Abstract
The severity of Hashimoto's disease (HD) and intractability of Graves' disease (GD) varies among patients. Severity of HD is associated with the functional +874A/T polymorphism for interferon-γ, an inflammatory cytokine. To clarify the association between functional polymorphisms in two other inflammatory cytokine genes [tumour necrosis factor (TNF)-α and interleukin (IL)-2] and the severity of autoimmune thyroid disease (AITD), we examined the TNF-α−1031T/C, TNF-α−857C/T and IL-2 −330T/G polymorphisms in genomic DNA samples. We genotyped 41 patients with intractable GD, 34 patients with GD in remission, 41 patients with severe HD, 36 patients with mild HD and 70 healthy controls. The frequency of carriers of TNF-α−1031C (CT + CC), which correlates with higher TNF-α production, was significantly higher in HD and GD patients than in controls, but was not associated with the severity of HD. In GD patients, the levels of anti-thyrotropin receptor antibody (TRAb) at onset of the disease was higher in patients with the TNF-α−857T (CT + TT) genotype, which correlates with higher TNF-α production, than in those with the −857CC genotype. We found no differences in the IL-2 −330T/G polymorphism among groups of AITD patients. In conclusion, the functional −1031T/C polymorphism of the TNFA gene is associated with the development of AITD and the functional −857C/T polymorphism is associated with the levels of TRAb in active GD patients.
Keywords: disease severity, IL-2, single nucleotide polymorphism, TNF-α, TRAb
Introduction
Autoimmune thyroid disease (AITD), such as Hashimoto's disease (HD) and Graves' disease (GD), are archetypes for organ-specific autoimmune disease [1,2]. The severity of HD and intractability of GD varies among patients. Some patients develop hypothyroidism in early life, and some maintain a euthyroid state in old age despite the passage of time [3,4]. On the other hand, some patients with GD achieve remission through medical treatment, while others do not. However, the severity of HD and the intractability of GD are difficult to predict at the first diagnosis. We have reported previously that the T allele of the interferon (IFN)-γ+874A/T polymorphism, which enhances the production of IFN-γ, a typical inflammatory cytokine, was more frequent in patients with severe HD [5]. This finding suggests that hypothyroidism is more likely to develop in HD patients with a genetically higher productivity of IFN-γ because IFN-γ increases the activity of cytotoxic T lymphocytes, which play an important role in thyroid destruction in thyroid autoimmunity [6].
Tumour necrosis factor (TNF)-α is another inflammatory cytokine that is produced by macrophage, monocytes, epithelial cells and lymphocytes, and induces the production of IFN-γ and interleukin (IL)-6 [7,8]. The serum concentration of TNF-α is elevated in patients with rheumatoid arthritis (RA), and mRNA levels of TNF-α are significantly higher in thyroid tissues obtained from HD patients than in tissue obtained from controls [9]. This finding suggests the involvement of TNF-α in autoimmune inflammatory diseases. Some polymorphisms (–1031T/C, −863C/A, −857C/T, −308G/A and −238G/A) in the TNFA gene promoter region have been reported to be associated with various diseases, such as RA and asthma [10–14]. The polymorphisms −857C/T, −863C/A and −1031T/C are frequent in the Japanese population, and −863C/A and −1031T/C are in significant linkage disequilibrium with each other [15,16]. Most Japanese have haplotypes consisting of either −1031T/−863C or −1031C/−863A [17]. The promoter sequence in individuals with the −1031C/−863A haplotype has a higher luciferase activity than that in individuals with the −1031T/−863C haplotype [15]. In the present study, we focused on the −1031T/C polymorphism to distinguish the two haplotypes observed commonly in the Japanese population. On the other hand, the −857C/T polymorphism, which is not linked to the −1031T/C polymorphism [15], has also been shown to be associated with the prognosis of RA [18]. The −857T allele of this polymorphism has significantly greater transcriptional activity than does the −857C allele in response to lipopolysaccharides [19].
