Abstract
Primary biliary cirrhosis (PBC) is an organ-specific autoimmune liver disease characterized by progressive loss of intrahepatic small bile ducts. Cellular immune mechanisms involving T cell reaction are thought to be involved significantly in the pathogenesis of PBC. Recent studies have independently revealed enhanced T helper type 17 (Th17) response and weakened T regulatory cell (Treg) response in some autoimmune diseases, indicating a role of Th17/Treg imbalance in the pathogenesis of autoimmunity. This prompted us to investigate whether the Th17/Treg balance was broken in the peripheral blood of patients with PBC and, if it was, what cytokine circumstances might contribute to this imbalance. The expression of 11 Th17/Treg differentiation-related genes and serum concentrations of the corresponding cytokines in 36 patients with PBC, 28 patients with chronic hepatitis B and 28 healthy controls were measured by real-time quantitative–polymerase chain reaction and enzyme-linked immunosorbent assay respectively. Peripheral Th17 and Treg cells were analysed by flow cytometry. Th17-related cytokines were increased significantly in patients with PBC. Consistent with the cytokine profile, the Th17 cell population and retinoid-related orphan receptor γt expression were increased markedly. In contrast, the Treg cell population and forkhead box P3 expression were decreased dramatically in the peripheral blood of patients with PBC. Our study revealed that the Th17/Treg imbalance, both cytokine profile and cell numbers, exists in patients with PBC, suggesting its potential role in the breakdown of immune self-tolerance in PBC. Interleukin-23, which characterized the imbalanced cytokine profile, may play an essential role in Th17-related human autoimmunity.
Keywords: autoimmunity, PBC, Th17 cells, Treg cells
Introduction
Primary biliary cirrhosis (PBC) is an organ-specific autoimmune liver disease characterized serologically by the presence of serum anti-mitochondrial antibodies (AMA) and histologically by progressive intrahepatic bile ducts destruction [1]. Studies of the pathogenesis of PBC have made great progress in past decades, but still deserve much more effort because of the substantial increase of the prevalence of PBC and the controversial therapeutic benefit of ursodeoxycholic acid, the only drug approved for PBC by the Food and Drug Administration [2–4]. The precise aetiology of PBC is largely unknown at present; however, several pathogenetic factors including genetic susceptibility [5], infection [6] and, in particular, environmental agents[7], have been reported to potentially trigger the onset of PBC.
Accumulating evidence has suggested that CD4+ T cells play a dominant role in immune-mediated cholangitis in PBC [4]. CD4+ T cells have been divided classically into two distinct subsets based on their cytokine production profile: T helper type 1 (Th1) and Th2. Th1 cells, characterized by secreting interferon-γ in response to interleukin (IL)-12, are involved in the enhanced eradication of intracellular pathogens, while Th2 cells, characterized by production of IL-4, IL-5 and IL-13, act as activators of B cells in immunoglobulin E (IgE) production, eosinophil recruitment and mucosal expulsion mechanisms [8]. More recently, two additional subsets, the forkhead box P3 (FoxP3) positive regulatory subset (Treg) and IL-17-producing subset (Th17) [9,10], have emerged and together with Th1 and Th2 have formed a functional quartet of CD4+ T cells which provides a closer insight into the mechanisms of immune-mediated disease and an enhanced opportunity to explore the causes of human autoimmune diseases such as PBC.
Reduced generation or deficient function of CD4+FoxP3+ Tregs has been found in a number of different human autoimmune diseases. Ablating Tregs in mice is associated with fatal autoimmune disease [9,11]. Another subset of CD4+IL-17+Th17 cells is now considered to be a major activator of autoimmune damage in mouse autoimmune models [12]. Taking all this evidence together, it is reasonable to hypothesize that an imbalance of enhanced Th17 and weakened Treg response might exist and play a role in the formation of autoimmunity in PBC.
It has been well described that cytokines produced by cells from the innate and adaptive immune system are the hallmarks of CD4+T cell development and lineage commitment [13]. Human Th17 cell differentiation was reported to be induced mainly by IL-1β and enhanced by IL-6 but, unlike its murine counterpart, was suppressed by transforming growth factor (TGF)-β1 [14,15]. In addition, IL-23, a heterodimer composed of a unique subunit p19 and a common subunit p40, was also identified to contribute to the differentiation and maintenance of Th17 cells [15,16]. Moreover, TGF-β1 and IL-2 were reported to be the dominant cytokines for the differentiation of human Treg cells [17]. These key contributors of Th17/Treg differentiation, together with retinoid-related orphan receptor (ROR)γt and FoxP3, the master regulatory transcription factors of Th17 and Treg[18,19], were chosen for our present study. In addition, given that Th1, Th2 and Th17 could inhibit each other's differentiation and the imbalance could cover all four subsets of CD4+T cells [20–22], T-box expressed in T cells (Tbet) and GATA binding protein 3 (GATA3), the master regulatory transcription factors of Th1 and Th2, were also enlisted [23,24].
