Abstract
In the small intestine members of both the α-defensin (DEFA5 and DEFA6) and β-defensin (DEFB1 and DEFB2) family contribute to the anti-microbial barrier against infection. The aim of this study was to determine whether Staphylococcal enterotoxin B (SEB)-mediated immune activation and proinflammatory cytokines play a role in the regulation of intestinal defensin expression. Defensin mRNA and peptide secretion was studied after ex vivo tissue culture of duodenal biopsies over 24 h. Immune (T cell and macrophage) activation was induced by SEB, and in separate experiments exogenous proinflammatory cytokines were added individually. Defensin mRNA levels were quantified by reverse transcription–polymerase chain reaction, and peptide release into culture supernatants was quantified by immuno dot blot or enzyme-linked immunosorbent assay. Increasing concentrations of SEB down-regulated DEFA5, DEFA6 and DEFB1 mRNA in a dose-dependent manner but increased DEFB2 simultaneously. The down-regulation of α-defensins was reversed by dexamethasone. DEFA5 and DEFB2 peptide secretion levels were altered in parallel with mRNA. Interferon-γ and interleukin (IL)-1β exhibited a dose-dependent down-regulation of α-defensin mRNA, IL-6 significantly down-regulated only DEFA6; in contrast, tumour necrosis factor-α and IL-4 had no significant effect. Immune cell activation and proinflammatory cytokines down-regulated the constitutively expressed DEFA5, DEFA6 and DEFB1 defensins, and up-regulated DEFB2 in intact human intestinal tissue explants in short-term culture. The effect of local immune activation on innate defence may explain the reduced α-defensin expression noted in inflammatory T cell-mediated enteropathies.
Keywords: defensin, inflammation, intestine, mucosal immunity
Introduction
Intestinal infectious disease is a major contributor to morbidity and mortality in tropical countries, estimated to have caused 1·5 million deaths in children in developing countries in 2002 [1]. Anti-microbial peptides secreted into the intestine constitute an important barrier to colonization of enterocytes by pathogens, but little is known about factors which regulate their expression [2–4]. Paneth cells and absorptive enterocytes in the intestine contain a rich armamentarium of anti-microbial peptides and proteins. Paneth cell-derived α-defensins, human defensin-5 and -6 (DEFA5 and DEFA6) and epithelial β-defensins (DEFB1–4) are two structurally related families implicated in providing intestinal anti-microbial defence, thus contributing to maintaining gut homeostasis [5–8].
Several studies have demonstrated a crucial role for these peptides in various models of gastrointestinal infection and inflammation [2,9–11]. It has been demonstrated that mice with a deletion of the matrilysin gene cannot process α-defensins to their active form and are therefore more susceptible to colonisation with Salmonella spp. [12]. Also, transgenic mice which express DEFA5 are protected from lethal challenge with S. typhimurium, indicating that DEFA5 can function as an anti-microbial molecule in vivo[13].
We have observed previously that adults living in a crowded township in Lusaka, Zambia, exhibit an approximate 10-fold decrease in DEFA5 and DEFA6 mRNA expression when compared with adults living in London [14]. This is a surprise finding, as one may speculate that increased expression of anti-microbial peptides might confer a survival advantage in tropical populations where exposure to intestinal pathogens is frequent and intense. In a previous study of a healthy control population in Zambia it was noted that tropical enteropathy appears to be the normal state for the small intestine in this setting [15]. The morphological changes in the pathogenesis of tropical enteropathy have been associated with T cell activation [16,17]. In view of these previous findings, we postulated that local immune activation through proinflammatory cytokine release and action may represent one mechanism that allows modulation of defensin gene expression during infection and inflammation.
We report here an analysis of the impact of local immune activation and proinflammatory cytokines on α- and β-defensin expression using an ex vivo model of immune cell activation in explants of adult human small intestine. In order to establish which cell-mediated mucosal factors may play a role in modulating defensin expression, the direct effects of individual cytokines on cultured biopsies were also examined.
Materials and methods
Study groups and tissue collection
Ex vivo tissue culture was performed on biopsies collected from adults (18 years of age or more) in London. These were obtained by direct vision from the distal duodenum from patients undergoing diagnostic gastrointestinal endoscopy, but only if they were endoscopically and histologically normal. Those with evidence of diarrhoea, inflammation, ulceration or lymphoma were excluded from the study, but patients of any age, sex or ethnic origin were included. All patients gave written informed consent and ethical approval for this work was obtained from the East London and City Health Authority Research Ethics Committee.
