Abstract
Although CD4+/CD25+ T regulatory cells (Tregs) are a potentially powerful tool in bone marrow transplantation, a prerequisite for clinical use is a cell-separation strategy complying with good manufacturing practice guidelines. We isolated Tregs from standard leukapheresis products using double-negative selection (anti-CD8 and anti-CD19 monoclonal antibodies) followed by positive selection (anti-CD25 monoclonal antibody). The final cell fraction (CD4+/CD25+) showed a mean purity of 93·6% ± 1·1. Recovery efficiency was 81·52% ± 7·4. The CD4+/CD25+bright cells were 28·4% ± 6·8. The CD4+/CD25+ fraction contained a mean of 51·9% ± 15·1 FoxP3 cells and a mean of 18·9% ± 11·5 CD127 cells. Increased FoxP3 and depleted CD127 mRNAs in CD4+CD25+FoxP3+ cells were in line with flow cytometric results. In Vβ spectratyping the complexity scores of CD4+/CD25+ cells and CD4+/CD25− cells were not significantly different, indicating that Tregs had a broad T cell receptor repertoire. The inhibition assay showed that CD4+/CD25+ cells inhibited CD4+/CD25− cells in a dose-dependent manner (mean inhibition percentages: 72·4 ± 8·9 [ratio of T responder (Tresp) to Tregs, 1:2]; 60·8% ± 20·5 (ratio of Tresp to Tregs, 1:1); 25·6 ± 19·6 (ratio of Tresp to Tregs, 1:0·1)). Our study shows that negative/positive Treg selection, performed using the CliniMACS device and reagents, enriches significantly CD4+CD25+FoxP3+ cells endowed with immunosuppressive capacities. The CD4+CD25+FoxP3+ population is a source of natural Treg cells that are depleted of CD8+ and CD4+/CD25− reacting clones which are potentially responsible for triggering graft-versus-host disease (GvHD). Cells isolated by means of this approach might be used in allogeneic haematopoietic cell transplantation to facilitate engraftment and reduce the incidence and severity of GvHD without abrogating the potential graft-versus-tumour effect.
Keywords: CD127, GvHD, FoxP3, immunosuppression, Tregs
Introduction
T regulatory cells (Tregs) play a pivotal role in controlling the adaptive immune response and in self-tolerance maintenance in mice and humans [1]. In mice Tregs constitute a phenotypically distinct population of CD25+bright cells that comprise 5–10% of the peripheral blood CD4+/CD8− population [2]. In humans up to 10% of peripheral blood CD4+ T cells express the CD25 antigen [3], but only 1–2% express a high level of CD25 (CD25 bright cells) and possess suppressor activity [4].
At present there is no unique repertoire of cell surface molecules which define Tregs in humans unambiguously. Although CD25 brightness appears to be an integral characteristic [4], it is not a specific marker for several reasons. CD25 expression is up-regulated in naive non-regulatory CD4+ T cells after T cell receptor (TCR) engagement; after in vitro clonal expansion CD4+/CD25− T cells also have the potential to exert regulatory activity; furthermore, when Tregs are stimulated antigenically and homeostatically in vivo, CD25 expression becomes unstable [5].
In the search for a specific marker of Tregs, FoxP3, a transcription factor member of the forkhead-winged helix family, was identified as the master gene controlling Treg development and function in mice and humans [6,7]. A sustained high level of FoxP3 expression is essential for induction of Treg phenotype and function [8,9]. As FoxP3 is a transcription factor which localizes to the nucleus, it cannot be used as a surface marker of Tregs.
Another potential discriminating marker for Tregs which has been proposed recently is low expression of CD127, the interleukin (IL)-7 receptor alpha chain. Interestingly, CD127 expression is associated with acquisition of Treg function [10]. It correlates inversely with FoxP3 expression and Treg suppressor activity [11]. Given the drawbacks of each of the above markers, the best way at present to identify human Tregs appears to be co-expression of high levels of CD25 and FoxP3 as well as low levels of CD127.
Several studies have demonstrated the potential benefits of Tregs in the allogeneic bone marrow transplantation (BMT) setting. They were reported to reduce the incidence of graft-versus-host disease (GvHD) and hasten immune reconstitution, which usually takes up to 1 year [12–15]. Adoptive transfer of donor Tregs controlled GvHD while maintaining the graft-versus-tumour responses [16–18], preserved thymic and lymph node architecture from GvHD and accelerated donor lymphoid reconstitution [19].