The IL-2 is another inflammatory cytokine and is produced by activated T cells. G/G homozygosity of the −330T/G polymorphism in the IL2 gene has been reported to result in a threefold increase in IL-2 production compared with the T/T or T/G genotypes [20]. The IL-2 −330T/G polymorphism is also associated with various autoimmune diseases such as multiple sclerosis [21].
Because we supposed that the development and severity of AITD is affected by these inflammatory cytokines, as well as by IFN-γ[5], we genotyped these polymorphisms in the TNFA and IL2 genes of HD and GD patients to clarify the association of these polymorphisms with the prognosis of HD and GD.
Materials and methods
Subjects
We obtained genomic DNA from 41 patients with HD who developed moderate to severe hypothyroidism before 50 years of age, and were treated daily with at least 1·5 µg thyroxine (T4) per kg body weight (severe HD) and from 36 untreated, euthyroid patients with HD who were more than 50 years of age (mild HD). All patients with HD were positive for anti-thyroid peroxidase antibody (TPOAb) or anti-thyroglobulin antibody (TgAb) and all patients with mild HD had palpable diffuse goitre. We also examined 41 euthyroid patients with GD who had been treated with methimazole or propylthiouracil for at least 5 years and were still positive for thyrotropin receptor antibody (TRAb) (intractable GD), 34 patients with GD in remission who had maintained a euthyroid state and had been negative for TRAb for more than 2 years without medication (GD in remission) and 70 healthy volunteers (control subjects) who were euthyroid and negative for thyroid autoantibodies. GD was diagnosed in most patients based on the presence of hyperthyroidism and serum TRAb, and in about 10% of hyperthyroid patients negative for TRAb based on the presence of thyroid-stimulating antibody or high radioactive iodine uptake by the thyroid gland. We excluded GD patients treated by thyroidectomy or radioiodine. Some GD patients were referred to our clinic after the initiation of treatment in other clinics, and hence serum TRAb could not be measured at the onset in our laboratory. All patients and control subjects were Japanese and unrelated to one another. All patients were followed-up closely for more than 5 years as out-patients at our thyroid clinic. Patients with AITD had no other autoimmune diseases such as coeliac disease, pernicious anaemia or vitiligo. Genomic DNA was isolated from ethylenediamine tetraacetic acid-treated peripheral blood mononuclear cells using a commercially available kit (Dr.GenTLE™; Takara Bio Inc., Shiga, Japan). Written informed consent was obtained from all patients and controls, and the study protocol was approved by the Ethics Committee of Osaka University.
Genotyping of −1031T/C and −857C/T polymorphisms in the TNFA gene
We used TaqMan SNP genotyping assays (Applied Biosystems, Tokyo, Japan) to genotype the −1031T/C and −857 C/T polymorphisms of the TNFA gene according to the manufacturer's instructions. Patients with the TNF-α−857C/T polymorphism were categorized into CC (lower production) and CT + TT (higher production) groups according to their production levels of TNF-α. Patients with the TNF-α−1031T/C polymorphism were categorized into TT (lower production) and CT + CC (higher production) groups using the same criterion.
Genotyping of −330T/G polymorphism in the IL2 gene
The target sequence of the IL2 gene was amplified using polymerase chain reaction (PCR). The forward primer was 5′-CTT GCT CTT GTT CAC ACA A-3′ and the reverse primer was 5′-AAT GGA TGT AGG TGA AAT CCC-3′. The protocol for PCR was as follows: 96°C for 60 sec, 30 cycles of denaturing at 96°C for 30 sec, annealing at 55°C for 30 sec, extension at 72°C for 60 sec and a single final extension at 72°C for 4 min. The genotype was determined by direct sequencing (Applied Biosystems). We categorized the patients into GG (higher production) and TG + TT (lower production) groups according to the production levels of IL-2.
