Abstract
The cell bodies of sensory neurons in the dorsal root ganglion (DRG) are enveloped by satellite glial cells (SGCs). In an animal model of intervertebral foraminal stenosis and low-back pain, a chronic compression of the DRG (CCD) increases the excitability of neuronal cell bodies in the compressed ganglion. The morphological and electrophysiological properties of SGCs were investigated in both CCD and uninjured, control lumbar DRGs. SGCs responded within 12 hours of the onset of CCD as indicated by an increased expression of glial fibrillary acidic protein (GFAP) in the compressed DRG but to lesser extent in neighboring or contralateral DRGs. Within one week, coupling through gap junctions between SGCs was significantly enhanced in the compressed ganglion. Under whole-cell patch clamp recordings, inward and outward potassium currents, but not sodium currents, were detected in individual SGCs. SGCs enveloping differently sized neurons had similar electrophysiological properties. SGCs in the compressed vs. control DRG exhibited significantly reduced inwardly rectifying potassium currents (Kir), increased input resistances and positively shifted resting membrane potentials. The reduction in Kir was greater for nociceptive medium-sized neurons compared to non-nociceptive neurons. Kir currents of SGCs around spontaneously active neurons were significantly reduced one day after compression but recovered by 7 days. These data demonstrate rapid alterations in glial membrane currents and GFAP expression in close temporal association with the development of neuronal hyperexcitability in the CCD model of europathic pain. However, these alterations are not fully sustained and suggest other mechanisms for the maintenance of the hyperexcitable state.
Keywords: GFAP, gap junction, Kir current, DRG, intraforaminal stenosis
INTRODUCTION
Satellite glial cells (SGCs) form a distinct sheath around the cell bodies of individual sensory neurons in the dorsal root ganglion (Hanani, 2005; Pannese, 1981). Inwardly rectifying potassium (Kir) channels in SGCs may play an important role in maintaining ionic homeostasis by taking up potassium ions released during action potential activity in sensory neurons (Hanani 2005). Such activity will differ according to the functional properties of the neuron, for example whether its peripheral receptors discharge frequently (e.g. muscle spindles) or infrequently (nociceptors). In addition, ectopic spontaneous activity can originate within the dorsal root ganglion (DRG) after peripheral axotomy or after an injury of the ganglion itself. For example, after a chronic compression of the DRG (CCD) in the rat, ectopic discharges are generated from the neuronal axon or cell body (Ma and LaMotte 2007). However, there is little information regarding the participation of satellite glial cells during the period of hyperexcitability or even the relationship between satellite glial cell electrophysiological parameters and the functional properties of the neuron enveloped by the glial cell.
In the present study, we examined the effects of CCD on the expression of GFAP in SGCs, using GFAP as a marker, on the formation of gap junctional connections in SGCs, and on the electrophysiological characteristics of SGCs including their expression of Kir currents. We also explored the possible relationships between the electrophysiological properties of the single SGC and its associated neuron.
MATERIALS AND METHODS
Animals
Adult, female Sprague-Dawley rats, weighing 120–150g, were housed in groups of three or four under a 12 hr light/dark cycle. The use and handling of animals were approved by the Institutional Animal Care and Use Committee of the Yale University School of Medicine and were in accordance with guidelines provided by the National Institute of Health and the International Association for the Study of Pain.
Rod Implantation
The procedure for a chronic compression of the DRG (CCD) has been described (Hu and Xing, 1998; Song et al., 1999; Zhang et al., 1999). Under pentobarbital sodium anesthesia (Nembutal, 50 mg/kg i.p.), the right transverse process and intervertebral foramina were exposed at L4 and L5. Two L-shaped stainless rods (4×2 mm length and 0.4 mm diameter) were inserted into the L4 and L5 foramina, separately. After each rod was in place, the muscle and skin were sutured.
Immunocytochemical Labeling
Glutamine synthetase (GS), which catalyzes the conversion of glutamate to glutamine (Norenberg and Martinez-Hermandez, 1979), is selective for SGCs in DRG and trigeminal ganglia (Hanani, 2005). Thus, GS staining was used in the present study as a marker for SGCs. GFAP was used as a marker for glial cell activation. Injured and control rats were deeply anesthetized with isoflurane and transcardially perfused with saline followed by 4% paraformaldehyde at 6 hrs, 12 hrs, 1 day, 3 days, 7 days, 10 days and 21 days after surgery (n = 2 for each group). The L3 and L4 DRGs from both sides were removed and post-fixed in the same fixative for 4 hrs and then cryoprotected in 30% sucrose overnight. The tissue was frozen and sectioned at 10 μm thickness on a cryostat. Tissuesections were washed three times with PBS, incubated with blocking buffer (10% normal horse serum and 0.2% Triton X-100 in PBS) for 1 hr, followed by overnight incubation with the primary antibodies (rabbit-anti-GFAP, 1:2000, Chemicon, Temecula, CA; goat-anti-GS, 1:200, Abcam, Cambridge, MA) at room temperature. After three washings with PBS, the sections were incubated with secondary antibodies (Cy3-conjugated donkey-anti-rabbit, 1:500; Alexa Fluor 488-conjugated donkey-anti-goat, 1:500; Invitrogen, Carlsbad, CA) for 1 hr. Bisbenzimide (H33258, Sigma-Aldrich, St. Louis, MO) was used to stain nuclear profiles. The slides were finally washed in PBS and cover-slipped with Fluoromount-G mounting media (Southern Biotech, Birmingham, AL). The cells were visualized, and the images captured, using a laser confocal microscopic imaging system (LMS 510, Carl Zeiss MicroImaging, Thornwood, NY). The number of immunofluorescent positive cells was counted using ImagePro Plus 5.0 (Media Cybernetics, Silver Spring, MD). The person who counted cells was blinded to the treatment conditions. The total number of SGCs was determined by counting cells with GS-IR and bisbenzimide-positive nuclear staining. The total number of GFAP positive SGCs was determined by counting cells with GFAP-IR and bisbenzimide-positive nuclear staining.
Patch-Clamp Recordings From SGCs in the Intact DRG
The ipsilateral L4 and L5 ganglia, with attached sciatic nerve, were removed after the rats were anesthetized with pentobarbital sodium. DRGs from unoperated rats were used as controls. Sham operations were not used as these were previously found to have insignificant effects on pain behavior or on the electrophysiological properties of DRG neurons (Song et al., 1999; Zhang et al., 1999). The ganglia were incubated in 1mg/ml Collagenase P (Roche) and 0.4mg/ml Protease Type XIV (Sigma) for 30 min at 37°C in oxygenated artificial cerebrospinal fluid (ACSF) containing (mM): 130 NaCl, 24 NaHCO3, 3.5 KCl, 1.25 NaH2PO4, 1.2 MgCl2, 1.2 CaCl2 and 10 Dextrose. After incubation, the ganglia were transferred to a recording chamber and superfused continuously with ACSF bubbled with 95% O2+5% CO2 at room temperature. The recording chamber was mounted on the stage of an upright microscope that was equipped with fluorescence illumination (BX50-WI, Olympus, Japan). DRG neurons and SGCs were viewed under differential interference contrast (DIC) with a 40× water immersion objective. All chemicals were purchased from Sigma except where noted.
