Abstract
Studies of human mucosal lymphoid follicles are rare and have been limited to children's Peyer's patches, which are visible at endoscopy. We investigated lymphoid follicles in ileum biopsies of 87 patients and surgical colon specimens from 66 cancer patients, and examined phenotype and function of isolated follicular immune cells. Two (0–10) and 12 (0–117) follicles per patient were found in ileum and colon samples respectively (P < 0·001). The number of lymphoid follicles mononuclear cells (LFMC) that could be isolated per patient was higher from colon compared with ileum specimens [725 000 (0–23 Mio) versus 100 000 (0–1·3 Mio), P < 0·001]. T cells were predominant in both LFMC and lamina propria mononuclear cells (LPMC), but B cells were more and plasma cells less frequent in LFMC. T cells from mucosal follicles were more frequently CD4-positive and CD62L-positive, but less frequently CD8-positive, CD103-positive and CD69-positive than lamina propria T cells. LFMC from ileum compared with colon showed no differences in mononuclear cell composition. Anti-CD3/CD28 stimulation induced similar proliferation of LFMC and LPMC from ileum and colon, as well as secretion of high levels of interferon-γ, tumour necrosis factor-α and interleukin (IL)-2, but lower levels of IL-4, IL-6 and IL-10. LFMC from colon secreted more IL-2 than those from ileum. Our study shows that mucosal lymphoid follicles can be identified clearly in adult human colon and yield viable immune cells sufficient for phenotypical and functional analysis. The cellular composition of LFMC from ileum and colon is similar, and both secrete predominantly T helper type 1 cytokines.
Keywords: cell surface molecules, comparative immunology, cytokines, human, mucosa, T cells
Introduction
The mucosal immune system of the gut has to maintain a delicate balance between tolerance to food antigens and an active immune response against invading pathogens and their products [1]. The induction of such diverse immune reactions has long been thought to take place in the organized secondary lymphoid tissue of the intestine, i.e. mucosal lymphoid follicles which aggregate as Peyer's patches (PP) in the human terminal ileum. This concept was developed from animal models [2,3] and has been challenged by recent data [4–7] demonstrating normal tolerance induction in rodents deficient of PP. On the other hand, PP were required for the induction of a rapid T helper type 1 (Th1) response in murine gut and mesenteric lymph nodes during enteric infection [8].
In humans, earlier studies revealed a predominant secretion of interferon (IFN)-γ by T cells from PP in response to food proteins [9], in marked contrast to data from rodents which produce predominantly regulatory cytokines [10]. However, functional studies of mucosal lymphoid follicles are rare and have been restricted to children so far, because PP are not easily visible at endoscopy in adults [11].
We therefore established a method to identify human mucosal lymphoid follicles from both small and large intestine in adults. We show that mononuclear cells (MC) (LFMC, lymphoid follicles mononuclear cells) isolated from colonic follicles are similar in phenotype and function to those isolated from PP.
Methods
Patients and samples
Biopsies from the terminal ileum were obtained from 87 patients (36 men, 51 women; median age 52 years, range 18–83 years) who had no endoscopic abnormalities at diagnostic colonoscopy to exclude colonic tumours. Macroscopically unaltered colon mucosa adjacent to resected colon cancer and not required for pathological assessment was obtained from 66 patients (32 men, 34 women; median age 64 years, range 27–99 years). Patients receiving corticosteroids or chemotherapy were excluded. Five surgical specimens were from the caecum/ascending colon, two from the transverse colon and 59 from the descending/sigmoid colon. The study was approved by the local ethics committee.
Identification of mucosal lymphoid follicles from ileum and colon
Biopsies from the terminal ileum were placed immediately in phosphate-buffered saline (PBS; Gibco, Berlin, Germany) and examined under a dissecting microscope at 10–50× magnification. To detect colonic mucosal follicles, resected colon was washed in PBS, the tissue area was measured, the muscularis propria removed with a scalpel and the mucosal surface stained with 1% methylene blue (Neopharma, Aschgau/Chiemgau, Germany). Follicles were cut free from adjacent tissue, which was used as follicle-free control lamina propria for parallel isolation of lamina propria mononuclear cells (LPMC). At the beginning of our study, the identification/dissecting procedure of follicles from ileum and colon was evaluated by histology. For this purpose dissected follicles were suspended in 0·9% NaCl and shock-frozen in liquid nitrogen. Ten µm cryosections were air-dried and stained with haematoxylin and eosin or with methylene blue.