A prerequisite for the clinical use of Tregs is a cell-separation strategy that enriches this rare cell population efficiently, ensures that all are characterized by the proposed phenotype and complies with good manufacturing practice (GMP) guidelines. In this study we isolated Tregs from standard leukapheresis products by magnetic cell-separation under GMP conditions, using double-negative selection (anti-CD8 and anti-CD19) followed by positive selection (anti-CD25). Phenotype, molecular and functional characteristics of the large-scale isolated Tregs are described and their suitability for clinical use is assessed.
Materials and methods
Apheresis procedures
After obtaining informed consent from donors, mononuclear cells were obtained by apheresis using a continuous-flow cell separator (Cobe SPECTRA, Cobe BCT Inc., Lakewood, CO, USA). Five procedures were performed from five different donors [median age 52 years (range 33–58)]. Two of the donors also underwent an additional procedure after 2 months (a total of seven procedures). Peripheral blood mononuclear cells were obtained by Ficoll density gradient centrifugation.
Large-scale Treg separation
CD4+CD25+ regulatory T cells were isolated using large-scale (CliniMACS TM Instruments, Miltenyi Biotec GmbH, Germany) separation systems. Clinical-grade reagents were used (GMP conditions). CD4+CD25+ Tregs were isolated in a two-step procedure. First, cells were washed, adjusted to 87·5 ml in phosphate-buffered saline (PBS), ethylenediamine tetraacetic acid (EDTA) and 2% human albumin (HA), labelled with CliniMACS CD8 and CD19 microBeads (Miltenyi Biotec) for 30 min at room temperature on an orbital shaker, re-washed and re-suspended in 100 ml PBS/EDTA/2% HA. CD8- and CD19-labelled cells were depleted by the 2·1 depletion programme on the CliniMACS instrument (Miltenyi Biotec).
The negative cell fraction was suspended in 190 ml PBS/EDTA/2% HA, labelled with 7·5 ml CD25 microbeads (CliniMACS) for 30 min at room temperature on an orbital shaker, washed and re-suspended in 100 ml PBS/EDTA/2% HA. CD25+ cells were isolated by automatic positive selection cycles using the 3·1 enrichment programme on the CliniMACS device. Aliquots before and after each labelling, depletion and enrichment step were immunophenotyped.
Immunophenotyping
Phenotypes were determined using direct immunofluorescence with a panel of monoclonal antibodies directed against the following antigens: CD45, CD3, CD4, CD8, CD14, CD19, CD16, CD25, CD56, CD11b, CCR7, CCR5, CD45RA, CD45RO, CD62L, CD127 (Coulter Corporation, Hialeah, FL, USA) and FoxP3 (Miltenyi Biotec). The control used for FoxP3 analysis was mouse immunoglobulin G 1-phycoerythrin (Miltenyi Biotec). Cells were analysed by Cytomics FC500 Cytometer (Coulter Corporation, Hialeah, FL, USA).
Suppression assay
Cultures of 105 irradiated stimulator cells, 2 × 104 T responder cells CD4+/CD25− (Tresp) and autologous suppressor subpopulations, i.e. CD4+/CD25+ (Tregs), in the presence of phytohaemagglutinin (4 µg/ml) (Biochrom AG, Berlin, Germany) were analysed. The final responder to suppressor ratios were 1:2; 1:1; 1:0·1. Cells were pulsed with 1 µCi/well [3H]-thymidine (Amersham Biosciences, Buckinghamshire, UK) for the last 16 h, and incorporated [3H]-thymidine was measured with a beta-counter. Data are presented as inhibition percentages calculated by the following formula: 100 − (counts per minute of activated Tresp cells alone/cmp Tresp+ Tregs cells).
T cell receptor Vβ CDR3 size spectratyping
Spectratyping was performed on CD4+/CD25+ and CD4+/CD25− cells. CDR3 size distribution of 26 TCR Vβ families was determined by reverse transcription–polymerase chain reaction (RT–PCR), as described previously [20]. In brief, RNA was extracted and cDNA synthesized using Moloney murine leukaemia virus–reverse transcriptase (Nanogen Advanced Diagnostic, Turin, Italy). PCR was performed with a specific forward primer for one of the TCR Vβ families along with a constant Cβ reverse primer labelled with fluorescent 5-carboxyfluorescein [21]. RT–PCR products were analysed on an ABI PRISM 3130 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) using GeneScan software. Normal TCR Vβ CDR3 was characterized by a Gaussian distribution, containing 8–10 peaks for each Vβ subfamily. The overall complexity of TCR Vβ subfamilies was determined by spectratype scoring as described previously [22].