We first investigated whether an imbalanced serum cytokine profile exists in patients with PBC at gene and protein level by real-time quantitative–polymerase chain reaction (RQ–PCR) and enzyme-linked immunosorbent assay (ELISA) respectively. Flow cytometry was then employed to determine whether the peripheral cell populations of Th17 and Treg in patients with PBC were changed correspondingly.
Materials and methods
Patients
Thirty-six patients with PBC according to the internationally accepted criteria [25] were recruited randomly from Changhai Hospital and Changzheng Hospital, Shanghai, China. The diagnosis of PBC required two out of three of the following: the presence of detectable AMA (titre = 1:40) in serum, liver enzymes elevation for more than 6 months and diagnostic or compatible liver biopsy. The exclusion criteria were: focal intrahepatic or extrahepatic lesions and obstruction, as well as primary sclerosing cholangitis; pregnancy; alcohol or drug-induced liver disease; and systemic use of corticosteroids or immunosuppressive medication; or any clinically significant concomitant liver disease. All patients with PBC were undergoing standard ursodeoxycholic acid treatment (15 mg/kg/day). The Child–Pugh stage of each patient was calculated using laboratory values and physical examination findings. The disease control group was composed of 28 age-, gender- and Child–Pugh stage-matched patients with chronic hepatitis B (CHB).The diagnostic criteria for CHB were: positive for hepatitis B surface antigen and hepatitis B e antigen for at least 6 months and detectable hepatitis B virus (HBV) DNA at the time of enrollment. Twenty-eight healthy adults (49·1 ± 11·8 years, 20 female and eight male) were included as healthy controls (HCs). The study was approved by local ethical committee and written informed consent was obtained from all subjects. The AMA was determined by indirect immunofluorescence kit (Euroimmun, Luebeck, Germany). Aspartate aminotransferase, alanine aminotransferase, γ-glutamyltranspeptidase, alkaline phosphatase, albumin, total bilirubin and IgM were measured using routine clinical chemical methods.
Peripheral blood mononuclear cells isolation, RNA extraction and complementary DNA synthesis
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized peripheral blood of the studied subjects by standard Ficoll–Paque (GE Healthcare, Uppsala, Sweden) density centrifugation. mRNA was extracted from PBMCs by means of the RNeasy kit (Qiagen, Valencia, CA, USA). All the samples were treated with DNaseI to eliminate potential genomic DNA contamination. The quality and quantity of the RNA were determined by ultraviolet spectrophotometer. Target RNA was reverse-transcribed using the Omniscript RT Kit (Qiagen, Valencia, CA, USA). All samples were treated according to identical protocols and in parallel. RNA and cDNA were stored at −80°C until further processing.
Quantification of gene expression
The panels of genes of interest (GOIs) are listed in Table 2. The ribosomal genes 18S (18S rRNA) were selected as endogenous reference. Candidate primer sets were first designed by Primer Express Software (Applied Biosystems, Foster City, CA, USA) and then validated manually to satisfy the following criteria: (i) the amplicon length varies between 50 and 150 base pairs; (ii) amplification efficiencies, determined by a template linear dilution methods as described previously [26], were approximately equal for the GOIs with 18S rRNA; and (iii) primers were verified to generate a single product specific to target genes by both blast algorithm (http://www.ncbi.nlm.nih.gov/blast/) and melting curve analysis. The expression of GOIs was determined by RQ–PCR using an ABI PRISM 7000 Sequence Detection System (Applied Biosystems). PCRs were performed using SYBRgreen I universal PCR master mix (Applied Biosystems) in a final volume of 50 µl with the following thermal conditions: 50°C for 2 min, 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Samples were assayed in triplicate. The expression ratio of each GOIs between different groups was analysed using the delta delta comparative cycle threshhold method [26].
Table 2.
T helper type 17 (Th17) and T regulatory (Treg) differentiation-related cytokines.