After the correct identification of lymphoid follicles had been established, dissected follicles were subject to isolation of MC.
Isolation of MC from lymphoid follicles (LFMC) and lamina propria (LPMC)
Follicle-free mucosa destined for LPMC isolation was cut into smaller pieces of approximately 1 mm2. These mucosa pieces and the mucosal lymphoid follicles were incubated separately for 30 min at 37°C in calcium- and magnesium-free Hanks's balanced salt solution, including 1 mM ethylenediamine tetraacetic acid (Sigma-Aldrich, München, Germany) to remove both epithelium and intraepithelial lymphocytes. After washing, the follicle-free mucosal tissue was disrupted mechanically into smaller pieces of approximately 0·1 mm2. Both fractions were incubated in RPMI-1640 medium supplemented with 30 mM HEPES, 10% fetal calf serum (FCS), 100 U/ml penicillin, 100 µg/ml streptomycin, 50 µg/ml gentamicin and 2·5 µg/ml amphotericin (all from Biochrom AG, Berlin, Germany), 0·1% collagenase type IV (Sigma-Aldrich, München, Germany), 0·05% DNase I (Roche Diagnostics GmbH, Mannheim, Germany) and 1 µl/ml 2-mercaptoethanol (Sigma-Aldrich, München, Germany) at 37 °C in a humidified 5% CO2 atmosphere for 3 h with continuous agitation and vortexed vigorously every 15 min. Remaining tissue aggregates were disrupted by slow aspiration through a 19Gx3 1/2 needle (BSN Medical GmbH, Hamburg, Germany) and finally removed by a 40 µm nylon cell strainer (Becton Dickinson GmbH, Heidelberg, Germany). The resulting cell suspension was centrifuged at 600 g for 10 min in a 30% isotonic Percoll solution (Amersham Biosciences, Uppsala, Sweden). The supernatant containing epithelial cells and debris was discarded; the cell pellet was washed and resuspended in RPMI-1640 medium. The LPMC fraction additionally underwent Ficoll (Amersham Biosciences) density centrifugation to remove erythrocytes. More than 96% of the isolated cell population were MC. The number of vital MC was counted in a Neubauer chamber with trypan blue dye exclusion. Viability of leukocytes was more than 90%. If sufficient cell numbers were obtained, generally one set of phenotypical or functional analyses was performed as described below.
Flow cytometry
For cytofluorometric analyses cells were preincubated in PBS supplemented with 2% FCS with 2 µl Beriglobin (Aventis, Strasbourg, France) and stained for 10 min at 4 °C using saturating concentrations of the following antibodies: Alexa 405-conjugated anti-CD3 antibody (generous gift from A. Scheffold, DRFZ, Berlin, Germany), allophycocyanin (APC)-Cy7-conjugated anti-CD45 antibody, phycoerythrin (PE)-Cy5-conjugated anti-CD4 antibody, PE-Cy7-conjugated anti-CD8 antibody, PE-Cy7-conjugated anti-CD25 antibody, APC-conjugated anti-CD62L antibody, APC-conjugated anti-CD69 antibody, PE-Cy5-conjugated anti-CD38 antibody, APC-conjugated anti-human leucocyte antigen (HLA)-DR antibody and fluorescein isothiocyanate (FITC)-conjugated anti-γδ T cell receptor antibody (all from Pharmingen, Franklin Lakes, NJ, USA); PE-conjugated anti-CD103 antibody and anti-CD45R0 antibody (all from DakoCytomation, Glostrup, Denmark); FITC-conjugated anti-CD33 antibody, PE-conjugated anti-CD1a antibody and PE-Cy7-conjugated anti-CD20 antibody from Beckman Coulter (Fullerton, CA, USA). Cell viability was assessed by 4,6-diamino-2-phenylindole (DAPI) exclusion (0·1 µg/ml; Calbiochem, Darmstadt, Germany), and viable leukocytes defined as DAPI-negative and CD45-positive cells were analysed in a Becton-Dickinson LSRII cytometer using FACS Diva software (Becton Dickinson).
