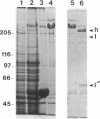
Abstract
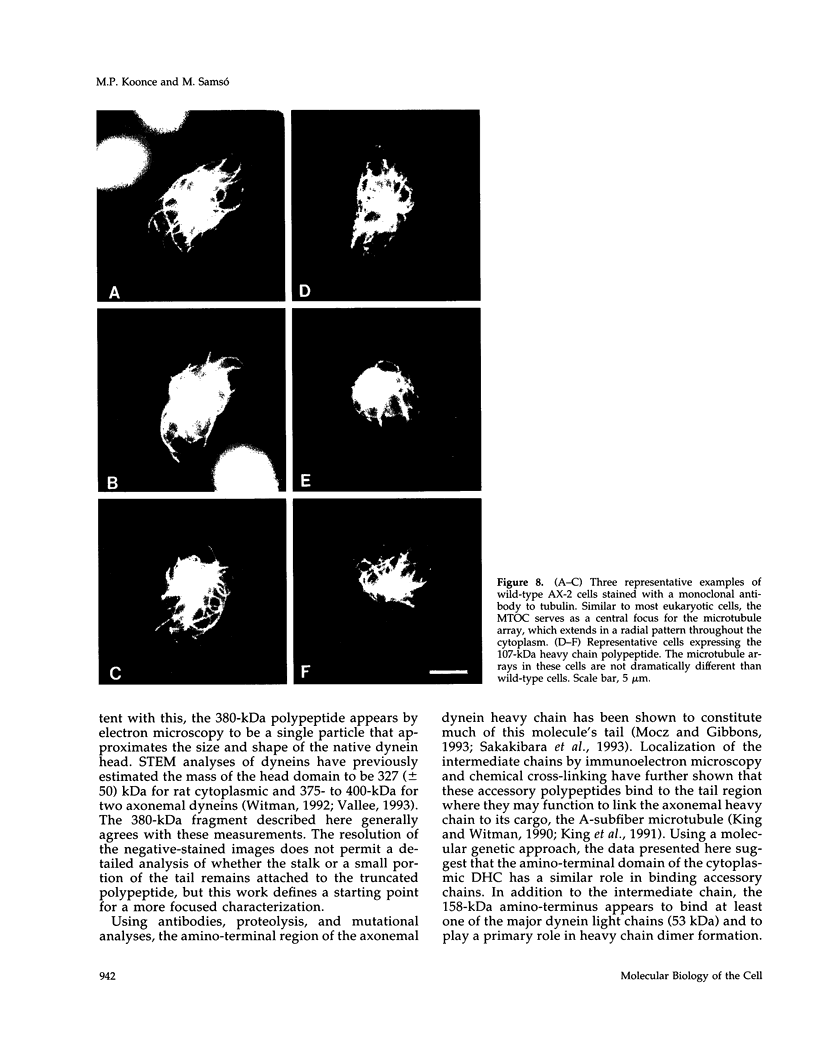
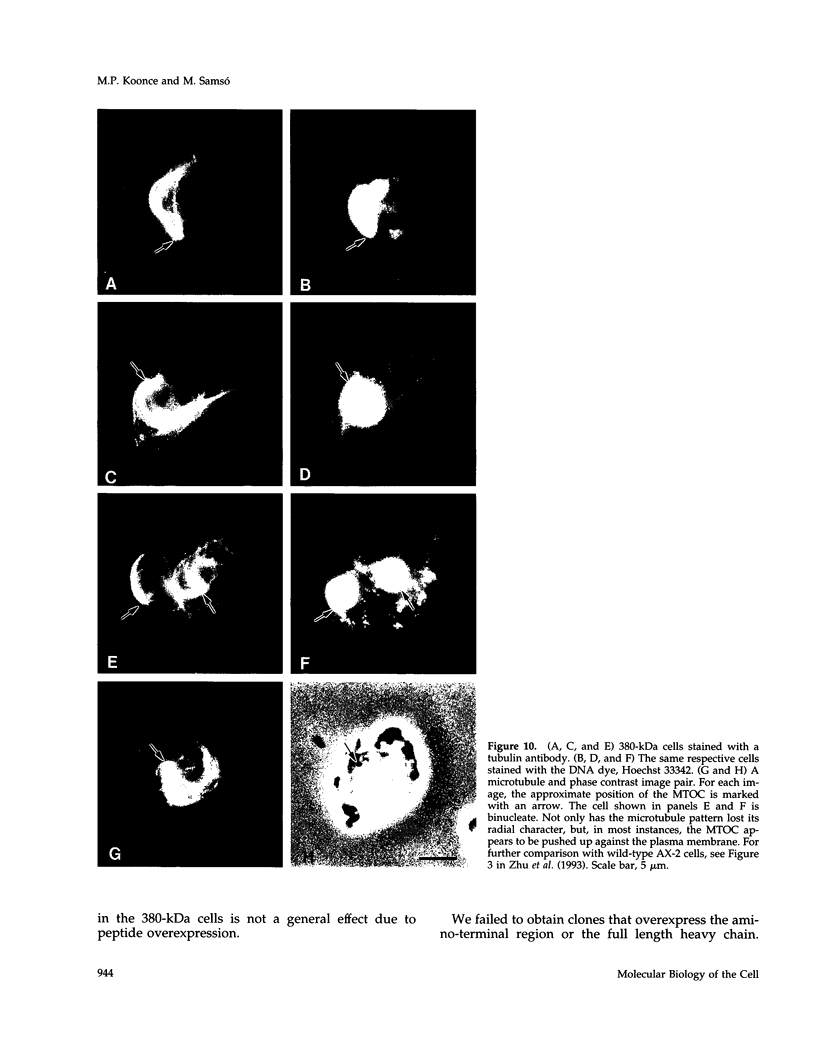
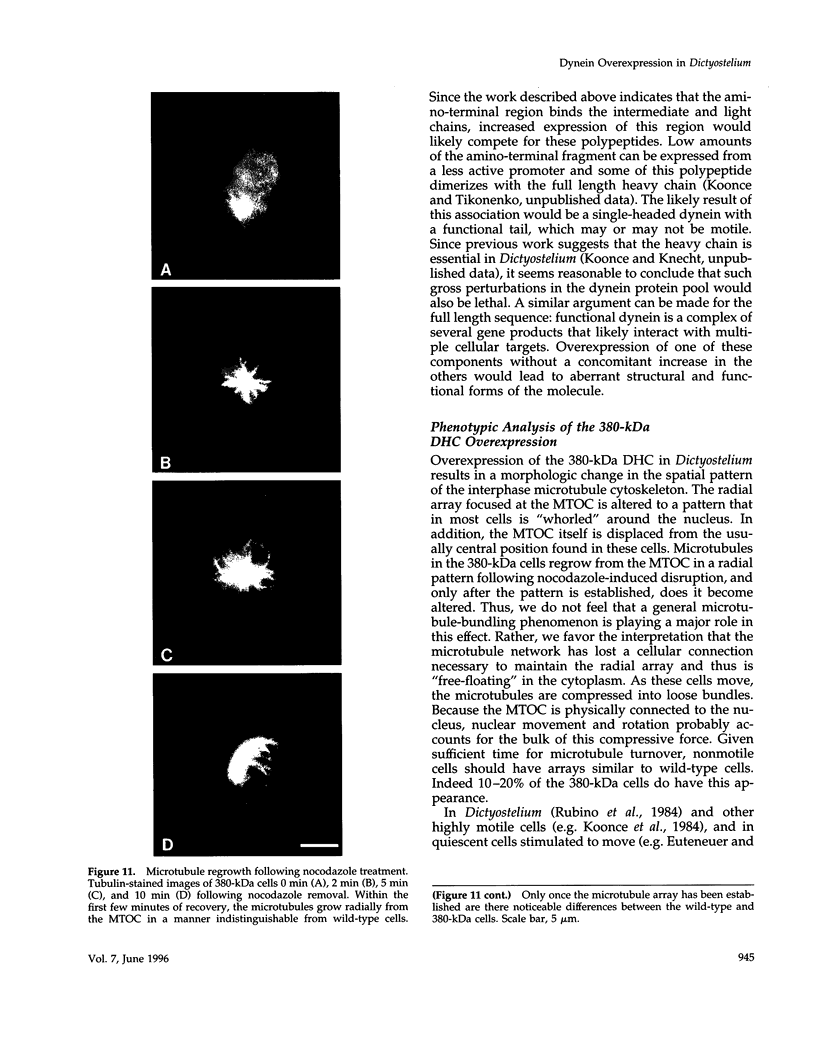
Cytoplasmic dynein is a minus-end directed microtubule-based motor. Using a molecular genetic approach, we have begun to dissect structure-function relationships of dynein in the cellular slime mold Dictyostelium. Expression of a carboxy-terminal 380-kDa fragment of the heavy chain produces a protein that approximates the size and shape of the globular, mechanochemical head of dynein. This polypeptide cosediments with microtubules in an ATP-sensitive fashion and undergoes a UV-vanadate cleavage reaction. The deleted amino-terminal region appears to participate in dimerization of the native protein and in binding the intermediate and light chains. Overexpression of the 380-kDa carboxy-terminal construct in Dictyostelium produces a distinct phenotype in which the interphase radial microtubule array appears collapsed. In many cells, the microtubules form loose bundles that are whorled around the nucleus. Similar expression of a central 107-kDa fragment of the heavy chain does not produce this result. The data presented here suggest that dynein may participate in maintaining the spatial pattern of the interphase microtubule network.
Full text
PDF

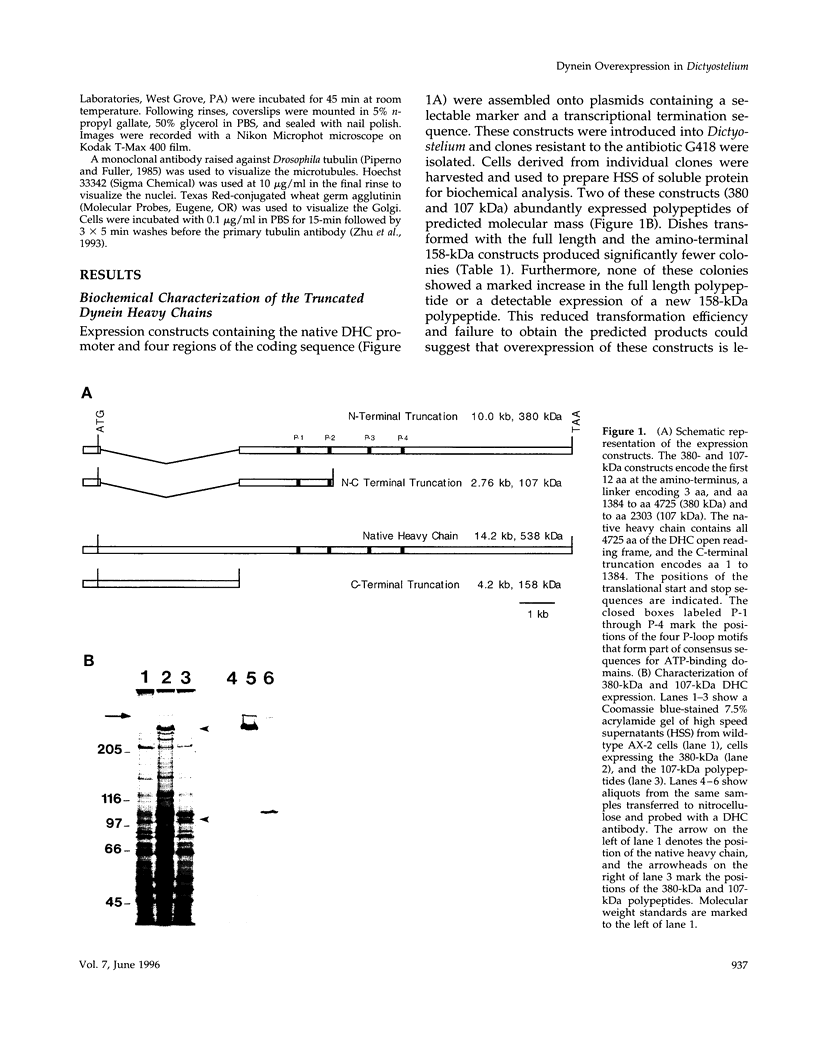
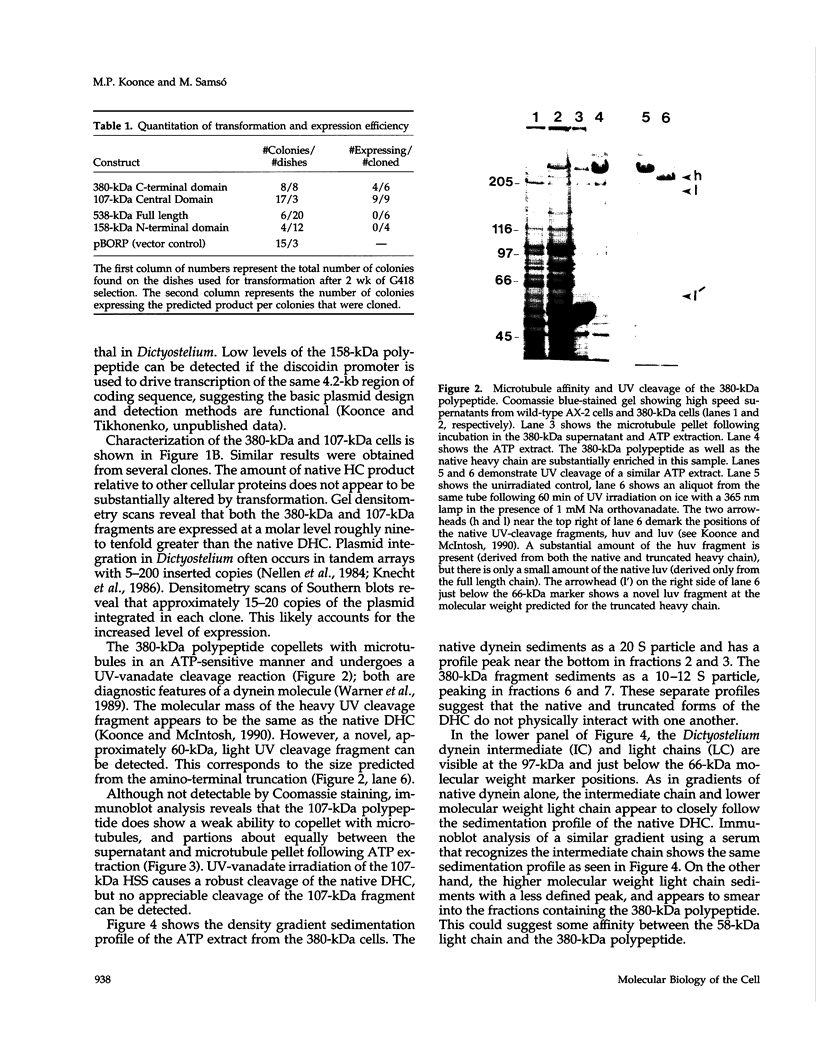







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ault J. G., Rieder C. L. Centrosome and kinetochore movement during mitosis. Curr Opin Cell Biol. 1994 Feb;6(1):41–49. doi: 10.1016/0955-0674(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Dillman J. F., 3rd, Pfister K. K. Differential phosphorylation in vivo of cytoplasmic dynein associated with anterogradely moving organelles. J Cell Biol. 1994 Dec;127(6 Pt 1):1671–1681. doi: 10.1083/jcb.127.6.