Ex vivo tissue culture
Duodenal biopsies (three or four from each patient) were collected into culture medium (5 vols National Cancer Tissue Culture-135 medium, 5 vols Dulbecco's modified Eagle's medium and 1 vol. newborn calf serum; all reagents from Sigma, Poole, UK) and established in culture within 2 h. For each set of biopsies a control biopsy was cultured for 24 h in the absence of any stimulus in 1·5 ml sterile culture medium supported on a sterile RNase-free metal mesh (Expanded Metal Co, Hartlepool, UK). For activation of resident immune cells, the superantigen Staphylococcus aureus enterotoxin B (SEB; Sigma) was added to the culture medium to final concentrations of 0·1, 1 and 10 µg/ml. Pokeweed mitogen (PWM; Sigma), an agent that also activates T cells, was used at a concentration of 10 µg/ml. To determine the reversibility of immune cell activation, 10 µg/ml SEB was used with or without 1 µM dexamethasone (Sigma).
In separate experiments, the following cytokines were added individually to the medium at a range of concentrations: interleukin (IL)-1β (20, 50 ng/ml), tumour necrosis factor (TNF)-α (1, 10, 20 ng/ml), IL-6 (10, 20 ng/ml), interferon (IFN)-γ (100, 1000 units/ml) or IL-4 (1, 10, 20 ng/ml) (all cytokines from Peprotech, London, UK). Biopsies were incubated for 24 h at 37°C in an atmosphere of 95% O2/5% CO2. Biopsies and culture supernatants were snap-frozen and stored for mRNA and peptide analyses respectively. The viability of all cultured biopsies was verified by confirming low concentrations of lactate dehydrogenase (cytotoxicity detection kit; Roche, Mannheim, Germany) in the supernatant at the end of the culture period (data not shown). In all experiments a minimum of six sets of biopsies was used for each experimental condition.
Reverse transcription–polymerase chain reaction
We have described the polymerase chain reaction (PCR) assays for DEFA5, DEFA6, DEFB1 and DEFB2 mRNA previously [14,18]. Briefly, biopsies were treated with TriZOL® (Invitrogen, Paisley, UK) for RNA extraction, treated with DNase (Promega, Southampton, UK) and co-reverse-transcribed with known quantities of a standard synthetic RNA prior to PCR amplification.
Dot blots for quantification of DEFA5 peptide
Supernatants from ex vivo culture experiments in which biopsies were treated with SEB (10 µg/ml) with or without dexamethasone (1 µM) were used to determine whether DEFA5 mRNA correlated with changes in DEFA5 peptide secretion using an immuno dot blot technique. Total protein was measured using the DC Protein Assay kit (BioRad, Herts, UK). Ten µg total protein of each supernatant was spotted onto polyvinylidene difluoride membrane (Pharmacia, Amersham, Bucks, UK). Membranes were incubated with rabbit polyclonal anti-DEFA5 antibody (1:3000 dilution; kindly provided by C. Bevins, University of California, Davis, CA, USA), followed by horseradish peroxidase (HRP)-conjugated secondary antibody (1:2000 dilution; Autogen BioClear, Wilts, UK). Blots were developed using the ECL-Plus kit (Amersham). Densitometric analysis was performed using 1D Image Analysis software, version 3·0·0 (Kodak, Rochester, MN, USA).
Enzyme-linked immunosorbent assay for quantification of DEFB2 peptide
Supernatants from ex vivo culture experiments in which biopsies were cultured either in the presence or absence of 10 µg/ml SEB were used to determine whether changes in DEFB2 peptide levels correlated with changes in DEFB2 mRNA expression. A DEFB2 enzyme-linked immunosorbent assay (ELISA) development kit (Peprotech) was used according to the manufacturer's instructions. Capture and detection antibodies were used at concentrations of 0·25 µg/ml and 0·5 µg/ml respectively. The detection range for this assay was 8–1000 pg/ml.
Data analysis
mRNA expression levels were expressed as log transcript per µg total RNA. Cuzick's non-parametric trend test was used to look for trends across ordered groups (for concentration-dependent effects), and the Kruskal–Wallis test and Wilcoxon's matched-pair rank sum test were used to look for differences between groups where trend effects were not relevant. Where the result of the reverse transcription (RT)–PCR was below threshold (< 104 transcripts/µg) the result was designated 4·0 for the purposes of analysis, which would not affect non-parametric test results. Analysis was performed using stata version 8·0 (StataCorp, College Station, TX, USA).
