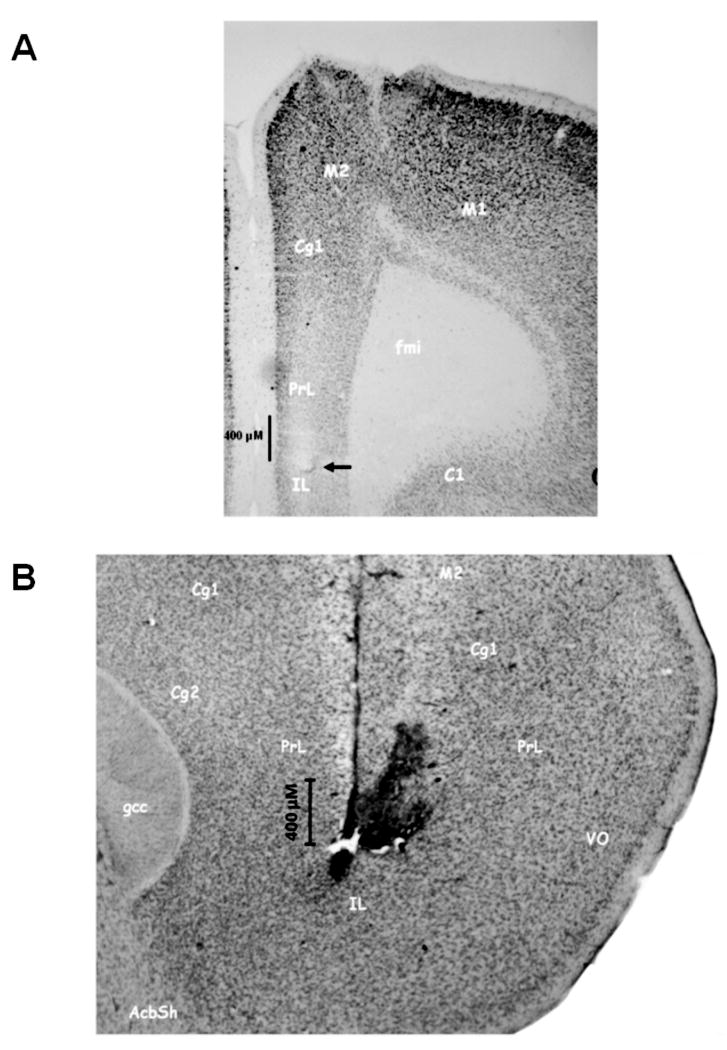
Figure 3 depicts a representative coronal (3A) and sagittal (3B) section revealing the placement of the MEA/cannula assemblage. Figure 3A illustrates that the tip of the MEA (indicated by the arrow) terminated in the prelimbic-infralimbic border of the PFC. This representative photomicrograph also illustrates the minimal tissue damage produced by the chronic placement of the MEA in brain [see also Rutherford et al., (2007) for a discussion of this topic]. The sagittal section in Figure 3B illustrates the relative placements of the MEA (vertical tract) and the adjacent infusion cannula (20° angle). The tissue disruption just to the right of the tip of the MEA placement was caused by the infusion cannula used to locally deliver drugs in close proximity (∼50 um) to the glutamate sensor. Only rats with microelectrodes and infusion cannula terminating in this region of PFC were used in these analyses.
Figure 3.
Photomicrographs illustrating a representative placement of MEA and infusion cannula within prefrontal cortex (PFC). (A) frontal section depicting the position of a microelectrode in the ventral prelimbic region of PFC. The termination of the Pt tip of the MEA is depicted by the arrow above the infra-limbic (IL) cortex. Note the modest tissue disruption produced by the implanted MEA. This is consistent with previous reports from our group (see Rutherford et al., 2007). (B) sagittal section highlighting the relative position of an MEA and the proximity of its infusion cannula. The vertical descent of the MEA can be clearly seen again within pre-limbic (PrL) and infra-limbic (IL) cortex. The modest tissue disruption to the right of the MEA reflects that produced by the infusion cannula and the subsequent infusions of drugs. The close proximity of the end of the infusion cannula and the recording channels of the MEA is evident.
Nicotine-induced glutamate release
Effects of saline (control) infusions on nicotine-induced signals
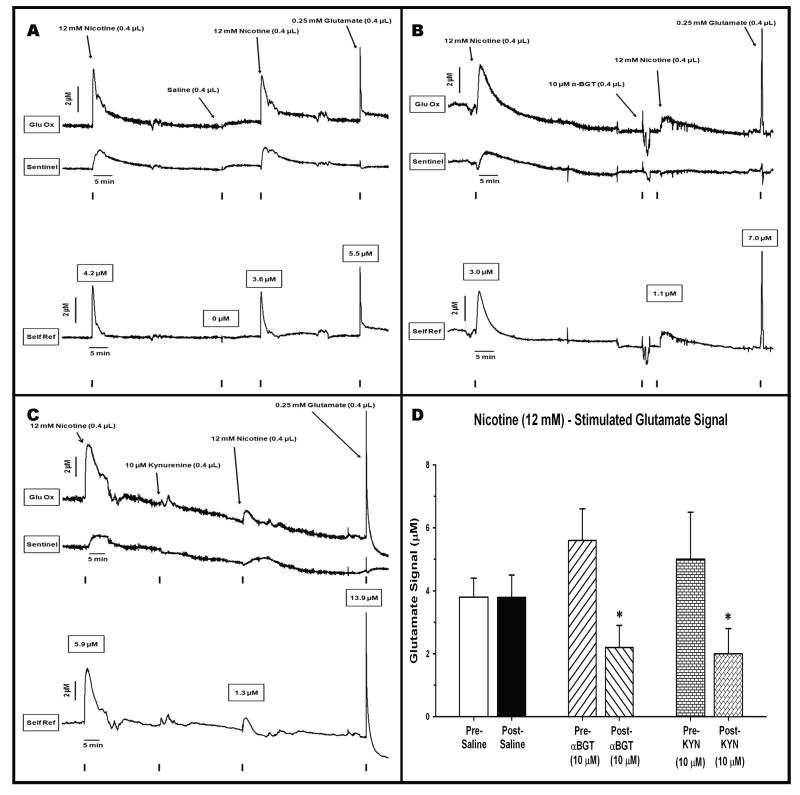
The intra-cortical infusion of nicotine (1.0 μg/0.4 μL) produced reliable increases in glutamate signal in PFC. Figure 4A illustrates a representative tracing of the electrochemical signals generated following two infusions of nicotine, separated by a control infusion of saline (0.9%). For this and subsequent figures, the top tracing is the signal generated from the glutamate-sensitive site. The middle tracing is recorded from the background or sentinel site. The bottom tracing represents the self-referenced difference between the top two, highlighting the signal generated exclusively from the oxidation of glutamate. A focus on the initial peaks of the top two tracings reveals that the infusion of nicotine resulted in a rapid increase in signal. The glutamate-sensitive site produced a peak with a rapid rise to maximum amplitude. The signal revealed two phases as it returned to baseline, a narrow, rapidly clearing signal followed by a broad, more slowly falling component. This gradual peak or dome was reproduced on the background or sentinel channel (middle) and presumably reflects the oxidation of compounds other than glutamate (see Discussion). The self-referencing procedure produced a glutamate signal that appeared more symmetrical in its rise and clearance. The initial self-referenced glutamate signal reached a maximum current amplitude equivalent to 4.2 μM glutamate in 13 sec. The stimulated glutamate signal was cleared by 80% (i.e T80) in 100 sec. The infusion of saline, 45 min later produced no appreciable changes in signal on either the glutamate or sentinel channels. This demonstrates that the initial change in glutamate signal was not the result of an infusion per se and it also provides a control infusion against which to assess the effects of α7 nACh receptor antagonists (see below). The nicotine-induced glutamate release was reproducible as a second infusion, delivered 5 - 40 minutes later (to reproduce the interval for α-BGT or kynurenine, respectively), resulted in a glutamate signal that was very similar to the first one (amplitude = 3.6 μM, time to peak = 13 sec, T80 = 89 sec). The final peak illustrates that a control infusion of a glutamate standard produced a glutamate signal similar in shape and amplitude (5.5 μM) to those produced by nicotine. An analysis of nicotine-saline-nicotine group data (Fig. 4D) revealed a consistency in the nicotine-induced glutamate release and that saline infusions had no effect on the amplitude of the drug-induced signal (t5 = -0.292, P = 0.78).