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egelhoff T. T., Titus M. A., Manstein D. J., Ruppel K. M., Spudich J. A. Molecular genetic tools for study of the cytoskeleton in Dictyostelium. Methods Enzymol. 1991;196:319–334. doi: 10.1016/0076-6879(91)96029-q. [DOI] [PubMed] [Google Scholar]
- Endow S. A., Titus M. A. Genetic approaches to molecular motors. Annu Rev Cell Biol. 1992;8:29–66. doi: 10.1146/annurev.cb.08.110192.000333. [DOI] [PubMed] [Google Scholar]
- Eshel D., Urrestarazu L. A., Vissers S., Jauniaux J. C., van Vliet-Reedijk J. C., Planta R. J., Gibbons I. R. Cytoplasmic dynein is required for normal nuclear segregation in yeast. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11172–11176. doi: 10.1073/pnas.90.23.11172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euteneuer U., Schliwa M. Mechanism of centrosome positioning during the wound response in BSC-1 cells. J Cell Biol. 1992 Mar;116(5):1157–1166. doi: 10.1083/jcb.116.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke J., Kessin R. A defined minimal medium for axenic strains of Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1977 May;74(5):2157–2161. doi: 10.1073/pnas.74.5.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Rosen D., Berke G. Spatial relationships of microtubule-organizing centers and the contact area of cytotoxic T lymphocytes and target cells. J Cell Biol. 1982 Oct;95(1):137–143. doi: 10.1083/jcb.95.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons I. R., Gibbons B. H., Mocz G., Asai D. J. Multiple nucleotide-binding sites in the sequence of dynein beta heavy chain. Nature. 1991 Aug 15;352(6336):640–643. doi: 10.1038/352640a0. [DOI] [PubMed] [Google Scholar]