Results
Down-regulation of α-defensin gene expression following local immune activation
Biopsies were cultured for 24 h with different concentrations of SEB, and the DEFA5 and DEFA6 mRNA content of the biopsy assayed by quantitative RT–PCR. A concentration-dependent down-regulation of mRNA levels of both DEFA5 and DEFA6 was observed, with a decrease of approximately 1·5 log mRNA transcripts per µg total RNA over the range 0–10 µg/ml SEB (Fig. 1a and b). This was statistically significant for DEFA5 (P = 0·02) and DEFA6 (P = 0·02) using the non-parametric trend test, and for both DEFA5 (P = 0·001) and DEFA6 (P = 0·001) using the Kruskal–Wallis test. Similar results for α-defensins were obtained after treatment with PWM (data not shown).
Fig. 1.
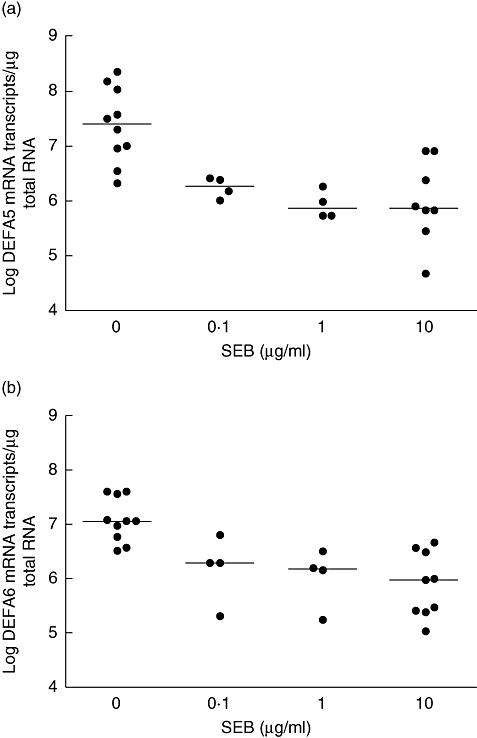
Effect of SEB on α-defensin mRNA expression; 24-h Staphylococcal enterotoxin B (SEB) stimulation resulted in a concentration-dependent down-regulation of α-defensin mRNA expression. Using the Kruskal–Wallis test there was a significant difference in mRNA expression levels across all the dose-groups for both α-defensin (DEFA5) (a; P = 0·0012) and DEFA6 (b; P = 0·0013). Cuzick's non-parametric trend test was also significant for both DEFA5 (P = 0·02) and DEFA6 (P = 0·02). Median bars are shown.
Dexamethasone reversed the effect of local immune activation on α-defensin gene expression
To test whether the observed decrease in α-defensin expression was due to SEB-mediated inflammation, a series of biopsies from a single individual (n = 6) were exposed to SEB in the presence and absence of dexamethasone. At a concentration of 1 µM dexamethasone completely abrogated the effect of 10 µg/ml SEB on both DEFA5 and DEFA6 expression (Fig. 2a and b). Using Wilcoxon's paired test, this reversal was statistically significant for DEFA5 (P = 0·03) and DEFA6 (P = 0·03). Interestingly, dexamethasone alone appeared to increase expression levels slightly to just above those of controls, although this was not statistically significant.
Fig. 2.

Dexamethasone abrogates Staphylococcal enterotoxin B (SEB)-mediated effects on α-defensin gene expression. Dexamethasone (1 µM) reversed the down-regulatory effect of SEB-stimulation (10 µg/ml) on (a) α-defensin (DEFA5) and (b) DEFA6 mRNA expression. Statistical significance was assessed in pairwise comparisons using the Wilcoxon matched-pairs rank sum test (*P = 0·03 compared with controls; n.s. not significant). Median bars are shown.
Secreted DEFA5 peptide levels paralleled mRNA levels
Secreted DEFA5 peptide was quantified by densitometry of immuno dot blots (Fig. 3a and b). DEFA5 immunoreactivity showed a decrease in peptide in biopsies incubated with SEB (P = 0·03) and, as shown for mRNA, this was reversible by dexamethasone. Dexamethasone alone increased DEFA5 peptide significantly compared with controls (P = 0·03). As no antibody for DEFA6 was available, peptide analyses were performed only on DEFA5.
Fig. 3.