Cell culture
The LFMC and LPMC were cultured at 100 000 cells per well in 96-well U-bottomed plates (Nunclon Surface, Nunc, Roskilde, Denmark) in 100 µl RPMI-1640 medium supplemented with 30 mM HEPES, 10% FCS, 100 U/ml penicillin, 100 µg/ml streptomycin, 50 µg/ml gentamicin and 2·5 µg/ml amphotericin. Cells were stimulated with 1 µg/ml anti-CD3 (Becton Dickinson GmbH), cross-linked by goat anti-mouse IgG (H+L) antibody (Caltag, Burlingame, CA, USA) and co-stimulated with 1 µg/ml anti-CD28 antibody (Becton Dickinson GmbH).
Proliferation assay
After 96 h incubation of LFMC and LPMC in duplicates as described above, [3H]-thymidine, 0·5 µCi/well, was added for another 6 h. Cells were lysed subsequently by freezing and harvested onto filters. Filters were immersed in scintillation fluid and incorporated radioactivity was quantified in a beta counter (Wallac, Perkin Elmer, Rodgau-Jügesheim, Germany).
Cytokine concentrations in culture supernatants
Supernatants from 48 h culture as described above were collected and stored at −80°C until analysis. Interleukin (IL)-2, IL-4, IL-6, IL-10, tumour necrosis factor (TNF)-α and IFN-γ were quantified simultaneously by human Th1/Th2 cytokine cytometric bead array kit according to the manufacturer's instructions using FACSCalibur cytometer and Cellquest software (all from Becton Dickinson).
Statistical analysis
Results are given as median and range; differences were tested for statistical significance by the Mann–Whitney U-test and Wilcoxon signed-rank test, as appropriate. For multi-parametric analyses, one-way ANOVA was used; P < 0·05 was considered significant.
Results
Dissection of mucosal lymphoid follicles and yield of LFMC from endoscopic ileum biopsies and resected colon mucosa
We obtained six (median; range 2–12) biopsies from the terminal ileum and 24 cm2 (3–200 cm2) resected colon mucosa per patient. In ileum biopsies lymphoid follicles were identified readily at 10–30× magnification as protruding hemispheres devoid of villi, approximately 0·2–1·5 mm in diameter (Fig. 1a). In the colon mucosa lymphoid follicles were identified at 10–50× magnification as circular structures of approximately 100–400 µm in diameter, which interrupted the otherwise regular pattern of crypts (Fig. 1c). To confirm correct identification and preparation several dissected mucosal follicles were cut and stained immediately instead of being processed for cell isolation. Follicles were found in seven of seven and eight of eight specimens of ileum and colon, respectively (Fig. 1b and d).
Fig. 1.
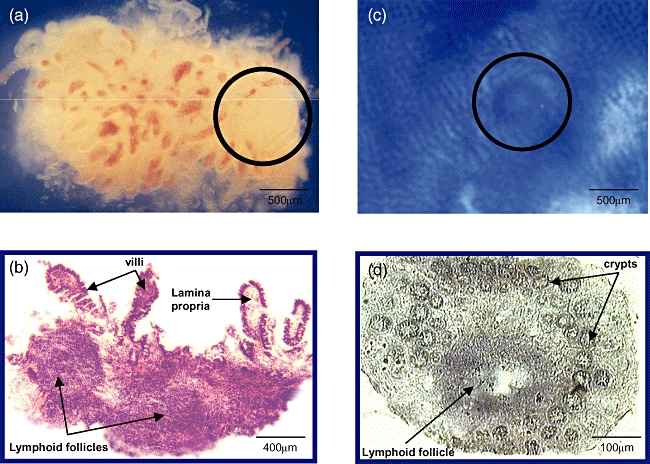
(a) Micrograph of an ileum biopsy (×20) containing a lymphoid follicle (circle); (b) histological confirmation (×25, haematoxylin and eosin stain). (c) Microscopic view of a colon sample after methylene blue staining (×20), the circle indicates a mucosal lymphoid follicle; (d) histological confirmation (×100).
The ileum biopsies yielded two (0–10) follicles per patient and the colon tissue 12 (0–117) follicles per patient (P < 0·001, Fig. 2). No follicles were found in ileum biopsies from 26 (30%) of 87 patients and in colon tissue from four (6%) of 66 patients. Cell isolation yielded similar numbers of LFMC per follicle from ileum or colon, but the number of LFMC isolated per patient was higher from colon specimens (Fig. 2), obviously because of the higher number of follicles in the larger samples. Cell yield was independent of sex and age of the patients (data not shown).
Fig. 2.

