Abstract
Natural killer T cells (NKT) are a regulatory subset of T lymphocytes whose frequency in peripheral blood is highly variable within the human population. Lower than normal NKT frequencies are associated with increased predisposition to a number of diseases, including type 1 diabetes and some forms of cancer, raising the possibility that an increased frequency may be protective. However, there is little or no understanding of how high NKT frequencies arise or, most importantly, whether the potential exists to boost and maintain NKT levels for therapeutic advantage. Here, we provide a detailed functional and phenotypic characterization of the NKT compartment of a human donor with NKT levels approximately 50 times greater than normal, including an analysis of NKT in her immediate family members. The study focuses upon the characteristics of this donor and her family, but demonstrates more broadly that the size and flexibility of the NKT niche is far greater than envisioned previously. This has important implications for understanding how the human NKT compartment is regulated, and supports the concept that the human NKT compartment might be expanded successfully for therapeutic benefit.
Keywords: autoimmunity, NKT, T cell, type 1 diabetes
Introduction
Natural killer T cells (NKT) are a relatively rare subset of immunoregulatory T lymphocytes found in mice and humans. They express a semi-invariant, CD1d-restricted T cell receptor and a variety of other receptors associated more commonly with natural killer cells [1,2]. NKT recognize glycolipid antigens rather than peptides [1,3] and antigen-induced activation causes a strong and rapid release of cytokines capable of influencing a wide array of disease settings [2–5]. The distinct cytokine profiles of different NKT subsets are thought to be an important contributor to their functional diversity [6–8].
The NKT frequencies in humans remain relatively stable over time, but can vary 50-fold or more between individuals [9,10]. There is accumulating evidence in humans and mice that a low NKT frequency predisposes to many different diseases (especially autoimmune) [5,11,12] which has, in turn, prompted speculation that increased NKT levels may be beneficial therapeutically [4,13]. In mice, there are many examples where NKT deficiencies can arise, resulting typically from problems during their development in the thymus [14,15]. However, there are few instances where abnormally high NKT frequencies exist in a stable manner. Humans appear to have a naturally broad range in NKT frequencies but the basis for this diversity is unclear, and while the lower limit appears to be essentially no NKT, the upper limit is not clear but considered generally to be less than 1% of peripheral blood mononuclear cells (PBMC) [9,16,17]. These are important issues to consider, because in mice, higher than normal NKT numbers induced through the antigen-driven expansion of mature NKT are typically transient [18,19] and the cells can subsequently become functionally unresponsive [20,21].
In humans, there have been a small number of Phase 1 trials where patients have been administered with the NKT agonist alpha galactosylceramide (αGC) to boost NKT function and numbers. The outcomes have been inconsistent, with one trial reporting a significant increase in NKT proportion that gradually fell over time [22], while other trials reported increases that were typically minor and/or transient [23–25]. One of these trials also found that the NKT were functionally defective compared with control cells from healthy donors, although it is unclear whether this was caused by the expansion process or was a symptom of the underlying disease [22]. To our knowledge, NKT from a donor with naturally high NKT levels have not been studied.
As part of our ongoing studies into the significance of NKT in human type 1 diabetes (T1D), we identified an otherwise healthy female donor at risk of T1D with NKT levels higher than ever recorded previously (referred to herein as ‘donor A’). We undertook a detailed functional and cell surface phenotypic characterization of the NKT compartment of donor A and her immediate family members. Our findings have important implications for understanding the circumstances under which an unusually high NKT frequency could be established and maintained, and the impact the high frequency may have upon the functional characteristics of the pool.
