Abstract
Regulatory T cells (Treg) have an essential role in maintaining immune tolerance in the gut. The functional CD4+ Treg express the transcription factor forkhead box protein 3 (FoxP3) or a CD25high in humans. Further, depletion of elevated granulocytes/monocytes by extracorporeal adsorption (GMA) induces immunomodulation in patients with ulcerative colitis (UC). We investigated the impact of GMA on Treg. Thirty-one UC patients, clinical activity index (CAI) 12·1 ± 2·97, refractory to conventional medications including intravenous corticosteroid and 13 healthy controls (HC), were included. Patients received five GMA sessions over 5 weeks. Biopsies from the rectal mucosa and blood samples at baseline and post-GMA were immunostained with anti-CD4/FoxP3 and anti-CD4/CD25 antibodies for immunohistochemistry and flow cytometry. Following GMA, 22 of 31 patients achieved remission (CAI ≤ 4, P < 0·01) and their endoscopic activity index decreased from 10·6 ± 2·32 to 4·75 ± 1·48 (P = 0·003). The circulating CD4+CD25high+ Treg level was low and increased markedly in responders (P < 0·02). In the nine non-responders, the baseline CD4+CD25high+ Treg level was about 50% of the level in the responders (P < 0·03) or in the HC (P < 0·01), and all nine had to undergo colectomy. Conversely, the number of CD4+/FoxP3+ mucosal Treg in GMA responders decreased significantly after the fifth GMA session compared with the baseline level (P < 0·05). It is believed that the CD4+ Treg has an essential role in the control of immune pathology in UC patients and a net influx of these cells from the circulation into the mucosa may proceed to suppress inflammation. GMA can impact the circulating as well as the mucosal levels of Treg.
Keywords: adsorptive granulocyte/monocyte apheresis, CD4+CD25high+, FoxP3+, Treg, UC
Introduction
Ulcerative colitis (UC) and Crohn's disease (CD) are the major phenotypes of the idiopathic inflammatory bowel diseases (IBD) of the gut. Both UC and CD are chronic relapsing–remitting inflammatory disorders that afflict millions of individuals throughout the world, causing symptoms which impair ability to function and quality of life [1]. Epidemiological studies show that the incidence of IBD is highest in most developed countries in the West, such as Scandinavia, and lowest in Asia, indicating an association with modern lifestyle [2]. The aetiology of IBD is poorly understood, but a dysregulated immune activity involving the CD4+ T cell component is suspected to be an initial or an additional factor for the development of IBD [3].
The CD4+ T cell phenotype expressing CD25high and forkhead box protein 3 (FoxP3) has been recognized as the functional representative of regulatory T cells (Treg), which constitute 5–10% of peripheral T cells in normal naive mice, and in humans [3]. The Treg is known to down-regulate immune responses to both foreign and self-antigens. The removal of CD4+ Treg from healthy animals can break self-tolerance, while repopulation of these cells can re-establish self-tolerance and prevent autoimmune diseases [3–8]. Accordingly, the immune pathology in patients with IBD is thought to reflect an inadequate Treg function in these patients [6–8].
Consistent with the above background, in an earlier study we found that the level of Treg in patients with active UC was about one-third of the level in patients with quiescent UC, suggesting that Treg are involved in the suppression of inflammation in the gut mucosa [9]. Further, selective depletion of peripheral granulocyte and monocyte/macrophage by extracorporeal adsorption (GMA) with an Adacolumn (JIMRO Co. Ltd., Takasaki, Gunma, Japan) has been used as a non-pharmacological strategy to regulate the inflammatory response in patients with IBD [10,11]. However, the primary target of GMA has been to deplete elevated/activated circulating myeloid leucocytes, which infiltrate the colonic mucosa in vast numbers during disease activity [1,11]. None the less, several studies have reported an immunological impact by GMA in patients with IBD [11–15]. With this background in mind, the present study was designed to achieve two major objectives: first, to evaluate the effects of GMA on peripheral blood levels of CD4+ Treg subsets in patients with active UC, and secondly, to determine what happens to the mucosal levels of CD4+ Treg, e.g. which are defined as the CD4/FoxP3 double-positive T cells (the migrating CD4+ Treg phenotype) following a course of GMA.
Materials and methods
Subjects
The subjects' background demography is presented in Table 1. Thirty-one patients with an acute UC flare, 13 men and 18 women, mean age 36·9 ± 12·7 years, were included together with 13 age- and gender-matched healthy volunteers as a control group (HC). The mean UC clinical activity index (CAI) was 12·1 ± 2·97, range 8–19, measured according to Lichtiger et al.[16]. All patients had been treated with a combination of 5-aminosalicylic acid and corticosteroid, with the exception of two patients who had experienced adverse side effects previously during prednisolone (PSL) therapy. Their concomitant medications, except PSL, were to be continued at the same dosage. The PSL dose was to be tapered if their CAI improved during the course of GMA treatment. Patients were excluded if they had other acute or chronic diseases with possible effects on the immune function or were taking immunosuppressants within 8 weeks before the initiation of this study. The study protocol was reviewed and approved by the institutional ethics committee and all subjects provided informed consent.