Recording electrodes were made from borosilicate glass (Sutter Instruments, Novato, CA), pulled on a Flaming/Brown micropipette puller (P-97, Sutter Instrument, Novato, CA) and had a resistance of 2–3 MN when filled with the following solution (mM): 131 KCl, 2 MgCl2, 1 CaCl2, 11 EGTA, 10 HEPES. The pH was adjusted to 7.2 with KOH. Patch clamp recordings were made from SGCs using the whole-cell configuration (Hamill et al. 1981). Membrane currents of each SGC were recorded using a Multiclamp 700A amplifier and pClamp 9.0 software (Axon Instruments, Sunnyvale, CA). Current signals were low-pass filtered at 5 kHz and digitized online at 25–100 kHz using a Digidata 1322A digitizing board (Axon Instruments, Sunnyvale, CA) interfaced with a computer system. Capacitance compensation and series resistance compensation were used to minimize voltage errors. The input resistance (Rin) of the cell was calculated based on the steady state current change during the application of a 10 mV depolarizing and/or 10 mV hyperpolarizing pulse. The membrane capacitance (Cm) of the SGC was calculated from the transient capacitive currents during the application of a 10 mV depolarizing or hyperpolarizing pulse, using a single, spherical, compartment model. The resting membrane potential (Vm) was recorded in current-clamp mode. The recordings were not corrected for leakage currents during recording unless otherwise noted.
The cell body of only one SGC per neuron was chosen as the site of patching. The associated neuron was classified by its diameter as large (≥ 45 μm), medium (31 to 44 μm) or small (≤ 30 μm). In a subset of patch-clamp recording experiments using control- and CCD ganglia, the patch clamp electrodes were filled with 0.1% Lucifer Yellow (LY) fluorescent dye in the pipette solution. The dye would fill the recorded glial cell and, in some cases, particularly after CCD, spread into other SGCs presumably via gap junctional connections. The number of dye-filled cells was counted at the end of each recording by changing the focus of the microscope in a series of steps from the top to the bottom of the associated neuron. The images were captured with a digital camera and stored in digital form. In some experiments, carbenoxolone (100 μM) was added to the extracellular solution at least 30 min prior to recording to block the gap junctions between SGCs.
Intracellular Recording of DRG Neurons
Intracellular recordings were obtained under current clamp (MultiClamp 700A, Axon Instruments) from the cell bodies of DRG neurons using sharp electrodes with impedances of 60–80 MΩ when filled with 3M KCl. Electrophysiological signals were filtered at 2 kHz, digitized at 20 kHz via a Digidata 1320A interface, and analyzed offline with pClamp 9.0 software (Axon Instruments, Sunnyvale, CA).
A neuron was accepted for study only when it exhibited a resting membrane potential (Vm) more negative than −45 mV. After obtaining a continuous recording for 2 min without the delivery of any external stimulus, a neuron was classified as spontaneously active if spontaneous discharges persisted through this period. If there were no spontaneous activity (SA), a series of 200-ms currents from −0.5 nA to 3 nA in increments of 0.05 nA was injected to induce one or more action potentials. The current threshold was defined as the minimal current that evoked an action potential. Repetitive discharges of each neuron were measured by counting the spikes evoked by injecting a 1-s depolarizing current at 2.5 times the threshold.
Mean values are presented with standard errors (SEM). The statistical significance of differences between means was determined by Student’s t-test or a one-way ANOVA followed by post hoc, pair-wise comparisons (Student-Newman-Keuls method). Logistic regression was used to determine differences in the percentages of different groups of cells. All Statistical analyses were performed with SAS/STAT software (SAS Institute, Cary, NC) with a level of significance set at p < 0.05.
RESULTS
CCD Increased GFAP Immunoreactivity in SGCs
SGCs were identified by immunofluorescent staining for GS and GFAP. The incidence of GFAP immunoreactivity (GFAP-IR) was determined by counting the number of GFAP positive SGCs per square millimeter of each section randomly selected from the ipsilateral L4, L3 and contralateral L4 DRGs. GFAP-IR was absent in control DRGs (Fig. 1A–C) but began to appear in the compressed DRGs as early as 12 hours postoperatively (Fig. 1D–F and Fig. 2A), reaching a peak expression by postoperative day seven (POD7) (Fig. 1G–I), then decreasing sharply at POD10 followed by a further decrease by POD21 (Fig. 2A).
Figure 1.
Triple labeling of GFAP, GS and nuclei in the control and compressed DRG. A, D, G. Upregulation of GFAP in SGCs after CCD. GFAP-IR (red) was absent in control DRGs (A) but was observed in the compressed DRG as early as 12 hrs after CCD (D, arrows), and peaked at about 7 days after CCD (G, arrows). B, E, H. GS (green) was expressed in all conditions as a marker for SGCs. C, F, I. Merges of GFAP, GS and a bisbenzimide (blue) stain for the nuclei of SGCs and neurons. The cell bodies (nuclei) for some GFAP-IR positive SGCs are marked with arrows. Scale bar = 40 μm.
Figure 2.
Time course of GFAP expression in the compressed DRG. A. GFAP-IR was absent in DRGs of naïve animals (control) and 6 hours after CCD but appeared as early as 12 hours after the onset of compression. The mean number of GFAP-IR positive SGCs per mm2 area of section (±SEM) reached a peak at 7 days thereafter decreasing during the next two weeks. B. The mean number (±SEM) of all SGCs in control and compressed DRGs (7 days after CCD), and GFAP positive SGCs (“GFAP+ SGCs”) in compressed DRGs associated with neuronal cell bodies of different size categories. In both control and compressed DRGs, the number of SGCs (total and GFAP positive) increased significantly with the size of the neuronal soma. The number of SGCs associated with each size category of neurons did not differ between control and CCD ganglia.
GFAP-IR was also detected in the non-compressed, contralateral L4 DRG at POD1 (153.5 ± 66.1 cells/mm2, n=11) and the adjacent L3 DRG at POD3 (121.5 ± 43.5 cells/mm2, n=22), but to a much less degree than observed in the compressed DRGs (797.5 ± 90.2 cells/mm2, n = 20 and 834.0 ± 135.3/mm2, n = 18 at POD1 and POD3, respectively).
For compressed DRGs obtained on POD7, the total number of SGCs enveloping each neuron of each size category was determined by counting cells with GS-IR and bisbenzimide-positive nuclear staining. To delineate what constituted a neuron and its affiliated SGCs, we first visualized the dimly labeled bisbenzimide-positive neuronal nucleus. GS-IR SGCs which ringed this nucleus were then quantified. In both control and POD7 DRGs, the total number of SGCs and the number of GFAP positive SGCs increased significantly with the size of the neuronal soma (F 2,126 = 107.1, p < 0.001 for control and F= 39.8, p < 0.001 for CCD, one-way ANOVA) (Fig. 2B). There was no significant difference between CCD and control neurons in the total number of SGCs within each size category. In addition, the percentage of GFAP positive SGCs surrounding each neuron of each size category was calculated by dividing the number of GFAP-IR positive SGCs by the number of GS-IR positive cells. The percentage of GFAP positive SGCs around small-sized neurons (28.4% ± 5.1, n=64) was significantly lower than those around medium- (58.0% ± 6.1, n=35) or large- (57.6% ± 7.9, n=14) sized neurons (F 2,110 = 8.2, p < 0.001, one-way ANOVA).