Yield of follicles and lymphoid follicles mononuclear cells (LFMC) isolated from ileum (filled boxes) and colon (open boxes). Although the numbers of LFMC per follicle were not different between colon and ileum, the total number of LFMC per patient that could be obtained from ileum was severely limited, mainly because of restricted sample size. Values from 87 ileum and 66 colon specimens are presented as medians, shown as black lines in the boxes. Lower and upper borders indicate 25th and 75th percentiles, lower and upper whiskers indicate the lowest and highest values respectively; n.s., not significant.
At least 200 000 LFMC were recovered from 39% of all ileum preparations and from 73% of colonic preparations; at least 500 000 LFMC were obtained from 11% and 50% of preparations from ileum and colon respectively. The number of ileum biopsies per patient was higher in the second half of the study period [seven (four to 12) versus four (two to eight); P < 0·001], but the proportion of experiments yielding more than 200 000 LFMC increased from 38% to only 43%, and the proportion of preparations yielding more than 500 000 LFMC increased from 7% to only 19%.
Similar amounts of resected colon tissue were available throughout the study, but in the second half of the study period the percentage of experiments yielding more than 200 000 LFMC increased from 64 to 86% and the proportion yielding more than 500 000 LFMC increased from 31 to 81% of preparations.
Cytofluorometric analysis of MC isolated from mucosal lymph follicles and adjacent lamina propria
In both LFMC and LPMC T cells were predominant, but B cells were more and plasma cells (CD38+CD20−CD3−) less frequent in LFMC compared with LPMC (Table 1); the proportion of macrophages (CD33-positive) and dendritic cells (CD1a-positive) was always below 1%. Additional staining for HLA-DR to detect antigen-presenting cells apart from B cells (HLA-DR+CD20−CD3−) confirmed the lack of macrophages and dendritic cells in the isolated cell population (data not shown). T cells from mucosal follicles were more frequently CD4-positive and CD62L-positive, but less frequently CD8-positive, CD103-positive and CD69-positive than lamina propria T cells (Table 1).
Table 1.
Cytofluorometric analysis of mononuclear cells (MC) isolated from intestinal mucosal lymphoid follicles (LFMC) and adjacent lamina propria (LPMC).
| LFMC (n = 17) | LPMC (n = 17) | |
|---|---|---|
| % of MC | ||
| T cells (CD3+) | 54 (20–69)† | 53 (14–76) |
| B cells (CD20+) | 42 (18–46) | 22 (10–47)* |
| Plasma cells (CD38+CD20−CD3−) | 2 (0–5) | 5 (2–18)* |
| % of T cells | ||
| CD4 | 61 (37–83) | 49 (38–87)* |
| CD8 | 12 (3–83) | 18 (6–50)* |
| γδ T cell receptor | 1 (0–6) | 3 (0–14) |
| CD62L | 6 (2–16) | 4 (0–10)* |
| CD103 | 12 (2–25) | 28 (3–51)* |
| CD69 | 57 (21–90) | 81 (20–100)* |
| CD25 | 8 (2–20) | 8 (3–35) |
| CD45RO | 69 (37–90) | 73 (66–95) |
P < 0·01.
Median (range).
Cell composition was similar in LFMC isolated from ileum and colon (Table 2). However, the proportion of T cells expressing CD103 was lower, and the proportion of T cells expressing CD69 was higher in colon compared with ileum LFMC.
Table 2.
Comparison of lymphoid follicles mononuclear cells (LFMC) isolated from ileum and colon mucosal lymphoid follicles.
| Ileum (n = 9) | Colon (n = 8) | |
|---|---|---|
| % of MC | ||
| T cells (CD3+) | 43 (20–69)† | 54 (41–62) |
| B cells (CD20+) | 45 (24–57) | 34 (18–64) |
| Plasma cells (CD38+CD20−CD3−) | 3 (0–5) | 2 (0–4) |
| % of T cells | ||
| CD4 | 59 (37–77) | 69 (55–83) |
| CD8 | 13 (9–38) | 12 (3–19) |
| γδ T cell receptor | 1 (0–3) | 3 (0–6) |
| CD62L | 6 (3–11) | 6 (3–16) |
| CD103 | 15 (11–26) | 8 (5–19)* |
| CD69 | 42 (21–84) | 62 (49–90)* |
| CD25 | 8 (5–20) | 10 (2–14) |
| CD45RO | 66 (37–83) | 71 (60–90) |
P < 0·05.