Table 1.
Entry demography of subjects included in this study.
| Demography | Patients with UC | Healthy controls |
|---|---|---|
| Number | 31 | 13 |
| Gender (male/female) | 13/18 | 4/9 |
| Age (years) | 36·9 ± 12·7 | 33·8 ± 5·32 |
| Duration of UC (years) | 7·58 ± 10·3 | |
| Clinical activity index (CAI) | 12·1 ± 2·97 | |
| Location of colitis† | Total colitis (16) | |
| Left-sided (15) | ||
| Concomitant medications† | 5-ASA (30) | |
| i.v. PSL (20) | ||
| Oral PSL (9) | ||
| Steroids free (2) | ||
| WBC (/µl) | 10821·3 ± 4237·9 | 5123·3 ± 1200·7 |
| CRP (mg/dl) | 1·8 ± 2·8 | 0·19 ± 0·13 |
| ESR (mm/h) | 32·8 ± 21·1 | 5·08 ± 3·04 |
| EI (score) | 11·0 ± 2·07 |
No patient had proctitis or received steroid enema. UC, ulcerative colitis; 5-ASA, 5-aminosalicylic acid (2·25 g/day); PSL, prednisolone (36·1 ± 24·1 mg/day, range 2·5–80 mg); i.v., intravenous administration; WBC, white blood cell count; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; EI, endoscopic index by Rachmilewitz (reference [19]).
The GMA procedures
The GMA was performed with the Adacolumn, as described previously [9–11,12–15,17]. Each of the 31 patients with active UC received one GMA session per week for 5 consecutive weeks. The duration of one GMA session was 60 min at 30 ml/min. The Adacolumn is filled with specially designed cellulose acetate beads, which serve as the column adsorptive leucocytapheresis carriers [10]. From blood in the column, the carriers adsorb selectively about 65% of granulocytes, 55% monocytes/macrophages and some platelets; lymphocytes are spared and increase subsequently [10,11]. The mechanisms for sparing lymphocytes have been described recently [11], and are summarized briefly here. The Adacolumn carriers adsorb immunoglobulin G (IgG) from the plasma [18], and upon adsorption the binding sites on IgG become available for the Fcγ receptors (FcγR) on neutrophils and monocytes/macrophages [11]. Further, the adsorbed IgG generates complement activation fragments including C3a and C5a [18]. The opsonins C3b/C3bi, derived from complement, also adsorb onto the carriers and serve as the binding sites for leucocyte complement receptors such as CR3 (CD11b/CD18). Expressions of FcγR and CR3 are common features of neutrophils and monocytes/macrophages, but not lymphocytes [11,18].
Immunophenotypic studies
Peripheral blood samples were obtained directly from the patients' antecubital veins before and immediately after each GMA session. This approach avoided dilution of specimens by the anti-coagulant solution added to prime the GMA system [10]. Test samples were double-stained immediately with fluorochrome-conjugated monoclonal antibodies for 20 min at room temperature (IOTest; Immunotech, Marseille, France). The antibody combination used for double-colour fluorescence, fluorescein isothiocyanate (FITC) and phycoerythrin (PE), was anti-CD4CD25. Isotype-matched irrelevant FITC-, PE-labelled monoclonal antibodies served as control. Labelled samples were then washed three times and fixed with 2% paraformaldehyde just prior to flow cytometry. Measurements were performed immediately after preparing the samples using a Beckman Coulter Epics XL flow cytometer and System II software (Beckman Coulter Inc., Fullerton, CA, USA). The analysis and sort gates were restricted to the population of lymphocytes by means of their forward- and side-scatter properties. As described previously [9], CD4+CD25+ T lymphocytes were sorted into three phenotypes: CD25high+, CD25intermediate+ and CD25low+. The functional Treg cells expressing high levels of the interleukin (IL)-2 receptor, also characterized by the expression of the transcription factor FoxP3, are shown as CD4+CD25high+. The flow cytometer was set to count and measure percentages and fluorescent intensities of the immunostained lymphocytes of aspirated samples, which contained 100 µl/sample of patients' peripheral whole blood. Each measurement was continued for either 300 s or until 20 × 104 cells were counted.