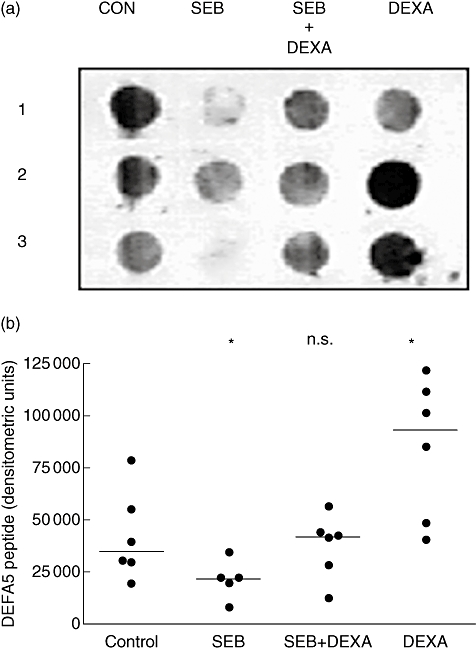
Dexamethasone (DEX) abrogates Staphylococcal enterotoxin B (SEB)-mediated effects on α-defensin (DEFA5) peptide expression. (a) Representative dot blot for HD5 peptide from ex vivo tissue culture supernatants showing down-regulation of HD5 peptide by SEB, reversal of the effect of SEB by the addition of dexamethasone and increase with dexamethasone alone (1, 2 and 3 denote experiments performed on biopsies from three individuals). (b) Densitometric analysis of dot blots (n = 6). Statistical significance was assessed in pairwise comparisons using the Wilcoxon matched-pairs rank sum test (*P = 0·03 compared with controls; n.s. not significant). Densitometric units (arbitrary) of measurement, and median bars are shown.
The IL-1β and IFN-γ down-regulated α-defensin gene expression
The SEB is a superantigen which induces T cell and macrophage activation without prior antigen presentation, with overall induction of a spectrum of cytokines. In subsequent experiments, the effect of individual cytokines known to drive T cell (IFN-γ, IL-4) and macrophage (IL-1β, TNF-α, IL-6) activation on α-defensin mRNA expression was tested. Stimulation with IL-1β (Fig. 4a and b) and IFN-γ (Fig. 4c and d) resulted in a concentration-dependent down-regulation of DEFA5 and DEFA6 (P = 0·03 by the trend test for each). IL-6 showed no effect on DEFA5 mRNA (data not shown), although a significant decrease for DEFA6 mRNA was noted (Fig. 4e; P = 0·03). Neither TNF-α nor IL-4 had a significant effect on DEFA5 or DEFA6 (data not shown).
Fig. 4.

Effect of proinflammatory cytokines on α-defensin gene expression. Interleukin (IL)-1β and interferon-γ down-regulated α-defensin (DEFA5) (a, c) and DEFA6 (b, d) gene expression in a dose-dependent manner. In contrast, IL-6 (20 ng/ml) showed a statistically significant effect only on DEFA6 (e) expression. Statistical significance was assessed in pairwise comparisons using the Wilcoxon matched-pairs rank sum test (*P = 0·03 compared with controls). Median bars are shown.
Differential effects of local immune activation on β-defensin gene expression
The down-regulation of α-defensin expression in SEB-stimulated biopsies at 24 h was paralleled by a decrease of 2 logs of DEFB1 mRNA transcripts (Fig. 5a) and accompanied by a concentration-dependent 2-log increase in DEFB2 mRNA expression (Fig. 5b) over the same range of SEB concentrations as described for assessment of α-defensins (Fig. 1). This was statistically significant for both DEFB1 (P = 0·03) and hBD2 (P = 0·01) by the trend test and for both hBD1 (P = 0·02) and DEFB2 (P = 0·02) by the Kruskal–Wallis test.
Fig. 5.

Effect of Staphylococcal enterotoxin B (SEB) on β-defensin gene expression; 24-h SEB stimulation resulted in a concentration-dependent decrease of β-defensin (DEFB1) gene expression (a) and a dose-dependent increase of DEFB2 gene expression (b). Using the Kruskal–Wallis test there was a significant difference in mRNA expression levels across all the dose-groups for both DEFB1 (P = 0·0171) and DEFB2 (P = 0·0192). Cuzick's trend test was also significant for both DEFB1 (P = 0·03) and DEFB2 (P = 0·01). Median bars are shown.
Secreted DEFB2 peptide levels correlated with mRNA during immune activation
Secreted hBD2 peptide was quantified by ELISA on biopsy culture supernatants. Peptide levels were increased significantly (P = 0·03) when biopsies were cultured with SEB (10 µg/ml) compared with controls (Fig. 6), thus paralleling mRNA expression. Because of limited sample availability, the effect of dexamethasone on DEFB2 peptide was not studied.
Fig. 6.

Effect of Staphylococcal enterotoxin B (SEB) on β-defensin (DEFB2) peptide expression. A significant increase in secreted DEFB2 peptide by SEB (10 µg/ml) was observed 24 h post-stimulation (*P = 0·03 using Wilcoxon matched-pairs rank sum test). Median bars are shown.