Thyroid function and autoantibodies
The serum concentration of free T4 (FT4) was measured using a commercial radio immunoassay kit (Eiken Chemical Co., Ltd., Tokyo, Japan). The normal range of serum FT4 is 1·0–1·6 ng/dl (12·9–20·6 pmol/l). The serum concentration of FT3 was measured using a radioimmunoassay kit (Japan Kodak Diagnostic Co., Ltd., Tokyo, Japan). The normal range of serum FT3 is 2·4–4·6 pg/ml (3·8–7·2 pmol/l). The serum thyrotropin (TSH) concentration was also measured using a radioimmunoassay kit (Daiichi Radioisotope Laboratories Ltd, Tokyo, Japan). The normal range of serum TSH is 0·6–5·4 µU/ml. Anti-TgAb and TPOAb were measured using a particle agglutination kit (Fujirebio Inc., Tokyo, Japan). A reciprocal titre of >1 : 100 was considered positive. Serum TRAb was measured using a radioreceptor assay using a commercial kit (Cosmic Corporation, Tokyo, Japan) and the results were expressed as percentage inhibition of binding of labelled TSH. The normal value is less than 10%.
Statistical analysis
We used the χ2 test and Fisher's exact test to evaluate the significance of differences in the frequencies of genotypes and alleles between groups. Student's t-test was used to analyse differences in goitre size. The Mann–Whitney U-test was used to analyse the differences in serum titres of TPOAb and TgAb. Data were analysed using JMP7 software (SAS Institute, Inc., Tokyo, Japan). Probability values of less than 0·05 were considered significant.
Results
The IL-2 −330T/G polymorphism
We found no differences in the frequencies of genotypes and alleles of the IL-2 −330T/G polymorphism between normal subjects and the patients in the HD or GD groups. These frequencies did not differ between patients with severe HD and those with mild HD or between patients with intractable GD and those with GD in remission (Table 1).
Table 1.
Genotype and allele frequencies of the interleukin (IL)-2 −330 T/G polymorphism, and the tumour necrosis factor (TNF)-α−857C/T and the −1031T/C polymorphisms in patients with Graves' disease (GD), Hashimoto's disease (HD) and in control subjects.
| GD |
Disease HD |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Productive ability | Control | All patients with GD | All patients with disease HD | Intractable | In remission | Severe | Mild | ||||||
| IL-2 −330 | TT | Low | 36 (62·1%) | 37 (46·3%) | 41 (55·4%) | 26 (52·0%) | 11 (36·7%) | 17 (42·5%) | 24 (70·6%) | ||||
| TG | Low | 17 (29·3%) | 38 (47·5%) | n.s.a | 25 (33·8%) | n.s.a | 20 (40·0%) | 18 (60·0%) | n.s.b | 17 (42·5%) | 8 (23·5%) | n.s.c | |
| GG | High | 5 (8·6%) | 5 (6·2%) | 8 (10·8%) | 4 (8·0%) | 1 (3·3%) | 6 (15·0%) | 2 (5·9%) | |||||
| TT + TG | Low | 53 (91·4%) | 75 (93·8%) | n.s.a† | 66 (89·2%) | n.s.a | 46 (92·0%) | 29 (96·7%) | n.s.b† | 34 (85·0%) | 32 (94·1%) | n.s.c† | |
| GG | High | 5 (8·6%) | 5 (6·2%) | 8 (10·8%) | 4 (8·0%) | 1 (3·3%) | 6 (15·0%) | 2 (5·9%) | |||||
| TNF-α −857 | CC | Low | 46 (65·7%) | 43 (57·3%) | 52 (67·5%) | 21 (51·2%) | 22 (64·7%) | 24 (58·5%) | 28 (77·8%) | ||||
| CT | High | 22 (31·4%) | 28 (37·3%) | n.s.a | 22 (28·6%) | n.s.a | 18 (43·9%) | 10 (29·4%) | n.s.b | 15 (36·6%) | 7 (19·4%) | n.s.c | |