| Genes | Accession no. | Primers (5′ to 3′) | Reported function |
|---|---|---|---|
| IL-1β | NM_000576 | Forward: GCTGATGGCCCTAAACAGATGAA | Induce human Th17 polarization [11] |
| Reverse: TGAAGCCCTTGCTGTAGTGGTG | |||
| IL-6 | NM_000600 | Forward: AAGCCAGAGCTGTGCAGATGAGTA | Induce human and mouse Th17 polarization [11] |
| Reverse: TGTCCTGCAGCCACTGGTTC | |||
| IL-23/p19 | NM_016584 | Forward: GCAGCCTGAGGGTCACCACT | Unique subunit of IL-23 |
| Reverse: GGCGGCTACAGCCACAAA | |||
| IL-23/p40 | NM_002187 | Forward: CGTGGCCATATGGGAACTGA | Shared subunit of IL-23 |
| Reverse: CTGGTCCAAGGTCCAGGTGATAC | |||
| IL-17A | NM_002190 | Forward: TGTCCACCATGTGGCCTAAGAG | Main effective cytokine of Th17 cells [7] |
| Reverse: GTCCGAAATGAGGCTGTCTTTGA | |||
| RORγt | NM_005060 | Forward: GCTGTGATCTTGCCCAGAACC | Master regulatory transcription factors of Th17 lineage [15] |
| Reverse: CTGCCCATCATTGCTGTTAATCC | |||
| TGF-β1 | NM_000660 | Forward: AGCGACTCGCCAGAGTGGTTA | Induce mouse Th17 and Treg polarization, suppress human Th17 polarization [11,12,14] |
| Reverse: GCAGTGTGTTATCCCTGCTGTCA | |||
| IL-2 | NM_000586 | Forward: CAACTCCTGTCTTGCATTGCACTAA | Induce human and mouse Treg polarization[14] |
| Reverse: AATGTGAGCATCCTGGTGAGTTTG | |||
| FoxP3 | NM_014009 | Forward: GTTCACACGCATGTTTGCCTTC | Master regulatory transcription factors of Treg lineage [16] |
| Reverse: CACAAAGCACTTGTGCAGACTCAG | |||
| Tbet | NM_013351 | Forward: TGTTGTGGTCCAAGTTTAATCAGCA | Master regulatory transcription factors of Th1 lineage [17] |
| Reverse: CCCGGCCACAGTAAATGACAG | |||
| GATA3 | NM_002051 | Forward: TGGCCTAAGGTGGTTGTGCTC | Master regulatory transcription factors of Th2 lineage [18] |
| Reverse: CTCAGCACAGGCTGCAGGAATA |
FoxP3, forkhead transcription factor protein 3; IL, interleukin; ROR, retinoid-related orphan receptor; TGF, transforming growth factor; Tbet, T-box expressed in T cells; GATA3, GATA binding protein 3.
The ELISA
Serum concentrations of IL-1β, IL-6, IL-23, IL-17A, TGF-β1 and IL-2 were measured by commercially available ELISA kits (eBioscience, San Diego, CA, USA), according to the protocols provided by the manufacturer. All samples were assessed in triplicate.
Flow cytometry
The PBMCs, 2 × 106, were isolated from peripheral blood of the studied subjects. For Th17 cell detection, PBMCs were stimulated additionally for 5 h with 50 ng/ml phorbol myristate acetate, 1 µM ionomycin (both from Sigma, St Louis, MO, USA) and 10 µg/ml brefeldin A (Tocris Cookson, Bristol, UK) in complete RPMI-1640 (Invitrogen, Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal bovine serum (Gibco, Grand Island, NY, USA). Upon harvest, cells were first surface-stained with fluorescein isothiocyanate-conjugated anti-human CD4 antibodies for 20 min, then fixed and permeabilized with Perm/Fix solution, and finally stained intracellularly with phycoerythrin (PE)-conjugated anti-human IL-17A and PE-conjugated anti-human FoxP3 antibodies respectively. Isotope controls were used to ensure antibody specificity. All antibodies and fixation/permeabilization agents were purchased from eBioscience (eBioscience).
Statistical analysis
Statistical analysis was performed using spss version 15·0 for Windows software (SPSS, Inc., Chicago, IL, USA). Normally distributed data were analysed using one-way anova followed by the Student–Newman–Keuls post-hoc test. For non-normally distributed data significant analyses between groups were performed using the Kruskall–Wallis test instead; significant differences between groups were analysed using the Mann–Whitney U-test. The significance level was set at P < 0·05.
Results
The clinical and biochemical details of studied patients are listed in Table 1. The expression of 11 genes and the serum concentrations of six corresponding cytokines, which have been reported previously to contribute pivotally to the differentiation of Th17 cells and Treg cells, were measured. The name, GenBank accession number, primer sequences and reported function of each gene are presented in Table 2.
Table 1.
Clinical details of the studied patients.
| PBC | CHB | |
|---|---|---|
| Number | 36 | 28 |
| Age (years) | 52·3 ± 13·2 | 50·2 ± 13·6 |
| Gender (male/female) | 6/30 | 8/20 |
| AMA (positive/negative) | 36/0 | – |
| AST (IU/l) | 62 (17–764) | 52 (23–183) |
| GGT (IU/l) | 171 (14–535) | 67 (19–258) |
| ALP (IU/l) | 257 (63–678) | 99 (22–572) |
| Albumin (g/l) | 35 (18–47) | 40 (29–56) |
| Total bilirubin (µmol/l) | 24 (4–238) | 32 (7–125) |
| IgM (g/l) | 2·69 (0·45–8·26) | 1·27 (0·36–2·11) |
| Child–Pugh (A/B/C) | 19/7/10 | 15/5/8 |
Age is expressed as mean ± standard deviation; other data are expressed as median (range). PBC, primary biliary cirrhosis; CHB, chronic hepatitis B; AMA, anti-mitochondrial antibodies; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, γ-glutamyltranspeptidase; IgM, immunoglobulin M. Normal values: AST, 0–40 U/l; GGT, 1–50 U/l; ALP, 15–112 U/l; albumin, 35–55 g/l; total bilirubin, 2–24 umol/l; IgM, 0·5–2·5 g/l.
