Abstract
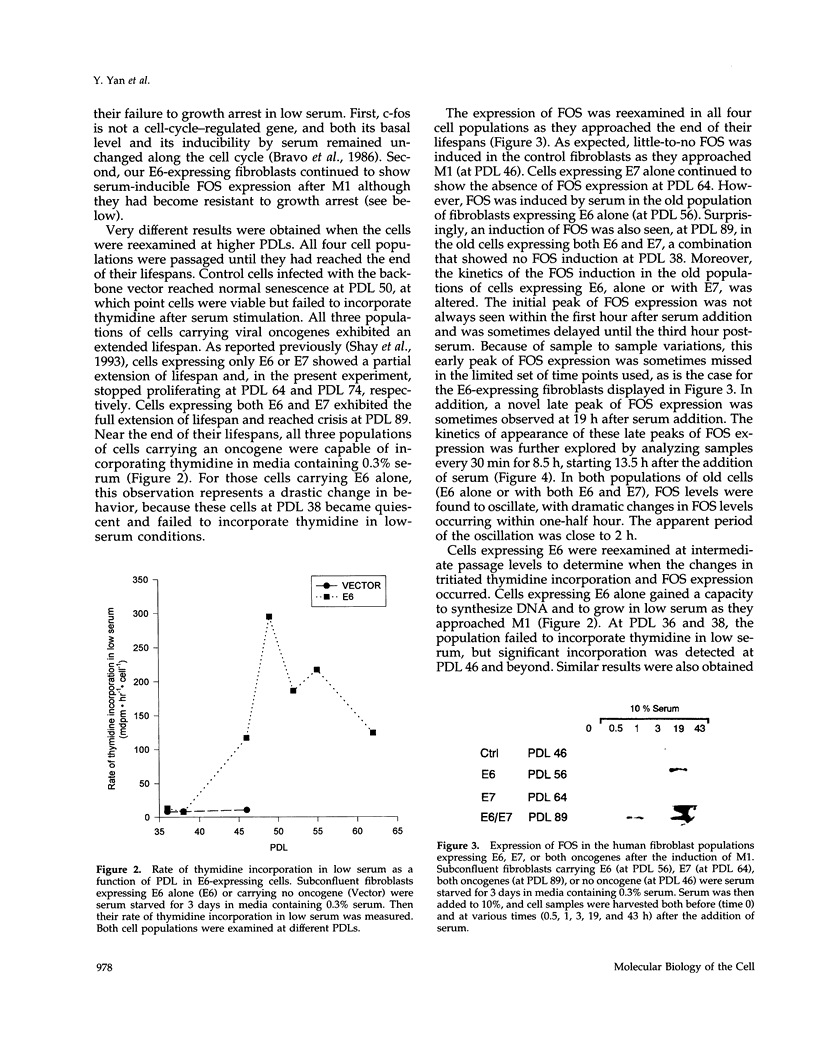
Normal human cells in culture become senescent after a limited number of population doublings. Senescent cells display characteristic changes in gene expression, among which is a repression of the ability to induce the c-fos gene. We have proposed a two-stage model for cellular senescence in which the mortality stage 1 (M1) mechanism can be overcome by agents that bind both the product of the retinoblastoma susceptibility gene (pRB)-like pocket proteins and p53. In this study we determined whether the repression of c-fos at M1 was downstream of the p53 or pRB-like "arms" of the M1 mechanism. We examined c-fos expression during the entire lifespan of normal human fibroblasts carrying E6 (which binds p53), E7 (which binds pRB), or both E6 and E7 of human papilloma virus type 16. The results indicate a dramatic change in cellular physiology at M1. Before M1, c-fos inducibility is controlled by an E6-independent mechanism that is blocked by E7. After M1, c-fos inducibility becomes dependent on E6 whereas E7 has no effect. In addition, a novel oscillation of c-fos expression with an approximately 2-h periodicity appears in E6-expressing fibroblasts post-M1. Accompanying this shift at M1 is a dramatic change in the ability to divide in low serum. Before M1, E6-expressing fibroblasts growth arrest in 0.3% serum, although they continue dividing under those conditions post-M1. These results demonstrate the unique physiology of fibroblasts during the extended lifespan between M1 and M2 and suggest that p53 might participate in the process that represses the c-fos gene at the onset of cellular senescence.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afshari C. A., Vojta P. J., Annab L. A., Futreal P. A., Willard T. B., Barrett J. C. Investigation of the role of G1/S cell cycle mediators in cellular senescence. Exp Cell Res. 1993 Dec;209(2):231–237. doi: 10.1006/excr.1993.1306. [DOI] [PubMed] [Google Scholar]
- Allsopp R. C., Vaziri H., Patterson C., Goldstein S., Younglai E. V., Futcher A. B., Greider C. W., Harley C. B. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991 Dec 10;1072(2-3):129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Atadja P., Wong H., Garkavtsev I., Veillette C., Riabowol K. Increased activity of p53 in senescing fibroblasts. Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8348–8352. doi: 10.1073/pnas.92.18.8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Burckhardt J., Curran T., Müller R. Expression of c-fos in NIH3T3 cells is very low but inducible throughout the cell cycle. EMBO J. 1986 Apr;5(4):695–700. doi: 10.1002/j.1460-2075.1986.tb04269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn S. M., Krawisz B. R., Dresler S. L., Lieberman M. W. Induction of replicative DNA synthesis in quiescent human fibroblasts by DNA damaging agents. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4828–4832. doi: 10.1073/pnas.81.15.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counter C. M., Avilion A. A., LeFeuvre C. E., Stewart N. G., Greider C. W., Harley C. B., Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992 May;11(5):1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etscheid B. G., Foster S. A., Galloway D. A. The E6 protein of human papillomavirus type 16 functions as a transcriptional repressor in a mechanism independent of the tumor suppressor protein, p53. Virology. 1994 Dec;205(2):583–585. doi: 10.1006/viro.1994.1684. [DOI] [PubMed] [Google Scholar]