Figure 4.
Representative recordings from animals receiving intra-cortical infusions of nicotine separated by infusion of saline or α7nACh receptor antagonists. (A) effects of saline: The two infusions of nicotine (marked by TTLs on bottom of graph) produced similar signals on the Glu Ox site (top tracing) as well as increased the non-glutamatergic background signal (middle tracing). The infusion of saline was without effect on any channel. Self-referencing isolated the signal due to glutamate (bottom tracing). This signal rose rapidly to maximum amplitude and then was cleared more gradually. The nicotine-induced signal was similar to that seen following the infusion of an exogenous glutamate standard at the end of the test session. (B) effects of α-BGT: the bottom, self-referenced tracing reveals that the initial infusion of nicotine produced a clear increase (3.0 μM) in glutamate. Infusion of α-BGT, 5 min earlier, markedly attenuated the second nicotine-induced glutamate signal by 64% (1.1 μM). This attenuation did not reflect a loss in the ability of the MEA to detect glutamate as a control infusion of exogenous glutamate still produced a robust (7.0 μM) signal. (C) effects of kynurenine: the bottom self-referenced tracing shows that the initial infusion of nicotine produced a clear increase (5.9 μM) in glutamate. Infusion of kynurenine 40, min earlier, significantly attenuated the second nicotine-induced glutamate signal by 78% (1.3 μM). Kynurenine did not impair the MEA's ability to detect glutamate as the infusion of a glutamate control results in a marked elevation (13.9 μM) in the glutamate signal. (D) Group data: maximum amplitude (μM; mean ± S.E.M.) is depicted following two infusions of nicotine (12 mM in 0.4 μL). The nicotine infusions were conducted both before and after the administration of saline (control), α-BGT, or kynurenine. Separate groups of rats (n = 6/group) were tested in each nicotine-drug combination. * = amplitudes significantly reduced post- relative to pre-α-BGT (P = 0.008) or pre-kynurenine (P = 0.017).
Effects of α7 antagonists on nicotine-induced glutamate signals
Figure 4B is a representative record depicting the attenuating effects of the α7 antagonist α-BGT on nicotine-induced glutamate release. A focus on the self-referenced bottom tracing reveals that, as in Figure 4A, intra-PFC infusion of nicotine produced a marked phasic increase in cortical glutamate signal (maximum amplitude = 3.0 μM; time to peak = 77 sec; T80 = 189 sec). The infusion of α-BGT prior to the second nicotine infusion resulted in a 60% reduction in peak amplitude (1.1 μM; rise time to peak = 90 sec; T80 = 393). The post-nicotine control infusion of glutamate revealed a clear signal (7.0 μM), albeit one that was cleared more rapidly than that seen following nicotine alone. In any event, it is clear that the addition of α-BGT did not interfere with the ability of the MEA to detect glutamate. An analysis of nicotine-α-BGT-nicotine group data (Fig. 4D) revealed that α-BGT produced a significant 61% decrease in the maximum signal amplitude (t5 = 4.31; P = 0.002).
Figure 4C illustrates the effects of infusing kynurenine, a precursor to the α7 antagonist kynurenic acid, on nicotine-induced glutamate release in PFC. As in previous tracings, the nicotine-induced signal on the glutamate-sensitive site (top trace) is considerably larger than that seen on the sentinel site (middle trace). A focus on the self-referenced, glutamate signal (bottom trace) reveals an initial nicotine-stimulated release that is comparable in maximal amplitude (5.9 μM), rise time (75 sec), and T80 (394 sec) as seen in Figures 4A,B. As was the case following α-BGT, pre-treatment with kynurenine led to a marked reduction (78%) in the peak amplitude (1.3 μM) of the glutamate signal (rise time = 94 sec; T80 = 134 sec). Again, the control infusion of glutamate demonstrated that kynurenine did not negatively affect the ability of the MEA to detect glutamate per se. An analysis of nicotine-kynurenine-nicotine group data (Fig. 4D) revealed that kynurenine infusion produced a significant 60% decrease in the maximum signal amplitude (t5 = 3.51; P = 0.017).