Pro-Th17 cytokines expression were increased significantly in patients with PBC
When compared with HCs the expression of pro-Th17 cytokines, IL-1β, IL-6 and IL-23/p19 were up-regulated significantly in patients with PBC (Fig. 1a and Table S1). However, only IL-23/p19 was up-regulated statistically when compared with patients with CHB (Fig. 1c and Table S1). RORγt and IL-17A were up-regulated markedly in patients with PBC compared with patients with both CHB and HCs (Fig. 1a and c and Table S1). Tbet and GATA3 did not appear to be expressed differentially between patients with PBC and HCs, but were clearly down-regulated in patients with CHB. The IL-12/p40 expression was not statistically significant different among all groups.
Fig. 1.
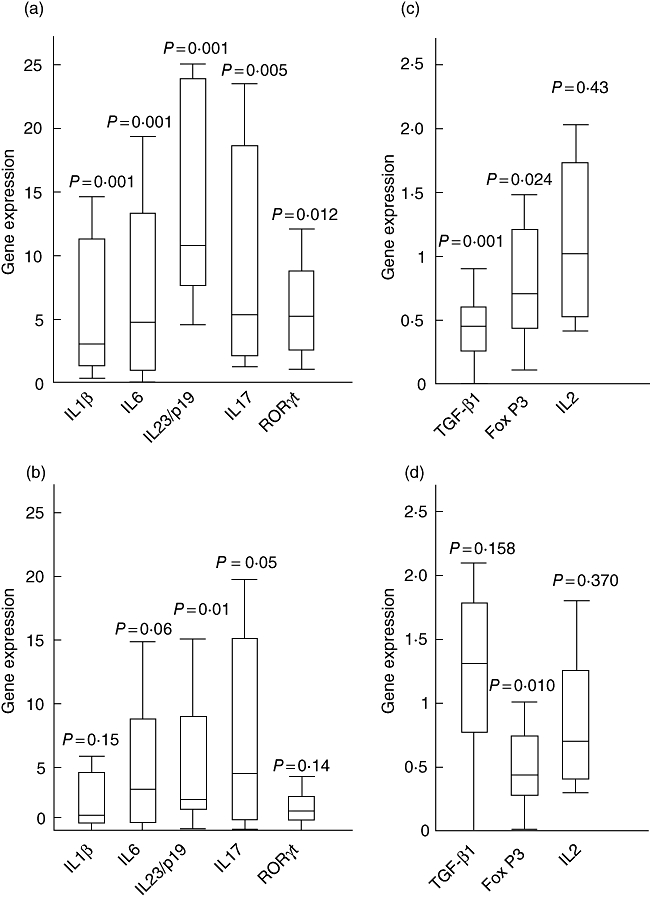
Expression ratio of T helper type 1 (Th17) and Treg-related gene in patients with primary biliary cirrhosis (PBC). When compared with healthy controls (HC), Th17-related genes were up-regulated significantly (a), whereas transforming growth factor (TGF)-β1 and forkhead box P3 (FoxP3) were obviously down-regulated (b) in patients with PBC. Comparison between patients with PBC and patients with chronic hepatitis B (CHB) is shown in (c) and (d). Data are presented as the whisker–box plot and analysed by Mann–Whitney U-test. Boxes represent the interquartile range, with the median represented by the line inside the box. Upper whisker, the highest value less than or equal to the 75 percentile plus 1·5 times interquartile range; lower whisker, the lowest value greater than or equal to the 25 percentile minus 1·5 times interquartile range. Outliers were excluded.
Expression of TGF-β1 and FoxP3 were down-regulated significantly in patients with PBC
The TGF-β1 and FoxP3 showed significant down-regulation in patients with PBC compared with HCs. IL-2 was not expressed differentially among all three groups (Fig. 1b and d and Table S1).
Pro-Th17 cytokines showed increased serum concentration in patients with PBC
The serum concentration of pro-Th17 cytokines, IL-1β, IL-6 and IL-23 were elevated significantly in patients with PBC compared with HCs, and again IL-23 was the only evaluated pro-Th17 cytokine in patients with PBC compared with those with CHB (Table 3 and Fig. 2a–c). IL-17A was also increased markedly in patients with PBC compared with patients with both CHB and HCs (Table 3 and Fig. 2d).
Table 3.