Discussion
Diarrhoeal disease remains a major cause of morbidity and mortality worldwide, and there is no doubt that greater understanding of host defence mechanism(s) against intestinal infections may yield novel targets for future therapeutic intervention. Accumulating evidence has begun to highlight a potential role for endogenous anti-microbial peptides in maintaining a dynamic defence shield at the intestinal mucosal surface and perturbation in this defence is most likely to be detrimental, as shown in animal model studies and a previous human cohort study from our laboratory [12,13,19]. In a separate study we found that Zambian adults living in impoverished conditions, with high exposure to enteropathogens, remain healthy despite having significantly lower α-defensin mRNA levels compared with adults from a European population, suggesting that greater complexity is at play [14]. In view of the high level of T cell activation prevalent in the tropical population, we explored the possibility that this interpopulation difference in defensin expression could be explained, at least in part, by the effects of continuous immune activation in the lamina propria. Major histocompatibility complex (MHC) class II represents the major receptor-binding site for superantigens such as SEB, therefore any cell expressing MHC class II is a potential target. Effects of SEB on T cell, macrophages and dendritic cell lineages have been documented [20–22]. Activation of resident immune cells by SEB in our ex vivo model of immune activation resulted in significant modulation of both constitutive and inducible defensins in response to stimuli. These studies suggest that the constitutive expression of anti-microbial peptides at the mucosal surface is not an inert passive defence mechanism, but has the ability to respond dynamically to the changing cytokine milieu during infection and/or inflammation.
Studies investigating the regulation of human α-defensins have been hampered because of the lack of a suitable Paneth cell-line. We therefore used an explant model which allows effects on Paneth cell expression to be explored ex vivo. The explant model system utilized in this study allowed us to investigate the effect of activation of resident immune cells, as opposed to migrating cells, on defensin expression. Further, in this near-sterile model the results are likely to be less prone to the difficulties of interpretation caused by intestinal microflora.
Activation of immune cells with either SEB or PWM resulted in a decrease of mRNA expression of both DEFA5 and DEFA6 in a dose-dependent manner (Fig. 1). The decrease in DEFA5 mRNA by SEB was paralleled by a decrease in the amount of peptide (Fig. 3). In recent years elegant studies by Wehkamp and colleagues have highlighted the reduced expression of α-defensins during gut inflammation, particularly in ileal Crohn's disease (CD) [23]. The authors found further significant reduction in α-defensin expression to be associated with nucleotide-binding oligomerization domain-2 (NOD2) mutation status, which was independent of the degree of inflammation [9]. These studies are therefore indicative of at least two regulatory mechanism(s) that modulate α-defensin expression, i.e. an inflammation-dependent and an inflammation-independent but NOD2-dependent mechanism. Evidence for inflammation-dependent modulation of Paneth cell α-defensin expression has been reported recently [11]. Our data, showing a dose-dependent decrease in DEFA5 and DEFA6 in response to SEB in biopsies from the same individual (Fig. 1), clearly add further support to the notion that α-defensin expression can be modulated by inflammation, a feature independent of the NOD2 status of an individual. T cell activation is the known trigger for the observed pathology in CD and our ex vivo studies clearly recapitulate in vivo findings. The glucocorticoid, dexamethasone, reversed the down-regulatory effects of SEB on α-defensin gene expression, thus suggesting that the modulation of defensin mRNA was due to immune cell activation and not to alteration in mucosal architecture per se, or because of the direct effects of SEB on Paneth cells. In this context, any potential direct effect of SEB on enterocytes and thus on β-defensin expression currently remains unresolved. Further, dexamethasone is a molecule with multiple effects, so effects on other cell lineages cannot be excluded as the explanation for the reversal of the SEB changes. The secretion of DEFA5 peptide into the supernatant was modulated in a manner similar to mRNA content under the same experimental conditions (Fig. 3). Interestingly, dexamethasone alone increased the secretion of DEFA5 peptide, although this was not significant for mRNA. This may indicate that as-yet unidentified factor(s) can modulate secretion of DEFA5. It may also indicate that the effects of dexamethasone are less pronounced at the transcriptional level when compared with translational control.