Choline-induced glutamate signals
Effects of saline (control) on choline-induced glutamate signals
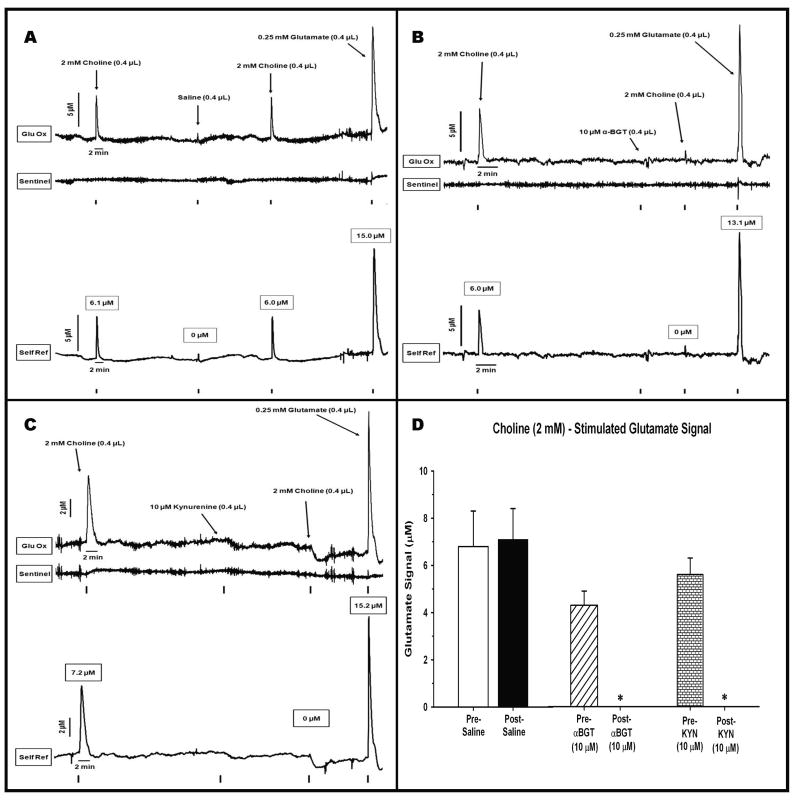
The ability of intra-cortical infusions of the α7 agonist choline to evoke glutamate release is depicted in Figure 5A. Choline, like nicotine, produced clear and rapid increases in current on the glutamate-sensitive channel (top) but, unlike nicotine, very little activity on the sentinel site (middle). The self-referenced initial choline infusion produced a maximum glutamate signal equivalent to 6.1 μM. As in Figure 4A, the control infusion of saline did not produce any appreciable change in current. The choline-induced glutamate release was highly reproducible as evident by the second infusion (peak amplitude 6.0 μM; time to peak = 8 sec; T80 = 32 sec). The control infusion of glutamate revealed a larger peak but one that was nearly identical in shape to the choline-induced signals. An analysis of choline-saline-choline group data (Fig. 5D) revealed a consistency in the choline-induced glutamate release and that saline infusions had no effect on the amplitude of the drug-induced signal (t5 = 0.188; P = 0.85). A comparison of the nicotine and choline group data, following the saline infusions in between drugs, revealed that the temporal properties of the glutamate signal (release and clearance) differed significantly between the two nACh receptor agonists. The rise time to maximum amplitude following nicotine (115 ± 32 sec) was slower than that seen following choline (6 ± 1 sec; t(22) = 3.40, P = 0.003). Likewise, the glutamate clearance (T80) following nicotine (493 ± 96) was slower than observed after choline infusions (22 ± 4; t(22) -4.88, P < 0.001).
Figure 5.
Representative recordings from animals receiving intra-cortical infusions of the α7 agonist choline separated by a control infusion of saline or α7nACh receptor antagonists. (A) effects of saline: choline infusions produced marked, symmetrical signals on the glutamate sensitive channel (top tracing). In contrast to nicotine infusions (Figs 4A-C) there was negligible choline-induced activity on the sentinel channel (middle tracing). Application of the self-referencing procedure (bottom tracing) produced highly reproducible choline-induced glutamate signals that rose quickly to maximum amplitude (6.1 μM) and were rapidly cleared to baseline. The infusion of saline did not evoke a glutamate peak nor did it affect the ability of the second choline infusion to stimulate glutamate (6.0 μM). The choline-evoked signals looked very similar to the signal stimulated following a glutamate control infusion (15.0 μM). (B) effects of α-BGT: the initial infusion of choline produced a clear increase (6.0 μM) in glutamate. The infusion of α-BGT, 5 min earlier, completely blocked the ability of a subsequent choline infusion to stimulate glutamate (0 μM). α-BGT had no detrimental effect on the ability of the MEA to detect glutamate as evident by the large signal (13.1 μM) evoked following an infusion of exogenous glutamate. (C) effects of kynurenine: the initial infusion of choline produced a clear increase (7.2 μM) in glutamate. The infusion of kynurenine, 40 min earlier, completely blocked the ability of a subsequent choline infusion to stimulate glutamate (0 μM). Again, kynurenine had no detrimental effect on the ability of the MEA to detect glutamate as evident by the large signal (15.2 μM) evoked following an infusion of exogenous glutamate. (D) Group data: maximum amplitude (μM; mean ± S.E.M.) is depicted following two infusions of choline (2 mM in 0.4 μL). The choline infusions were conducted both before and after the administration of saline (control), α-BGT, or kynurenine. Separate groups of rats (n = 6/group) were tested in each choline-drug combination. * = amplitudes significantly reduced post- relative to pre-α-BGT (P = 0.002) or pre-kynurenine (P = 0.001).