- Futreal P. A., Barrett J. C. Failure of senescent cells to phosphorylate the RB protein. Oncogene. 1991 Jul;6(7):1109–1113. [PubMed] [Google Scholar]
- Ginsberg D., Mechta F., Yaniv M., Oren M. Wild-type p53 can down-modulate the activity of various promoters. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):9979–9983. doi: 10.1073/pnas.88.22.9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYFLICK L., MOORHEAD P. S. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961 Dec;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- HAYFLICK L. THE LIMITED IN VITRO LIFETIME OF HUMAN DIPLOID CELL STRAINS. Exp Cell Res. 1965 Mar;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Hara E., Tsurui H., Shinozaki A., Nakada S., Oda K. Cooperative effect of antisense-Rb and antisense-p53 oligomers on the extension of life span in human diploid fibroblasts, TIG-1. Biochem Biophys Res Commun. 1991 Aug 30;179(1):528–534. doi: 10.1016/0006-291x(91)91403-y. [DOI] [PubMed] [Google Scholar]
- Harley C. B., Futcher A. B., Greider C. W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990 May 31;345(6274):458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Harley C. B. Telomere loss: mitotic clock or genetic time bomb? Mutat Res. 1991 Mar-Nov;256(2-6):271–282. doi: 10.1016/0921-8734(91)90018-7. [DOI] [PubMed] [Google Scholar]
- Holt J. T., Gopal T. V., Moulton A. D., Nienhuis A. W. Inducible production of c-fos antisense RNA inhibits 3T3 cell proliferation. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4794–4798. doi: 10.1073/pnas.83.13.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu E., Mueller E., Oliviero S., Papaioannou V. E., Johnson R., Spiegelman B. M. Targeted disruption of the c-fos gene demonstrates c-fos-dependent and -independent pathways for gene expression stimulated by growth factors or oncogenes. EMBO J. 1994 Jul 1;13(13):3094–3103. doi: 10.1002/j.1460-2075.1994.tb06608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
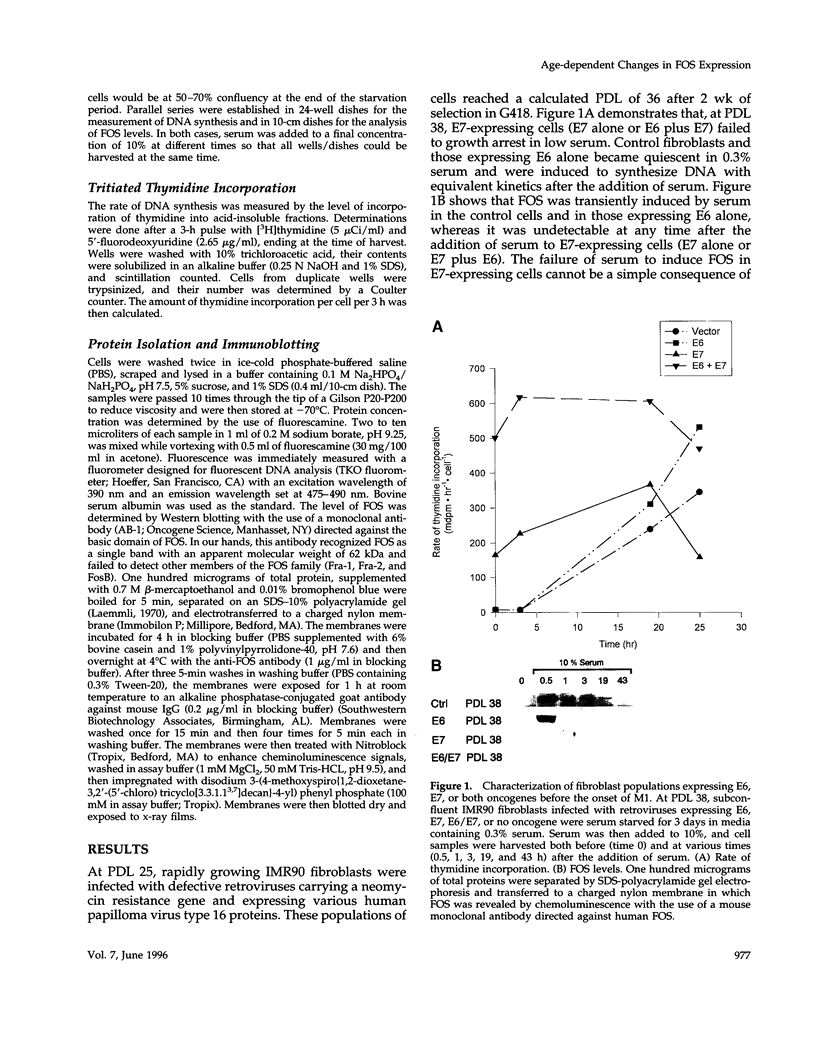
- Huibregtse J. M., Scheffner M., Howley P. M. Cloning and expression of the cDNA for E6-AP, a protein that mediates the interaction of the human papillomavirus E6 oncoprotein with p53. Mol Cell Biol. 1993 Feb;13(2):775–784. doi: 10.1128/mcb.13.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving J., Feng J., Wistrom C., Pikaart M., Villeponteau B. An altered repertoire of fos/jun (AP-1) at the onset of replicative senescence. Exp Cell Res. 1992 Sep;202(1):161–166. doi: 10.1016/0014-4827(92)90415-5. [DOI] [PubMed] [Google Scholar]
- Keen N., Elston R., Crawford L. Interaction of the E6 protein of human papillomavirus with cellular proteins. Oncogene. 1994 May;9(5):1493–1499. [PubMed] [Google Scholar]