At present, glucocorticoids provide the most effective treatment for various chronic inflammatory diseases, although intensive use may be accompanied by increased susceptibility to infections. Although there have been no studies examining the effects of glucocorticoids on the modulation of Paneth cell α-defensin synthesis, van Wetering and colleagues demonstrated that dexamethasone, a synthetic glucocorticoid receptor agonist, reduced neutrophil defensin-induced cytokine synthesis in airway epithelial cells, notably that of IL-8 [24]. The biopsy ex vivo culture system employed in the present study allowed only limited measurements and the effect of dexamethasone on β-defensin gene regulation was not investigated. Dexamethasone, however, has been shown previously to inhibit IL-1β-induced expression of β-defensin mRNA [25,26]; it may therefore be hypothesized that similar mechanism(s) are likely to be operative in our model system.
Among the proinflammatory cytokines tested, IL-1β and IFN-γ both decreased DEFA5 and -6 mRNA expression significantly. Decrease in expression was less (Fig. 4) than that observed after SEB-mediated activation (Fig. 1), which would be expected to release a multitude of proinflammatory mediators. IL-6 induced a significant down-regulation of DEFA6 but not DEFA5 (Fig. 4e). The 1·3 kb 5′ flanking DNA sequence upstream of exon 1 of DEFA5 is sufficient for Paneth cell-specific expression in a transgenic mouse model [13]. Also, this region of DEFA5 and six promoters contain AP2 and nuclear factor (NF)-IL-6 transcription factor binding motifs, but no NF-κB binding sequence, suggesting specific transcriptional regulation of these molecules [27]. More recent studies suggest a NOD2-independent association between a member of the canonical Wnt signalling pathway, the T cell transcription factor (TCF-4) and α-defensin expression [28]. How immune activation, if at all, may modulate TCF-4 expression and/or nuclear localization in regulating α-defensin expression during infection and inflammation warrants further attention.
No significant differences in α-defensin expression were observed with the addition of exogenous TNF-α (data not shown). In a study by Veitch and colleagues the Zambian population did not show elevated serum TNF-α levels when compared with the non-tropical populations [16]. However, we have shown previously that α-defensin levels varied between tropical and non-tropical populations [14]. Collectively, these findings suggest that TNF-α is unlikely to be directly responsible for modulating α-defensin expression.
There was no effect on α-defensin expression by the T helper type 2 (Th2) cytokine IL-4, and although the present study did not extend to include effects of anti-inflammatory cytokines such as IL-10, the effects of the latter in limiting T cell-mediated activation by SEB and PWM on gut responses are well documented [20].
The down-regulation of DEFB1 mRNA by SEB paralleled that observed for the constitutively expressed α-defensins, and was in contrast to the increase in DEFB2 mRNA and peptide expression (Fig. 5). There is very limited information with regard to the identity of factor(s) that may modulate DEFB1 expression during homeostasis. DEFB1 expression is dependent upon cell differentiation [29]. Recent studies suggest that DEFB1 may act as a tumour suppressor molecule with Paired box 2, a potential transcriptional factor modulating DEFB1 [30]. Shigella DNA and Toll-like receptor-9 cross-talk has been implicated as one potential mechanism involved in DEFB1 down-regulation observed during Shigella infection [31]. How SEB mediates reduction of DEFB1 mRNA in our model system remains unclear, as its regulation may involve more than one cell type. This would be analogous to the in vivo down-regulation of mouse β-defensin-1 in murine cryptosporidiosis, which is IFN-γ-dependent, and appears to require the activation of non-epithelial cells [32]. Further studies are required to delineate the molecular nature of DEFB1 gene regulation.
Microbial and cytokine-driven signalling events that regulate the expression of inducible DEFB2 are now well established. IL-1β remains the most potent agonist with TNF-α inducing DEFB2 in some cells [33]. IL-12/IL-23/IL-27, which are derived mainly from dendritic cells, have the ability to modulate IL-1β-driven DEFB2 expression in the skin via increased NF-κB, signal transducer and activation of transcription-1 (STAT-1) and STAT-3 activities [34]. It is most likely that these novel cytokines modulate innate defence in the intestine. IL-17A, a downstream effector of the IL-12/IL-23 immune axis also induces DEFB2 independently [35]. In contrast, we have found IL-17A to be 100-fold less potent than IL-1β in its ability to induce DEFB2 in human intestinal cell lines (M. Bajaj-Elliott, unpublished data). Therefore the biological significance of the novel IL-12/IL-23/Th17 immune axis in modulating human intestinal defensin expression needs further investigation. What is clear is that the final magnitude of the host innate defence response will depend critically upon the cytokine milieu of the corresponding infection or inflammatory stimuli.
Although our understanding of the regulation of innate defence at the gastrointestinal mucosal surface is increasing, many fundamental mechanism(s) remain unclear. Further work on these interactions may open up new avenues for therapy of intestinal infection through modulation of defensin expression and function.