Sigmoidoscopic examinations of the rectal mucosa
Sigmoidoscopic evaluations were performed within a week before starting GMA and after the 5th GMA session. A total of 24 patients were included for this evaluation study. Patients were excluded if they were unwilling or had severe episodes of bloody diarrhoea or abdominal discomfort at the beginning of GMA therapy. At least two biopsy specimens were sampled from the inflamed rectal mucosa (avoiding the ulcerated sites during every sigmoidoscopy). Sigmoidoscopic findings were evaluated by using an endoscopic index (EI), according to Rachmilewitz [19].
Immunohistochemical evaluation
For double-colour staining of CD4 and FoxP3, paraffin sections were first incubated with mouse monoclonal anti-CD4 antibody (NCL-CD4-1F6; Novocastra, Newcastle upon Tyne, UK) for 30 min after the antigen retrieval procedure with 1 × TE solution (0·01 M Tris with 0·001 M ethylenediamine tetraacetic acid; pH 9·0) for 40 min at 95°C. The tests were then treated with Envision-horseradish peroxidase solution (Dako, Tokyo, Japan) for 30 min followed by incubation with nickel-3′3′-diaminobenzidine substrate for 15 min. After washing with water, the sections were treated with 0·01 M citrate buffer (pH 6·0) for 5 min at 95°C and were incubated with mouse monoclonal anti-FoxP3 antibody (236A/E7; Abcam, Cambridge, UK) for 30 min. The tests were then treated with secondary antibody (rabbit polyclonal anti-mouse Ig) conjugated with alkaline phosphatase (Dako) for 15 min followed by counterstaining with or without haematoxylin. Finally, tests were washed with water, dried and enclosed by non-aqueous medium. Microscopic observations were performed at a magnification of 400×. The number of FoxP3/CD4 double-positive lymphocytes was counted by two physicians who were not aware of the patients' condition at that time.
Statistics
Where appropriate, data are presented as the mean ± standard deviation values. Comparison of the percentages and the mean fluorescent intensities of T cell surface markers in groups were made by using the Mann–Whitney U-test. For comparisons between pre- and post-GMA, the Wilcoxon signed-rank test was used. Statistical analysis was performed using spss version 11·0J software (SPSS Co., Tokyo, Japan). P < 0·05 was considered statistically significant.
Results
Clinical outcomes
In Table 1, clinical and biochemical measurements at baseline in 31 patients with UC and 13 controls are presented. At entry all 31 patients had active UC that was refractory to conventional medications, including corticosteroids. The CAI score in the patients was 12·1 ± 2·97, range 8–19. Similarly, the entry EI score was 11·0 ± 2·07, range 5–12. Total white cell count (×103/µl) in the patient group was 10·82 ± 4·24, more than double the level in the HC group, 5·12 ± 1·20. At the end of the five GMA treatment courses, 22 of 31 patients (71·0%) had achieved clinical remission (CAI ≤ 4, P < 0·01) together with a significant improvement in the EI score, from 10·6 ± 2·32 to 4·75 ± 1·48 (P = 0·003). The nine non-responders to GMA had to undergo colectomy because of worsening UC. Table 2 shows the demographic comparison at entry between the subgroup who responded subsequently to GMA (GMA responders) and the GMA non-responders. A significantly higher initial CAI score (P = 0·002) and C-reactive protein (P = 0·007) were seen in the two subgroups of patients. The initial EI score showed a weak and statistically insignificant (P = 0·169) association with lack of response to GMA. Because of unremitting severe inflammation, all non-responders had received intravenous (i.v.) PSL, 54·3 ± 15·1 mg/day even during the GMA course, but the need for i.v. PSL was not an indication for lack of response to GMA, as the dosage of PSL was not different between responders and non-responders in patients who had received i.v. PSL.
Table 2.
Demographic comparison at entry between granulocyte and monocyte/macrophage by extracorporeal adsorption (GMA) responders and non-responders.
| Demography | Responder | Non-responder | P-value* |
|---|---|---|---|
| Number | 22 | 9 | |
| Gender (male/female) | 10/12 | 3/6 | |
| Age (years) | 38·1 ± 12·3 | 34·0 ± 14·0 | 0·356 |
| Duration of UC (years) | 9·14 ± 11·7 | 3·78 ± 4·32 | 0·273 |
| Clinical activity index (CAI) | 11·05 ± 2·478 | 14·7 ± 2·55 | 0·002** |
| Location of colitis | Total colitis (13) | Total colitis (3) | |
| Left-sided (9) | Left-sided (6) | ||
| Concomitant steroid (mean ± s.d.) mg/day | i.v. PSL (11) | i.v. PSL (9) | 0·710 |
| 55·5 ± 14·4 | 57·8 ± 15·6 | ||
| Oral PSL (9) | Oral (0) | ||
| 24·4 ± 8·46 | |||
| Steroid free (2) | Steroid free (0) | ||
| WBC (/µl) | 9873·2 ± 3517·9 | 13138·9 ± 5133·0 | 0·147 |
| CRP (mg/dl) | 1·03 ± 1·80 | 3·61 ± 3·91 | 0·007** |
| ESR (mm/h) | 30·4 ± 19·4 | 38·2 ± 25·1 | 0·657 |
| EI (score) | 10·6 ± 2·32† | 12·0 ± 0·00‡ | 0·169 |
P-values by Mann–Whitney U-test;
compared with responders;
n = 19;
n = 7. UC, ulcerative colitis; 5-ASA, 5-aminosalicylic acid (2·25 g/day); PSL, prednisolone (36·1 ± 24·1 mg/day, range 2·5–80 mg); i.v., intravenous administration; WBC, white blood cell count; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; EI, endoscopic index by Rachmilewitz (reference [19]).