Serum concentration of T helper type 1 (Th17) and T regulatory (Treg)-related cytokines in patients with primary biliary cirrhosis (PBC).
|
P-values |
|||||
|---|---|---|---|---|---|
| Cytokine (pg/ml) | PBC | CHB | HCs | P versus H | P versus C |
| IL-1β | 57 ± 71 | 71 ± 75 | 28 ± 28 | 0·001 | 0·339 |
| IL-6 | 18·5 ± 22·7 | 21·5 ± 27 | 3·5 ± 4 | 0·001 | 0·473 |
| IL-23 | 164 ± 127 | 78 ± 5 | 81 ± 16 | 0·009 | 0·001 |
| IL-17 | 25 ± 10·7 | 16 ± 8·8 | 12 ± 6·4 | 0·001 | 0·001 |
| TGF-β1 | 3834 ± 1546 | 5227 ± 2409 | 4103 ± 1672 | 0·734 | 0·026 |
| IL-2 | 47 ± 20·5 | 49 ± 19·5 | 38 ± 17·1 | 0·092 | 0·636 |
Data are presented as mean ± standard deviation and analysed statistically by Mann–Whitney U-test. P versus H, PBC versus healthy controls (HCs), P versus C, PBC versus chronic hepatitis B (CHB). IL, interleukin; TGF, transforming growth factor.
Fig. 2.
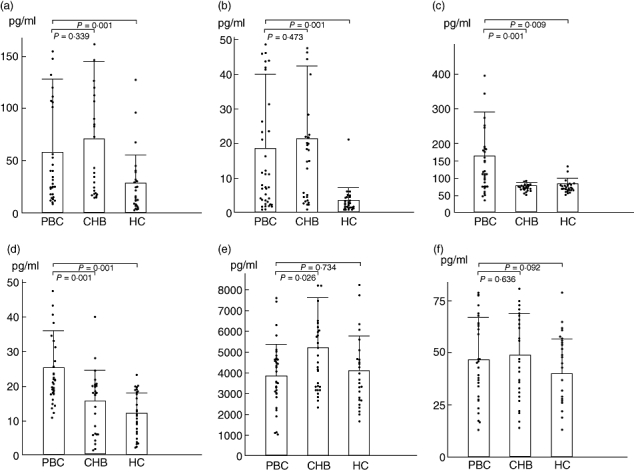
Serum concentration of T helper type 1 (Th17) and Treg-related cytokines. Interleukin (IL)-1β (a), IL-6 (b), IL-23 (c) and IL-17A (d) were increased in patients with primary biliary cirrhosis (PBC) while transforming growth factor (TGF)-β1 (e) and IL-2 (f) were not changed statistically. Data are presented as mean ± standard deviation and analysed by Mann–Whitney U-test. CHB, chronic hepatitis B; HC, healthy control.
Serum concentrations of pro-Treg cytokines were not changed statistically in patients with PBC, but TGF-β1 raised in patients with CHB
The serum concentration of TGF-β1 was mildly decreased without statistically significant difference in patients with PBC, but raised significantly in patients with CHB. IL-2 was not raised or lowered statistically among all three groups (Table 3 and Fig. 2e and f).
Imbalanced cytokine milieu in patients with PBC
The simultaneously presented up-regulation of pro-Th17 genes and down-regulation of pro-Treg genes indicated, at least at gene level, an imbalance between Th17 and Treg in patients with PBC. The serum ELISA data, except TGF-β1, showed an explicit correlation with the gene expression data, which further confirmed the imbalance at protein level.
The IL-23 was the distinctive and characteristic cytokine of the imbalanced cytokine milieu in patients with PBC
Compared either with patients with CHB or with HCs, IL-23 was always increased both in gene expression and serum concentration in patients with PBC, which indicated that IL-23 was the distinctive and characteristic component of the imbalanced cytokine milieu and may play a vital role in the differentiation of Th17 cells.
Increased Th17 population and decreased Treg population in patients with PBC
The PBMCs from patients with PBC (n = 36), patients with CHB (n = 28) and HCs (n = 28) were examined for the subset population, defined as the percentage of total CD4+ T cells, of Th17 cells and Treg cells using flow cytometry. The Th17 cell population was increased in patients with PBC (3·0 ± 1·9%,Figs 3a and 4a) compared with patients with CHB (1·4 ± 0·9%, P < 0·01, Figs 3b and 4a) and HCs (1·3 ± 0·9%, P < 0·01, Figs 3c and 4a), whereas CD4+FoxP3+Tregs were found to be decreased in PBC patients (3·9 ± 1·6%, Figs 3d and 4b) versus CHB patients (7·4 ± 2·1%, P < 0·01, Figs 3e and 4b) and HCs (5·2 ±1·5%, P < 0·05, Fig. 3f and 4b).
Fig. 3.