Acknowledgments
We are grateful to David Rampton, Kousay Al-Zoubi and Salem Abdalla of the Royal London Hospital endoscopy unit, and to Charles Bevins for the DEFA5 antibody. Financial support was obtained from the Wellcome Trust.
References
- 1.World Health Organization. Global health – today's challenges. Geneva: The World Health Report; 2003. [Google Scholar]
- 2.Dommett R, Zilbauer M, George JT, Bajaj-Elliott M. Innate immune defence in the human gastrointestinal tract. Mol Immunol. 2005;42:903–12. doi: 10.1016/j.molimm.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Menendez A, Brett Finlay B. Defensins in the immunology of bacterial infections. Curr Opin Immunol. 2007;19:385–91. doi: 10.1016/j.coi.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Wehkamp J, Schmid M, Stange EF. Defensins and other antimicrobial peptides in inflammatory bowel disease. Curr Opin Gastroenterol. 2007;23:370–78. doi: 10.1097/MOG.0b013e328136c580. [DOI] [PubMed] [Google Scholar]
- 5.Jones DE, Bevins CL. Paneth cells of the human small intestine express an antimicrobial peptide gene. J Biol Chem. 1992;267:23216–25. [PubMed] [Google Scholar]
- 6.Jones DE, Bevins CL. Defensin-6 mRNA in human Paneth cells: implications for antimicrobial peptides in host defence of the human bowel. FEBS Lett. 1993;315:187–92. doi: 10.1016/0014-5793(93)81160-2. [DOI] [PubMed] [Google Scholar]
- 7.Harder J, Bartels J, Christophers E, Schroder JM. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 8.Garcia JR, Krause A, Schulz S, et al. Human beta-defensin 4: a novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. FASEB J. 2001;15:1819–21. [PubMed] [Google Scholar]
- 9.Wehkamp J, Salzman NH, Porter E, et al. Reduced Paneth cell alpha-defensins in ileal Crohn's disease. Proc Natl Acad Sci USA. 2005;102:18129–34. doi: 10.1073/pnas.0505256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salzman NH, Underwood MA, Bevins CL. Paneth cells, defensins, and the commensal microbiota: a hypothesis on intimate interplay at the intestinal mucosa. Semin Immunol. 2007;19:70–83. doi: 10.1016/j.smim.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Simms LA, Doecke JD, Walsh MD, Huang N, Fowler EV, Radford-Smith GL. Reduced alpha-defensin expression is associated with inflammation and not NOD2 mutation status in ileal Crohn's disease. Gut. 2008;57:903–10. doi: 10.1136/gut.2007.142588. [DOI] [PubMed] [Google Scholar]
- 12.Wilson CL, Ouellette AJ, Satchell DP, et al. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defence. Science. 1999;286:113–7. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- 13.Salzman NH, Ghosh D, Huttner KM, Paterson Y, Bevins CL. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature. 2003;422:522–6. doi: 10.1038/nature01520. [DOI] [PubMed] [Google Scholar]
- 14.Dhaliwal W, Bajaj-Elliott M, Kelly P. Intestinal defensin gene expression in human populations. Mol Immunol. 2003;40:469–75. doi: 10.1016/s0161-5890(03)00156-1. [DOI] [PubMed] [Google Scholar]
- 15.Kelly P, Menzies I, Crane R, et al. Responses of small intestinal architecture and function over time to environmental factors in a tropical population. Am J Trop Med Hyg. 2004;70:412–9. [PubMed] [Google Scholar]
- 16.Veitch AM, Kelly P, Zulu IS, Segal I, Farthing MJ. Tropical enteropathy: a T-cell-mediated crypt hyperplastic enteropathy. Eur J Gastroenterol Hepatol. 2001;13:1175–81. doi: 10.1097/00042737-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Campbell DI, Murch SH, Elia M, et al. T cell-mediated enteropathy in rural West African children: relationship with nutritional status and small bowel function. Pediatr Res. 2003;54:306–11. doi: 10.1203/01.PDR.0000076666.16021.5E. [DOI] [PubMed] [Google Scholar]