- Holzbaur E. L., Vallee R. B. DYNEINS: molecular structure and cellular function. Annu Rev Cell Biol. 1994;10:339–372. doi: 10.1146/annurev.cb.10.110194.002011. [DOI] [PubMed] [Google Scholar]
- Hyman A. A. Centrosome movement in the early divisions of Caenorhabditis elegans: a cortical site determining centrosome position. J Cell Biol. 1989 Sep;109(3):1185–1193. doi: 10.1083/jcb.109.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoué S., Salmon E. D. Force generation by microtubule assembly/disassembly in mitosis and related movements. Mol Biol Cell. 1995 Dec;6(12):1619–1640. doi: 10.1091/mbc.6.12.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg D. R., Moritz M., Alberts B. M. The centrosome and cellular organization. Annu Rev Biochem. 1994;63:639–674. doi: 10.1146/annurev.bi.63.070194.003231. [DOI] [PubMed] [Google Scholar]
- King S. M., Patel-King R. S. The M(r) = 8,000 and 11,000 outer arm dynein light chains from Chlamydomonas flagella have cytoplasmic homologues. J Biol Chem. 1995 May 12;270(19):11445–11452. doi: 10.1074/jbc.270.19.11445. [DOI] [PubMed] [Google Scholar]
- King S. M., Wilkerson C. G., Witman G. B. The Mr 78,000 intermediate chain of Chlamydomonas outer arm dynein interacts with alpha-tubulin in situ. J Biol Chem. 1991 May 5;266(13):8401–8407. [PubMed] [Google Scholar]
- King S. M., Witman G. B. Localization of an intermediate chain of outer arm dynein by immunoelectron microscopy. J Biol Chem. 1990 Nov 15;265(32):19807–19811. [PubMed] [Google Scholar]
- Kirschner M., Mitchison T. Beyond self-assembly: from microtubules to morphogenesis. Cell. 1986 May 9;45(3):329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- Knecht D. A., Cohen S. M., Loomis W. F., Lodish H. F. Developmental regulation of Dictyostelium discoideum actin gene fusions carried on low-copy and high-copy transformation vectors. Mol Cell Biol. 1986 Nov;6(11):3973–3983. doi: 10.1128/mcb.6.11.