Effects of α7 antagonists on choline-induced glutamate signals
The selectivity of the choline effect for α7nACh receptors is confirmed in Figures 5B,C. Figure 5B depicts a representative recording from an animal receiving an infusion of α-BGT prior to the second choline infusion. The self-referenced tracing reveals that the initial infusion of choline produced a rapid (time to peak = 11 sec; T80 = 22 sec) and significant increase in glutamate release (6.0 μM) that was nearly identical to that seen in Figure 5A. Infusion of α-BGT completely eliminated the ability of a subsequent choline infusion to evoke glutamate (0 μM). The absence of a signal was not the result of loss of sensitivity of the MEA as a control infusion of glutamate continued to produce the expected changes in current output (13.1 μM). An analysis of choline-α-BGT-choline group data (Fig. 5D) revealed that α-BGT produced a significant 100% decrease in the maximum signal amplitude (t5 = 6.18; P = 0.002).
Figure 5C illustrates the effects of infusing kynurenine, a precursor to the α7 antagonist kynurenic acid, on choline-induced glutamate release in PFC. The initial infusion results look very similar to those seen in Figure 9 with a peak amplitude of 7.2 μM (rise time = 28 sec; T80 = 66 sec). Again, pre-treatment with kynurenine completely blocked the subsequent ability of choline to stimulate glutamate release even though the MEA remained sensitive to a control infusion of exogenous glutamate (15.2 μM). These data were further confirmed by an analysis of choline-kynurenine-choline group data (Fig. 5D) revealing that kynurenine produced a significant 100% decrease in the maximum signal amplitude (t5 = 7.66; P = 0.001).
Discussion
The microelectrode array (MEA) provides, for the first time, the ability to determine the nicotinic modulation of cortical glutamate release, with second-by-second resolution, in freely moving rats. The results generated by these experiments led to several novel and important findings. First, this is the first study to directly measure the effects of local stimulation of nACh receptors on evoked cortical glutamate release and clearance in freely moving, awake rats. The non-subtype selective agonist nicotine produced clear increases in glutamate release in prefrontal cortex (PFC). Second, the ability of nicotine to stimulate glutamate release in awake rats depends upon activation of both α7 and non-α7 nACh receptors as pretreatment with the selective α7 antagonist α-BGT attenuated (∼ 60%) but did not eliminate the nicotine-induced glutamate signal. Third, activation of α7 nACh receptors is a sufficient condition for stimulating prefrontal glutamate release as intra-cortical infusion of the selective α7 receptor agonist choline (Aldondon et al., 2000, 2006) produced a clear, phasic increase in glutamate signal that was completely blocked by pre-treatment with α-BGT. Finally, the attenuating effects of α-BGT on nicotine- and choline-stimulated glutamate relase was reproduced following intra-cortical infusions of kynurenine – a precursor to kynurenic acid, an astrocyte-derived, endogenous negative modulator of α7 nACh receptors (Hilmas et al., 2001; Turski et al., 1989;). The discussion below focuses on three topics surrounding the interpretation of these findings; a) methodological issues related to the isolation of the glutamate electrochemical signal, b) mechanisms linking nACh receptor subtypes to cortical glutamatergic transmission, and c) functional implications of nACh receptor-based therapeutics and the treatment of cortically-mediated cognitive deficits in disorders such as schizophrenia.
Methodological Issues
The electrochemical signal from the microelectrode array can be used to determine basal glutamate levels (Day et al., 2006, Rutherford et al., 2007; van der Zeyden et al., 2008) or phasic changes in evoked glutamate (Burmeister and Gerhardt, 2001; Pomerleau et al., 2003; Rutherford et al., 2007). The selectivity of the glutamate signal is supported by a convergence of several observations. First, the self-referencing technique allows for the isolation of a signal derived by the presence of glutamate oxidase on the recording site. All electrochemical signals not generated by glutamate oxidase (i.e. the sentinel background) are subtracted from signals recorded on the GluOx-coated channels. Second, both basal and stimulated glutamate signals are eliminated following the reduction of the MEA's potential from +0.7 to +0.25V (Day et al., 2006; Rutherford et al., 2007). At this lower potential, the reporting molecule H2O2 is no longer oxidized but other potential sources to the sentinel signal (i.e. DA) still may contribute to a signal (although these non-glutamatergic sources are eliminated via self-referencing). Third, administration of D,L,-threo-β-benzyloxyaspartate (TBOA), an inhibitor of glial and neuronal glutamate uptake (Shimamoto et al., 1998), potentiates both the maximum amplitude and the time for clearance of the glutamate signal (Day et al., 2006; van der Zeyden et al., 2008). Finally, the infusion of an exogenous glutamate control solution at the end of our recording sessions yielded a peak on the glutamate-sensitive but not sentinel sites.
The rise times of our signals revealed that stimulated glutamate release reached peak amplitude within 13 and 60 sec following the infusion of choline and nicotine, respectively. These stimulated peaks were cleared back toward baseline (T80) within 4-22 sec (choline) and 100-400 sec (nicotine). The impressive temporal resolution of the MEA is highlighted by the fact that the phasic components of drug-induced glutamate release emerge and are terminated in less time than a single fraction collection interval using traditional microdialysis techniques. Nevertheless, even our own data reveal considerable variability in rise times and T80 values. We believe that the variability within a drug condition (i.e. nicotine or choline) is primarily due to a) small differences in the regions of PFC where the cannula/MEA assembly is implanted (yielding differences in the density of nACh receptors and glutamate terminals) and b) slight deviations in the distance between the tip of the infusion cannula and the recording site of the MEA. Our results also indicated a systematic difference in the clearance (T80) of the glutamate signal following nicotine and choline. Nicotine-induced glutamate signals were consistently cleared more slowly than those seen following choline, despite the fact that the amplitudes of the peaks were similar. Several factors may contribute to the slower clearance rates following nicotine. First, unlike the case with choline, activation of both α7 vs non-α7 nACh receptors will contribute to the effects of nicotine on glutamate signals (Gioanni et al., 1999). Given differences in the distribution densities of α7 vs non-α7 nACh receptors relative to the infusion cannula/MEA assembly in prefrontal cortex (see Clarke et al., 1985), there simply may be more neuronal targets available to the mixed agonist and, thus, extracellular glutamate levels persist for longer periods of time. Second, the protracted clearance of the nicotine-induced glutamate signal, relative to that seen following choline, may reflect nicotine's greater affinity for nACh receptors (Xiao and Kellar, 2004). Finally, differences in the intracellular transduction mechanisms associated with stimulation of α7 vs non-α7 nACh receptors (see below) may contribute to stimulation effects of varied duration. In this regard, nicotine evoked glutamate-driven EPSPs in hippocampus have been shown to occur along two distinct time phases, one that lasts for seconds and the other that lasts for minutes (Radcliffe & Dani, 1998). Perhaps the protracted clearance of nicotine-induced extracellular glutamate in PFC is an overlapping of these two time phases that is not seen following the more selective α7 agonist choline.