CD4+ T cell subsets in patients with UC and controls
In Fig. 1, typical data on CD4+ T cell subsets in a healthy control and a single patient with active UC, as revealed by flow cytometry, are presented. This patient had UC that was refractory to conventional medications as well as to GMA (see Tables 3 and 4). It can be seen that among the three subsets of CD4+CD25+ T cells, the major regulatory phenotype (CD4+CD25high+ Treg) in patients with refractory UC is depleted (Fig. 1 and Table 3), as measured at baseline. The baseline CD4+CD25high+ Treg data measured in all 31 patients with UC, subgrouped into GMA responders and GMA non-responders, are presented in Fig. 2, which show overlap with those of low-level CD4+CD25high+ Treg in the responder subgroup, although a significant up-regulation of mean Treg expression was seen in GMA responders when compared with non-responders (P < 0·03). Additionally, in Table 3, CD4+CD25+ T cell data from the two UC subgroups and the control group at baseline are presented. With regard to the total CD4+CD25+ T cells, the GMA responder UC subgroup showed higher levels compared with the GMA non-responder subgroup (P < 0·04) or the control group (P < 0·05). In line with the data seen in Figs 1 and 2, in the refractory UC subgroup the essential CD4+CD25high+ Treg phenotype was depleted compared with the control group (P < 0·01). A similar trend in the other two CD4+CD25+ subsets (CD4+CD25intermediate and CD4+CD25low) can also be seen in Table 3, both being significantly lower in the refractory subgroup compared with the responder subgroup or the control group. The naive, CD4+CD25− phenotype was also significantly lower in the refractory UC subgroup compared with the responder subgroup (P < 0·03) or the control group (P < 0·01).
Fig. 1.
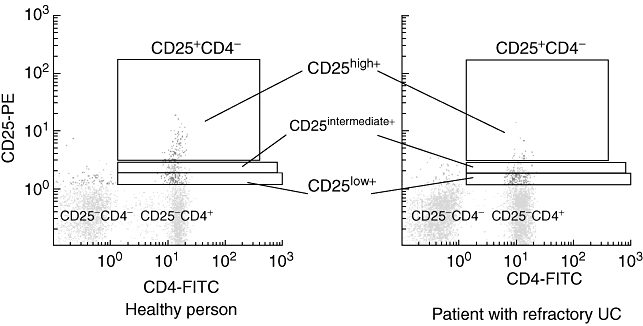
Flow cytometry profiles showing regulatory T cells (Treg): CD4+CD25high+, CD4+CD25intermediate+, CD4+CD25low+ phenotypes within the CD4+CD25+ T cells. The principal feature seen in this flow cytometry analysis is the very low level of the essential CD4+CD25high+ Treg phenotype in a patient with active ulcerative colitis (UC) compared with a healthy control subject (see Table 3 for group data). FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Table 3.
Entry levels of circulating CD4+ lymphocytes expressing CD25 surface marker in controls, granulocyte and monocyte/macrophage by extracorporeal adsorption (GMA) responders and non-responders.
| Active UC |
||||
|---|---|---|---|---|
| Cell surface marker | Total (n = 31) | Responder (n = 22) | Non-responder (n = 9) | Controls (n = 13) |
| CD25+ | 4·85 ± 2·40 | 5·47 ± 2·33*† | 3·33 ± 1·90 | 3·86 ± 1·19 |
| CD25high+ | 0·77 ± 0·48 | 0·89 ± 0·49‡ | 0·47 ± 0·26** | 0·96 ± 0·36 |
| CD25intermediate+ | 1·09 ± 0·56 | 1·23 ± 0·52‡ | 0·74 ± 0·51 | 1·10 ± 0·41 |
| CD25low+ | 2·74 ± 1·33 | 3·07 ± 1·30*† | 1·94 ± 1·08 | 2·23 ± 0·69 |
| CD25− | 31·1 ± 8·58* | 33·6 ± 8·14‡ | 25·0 ± 6·5** | 38·5 ± 7·9 |
P < 0·05,
P < 0·01 compared with controls;
P < 0·04,
P < 0·03 for GMA responders versus non-responders. Data (%) as the mean ± standard deviation values.