Materials and methods
Subjects
Donor A was a healthy 16-year-old female at risk for T1D, whose mother had childhood-onset T1D and who, on screening, had autoantibodies to the pancreatic islet antigen glutamic acid decarboxylase molecular weight isoform 65 000 (GAD65). Fifty ml blood samples were obtained from donor A and her family members, consisting of: donor A's 50-year-old mother (an otherwise healthy type 1 diabetic with high anti-insulin serum titres); her 55-year-old father (an otherwise healthy T2D); her 22-year-old brother (healthy, no diabetes); her 45-year-old uncle (an otherwise healthy T2D); and her 77-year-old grandfather (an otherwise healthy T2D). Each family member was in good health at the time of analysis. As controls, cord blood and blood from otherwise healthy neonates undergoing cardiac surgery was obtained from the Royal Children's Hospital (Melbourne, Australia) and adult blood samples from the Red Cross Blood Bank (Melbourne, Australia). In addition, blood samples from children with a family history of T1D and with autoantibodies to at least one pancreatic islet antigen were obtained from the Burnet Clinical Research Unit, Royal Melbourne Hospital (Melbourne, Australia). PBMC were isolated by gradient centrifugation in Ficoll-Histopaque (Sigma-Aldrich, St Louis, MO, USA). Informed consent was obtained from all donors or their legal guardian. The research was approved by the Health Sciences Human Ethics Committee (University of Melbourne), the Ethics in Human Research Committee (Royal Children's Hospital), the Human Research Ethics Committee (Royal Melbourne Hospital) and the Human Research Ethics Committee (Walter and Eliza Hall Institute of Medical Research).
Antibodies
Human antibodies [phycoerythrin (PE)-conjugated anti-CD19, PE-Cy7-conjugated anti-CD3 and anti-CCR7, Pacific Blue-conjugated anti-CD4, fluorescein isothiocyanate (FITC)-conjugated anti-CD25, anti-CD45RA, anti-CD62L and anti-immunoglobulin 1 (IgG1), allophycocyanin (APC)-H7-conjugated anti-CD8, APC-conjugated anti-CD94 and anti-interferon (IFN)-γ used for fluorescence activated cell sorter staining] were all purchased from BD Biosciences (San Diego, CA, USA). FITC-conjugated anti-CD161 was purchased from Miltenyi Biotech (Bergisch Gladbach, Germany). The generation of PE-conjugated αGalCer-loaded and unloaded CD1d tetramer has been described previously [16]. Intracellular staining for IFN-γ was performed using a BD Cytofix/Cytoperm Plus Kit (BD Biosciences), as per the manufacturer's instructions. Flow cytometry data was acquired by a LSRII flow cytometer (BD) and analysed using FlowJo software (TreeStar, Ashland, OR, USA).
Flow cytometry analysis of IFN-γ production
The PBMCs were cultured in RPMI-1640 medium (Invitrogen Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal calf serum (FCS) (JRH), 100 U/ml penicillin (Invitrogen Life Technologies), 100 µg/ml streptomycin (Invitrogen Life Technologies), 2 mM glutamax (Invitrogen Life Technologies), 1 mM sodium pyruvate (Invitrogen Life Technologies), 50 µM 2-mercaptoethanol (Sigma-Aldrich), 0·1 mM non-essential amino acids (Invitrogen Life Technologies) and 15 mM HEPES buffer (Invitrogen Life Technologies) with 10 ng/ml phorbol myristate acetate (PMA) (Sigma-Aldrich) and 1 µg/ml ionomycin (Sigma-Aldrich) for 4 h; 2 µM monensin (Golgistop; BD Biosciences) was added 3 h before end of culture. Cells were prepared for flow cytometric analysis of intracellular IFN-γ using the Cytofix/Cytoperm staining kit (BD Biosciences).
Results
The NKT frequency
As part of an ongoing analysis of NKT characteristics among different human subject groups, we identified a donor (donor A) whose NKT proportion among PBMCs was more than 3% of total PBMCs and more than 5% of total T cells (Fig. 1a and b). This is higher than any previously published proportion from a human donor where αGC-loaded CD1d tetramer or 6B11 monoclonal antibody has been used to identify NKT specifically and is approximately 50 times higher than average [7,16,17,26]. For context, we provide details of NKT proportions from our own ongoing studies of donor groups separated according to age and/or susceptibility to T1D (Fig. 1a). NKT proportions are lowest in infancy, with the mean NKT frequency from cord blood or the peripheral blood of neonates (< 2 years old) being less than 0·1%. Proportions are marginally higher in peripheral blood of older donors, with no significant difference between mean NKT frequencies in peripheral blood from healthy donors (Fig. 1a; mean = 0·16%) and those, such as donor A, deemed to be at increased risk of T1D on the basis of autoantibody reactivity to pancreatic antigens (Fig. 1a; mean = 0·21%). This latter comparison is significant because it is consistent with an array of independent studies that report individuals ‘at risk’ of T1D often have reduced or normal proportions of NKT [5,11,12], thereby highlighting the unusual nature of a very high NKT proportions in donor A and indicating that it is unlikely to be related to her being ‘at risk’ of T1D.