| TT | High | 2 (2·9%) | 4 (5·3%) | 3 (3·9%) | 2 (4·9%) | 2 (5·9%) | 2 (4·9%) | 1 (2·8%) | |||||
| CC | Low | 46 (65·7%) | 43 (57·3%) | n.s.a | 52 (67·5%) | n.s.a | 21 (51·2%) | 22 (64·7%) | n.s.b | 24 (58·5%) | 28 (77·8%) | n.s.c | |
| CT + TT | High | 24 (34·3%) | 32 (42·6%) | 25 (32·5%) | 20 (48·8%) | 12 (35·3%) | 17 (41·5%) | 8 (22·2%) | |||||
| TNF-α −1031 | TT | Low | 53 (81·5%) | 54 (65·9%) | 31 (65·4%) | 33 (66·0%) | 21 (65·6%) | 22 (61·1%) | 9 (47·4%) | ||||
| CT | High | 9 (13·6%) | 27 (32·9%) | 0·0170a | 22 (40·0%) | 0·0049a | 17 (34·0%) | 10 (31·3%) | n.s.b | 14 (38·9%) | 8 (42·1%) | n.s.c | |
| CC | High | 3 (4·6%) | 1 (1·2%) | 2 (3·6%) | 0 (0%) | 1 (3·1%) | 0 (0%) | 2 (10·5%) | |||||
| TT | Low | 53 (81·5%) | 54 (65·9%) | 0·0338a | 31 (56·4%) | 0·0027a | 33 (66·0%) | 21 (65·6%) | n.s.b | 22 (61·1%) | 9 (47·4%) | n.s.c | |
| CT + CC | High | 12 (18·5%) | 28 (34·1%) | 24 (43·6%) | 17 (34·0%) | 11 (34·4%) | 14 (38·9%) | 10 (52·6%) | |||||
Analysed by χ2 tests or
Fisher's exact test.
Versus control;
intractable GD versus GD in remission;
severe HD versus mild HD; n.s., not significant.
The TNF-α−857C/T polymorphism
We found no differences in the frequencies of genotypes and alleles of the TNF-α−857C/T polymorphism between normal subjects and the patients in the GD or HD groups. These frequencies did not differ between patients with severe HD and those with mild HD, or between patients with intractable GD and those with GD in remission (Table 1).
The TNF-α−1031T/C polymorphism
The frequency of carriers of the C allele (CT + CC) of the TNF-α−1031T/C polymorphism was significantly higher in all HD patients than in controls. This frequency was also significantly higher in all GD patients than in controls. However, there was no significant difference in genotype and allele frequencies between the two HD and two GD groups (Table 1).
Combined analysis between TNF-α and IL-2 polymorphisms
We examined the inner correlation between the polymorphisms of TNF-α and IL-2, and found that the frequency of the C allele (CT + CC) of the TNF −1031T/C polymorphism was significantly higher in HD patients than in controls among subjects with the T allele (TG + TT) of the IL-2 −330T/G polymorphism. There were no significant correlations between the other polymorphism combinations (P = 0·0018).
Clinical characteristics and genotype frequencies
We measured TRAb levels at the onset of the disease in 32 GD patients (20 patients with intractable GD and 12 patients with GD in remission). The proportion of GD patients whose TRAb level at the onset of disease was >37% was significantly higher in TNF-α−857T carriers (CT + TT) (85·7%) than in patients with the CC genotype (44·4%) (P = 0·0276) (Fig. 1).
Fig. 1.
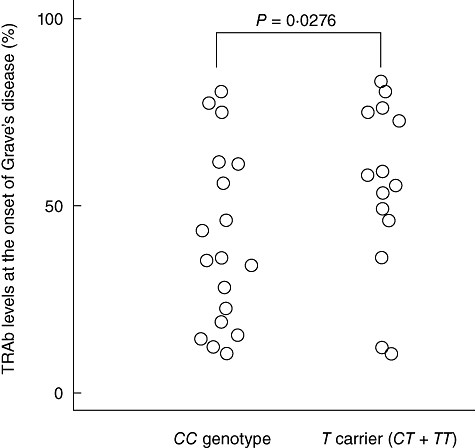
Serum levels of anti-thyrotropin receptor antibody (TRAb) at the onset of the disease in Graves' disease patients with different tumour necrosis factor (TNF)-α−857C/T genotypes.
We found no association between IL-2 −330T/G and TNF-α−1031T/C polymorphisms and TRAb levels. Moreover, we found no association between any of the polymorphisms and TPOAb titre, TgAb titre or goitre size.