CCD Increased Functional Connections between SGCs through Gap Junctions
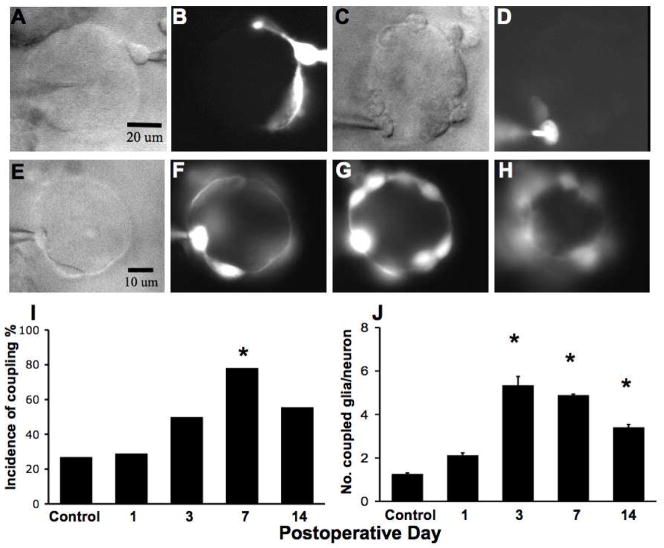
The round or fusiform-shaped cell bodies of SGCs were small (<10 μM), but clearly visible near the edges of superficial DRG neurons under differential interference contrast (DIC) (Fig. 3A, C, and E). In the following experiments, patch-clamp recordings were obtained from only one SGC of each neuron. The neurons were of medium- or large size. The pipette solution contained Lucifer Yellow (0.1%). The spread of the dye was noted after completion of electrophysiological recording. When filled with Lucifer Yellow dye, the thinner portions of the SGC, extending beyond its cell body along the edges of the neuron, were often visible under epifluorescence (Fig. 3B and F). In control DRGs, the labeling was confined to the patched SGC in 22 of 30 recordings (Fig. 3D) or spread to a single, adjacent SGC of the same neuron in 8 of 30 cases (Fig. 3B). In contrast, by POD7 after CCD, SGCs around a given neuron (Fig. 3E) were more likely to be coupled (Fig. 3F–H showing successively deeper planes of view through the same neuron). The passage of the dye between SGCs of different neurons or between SGCs and neurons was never observed in either control or CCD ganglia.
Figure 3.
Effects of CCD on coupling between SGCs. A, C, E. SGC patch-clamped, each on a different neuronal soma, and viewed under differential interference contrast. B, D, F. The same SGC now filled with Lucifer Yellow fluorescent dye from the pipette and viewed under epifluorescence. Most SGCs of control neurons were not coupled (B) or were coupled only to one other, adjacent, SGC on the same neuron (D) but never to one on another neuron. In contrast, after CCD by POD7, multiple SGCs around the same neuron were coupled together as viewed in F and at successively deeper planes through the neuron in steps of 10 um (G and H). For this neuron, 8 SGCs were coupled together. I. The time course of coupling in SGCs per neuron after CCD. The incidence of coupling increased significantly on CCD7 (p < 0.01) and increased but comparable to the control on CCD3 and CCD14 (p = 0.9 and 0.4, respectively). The incidence of coupling on CCD14 is similar to that on CCD3 (p = 0.4). J. The mean number of coupled SGCs (beyond the original one that was patch-clamped) per neuron was significantly increased from POD3 compared to control and significantly decreased from POD7 to POD14. (n= 8, 9, 6, 25, 15 for control, POD1, POD3, POD7 and POD14, respectively. * P < 0.05, Truncated Poisson distribution)
The incidence of coupling between SGCs was defined as the percentage of recordings in which the dye was present in more than one SGC (Fig. 3I). We examined the incidence of coupling using logistic regression. The incidence of coupling was the same as the control on POD1, elevated on POD7 (p < 0.01) and elevated but comparable to the control on POD3 and POD14 (p = 0.9 and p = 0.4, respectively). The incidence of coupling on POD14 was similar to that on POD3 (p = 0.4).
The number of coupled SGCs per neuron (Fig. 3 J) was determined by counting the number of fluorescently labeled cells, beyond the original one that was patched, at different focal planes each separated by 10 μM (Fig. 3F, G, and H). The mean number of coupled cells before and 1, 3, 7 and 14 days after CCD was modeled using a truncated Poisson distribution, omitting unobservable values of zero (Zelterman 2006). In control DRGs, the average number of coupled SGCs per recording was 1.3 ± 0.1. The number obtained on POD1 was not different from control (p = 0.15) but significantly increased on POD3, 7 and 14 (p < 0.01, p < 0.01, and p = 0.02, respectively). The number of coupled cells on POD14 was significantly decreased compared with that on POD7 (p = 0.02) but did not return to the numbers obtained for control or POD3.
The Electrophysiological Properties of SGCs in the Absence of a Gap Junctional Blocker
Successful patch-clamp recordings were obtained from most of the SGCs associated with neuronal somata of medium or large size in the aforementioned studies of gap junctional coupling using dye-filled pipettes. Passive and active membrane properties of SGCs in control DRGs were compared with those obtained from CCD ganglia 7 days after surgery (“CCD7”) when the number of coupled glia had reached a maximum. In comparison with SGCs of control DRGs, SGCs in CCD7 ganglia had a significantly lower mean input resistance (Rin, 14.4 ± 1.8, n = 25 vs. 37.9 ± 2.1 MΩ, n = 28) and greater mean membrane capacitance (Cm, 37.8 ± 8.2 vs. 21.6 ± 3.0 pF) but did not differ in resting membrane potential (Vm, −81.1 ± 1.1 vs. −79.9 ± 0.4 mV) (Student’s t-tests). These differences were attributed to the greater coupling between SGCs in CCD7- than in control ganglia.
The Electrophysiological Properties of Single (non-coupled) SGCs
In following experiments, the gap junctional blocker, carbenoxolone (100 μM), was added to the perfusate 30 min before the start of recording. This allowed electrophysiological measurements to be confined to a single SGC rather than two or more SGCs coupled together. Whole cell patch-clamp recordings were obtained from SGCs around small-, medium-, and large-sized neurons in control and CCD7 DRGs. Within each group (control or CCD7) there were no significant differences in the electrophysiological properties of SGCs associated with neurons of different sizes. SGCs of small, medium, or large sized neurons had similar input resistance, resting membrane potential and membrane capacitance (Table 1). However, there were differences in certain properties between control- and CCD7 SGCs. In comparison with single SGCs of control DRGs, those in CCD7 ganglia, regardless of the size of the neuronal soma, exhibited a significantly greater Rin and a more depolarized Vm (Table 1). Because the strongly negative Vm of glia is maintained primarily by Kir channels (Verkhratsky and Steinhauser, 2000), the positive shift of Vm and increase in Rin in single SGCs after CCD were consistent with a decrease in the expression of Kir channels as subsequently investigated in the present study. In contrast, there were no differences in membrane capacitance between SGCs of control- and CCD7 ganglia, suggesting that CCD produced no major change in the size of the single SGC.
TABLE 1.
Passive membrane properties of individual satellite glia associated with small-, medium-, and large- sized DRG neurons
| Small | Medium | Large | ||||
|---|---|---|---|---|---|---|
| Control | CCD7 | Control | CCD7 | Control | CCD7 | |
| Cm, pF | 13.0 ± 2.3 | 12.0 ± 2.0 | 14.0 ± 2.5 | 13.5 ± 1.2 | 13.9 ± 1.5 | 13.1 ± 0.6 |
| Rin, MΩ | 48.8 ± 5.4 | 55.7 ± 6.6* | 43.2 ± 4.8 | 59.9 ± 3.3* | 47.3 ± 4.2 | 66.9 ± 4.7* |
| Vm, mV | −84.7 ± 1.1 | −81.1 ± 0.6* | −86.0 ± 0.7 | −80.8 ± 1.7* | −84.2 ± 0.6 | −80.2 ± 0.6* |
| N | 10 | 10 | 12 | 11 | 10 | 12 |
Values are mean ± SEM. Small: small-sized neurons; Medium: medium-sized neurons; Large: large-sized neurons. Cm: membrane capacitance; Rin: input resistance; Vm: resting membrane potential.