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht D. A., Jung J., Matthews L. Quantification of transformation efficiency using a new method for clonal growth and selection of axenic Dictyostelium cells. Dev Genet. 1990;11(5-6):403–409. doi: 10.1002/dvg.1020110513. [DOI] [PubMed] [Google Scholar]
- Koonce M. P., Cloney R. A., Berns M. W. Laser irradiation of centrosomes in newt eosinophils: evidence of centriole role in motility. J Cell Biol. 1984 Jun;98(6):1999–2010. doi: 10.1083/jcb.98.6.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonce M. P., Grissom P. M., Lyon M., Pope T., McIntosh J. R. Molecular characterization of a cytoplasmic dynein from Dictyostelium. J Eukaryot Microbiol. 1994 Nov-Dec;41(6):645–651. doi: 10.1111/j.1550-7408.1994.tb01528.x. [DOI] [PubMed] [Google Scholar]
- Koonce M. P., Grissom P. M., McIntosh J. R. Dynein from Dictyostelium: primary structure comparisons between a cytoplasmic motor enzyme and flagellar dynein. J Cell Biol. 1992 Dec;119(6):1597–1604. doi: 10.1083/jcb.119.6.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonce M. P., McIntosh J. R. Identification and immunolocalization of cytoplasmic dynein in Dictyostelium. Cell Motil Cytoskeleton. 1990;15(1):51–62. doi: 10.1002/cm.970150108. [DOI] [PubMed] [Google Scholar]
- Li Y. Y., Yeh E., Hays T., Bloom K. Disruption of mitotic spindle orientation in a yeast dynein mutant. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10096–10100. doi: 10.1073/pnas.90.21.10096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. X., Ferro K. L., Collins C. A. Cytoplasmic dynein undergoes intracellular redistribution concomitant with phosphorylation of the heavy chain in response to serum starvation and okadaic acid. J Cell Biol. 1994 Nov;127(4):1009–1019. doi: 10.1083/jcb.127.