The attenuation of the nicotine- and choline-induced glutamate signals following pre-infusion of α-BGT or kynurenine did not reflect drug-induced changes in the ability of the MEA to detect the second infusion of the nACh receptor agonist. Control infusions of glutamate at the end of each recording session consistently revealed that the MEAs remained highly sensitive to glutamate at the surface of the electrode. Variations in the maximum amplitude of the glutamate control signal, as discussed above in terms of variance associated with the agonist-induced signals, may have reflected differences in the gap between the infusion cannula and the MEA as well as the density of astrocytes and glutamate terminals that would contribute to the clearance of infused glutamate.
Potential Mechanisms Underlying Nicotine- and Choline-Induced Glutamate Release
Nicotinic receptors have been demonstrated on glutamatergic nerve endings (Dickinson et al., 2008; Gioanni et al., 1999; Lambe et al., 2003; Marchi et al., 2002; Rousseau et al., 2005; Wang et al., 2006), interneurons (Alkondon et al., 2000), and astrocytes (Patti et al., 2007) in rat and human cortex. The present electrochemical evidence of a nicotinic-based modulation of in vivo cortical glutamate release is consistent with several reports using indirect and direct analyses from in vitro and in vivo protocols. Superfusion of nicotine stimulated [3H]-Asp release (an index of glutamate release) in cortical synaptosomes and this effect was antagonized by addition of the α4β2 or α7 antagonists, DHβE and MLA (or α-BGT), respectively (Wang et al., 2006). Interestingly, in this slice preparation, the nicotine effect was completely blocked by MLA or DHβE. The contribution of α7 receptors to the control of cortical glutamate release is also supported by the demonstration that nicotine's effect on glutamate release was mimicked by application of the α7 agonist choline and antagonized by α-BGT in cortical synaptosomes and minces (Rousseau et al., 2005) as well as in our own results above. Our own data, demonstrating that α-BGT reduced nicotine-induced glutamate release by ∼60%, suggests significant contributions of both α7 and non-α7 receptors to the stimulatory effects of nicotine in PFC. Finally, our recent data indicate that cortical α7 receptors mediate basal as well as evoked glutamate release in prefrontal cortex (Wu et al., 2008).
Nicotine has also been shown to facilitate glutamate-mediated sEPSCs in prefrontal cortex (Lambe et al., 2003). This increase in cortical activity depends upon nicotine's actions on thalamocortical terminals as thalamic lesions eliminated the effect. Nicotine's facilitation of cortical activity in this preparation probably reflected stimulation of high affinity α4β2 receptors given the low concentration of nicotine and the demonstration that the effect was not evident in rats lacking α4β2 receptors. The contribution of nACh receptors to glutamate-based prefrontal cortical activity has also been demonstrated in vivo (Gioanni et al., 1999). Administration of nicotine facilitated short latency responses evoked by stimulation of the medial dorsal thalamus. The nicotine facilitation was antagonized by both MLA and DHβE, suggesting a role for both α7 and non-α7 receptors. These authors also used microdialysis to measure the effects of nicotine on extracellular glutamate levels. Intra-cortical perfusion of nicotine increased glutamate levels and this effect was antagonized by DHβE (the effects of an α7 antagonist were not studied). It should be noted that the source of extracellular glutamate measured directly via microdialysis or the selective MEA or indirectly using electrophysiology need not originate solely from thalamocortical afferents. Glutamatergic inputs to cortex from hippocampus (Ishikawa and Nakamura, 2003), amygdala (Chiba, 2000), and basal forebrain (Henny and Jones, 2008) may contribute to these effects. We stress that the rise times to peak amplitude and the clearance to baseline were of sufficient duration that the effects of nACh receptor stimulation on glutamate release may involve polysynaptic and even rather extensive neuronal circuits. Moreover, glutamate release need not be limited to neurons as the nACh receptor agonist epibatidine has been reported to elevate basal levels of [3H]-Asp from mouse gliosomes and this effect was blocked by MLA or α-BGT (Patti et al., 2007).
Implications for Normal and Abnormal Cognition
The modulation of cortical glutamate release by α7 and non-α7 nACh receptors has implications for both normal cognition and the development of pharmacotherapeutics for treating cognitive deficits in neuropsychiatric disorders such as schizophrenia. Nicotine has been shown to enhance cognitive function in human smokers as well as non-smokers (Foulds et al., 1996; Wesnes and Warburton, 1994). The ability of nicotine and its agonists to facilitate information processing (e.g. attention, memory) in laboratory animals has been repeatedly demonstrated (for review see Mansvelder et al., 2006). Infusions of nicotine into the PFC, at the same concentration as employed in the present study, increased accuracy measures in the five choice serial reaction test of spatial attention (Hahn et al., 2003). Recent studies have focused on a differentiation of the roles of nACh receptor subtypes in cognition. Intra-cortical infusions of an α7 antagonist impaired both working and reference memory in rats performing in a radial arm maze whereas infusions of an α4β2 antagonist impaired only working memory (Chan et al., 2007). Systemic and intra-cerebroventricular injections of a novel α7 agonist improved working memory in monkeys, social recognition in rats, and long-term memory consolidation in mice. These behaviorally efficacious doses of the α7 agonist were shown to increase ERK1/2 phosphorylation in mouse cortical and hippocampal cells (Bitner et al., 2007).