P < 0.05, control vs. CCD7, student’s t-test.
Electrophysiological Properties of Single SGCs associated with Different Types of Medium-sized Neurons
In the following experiment, we asked whether the electrophysiological properties of SGCs associated with neuronal cell bodies of the same, medium-sized, diameter might differ according to the expected level of action-potential activity of the neuron. Based on in vivo electrophysiological recordings, neuronal somata of medium size have peripheral nociceptors if they exhibit an inflection on the falling phase of the action potential (“hump”) or low threshold mechanoreceptors if they do not (Koerber et al., 1988). In comparison with nociceptive neurons, the non-nociceptive medium-sized neurons would be expected to fire more often and produce greater fluxes of potassium ions in the vicinity of SGCs. In addition, after CCD, some non-nociceptive neurons were spontaneously active (SA) and would thus have the highest level of neuronal activity.
To obtain electrophysiological properties of DRG neurons, intracellular, sharp-electrode recordings were first made from each medium-sized neuron followed by patch-clamp recording from only one of its associated SGCs. The neurons were classified into three types according to the level of action-potential activity. The first two were classified as “nociceptive” or “non-nociceptive” depending on the presence or absence of a “hump” during the electrically evoked somal action potential, respectively. A third category, found only after CCD, was the neuron with SA. The samples of neurons in the SA category were all of the non-nociceptive type. Recordings were obtained from SGCs, in the presence of carbenoxolone, on POD1 and POD7 and from SGCs of control ganglia.
For a given experimental condition (control or CCD), there were no significant differences in the passive membrane properties of single SGCs associated with nociceptive and non-nociceptive medium sized neurons (Table 2). This finding indicated that SGCs have uniform passive characteristics even when the neurons they envelope have different levels of action-potential activity. However, SGCs of the compressed DRG differed in certain properties from those of the control ganglion. In comparison with SGCs from control ganglia, SGCs recorded one day after CCD (“CCD1”), had a significantly increased mean Rin and a more depolarized mean Vm (Table 2) though not differing in Cm. In CCD1 ganglia, the SGCs associated with the SA (non-nociceptive) neurons were significantly more depolarized and had a higher input resistance than those associated with silent neurons. The CCD1 neurons were more excitable than control neurons, i.e., they exhibited lower current thresholds, responded with more action potentials to injected current (at 2× threshold), and exhibited a higher incidence of SA (9/63 tested or 14.3% had SA vs. 0/61 from control) (Table 3). These findings suggest that at one day after CCD, SGCs alter their electrophysiological properties. In addition, this alteration occurs at the same time as the development of neuronal hyperexcitability, which precedes the increase in glial coupling. The mean values of Rin and Vm of SGCs associated with SA neurons (but not those for SGCs of silent CCD neurons) returned to control values by 7 days after the onset of CCD. The significantly increased input resistance of SGCs associated with SA- vs. silent neurons at CCD1 suggests that glia quickly lost a membrane conductance one day after CCD, but regained it 6 days later.
TABLE 2.
Passive membrane properties of SGCs associated with medium-sized neurons that differ in functional properties
| Nociceptive | Non-nociceptive | Non-nociceptive with SA | |||||
|---|---|---|---|---|---|---|---|
| Control | CCD7 | Control | CCD1 | CCD7 | CCD1 | CCD7 | |
| Cm, pF | 14.1 ± 1.1 | 14.3 ± 1.2 | 13.0 ± 2.4 | 13.8 ± 0.9 | 12.8 ± 1.2 | 13.8 ± 1.7 | 11.5 ± 1.5 |
| Rin, MΩ | 44.7 ± 4.6 | 61.3 ± 4.7* | 45.5 ± 7.5 | 68.4 ± 7.1* | 63.2 ± 5.2* | 159.2 ± 15.3Φ | 48.4 ± 5.9† |
| Vm, mV | −84.7 ± 0.8 | −80.6 ± 1.0* | −85.6 ± 0.8 | −78.8 ± 1.0* | −82.1 ± 1.4* | −74.1 ± 1.1Φ | −84.6 ± 0.8† |
| N | 15 | 14 | 12 | 12 | 20 | 9 | 10 |
Values are mean ± SEM. SA: spontaneous activity; Cm: membrane capacitance; Rin: input resistance; Vm: resting membrane potential. The nociceptive medium sized neuron is defined by the presence of an inflection on the falling phase of the action potential.
P < 0.05, CCD7 vs. control or CCD1 vs. control in the same category (F2,41 = 4.18, p < 0.05 for Rin; F2,41 = 6.20, p < 0.01 for Vm);
P < 0.01, CCD1 non-nociceptive with SA vs. control non-nociceptive;
P < 0.01 non-nociceptive with SA CCD1 vs. CCD7. One-way ANOVA followed by post hoc pair-wise comparisons (Student-Newman-Keuls test) was used when comparing control, CCD1 and CCD7 in non-nociceptive category, for others Student’s t-test was used.
TABLE 3.
Electrophysiological properties of DRG neurons
| Control | CCD1 | |||||
|---|---|---|---|---|---|---|
| Small | Medium | Large | Small | Medium | Large | |
| Diameter, um | 30.0 ± 0.4 | 38.2 ± 0.6 | 52.0 ± 0.8 | 29.2 ± 0.3 | 36.3 ± 0.6 | 50.7 ± 0.7 |
| CV, m/s | 0.8 ± 0.1 | 9.8 ± 1.5 | 19.7 ± 1.6 | 1.2 ± 0.4 | 6.6 ± 1.0 | 17.6 ± 1.3 |
| RMP, mV | −60.6 ±1.5 | −61.1 ±1.4 | −63.8 ±0.9 | −57.0 ± 1.6 | −59.4 ± 1.1 | −62.0 ± 1.6 |
| Input Resistance, MΩ | 72.6 ± 7.0 | 38.6 ± 3.4 | 27.3 ± 2.7 | 113.1 ± 13.0** | 48.6 ± 5.4 | 38.3 ± 5.9 |
| Current Threshold, nA | 0.8 ± 0.1 | 1.1 ± 0.2 | 1.5 ± 0.2 | 0.6 ± 0.1 | 0.6 ± 0.1** | 0.7 ± 0.1** |
| No. of APs at 2XCT | 1.9 ± 0.4 | 1.6 ± 0.3 | 4.9 ± 1.3 | 6.7 ± 0.2* | 10.7 ± 1.6** | 52.9 ± 17.3** |
| No. of neurons with | ||||||
| SA | 0 | 0 | 0 | 1 | 3 | 5 |
| n | 16 | 18 | 27 | 16 | 25 | 22 |
Values are mean ± SEM. CV: conducting velocity; RMP: resting membrane potential; AP: action potential; SA: spontaneous activity.
P < 0.01,
P < 0.05, CCD1 vs. control in the same category (Student’s t-test).