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombillo V. A., Nislow C., Yen T. J., Gelfand V. I., McIntosh J. R. Antibodies to the kinesin motor domain and CENP-E inhibit microtubule depolymerization-dependent motion of chromosomes in vitro. J Cell Biol. 1995 Jan;128(1-2):107–115. doi: 10.1083/jcb.128.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz D. A., Hamaguchi Y., Inoué S. Micromanipulation studies of the asymmetric positioning of the maturation spindle in Chaetopterus sp. oocytes: I. Anchorage of the spindle to the cortex and migration of a displaced spindle. Cell Motil Cytoskeleton. 1988;11(2):83–96. doi: 10.1002/cm.970110202. [DOI] [PubMed] [Google Scholar]
- Maniotis A., Schliwa M. Microsurgical removal of centrosomes blocks cell reproduction and centriole generation in BSC-1 cells. Cell. 1991 Nov 1;67(3):495–504. doi: 10.1016/0092-8674(91)90524-3. [DOI] [PubMed] [Google Scholar]
- McCaffrey G., Vale R. D. Identification of a kinesin-like microtubule-based motor protein in Dictyostelium discoideum. EMBO J. 1989 Nov;8(11):3229–3234. doi: 10.1002/j.1460-2075.1989.tb08482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNiven M. A., Porter K. R. Organization of microtubules in centrosome-free cytoplasm. J Cell Biol. 1988 May;106(5):1593–1605. doi: 10.1083/jcb.106.5.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocz G., Gibbons I. R. ATP-insensitive interaction of the amino-terminal region of the beta heavy chain of dynein with microtubules. Biochemistry. 1993 Apr 6;32(13):3456–3460. doi: 10.1021/bi00064a032. [DOI] [PubMed] [Google Scholar]