Discussion
Because the destruction of thyroid follicles by autoimmune inflammation is the cause of HD, and TNF-α, IL-2 and IFN-γ are known to promote inflammation, we expected that functional polymorphisms in the TNFA and IL2 genes might be associated with the severity of HD. Some studies have examined the association between AITD and the gene polymorphisms of TNF-α; however, the association with −308A/G polymorphisms was examined more frequently, and the results differ between studies [22–24]. Furthermore, the frequency of the A allele in the −308A/G polymorphism is less than 2% in the Japanese [15]. In this study, therefore, we examined the association of the functional TNF-α−1031T/C and −857C/T polymorphisms with both the susceptibility and the severity of AITD.
In contrast with our expectation, we found no significant association between these polymorphisms with the severity of HD in the present study. On the other hand, in our previous study, the +874T allele of the IFNG gene polymorphism, which correlates with high IFN-γ production, the +869T allele of the TGFB gene polymorphism, which correlates with low transforming growth factor-β production and the −590CC genotype of the IL4 gene polymorphism, which correlates with low IL-4 production, were associated with severe destruction of the thyroid gland in HD [5,25,26]. This suggests that genetic differences in the production of various cytokines affect the severity of HD, but the production of TNF-α and IL-2 did not. IFN-γ, TNF-α and IL-2 are inflammatory cytokines and promote tissue inflammation. However, we found that the genetic difference in IFN-γ productivity was related to the severity of HD but not with the development of AITD in our previous study, and for the first time in this study, the genetic difference in TNF-α productivity was associated strongly with the development of AITD but not with the severity of HD. The genetic difference in IL-2 productivity was not associated with the development of AITD or with the severity of HD. The reason why such differences existed between similar inflammatory cytokines remains to be elucidated, but the results suggest that TNF-α plays an important role in the development of AITD, that IFN-γ affects the severity of HD and that IL-2 may not be critical for the development of AITD and the severity of HD.
The TNF-α−857C/T polymorphism has been reported to be associated with the susceptibility of GD in the Japanese population [27]. However, no significant difference in the frequency of this polymorphism between patients with GD and controls was observed in the present study (P = 0·51). This may have been a result of the smaller numbers of GD patients and controls in our study than those in previous studies [27], but even if this were the case the association would probably not be as strong. On the other hand, in our study the frequency of C carriers (CC + CT) of the −1031T/C polymorphism in TNF-α promoter gene, which correlates with high TNF-α production, was higher in all GD and HD patients than in controls. Although the −1031T/C polymorphism in the TNF-α promoter gene has been examined only in GD patients and clarified to be associated with the development of GD [28], we examined the association of this polymorphism not only with the development of AITD (GD and HD) but also with the prognosis of AITD, i.e. with the severity of HD and the intractability of GD. We found for the first time a strong association of this polymorphism with the development of HD. Therefore, this polymorphism may be associated strongly with the pathogenesis of AITD. Moreover, this polymorphism has also been reported to be linked with thyroid-associated ophthalmopathy and Behçet's disease [27,29] and the −863C/A polymorphism, which is in very strong linkage disequilibrium with −1031T/C [15,16] and is associated with systemic lupus erythematosus, RA and Guillain–Barré syndrome [30–32]. Therefore, the −1031T/C polymorphism has been linked with the development of not only AITD, but also with many other autoimmune diseases.
Interestingly, we found that significantly more GD patients with the −857T allele than GD patients with the −857CC genotype had high TRAb levels at the onset of disease. This suggests that the −857T allele of the TNFA gene, which is associated with greater TNF-α production, may be associated with a higher production of TRAb in active GD. Most TRAb is believed to be of the immunoglobulin 1 (IgG1) subclass [33], and it has been reported that TNF-α promotes IgG1 production and that IFN-γ suppresses it [34]. Therefore, production of the IgG1 subclass of TRAb may be relatively high in GD patients carrying the −857T allele, who have a greater ability to produce TNF-α, than in GD patients with the −857CC genotype. In support of this idea, individuals who have a greater genetic ability to produce TNF-α are also susceptible to asthma, in which antibodies play an important role in the pathogenesis, as well as in GD [10]. Patients with severe and intractable asthma had significantly higher serum IgG1 levels than did patients with mild asthma [35]. Furthermore, we have reported previously that the number of B cells, especially CD5+ B cells, increases in patients with active GD [36] and it has been reported that TNF-α contributes to the induction of B lymphocyte proliferation, especially CD5+ B cells, in cattle [37].