CCD Reduced Kir Currents in SGCs
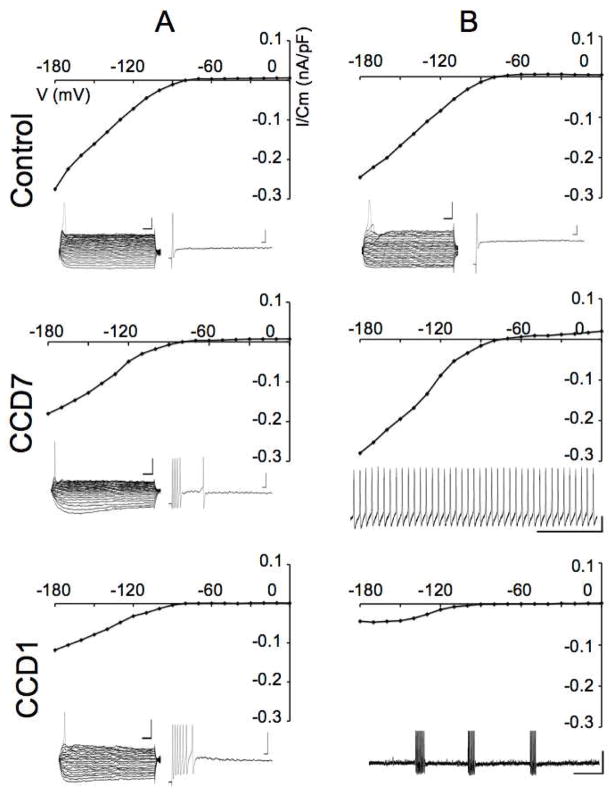
In the presence of glial coupling (absence of the gap junctional blocker), inward and outward currents in SGCs were obtained in response to depolarizing and hyperpolarizing voltage steps in voltage-clamp from a holding potential of −80 mV (Fig. 4A–C). In control DRGs, time- and voltage-dependent currents were observed particularly during very negative voltage steps (Fig. 4A). At CCD7, the current accompanying depolarizing or hyperpolarizing steps was significantly larger, likely due to the increased gap junctional coupling (Fig. 4B). The current-voltage relationship obtained by plotting the mean peak current at each voltage exhibited a slight rectification of inward currents for the control SGCs (n=28, Fig. 4C, inset) but was nearly linear for the coupled CCD7 SGCs (n=25) (Fig. 4C). After blocking gap-junctions with carbenoxolone (Fig. 4D–E), time- and voltage-dependent inward and outward currents were induced in both the control- and CCD7-SGCs (Figs. 4D and E, respectively) in response to the same voltage steps. The I-V curve exhibited a slight rectification of inward and outward currents in both the control- (n = 32) and CCD7 (n = 33) SGCs (Fig. 4F). Additionally, in comparison with control SGCs, CCD7 SGCs exhibited significantly less inward and outward currents (p < 0.01, one-way ANOVA followed by Student-Newman-Keuls method), which was consistent with the increase in input resistance after CCD.
Figure 4.
Effects of CCD, and a gap junctional blocker, carbenoxolone, on whole-cell currents in SGCs. A. Under voltage clamp, inward and outward currents were evoked in an uncoupled control SGC by depolarizing and hyperpolarizing voltage steps from a holding potential of −80 mV (inset). The typical responses of control SGCs consisted of time- and voltage-dependent currents, particularly during very negative voltage steps. B. Responses of a SGC to the same voltage steps on POD7 after CCD. Note the high degree of coupling between SGCs (inset) resulting in passive and non-inactivating currents. C. Current-voltage relationship, obtained by plotting the peak currents at each voltage in A and B. The relationship exhibited a slight rectification of inward currents for the control SGC (n=28, inset, the same I-V curve in C except only data of control SGCs was presented) but was nearly linear for the coupled CCD SGC (n=25). D–F. Responses to the same voltage steps of two other SGCs, one control and the other 7 days, after CCD, recorded in the presence of carbenoxolone. Note the absence of coupling, due to the blocker, in CCD SGCs (E, inset). Both control- (D) and CCD-SGCs (E) exhibited time- and voltage-dependent currents under carbenoxolone. Note the effects of CCD in decreasing both inward and outward currents of SGCs in control (n=32) and CCD7 (n=33) (F). Note differences in vertical scale in C and F.
The pronounced inactivation of inward currents induced by strong negative voltage steps suggested that Kir currents were expressed in these cells (Ransom and Sontheimer, 1995). To isolate the contribution of Kir, whole-cell recordings were obtained in which Kir channels were initially inactivated by a pre-pulse potential to +40 mV followed by voltage steps from −180 mV to 0 mV (Fig. 5A and inset). An application of 1 mM Cs+ in the bath effectively blocked the inward currents while leaving the outward currents – an indication that the inward currents were produced by K+ channel activation (Fig. 5B) (McKhann et al., 1997; Ransom and Sontheimer, 1995). The Cs+-sensitive currents were obtained by subtracting induced currents before and after application of Cs+ (Fig. 5C). The peak and the steady-state Kir currents, plotted as a function of voltage, indicated that current amplitudes increased with increasing hyperpolarizations and reversed near the K+ equilibrium potential (Fig. 5D). At potentials more negative than −150 mV, the currents exhibited time- and voltage-dependent inactivation, a characteristic of Kir currents (Ransom and Sontheimer, 1995). The depolarization of SGCs provided no evidence for the presence of Na+ currents.
Figure 5.
Effect of Cs+ on inwardly rectifying K+ currents (Kir) in a single (non-coupled) SGC from a control DRG in the presence of carbenoxolone. A. Whole-cell inward currents recorded in response to voltage steps of −180 to 0 mV each delivered following a pre-pulse to +40 mV (inset). B. Bath application of Cs+ (1 mM) blocked inward currents while leaving outward currents intact. C. Cs+-sensitive Kir currents were obtained by subtracting traces recorded in B from those in A. D. Current-voltage relationship obtained by plotting the peak Cs+-sensitive currents (circles) and the steady-state currents, taken at the end of each voltage step (triangles) against command voltages from traces in C. The Kir currents displayed a typical inactivation at voltages more negative than −150 mV.
Kir currents were recorded in the SGCs of neurons classified by size as small, medium or large. The Kir density was computed by dividing the peak current amplitude by the membrane capacitance to normalize for the size of the SGC (Fig. 6A). For a given experimental condition (control or CCD7), there was no significant difference in Kir current of SGCs of different sized neurons (Fig. 6A, B).
Figure 6.
Effect of CCD on peak Kir currents in SGCs encircling small, medium and large sized neurons at POD7 (“CCD7”). A. Kir currents of SGCs associated with different sized neurons in control and CCD neurons recorded at POD7 (“CCD7”). Currents were obtained in response to membrane potentials of −180 to 0 mV. SGCs associated with small, medium and large neurons (square, circle, and triangle, respectively) had similar densities of Kir currents in either control or CCD7 ganglia (closed and open symbols, respectively). For neurons of each size category, SGCs had significantly reduced Kir currents 7 days after onset of CCD. B. Mean peak Kir currents obtained in response to depolarizations to −180 mV for small- (n=10 and 10 for control and CCD7), medium- (n=12 and 11 for control and CCD7), and large-sized (n=10 and 12 for control and CCD7) neurons. C. At −180 mV, SGCs encircling non-nociceptive neurons (n= 12 and 20 for control and CCD7) exhibited a higher density of Kir current than those associated with nociceptive neurons (n=15 and 14 for control and CCD7) in either control- or CCD7 ganglia (* P < 0.01, student’s t-test).