Fig. 1.
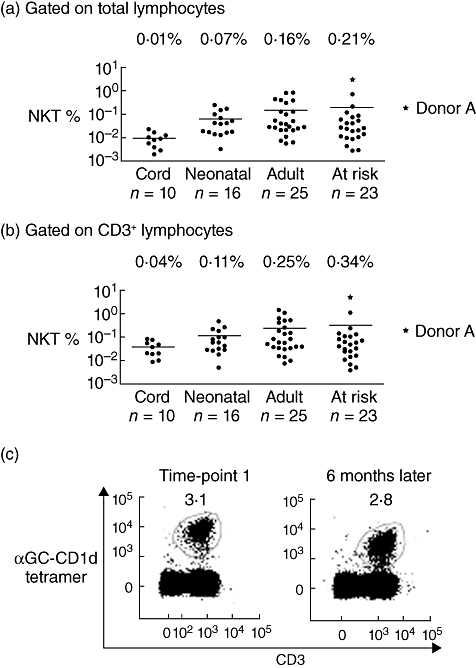
Donor with very high proportion of peripheral blood natural killer T cells (NKT). The proportion of NKT among human peripheral blood lymphocytes (a), and CD3+ T cells (b) is shown for healthy newborns (cord blood), neonates, adults and adolescents at risk of developing type 1 diabetes (n values beneath graph). Each filled circle represents one donor. Donor A is marked by a star. Lines indicate the mean for each group (numerical mean above graphs). (c) The proportion of NKT in donor A's blood was assessed at two time-points 6 months apart by staining with α-galactosylceramide-loaded CD1d tetramer and anti-CD3. Each profile has been gated on total lymphocytes.
The high NKT proportions of donor A prompted us to conduct a comprehensive follow-up study 6 months later that included analysis of her immediate family members. Donor A's NKT proportion at the second time-point was similar to that of 6 months earlier, suggesting that her high NKT frequency was not a transient event (Fig. 1c). We also examined the NKT compartments of donor A's mother, father, brother, maternal uncle and maternal grandfather. The NKT frequency varied between each family member, but was consistently at least 10-fold lower than for donor A (Fig. 2a), suggesting that Donor A's very high levels was not a dominant genetic trait.
Fig. 2.
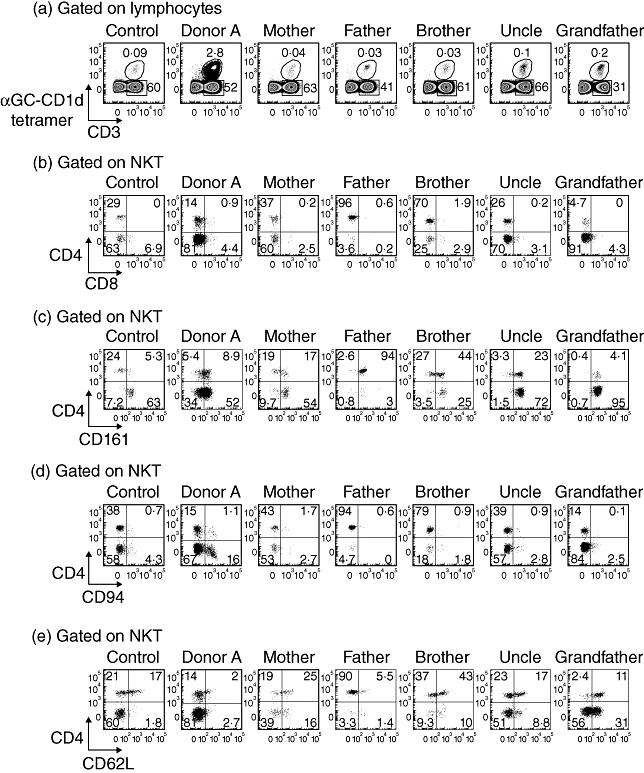
Differential expression of natural killer cell markers by natural killer T cells (NKT) from donor A and family members. (a) The proportion of peripheral blood NKT is shown for a representative healthy control donor, donor A and family members. Fluorescence activated cell sorter (FACS) profiles are gated on total lymphocytes. (b–e) NKT from a representative healthy control donor, donor A and each family member were analysed by FACS for expression of CD4 versus CD8, CD161, CD94 and CD62L. The profiles are gated on NKT.