In conclusion, functional polymorphisms in the TNFA gene are associated with the development of AITD and with TRAb levels in patients with active GD.
References
- 1.Davies TF. Pathogenesis of Graves' disease. In: Braverman LE, Utiger RD, editors. The thyroid: a fundamental and clinical text. Philadelphia, PA: Lippincott Williams & Wilkins; 2000. pp. 518–31. [Google Scholar]
- 2.Volpe R. The immune system and its role in endocrine function. In: Becker KL, editor. Principles and practice of endocrinology and metabolism. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. pp. 1770–81. [Google Scholar]
- 3.Amino N, Hagen SR, Yamada N, Refetoff S. Measurement of circulating thyroid microsomal antibodies by the tanned red cell haemagglutination technique: its usefulness in the diagnosis of autoimmune thyroid diseases. Clin Endocrinol (Oxf) 1976;5:115–25. doi: 10.1111/j.1365-2265.1976.tb02822.x. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida H, Amino N, Yagawa K, et al. Association of serum antithyroid antibodies with lymphocytic infiltration of the thyroid gland: studies of seventy autopsied cases. J Clin Endocrinol Metab. 1978;46:859–62. doi: 10.1210/jcem-46-6-859. [DOI] [PubMed] [Google Scholar]
- 5.Ito C, Watanabe M, Okuda N, Watanabe C, Iwatani Y. Association between the severity of Hashimoto's disease and the functional +874A/T polymorphism in the interferon-gamma gene. Endocr J. 2006;53:473–8. doi: 10.1507/endocrj.k06-015. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe M, Yamamoto N, Maruoka H, et al. Independent involvement of CD8+ CD25+ cells and thyroid autoantibodies in disease severity of Hashimoto's disease. Thyroid. 2002;12:801–8. doi: 10.1089/105072502760339370. [DOI] [PubMed] [Google Scholar]
- 7.Matsuno H, Yudoh K, Katayama R, et al. The role of TNF-alpha in the pathogenesis of inflammation and joint destruction in rheumatoid arthritis (RA): a study using a human RA/SCID mouse chimera. Rheumatology (Oxford) 2002;41:329–37. doi: 10.1093/rheumatology/41.3.329. [DOI] [PubMed] [Google Scholar]
- 8.Scheurich P, Thoma B, Ucer U, Pfizenmaier K. Immunoregulatory activity of recombinant human tumor necrosis factor (TNF)-alpha: induction of TNF receptors on human T cells and TNF-alpha-mediated enhancement of T cell responses. J Immunol. 1987;138:1786–90. [PubMed] [Google Scholar]
- 9.Aust G, Heuer M, Laue S, et al. Expression of tumour necrosis factor-alpha (TNF-alpha) mRNA and protein in pathological thyroid tissue and carcinoma cell lines. Clin Exp Immunol. 1996;105:148–54. doi: 10.1046/j.1365-2249.1996.d01-726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noguchi E, Yokouchi Y, Shibasaki M, et al. Association between TNFA polymorphism and the development of asthma in the Japanese population. Am J Respir Crit Care Med. 2002;166:43–6. doi: 10.1164/rccm.2110052. [DOI] [PubMed] [Google Scholar]
- 11.Negoro K, Kinouchi Y, Hiwatashi N, et al. Crohn's disease is associated with novel polymorphisms in the 5′-flanking region of the tumor necrosis factor gene. Gastroenterology. 1999;117:1062–8. doi: 10.1016/s0016-5085(99)70390-2. [DOI] [PubMed] [Google Scholar]
- 12.Kuo NW, Lympany PA, Menezo V, et al. TNF-857T, a genetic risk marker for acute anterior uveitis. Invest Ophthalmol Vis Sci. 2005;46:1565–71. doi: 10.1167/iovs.04-0932. [DOI] [PubMed] [Google Scholar]