One might have expected a greater Kir current in SGCs of larger-sized neurons that presumably fire more action potentials and, thus have a greater K+ flux than small, mostly nociceptive, neurons. However, nociceptive neurons have a wider action potential and may have a greater K+ flux per action potential than non-nociceptive neurons. Thus, we compared Kir currents for SGCs of nociceptive and non-nociceptive neurons, each of medium size. The neurons were classified as nociceptive or non-nociceptive according to the presence or absence of a “hump” on the falling phase of the action potential. In comparison with SGCs of non-nociceptive neurons, those of nociceptive neurons had a significantly lower Kir current (Fig. 6C). This was true for SGCs in control and CCD7 ganglia. The implication is that Kir current in SGCs is related to the frequency of neuronal firing (perhaps to accommodate requirements for buffering larger amounts of potassium released during firing) assuming that non-nociceptive neurons fire more often than nociceptive. In addition, Kir current was significantly less in CCD than in control ganglia for SGCs of neurons in each size category and for both nociceptive and non-nociceptive medium-sized neurons (p < 0.01, one-way ANOVA followed by Student-Newman-Keuls method).
To study further whether Kir currents of SGCs after CCD are accompanied by changes in neuronal activity, we compared the densities of Kir current of SGCs of two types of non-nociceptive medium-sized CCD neurons – those that were spontaneously active and those that were silent. The I/V curves of representative SGCs and the action potential activity of their neurons are shown in Fig. 7, the mean I/V curves in Fig 8A, and the mean peak current densities in Fig 8B. In Fig. 7, the voltage recordings from the neurons represent the firing patterns of DRG neurons after CCD in each group. The classification of SGCs was based on these properties of the neurons they enveloped. The Kir in SGCs was significantly reduced at CCD1 regardless of whether their neurons were SA or silent (Fig. 8A, p < 0.001, one-way ANOVA with posthoc tests for currents at voltages from −160 to −90 mV in control and CCD1 group). The reduction continued through CCD7 for SGCs of silent neurons but had increased back to control values for SGCs of SA neurons (Fig. 8B). Thus, Kir currents in SGCs of SA neurons exhibited dynamic changes after CCD. The significant reduction of Kir currents one day after CCD may indicate an impaired capacity of SGCs to buffer K+ that might contribute to the development of neuronal SA in the early phases after CCD but which clearly are not responsible for the maintenance of SA once the deficit is corrected at CCD7.
Figure 7.
Representative recordings of Kir currents from SGCs (upper panel) and action potentials (AP) from their associated neurons (lower panel) in control, CCD7 and CCD1 ganglia. For each neuron, a continuous recording was obtained intracellularly for 2 min, without the delivery of any external stimulus, to detect the presence of ongoing, spontaneous discharges (SA). The neurons were classified as spontaneously active (SA) if spontaneous discharges persisted during this period (B in CCD7 and CCD1, lower panel), or categorized as silent. For silent neurons, a series of 200-ms depolarizing currents from −0.5 nA to 3 nA in increments of 0.05 nA was injected to induce APs (left lower panel in column A and control B). The current threshold (CT) was defined as the minimal current required to evoke an AP. Repetitive discharges of each neuron were measured by counting the spikes evoked by injection of a 1-s depolarizing current at 2.5 times of CT (right lower panel in column A and control B). Scale bar is 100 ms in CCD7 B and 1 s in CCD1 B, otherwise is 10 ms and 20 mV.
Figure 8.
Kir currents of SGCs associated with non-nociceptive, medium-sized neurons. A. Kir currents were significantly reduced in CCD1 ganglia (n=21) but recovered after 7 days (CCD7, n=30). B. SGCs associated with spontaneously active (SA) neurons in CCD1 ganglia (n=9) had significantly decreased Kir currents at −180 mV but comparable Kir currents in CCD7 ganglia (n=10) when compared to those of silent neurons in control (n= 12, p < 0.01, student’s t-test). SGCs associated with SA neurons have less Kir currents in CCD1 ganglia but more currents in CCD7 ganglia when compared to SGCs associated with silent neurons (* P < 0.01, student’s t-test, n= 12 and 20 for SGCs associated with silent neurons in CCD1 and CCD7, respectively).
DISCUSSION
The present findings extend our knowledge of the functional properties of satellite glial cells in the uninjured DRG and provide novel information about the dynamic changes that occur after a compression injury of the ganglion that causes signs of neuronal hyperexcitability in the absence of axotomy.
Increased GFAP Expression in SGCs after CCD
We confirm the results of previous studies of sensory ganglia indicating the absence of GFAP labeling of SGCs in naïve, uninjured sensory ganglia (Stephenson and Byers, 1995; Woodham et al., 1989). The rapid increase of GFAP-IR in SGCs within the first day after the onset of the compression injury coincided with the appearance of neuronal hyperexcitability as measured GLIA-00398-2008 R2 electrophysiologically. This result is consistent with a previous observation of a temporal correlation between an injury of the inferior alveolar nerve and the changes in GFAP expression in the trigeminal ganglion (Chudler et al., 1997; Stephenson and Byers, 1995; Woodham et al., 1989; Zhou et al., 1996). Although the function of GFAP is uncertain, the increase of GFAP might be interpreted as indicating changes in cell shape or motility during glial hypertrophy. However, we found neither an increase in the number of SGCs (labeled as such by the glutamine synthetase immunoreactivity) nor an increase in the size of each SGC as indicated by measurements of membrane capacitance during patch clamp recording. Thus, the SGCs responded to CCD as evidenced by the novel expression of GFAP but did not exhibit other properties, such as hypertrophy and proliferation occurring after sciatic nerve axotomy (Shinder V et al., 1999) that are indicative of glial activation.
Increased Coupling Between SGCs after CCD
Glial cells are mutually coupled by gap junctions in most regions of the nervous system (Rozental et al., 2001, review). In our study, both the incidence of coupling and the number of coupled SGCs in the control DRG are comparable to values obtained in previous studies of sensory ganglia (Cherkas et al., 2004; Huang et al., 2005), confirming the low level of mutual coupling among SGCs in normal ganglia. After CCD, the coupling among SGCs via gap junctions was significantly enhanced similar to that occurring after peripheral inflammation or peripheral nerve injury (Cherkas et al., 2004; Dublin and Hanani, 2007; Hanani et al., 2002). We did not find coupling between SGCs of different neuronal somata as observed for 2.3% – 6.6% of coupling in SGCs around neighboring neurons in mouse DRG by injecting dye using sharp electrodes (Dublin and Hanani, 2007; Hanani et al., 2002; Huang et al., 2005; Huang and Hanani, 2005). Possibly some degree of coupling between SGCs of different neurons might have been observed in the present study had we recorded from a much larger sample of glia.
The functional significance of increased connections in SGCs in response to injury is still uncertain. As SGCs are rarely coupled in normal DRGs, the increased coupling after injury may enhance the ability of SGCs to communicate over longer distances, allowing them to function as a larger network. It is suggested that the gap junctional connections in glial cells play a unique role in the spatial buffering of K+ remote from the site of release (Kettenmann and Ransom, 1988). An increase in coupling between SGCs in the compressed DRG may aid in the buffering of excessive K+ possibly released due to the increased neuronal excitability and increased incidence of spontaneous activity.
Electrophysiological Properties of SGCs in the Naïve, Uninjured DRG
In comparison with the well documented electrophysiological properties of astrocytes (Barres et al., 1990; Bordey and Sontheimer, 2000; Sontheimer 1994) and Schwann cells (Baker and Ritchie, 1996; Konishi 1989a and 1989b;Wilson and Chiu, 1990), those of SGCs in the DRG have received much less attention. The present findings indicate that SGCs in DRG primarily express K+-selective channels, including outward and inward K+ channels as shown on SGCs in trigeminal ganglia (Cherkas et al., 2004). The inward K+ currents display inwardly rectifying properties and are blocked by Cs+. We did not record any signs of transient K+ currents or sodium currents from SGCs in our preparation. Our results are also in line with those of SGCs in sympathetic ganglia (Gola et al., 1993; Gommerat and Gola, 1996; Konishi, 1996).