Table 4.
(a) Transition of the circulating peripheral CD4+CD25high+ Treg expressions during weekly granulocyte and monocyte/macrophage by extracorporeal adsorption (GMA) in the responder group (n = 22).
| Weekly GMA† | Pre-GMA | Post-GMA | P-value* |
|---|---|---|---|
| 1st session | 0·89 ± 0·49 | 1·06 ± 0·50 | 0·006 |
| 2nd session | 0·88 ± 0·49 | 1·02 ± 0·49 | 0·001 |
| 3rd session | 0·92 ± 0·60 | 1·05 ± 0·70 | 0·046 |
| 4th session | 0·93 ± 0·61 | 1·06 ± 0·67 | 0·017 |
| 5th session | 0·92 ± 0·53 | 1·07 ± 0·69 | 0·017 |
Compared with pre-GMA by Wilcoxon signed-rank test.
Data (%) as the mean ± standard deviation values.
Fig. 2.
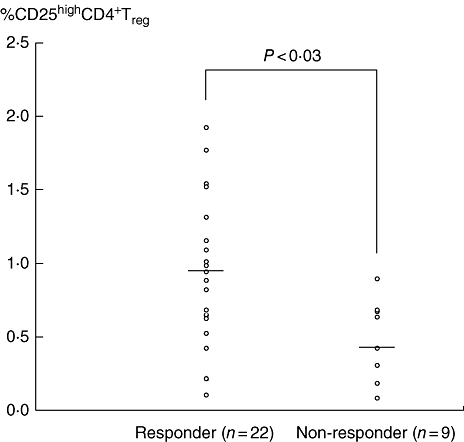
The baseline levels of Treg phenotype expressing CD4+CD25high+ in the peripheral blood of granulocyte and monocyte/macrophage by extracorporeal adsorption (GMA) responder and non-responder patients. It is inferred that patients with compromised CD4+CD25high+ Treg at baseline are not likely to respond to the Adacolumn GMA, while patients with a fair level of CD4+CD25high+ Treg at baseline are likely to respond to GMA.
The impact of GMA on peripheral and mucosal Treg phenotypes
As stated above, 22 of 31 patients with drug-refractory UC responded to GMA and nine did not respond. Table 4 has been divided into two parts (a and b); the former shows changes in the peripheral CD4+CD25high+ Treg phenotype measured after each weekly GMA session in the responder UC subgroup, while the latter shows the corresponding data in the refractory UC subgroup. Table 4 shows that in the responder subgroup, after each GMA session, there was a significant increase in CD4+CD25high+ Treg, but the increase of CD4+CD25high+ was not sustained until the subsequent GMA session (Table 4A), bearing in mind that the CD4+CD25high+ Treg phenotype in the responder subgroup was similar to or even better than the levels in healthy controls shown in Table 3. In part (b) of Table 4, it can be seen that in the refractory subgroup, CD4+CD25high+ Treg phenotype increased after the first and the second GMA sessions, but not after the third or the fourth, but between the third and the fourth GMA sessions the CD4+CD25high+ Treg had increased to the levels seen in the HC or the responder subgroup shown in part (a) and in Table 3.
Table 4.
(b) Transition of the circulating peripheral CD4+CD25high+ Treg expressions during weekly granulocyte and monocyte/macrophage by extracorporeal adsorption (GMA) in the non-responder group (n = 2–9).
| Weekly GMA (n) | Pre-GMA | Post-GMA | P-value* |
|---|---|---|---|
| 1st session (9) | 0·47 ± 0·26 | 0·67 ± 0·43 | 0·021** |
| 2nd session (6) | 0·59 ± 0·36 | 0·86 ± 0·41 | 0·046** |
| 3rd session (3) | 0·69 ± 0·34 | 0·78 ± 0·53 | 0·593 |
| 4th session (2) | 0·92 ± 0·00 | 0·81 ± 0·12 | 0·180− |
P-values by Wilcoxon signed-rank test.
Compared with pre-GMA. Data (%) as the mean ± standard deviation values.
We were particularly interested to see the time–course of the changes in the peripheral blood levels of CD4+CD25+ T cells. Figure 3 shows the rise in the levels of CD4+CD25+ phenotypes in six patients of GMA responders who could be traced, until 10 weeks after their final GMA procedure. It can be seen that at week 10 after the last GMA session, when the patients' UC had improved to quiescent phase, the levels of CD4+CD25+ phenotypes had increased to the levels in the HC subjects. Further, among the three CD4+CD25+ phenotypes, only the increase in the expression frequency of the CD4+CD25high+ Treg was significant compared with the baseline level (P < 0·02).