Median (range); MC, mononuclear cells.
Functional analysis of MC isolated from mucosal lymph follicles and adjacent lamina propria
The LFMC and LPMC isolated from colon specimens were cultured for 96 h with and without anti-CD3/CD28. Anti-CD3/CD28-induced proliferation was not significantly different between LFMC and LPMC [stimulation index 4 (2–59) versus 9 (3–69); n = 5 respectively].
The LFMC and LPMC isolated from ileum and colon specimens were cultured for 48 h with and without anti-CD3/CD28 stimulation and cytokine secretion was measured in culture supernatants (Table 3). Basal cytokine secretion was not different between ileum LFMC and LPMC, but colon LFMC produced more IL-2 and less IL-4, IL-6 and IL-10 than colon LPMC. Following anti-CD3/CD28 stimulation LFMC and LPMC secreted comparably high amounts of IFN-γ, TNF-α and IL-2 and lesser amounts of IL-4, IL-6 and IL-10. Ileum LFMC produced less IL-2 than colon LFMC both with and without anti-CD3/CD28 stimulation, but secretion of other cytokines was not different between ileum and colon LFMC (Table 3).
Table 3.
Cytokine secretion by lamina propria mononuclear cells (LPMC) and lymphoid follicles mononuclear cells (LFMC).
| Basal secretion |
Anti-CD3/CD28 stimulation |
|||||||
|---|---|---|---|---|---|---|---|---|
| LFMC |
LPMC |
LFMC |
LPMC |
|||||
| Ileum | Colon | Ileum | Colon | Ileum | Colon | Ileum | Colon | |
| IFN-γ | 12 (2–47)† | 52 (0–170) | 9 (3–391) | 6 (0–677) | 3017 (1794–>5000) | >5000 (1850–>5000) | >5000 (4160–>5000) | >5000 (1587–>5000) |
| TNF-α | 11 (3–27) | 28 (6–84) | 10 (3–68) | 12 (4–60) | 956 (600–2674) | >5000 (1644–>5000) | 1465 (470–>5000) | 1570 (365–>5000) |
| IL-2 | 2 (1–16)* | 28 (2–59)** | 6 (0–36) | 4 (1–38) | 1496 (794–2562)* | >5000 (1850–>5000) | 3591 (1347–>5000) | 3397 (1719–>5000) |
| IL-4 | 17 (0–250) | 4 (2–39)** | 21 (0–682) | 10 (3–96) | 419 (147–2761) | 737 (83–>5000) | 474 (124–>5000) | 500 (44–>5000) |
| IL-6 | 47 (8–3032) | 90 (33–709)** | 49 (19–4900)* | 1182 (77–2265) | 124 (93–4801) | 570 (106–>5000) | 204 (121–>5000) | 2724 (124–>5000) |
| IL-10 | 6 (3–14) | 13 (3–37)** | 6 (2–42) | 29 (2–330) | 238 (98–531) | 115 (10–>5000) | 322 (89–634) | 122 (9–>5000) |
P < 0·05 ileum (n = 6) versus colon (n = 9);
P < 0·05, LFMC versus LPMC.
Median (range) in pg/ml. IL, interleukin; IFN, interferon; TNF, tumour necrosis factor.
Discussion
The secondary lymphoid tissue of the gut, i.e. lymphoid follicles in the large and small intestine, has long been considered essential for the induction of mucosal immune responses [12]. Human studies are rare because access to lymphoid follicles in adults has not been established so far. Here we report the identification and preparation of lymphoid follicles from human ileal biopsies and colonic specimens. Cell yield from ileum follicles was often low, but MC isolated from colon follicles were similar in phenotype and function to those from ileum.
If present, lymphoid follicles were easily visible at microscopic examination of ileum biopsies. Unfortunately, many biopsies did not contain organized lymphoid tissue, and neither intravital staining nor experience improved the endoscopic detection of follicles. Even the collection of more than six ileum biopsies could not guarantee the recovery of lymphoid follicles, and cell yield after isolation from dissected follicles exceeded 500 000 in fewer than 20% of the patients. Overall, ileum biopsies from adults were no reliable source of follicular immune cells. We therefore examined specimens of resected colon for the presence of lymphoid follicles. Follicles were not visible in native colon, but could be identified microscopically in stripped colon mucosa after methylene blue staining. Cell numbers isolated from colon but not from ileum specimens improved over time because of better detection of colon follicles with experience, and at the end of our study we recovered routinely more than 500 000 follicular cells from resected colon specimens.