We found that SGCs associated with different types of neurons such as those differing in size or action-potential characteristics, had similar passive membrane properties including input resistance, membrane capacitance and resting membrane potential. SGCs associated with medium-sized neurons classified as “non-nociceptive” (absence of an inflection on the falling phase of the action potential) had a greater density of Kir current than those of medium-sized neurons that were “nociceptive”. The expression of Kir currents of SGCs in sympathetic ganglia has been found to depend on the activity of the neurons in the ganglia (Konishi, 1996) and is therefore possibly regulated by exogenous K+ released during neuronal activities (Barres, 1991). Because non-nociceptive neurons would be expected to fire more often than nociceptive, the higher density of Kir currents in SGCs around non-nociceptive neurons suggested that the SGCs adapted in response to neuronal activity. Thus, it is possible the DRG neurons set the tone of Kir expression of the SGCs. On the other hand, SGCs around small sized neurons had similar Kir currents with those around large sized neurons even though most small neurons fire less than large neurons. However, because small neurons have much broader action potentials than large neurons they may release as much K+ as the large neurons.
Altered Electrophysiological Properties of SGCs after CCD
In comparison with single SGCs of control ganglia, those in compressed DRGs had more depolarized resting membrane potentials, higher input resistances and less passive current especially Kir current. Indeed, the reduction in the density of Kir currents in single SGCs accompanied the increased excitability of DRG neurons. The SGCs around all types of DRG neurons exhibited significantly reduced Kir currents after CCD. Our result is in line with previous findings that glial cells, including astrocytes and Müller cells, have exhibited largely decreased Kir currents under pathological conditions (Bordey et al., 2001; D’Ambrosio et al., 1999; Francke et al., 1997; Schröder et al., 1999). The reduction in Kir was associated with impaired K+ buffering and enhanced neuronal activity (D’Ambrosio et al., 1999; Janigro et al., 1997). It is possible that the reduction of Kir currents in SGCs also compromised their ability at buffering K+ and promoted the accumulation of extracellular K+. Although a real time measurement of the concentration of K+ by potassium sensitive electrodes would provide more detailed information on K+ buffering, the very limited space between the DRG neuron and its SGCs (Pannese et al, 1996) makes such a test unfeasible. It was recently discovered that mice without Kir4.1 in SGCs of trigeminal ganglion show facial pain-like behavior, suggesting a correlation between altered glial Kir channels in the development of neuropathic pain (Vit et al., 2008). Similarly, our findings suggest the possibility that a compromised K+ buffering of SGCs might contribute to the neuronal hyperexcitability in an early phase after CCD prior to the onset of increased coupling between SGCs that begins a few days later.
Although the SGCs associated with spontaneously active neurons, had less Kir currents on POD1 when compared to SGCs of control or silent CCD neurons, they regained their Kir currents on POD7. Conversely, excessive neuronal firing may initiate an upregulation of Kir expression in SGCs as demonstrated for other types of neurons (Barres et al., 1990; Konishi, 1994). Thus, SGCs of neurons that became spontaneously active on POD1, and that exhibited a greater decrease in Kir than SGCs of silent neurons, responded (possibly due to the excessive neuronal activity) with an upregulation and restoration of the density of Kir current within the next six days. SGCs of silent but hyperexcitable neurons exhibited a trend toward, but not completion of, such restoration by POD7. This also parallels our finding that SGCs around non-nociceptive neurons have more Kir currents than those around nociceptive neurons.
Acknowledgments
We appreciate the statistical assistance of Daniel Zelterman
This work was supported by NIH Grants NS053491 and NS014624.
References
- Anderson LC, von Bartheld CS, Byers MR. NGF depletion reduces ipsilateral and contralateral trigeminal satellite cell reactions after inferior alveolar nerve injury in adult rats. Exp Neurol. 1998;150:312–320. doi: 10.1006/exnr.1997.6769. [DOI] [PubMed] [Google Scholar]
- Baker MD, Ritchie JM. Characteristics of type I and type II K+ channels in rabbit and Schwann cells. J Physiol. 1996;490:79–95. doi: 10.1113/jphysiol.1996.sp021128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Chun LL, Corey DP. Ion channels in vertebrate glia. Annu Rev Neurosci. 1990;13:441–474. doi: 10.1146/annurev.ne.13.030190.002301. [DOI] [PubMed] [Google Scholar]
- Barres BA. Glia ion channels. Curr Opin Neurobiol. 1991;1:354–359. doi: 10.1016/0959-4388(91)90052-9. [DOI] [PubMed] [Google Scholar]
- Bordey A, Lyons SA, Hablitz JJ, Sontheimer H. Electrophysiological characteristics of reactive astrocytes in experimental cortical dysplasia. J Neurophysiol. 2001;85:1719–1731. doi: 10.1152/jn.2001.85.4.1719. [DOI] [PubMed] [Google Scholar]
- Bordey A, Sontheimer H. Ion channel expression by astrocytes in situ: comparison of different CNS regions. Glia. 2000;30:27–38. doi: 10.1002/(sici)1098-1136(200003)30:1<27::aid-glia4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Cherkas PS, Huang TY, Pannicke T, Tal M, Reichenbach A, Hanani M. The effects of axotomy on neurons and satellite glial cells in mouse trigeminal ganglion. Pain. 2004;110:290–298. doi: 10.1016/j.pain.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Chudler EH, Anderson LC, Byers MR. Trigeminal ganglion neuronal activity and glial fibrillary acidic protein immunoreactivity after inferior alveolar nerve crush in the adult rat. Pain. 1997;73:141–149. doi: 10.1016/S0304-3959(97)00088-2. [DOI] [PubMed] [Google Scholar]
- D’Ambrosio R, Maris DO, Grady MS, Winn HR, Janigro D. Impaired K(+) homeostasis and altered electrophysiological properties of post-traumatic hippocampal glia. J Neurosci. 1999;19:8152–8162. doi: 10.1523/JNEUROSCI.19-18-08152.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dublin P, Hanani M. Satellite glial cells in sensory ganglia: their possible contribution to inflammatory pain. Brain Behav Immun. 2007;21:592–598. doi: 10.1016/j.bbi.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Francke M, Pannicke T, Biedermann B, Faude F, Wiedemann P, Reichenbach A, Reichelt W. Loss of inwardly rectifying potassium currents by human retinal glial cells in disease of the eye. Glia. 1997;20:210–218. doi: 10.1002/(sici)1098-1136(199707)20:3<210::aid-glia5>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Gola M, Niel JP, Delmas P, Jacquet G. Satellite glial cells in situ within mammalian prevertebral ganglia express K+ channels active at rest potential. J Membr Biol. 1993;136:75–84. doi: 10.1007/BF00241491. [DOI] [PubMed] [Google Scholar]