Fig. 3.
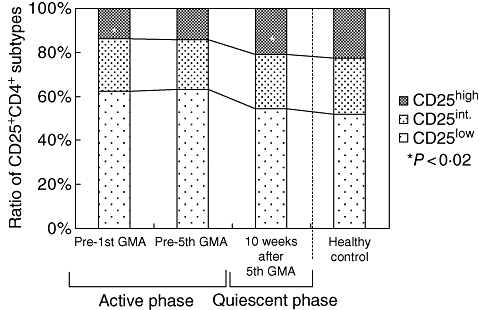
Time–course of the increase in the circulating levels of CD25+CD4+ Treg phenotypes in patients with refractory UC (n = 6) who achieved remission following a course of granulocyte and monocyte/macrophage by extracorporeal adsorption (GMA). It can be seen that at week 10, after the last GMA session when the patients' UC had improved to quiescent condition, the levels of CD25+CD4+ Treg phenotypes has increased to the levels in healthy control subjects (n = 13). Further, among the three CD25+CD4+ phenotypes, only the increase in the expression of CD4+CD25high+ was significant compared with the baseline level (P < 0·02).
As the site of action of Treg is within the mucosal tissue, we were extremely interested to examine whether GMA impacts the mucosal levels of the CD4+/FoxP3+ phenotype. In Figure 4, typical immunohistochemical findings are presented in the rectal mucosa of a patient with refractory UC who responded to GMA. The micrographs show that the presence of CD4 T cells stained dark brown with the FoxP3 in the nuclei seen stained pale red. The average number of CD4/FoxP3 double-positive staining mucosal CD4+Treg in the GMA responder UC subgroup was decreased significantly after the fifth GMA session compared with the baseline level (5·98 ± 5·63 versus 1·11 ± 1·73; P < 0·05, n = 19).
Fig. 4.
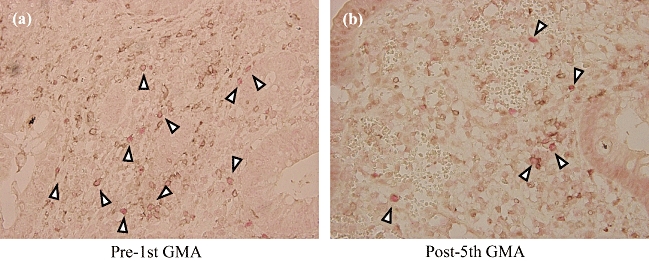
Typical immunohistological findings in the rectal mucosa of a patient with refractory ulcerative colitis (UC) who responded to granulocyte and monocyte/macrophage by extracorporeal adsorption (GMA). The micrographs show a marked decrease (b) of CD4+ T cells stained dark brown with the forkhead box protein 3 in the nuclei seen stained pale red (arrowheads) compared with before GMA (a).
Discussion
CD4+CD25+ has been recognized as the naturally occurring Treg with an essential role in maintaining immune tolerance in the intestinal mucosa [20–22]. Unequivocal evidence for this perception has been provided by animal models of colitis, which showed that depletion of CD4+CD25+ from the circulation leads to an inflammatory colitis similar to the UC seen in human IBD [20,21,23,24]. In humans, the functional Treg cells express high levels of the IL-2 receptor (CD25) and are characterized by expression of the transcription factor FoxP3 (CD4+CD25high+/FoxP3+ phenotype) [25,26]. Both IL-10 and transforming growth factor (TGF)-β are reported to be involved in the regulatory activities of the CD4+CD25high+ Treg[21,23,27,28]. Therefore, we were interested to see the status of the functional Treg both in patients with refractory UC during active disease and when the UC in the same patients had improved to a quiescent phase. For comparison, we included a healthy control group who had no known inflammatory disorders.
The findings of the present study may be summarized as follows. Following a course of GMA therapy, 22 of 31 patients achieved clinical remission. Given that this was a cohort of patients in whom the UC had not responded to conventional drug-based medications, including i.v. corticosteroid, the efficacy level we achieved was very significant. Further, we thought that this cohort of drug-refractory patients is an appropriate patient group to investigate the status of CD4+CD25high+/FoxP3+ Treg. All nine non-responders to GMA needed colectomies because of worsening UC (see Results section).