- 13.Grutters JC, Sato H, Pantelidis P, et al. Increased frequency of the uncommon tumor necrosis factor −857T allele in British and Dutch patients with sarcoidosis. Am J Respir Crit Care Med. 2002;165:1119–24. doi: 10.1164/ajrccm.165.8.200110-0320. [DOI] [PubMed] [Google Scholar]
- 14.Tsuchiya N, Tokushige K, Yamaguchi N, et al. Influence of TNF gene polymorphism in patients with acute and fulminant hepatitis. J Gastroenterol. 2004;39:859–66. doi: 10.1007/s00535-004-1402-1. [DOI] [PubMed] [Google Scholar]
- 15.Higuchi T, Seki N, Kamizono S, et al. Polymorphism of the 5′-flanking region of the human tumor necrosis factor (TNF)-alpha gene in Japanese. Tissue Antigens. 1998;51:605–12. doi: 10.1111/j.1399-0039.1998.tb03002.x. [DOI] [PubMed] [Google Scholar]
- 16.Nishimura M, Obayashi H, Mizuta I, et al. TNF receptor type 1, and allograft inflammatory factor-1 gene polymorphisms in Japanese patients with type 1 diabetes. Hum Immunol. 2003;64:302–9. doi: 10.1016/s0198-8859(02)00799-1. [DOI] [PubMed] [Google Scholar]
- 17.Soga Y, Nishimura F, Ohyama H, Maeda H, Takashiba S, Murayama Y. Tumor necrosis factor-alpha gene (TNF-alpha) −1031/-863, −857 single-nucleotide polymorphisms (SNPs) are associated with severe adult periodontitis in Japanese. J Clin Periodontol. 2003;30:524–31. doi: 10.1034/j.1600-051x.2003.00287.x. [DOI] [PubMed] [Google Scholar]
- 18.Fox DA. Cytokine blockade as a new strategy to treat rheumatoid arthritis: inhibition of tumor necrosis factor. Arch Intern Med. 2000;160:437–44. doi: 10.1001/archinte.160.4.437. [DOI] [PubMed] [Google Scholar]
- 19.Lv K, Chen R, Cai Q, Fang M, Sun S. Effects of a single nucleotide polymorphism on the expression of human tumor necrosis factor-alpha. Scand J Immunol. 2006;64:164–9. doi: 10.1111/j.1365-3083.2006.01786.x. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann SC, Stanley EM, Darrin Cox E, et al. Association of cytokine polymorphic inheritance and in vitro cytokine production in anti-CD3/CD28-stimulated peripheral blood lymphocytes. Transplantation. 2001;72:1444–50. doi: 10.1097/00007890-200110270-00019. [DOI] [PubMed] [Google Scholar]
- 21.Matesanz F, Fedetz M, Leyva L, Delgado C, Fernandez O, Alcina A. Effects of the multiple sclerosis associated −330 promoter polymorphism in IL2 allelic expression. J Neuroimmunol. 2004;148:212–17. doi: 10.1016/j.jneuroim.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Bougacha-Elleuch N, Rebai A, Mnif M, et al. Analysis of MHC genes in a Tunisian isolate with autoimmune thyroid diseases: implication of TNF −308 gene polymorphism. J Autoimmun. 2004;23:75–80. doi: 10.1016/j.jaut.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Chen RH, Chen WC, Wang TY, Tsai CH, Tsai FJ. Lack of association between pro-inflammatory cytokine (IL-6, IL-8 and TNF-alpha) gene polymorphisms and Graves' disease. Int J Immunogenet. 2005;32:343–7. doi: 10.1111/j.1744-313X.2005.00536.x. [DOI] [PubMed] [Google Scholar]
- 24.Hunt PJ, Marshall SE, Weetman AP, et al. Histocompatibility leucocyte antigens and closely linked immunomodulatory genes in autoimmune thyroid disease. Clin Endocrinol (Oxf) 2001;55:491–9. doi: 10.1046/j.1365-2265.2001.01356.x. [DOI] [PubMed] [Google Scholar]