- 18.Bajaj-Elliott M, Fedeli P, Smith GV, et al. Modulation of host antimicrobial peptide (beta-defensins 1 and 2) expression during gastritis. Gut. 2002;51:356–61. doi: 10.1136/gut.51.3.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly P, Bajaj-Elliott M, Katubulushi M, et al. Reduced gene expression of intestinal alpha-defensins predicts diarrhea in a cohort of African adults. J Infect Dis. 2006;193:1464–70. doi: 10.1086/503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pender SL, Breese EJ, Gunther U, et al. Suppression of T cell-mediated injury in human gut by interleukin 10: role of matrix metalloproteinases. Gastroenterology. 1998;115:573–83. doi: 10.1016/s0016-5085(98)70136-2. [DOI] [PubMed] [Google Scholar]
- 21.Khan AA, Martin S, Saha B. SEB-induced signalling in macrophages leads to biphasic TNFα. J Leukoc Biol. 2008;83:1363–9. doi: 10.1189/jlb.1007686. [DOI] [PubMed] [Google Scholar]
- 22.Yang PC, Xing Z, Berin CM, et al. TIM-4 expressed by mucosal dendritic cells plays a critical role in food-antigen specific Th2 differentiation and intestinal allergy. Gastroenterology. 2007;133:1522–33. doi: 10.1053/j.gastro.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Wehkamp J, Harder J, Weichenthal M, et al. NOD2 (CARD15) mutations in Crohn's disease are associated with diminished mucosal alpha-defensin expression. Gut. 2004;53:1658–64. doi: 10.1136/gut.2003.032805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Wetering S, Mannesse-Lazeroms SP, van Sterkenburg MA, Hiemstra PS. Neutrophil defensins stimulate the release of cytokines by airway epithelial cells: modulation by dexamethasone. Inflamm Res. 2002;51:8–15. doi: 10.1007/pl00000282. [DOI] [PubMed] [Google Scholar]
- 25.Duits LA, Rademaker M, Ravensbergen B, et al. Inhibition of hBD-3, but not hBD-1 and hBD-2, mRNA expression by corticosteroids. Biochem Biophys Res Commun. 2001;280:522–5. doi: 10.1006/bbrc.2000.4157. [DOI] [PubMed] [Google Scholar]
- 26.Jang BC, Lim KJ, Suh MH, Park JG, Suh SI. Dexamethasone suppresses interleukin-1beta-induced human beta-defensin 2 mRNA expression: involvement of p38 MAPK, JNK, MKP-1, and NF-kappaB transcriptional factor in A549 cells. FEMS Immunol Med Microbiol. 2007;51:171–84. doi: 10.1111/j.1574-695X.2007.00293.x. [DOI] [PubMed] [Google Scholar]
- 27.Mallow EB, Harris A, Salzman N, et al. Human enteric defensins. Gene structure and developmental expression. J Biol Chem. 1996;271:4038–45. doi: 10.1074/jbc.271.8.4038. [DOI] [PubMed] [Google Scholar]
- 28.Wehkamp J, Wang G, Kübler I, et al. The Paneth cell alpha-defensin deficiency of ileal Crohn's disease is linked to Wnt/Tcf-4. J Immunol. 2007;179:3109–18. doi: 10.4049/jimmunol.179.5.3109. [DOI] [PubMed] [Google Scholar]
- 29.Sayama K, Komatsuzawa H, Yamasaki K, et al. New mechanisms of skin innate immunity: ASK1-mediated keratinocyte differentiation regulates the expression of beta-defensins, LL37, and TLR2. Eur J Immunol. 2005;35:1886–95. doi: 10.1002/eji.200425827. [DOI] [PubMed] [Google Scholar]
- 30.Bullard RS, Gibson W, Bose SK, et al. Functional analysis of the host defensin peptide human ß-defensin 1: new insight into its potential role in cancer. Mol Immunol. 2008;45:839–48. doi: 10.1016/j.molimm.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Islam D, Bandholtz L, Nilsson J, et al. Downregulation of bactericidal peptides in enteric infections: a novel immune escape mechanism with bacterial DNA as a potential regulator. Nat Med. 2001;7:180–5. doi: 10.1038/84627. [DOI] [PubMed] [Google Scholar]
- 32.Zaalouk TK, Bajaj-Elliott M, George JT, McDonald V. Differential regulation of beta-defensin gene expression during Cryptosporidium parvum infection. Infect Immun. 2004;72:2772–9. doi: 10.1128/IAI.72.5.2772-2779.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Neil DA, Porter EM, Elewaut D, et al. Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. J Immunol. 1999;163:6718–24. [PubMed] [Google Scholar]
- 34.Kanda N, Watanabe S. IL-12, IL-23 and IL-27 enhance human beta defensin 2 production in human keratinocytes. Eur J Immunol. 2008;38:1287–96. doi: 10.1002/eji.200738051. [DOI] [PubMed] [Google Scholar]
- 35.Kao CY, Chen Y, Thai P, et al. IL-17 markedly upregulates beta defensin 2 expression in human airway epithelium via JAK and NF-κB signalling pathways. J Immunol. 2004;173:3482–91. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]