The NKT subset distribution
Human NKT express a range of cell surface antigens that include CD4, CD8, CD161, CD94 and CD62L. The differential expression of these markers is thought to indicate, and in some instances impart, functional and/or developmental diversity within the NKT pool. The clearest examples of this are CD4− and CD4+ NKT subsets, which display different cytokine profiles after stimulation [7,27,28], and CD161− and CD161+ subsets, which broadly identify immature (particularly CD4+CD161−) and mature NKT respectively [16,29,30]. The frequency of these subsets varies between individuals, but we were particularly interested in whether the high frequency of total NKT was accompanied by a clear over-representation of one particular NKT subset.
Approximately 15% of donor A's NKT were CD4+, which was within the range of healthy controls, although in our experience somewhat lower than normal for a young adult (Fig. 2b). Donor A also had a lower proportion of CD161+ NKT than other family members, but the differences were not great, and the proportion was similar to that of healthy controls (Fig. 2c). Interestingly, although the putatively ‘immature’ CD4+CD161− subset accounted for only 5·4% of NKT from donor A, 34% of NKT were CD4−CD161−. This subset is regarded as being mature but usually represents less than 10% of NKT, as was the case among healthy control donors and all donor A's other family members. The proportions of CD4+CD161+ (9%) and CD4−CD161+ (52%) NKT subsets from donor A were consistent with that of control donors (means of 13% and 69% respectively) and family members (Fig. 2c), as was the expression of CD94 and CD62L (Fig. 2d and e). In summary, the distribution of some subsets within the NKT compartment of donor A had some unusual attributes [e.g. more NKT from donor A (17%) expressed CD94 than from other family members], but there was no indication that one particular subset was responsible for the high overall proportion of NKT. This suggested that there was an expansion of NKT at a precursor stage prior to subset differentiation, or that the factors responsible for the high NKT frequency impacted to a similar extent on all mature subsets.
Conventional lymphocytes
We next undertook a careful analysis of conventional lymphocytes from donor A and family members to investigate the possibility that the high NKT frequency of donor A reflected (or accompanied) broader immune abnormalities (Fig. 3). For example, a significant increase in overall T cell numbers or a corresponding deficit among other cell types (e.g. B cells) could result conceivably in a secondary change in the overall proportion of NKT. We examined T and B cells using a panel of antibodies (CD3, CD19, CD4, CD8, CD25, CD45RA and CCR7) that also allowed for a broad analysis of their activation and memory. The proportions of B cells and of the major T cell subsets (defined by CD3, CD4 and CD8) among PBMCs from donor A and her family were similar to healthy controls (Fig. 3), ruling out the possibility that the high NKT frequency of donor A was a secondary effect of changes to another lymphocyte subset.
Fig. 3.
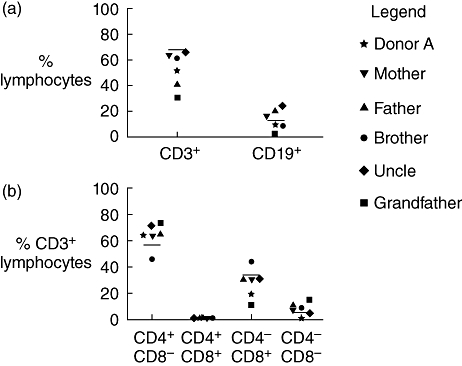
Donor A and family members have normal B cell and T cell compartments. (a) The proportion of peripheral blood lymphocytes expressing CD3 or CD19 is shown for donor A and family members. Lines indicate the means of healthy controls (T cells; n = 18, B cells; n = 12) and symbols represent individual family members. (b) The distribution of conventional CD4 and CD8 T cell subsets is shown as a proportion of CD3+ peripheral blood lymphocytes for donor A and family members. Means of control groups are represented by lines (n = 18).