- Kim N. W., Piatyszek M. A., Prowse K. R., Harley C. B., West M. D., Ho P. L., Coviello G. M., Wright W. E., Weinrich S. L., Shay J. W. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994 Dec 23;266(5193):2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- Kley N., Chung R. Y., Fay S., Loeffler J. P., Seizinger B. R. Repression of the basal c-fos promoter by wild-type p53. Nucleic Acids Res. 1992 Aug 11;20(15):4083–4087. doi: 10.1093/nar/20.15.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovary K., Bravo R. The jun and fos protein families are both required for cell cycle progression in fibroblasts. Mol Cell Biol. 1991 Sep;11(9):4466–4472. doi: 10.1128/mcb.11.9.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levy M. Z., Allsopp R. C., Futcher A. B., Greider C. W., Harley C. B. Telomere end-replication problem and cell aging. J Mol Biol. 1992 Jun 20;225(4):951–960. doi: 10.1016/0022-2836(92)90096-3. [DOI] [PubMed] [Google Scholar]
- Martin G. M. Cellular aging--clonal senescence. A review (Part I). Am J Pathol. 1977 Nov;89(2):484–512. [PMC free article] [PubMed] [Google Scholar]
- Martin G. M., Sprague C. A., Epstein C. J. Replicative life-span of cultivated human cells. Effects of donor's age, tissue, and genotype. Lab Invest. 1970 Jul;23(1):86–92. [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Morosov A., Phelps W. C., Raychaudhuri P. Activation of the c-fos gene by the HPV16 oncoproteins depends upon the cAMP-response element at -60. J Biol Chem. 1994 Jul 15;269(28):18434–18440. [PubMed] [Google Scholar]
- Naranjo J. R., Mellström B., Auwerx J., Mollinedo F., Sassone-Corsi P. Unusual c-fos induction upon chromaffin PC12 differentiation by sodium butyrate: loss of fos autoregulatory function. Nucleic Acids Res. 1990 Jun 25;18(12):3605–3610. doi: 10.1093/nar/18.12.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikura K., Murray J. M. Antisense RNA of proto-oncogene c-fos blocks renewed growth of quiescent 3T3 cells. Mol Cell Biol. 1987 Feb;7(2):639–649. doi: 10.1128/mcb.7.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips P. D., Pignolo R. J., Nishikura K., Cristofalo V. J. Renewed DNA synthesis in senescent WI-38 cells by expression of an inducible chimeric c-fos construct. J Cell Physiol. 1992 Apr;151(1):206–212. doi: 10.1002/jcp.1041510126. [DOI] [PubMed] [Google Scholar]
- Radna R. L., Caton Y., Jha K. K., Kaplan P., Li G., Traganos F., Ozer H. L. Growth of immortal simian virus 40 tsA-transformed human fibroblasts is temperature dependent. Mol Cell Biol. 1989 Jul;9(7):3093–3096. doi: 10.1128/mcb.9.7.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riabowol K. T., Vosatka R. J., Ziff E. B., Lamb N. J., Feramisco J. R. Microinjection of fos-specific antibodies blocks DNA synthesis in fibroblast cells. Mol Cell Biol. 1988 Apr;8(4):1670–1676. doi: 10.1128/mcb.8.4.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins P. D., Horowitz J. M., Mulligan R. C. Negative regulation of human c-fos expression by the retinoblastoma gene product. Nature. 1990 Aug 16;346(6285):668–671. doi: 10.1038/346668a0. [DOI] [PubMed] [Google Scholar]