- 25.Yamada H, Watanabe M, Nanba T, Akamizu T, Iwatani Y. The +869T/C polymorphism in the transforming growth factor-beta1 gene is associated with the severity and intractability of autoimmune thyroid disease. Clin Exp Immunol. 2008;151:379–82. doi: 10.1111/j.1365-2249.2007.03575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nanba T, Watanabe M, Akamizu T, Iwatani Y. The −590CC genotype in the IL4 gene as a strong predictive factor for the development of hypothyroidism in Hashimoto disease. Clin Chem. 2008;54:621–3. doi: 10.1373/clinchem.2007.099739. [DOI] [PubMed] [Google Scholar]
- 27.Kamizono S, Hiromatsu Y, Seki N, et al. A polymorphism of the 5′ flanking region of tumour necrosis factor alpha gene is associated with thyroid-associated ophthalmopathy in Japanese. Clin Endocrinol (Oxf) 2000;52:759–64. [PubMed] [Google Scholar]
- 28.Li N, Zhou Z, Liu X, et al. Association of tumour necrosis factor alpha (TNF-alpha) polymorphisms with Graves' disease: a meta-analysis. Clin Biochem. 2008;41:881–6. doi: 10.1016/j.clinbiochem.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Akman A, Sallakci N, Coskun M, et al. F-alpha gene 1031 T/C polymorphism in Turkish patients with Behcet's disease. Br J Dermatol. 2006;155:350–6. doi: 10.1111/j.1365-2133.2006.07348.x. [DOI] [PubMed] [Google Scholar]
- 30.Hirankarn N, Avihingsanon Y, Wongpiyabovorn J. Genetic susceptibility to SLE is associated with TNF-alpha gene polymorphism −863, but not −308 and −238, in Thai population. Int J Immunogenet. 2007;34:425–30. doi: 10.1111/j.1744-313X.2007.00715.x. [DOI] [PubMed] [Google Scholar]
- 31.Hirankarn N, Nakkuntod J, Duangchalermwong P, Deesomchok U, Charoenwongse P. The association of DRB1*04 share epitope alleles and tumor necrosis factor-alpha gene polymorphism (−863) with susceptibility to rheumatoid arthritis in Thai. Rheumatol Int. 2007;28:161–5. doi: 10.1007/s00296-007-0392-8. [DOI] [PubMed] [Google Scholar]
- 32.Geleijns K, Emonts M, Laman JD, et al. Genetic polymorphisms of macrophage-mediators in Guillain–Barre syndrome. J Neuroimmunol. 2007;190:127–30. doi: 10.1016/j.jneuroim.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Weetman AP, Yateman ME, Ealey PA, et al. Thyroid-stimulating antibody activity between different immunoglobulin G subclasses. J Clin Invest. 1990;86:723–7. doi: 10.1172/JCI114768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hussain R, Kifayet A, Dojki M, Dockrell HM. Selective correlation of interferon-gamma, tumour necrosis factor-alpha and granulocyte-macrophage colony-stimulating factor with immunoglobulin G1 and immunoglobulin G3 subclass antibody in leprosy. Immunology. 1999;98:238–43. doi: 10.1046/j.1365-2567.1999.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogurusu K, Takahashi K, Soda R, et al. Studies on IgG subclass antibodies in adult asthma. 2. Changes in serum antigen specific IgG subclass antibodies for aging and intractability in asthmatics. Arerugi. 1992;41:7–14. [PubMed] [Google Scholar]
- 36.Iwatani Y, Amino N, Kaneda T, et al. Marked increase of CD5+ B cells in hyperthyroid Graves' disease. Clin Exp Immunol. 1989;78:196–200. [PMC free article] [PubMed] [Google Scholar]
- 37.Konnai S, Usui T, Ikeda M, et al. Tumor necrosis factor-alpha up-regulation in spontaneously proliferating cells derived from bovine leukemia virus-infected cattle. Arch Virol. 2006;151:347–60. doi: 10.1007/s00705-005-0622-x. [DOI] [PubMed] [Google Scholar]