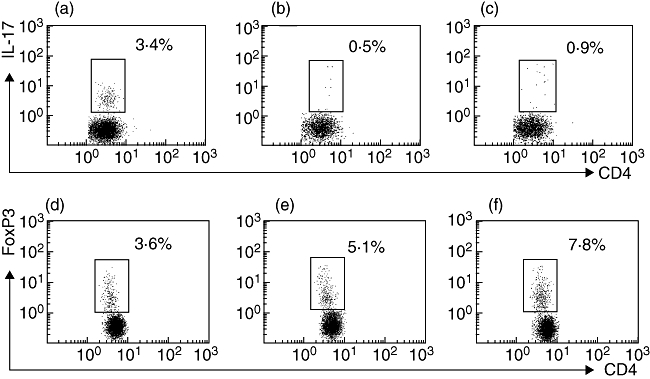
The population of T helper type 1 (Th17) cells as a percentage of total CD4+ cells in the peripheral blood mononuclear cells was evaluated by flow cytometry. Representative plots in patients with primary biliary cirrhosis (PBC), chronic hepatitis B (CHB) and healthy control (HCs) are shown in (a), (b) and (c) respectively. Representative plots of peripheral populations of T regulatory cells in patients with PBC, CHB and HC are shown in (d), (e) and (f) respectively.
Fig. 4.
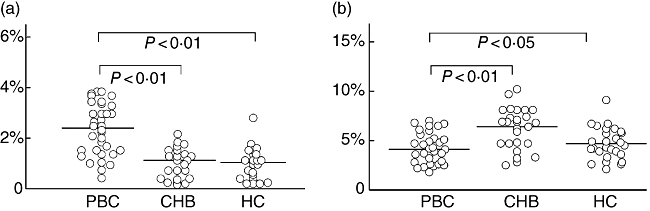
Distribution of T helper type 1 (Th17) (a) and T regulatory cells (b) subset population of subjects in each studied group. The mean cell population for each group is represented by a line. PBC, primary biliary cirrhosis; CHB, chronic hepatitis B; HC, healthy control.
Neither the population of CD4+IL-17+Th17 cells nor that of CD4+FoxP3+Tregs in peripheral blood was correlated with the severity of disease
Given that the Child–Pugh score system had been proved to predict clearly the prognosis of patients with PBC and had been used to assess the severity of disease in PBC and alcoholic cirrhosis [27,28], patients with PBC in our study were divided into two groups according to their Child–Pugh stage: patients with Child–Pugh stage A and patients with Child–Pugh stage B or C. The mean population of each subset were calculated and compared between the two groups. No correlation was found between disease stage and subset population (data not shown).
Discussion
In the early immune response, cross-talk between T cells and antigen-presenting cells initiate the production of certain patterns of cytokines, which was dictated by the type of invaded antigen, and orchestrates the subsequent development of CD4+T cell subsets [13]. Each subset plays a unique role in the elimination of invaded antigen, and dysregulated subset differentiation has long been associated with disease [29,30]. In the present study we demonstrated a Th17/Treg-related cytokine profile in patients with PBC with significant increases in IL-1β, IL-6 and IL-23, which were theoretically prone to Th17 differentiations and averse to Treg differentiation. Under these cytokine circumstances, the peripheral population of Th17 and Treg cells was changed accordingly.
Unlike the relatively conclusive role that murine Th17 cells played in a murine autoimmune disease model, little evidence has been proposed to illustrate the precise profile of human Th17 cells. According to published reports, this newly founded CD4+ T cell subset seems to have discordant activity in various human autoimmune diseases. Yamada [31]et al. reported a scarcely detected Th17 population in the joints of patients with rheumatoid arthritis and considered Th1 but not Th17 predominate the pathogenesis of rheumatoid arthritis; however, other autoimmune disorders such as multiple sclerosis and inflammatory bowel disease were reported to show a significantly increased population of Th17 cells in the target organs, which indicated that Th17 cells were pivotal mediators during the onset of autoimmune damage in humans [32,33]. Hence, with regard to certain diseases, perhaps one important question needs to be answered: is the disease related mainly to Th17, or Th1, or both? Our present study provides the first evidence, to our knowledge, of human Th17 cells in the pathogenesis of PBC by identifying a significantly increased peripheral Th17 population and Th17-related cytokines; these findings suggest that PBC, at least, is a Th17-related autoimmune disease. We also observed a simultaneously decreased Treg population in the same subjects. If the importance of Treg cells in the maintenance of self-tolerance is taken into consideration, it is rational to propose a potential role of Th17/Treg imbalance in the breakdown of immune homeostasis in PBC, providing new clues to the aetiology of PBC.
The TGF-β1 is involved pleiotropically in a variety of immunological processes, and has been in focus recently for its reciprocal effect in the differentiation of human and murine Th17 cells [14,15]. Littman [34,35] and colleagues have explained this paradox in their latest reports by proposing that TGF-β1 was indeed required for Th17 cell differentiation in both humans and mice; their work also showed that TGF-β1 at low levels induced differentiation of Th17 cells in the presence of inflammatory cytokines, whereas differentiation of Treg cells was induced at high levels. Furthermore, TGF-β1 combined with IL-4 induced the generation of a new IL-9-producing effector CD4+T cell subgroup, termed Th9 cells, which could also cause murine autoimmunity [36,37]. In our study the PBC and CHB groups, with the lowest and the highest concentrations of TGF-β1, respectively, were shown to correspond to the highest population of Th17 cells and Treg cells. Therefore, TGF-β1 might no longer be treated as merely an anti-inflammatory or pro-Treg cytokine, and its concentration and immune context may be a key regulator of local tissue construction and destruction.