Histology confirmed the identification of follicles in all cases examined. Although smaller follicles could be missed in the colon, this is highly unlikely for the always prominent follicles of the ileum. The composition of the isolated LFMC and LPMC and the distribution of T cell subpopulations confirm earlier, mainly histological data [11,13–15], reviewed by Kelsall and Strober [16]. The higher proportions of B cells and of CD4- and CD62L-positive T cells, as well as the lower proportions of plasma cells, and of CD8- and CD103-positive T cells in LFMC compared with LPMC, support their separate isolation by our protocol.
Immunohistochemical studies suggest that solitary and aggregated mucosal follicles in the ileum are similar structures [17]. Both are not anatomically significantly different from the solitary scattered mucosal follicles of the colon indicating similar functions [16]. We found only minor phenotypical differences in LFMC from colon or ileum, and both secreted predominantly Th1-type cytokines after CD3/CD28 stimulation, with only IL-2 secretion higher in colon compared with ileum LFMC. Increased IL-2 secretion could be due to pre-activation by higher levels of luminal antigens, which could also explain the increased expression of CD69 on colonic LFMC [18]. Although MC isolation was highly effective, we cannot exclude that stromal or epithelial cells may have contributed to the high levels of IL-6 secretion measured in colon LPMC. Overall, we found no evidence for strikingly different functions of LFMC between the small or large intestine.
In contrast to mouse models [19–21], cells isolated from children's PP and restimulated with β-lactoglobulin or casein exhibit a Th1 response [9], probably because of the presence of IL-12 [22]. The predominant secretion of Th1 cytokines after CD3/CD28 stimulation in our study suggests that LFMC from both ileum and colon follicles in human adults are similarly skewed. These findings are in line with the recent report that Th1 responses to enteric infections in mice require the presence of PP [8]. On the other hand, it remains to be seen whether, as in mouse models [4,7], the induction of oral tolerance in humans is independent from lymphoid follicles.
In conclusion, we found that mucosal lymphoid follicles can be identified clearly in adult human colon and yield viable immune cells sufficient for phenotypical and functional analysis. The cellular composition of LFMC from ileum and colon is similar, and both secrete predominantly Th1 cytokines.
Acknowledgments
The excellent assistance of Ulrike Dethlefs, Katrin Pittasch and Sylvia Münchow is acknowledged gratefully. We thank Katarina Raba and Thoralf Kaiser for their support in establishing the multi-parametric FACS stains. Supported by the DFG, SFB 633 and the BMBF, Competence Network on HIV/AIDS, FKZ 01 KI 0511.
References
- 1.Strobel S, Mowat AM. Oral tolerance and allergic responses to food proteins. Curr Opin Allergy Clin Immunol. 2006;6:207–13. doi: 10.1097/01.all.0000225162.98391.81. [DOI] [PubMed] [Google Scholar]
- 2.Fujihashi K, Dohi T, Rennert PD, et al. Peyer's patches are required for oral tolerance to proteins. Proc Natl Acad Sci USA. 2001;98:3310–15. doi: 10.1073/pnas.061412598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsuji NM, Mizumachi K, Kurisaki J. Interleukin-10-secreting Peyer's patch cells are responsible for active suppression in low-dose oral tolerance. Immunology. 2001;103:458–64. doi: 10.1046/j.1365-2567.2001.01265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Worbs T, Bode U, Yan S, et al. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203:519–27. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kunkel D, Kirchhoff D, Nishikawa S, et al. Visualization of peptide presentation following oral application of antigen in normal and Peyer's patches-deficient mice. Eur J Immunol. 2003;33:1292–301. doi: 10.1002/eji.200323383. [DOI] [PubMed] [Google Scholar]