Real-time–quantitative PCR
Total RNA from CD4+/CD25+ and CD4+/CD25− cells was reverse-transcribed using a random primer, high-capacity cDNA RT kit (Applied Biosystems). cDNA was amplified with TaqMan® Universal PCR Master Mix (Applied Biosystems) using the TaqMan Gene Expression Assay for analysis of FoxP3 gene expression (Hs01085835_m1) and CD127 gene expression (Hs00902338_g1). mRNA was analysed on an ABI PRISM® 7900 Sequence Detection System (Applied Biosystems). Gene expression level in a given sample was represented as 2−ΔΔCt where ΔΔCt = [ΔCt (sample) − ΔCt (calibrator)] and ΔCt = [Ct (sample) − Ct (housekeeping)]. Relative expression was determined by normalization to Abelson gene.
Results
Large-scale selection of Tregs: phenotypic features
The initial leukapheresis products contained a median of 7·1 × 109 (range 4·2–9·2 × 109) nucleated cells. On average, 3·5% (range 2·5–7) of the cells expressed CD4 and CD25, and an average of 0·7% (range 0·5–1·75) were CD4+/CD25+bright. In the starting fraction the median number of CD4+/CD25+ was 238 × 106 (range 157–509), while the median number of CD4+/CD25+bright was 102 × 106 (range 52–184). After magnetic cell separation a median of 186 × 106 (range 117–381 × 106) were recovered in the final cell fraction (CD4+/CD25+) with a mean purity of 93·6% ± 1·1 (Table 1). The efficiency of recovery was 81·52% ± 7·4. When CD4+/CD25+bright are considered, a median of 55 × 106 (range 46–170 × 106) were recovered in the final cell fraction with a mean purity of 28·4% ± 6·8 (Table 1). The efficiency of recovery was 78% ± 20·9. The final fraction contained no B cells and CD8+ cells; CD14+ cells were 0·4% ± 0·4, CD56+ 0·9% ± 0·6 and CD11b 0·7% ± 0·5.
Table 1.
Phenotypical and functional features of isolated T regulatory cells (Tregs).
| % CD4/CD25 |
% CD4/CD25FoxP3 |
% CD4/CD25 CD127 |
|||||
|---|---|---|---|---|---|---|---|
| Sample | Bright | Bright | Bright | % of Inhibition | |||
| A | 95·2 | 29·3 | 32·3 | 81·1 | 16·8 | 0·0 | 76·3 |
| B | 92·7 | 20·5 | 29·9 | 85 | 16·3 | 0·3 | 69·3 |
| C1 | 93·2 | 25·2 | 57·6 | 92·6 | 43·2 | 3·9 | 0 |
| C2 | 94 | 26 | 57·4 | 93·6 | 19·1 | 2·7 | 70·5 |
| D1 | 91·9 | 24·5 | 52·6 | 87·7 | 14·7 | 1·1 | 20 |
| D2 | 94·4 | 31·3 | 52 | 87·4 | 16·1 | 1·1 | 64·7 |
| E | 93·7 | 41·68 | 76·6 | 91·6 | 6·19 | 0·28 | 63·7 |
| Mean | 93·6 | 28·4 | 51·2 | 88·4 | 18·9 | 1·3 | 52·1 |
| s.d. | 1·1 | 6·8 | 16·0 | 4·5 | 11·5 | 1·4 | 29·6 |
Regulatory cells were identified not only by their high CD25 expression, but also by their high FoxP3 and low CD127 expressions compared with CD4/CD25− fraction. In the starting fraction the median number of FoxP3+ was 171 × 106 (range 84–1002 × 106). After magnetic cell separation a median of 97 × 106 (range 37–285 × 106) were recovered in the final cell fraction. The efficiency of recovery for FoxP3 was 66·3% ± 38·2. The CD4/CD25+ fraction contained a mean of FoxP3 cells equal to 51·2% ± 16; within the CD4/CD25+bright fraction the mean of FoxP3 cells was 88·4% ± 4·5. The CD4/CD25− fraction contained a mean of FoxP3+ cells of 4·4% ± 2·3 (Fig. 1).
Fig. 1.