Markers of activation and memory
We examined T and NKT from donor A and her family for expression of the activation marker CD25 to establish whether NKT levels might be maintained at high levels through stimulation. However, this was not the case, as fewer than 2% of donor A's NKT expressed CD25 and forward-scatter analysis suggested that the cells were not blasting. Curiously, the proportion of CD25+ NKT was higher among other family members ranging from 8% to 27% (Fig. 4a). The reason for the variability within the family is unclear, but the fact that donor A's levels are so low suggests that the NKT levels are not being supported artificially through activation. Variable CD25 expression by NKT has been reported previously, but its significance is not known [16]. Of note, donor A and other family members had only a low proportion (< 6%) of conventional T cells that expressed CD25, confirming no broad activation of the T cell compartment that might impact upon NKT. The proportions of CD4+ T cells expressing CD25 were also normal for donor A and her family, suggesting that forkhead box P3-positive (FoxP3+) regulatory T cells (which are greatly enriched among CD25+CD4+ T cells) other than NKT were represented normally (Fig. 4c). We also excluded the possibility that an earlier compartment-wide activation of T cells contributed to the NKT abnormality by finding relatively normal levels of naive T cells, with CD45RA and CCR7 co-expressed (indicative of naive phenotype) on 65% of CD8+ T cells (Fig. 4d).
Fig. 4.
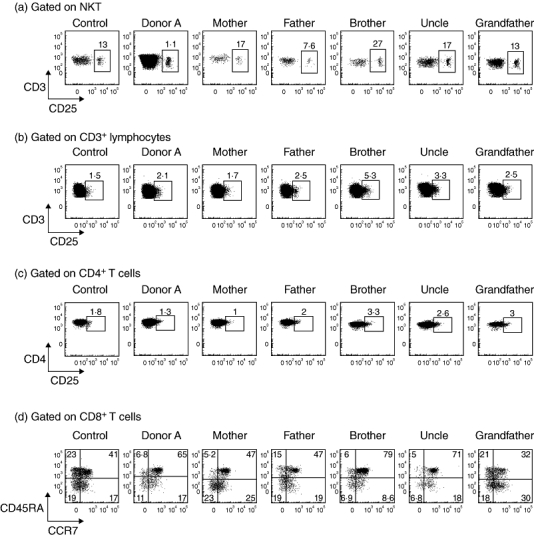
Activation status of the natural killer (NK) T and T cell compartments in donor A and family members. (a) CD25 expression on natural killer T cells (NKT) and (b) CD3+ conventional T cells, (c) CD3+ CD4+ conventional T cells of a healthy control donor, donor A and family members. (d) The proportion of peripheral blood naive CD8 T cells co-expressing CD45RA and CCR7 is shown for a healthy control donor, donor A and family members.
The NKT cytokine production
The NKT are potent producers of cytokines, and we were particularly interested in whether the NKT from donor A responded normally to stimulation by rapidly releasing IFN-γ or if there were signs of dysfunction, such as spontaneous cytokine release or anergy, both of which can result from prolonged stimulation. After stimulation with PMA and ionomycin for 4 h, intracellular staining revealed clear IFN-γ production by NKT from donor A and each family member (Fig. 5). The extent of cytokine production varied between family members, but there was no evidence of anergy among NKT from donor A and no spontaneous cytokine release in the absence of stimulation that might have indicated ongoing stimulation. A small proportion (10–25%) of conventional T cells from each family member also produced IFN-γ (Fig. 5). In summary, NKT from donor A exhibited a normal IFN-γ response to stimulation.
Fig. 5.