As stated above, we were particularly interested to examine the expression frequencies of the Treg in these two groups of subjects, UC and HC with contrasting demography. Our flow cytometric analyses revealed that among the three subsets of CD4+CD25+ T cells, the major regulatory phenotype (CD4+CD25high+) in patients with UC was compromised compared with healthy controls. Additionally, at baseline, the expression frequency of the CD4+CD25high+ Treg in the subgroup who did not respond to GMA was about half the level in the GMA responder subgroup. Even the non-regulatory naive CD4+CD25− phenotype, which is known to induce colitis [29], was significantly lower in the refractory UC subgroup compared with the responder subgroup or the HC group. This observation, while interesting, may not necessarily mean a definite lack of response to GMA, because a few responders also fell into this low CD4+CD25high+ Treg range. However, what is unequivocally clear is that patients with refractory UC who subsequently need to undergo colectomy have a compromised expression of circulating CD4+CD25high+ Treg and that GMA impacts this phenotype in GMA responders. However, at present this is of academic importance until an association between the clinical efficacy of GMA and its contribution to an increased CD4+CD25high+ Treg has been established firmly by additional studies.
We were equally interested to see if GMA also impacts the mucosal levels of the CD4+FoxP3+ Treg phenotype. Immunohistological investigations on the mucosal tissues sampled during active UC and after the UC had improved to a quiescent condition revealed intriguing information on the migrated Treg (see Results section and Fig. 4). Our data indicated that during active UC, the number of Treg in the rectal mucosa had increased while the level in the peripheral circulation had decreased significantly. This change in Treg expression between peripheral blood and rectal mucosa might suggest a net influx of these cells from the peripheral circulation into the inflammatory lesions in the rectal mucosa. It could be assumed that the Treg mechanism in patients with UC is set to send CD4+ Treg from the peripheral pool to the colonic mucosa in an attempt to suppress inflammation. Accordingly, the expression of CD4+CD25high+ Treg during active UC might indicate the circulating level of the Treg reservoir, and GMA appears to up-regulate the CD4+CD25high+ Treg during an hour of extracorporeal procedure.
In conclusion, there is solid evidence that the CD4+CD25high+/FoxP3+ Treg play an essential role in the control of immune pathology in patients with IBD. In other words, compromised Treg is seen in patients with refractory UC. GMA can impact the circulating as well as the mucosal levels of Treg phenotype. Further studies are warranted to establish an association between GMA-dependent recovery of migrating CD4+CD25high+/ FoxP3 phenotype and its clinical efficacy in patients with UC.
Disclosure
This study was equally supported by Grants-in-Aid for the Research Committee of Inflammatory Bowel Disease, Research on Intractable Diseases, the Health and Labour Sciences Research Grants from Ministry of Health, Labor and Welfare and Grant-in-Aid for Graduate Students, Hyogo College of Medicine.
References
- 1.Allison MC, Dhillon AP, Lewis WG. Inflammatory bowel disease. London: Mosby; 1998. pp. 9–95. [Google Scholar]
- 2.Gismera CS, Aladrén BS. Inflammatory bowel disease: a disease(s) of modern times? Is incidence still increasing? World J Gastroenterol. 2008;14:5491–98. doi: 10.3748/wjg.14.5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanai K, Watanabe M. Clinical application of human CD4+CD25+ regulatory T cells for the treatment of inflammatory bowel disease. Expert Opin Biol Ther. 2005;5:451–62. doi: 10.1517/14712598.5.4.451. [DOI] [PubMed] [Google Scholar]
- 4.Markovic-Plese S, Cortese I, Wandinger KP, et al. CD4+CD28- costimulation-independent T cells in multiple sclerosis. J Clin Invest. 2001;108:1185–94. doi: 10.1172/JCI12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gershon RK. A disquisition on suppressor T cells. Transplant Rev. 1975;26:170–85. doi: 10.1111/j.1600-065x.1975.tb00179.x. [DOI] [PubMed] [Google Scholar]
- 6.Sakaguchi S, Sakaguchi N, Shimizu J, et al. Immunologic tolerance maintained by CD25+CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 7.Curotto de Lafaille MA, Lafaille JJ. CD4+ regulatory T cells in autoimmunity and allergy. Curr Opin Immunol. 2002;14:771–8. doi: 10.1016/s0952-7915(02)00408-9. [DOI] [PubMed] [Google Scholar]
- 8.Annacker O, Pimenta-Araujo R, Burlen-Defranoux O, et al. CD25+CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J Immunol. 2001;166:3008–18. doi: 10.4049/jimmunol.166.5.3008. [DOI] [PubMed] [Google Scholar]