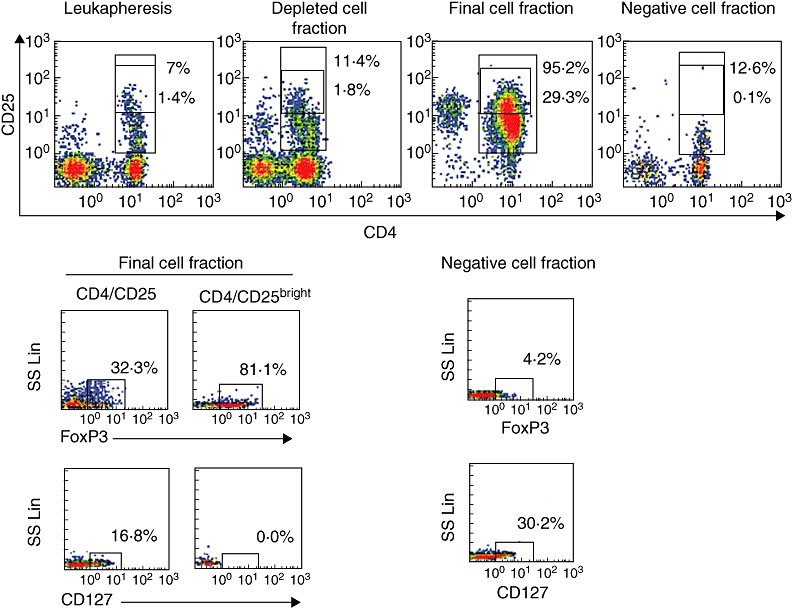
CD4+/CD25+ and CD4+/CD25bright cell fraction within starting fraction, depleted cell fraction, final cell fraction and negative cell fraction. FoxP3 and CD127 expressions in the CD4+/CD25+, CD4+/CD25bright cell fractions and in the negative cell fraction are also shown. Positive cells are boxed and the percentage of cells in the box is shown.
The percentage of CD127 was also analysed. The CD4/CD25+ fraction contained a mean of CD127 cells of 18·9% ± 11·5; within the CD4/CD25+bright fraction the mean of CD127 cells was of 1·4% ± 1·4 (Fig. 1).
The distribution of CD45 isoforms within the CD4+/CD25+ fraction was 95·3 ± 2·3 for CD45RO and 5·3% ± 0·25 for CD45RA. The distribution of CD45 isoforms within the CD4+/CD25+ bright fraction was 99·4% ± 0·1 for CD45RO and 1·4% ± 0·4 for CD45RA (Fig. 2).
Fig. 2.
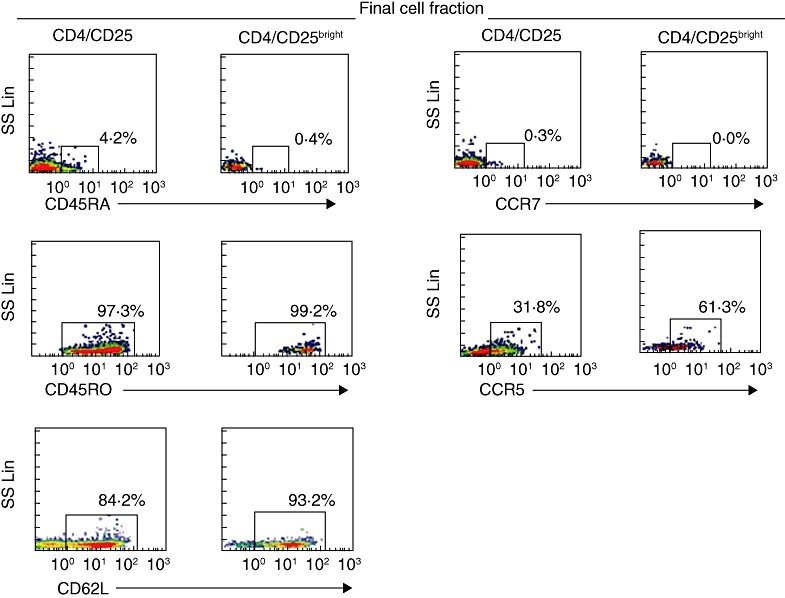
CD45RA, CD45RO, CCR7, CCR5 and CD62L expressions in the CD4+/CD25+, CD4+/CD25bright cell fractions and in the negative cell fraction are also shown. Positive cells are boxed and the percentage of cells in the box is shown.
CCR5 constituted 21% ± 14·8 of the CD4/CD25+ final fraction and 42·5% ± 17·7 of the CD4/CD25+bright fraction. CCR7 constituted 0·5% ± 0·3 of the CD4/CD25+ final fraction and 0·2% ± 0.2 of the CD4/CD25+bright fraction. The percentage of CD62L was 79·5 ± 6·1 on CD4+/CD25+ and 85·8% ± 4·5 on the CD4/CD25+bright fraction (Fig. 2).