- Nellen W., Silan C., Firtel R. A. DNA-mediated transformation in Dictyostelium discoideum: regulated expression of an actin gene fusion. Mol Cell Biol. 1984 Dec;4(12):2890–2898. doi: 10.1128/mcb.4.12.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K. Four ATP-binding sites in the midregion of the beta heavy chain of dynein. Nature. 1991 Aug 15;352(6336):643–645. doi: 10.1038/352643a0. [DOI] [PubMed] [Google Scholar]
- Ostrow B. D., Chen P., Chisholm R. L. Expression of a myosin regulatory light chain phosphorylation site mutant complements the cytokinesis and developmental defects of Dictyostelium RMLC null cells. J Cell Biol. 1994 Dec;127(6 Pt 2):1945–1955. doi: 10.1083/jcb.127.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G., Fuller M. T. Monoclonal antibodies specific for an acetylated form of alpha-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J Cell Biol. 1985 Dec;101(6):2085–2094. doi: 10.1083/jcb.101.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plamann M., Minke P. F., Tinsley J. H., Bruno K. S. Cytoplasmic dynein and actin-related protein Arp1 are required for normal nuclear distribution in filamentous fungi. J Cell Biol. 1994 Oct;127(1):139–149. doi: 10.1083/jcb.127.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino S., Fighetti M., Unger E., Cappuccinelli P. Location of actin, myosin, and microtubular structures during directed locomotion of Dictyostelium amebae. J Cell Biol. 1984 Feb;98(2):382–390. doi: 10.1083/jcb.98.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara H., Takada S., King S. M., Witman G. B., Kamiya R. A Chlamydomonas outer arm dynein mutant with a truncated beta heavy chain. J Cell Biol. 1993 Aug;122(3):653–661. doi: 10.1083/jcb.122.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer T. A. Structure, function and regulation of cytoplasmic dynein. Curr Opin Cell Biol. 1994 Feb;6(1):69–73. doi: 10.1016/0955-0674(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Scliwa M., Höner B. Microtubules, centrosomes and intermediate filaments in directed cell movement. Trends Cell Biol. 1993 Nov;3(11):377–380. doi: 10.1016/0962-8924(93)90086-g. [DOI] [PubMed] [Google Scholar]