Finally, the dysregulation of cortical glutamate transmission is believed to contribute significantly to the cognitive deficits seen in schizophrenia (Krystal, 2003; Moghaddam, 2003; Tan et al., 2007). Impairments in nicotinic transmission are proposed to play a role in these cognitive deficits (Hyde and Crook, 2001; Sarter et al., 2005) and this may, in part, reflect an exacerbation an already dysfunctional release of cortical glutamate. Non-selective nACh receptor agonists have been shown to attenuate several of the neurophysiological and neurocognitive impairments seen in schizophrenia (Levin and Rezvani, 2007; Mansvelder et al., 2006). More recently, attention has been directed toward the ability of partial α7 agonists to reduce some of the negative and cognitive symptoms in schizophrenia. The partial α7 agonist DMXB-A improves scores on a composite neuropsychological scale and facilitates the P50 inhibitory gating component of auditory processing in schizophrenics better than nicotine or placebo (Olincy et al., 2006). A treatment regimen of DMXB-A has recently been shown to improve negative symptoms in schizophrenics (Freedman et al., 2008). Specific cognitive improvements in schizophrenic patients have recently been reported following administration of the positive α7 allosteric modulator galantamine (Schubert et al., 2006; Buchanan et al., 2008). Our in vivo data provide a mechanism by which drugs that increases cortical nACh receptor activity, including a significant contribution by the α7 subtype, could normalize cortical glutamate release, particularly in light of diminished α7 receptor expression (Guan et al., 1999), and be efficacious in the alleviation of cognitive deficits in schizophrenia.
Acknowledgments
This research was supported by PHS grant DA019502 to J.P.B. and G.G. The authors wish to acknowledge the continued support and technical expertise of Jason Burmeister on issues related to the coating of the microelectrode array and associated data analysis.
References
- Alkondon M, Pereira E, Cortes WS, Maelicke A, Albuquergue EX. Choline is a selective agonsit of alpha7 nicotinic acetylcholine receptors in the rat brain neurons. European Journal of Neuroscience. 1997;9(12):2734–2742. doi: 10.1111/j.1460-9568.1997.tb01702.x. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque E. Subtype-specific inhibition of nicotinic acetylcholine receptors by choline: a regulatory pathway. Journal of Pharmacology and Experimental Therapeutics. 2006;318(1):268–275. doi: 10.1124/jpet.106.103135. [DOI] [PubMed] [Google Scholar]
- Bitner RS, Bunnelle WH, Anderson DJ, Briggs CA, Buccafusco J, Curzon P, Decker MW, Frost JM, Gronlien J, Gubbins E, Li J, Malysz J, Markosyan S, Marsch K, Meyer MD, Nikkel AL, Radek RJ, Robb HM, Timmerman D, Sullivan JP, Gopalakrishnan M. Broad-spectrum efficacy across cognitive domains by α7 nicotinic acetylcholine receptor agonism correlates with activation of ERK1/2 and CREB phosphorylation pathways. Journal of Neuroscience. 2007;27(39):10578–10587. doi: 10.1523/JNEUROSCI.2444-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno JP, Gash C, Martin B, Zmarowski A, Pomerleau F, Burmeister J, Huettl P, Gerhardt GA. Second-by-second measurement of acetylcholine release in prefrontal cortex. European Journal of Neuroscience. 2006;24(10):2749–2757. doi: 10.1111/j.1460-9568.2006.05176.x. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Conley RR, Dickinson D, Ball MP, Feldman S, Gold JM, McMahon RP. Galantamine for the treatment of cognitive impairments in people with schizophrenia. American Journal of Psychiatry. 2008;165:82–89. doi: 10.1176/appi.ajp.2007.07050724. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Gerhardt GA. Self referencing ceramic based multisite microelectrodes for the detection and elimination of interferences from the measurement of L-glutamate and other analytes. Analytical Chemistry. 2001;73:1037–1042. doi: 10.1021/ac0010429. [DOI] [PubMed] [Google Scholar]
- Cao YJ, Surowy CS, Puttfarcken PS. Different nicotinic acetylcholine receptor subtypes mediating striatal and prefrontal cortical [3H]-dopamine release. Neuropharmacology. 2005;48:7279. doi: 10.1016/j.neuropharm.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Castro NG, Albuquerque EX. alpha-Bungarotoxin-sensitive hippocampal nicotinic receptor channel has a high calcium permeability. Biophysics Journal. 1995;68(2):516–524. doi: 10.1016/S0006-3495(95)80213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WK, Wong PT, Sheu F. Frontal cortical α7 and α4β2 nicotinic acetylcholine receptors in working and reference memory. Neuropharmacology. 2007;52:1641–1649. doi: 10.1016/j.neuropharm.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Chiba T. Collateral projection from the amygdalo-hippocampal transition area and CA1 to the hypothalamus and medial prefrontal cortex in the rat. Neuroscience Research. 2000;38(4):373–383. doi: 10.1016/s0168-0102(00)00183-8. [DOI] [PubMed] [Google Scholar]
- Clarke PBS, Schwartz R, Paul S, Pert C, Pert A. Nicotinic binding in rat brain: autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-α-bungarotoxin. Journal of Neuroscience. 1985;5(5):1307–1315. doi: 10.1523/JNEUROSCI.05-05-01307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BK, Pomerleau F, Burmesiter J, Huettl P, Gerhardt GA. Microelectrode array studies of basal and potassium-evoked release of L-glutamate in the anesthetized rat brain. Journal of Neurochemistry. 2006;96:1626–1635. doi: 10.1111/j.1471-4159.2006.03673.x. [DOI] [PubMed] [Google Scholar]
- Dickinson JA, Kew JN, Wonnacott S. Presynaptic α7- and β2-containing nicotinic acetylcholine receptors modulate excitatory amino acid release from rat prefrontal cortex nerve terminals via distinct cellular mechanisms. Molecular Pharmacology. 2008;74(2):348–359. doi: 10.1124/mol.108.046623. [DOI] [PubMed] [Google Scholar]