FoxP3 and CD127 mRNA quantification
The qRT–PCR on Tregs showed that FoxP3 expression was increased compared with the CD4/CD25− fraction (4·06 ± 2·6-fold in CD4+/CD25+). The CD127 expression was decreased by –2·27 ± 0·68-fold in CD4+/CD25+, in line with the results of immunophenotyping.
CD4/CD25+ and CD4/CD25− spectratyping analysis
Vβ spectratyping was performed on CD4+/CD25+ cells and CD4+/CD25− cells. Complexity scores in T cells did not differ significantly (194 ± 2 versus 187 ± 2) (Fig. 3), indicating that a broad TCR repertoire had been maintained.
Fig. 3.
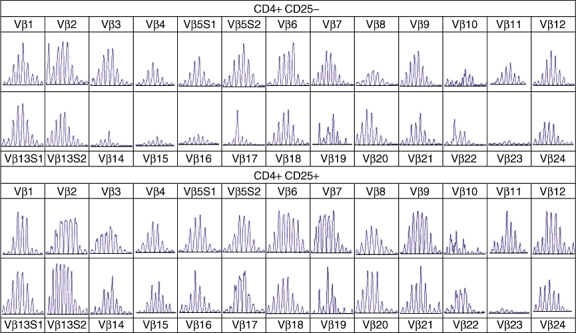
T cell receptor (TCR) Vβ spectratyping of the CD4+/CD25+ and CD4+/CD25− cell fractions are represented.
Immunosuppressive assay
The immune suppressive capacity of purified Treg populations was tested in an inhibition assay. CD4/CD25+ cells inhibited CD4+/CD25− cells in a dose-dependent manner. In particular the mean inhibition percentage was 72·4 ± 8·9 (ratio of Tresp to Tregs, 1:2); 60·8% ± 20·5. (ratio of Tresp to Tregs, 1:1); 25·6 ± 19·6 (ratio of Tresp to Tregs, 1:0·1) (Fig. 4). Interestingly, in two cases the inhibition capacity of the isolated Tregs was particularly low (cases C1 and D1 with 0% and 20% of inhibition respectively) (Table 1).
Fig. 4.
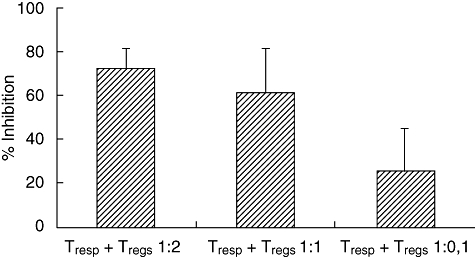
Suppression assays: at T responder (Tresp) to T regulatory (Treg) ratios of 1:2, 1:1 and 1:0·1, suppression capacity is indicated by the percentage of the inhibition of responder cells.
Correlation between phenotypes and inhibition assay
To investigate whether the CD127+-contaminating population played a role in the functional properties of purified Treg populations, we plotted CD127 versus CD25 in all cases. As shown in Fig. 5, case C1 had 43% double-positive cells (CD25+/CD127+). Cases A, B, D1 and E had between 6·2% and 16·3%. In case D1, even with a 20% inhibition rate, there were only 14·1% CD25+/CD127+ cells.
Fig. 5.
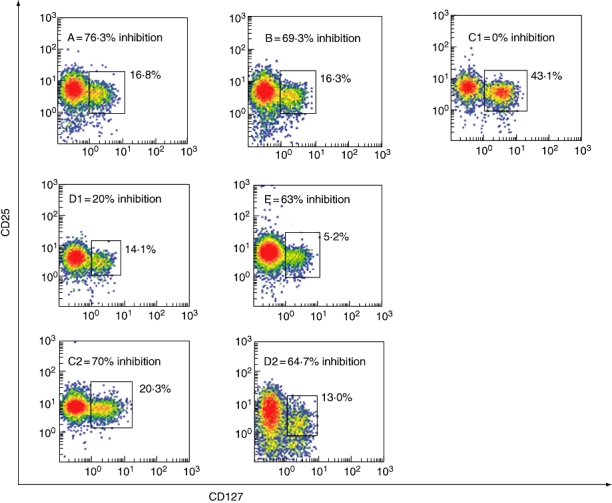
Double-positive CD25/CD127 cells within the seven different CD4+/CD25+ final fractions are indicated. Positive cells are boxed and the percentage of cells in the box is shown. Letters indicate the different procedures as marked in Table 1. The inhibition capacity of each different cell product is also shown.