- Scheffner M., Huibregtse J. M., Vierstra R. D., Howley P. M. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993 Nov 5;75(3):495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- Schönthal A., Feramisco J. R. Inhibition of histone H1 kinase expression, retinoblastoma protein phosphorylation, and cell proliferation by the phosphatase inhibitor okadaic acid. Oncogene. 1993 Feb;8(2):433–441. [PubMed] [Google Scholar]
- Sedman S. A., Hubbert N. L., Vass W. C., Lowy D. R., Schiller J. T. Mutant p53 can substitute for human papillomavirus type 16 E6 in immortalization of human keratinocytes but does not have E6-associated trans-activation or transforming activity. J Virol. 1992 Jul;66(7):4201–4208. doi: 10.1128/jvi.66.7.4201-4208.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri T., Campisi J. Repression of c-fos transcription and an altered genetic program in senescent human fibroblasts. Science. 1990 Jan 12;247(4939):205–209. doi: 10.1126/science.2104680. [DOI] [PubMed] [Google Scholar]
- Shay J. W., Pereira-Smith O. M., Wright W. E. A role for both RB and p53 in the regulation of human cellular senescence. Exp Cell Res. 1991 Sep;196(1):33–39. doi: 10.1016/0014-4827(91)90453-2. [DOI] [PubMed] [Google Scholar]
- Shay J. W., West M. D., Wright W. E. Re-expression of senescent markers in deinduced reversibly immortalized cells. Exp Gerontol. 1992 Sep-Dec;27(5-6):477–492. doi: 10.1016/0531-5565(92)90003-i. [DOI] [PubMed] [Google Scholar]
- Shay J. W., Wright W. E., Brasiskyte D., Van der Haegen B. A. E6 of human papillomavirus type 16 can overcome the M1 stage of immortalization in human mammary epithelial cells but not in human fibroblasts. Oncogene. 1993 Jun;8(6):1407–1413. [PubMed] [Google Scholar]
- Shay J. W., Wright W. E. Quantitation of the frequency of immortalization of normal human diploid fibroblasts by SV40 large T-antigen. Exp Cell Res. 1989 Sep;184(1):109–118. doi: 10.1016/0014-4827(89)90369-8. [DOI] [PubMed] [Google Scholar]
- Stein G. H., Beeson M., Gordon L. Failure to phosphorylate the retinoblastoma gene product in senescent human fibroblasts. Science. 1990 Aug 10;249(4969):666–669. doi: 10.1126/science.2166342. [DOI] [PubMed] [Google Scholar]
- Stein R. W., Corrigan M., Yaciuk P., Whelan J., Moran E. Analysis of E1A-mediated growth regulation functions: binding of the 300-kilodalton cellular product correlates with E1A enhancer repression function and DNA synthesis-inducing activity. J Virol. 1990 Sep;64(9):4421–4427. doi: 10.1128/jvi.64.9.4421-4427.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright W. E., Pereira-Smith O. M., Shay J. W. Reversible cellular senescence: implications for immortalization of normal human diploid fibroblasts. Mol Cell Biol. 1989 Jul;9(7):3088–3092. doi: 10.1128/mcb.9.7.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright W. E., Shay J. W. Telomere positional effects and the regulation of cellular senescence. Trends Genet. 1992 Jun;8(6):193–197. doi: 10.1016/0168-9525(92)90232-s. [DOI] [PubMed] [Google Scholar]
- Wright W. E., Shay J. W. Time, telomeres and tumours: is cellular senescence more than an anticancer mechanism? Trends Cell Biol. 1995 Aug;5(8):293–297. doi: 10.1016/s0962-8924(00)89044-3. [DOI] [PubMed] [Google Scholar]