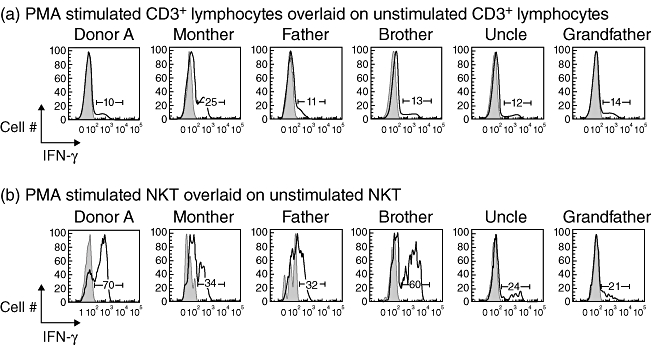
Natural killer T cells (NKT) from donor A produce interferon (IFN)-γ when stimulated in vitro with phorbol myristate acetate (PMA) and ionomycin. (a) Histograms show IFN-γ production by CD3+ lymphocytes from each family member after 4 h stimulation by PMA and ionomycin (black line) overlaid onto unstimulated CD3+ lymphocytes (grey filled area). (b) Histograms show IFN-γ production by NKT (defined by labelling with α-galactosylceramide-loaded CD1d-tetramer and anti-CD3) from each family member after 4 h stimulation by PMA and ionomycin (black line) overlaid onto unstimulated NKT (grey filled area).
Discussion
This study shows that the exceptionally high NKT frequency of donor A was neither transient nor the result of activation. The quiescent nature of donor A's NKT, coupled with a normal distribution of subsets, was consistent with earlier reports of genetic control of the NKT compartment [9,17], but the varied and much lower frequencies of the immediate family group suggests that there was no dominant genetic factor responsible for maintaining the size of the pool at this high level. The current study also suggests that the size of the niche that NKT can occupy appears to be greater and more flexible than envisaged previously – a finding implied in earlier clinical studies where patients treated with αGC showed increases in their NKT proportions [23–25].
The potential to increase the NKT proportion has important implications for the treatment of individuals who are ‘at risk’ of diseases where low NKT numbers may have an impact, because it suggests that the proportion could be raised and maintained at a level where the protective role of the NKT pool is restored. Certainly, our finding that a frequency above 3% was sustained in donor A without stimulation supports the idea that very significant increases are feasible, but further longer-term studies are required. For example, given the large number of studies that associate low NKT levels with a predisposition to T1D, it will be of interest to monitor the ongoing health and NKT pool characteristics of donor A, given her high NKT levels in the context of a family predisposition to T1D and her own signs of preclinical diabetes in the form of autoantibodies directed against islet antigens. However, the rarity of such individuals is a clear obstacle to broader studies and other approaches will be required to assess fully whether higher NKT levels are achievable, whether this confers any benefit and whether the function of the expanded NKT pool is changed, particularly in relation to cytokine production.
One potential approach may be to monitor patients who have received therapeutic doses of NKT agonists such as αGC [23–25]. This could also help to determine how tightly the composition of the human NKT compartment is regulated and its potential to be manipulated. A Phase 1 clinical trial using αGC-pulsed dendritic cells as a cancer immune therapy found the proportion of NKT could be maintained above pretreatment levels for many weeks, but this occurred only after multiple rounds of NKT stimulation and the increases were relatively small [23]. Such results are intriguing and might be improved upon as our understanding of the factors contributing to NKT expansion and survival increases, but a significant complicating factor is that the patients in these trials are typically seriously ill with immune irregularities and have already been undergoing various forms of therapy to treat their cancer. An alternative approach could be to monitor the frequency and functional activity of NKT from groups who have been exposed to environmental antigens known to promote NK T responses, such as Sphingomonas, or Borrelia burgdorferi (associated with Lyme disease) [31–34].
Regardless of the form of stimulation, monitoring patient health and the composition of the NKT compartment after NKT activation presents a number of problems. First, the systemic administration of any reagent that activates NKT (be they environmental or otherwise) may have unpredictable outcomes, given the likelihood of widespread cytokine release and the complexity of NKT subsets [3–5]. Secondly, any such studies would need to address reports from mice that multiple rounds of stimulation lead to an anergic-like state for NKT, which would presumably negate many of the potential advantages afforded by increased NKT frequency [20,21]. One group reported that this could potentially be overcome in mice by using dendritic cells pulsed with αGC [35], although at least one clinical study suggested this approach still results in NKT anergy in humans [22].
The fact that the high NKT frequency of donor A did not appear to result from activation within the NKT or T cell compartments means that the high proportion of peripheral NKT arose probably from abnormally high NKT development in the thymus or an increased rate of basal proliferation or survival in the periphery. This is consistent with the quiescent nature of the NKT from donor A, and the relative stability and normal subset distribution of the pool. It suggests that NKT numbers could be boosted by adoptive transfer of additional cells, potentially through the transfer of autologous NKT expanded in vitro from blood. Provided that cell function was maintained, this would help to avoid many of the potential drawbacks to in vivo expansion.