In donors C and D we repeated the separation procedure after 60 days (procedures C2 and D2). In case C2 double-positive cells (CD25/CD127) decreased to 20·3%. The inhibition capacity was restored (70·5%). Interestingly, the donor's case history revealed an influenza-like syndrome immediately before the first apheresis (procedure C1).
When donor D repeated the separation procedure (D2) no significant difference emerged in the phenotypes (14·1% versus 13% CD25/CD127). The inhibition capacity increased from 20% to 64·7%. No clinically relevant episodes were found in donor D's history before either procedure.
Discussion
In this study magnetic cell-separation, i.e. double-negative selection (anti-CD8 and anti-CD19) followed by positive selection (anti-CD25), served to isolate Tregs from standard leukapheresis products under GMP conditions.
Hoffmann et al.[23] reported an alternative approach using one cycle of B cell depletion with anti-human CD19 beads followed by three repetitive enrichment cycles for CD25+ cells had ensured efficient enrichment of human T regulatory cells from leukapheresis products but admitted a low percentage of activated CD8+ T cells, and a few natural killer cells still contaminated the cell products. In a recently described population of CD8 regulatory cells [24] CD25 expression was not clarified completely [25], but it could have been a potential signal of activated CD8+ cells. As alloreactive CD8+ T cells may trigger GvHD, we decided to perform concomitant B and CD8 cell depletion because complete depletion of activated cells is a fundamental prerequisite for clinical purposes in BMT patients. B cell depletion eliminated activated CD25+ B cells that could enhance the risk of B cell type-associated lymphoproliferative disorders in the setting of BMT [26].
Because Tregs are characterized not only by CD25 expression, but also by FoxP3 high expression and low CD127 expression, our system provided enrichment of FoxP3-expressing cells together with depletion of CD127-positive cells in the final product.
In a previous study [27] we isolated CD4+/CD25+ regulatory cells under non-GMP conditions using an isolation kit (Miltenyi). Compared with non-GMP small-scale selection [27], this large-scale separation system maintained purity of the final product, although it was associated with loss of CD25bright and a slight increase in CD127+ cells. Despite these slight differences in small- and large-scale systems, the final products of both exerted the same suppressive activity. Indeed, CD4/CD25 with intermediate expression of CD25 also showed T regulatory function [4]. In both small- and large-scale selected cells mRNA expression confirmed that CD127 correlated inversely with FoxP3 expression, providing evidence that cell populations deriving from large-scale selection were Tregs[10]. Continued expression of FoxP3 maintains lineage identity and function of peripheral mature Treg cells [7], while low CD127 expression is associated with acquisition of regulatory functions [28]. The mechanism underlying CD127 inhibition was hypothesized to be IL-2-related, as it regulates CD127 negatively through defective downstream PI3K signalling [29].
Of interest, two of seven procedures exhibited, respectively, 0 and 20% inhibition capacity and one of these (C1) had 43% double-positive CD25+/CD127+ cells. In our view this phenotype can explain the lack of regulatory function in the C1 procedure. We speculate that an influenza-like syndrome before the separation procedure might have triggered increased expression for the CD25 antigen in the CliniMACS system which, consequently, also selected the double-positive CD25+/CD127+ contaminating effector cells, causing the low inhibition rate. Interestingly, repeating the procedure (C2) 2 months later generated many fewer contaminating effectors and was associated with an increased inhibition rate in the suppression assay. Therefore, before large-scale selection accurate screening of donors is advisable and/or performance of a small-scale test to predict the inhibition capacity of each potential donor.
Purity of Treg preparations is a fundamental issue when expansion is needed for clinical purposes. Interestingly, in attempting to improve the purity of Tregs obtained under GMP conditions, Peters et al.[30] recently reported enrichment of functional Tregs after CD127 depletion by fluorescence activated cell sorter under non-GMP conditions, as anti-CD127 microbeads for use in CliniMacs are not currently available. It must be admitted that high-level purity was achieved compared with the present results of 18·9% ± 11·5 CD127+-contaminating cells obtained, however, under GMP conditions. Indeed, our preliminary experiments in expanding Treg populations after GMP separation showed reduced immunosuppressive capacity because of the outgrowth of unwanted CD127+ cells (data not given), which could become a risk factor in the clinical setting. Furthermore, double-positive CD25+/CD127+-contaminating effector cells might be only one of the factors influencing the suppressive capacity of selected Tregs. In fact, the low inhibition rate of the D1 procedure remains unaccounted for. Further studies are needed to address the phenomenon.