- 9.Yokoyama Y, Fukunaga K, Fukuda Y, et al. Demonstration of low CD25High+CD4+ and high CD28−CD4+ T-cell subsets in patients with ulcerative colitis: modified by selective leucocytapheresis. Dig Dis Sci. 2007;52:2725–31. doi: 10.1007/s10620-006-9560-z. [DOI] [PubMed] [Google Scholar]
- 10.Saniabadi AR, Hanai H, Bjarnason I, et al. Adacolumn, an adsorptive carrier based granulocyte and monocyte apheresis device for the treatment of inflammatory and refractory diseases associated with leukocytes. Ther Apheres Dial. 2003;7:48–59. doi: 10.1046/j.1526-0968.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 11.Saniabadi AR, Hanai H, Fukunaga K, et al. Therapeutic leucocytapheresis for inflammatory bowel disease. Transf Apher Sci. 2007;37:191–200. doi: 10.1016/j.transci.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Muratov V, Saniabadi A, Lofberg R, et al. Downregulation of interferon-γ parallels clinical response to selective leukocyte apheresis in patients with inflammatory bowel disease. A 12-month follow-up study. Int J Colorect Dis. 2006;21:493–504. doi: 10.1007/s00384-005-0069-2. [DOI] [PubMed] [Google Scholar]
- 13.Hanai H. Position of selective leucocytapheresis in the medical therapy of ulcerative colitis. World J Gastroenterol. 2006;12:7568–77. doi: 10.3748/wjg.v12.i47.7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto T, Saniabadi AR, Umegae S, et al. Impact of selective leukocytapheresis on mucosal inflammation and ulcerative colitis: cytokine profiles and endoscopic findings. Inflamm Bowel Dis. 2006;12:719–26. doi: 10.1097/00054725-200608000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Hanai H, Watanabe F, Yamada M, et al. Correlation of serum soluble TNF-alpha receptors I and II levels with disease activity in patients with ulcerative colitis. Am J Gastroenterol. 2004;99:1532–8. doi: 10.1111/j.1572-0241.2004.30432.x. [DOI] [PubMed] [Google Scholar]
- 16.Lichtiger S, Present DH, Kornbluth A. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med. 1994;330:1841–5. doi: 10.1056/NEJM199406303302601. [DOI] [PubMed] [Google Scholar]
- 17.Shimoyama T, Sawada K, Hiwatashi N, et al. Safety and efficacy of granulocytes and monocyte adsorption apheresis in patients with active ulcerative colitis: a multicenter study. J Clin Apher. 2001;16:1–9. doi: 10.1002/jca.1000. [DOI] [PubMed] [Google Scholar]
- 18.Hiraishi K, Takeda Y, Shiobara N, et al. Studies on the mechanisms of leukocyte adhesion to cellulose acetate beads: an in vitro model to assess the efficacy of cellulose acetate carrier-based granulocyte and monocyte adsorptive apheresis. Ther Apher Dial. 2003;7:334–40. doi: 10.1046/j.1526-0968.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- 19.Rachmilewitz D on behalf of an International Study Group. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomized trial. BMJ. 1989;298:82–6. doi: 10.1136/bmj.298.6666.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Powrie F, Read S, Mottet C, et al. Control of immune pathology by regulatory T cells. Novartis Found Symp. 2003;252:92–8. [PubMed] [Google Scholar]
- 21.Liu H, Hu B, Xu D, et al. CD4+CD25+ regulatory T cells cure murine colitis: the role of IL-10, TGF-beta, and CTLA4. J Immunol. 2003;171:5012–17. doi: 10.4049/jimmunol.171.10.5012. [DOI] [PubMed] [Google Scholar]
- 22.Sitohy B, Hammarström S, Danielsson A, et al. Basal lymphoid aggregates in ulcerative colitis colon: a site for regulatory T cell action. Clin Exp Immunol. 2008;151:326–33. doi: 10.1111/j.1365-2249.2007.03566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fahlen L, Read S, Gorelik L, et al. T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2005;201:737–46. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh B, Read S, Asseman C, et al. Control of intestinal inflammation by regulatory T cells. Immunol Rev. 2001;182:190–200. doi: 10.1034/j.1600-065x.2001.1820115.x. [DOI] [PubMed] [Google Scholar]
- 25.Maul J, Loddenkemper C, Mundt P, et al. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–78. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 26.Baecher-Allan C, Brown JA, Freeman GJ, et al. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 27.Huber S, Schramm C, Lehr HA, et al. Cutting edge: TGF-beta signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells. J Immunol. 2004;173:6526–31. doi: 10.4049/jimmunol.173.11.6526. [DOI] [PubMed] [Google Scholar]
- 28.Melgar S, Yeung MM, Bas A, et al. Over-expression of interleukin 10 in mucosal T cells of patients with active ulcerative colitis. Clin Exp Immunol. 2003;134:127–37. doi: 10.1046/j.1365-2249.2003.02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piccirillo CA, Shevach EM. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J Immunol. 2001;167:1137–40. doi: 10.4049/jimmunol.167.3.1137. [DOI] [PubMed] [Google Scholar]