In summary, the detailed characterization of donor A has identified an instance where a stable, very high overall proportion of NKT in the peripheral blood has been maintained in the absence of stimulation, while maintaining normal subset distribution and cytokine production. The premise of an expandable NKT niche provides a basis for encouraging a more deliberate exploration of the potential benefits of therapeutic manipulation of the NKT compartment in humans.
Acknowledgments
The authors acknowledge the following support: S. P. B. is supported by a National Health and Medical Research Council (NHMRC) Career Development Award and an NHMRC Project grant (no. 454363); D. I. G. is supported by an NHMRC Program grant (no. 251608, renewed as no. 454569) and an NHMRC Research Fellowship. L. C. H. is an NHMRC Senior Principal Research Fellow supported by an NHMRC Program grant. We acknowledge the generous assistance of the research team involved in the INIT 2 project at The Burnet Clinical Research Unit and The Walter and Eliza Hall Institute. We also thank ‘donor A’ and her family for their willingness to participate in this research.
References
- 1.Godfrey DI, MacDonald HR, Kronenberg M, et al. NKT cells: what's in a name? Nat Rev Immunol. 2004;4:231–7. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 2.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 3.Van Kaer L. NKT cells: T lymphocytes with innate effector functions. Curr Opin Immunol. 2007;19:354–64. doi: 10.1016/j.coi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379–88. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 6.Coquet JM, Chakravarti S, Kyparissoudis K, et al. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc Natl Acad Sci USA. 2008;105:11287–92. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee PT, Benlagha K, Teyton L, et al. Distinct functional lineages of human V(alpha)24 natural killer T cells. J Exp Med. 2002;195:637–41. doi: 10.1084/jem.20011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gumperz JE, Miyake S, Yamamura T, et al. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002;195:625–36. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee PT, Putnam A, Benlagha K, et al. Testing the NKT cell hypothesis of human IDDM pathogenesis. J Clin Invest. 2002;110:793–800. doi: 10.1172/JCI15832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berzins SP, Uldrich AP, Pellicci DG, et al. Parallels and distinctions between T and NKT cell development in the thymus. Immunol Cell Biol. 2004;82:269–75. doi: 10.1111/j.0818-9641.2004.01256.x. [DOI] [PubMed] [Google Scholar]
- 11.Hammond KJ, Godfrey DI. NKT cells: potential targets for autoimmune disease therapy? Tissue Antigens. 2002;59:353–63. doi: 10.1034/j.1399-0039.2002.590501.x. [DOI] [PubMed] [Google Scholar]
- 12.Wilson SB, Delovitch TL. Janus-like role of regulatory iNKT cells in autoimmune disease and tumour immunity. Nat Rev Immunol. 2003;3:211–22. doi: 10.1038/nri1028. [DOI] [PubMed] [Google Scholar]
- 13.Hammond KJ, Kronenberg M. Natural killer T cells natural or unnatural regulators of autoimmunity? Curr Opin Immunol. 2003;15:683–9. doi: 10.1016/j.coi.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Hammond KJ, Pellicci DG, Poulton LD, et al. CD1d-restricted NKT cells: an interstrain comparison. J Immunol. 2001;167:1164–73. doi: 10.4049/jimmunol.167.3.1164. [DOI] [PubMed] [Google Scholar]
- 15.McNab FW, Berzins SP, Pellicci DG, et al. The influence of CD1d in postselection NKT cell maturation and homeostasis. J Immunol. 2005;175:3762–8. doi: 10.4049/jimmunol.175.6.3762. [DOI] [PubMed] [Google Scholar]
- 16.Berzins SP, Cochrane AD, Pellicci DG, et al. Limited correlation between human thymus and blood NKT cell content revealed by an ontogeny study of paired tissue samples. Eur J Immunol. 2005;35:1399–407. doi: 10.1002/eji.200425958. [DOI] [PubMed] [Google Scholar]
- 17.Montoya CJ, Pollard D, Martinson J, et al. Characterization of human invariant natural killer T subsets in health and disease using a novel invariant natural killer T cell-clonotypic monoclonal antibody, 6B11. Immunology. 2007;122:1–14. doi: 10.1111/j.1365-2567.2007.02647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crowe NY, Uldrich AP, Kyparissoudis K, et al. Glycolipid antigen drives rapid expansion and sustained cytokine production by NK T cells. J Immunol. 2003;171:4020–7. doi: 10.4049/jimmunol.171.8.4020. [DOI] [PubMed] [Google Scholar]