Large-scale selection maintained the same CD45 RO/RA and the CCR5/CCR7 ratios that we obtained in small-scale selection [27]. Hoffmann et al.[31] demonstrated recently that only the CD45RA+/CD62L+/CCR7+ subset maintains the Treg cell phenotype and function throughout culture. On the other hand, CCR5+ cell enrichment by immunoselection and cell sorting within the CD4+/CD25+/CD45RO+ fraction was associated with stronger suppressive activity [32].
Although it is assumed Treg cells are not selected against any specific virus or fungal infectious agent, specific clones against such pathogens might be present, allowing a potential in vivo response against the infections [33]. Evidence in support of this hypothesis derives from TCR Vβ spectratyping which showed that a broad repertoire had been maintained in Treg lymphocytes in both small- and large-scale procedures. Further qualitative and quantitative studies are in progress to define these repertoires.
The Tregs appear to be a good candidate for cellular therapy, as they can prevent development of autoimmune diseases [34], tumour immunity [34], graft rejection [35] and GvHD [35]. Although several studies have described strategies for expanding Tregsin vitro, largely by using co-stimulatory antibodies against CD3 and CD28 in conjunction with an extremely high dose of IL-2 [36], they may be associated with the risk of triggering CD4+/CD25+ effector cell expansion and consequent T cell-mediated alloreactions when used as adoptive therapy. The results of the present in vitro studies show how the difficulties in obtaining a sufficient number of Tregs for clinical purposes can be overcome using our GMP selection system, but some issues still need to be investigated in depth to ensure optimal yields.
Acknowledgments
We would like to thank Dr Geraldine Anne Boyd for her help. This work was supported by ‘Associazione Umbra Leucemie e Linfomi’, Perugia, Italy and by ‘Associazione Italiana Leucemie, Linfomi e Mielomi’, L'Aquila Section, L'Aquila, Italy.
References
- 1.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Ann Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 2.Itoh M, Takahashi T, Sakaguchi N, et al. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining self-tolerance. J. Immunol. 1999;162:5317–26. [PubMed] [Google Scholar]
- 3.Stephens LA, Mottet C, Mason D, Powrie F. Human CD4+CD25+ thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur J Immunol. 2001;31:1247–54. doi: 10.1002/1521-4141(200104)31:4<1247::aid-immu1247>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 4.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 5.Zelenay S, Lopes-Carvalho T, Caramalho I, Moraes-Fontes MF, Rebelo M, Demengeot J. Fox P3+CD25-CD4 T cells constitute a reservoir of committed regulatory cells that regain CD25 expression upon homeostatic expansion. Proc Natl Acad Sci USA. 2005;102:4091–6. doi: 10.1073/pnas.0408679102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fontenot JD, Rasmussen JP, Willliams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor FoxP3. Immunity. 2005;22:329–41. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Williams LM, Rudensky A. Maintenance of the FoxP3-dependent developmental program in mature regulatory T cells requires continued expression of FoxP3. Nat Immunol. 2007;8:277–84. doi: 10.1038/ni1437. [DOI] [PubMed] [Google Scholar]
- 8.Gavin MA, Torgerson TR, Houston E, et al. Single-cell analysis of normal and FoxP3-mutant human T cells: FoxP3 expression without regulatory T cell development. Proc Natl Acad Sci USA. 2006;103:6659–64. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–27. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 10.Seddiki N, Santner-Nanan B, Martinson J, et al. Expression of interleukin (IL)-2 and Il-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;7:1693–700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ Treg cells. J Exp Med. 2006;203:1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davison GM, Novitzky N, Kline A, et al. Immune reconstitution after allogeneic bone marrow transplantation depleted of T cells. Transplantation. 2000;69:1341–7. doi: 10.1097/00007890-200004150-00022. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389–99. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen JL, Trenado A, Vasey D, Klatzmann D, Salomon BL. CD4(+)CD25(+) immunoregulatory T Cells: new therapeutics for graft-versus-host disease. J Exp Med. 2002;196:401–6. doi: 10.1084/jem.20020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–9. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 16.Edinger M, Hoffmann P, Ermann J, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–50. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 17.Trenado A, Charlotte F, Fisson S, et al. Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J Clin Invest. 2003;112:1688–96. doi: 10.1172/JCI17702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones SC, Murphy GF, Korngold R. Post-hematopoietic cell transplantation control of graft-versus-host disease by donor CD425 T cells to allow an effective graft-versus-leukemia response. Biol Blood Marrow Transplant. 2003;9:243–56. doi: 10.1053/bbmt.2003.50027. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen VH, Shashidhar S, Chanq DS, et al. The impact of regulatory T cells on T cell immunity following hematopoeitic cell transplantation. Blood. 2008;111:945–53. doi: 10.1182/blood-2007-07-103895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eiraku N, Hingorani R, Ijichi S. Clonal expansion within CD4+ and CD8+ T cell subsets in human T lymphotropic virus type I-infected individuals. J Immunol. 1998;161:6674–80. [PubMed] [Google Scholar]
- 21.Gorski J, Piatek T, Yassai M, Gorski J, Maslanka K. Improvements in repertoire analysis by CDR3 size spectratyping: bifamily PCR. Ann NY Acad Sci. 1995;756:99–102. doi: 10.1111/j.1749-6632.1995.tb44490.x. [DOI] [PubMed] [Google Scholar]
- 22.Wu JC, Chillemi A, Alyea EP, et al. Reconstitution of T-cell receptor repertoire diversity following T-cell depleted allogeneic bone marrow transplantation is related to hematopoietic chimerism. Blood. 2000;95:352–59. [PubMed] [Google Scholar]
- 23.Hoffmann P, Boeld TJ, Eder R, et al. Isolation of CD4+CD25+ regulatory T cells for clinical trials. Biol Blood Marrow Transplant. 2006;12:267–74. doi: 10.1016/j.bbmt.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Joosten SA, van Meijgaarden KE, Savage ND, et al. Identification of a human CD8+ regulatory T cell subset that mediates suppression through the chemokine CC chemokine ligand 4. Proc Natl Acad Sci USA. 2007;104:8029–34. doi: 10.1073/pnas.0702257104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herndler-Brandstetter D, Veel E, Laschober GR, et al. Non-regulatory CD8+CD45RO+CD25+ T lymphocytes may compensate for the loss of antigen-inexperienced CD8+CD45RA+ in old age. Biol Chem. 2008;389:561–8. doi: 10.1515/bc.2008.052. [DOI] [PubMed] [Google Scholar]
- 26.Weinstock DM, Ambrossi GG, Brennan C, Kiehn TE, Jakubowski A. Preemptive diagnosis and treatment of Epstein–Barr virus associated post transplant lymphoproliferative disorder after hematopoietic stem cell transplantation: an approach in development. Bone Marrow Transplant. 2006;37:539–46. doi: 10.1038/sj.bmt.1705289. [DOI] [PubMed] [Google Scholar]
- 27.Di Ianni M, Del Papa B, De Ioanni M, et al. Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol. 2008;36:309–18. doi: 10.1016/j.exphem.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Hartigan-O'Connor DJ, Poon C, Sinclair E, Mccune JM. Human CD4+ regulatory T cells express lower levels of the IL-7 receptor alpha chain (CD127), allowing consistent identification and sorting of live cells. J Immunol Methods. 2007;319:41–52. doi: 10.1016/j.jim.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Bensinger SJ, Walsh PT, Zhang J, et al. Distinct IL-2 receptor signalling patterns in CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5287–96. doi: 10.4049/jimmunol.172.9.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peters JH, Fw P, Woestenenk R, Hilbrands LB, Koenen HJPM, Joosten I. Clinical grade Treg: GMP isolation, improvement of purity by CD127pos depletion, Treg expansion, and Treg cryopreservation. PLoS ONE. 2008;3:1–9. doi: 10.1371/journal.pone.0003161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffmann P, Eder R, Boeld TJ, et al. Only the CD45Ra+ subpopulation of CD4+CD25high T cells gives rise to homogeneous regulatory T cell lines upon in vitro expansion. Blood. 2006;108:4260–7. doi: 10.1182/blood-2006-06-027409. [DOI] [PubMed] [Google Scholar]
- 32.Kallikourdis M, Andersen KG, Welch KA, Betz AG. Alloantigen-enhanced accumulation of CCR5+‘effector’ regulatory T cells in the gravid uterus. Proc Natl Acad Sci USA. 2007;104:594–9. doi: 10.1073/pnas.0604268104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perruccio K, Tosti A, Burchielli E, et al. Transferring functional immune responses to pathogens after haploidentical hematopoietic transplantation. Blood. 2005;106:4397–406. doi: 10.1182/blood-2005-05-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–18. [PubMed] [Google Scholar]
- 35.Le Blanc K, Rasmusson I, Sunberg B, et al. Treatment of severe graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 36.Levings M, Sangregorio R, Roncarolo M. Human CD4+CD25+ T regulatory cells suppress naïve and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med. 2001;193:1295–301. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]