- Gommerat I, Gola M. Satellite glial cell responses to neuronal firing in the nervous system of Helix pomatia. J Membr Biol. 1994;138:209–219. doi: 10.1007/BF00232793. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell free membrane patches. Pfluegers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hanani M, Huang TY, Cherkas PS, Ledda M, Pannese E. Glial cell plasticity in sensory ganglia induced by nerve damage. Neuroscience. 2002;114:279–283. doi: 10.1016/s0306-4522(02)00279-8. [DOI] [PubMed] [Google Scholar]
- Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Res Rev. 2005;48:457–476. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Hu SJ, Xing JL. An experimental model for chronic compression of dorsal root ganglion produced by intervertebral foramen stenosis in the rat. Pain. 1998;77:15–23. doi: 10.1016/S0304-3959(98)00067-0. [DOI] [PubMed] [Google Scholar]
- Huang TY, Cherkas PS, Rosenthal DW, Hanani M. Dye coupling among satellite glial cells in mammalian dorsal root ganglia. Brain Res. 2005;1036:42–49. doi: 10.1016/j.brainres.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Janigro D, Gasparini S, D’Ambrosio R, McKhann G, DiFrancesco D. Reduction of K+ uptake I glia prevents long-term depression maintenance and causes epileptiform activity. J Neurosci. 1997;17:2813–2824. doi: 10.1523/JNEUROSCI.17-08-02813.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Ransom BR. Electrical coupling between astrocytes and between oligodendrocytes studied in mammalian cell cultures. Glia. 1988;1:64–73. doi: 10.1002/glia.440010108. [DOI] [PubMed] [Google Scholar]
- Koerber HR, Druzinsky RE, Mendell LM. Properties of somata of spinal dorsal root ganglion cells differ according to peripheral receptor innervated. J Neurophysiol. 1988;60:1584–1596. doi: 10.1152/jn.1988.60.5.1584. [DOI] [PubMed] [Google Scholar]
- Konishi T. Activity-dependent regulation of inwardly rectifying potassium currents in non-myelinating Schwann cells in mice. J Physiol. 1994;474:193–202. doi: 10.1113/jphysiol.1994.sp020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi T. Developmental and activity-dependent changes in K+ currents in satellite glial cells in the mouse superior cervical ganglion. Brain Res. 1996;708:7–15. doi: 10.1016/0006-8993(95)01221-4. [DOI] [PubMed] [Google Scholar]
- Konishi T. Voltage-dependent potassium channels in cultured mammalian Schwann cells. Brain Res. 1989a;499:273–280. doi: 10.1016/0006-8993(89)90775-0. [DOI] [PubMed] [Google Scholar]
- Konishi T. Voltage-dependent potassium channels in mouse Schwann cells. J Physiol. 1989b;411:115–130. doi: 10.1113/jphysiol.1989.sp017564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, LaMotte RH. Enhanced excitability of dissociated primary sensory neurons after chronic compression of the dorsal root ganglion in the rat. Pain. 2005;113:106–112. doi: 10.1016/j.pain.2004.10.001. [DOI] [PubMed] [Google Scholar]
- McKhann GM, D’Ambrosio R, Janigro D. Heterogeneity of astrocyte resting membrane potentials and intercellular coupling revealed by whole-cell and gramicidin-perforated patch recordings from cultured neocortical and hippocampal slice astrocytes. J Neurosci. 1997;17:6850–6863. doi: 10.1523/JNEUROSCI.17-18-06850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norenberg MD, Martinez-Hernandez A. Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res. 1979;161:303–310. doi: 10.1016/0006-8993(79)90071-4. [DOI] [PubMed] [Google Scholar]
- Pannese E, Ledda M, Cherkas PS, Huang TY, Hanani M. Satellite cell reactions to axon injury of sensory ganglion neurons: increase in number of gap junctions and formation of bridges connecting previously separate perineuronal sheaths. Anatomy & Embryology. 2003;206:337–347. doi: 10.1007/s00429-002-0301-6. [DOI] [PubMed] [Google Scholar]
- Pannese E. The satellite cells of sensory ganglia. Adv Anat Embryol Cell Biol. 1981;65:1–111. doi: 10.1007/978-3-642-67750-2. [DOI] [PubMed] [Google Scholar]
- Ransom CB, Sontheimer H. Biophysical and pharmacological characterization of inwardly rectifying K+ currents in rat spinal cord astrocytes. J Neurophysiol. 1995;73:333–346. doi: 10.1152/jn.1995.73.1.333. [DOI] [PubMed] [Google Scholar]
- Rozental R, Srinivas M, Spray DC. How to close a gap junction channel. Efficacies and potencies of uncoupling agents. Methods Mol Biol. 2001;154:447–476. doi: 10.1385/1-59259-043-8:447. [DOI] [PubMed] [Google Scholar]
- Schröder W, Hager G, Kouprijanova E, Weber M, Schmitt AB, Seifert G, Steinhäuser C. Lesion-induced changes of electrophysiological properties in astrocytes of the rat dentate gyrus. Glia. 1999;28:166–174. [PubMed] [Google Scholar]
- Shinder V, Govrin-Lippmann R, Cohen S, Belenky M, Ilin P, Fried K, Wilkinson HA, Devor M. Structural basis of sympathetic-sensory coupling in rat and human dorsal root ganglia following peripheral nerve injury. J Neurocytol. 1999;28:743–761. doi: 10.1023/a:1007090105840. [DOI] [PubMed] [Google Scholar]
- Sontheimer H. Voltage-dependent ion channels in glial cells. Glia. 1994;11:156–172. doi: 10.1002/glia.440110210. [DOI] [PubMed] [Google Scholar]
- Stephenson JL, Byers MR. GFAP immunoreactivity in trigeminal ganglion satellite cells after tooth injury in rats. Exp Neurol. 1995;131:11–22. doi: 10.1016/0014-4886(95)90003-9. [DOI] [PubMed] [Google Scholar]
- Takeda M, Tanimoto T, Kadoi J, Nasu M, Takabashi M, Kitagawa J, Matsumoto S. Enhanced excitability of nociceptive trigeminal ganglion neurons by satellite glial cytokine following peripheral inflammation. Pain. 2007;129:155–166. doi: 10.1016/j.pain.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Steinhäuser C. Ion channels in glial cells. Brain Res Brain Res Rev. 2000;32:380–412. doi: 10.1016/s0165-0173(99)00093-4. [DOI] [PubMed] [Google Scholar]
- Vit JP, Ohara PT, Bhargava A, Kelley K, Jasmin L. Silencing the Kir4.1 potassium channel subunit in satellite glial cells of the rat trigeminal ganglion results in pain-like behavior in the absence of nerve injury. J Neurosci. 2008;28:4161–4171. doi: 10.1523/JNEUROSCI.5053-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson GF, Chiu SY. Potassium channel regulation in Schwann cells during early developmental myelinogenesis. J Neurosci. 1990;10:1615–1625. doi: 10.1523/JNEUROSCI.10-05-01615.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodham P, Anderson PN, Nadim W, Turmaine M. Satellite cells surrounding axotomized rat dorsal root ganglion cells increase expression of GFAP-like protein. Neurosci Lett. 1989;98:8–12. doi: 10.1016/0304-3940(89)90364-9. [DOI] [PubMed] [Google Scholar]
- Zelterman D. Models for discrete data. New York: Oxford University Press Inc; 2006. [Google Scholar]
- Zhang JM, Song XJ, LaMotte RH. Enhanced excitability of sensory neurons in rats with cutaneous hyperalgesia produced by chronic compression of the dorsal root ganglion. J Neurophysiol. 1999;82:3359–3366. doi: 10.1152/jn.1999.82.6.3359. [DOI] [PubMed] [Google Scholar]
- Zhou XF, Rush RA, McLachlan EM. Differential expression of the p75 nerve growth factor receptor in glia and neurons of the dorsal root ganglia after peripheral nerve transection. J Neurosci. 1996;16:2901–2911. doi: 10.1523/JNEUROSCI.16-09-02901.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]