- Fallon S, Shearman E, Sershen JH, Lajtha A. The effects of glutamate and GABA receptor antagonists on nicotine-induced neurotransmitter changes in cognitive areas. Neurochemistry Research. 2007;32(4-5):535–553. doi: 10.1007/s11064-006-9113-z. [DOI] [PubMed] [Google Scholar]
- Foulds J, Stapleton J, Swettenham J, Bell N, McSorley K, Russell MA. Cognitive performance effects of subcutaneous nicotine in smokers and never-smokers. Psychopharmacology. 1996;127:31–38. doi: 10.1007/BF02805972. [DOI] [PubMed] [Google Scholar]
- Freedman R, Olincy A, Buchanan RW, Harris JG, Johnson L, Allensworth D, Guzman-Bonilla A, Clement B, Ball M, Kutnick J, Pender V, Martin L, Stevens K, Wagner B, Zerbe G, Soti F, Kem W. Initial phase 2 trial of a nicotinic agonist in schizophrenia. American Journal of Psychiatry. 2008;165:1040–1047. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioanni Y, Rougeot C, Clarke PBS, Lepousé C, Thierry M, Vidal C. Nicotinic receptors in the rat prefrontal cortex: increase in glutamate release and facilitationo f mediodorsal thalmo-cortical transmission. European Journal of Neuroscience. 1999;11:18–30. doi: 10.1046/j.1460-9568.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- Gray R, Rajann AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low conentrations of nicotine. Nature. 1996;383(6602):713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- Guan ZZ, Zhang X, Blennow K, Nordberg A. Decreased protein level of nicotinic receptor alpha7 subunit in the frontal cortex from schizophrenic brain. Neuroreport. 1999;10(8):1779–1782. doi: 10.1097/00001756-199906030-00028. [DOI] [PubMed] [Google Scholar]
- Hahn B, Shoaib M, Stolerman I. Involvement of the prefrontal cortex but not the dorsal hippocampus in the attention-enhancing effects of nicotine in rats. Psychopharmacology. 2003;168:271–279. doi: 10.1007/s00213-003-1438-6. [DOI] [PubMed] [Google Scholar]
- Henny P, Jones BE. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic, and glutamatergic inputs to pyramidal cells or interneurons. European Journal of Neuroscience. 2008;27(3):654–670. doi: 10.1111/j.1460-9568.2008.06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits α7 nicotinic receptor activity and increases non-α7 nicotinic receptor expression: physiopathological implications. Journal of Neuroscience. 2001;21:7463–7473. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde TM, Crook JM. Cholinergic systems and schizophrenia: primary pathology or epiphenomena? Journal of Chemical Neuroanatomy. 2001;22(1-2):53–63. doi: 10.1016/s0891-0618(01)00101-6. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Nakamura S. Convergence and interaction of hippocampal and amygdalar projections within the prefrontal cortex in the rat. Journal of Neuroscience. 2003;23(31):9987–9995. doi: 10.1523/JNEUROSCI.23-31-09987.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson A, Olin K, Yoshitake T, Hagman B, Herrington MK, Kehr J, Permert J. Effects of isoflurane on prefrontal acetylcholine release and hypothalamic Fos response in young adult and aged rats. Experimental Neurology. 2004;190(2):535–543. doi: 10.1016/j.expneurol.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Kozak R, Martinez V, Young D, Brown H, Bruno JP, Sarter M. toward a neuro-cognitive animal model of the cognitive symptoms of schizophrenia: disruption of cortical cholinergic neurotransmission following repeated amphetamine exposure in attentional task-performing but not non-performing rats. Neuropyschopharmacology. 2007;32(10):2074–2086. doi: 10.1038/sj.npp.1301352. [DOI] [PubMed] [Google Scholar]
- Krenz I, Kalkan D, Wevers A, de Vos RA, Steur EN, Lindstrom J, Pilz K, Nowacki S, SchÜtz U, Moser N, Witter B, Schröder H. Parvalbumin-containing interneurons of the human cerebral cortex express nicotinic acetylcholine receptor proteins. Journal Chemical Neuroanatomy. 2001;21(3):239–246. doi: 10.1016/s0891-0618(01)00112-0. [DOI] [PubMed] [Google Scholar]
- Krystal JH. NMDA receptor antagnist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology. 2003;169:215–233. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- Kulagina NV, Shankar L, Michael AC. Monitoring glutamate and ascorbate in the extracellular space of brain tissue with electrochemical microsensors. Analytical Chemistry. 1999;71(22):5093–5100. doi: 10.1021/ac990636c. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Picciotto MR, Aghajanian GK. Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology. 2003;28:216–225. doi: 10.1038/sj.npp.1300032. [DOI] [PubMed] [Google Scholar]
- Levin ED, Simon BB. Nicotinic acetylcholine involvement in cognitive function in animals. Psychopharmacology. 1998;138(3-4):217–230. doi: 10.1007/s002130050667. [DOI] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH. Nicotinic interactions with antipsychotic drugs, models of schizophrenia, and impacts on cognitive function. Biochemical Pharmacology. 2007;74(8):1182–1191. doi: 10.1016/j.bcp.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López E, Arce C, Vicente S, Oset-Gasque MJ, González MP. Nicotinic receptors mediate the release of amino acid neurotransmitters in cultured cortical neurons. Cerebral Cortex. 2001;11(2):158–163. doi: 10.1093/cercor/11.2.158. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, van Aerde KI, Couey JJ, Brussard AB. Nicotinic modulation of neuronal networks: from receptors to cognition. Psychopharmacology. 2006;184:292–305. doi: 10.1007/s00213-005-0070-z. [DOI] [PubMed] [Google Scholar]
- Marchi M, Risso F, Viola C, Cavazzani P, Raiteri M. Direct evidence that release-stimulating alpha7* nicotinic cholinergic receptors are localized on human and rat brain glutamatergic axon terminals. Journal of Neurochemistry. 2002;80(6):1071–1078. doi: 10.1046/j.0022-3042.2002.00805.x. [DOI] [PubMed] [Google Scholar]