- Stöffler-Meilicke M., Stöffler G. Localization of ribosomal proteins on the surface of ribosomal subunits from Escherichia coli using immunoelectron microscopy. Methods Enzymol. 1988;164:503–520. doi: 10.1016/s0076-6879(88)64066-3. [DOI] [PubMed] [Google Scholar]
- Vaisberg E. A., Koonce M. P., McIntosh J. R. Cytoplasmic dynein plays a role in mammalian mitotic spindle formation. J Cell Biol. 1993 Nov;123(4):849–858. doi: 10.1083/jcb.123.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R. Molecular analysis of the microtubule motor dynein. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8769–8772. doi: 10.1073/pnas.90.19.8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J. C., Cole R. W., Rieder C. L. The force-producing mechanism for centrosome separation during spindle formation in vertebrates is intrinsic to each aster. J Cell Biol. 1993 Jul;122(2):361–372. doi: 10.1083/jcb.122.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witman G. B. Axonemal dyneins. Curr Opin Cell Biol. 1992 Feb;4(1):74–79. doi: 10.1016/0955-0674(92)90061-g. [DOI] [PubMed] [Google Scholar]
- Xiang X., Beckwith S. M., Morris N. R. Cytoplasmic dynein is involved in nuclear migration in Aspergillus nidulans. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2100–2104. doi: 10.1073/pnas.91.6.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q., Liu T., Clarke M. Calmodulin and the contractile vacuole complex in mitotic cells of Dictyostelium discoideum. J Cell Sci. 1993 Apr;104(Pt 4):1119–1127. doi: 10.1242/jcs.104.4.1119. [DOI] [PubMed] [Google Scholar]