The expression of IL-23/p19 and serum IL-23 levels was markedly higher in patients with PBC than that of the HCs. However, IL-23 increased uniquely in the patients with PBC compared with the patients with CHB. We therefore propose IL-23 as a distinctive and characteristic component of human pro-Th17 cytokines. Without IL-23, the increase of IL-1β and IL-6 may not be sufficient to induce human Th17 cell differentiation or to maintain its population. In fact, it has been demonstrated that IL-23 bridging the IL-12 cytokine family and the IL-17 cytokine family leads to identification of the Th17 lineage [38,39]. In the early studies, this veteran cytokine was the only cytokine to induce Th17 cell differentiation [40]. However, after identification of TGF-β1 and IL-6, the importance of IL-23 was diminished and is considered currently to play a role in maintaining Th17 cell survival [15,16]. Our findings suggest strongly a non-redundant role of IL-23 in Th17-induced pathogenesis in humans.
The main effector cytokines and master regulatory transcription factors were, to some extent, highly specific to their lineages. Therefore, in our present study, the increase in serum IL-17A and PBMC RORγt expression, as well as the decrease in FoxP3 expression in patients with PBC, can be traced to an imbalanced Th17/Treg population, and this imbalance may be limited within Th17 and Treg subsets, as there is no dysregulation in Tbet and GATA3 expression. Similarly, down-regulated Tbet and GATA3 expression in patients with CHB can be attributed to a reduced HBV-specific immune response [41].
It is interesting to note that some drugs and cytokines have been reported recently to suppress simultaneously the differentiation of Th17 cells and promote the generation of Treg cells in mice [42–45]. Therefore, the Th17/Treg imbalance we have reported here in PBC might be corrected easily with those drugs. Thus, the Th17/Treg imbalance appears to be a novel target of chemotherapy or immunotherapy for PBC or other autoimmune diseases.
Acknowledgments
We thank Dr. Linzhen Zhang and Dr. Huafeng Wei for their help and encouragement. We also thank Dr Cheng Wu and Dr Cen Wei for their assistance in sample collection. The study was funded by the China National High-tech R&D Program (863 Program), code no: 2006AA02Z496 and Funds for Excellent Department Leader, Science and Technology Commission of Shanghai Municipality, Code no: 07XD14013.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Expression ratios of T helper type 17 (Th17) and T regulatory (Treg) differentiation-related genes in patients with primary biliary cirrhosis (PBC).
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261–73. doi: 10.1056/NEJMra043898. [DOI] [PubMed] [Google Scholar]
- 2.Gong Y, Huang Z, Christensen E, Gluud C. Ursodeoxycholic acid for patients with primary biliary cirrhosis: an updated systematic review and meta-analysis of randomized clinical trials using Bayesian approach as sensitivity analyses. Am J Gastroenterol. 2007;102:1799–807. doi: 10.1111/j.1572-0241.2007.01235.x. [DOI] [PubMed] [Google Scholar]
- 3.Lindor K. Ursodeoxycholic acid for the treatment of primary biliary cirrhosis. N Engl J Med. 2007;357:1524–9. doi: 10.1056/NEJMct074694. [DOI] [PubMed] [Google Scholar]
- 4.Jones DE. Pathogenesis of primary biliary cirrhosis. Postgrad Med J. 2008;84:23–33. doi: 10.1136/gut.2007.122150. [DOI] [PubMed] [Google Scholar]
- 5.Selmi C, Mayo MJ, Bach N, et al. Primary biliary cirrhosis in monozygotic and dizygotic twins: genetics, epigenetics, and environment. Gastroenterology. 2004;127:485–92. doi: 10.1053/j.gastro.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Xu L, Shen Z, Guo L, et al. Does a betaretrovirus infection trigger primary biliary cirrhosis? Proc Natl Acad Sci USA. 2003;100:8454–9. doi: 10.1073/pnas.1433063100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amano K, Leung PSC, Rieger R, et al. Chemical xenobiotics and mitochondrial autoantigens in primary biliary cirrhosis: identification of antibodies against a common environmental, cosmetic, and food additive, 2-octynoic acid. J Immunol. 2005;174:5874–83. doi: 10.4049/jimmunol.174.9.5874. [DOI] [PubMed] [Google Scholar]
- 8.Foo YL. Th1 and Th2 cells: a historical perspective. Nat Rev Immunol. 2002;2:55–60. doi: 10.1038/nri705. [DOI] [PubMed] [Google Scholar]
- 9.Costantino CM, Baecher-Allan CM, Hafler DA. Human regulatory T cells and autoimmunity. Eur J Immunol. 2008;38:921–4. doi: 10.1002/eji.200738104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–67. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2:816–22. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 12.Bettelli E, Oukka M, Kuchroo VK. Th-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–50. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 13.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–44. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 14.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1 beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–9. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 15.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGF beta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–89. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 17.Horwitz DA, Zheng SG, Wang J, Gray JD. Critical role of IL-2 and TGF-beta in generation, function and stabilization of Foxp3+CD4+ Treg. Eur J Immunol. 2008;38:912–15. doi: 10.1002/eji.200738109. [DOI] [PubMed] [Google Scholar]