- 6.Spahn TW, Fontana A, Faria AM, et al. Induction of oral tolerance to cellular immune responses in the absence of Peyer's patches. Eur J Immunol. 2001;31:1278–87. doi: 10.1002/1521-4141(200104)31:4<1278::aid-immu1278>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 7.Kraus TA, Brimnes J, Muong C, et al. Induction of mucosal tolerance in Peyer's patch-deficient, ligated small bowel loops. J Clin Invest. 2005;115:2234–43. doi: 10.1172/JCI19102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwa SF, Beverley P, Smith AL. Peyer's patches are required for the induction of rapid Th1 responses in the gut and mesenteric lymph nodes during an enteric infection. J Immunol. 2006;176:7533–41. doi: 10.4049/jimmunol.176.12.7533. [DOI] [PubMed] [Google Scholar]
- 9.Nagata S, McKenzie C, Pender SL, et al. Human Peyer's patch T cells are sensitized to dietary antigen and display a Th cell type 1 cytokine profile. J Immunol. 2000;165:5315–21. doi: 10.4049/jimmunol.165.9.5315. [DOI] [PubMed] [Google Scholar]
- 10.Lefrancois L, Puddington L. Intestinal and pulmonary mucosal T cells: local heroes fight to maintain the status quo. Annu Rev Immunol. 2006;24:681–704. doi: 10.1146/annurev.immunol.24.021605.090650. [DOI] [PubMed] [Google Scholar]
- 11.MacDonald TT, Spencer J, Viney JL, et al. Selective biopsy of human Peyer's patches during ileal endoscopy. Gastroenterology. 1987;93:1356–62. doi: 10.1016/0016-5085(87)90266-6. [DOI] [PubMed] [Google Scholar]
- 12.Wittig BM, Zeitz M. The gut as an organ of immunology. Int J Colorectal Dis. 2003;18:181–7. doi: 10.1007/s00384-002-0444-1. [DOI] [PubMed] [Google Scholar]
- 13.Farstad IN, Halstensen TS, Lien B, et al. Distribution of beta 7 integrins in human intestinal mucosa and organized gut-associated lymphoid tissue. Immunology. 1996;89:227–37. doi: 10.1046/j.1365-2567.1996.d01-727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farstad IN, Halstensen TS, Fausa O, et al. Do human Peyer's patches contribute to the intestinal intraepithelial gamma/delta T-cell population? Scand J Immunol. 1993;38:451–8. doi: 10.1111/j.1365-3083.1993.tb02587.x. [DOI] [PubMed] [Google Scholar]
- 15.Brandtzaeg P, Bjerke K. Immunomorphological characteristics of human Peyer's patches. Digestion. 1990;46(Suppl. 2):262–73. doi: 10.1159/000200396. [DOI] [PubMed] [Google Scholar]
- 16.Kelsall B, Strober W. Gut-associated lymphoid tissue: antigen handling and T-lymphocyte responses. In: Orgra PL, Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, editors. Mucosal immunology. 2nd edn. San Diego, CA: Academic Press; 1999. pp. 293–317. [Google Scholar]
- 17.Moghaddami M, Cummins A, Mayrhofer G. Lymphocyte-filled villi: comparison with other lymphoid aggregations in the mucosa of the human small intestine. Gastroenterology. 1998;115:1414–25. doi: 10.1016/s0016-5085(98)70020-4. [DOI] [PubMed] [Google Scholar]
- 18.Niess JH, Leithauser F, Adler G, et al. Commensal gut flora drives the expansion of proinflammatory CD4 T cells in the colonic lamina propria under normal and inflammatory conditions. J Immunol. 2008;180:559–68. doi: 10.4049/jimmunol.180.1.559. [DOI] [PubMed] [Google Scholar]
- 19.Santos LM, al-Sabbagh A, Londono A, et al. Oral tolerance to myelin basic protein induces regulatory TGF-beta-secreting T cells in Peyer's patches of SJL mice. Cell Immunol. 1994;157:439–47. doi: 10.1006/cimm.1994.1240. [DOI] [PubMed] [Google Scholar]
- 20.Gonnella PA, Chen Y, Inobe J, et al. In situ immune response in gut-associated lymphoid tissue (GALT) following oral antigen in TCR-transgenic mice. J Immunol. 1998;160:4708–18. [PubMed] [Google Scholar]
- 21.MacDonald TT. The mucosal immune system. Parasite Immunol. 2003;25:235–46. doi: 10.1046/j.1365-3024.2003.00632.x. [DOI] [PubMed] [Google Scholar]
- 22.Monteleone G, Holloway J, Salvati VM, et al. Activated STAT4 and a functional role for IL-12 in human Peyer's patches. J Immunol. 2003;170:300–7. doi: 10.4049/jimmunol.170.1.300. [DOI] [PubMed] [Google Scholar]