- 19.Wilson MT, Johansson C, Olivares-Villagomez D, et al. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc Natl Acad Sci USA. 2003;100:10913–18. doi: 10.1073/pnas.1833166100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uldrich AP, Crowe NY, Kyparissoudis K, et al. NKT cell stimulation with glycolipid antigen in vivo: costimulation-dependent expansion, Bim-dependent contraction, and hyporesponsiveness to further antigenic challenge. J Immunol. 2005;175:3092–101. doi: 10.4049/jimmunol.175.5.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parekh VV, Wilson MT, Olivares-Villagomez D, et al. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115:2572–83. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang DH, Osman K, Connolly J, et al. Sustained expansion of NKT cells and antigen-specific T cells after injection of alpha-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005;201:1503–17. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nieda M, Okai M, Tazbirkova A, et al. Therapeutic activation of Valpha24+Vbeta11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood. 2004;103:383–9. doi: 10.1182/blood-2003-04-1155. [DOI] [PubMed] [Google Scholar]
- 24.Motohashi S, Ishikawa A, Ishikawa E, et al. A phase I study of in vitro expanded natural killer T cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2006;12:6079–86. doi: 10.1158/1078-0432.CCR-06-0114. [DOI] [PubMed] [Google Scholar]
- 25.Uchida T, Horiguchi S, Tanaka Y, et al. Phase I study of alpha-galactosylceramide-pulsed antigen presenting cells administration to the nasal submucosa in unresectable or recurrent head and neck cancer. Cancer Immunol Immunother. 2008;57:337–45. doi: 10.1007/s00262-007-0373-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jing Y, Gravenstein S, Chaganty NR, et al. Aging is associated with a rapid decline in frequency, alterations in subset composition, and enhanced Th2 response in CD1d-restricted NKT cells from human peripheral blood. Exp Gerontol. 2007;42:719–32. doi: 10.1016/j.exger.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Kim CH, Butcher EC, Johnston B. Distinct subsets of human Valpha24-invariant NKT cells: cytokine responses and chemokine receptor expression. Trends Immunol. 2002;23:516–19. doi: 10.1016/s1471-4906(02)02323-2. [DOI] [PubMed] [Google Scholar]
- 28.Sandberg JK, Bhardwaj N, Nixon DF. Dominant effector memory characteristics, capacity for dynamic adaptive expansion, and sex bias in the innate Valpha24 NKT cell compartment. Eur J Immunol. 2003;33:588–96. doi: 10.1002/eji.200323707. [DOI] [PubMed] [Google Scholar]
- 29.Baev DV, Peng XH, Song L, Barnhart JR, et al. Distinct homeostatic requirements of CD4+ and CD4– subsets of V{alpha}24-invariant natural killer T cells in humans. Blood. 2004;104:4150–6. doi: 10.1182/blood-2004-04-1629. [DOI] [PubMed] [Google Scholar]
- 30.Sandberg JK, Stoddart CA, Brilot F, et al. Development of innate CD4+ alpha-chain variable gene segment 24 (Valpha24) natural killer T cells in the early human fetal thymus is regulated by IL-7. Proc Natl Acad Sci USA. 2004;101:7058–63. doi: 10.1073/pnas.0305986101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinjo Y, Wu D, Kim G, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–5. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 32.Mattner J, Debord KL, Ismail N, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–9. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 33.Sriram V, Du W, Gervay-Hague J, et al. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur J Immunol. 2005;35:1692–701. doi: 10.1002/eji.200526157. [DOI] [PubMed] [Google Scholar]
- 34.Kinjo Y, Tupin E, Wu D, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–86. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 35.Fujii S, Shimizu K, Kronenberg M, et al. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nat Immunol. 2002;3:867–74. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]