- 18.Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 19.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 20.Mullen AC, High FA, Hutchins AS, et al. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292:1907–10. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 21.Ouyang W, Ranganath SH, Weindel K, et al. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 1998;9:745–55. doi: 10.1016/s1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- 22.Nakae S, Iwakura Y, Suto H, Galli SJ. Phenotypic differences between Th1 and Th17 cells and negative regulation of Th1 cell differentiation by IL-17. J Leukoc Biol. 2007;81:1258–68. doi: 10.1189/jlb.1006610. [DOI] [PubMed] [Google Scholar]
- 23.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–69. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 24.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–96. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 25.Heathcoat EJ. Management of primary biliary cirrhosis. The American Association for the Study of Liver Diseases practice guidelines. Hepatology. 2000;31:1005–13. doi: 10.1053/he.2000.5984. [DOI] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCt method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Su CW, Hung HH, Huo TI, et al. Natural history and prognostic factors of primary biliary cirrhosis in Taiwan: a follow-up study up to 18 years. Liver Int. 2008;28:1305–13. doi: 10.1111/j.1478-3231.2008.01715.x. [DOI] [PubMed] [Google Scholar]
- 28.Hanck C, Manigold T, Bocker U, et al. Gene expression of interleukin 18 in unstimulated peripheral blood mononuclear cells of patients with alcoholic cirrhosis. Gut. 2001;49:106–11. doi: 10.1136/gut.49.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Becker H, Langrock A, Federlin K. Imbalance of CD4+ lymphocyte subsets in patients with mixed connective tissue disease. Clin Exp Immunol. 1992;88:91–5. doi: 10.1111/j.1365-2249.1992.tb03044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen DY, Lan JL, Lin FJ, Hsieh TY, Wen MC. Predominance of Th1 cytokine in peripheral blood and pathological tissues of patients with active untreated adult onset Still's disease. Ann Rheum Dis. 2004;63:1300–06. doi: 10.1136/ard.2003.013680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamada H, Nakashima Y, Okazaki K, et al. Th1 but not Th17 cells predominate in the joints of patients with rheumatoid arthritis. Ann Rheum Dis. 2008;67:1299–304. doi: 10.1136/ard.2007.080341. [DOI] [PubMed] [Google Scholar]
- 32.Tzartos JS, Friese MA, Craner MJ, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172:146–55. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Annunziato F, Cosmi L, Santarlasci V, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–61. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou L, Lopes JE, Chong MM, et al. TGF-beta-induced Foxp3 inhibits Th17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–40. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manel N, Unutmaz D, Littman DR. The differentiation of human Th17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–9. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veldhoen M, Uyttenhove C, van SJ, et al. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–6. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 37.Dardalhon V, Awasthi A, Kwon H, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL9+ IL10+ Foxp3(–) effector T cells. Nat Immunol. 2008;9:1347–55. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–4. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 40.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 41.Ferrari C, Penna A, Sansoni P, Giuberti T, Fiaccadori F. Clonal analysis of intrahepatic T lymphocytes in chronic active hepatitis: isolation of a T-cell line specific for hepatitis B core antigen from a patient with serological evidence of exposure to HBV. J Hepatol. 1986;3:384–92. doi: 10.1016/s0168-8278(86)80493-7. [DOI] [PubMed] [Google Scholar]
- 42.Niedbala W, Wei X, Cai B, et al. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol. 2007;37:3021–9. doi: 10.1002/eji.200737810. [DOI] [PubMed] [Google Scholar]
- 43.Schambach F, Schupp M, Lazar MA, Reiner SL. Activation of retinoic acid receptor-alpha favours regulatory T cell induction at the expense of IL-17-secreting T helper cell differentiation. Eur J Immunol. 2007;37:2396–9. doi: 10.1002/eji.200737621. [DOI] [PubMed] [Google Scholar]
- 44.Mucida D, Park Y, Kim G, et al. Reciprocal Th17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–60. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 45.Kopf H, de la Rosa GM, Howard OMZ, Chen X. Rapamycin inhibits differentiation of Th17 cells and promotes generation of FoxP3+ T regulatory cells. Int Immunopharmacol. 2007;7:1819–24. doi: 10.1016/j.intimp.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