- Martin LF, Kem WR, Freedman R. Alpha-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacology. 2004;174:54–64. doi: 10.1007/s00213-003-1750-1. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Bringing order to the glutamate chaos in schizophrenia. Neuron. 2003;40:881–884. doi: 10.1016/s0896-6273(03)00757-8. [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Schilström B, Hildebrand BE, Panagis G, Grenhoff J, Svensson TH. Role of alpha7 nicotinic receptors in nicotine dependence and implications for psychiatric illness. Behavioral Brain Research. 2000;113(1-2):97–103. doi: 10.1016/s0166-4328(00)00204-7. [DOI] [PubMed] [Google Scholar]
- Olincy A, Harris JG, Johnson LL, Pender V, Kongs S, Allensworth D, Ellis J, Zerbe GO, Leonard S, Stevens KE, Stevens JO, Martin L, Adler LE, Soti F, Kem WR, Freedman R. Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Archives General Psychiatry. 2006;63(6):630–638. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- Parikh V, Man K, Decker MW, Sarter M. Glutamatergic contributions to nicotinic acetylcholine receptor agonist-evoked cholinergic transients in the prefrontal cortex. Journal of Neuroscience. 2008;28:3769–3780. doi: 10.1523/JNEUROSCI.5251-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patti L, Raiteri L, Grilli M, Zappettini S, Bonanno G, Marchi M. Evidence that α7 nicotinic receptor modulates glutamate release from mouse neocortical gliosomes. Neurochemistry International. 2007;51:1–7. doi: 10.1016/j.neuint.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Pomerleau F, Day BK, Huettl P, Burmeister JJ, Gerhardt GA. Real time in vivo measures of L-glutamate in the rat central nervous system using ceramic-based multisite microelectrode arrays. Annals New York Academy Science. 2003;1003:454–457. doi: 10.1196/annals.1300.051. [DOI] [PubMed] [Google Scholar]
- Radcliffe KA, Dani JA. Nicotinic stimulation produces multiple forms of increased glutamatergic synaptic transmission. Journal of Neuroscience. 1998;18(18):70775–7083. doi: 10.1523/JNEUROSCI.18-18-07075.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau SJ, Jones WW, Pullar IA, Wonnacott S. Presynaptic α7 and non-α7 nicotinic acetylcholine receptors modulate [3H]D-aspartate release from rat frontal cortex in vitro. Neuropharmacology. 2005;49:59–72. doi: 10.1016/j.neuropharm.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Rutherford EC, Pomerleau F, Huettl P, Strömberg, Gerhardt GA. Chronic second-by-second measures of L-glutamate in the central nervous system of freely moving rats. Journal of Neurochemistry. 2007;102:712–722. doi: 10.1111/j.1471-4159.2007.04596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Nelson CL, Bruno JP. Cortical cholinergic transmission and cortical information processing in schizophrenia. Schizophrenia Bulletin. 2005;31(1):117–138. doi: 10.1093/schbul/sbi006. [DOI] [PubMed] [Google Scholar]
- Sherman E, Rossi S, Sershen H, Hashim A, Lajtha A. Locally administered low nicotine-induced neurotransmitter changes in area of cognitive function. Neurochemical Research. 2005;30(8):1055–1066. doi: 10.1007/s11064-005-7132-9. [DOI] [PubMed] [Google Scholar]
- Schröder H, Zilles K, Luiten PG, Strosberg AD, Aghchi A. Human cortical neurons contain both nicotinic and muscarinic acetylcholine receptors: an immunocytochemical double-labeling study. Synapse. 1989;4(4):319–326. doi: 10.1002/syn.890040408. [DOI] [PubMed] [Google Scholar]
- Schubert MH, Young KA, Hicks PB. Galantamine improves cognition in schizophrenic patients stabilized on risperidone. Biological Psychiatry. 2006;60:530–533. doi: 10.1016/j.biopsych.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, Nakajima T. DL-threo-best-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Molecular Pharmacology. 1998;53(2):195–201. doi: 10.1124/mol.53.2.195. [DOI] [PubMed] [Google Scholar]
- Tan HY, Callicott JH, Weinberger DR. Dysfunctional and compensatory prefrontal cortical systems, genes, and the pathogenesis of schizophrenia. Cerebral Cortex. 2007;17(Suppl 1):171–181. doi: 10.1093/cercor/bhm069. [DOI] [PubMed] [Google Scholar]
- Tani Y, Saito K, Imoto M, Ohno T. Pharmacological characterization of nicotinic receptor-mediated acetylcholine release in rat brain – an in vivo microdialysis study. European Journal of Pharmacology. 1998;351(2):181–188. doi: 10.1016/s0014-2999(98)00314-8. [DOI] [PubMed] [Google Scholar]
- Turski WA, Gramsbergen JBP, Traitler H, Schwarcz R. Rat brain slices produce and liberate kynurenic acid upon exposure to L-kynurenine. Journal of Neurochemistry. 1989;52:1629–1636. doi: 10.1111/j.1471-4159.1989.tb09218.x. [DOI] [PubMed] [Google Scholar]
- vad ner Zeyden M, Oldenziel WH, Rea K, Cremers TI, Westerink BH. Microdialysis of GABA and glutamate: analysis, interpretation, and comparison with microsensors. Pharmacology, Biochemistry, and Behavior. 2008;90:135–147. doi: 10.1016/j.pbb.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Wang HW, Liao WN, Chang CT, Wang SJ. Facilitation of glutamate release by nicotine involves the activation of a Ca2+/calmodulin signaling pathway in rat prefrontal cortex nerve terminals. Synapse. 2006;59:491–501. doi: 10.1002/syn.20267. [DOI] [PubMed] [Google Scholar]
- Wesnes K, Warburton DM. Effects of scopolamine and nicotine on human rapid information processing performance. Psychopharmacology. 1984;82:147–150. doi: 10.1007/BF00427761. [DOI] [PubMed] [Google Scholar]
- Westphalen RI, Hemmings HC. Volatile anesthetic effects on glutamate versus GABA release from isolated rat cortical nerve terminals: 4-aminopyridine-evoked release. Journal of Pharmacological and Experimental Therapeutics. 2006;316(1):216–223. doi: 10.1124/jpet.105.090662. [DOI] [PubMed] [Google Scholar]
- Wu HQ, Pellicciari R, Bruno JP, Schwarcz R. Endogenous kynurenate modulates extracellular glutamate levels in the rat medial prefrontal cortex. Society for Neuroscience Abstracts. 2008;33 #657.50. [Google Scholar]
- Xiao Y, Kellar KJ. The comparative pharmacology and up-regulation of rat neuronal nicotinic receptor subtype binding sties stably expressed in transfected mammalian cells. Journal of Pharmacological and Experimental Therapeutics. 2004;310(1):98–107. doi: 10.1124/jpet.104.066787. [DOI] [PubMed] [Google Scholar]